Abstract
A reduced response to progesterone in the eutopic endometrium with endometriosis and in endometriotic tissues is considered to be the underlying factor for endometriosis. CD10 is known to be expressed by endometrial and endometriotic stromal cells and may be induced by progestins, although the function of CD10 is not fully revealed in endometrial or endometriotic tissues. In the current study, the expression of CD10 was significantly increased by treatment of the cells with progesterone, 17β-estradiol, and dibutyryl cyclic adenosine monophosphate (cAMP) in the endometrial stromal cells. On the other hand, the expression of CD10 following treatment with progesterone, 17β-estradiol, and dibutyryl cAMP was not significantly increased in endometriotic stromal cells. The adhesion assay for endometrial and endometriotic stromal cells to hyaluronan using 5- or 6-(N-succinimidyloxycarbonyl)-fluorescein 3', 6'-diacetate–labeled cells demonstrated that the CD44-dependent adhesion of stromal cells was inhibited by CD10. As far as the induction of CD10 is concerned, the effect of progesterone was different between endometrial stromal cells and endometriotic stromal cells. CD10 might be involved in the development of endometriosis due to its influence on CD44-dependent cell adhesion.
Keywords: adhesion, CD10, CD44, endometriosis, stromal cells.
Introduction
Endometriosis is a common gynecological disease, which affects 0.5% to 5% of fertile females and 25% to 40% of infertile females of reproductive age.1 It is an enigmatic disease that deteriorates the health of patients by causing pain, infertility, and ovarian cysts. Endometriosis is clinically defined by the presence of visible endometrium-like tissue outside the uterus, and the implantation of endometrial tissues in retrograde menstrual flux is widely accepted as an etiology of the disease.2 ,3 The pathogenesis of endometriosis is not fully understood, although decades of research have revealed the involvement of estrogen dependency, inflammatory mediators, and the immune response in the development of endometriosis and its symptoms.4–6
Progestins have been used for endometriosis, especially for endometriosis-related pain, as have gonadotropin-releasing hormone agonists. However, the development of therapeutic agents for endometriosis has been limited due to the unclear pathogenesis of the disease.7 Recently, molecular evidence has emerged, indicating that the cellular responses to progesterone differ among eutopic endometrium without endometriosis, eutopic endometrium with endometriosis, and endometriotic tissues.5,8,9 Progesterone resistance, which describes a reduced response to progesterone in the eutopic endometrium with endometriosis and in endometriotic tissues, is considered to be the underlying factor for endometriosis.10 Dysregulation of coactivators or the expression of the progesterone receptor and progesterone-induced molecules may be involved in the pathogenesis of endometriosis.11–15 Therefore, progesterone resistance-related molecules might be implicated as possible molecular targets for the treatment of endometriosis.
Neutral endopeptidase 24.11 (CD10, EC 3.4.24.11) is a cell surface metallopeptidase expressed by various tissues that consists of an N-terminal short cytoplasmic domain and a large extracellular catalytic domain. CD10 has been demonstrated to play important roles in the proliferation and adhesion of cells.16–18 In the human endometrium, CD10 regulates the local function by inducing the catalytic inactivation of bioactive peptides, such as endothelin 1.19 CD10 is also known to be expressed by endometrial and endometriotic stromal cells20 and is upregulated by progestins during decidualization. On the other hand, the function of CD10 in endometriotic tissues has not been explored.
CD44 is another membrane protein expressed by human endometrial cells, and it has been shown to promote the adhesion of endometrial cells.21 ,22 In other types of cells, CD44 and CD10 have been demonstrated to bind competitively to ezrin/radixin/moesin (ERM) proteins, linker proteins of the cytoskeleton, and membrane proteins. Therefore, in both human endometrial cells and endometriotic cells, the expression of CD10 induced by progesterone might affect the cell function by altering the CD44-mediated cell adhesion.
In the present study, we demonstrate that progestins induce the expression of CD10 in stromal cells of the eutopic endometrium but not in endometriotic tissues and that CD10 inhibits the CD44-mediated adhesion of stromal cells to hyaluronan. CD10 may be involved in the pathogenesis of endometriosis as a key molecule that responds to progesterone stimulation.
