Abstract
Pituitary adenylate cyclase-activating polypeptide (PACAP) and its receptors are expressed in the hypothalamus, the gonadotrope cells of the anterior pituitary gland, and the gonads, forming an autocrine–paracrine system in these tissues. Within the pituitary, PACAP functions either alone or synergistically with gonadotropin-releasing hormone (GnRH) to stimulate gonadotropin gene expression and secretion. Our goal was to define the hormonal regulation of pituitary PACAP and PACAP receptor (PAC1) gene expression by dihydrotestosterone (DHT), estradiol, and progesterone alone or in conjunction with GnRH. Treatment of adult male rat pituitary cell cultures with DHT or progesterone augmented GnRH-mediated increase in PACAP messenger RNA (mRNA) levels, but neither had an effect when present alone. Conversely, estradiol treatment blunted PACAP gene expression but did not alter GnRH effects on PACAP expression. Expression of PACAP receptor mRNA was decreased by GnRH treatment, minimally increased by DHT treatment, but not altered by the addition of estradiol or progesterone. DHT and GnRH together blunted PACAP receptor gene expression. Taken together, these results suggest that the activity of the intrapituitary PACAP-PAC1 system is regulated via the complex interaction of gonadal steroids and hypothalamic GnRH.
Keywords: anterior pituitary, PACAP, dihydrotestosterone, estradiol, progesterone, GnRH
Introduction
Sexual maturation and reproduction in mammals depend on the normal development and function of the hypothalamic–pituitary–gonadal axis. The hypothalamic decapeptide, gonado-tropin-releasing hormone (GnRH), stimulates the gonadotrope subpopulation of the anterior pituitary gland to synthesize and secrete the gonadotropins, luteinizing hormone (LH), and follicle-stimulating hormone (FSH). These gonadotropins then regulate the activity of the gonads to produce mature gametes and gonadal steroids, namely, estrogen, progesterone, and androgens. The gonadal steroids modulate gonadotropin gene expression by feedback actions at both the anterior pituitary and the hypothalamus.
Although gonadotropin biosynthesis and secretion are strongly regulated by GnRH,1 accumulating evidence indicates that the neuropeptide pituitary adenylate cyclase-activating polypeptide (PACAP) also modulates gonadotrope function, acting either alone or in conjunction with GnRH. The PACAP has been demonstrated to increase the α-subunit messenger RNA (mRNA) levels and to increase the length of LHβ transcript poly(A) tails and LH secretion in vivo in rats.2–5 In cultured primary rat pituitary cells, PACAP stimulates the transcription of follistatin by the folliculostellate cells, which in turn inhibits activin signaling leading to the repression of FSHβ gene expression.2 Pituitary PACAP transcript number has been shown to vary across the rat estrus cycle with peak levels occurring at 24 hours on proestrus, supporting the notion that locally derived PACAP may play an important role in modulating gonadotrope function.6,7
The PACAP was first isolated from sheep hypothalamic extracts and named for its ability to enhance cyclic adenosine monophosphate production by rat anterior pituitary cells.8 Since its discovery, numerous studies have investigated the structure, distribution, and developmental expression of PACAP and its receptors in a wide array of cell types and body systems, including the reproductive, neural, and gastrointestinal systems.7,9–11 Although initially isolated as a hypothalamic-releasing factor, PACAP and its receptors have been found more recently at all the levels of the hypothalamic–pituitary–gonadal axis as well as in the endometrium and placenta.12–14 The PACAP mRNA and protein have been detected in the germ cells of the rat and human testis as well as in the ovarian granulosa, theca-interstitial, and luteal cells.12,15 As PACAP receptors are also present in the gonads, PACAP appears to play a local role in regulating gametogenesis and steroidogenesis. In the anterior pituitary gland, PACAP expression has been localized to the gonadotropes and folliculostellate cells.16,17 The importance of PACAP to reproductive function is highlighted by reports that both PACAP and PACAP receptor (PAC1) null female mice have decreased fertility.18–20
Three distinct subtypes of PACAP receptors have been cloned. The PACAP receptors are G protein-coupled receptors that activate a number of signaling pathways, most importantly the cyclic AMP/protein kinase A signaling pathway.9 The PAC1 receptor binds PACAP with high affinity and the closely related peptide vasoactive intestinal peptide (VIP) with a much lower affinity. The vasoactive intestinal polypeptide receptor 1 (VPAC1) and receptor 2 (VPAC2) receptors bind PACAP and VIP with equal affinity. All anterior pituitary cells express at least one of the PACAP receptor subtypes, with PAC1 the most prevalent. Therefore, PACAP both acts on and is secreted by the pituitary cells, forming a functional autocrine–paracrine loop within this tissue.21,22
Prior studies have clearly demonstrated that gonadotropin gene expression is modulated by gonadal steroids in addition to the neuropeptides GnRH and PACAP. For example, androgens specifically increase FSHβ but not α-subunit or LHβ mRNA levels in both male and female rat pituitary cell cultures.23 Progesterone and estrogen have been shown to potentiate GnRH-induced increase in LHβ gene expression.24,25
The GnRH and gonadal steroids act via binding to unique receptors at the target cell. The GnRH binds to a specific G-protein-coupled membrane receptor, GnRH receptor (GnRHR), to activate the protein kinase C, protein kinase A, and calcium intracellular signaling pathways and thereby increase the gonadotropin gene expression.26–28 In contrast, gonadal steroids exert their effects primarily via association with corresponding nuclear receptors, the estrogen receptors (ERα or ERβ), progesterone receptors (PRA or PRB), and androgen receptor (AR).29,30 These activated nuclear receptors then bind to the promoter elements of their target genes directly or indirectly via tethering through other DNA-bound transcription factors.31 Although less well understood, estrogen and progesterone can also bind with high affinity to other cell components, including the cell membrane, to produce rapid effects on target cell activity.32,33
A limited number of studies have investigated the hormonal regulation of PACAP and PAC1 receptor gene expression. Outside the pituitary, estradiol has been reported to stimulate PACAP mRNA levels in the hypothalamic ventromedial and arcuate nuclei, and progesterone has been shown to increase PACAP and PAC1 mRNA levels in the rat hypothalamus and ovary.34–36 Gonadectomy leads to decreased PACAP levels in both brain and pituitary tissues of male and female Wistar rats.37 In addition, LH and FSH have been shown to induce PACAP transcript number in rat preovulatory follicles and in human granulosa–luteal cells obtained from patients undergoing in vitro fertilization.38,39
The regulation of pituitary PACAP and PAC1 expression by GnRH has been studied in the LβT2 gonadotrope cell line. Work in our laboratory demonstrated the ability of GnRH to increase PACAP promoter activity and mRNA expression via the PKC, PKA, and MAPK pathways.26 Purwana and colleagues subsequently confirmed the stimulatory effect of GnRH on PACAP transcript levels.40 This group also reported a GnRH-mediated increase in PACAP receptor mRNA levels, which we had not observed in our prior study. To our knowledge, the effect of GnRH on PACAP and PAC1 expression in primary pituitary cells has not been reported. Furthermore, nothing is known about the effects of gonadal steroids on pituitary PACAP expression, despite the fact that these steroids are known to play a pivotal role in feedback regulation of anterior pituitary function.
The overall goal of the studies reported here was to further define the hormonal factors that regulate PACAP and PAC1 receptor expression in the anterior pituitary gland. Specifically, we characterized the effects of dihydrotestosterone (DHT), estradiol, and progesterone alone or in combination with GnRH on PACAP and PAC1 mRNA levels in dispersed, cultured adult male rat anterior pituitary cells. Our findings suggest that, in addition to direct actions on the gonadotropin genes, GnRH and gonadal steroids may regulate gonadotropin expression indirectly via alterations in the local PACAP-PAC1 system.
Materials and Methods
Animals and Pituitary Tissue Collection
Male Sprague-Dawley rats were purchased from Charles River Breeding Laboratories (Wilmington, Massachusetts) at 57 to 70 days of age. Rats were housed in the University of Texas Southwestern Medical Center Animal Resource Center on a 14 light–10 dark cycle. Food and water were available ad libitum. After brief CO2 exposure, the animals were decapitated, and the anterior pituitaries were used for dispersion and culture as described below. All animal procedures were performed in accordance with the guidelines established by the UT Southwestern Institutional Animal Care and Use Committee.