Materials and Methods
Primary Culture of Endometrial Stromal Cells and Endometriotic Cyst Stromal Cells and Decidualization
The stromal cells were taken from the eutopic endometrium of patients without endometriosis (ESCs, n = 16, pt 1-16), and the eutopic endometrium (eESCs) and the chocolate cyst linings of the ovaries (CSCs) of patients with endometriosis (pt e1-e12) who had undergone surgery at Nagoya University Hospital as described previously.19 In brief, the endometriotic cysts and endometrial tissue were minced into small pieces (∼1 mm3), and these pieces were filtered through a cell strainer consisting of a 100-μm pore size nylon mesh (Becton Dickinson, Franklin Lakes, NJ) to remove the blood cells. Then, the minced tissue was incubated by stirring at 37°C for 20 minutes in phosphate-buffered saline (PBS) and 0.5% collagenase (Wako, Osaka, Japan). The tissue digest was vigorously pipetted and passed over a cell strainer consisting of 70-μm pore size nylon mesh (Becton Dickinson). The ESCs, eESCs, and CSCs collected from the lower receptacle were suspended and plated onto 60-mm sterile dishes. The ESCs, eESCs, and CSCs were cultured in Dulbecco-modified Eagle medium (DMEM; Sigma, St Louis, Missouri) containing 10% fetal bovine serum (FBS; Sigma), 100 IU/mL penicillin, and 100 μg/mL streptomycin. The purity of the ESCs, eESCs, and CSCs was assessed by a morphological determination using light microscopy. Each cell population was routinely 98% pure, as assessed by phase contrast microscopy. The ESCs, eESCs, and CSCs were cultured with progesterone (10−6 mol/L; Sigma), 17β-estradiol (10-8mol/L; Sigma), and dibutyryl cyclic adenosine monophosphate (cAMP; 1 mmol/L; Sigma) for 2 days (Western blotting), 4 days (Western blotting and cell adhesion assay), and 4 to 6 days (prolactin measurement) at 37°C in a humidified atmosphere of 5% CO2 in air to induce in vitro decidualization. In vitro decidualization was assessed by evaluating any morphological changes. The levels of prolactin in the supernatants of endometrial and endometriotic stromal cells were determined using a chemiluminescence immunoassay (Architect prolactin; Abbott Laboratories, North Chicago, IL). This study was approved by the ethics committee of Nagoya University Graduate School of Medicine.
Western Blotting Analysis
Cultured ESCs, eESCs, and CSCs with or without decidualization within the 5 passages were lysed in a radioimmunoprecipitation buffer (10 mmol/L Tris-HCl, pH 7.4, 150 mmol/L NaCl, 1% Nonidet P-40, 5 mmol/L EDTA, 1% sodium deoxycholate, 0.1% sodium dodecyl sulfate [SDS], 1.2% aprotinin, 5 μmol/L leupeptine, 4 μmol/L antipain, 1 mmol/L phenylmethylsulfonylfluoride, and 0.1 mmol/L Na3VO4). The cell lysates were clarified by centrifugation at 13 000g at 4°C for 15 minutes, diluted in 2× sample buffer (125 mmol/L Tris-HCl, pH 6.8, 4% SDS, 10% glycerol, 0.2% bromphenol blue, and 4% 2-mercaptoethanol), resolved by 10% SDS-polyacrylamide gel electrophoresis, and immunoblotted with an anti-CD10 antibody (Ab; NCL; NovocastraLaboratories Ltd, Newcastle, United Kingdom; 1:100), anti-CD44s Ab (Ab-4; Thermo Fisher Scientific, Waltham, Massachusetts; 1:200), or anti-β-actin Ab (Santa Cruz Biotechnology, Inc, Santa Cruz, California). The relative band density normalized to β-actin was determined from light scans of the resulting films using a densitometric analysis software program.
Small Interfering RNA
A small interfering RNA (siRNA) for CD10 was purchased from Santa Cruz Biotechnology. The ESCs, eESCs, and CSCs with or without decidualization were transfected with the siRNA for CD10 or negative control siRNA (AF 488) at a final concentration of 20 nmol/L using Lipofectamine RNAiMax (Life Technologies/Invirtogen, Carlsbad, California), according to the manufacturer’s instructions.
5- or 6-(N-Succinimidyloxycarbonyl)-Fluorescein 3', 6'-Diacetate Labeling and Adhesion Assay
The ESCs, eESCs, and CSCs were harvested, washed with PBS, and labeled with 5- or 6-(N-Succinimidyloxycarbonyl)-fluorescein 3', 6'-diacetate (CFSE, according to the manufacturer’s protocol (Vybrant CFDA SE Cell Trace Kit, Life Technologies/Molecular Probes).23 Briefly, the target cell suspensions were resuspended at 2 × 105 cells/100 μL and labeled with 1 μL of 1 mmol/L CFSE for 30 minutes at 37°C with rotation. After 2 washes, the CFSE-labeled cells were resuspended in DMEM for an adhesion assay.
An assay of the adhesion of the cells to hyaluronic acid was performed as described previously,24 with some modifications. In brief, flat bottom 96-well plates were coated with 100 μL of hyaluronic acid (1 mg/mL in PBS) with or without 5 μL of 1.0 mg/mL CD44-neutralizing Ab (BU75, Ancell Corporation, Bayport, Minnesota) for 1 hour at 37°C, followed by rinsing with PBS. The CFSE-labeled cells were seeded at 10 000 cells per well, and the fluorescent intensity was measured immediately (F0) at an excitation wavelength of 485 nm and an emission wavelength of 527 nm with a microplate fluorometer (Fluoroskan Ascent CF, Thermo-Labsystems, Finland). Nonadherent cells were removed by gentle washing with PBS after 2 hours, and the fluorescent intensity was measured (F2). The percentage of adherent cells (F2/F0 × 100) was calculated.
Statistical Analysis
One-way repeated-measure analysis of variance (ANOVA) with Dunnett posttest was used to determine the differences in the densitometric analysis of the results of the Western blotting analysis. One-way ANOVA with the Holm-Sidak test was used to analyze the differences in the concentrations of prolactin. The statistical analyses were performed using the SigmaPlot software program (Systat Software, Inc, San Jose, California).
Results
The CSCs Demonstrated Reduced Induction of CD10 and Prolactin Secretion by Progesterone
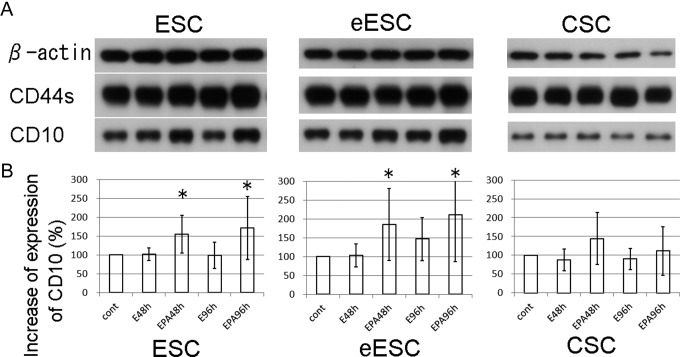
Endometrial stromal cells and endometriotic stromal cells undergo decidualization in response to sex steroid hormones. CD10 is a well-known marker of decidualization as are prolactin and insulin-like–growth factor binding protein 1. We investigated the expression of CD10 and CD44 in ESCs (n = 16), eESCs (n =12), and CSCs (n = 12) by immunoblotting. Figure 1A shows representative images of the Western blotting analysis. We performed a densitometric analysis to assess the expression of CD10 quantitatively (Figure 1B). We found that the expression of CD10 was significantly increased by progesterone, 17β-estradiol, and dibutyryl cAMP but not 17β-estradiol alone in ESCs and eESCs. On the contrary, the expression of CD10 after exposure of the CSCs to progesterone, 17β-estradiol, and dibutyryl cAMP was not increased significantly. CD44 did not show any change in expression following the exposure of cells to progesterone, 17β-estradiol, or dibutyryl cAMP.
Figure 1.
A, Representative images of immunoblotting against β-actin, CD44s, and CD10 expressed by ESCs, eESCs and CSCs. B, The results of the densitometric analysis of the expression of CD10. CD10 was significantly induced by stimulation of cells with progesterone, 17β-estradiol, and dibutyryl cAMP for 48 hours and 96 hours (EPA48 h and EPA96 h) in ESCs and eESCs, but not in CSCs, compared with control (cont) cells. The stimulation with 17β-estradiol for 48 hours and 96 hours (E48 h and E96 h) did not lead to a significant increase in CD10. The boxes and bars represent the means and SD of 12 and 16 samples of ESC and eESC/CSC, respectively. Asterisks show a significant increase compared with each cont (P < .05). ESCs indicates the stromal cells taken from the eutopic endometrium of patients without endometriosis; eESCs, the stromal cells taken from the eutopic endometrium of patients with endometriosis; CSCs, the stromal cells taken from the chocolate cyst linings of the ovaries of patients with endometriosis ; cAMP, cyclic adenosine monophosphate; SD, standard deviation.
We next assayed the concentration of prolactin in the media from 2 cultured cell lines of each type of stromal cells. The concentrations of prolactin were significantly decreased in the culture media of CSCs compared to that of eESCs (96 hours) and of eESCs and ESCs (144 hours; Figure 2).
Figure 2.

The secretion of prolactin by ESCs, eESCs, and CSCs. The concentrations of prolactin in the culture media were significantly increased by stimulation with progesterone, 17β-estradiol, and dibutyryl cAMP for 96 hours in eESCs and for 144 hours in ESCs and eESCs, compared to CSCs (*P < .05). Bars represent the SEM. ESCs indicates the stromal cells taken from the eutopic endometrium of patients without endometriosis; eESCs, the stromal cells taken from the eutopic endometrium of patients with endometriosis ; CSCs, the stromal cells taken from the chocolate cyst linings of the ovaries of patients with endometriosis ; cAMP, cyclic adenosine monophosphate; SEM, standard error of the mean.
CD10 Inhibits the CD44-Dependent Adhesion of Stromal Cells to Hyaluronic Acid
To investigate the effects of CD10 on cell adhesion, we suppressed the expression of CD10 induced by progesterone using siRNA. As shown in Figure 3A, the expression of CD10 was suppressed by the siRNA, while the expression of CD44 did not show any significant changes.
Figure 3.
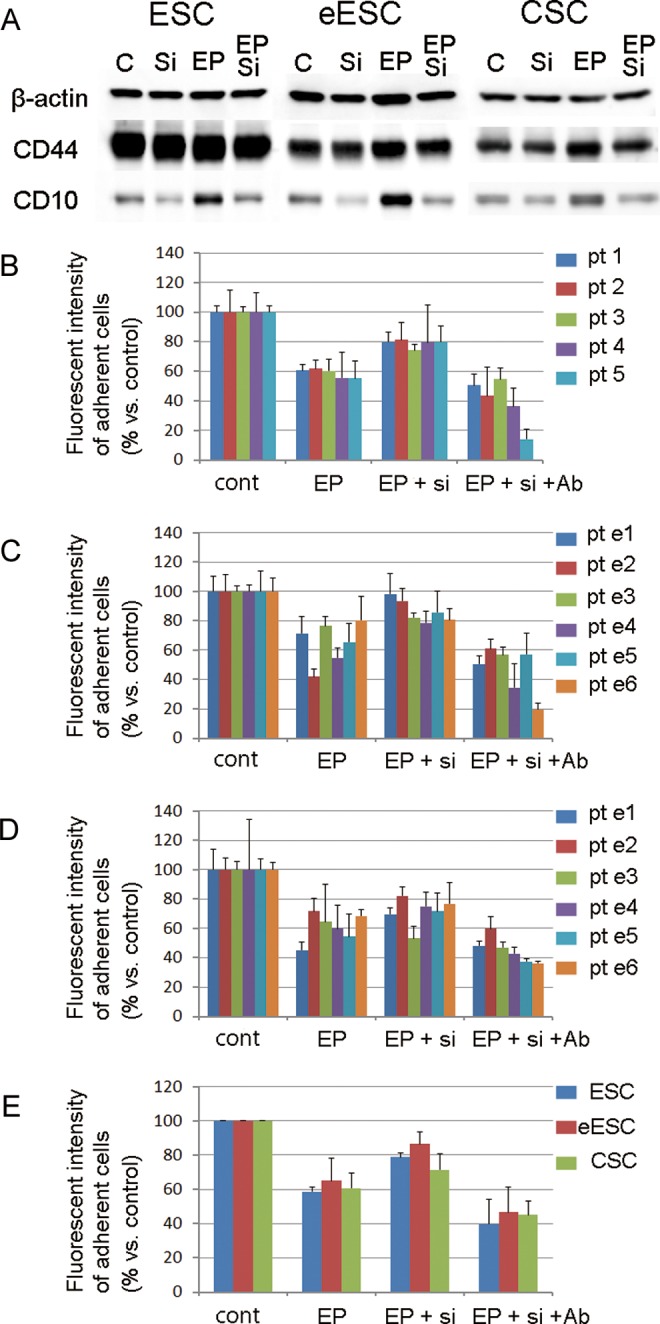
A, Representative images of immunoblotting against β-actin, CD44s, and CD10 expressed by ESCs, eESCs, and CSCs. The expression of CD10 was suppressed by the siRNA. B-D, Adhesion assays using ESCs (B), eESCs (C) and CSCs (D) to hyaluronan. Each ESC from patients 1 to 5 (pt 1-5), and each eESC and CSC from the patients with endometriosis (pt e1-e5) showed decreased adhesion following treatment with progesterone, 17β-estradiol, and dibutyryl cAMP (EP), which was partially recovered by the suppression of CD10 expression using siRNA (EP+si). A neutralizing antibody targeting CD44 attenuated the adhesion of cells (EP+si+Ab). E, A comparison of the adhesion rates in ESCs, eESCs, and CSCs. ESCs indicates the stromal cells taken from the eutopic endometrium of patients without endometriosis; eESCs, the stromal cells taken from the eutopic endometrium of patients with endometriosis ; CSCs, the stromal cells taken from the chocolate cyst linings of the ovaries of patients with endometriosis ; cAMP, cyclic adenosine monophosphate; siRNA, small interfering RNA.
The adhesion of ESCs, eESCs, and CSCs to hyaluronic acid, one of the extracellular matrix molecules, was evaluated by an in vitro cell adhesion assay using CFSE. Decidualization treatment using progesterone, 17β-estradiol, and dibutyryl cAMP inhibited the adhesion of all types of stromal cells to approximately 60% of the level of controls (Figures 3B-E). The cell adhesion to hyaluronic acid was partially recovered in the ESCs, eESCs, and CSCs in which the CD10 expression was suppressed using siRNA. A neutralizing Ab targeting CD44 attenuated the cell adhesion capacity to approximately 40%. These results showed that the CD10 induced by progesterone inhibited the CD44-dependent adhesion of endometrial and endometriotic stromal cells.
Discussion
Decidualization is induced by progestins and is characterized by morphological and functional changes in endometrial stromal cells.25 CD10 is one of the frequently used markers of decidualization, in addition to prolactin and insulin-like growth factor–binding protein 1. However, the function of CD10 has not been investigated in association with the pathogenesis of endometriosis nor has its progestin-inducible expression been fully examined. However, the transcriptional regulation of the CD10 gene has been well explored using prostate cancer cell lines. Two androgen-responsive elements (ARE1 and ARE2) exist in the promoter region of the CD10 gene, and their activities have been reported to be increased 3.6-fold (ARE1) and 5-fold (ARE2) by androgen, 4.2-fold (ARE1) and 8.2-fold (ARE2) by dexamethasone, and 3-fold (ARE1) and 4.1-fold (ARE2) by progesterone, respectively.26 We confirmed that androgens also upregulated CD10 in the endometrial and ESCs (data not shown). Taken together, these findings suggest that androgen-responsive elements might be involved in the induction of CD10 by progestins in the endometrial and endometriotic stromal cells.
We herein demonstrated that there was reduced induction of CD10 by progesterone in ESCs in comparison with eESCs. The decidualization of endometrial stromal cells is an example of the mesenchymal–epithelial transition (MET), which is a biological process that bestows an epitheloid phenotype on mesenchymal cells with a spindle shape. The endometrial stromal cells that undergo decidualization become dependent on continuous progesterone signaling. We simultaneously added cAMP to increase the decidualization. The cAMP has been used to induce decidualization by itself, or in combination with progesterone, due to its bypass of progesterone signaling. Klemmt et al have shown that stromal cells derived from different endometriotic lesions exhibited a decreased capacity for decidualization, which suggests that the signaling cascade leading to decidualization might be impaired.27 In the current study, we found that the concentration of prolactin, a marker of decidualization, was significantly decreased in the endometriotic stromal cells compared to the endometrial stromal cells. Therefore, the decrease in or disappearance of the induction of CD10 expression might be attributed not only to the decrease in progesterone responsiveness but also to a decrease in the decidualization capacity in endometriotic stromal cells. All things considered, the CD10 in endometriotic stromal cells, which might be relevant to the decreased capacity for decidualization, may be involved in the pathogenesis of endometriosis.
Decidualization affects cell characteristics, such as their capacity for adhesion and migration and their resistance to apoptosis and oxidative stress,28–31 which are possibly involved in the formation of ectopic endometrial implants due to retrograde menstrual flux.27 CD10, a well-known marker of decidualization, has not been fully characterized with regard to its function in endometrial and endometriotic stromal cells. We found that the expression of CD10 influences the CD44-mediated adhesion of endometrial and endometriotic stromal cells to hyaluronan. It is also known that the interaction between hyaluronan and its receptor, CD44, plays an important role in various physiological and pathological processes, such as lymphocyte homing and cancer cell metastasis.32 Peritoneal cells secrete hyaluronan, and, therefore, the attachment of endometrial cells expressing CD44 to peritoneal mesothelial cells might be an initial step in the development of endometriosis following retrograde menstruation.21 Taken together, our results suggest the possible involvement of CD10, which is not significantly induced by progesterone in endometriotic stromal cells, in the pathogenesis of endometriosis due to its regulation of cell adhesion.
We investigated the cell adhesion using ESCs from 5 patients and eESCs and CSCs from 6 patients and demonstrated that there was a similar tendency, regardless of the cell types; that is the decidualization treatment impaired cell adhesion to hyaluronan without significant differences among ESCs, eESCs, and CSCs. The recovery of cell adhesion using CD10 siRNA was up to 30% in the all types of stromal cells. In addition, the decrease in or disappearance of the induction of CD10 by progesterone did not cause any significant difference in the CD10-meidated suppression of cell adhesion. These results suggest that CD10 suppresses the CD44-mediated cell adhesion only partially and that other cell adhesion molecules, which are not affected by CD10, might be involved in cell adhesion.
CD44 can promote cell adhesion, migration, and invasiveness by binding to and localizing with hyaluronan and matrix metalloproteinases at the cell surface33 ,34 and by interacting with ERM proteins that are linker proteins to the cytoskeleton.35 ,36 CD10 has a positively charged amino acid cluster, similar to a previously identified region located in the intracellular portion of CD44, which serves as the binding domain to ERM proteins.24 The overlap of the binding sites in CD10 and CD44 provides the structural basis for the suppression of cell adhesion through the interaction between CD10 and ERM proteins.37 Further studies are needed to investigate the possible competitive binding of ERM proteins to CD10 and CD44 in endometrial and endometriotic stromal cells.
Recent studies have demonstrated that there are different characteristics between the eutopic endometrial cells of patients with endometriosis and those of patients without endometriosis. These differences, such as reduced progesterone responsiveness,8 ,13 increased resistance to apoptosis,38 and increased cell adhesion capacity,39 are considered to increase the survival of endometriotic tissues developed as a result of retrograde menstrual flux. In the current study, as far as the induction of CD10 by progesterone is concerned, we did not find any differences between the endometrial stromal cells associated with endometriosis and those not associated with endometriosis, although we demonstrated that there was a significant difference between endometrial stromal cells and endometriotic stromal cells. We speculate that the induction of CD10 by progesterone might be decreased or may completely disappear during the development of endometriotic lesions.
In the present study, we demonstrated that CD10 inhibits CD44-mediated adhesion of endometrial and endometriotic stromal cells and that the progesterone-inducible CD10 expression is decreased in endometriotic stromal cells compared to endometrial stromal cells. CD10 possesses antiproliferative functions through both kinase-dependent and kinase-independent actions40 in addition to its influence on cell adhesion. Moreover, it has been reported that dexamethasone stimulates the promoter activity of the CD10 gene through androgen-responsive elements similar to progesterone and androgen26 which have been used as therapeutic agents for endometriosis. The induction of CD10 and the CD10 by itself might be promising molecular targets for the development of novel treatments for endometriosis.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by Grant-in-Aid for Scientific Research 20591912 (A.I).
References
- 1. Ozkan S, Murk W, Arici A. Endometriosis and infertility: epidemiology and evidence-based treatments. Ann N Y Acad Sci. 2008;1127:92–100. [DOI] [PubMed] [Google Scholar]
- 2. de Ziegler D, Borghese B, Chapron C. Endometriosis and infertility: pathophysiology and management. Lancet. 2010;376(9742):730–738. [DOI] [PubMed] [Google Scholar]
- 3. Sampson JA. Metastatic or Embolic Endometriosis, due to the Menstrual Dissemination of Endometrial Tissue into the Venous Circulation. Am J Pathol. 1927;3(2):93–110.43. [PMC free article] [PubMed] [Google Scholar]
- 4. Agic A, Xu H, Finas D, Banz C, Diedrich K, Hornung D. Is endometriosis associated with systemic subclinical inflammation? Gynecol Obstet Invest. 2006;62(3):139–147. [DOI] [PubMed] [Google Scholar]
- 5. Bulun SE. Endometriosis. N Engl J Med. 2009;360(3):268–279. [DOI] [PubMed] [Google Scholar]
- 6. Osuga Y. Novel therapeutic strategies for endometriosis: a pathophysiological perspective. Gynecol Obstet Invest. 2008;66(suppl 1):3–9. [DOI] [PubMed] [Google Scholar]
- 7. Kennedy S, Bergqvist A, Chapron C, et al. ESHRE guideline for the diagnosis and treatment of endometriosis. Hum Reprod. 2005;20(10):2698–2704. [DOI] [PubMed] [Google Scholar]
- 8. Aghajanova L, Velarde MC, Giudice LC. Altered gene expression profiling in endometrium: evidence for progesterone resistance. Semin Reprod Med. 2010;28(1):51–58. [DOI] [PubMed] [Google Scholar]
- 9. Bulun SE, Cheng YH, Yin P, et al. Progesterone resistance in endometriosis: link to failure to metabolize estradiol. Mol Cell Endocrinol. 2006;248(1-2):94–103. [DOI] [PubMed] [Google Scholar]
- 10. Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364(9447):1789–1799. [DOI] [PubMed] [Google Scholar]
- 11. Aghajanova L, Velarde MC, Giudice LC. The progesterone receptor coactivator Hic-5 is involved in the pathophysiology of endometriosis. Endocrinology. 2009;150(8):3863–3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Attia GR, Zeitoun K, Edwards D, Johns A, Carr BR, Bulun SE. Progesterone receptor isoform A but not B is expressed in endometriosis. J Clin Endocrinol Metab. 2000;85(8):2897–2902. [DOI] [PubMed] [Google Scholar]
- 13. Burney RO, Talbi S, Hamilton AE, et al. Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology. 2007;148(8):3814–3826. [DOI] [PubMed] [Google Scholar]
- 14. Igarashi TM, Bruner-Tran KL, Yeaman GR, et al. Reduced expression of progesterone receptor-B in the endometrium of women with endometriosis and in cocultures of endometrial cells exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Fertil Steril. 2005;84(1):67–74. [DOI] [PubMed] [Google Scholar]
- 15. Taylor HS, Bagot C, Kardana A, Olive D, Arici A. HOX gene expression is altered in the endometrium of women with endometriosis. Hum Reprod. 1999;14(5):1328–1331. [DOI] [PubMed] [Google Scholar]
- 16. Papandreou CN, Usmani B, Geng Y, et al. Neutral endopeptidase 24.11 loss in metastatic human prostate cancer contributes to androgen-independent progression. Nat Med. 1998;4(1):50–57. [DOI] [PubMed] [Google Scholar]
- 17. Sumitomo M, Iwase A, Zheng R, et al. Synergy in tumor suppression by direct interaction of neutral endopeptidase with PTEN. Cancer Cell. 2004;5(1):67–78. [DOI] [PubMed] [Google Scholar]
- 18. Zheng R, Iwase A, Shen R, et al. Neuropeptide-stimulated cell migration in prostate cancer cells is mediated by RhoA kinase signaling and inhibited by neutral endopeptidase. Oncogene. 2006;25(44):5942–5952. [DOI] [PubMed] [Google Scholar]
- 19. Iwase A, Ando H, Nagasaka T, et al. Neutral endopeptidase expressed by decidualized stromal cells suppresses akt phosphorylation and deoxyribonucleic acid synthesis induced by endothelin-1 in human endometrium. Endocrinology. 2006;147(11):5153–5159. [DOI] [PubMed] [Google Scholar]
- 20. Potlog-Nahari C, Feldman AL, Stratton P, et al. CD10 immunohistochemical staining enhances the histological detection of endometriosis. Fertil Steril. 2004;82(1):86–92. [DOI] [PubMed] [Google Scholar]
- 21. Dechaud H, Witz CA, Montoya-Rodriguez IA, Degraffenreid LA, Schenken RS. Mesothelial cell-associated hyaluronic acid promotes adhesion of endometrial cells to mesothelium. Fertil Steril. 2001;76(5):1012–1018. [DOI] [PubMed] [Google Scholar]
- 22. Poncelet C, Leblanc M, Walker-Combrouze F, et al. Expression of cadherins and CD44 isoforms in human endometrium and peritoneal endometriosis. Acta Obstet Gynecol Scand. 2002;81(3):195–203. [DOI] [PubMed] [Google Scholar]
- 23. Obermajer N, Svajger U, Jeras M, Sattin S, Bernardi A, Anderluh M. An assay for functional dendritic cell-specific ICAM-3-grabbing nonintegrin (DC-SIGN) inhibitors of human dendritic cell adhesion. Anal Biochem. 2010;406(2):222–229. [DOI] [PubMed] [Google Scholar]
- 24. Iwase A, Shen R, Navarro D, Nanus DM. Direct binding of neutral endopeptidase 24.11 to ezrin/radixin/moesin (ERM) proteins competes with the interaction of CD44 with ERM proteins. J Biol Chem. 2004;279(12):11898–11905. [DOI] [PubMed] [Google Scholar]
- 25. Takano M, Lu Z, Goto T, et al. Transcriptional cross talk between the forkhead transcription factor forkhead box O1A and the progesterone receptor coordinates cell cycle regulation and differentiation in human endometrial stromal cells. Mol Endocrinol. 2007;21(10):2334–2349. [DOI] [PubMed] [Google Scholar]
- 26. Zheng R, Shen R, Goodman OB, Jr, , Nanus DM. Multiple androgen response elements cooperate in androgen regulated activity of the type 1 neutral endopeptidase promoter. Mol Cell Endocrinol. 2006;259(1-2):10–21. [DOI] [PubMed] [Google Scholar]
- 27. Klemmt PA, Carver JG, Kennedy SH, Koninckx PR, Mardon HJ. Stromal cells from endometriotic lesions and endometrium from women with endometriosis have reduced decidualization capacity. Fertil Steril. 2006;85(3):564–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gellersen B, Reimann K, Samalecos A, Aupers S, Bamberger AM. Invasiveness of human endometrial stromal cells is promoted by decidualization and by trophoblast-derived signals. Hum Reprod. 2010;25(4):862–873. [DOI] [PubMed] [Google Scholar]
- 29. Labied S, Kajihara T, Madureira PA, et al. Progestins regulate the expression and activity of the forkhead transcription factor FOXO1 in differentiating human endometrium. Mol Endocrinol. 2006;20(1):35–44. [DOI] [PubMed] [Google Scholar]
- 30. Leitao B, Jones MC, Fusi L, et al. Silencing of the JNK pathway maintains progesterone receptor activity in decidualizing human endometrial stromal cells exposed to oxidative stress signals. FASEB J. 2010;24(5):1541–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Leitao BB, Jones MC, Brosens JJ. The SUMO E3-ligase PIAS1 couples reactive oxygen species-dependent JNK activation to oxidative cell death. FASEB J. 2011;25(10):3416–3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nagano O, Saya H. Mechanism and biological significance of CD44 cleavage. Cancer Sci. 2004;95(12):930–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Herrlich P, Morrison H, Sleeman J, et al. CD44 acts both as a growth- and invasiveness-promoting molecule and as a tumor-suppressing cofactor. Ann N Y Acad Sci. 2000;910:106–18; discussion 118-120. [DOI] [PubMed] [Google Scholar]
- 34. Isacke CM, Yarwood H. The hyaluronan receptor, CD44. Int J Biochem Cell Biol. 2002;34(7):718–721. [DOI] [PubMed] [Google Scholar]
- 35. Louvet-Vallee S. ERM proteins: from cellular architecture to cell signaling. Biol Cell. 2000;92(5):305–316. [DOI] [PubMed] [Google Scholar]
- 36. Yonemura S, Hirao M, Doi Y, Takahashi N, Kondo T, Tsukita S. Ezrin/radixin/moesin (ERM) proteins bind to a positively charged amino acid cluster in the juxta-membrane cytoplasmic domain of CD44, CD43, and ICAM-2. J Cell Biol. 1998;140(4):885–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Terawaki S, Maesaki R, Hakoshima T. Structural basis for NHERF recognition by ERM proteins. Structure. 2006;14(4):777–789. [DOI] [PubMed] [Google Scholar]
- 38. Gonzalez-Ramos R, Defrere S, Devoto L. Nuclear factor-kappaB: a main regulator of inflammation and cell survival in endometriosis pathophysiology. Fertil Steril. 2012;98(3):520–528. [DOI] [PubMed] [Google Scholar]
- 39. Adachi M, Nasu K, Tsuno A, Yuge A, Kawano Y, Narahara H. Attachment to extracellular matrices is enhanced in human endometriotic stromal cells: a possible mechanism underlying the pathogenesis of endometriosis. Eur J Obstet Gynecol Reprod Biol. 2011;155(1):85–88. [DOI] [PubMed] [Google Scholar]
- 40. Sumitomo M, Shen R, Nanus DM. Involvement of neutral endopeptidase in neoplastic progression. Biochim Biophys Acta. 2005;1751(1):52–59. [DOI] [PubMed] [Google Scholar]