Pituitary Dispersion and Culture
The anterior pituitaries were washed twice in Hanks balanced salts solution (HBSS) supplemented with 0.1% fetal bovine serum (FBS) and 15 mmol/L 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES). The gland was cut into 1 to 2 mm fragments inside a Petri dish using a sterile blade while immersing in HBSS washing medium. The tissue fragments were collected into a conical tube, washed in 10 mL HBSS washing medium, and gently centrifuged. The tissue pellet was suspended in a 50-mL flask containing 5 mL HBSS dissociation medium consisting of 0.3% trypsin, 2 μg/mL deoxyribonuclease (DNase), 15 mmol/L HEPES, 1.2 mmol/L EDTA, and 0.3% bovine serum albumin. The tissue and enzymatic mixture was incubated in a water bath at 30°C with gently shaking for 20 minutes. The tissue blocks were gently triturated approximately 20 times with a disposable Pasteur pipette. The cell suspension was transferred to a new conical tube containing an equal volume of Dulbecco-modified Eagle medium (DMEM; Invitrogen, Carlsbad, California), with high glucose and 10% FBS, 23 mmol/L HEPES, 1 mmol/L sodium pyruvate, and 1× penicillin/streptomycin (DMEM culture medium). The undissociated tissue was allowed to settle at the bottom of the flask, followed by further enzymatic dissociation and trituration as described above. The gland was fully dispersed after 3 dissociation periods. The cell suspensions obtained from each dissociation were combined and centrifuged at 1000 rpm for 5 minutes. The supernatant was discarded, and the cells were resuspended in DMEM culture medium.
The dispersed primary cells were counted and plated in 48-well tissue culture plates at a density of 2 50 000 cells/well. After overnight culture in DMEM culture medium, the cells were gently washed and cultured with phenol red-free Opti-MEM I (Invitrogen) for 2 hours. Next, the cells were treated with DHT (5α-androstan-17β-ol-3-one, A8380; Sigma-Aldrich Inc, Saint Louis, MO), estradiol (17β-estradiol, 3301; Calbiochem, EMD Millipore, Billerica, MA), or progesterone (P6149; Sigma-Aldrich), with or without GnRH analog (L4897; Sigma-Aldrich). The concentration and duration of each hormone treatment are indicated in the corresponding figure legend. Control cell cultures were treated with equal amount of vehicles (ethanol for steroids and H2O for GnRH). Duplicate cell wells were used for every treatment in each experiment, which was conducted a minimum of 3 times.
RNA Extraction and Reverse Transcription
Total RNA was prepared from primary pituitary cells using TRI Reagent (Ambion, Austin, TX), according to the manufacturer’s instructions. Total RNA samples were DNase treated using the Turbo DNA-free kit (Ambion). The DNase-treated total RNA was reverse transcribed at 50°C for 50 minutes in the presence of 250 ng of random hexamers and 200 units Superscript III reverse transcriptase in 1× first strand buffer containing 0.5 mmol/L deoxyribonucleotide triphosphate and 40 units RNase Out (Invitrogen). A parallel reaction lacking the reverse transcriptase was prepared as a negative control.
Polymerase Chain Reactions
Quantitative real-time polymerase chain reaction (qPCR) was performed with the above reverse transcribed complementary DNA in a 384-well plate on a 7900HT Sequence Detection System (Applied Biosystems, Foster City, California) using Taqman Universal PCR Master Mix and gene-specific Taqman Gene Expression Assays (Table 1) with universal cycling conditions. Each reaction was run in 15 μL total volume, and each sample was run in triplicates. The expression of each target gene was normalized to 18S transcript expression in the sample. The relative target gene expression levels were calculated using the Comparative CT method as described in Applied Biosystems User Bulletin No. 2.
Table 1.
Quantitative PCR Primer Sequences.
| Genes | Assay ID/Primer Sequences |
|---|---|
| Tagman assays | |
| AR | Rn00560747_m1 |
| FSHβ | Rn01484594_m1 |
| GnRHR | Rn00578981_m1 |
| PACAP | Rn00566438_m1 |
| PAC1 | Rn00591653_m1 |
| SYBR green method | |
| PRB | Forward: 5′-CCAATACCGATCTCCCTGGAC-3′ |
| Reverse: 5′-CTTCCACTCCAGAGAAAGCTCC-3′ | |
Abbreviations: AR, androgen receptor; GnRHR, GnRH receptor; FSHβ, follicle-stimulating hormone β; PACAP, pituitary adenylate cyclase-activating polypeptide; PAC1, PACAP receptor; PCR, polymerase chain reaction; PRB, progesterone receptor.
Statistical Analysis
For each experiment, the result from one of the control wells was arbitrarily set at 1, and the remainder of the results corrected against that value. The experimental results were averaged, and the standard error of the mean was calculated counting each independent experiment as a single N. Statistical analysis was performed using the SigmaStat Software package (SPSS Science, Chicago, Illinois). Data were analyzed for normality followed by analysis of variance. The Tukey test was used for post hoc comparison. The student t test was used, where only 2 groups were involved. Statistical significance was set at P < .05.
Results
The GnRH Alters PACAP and PAC1 mRNA Levels in Rat Primary Pituitary Cells
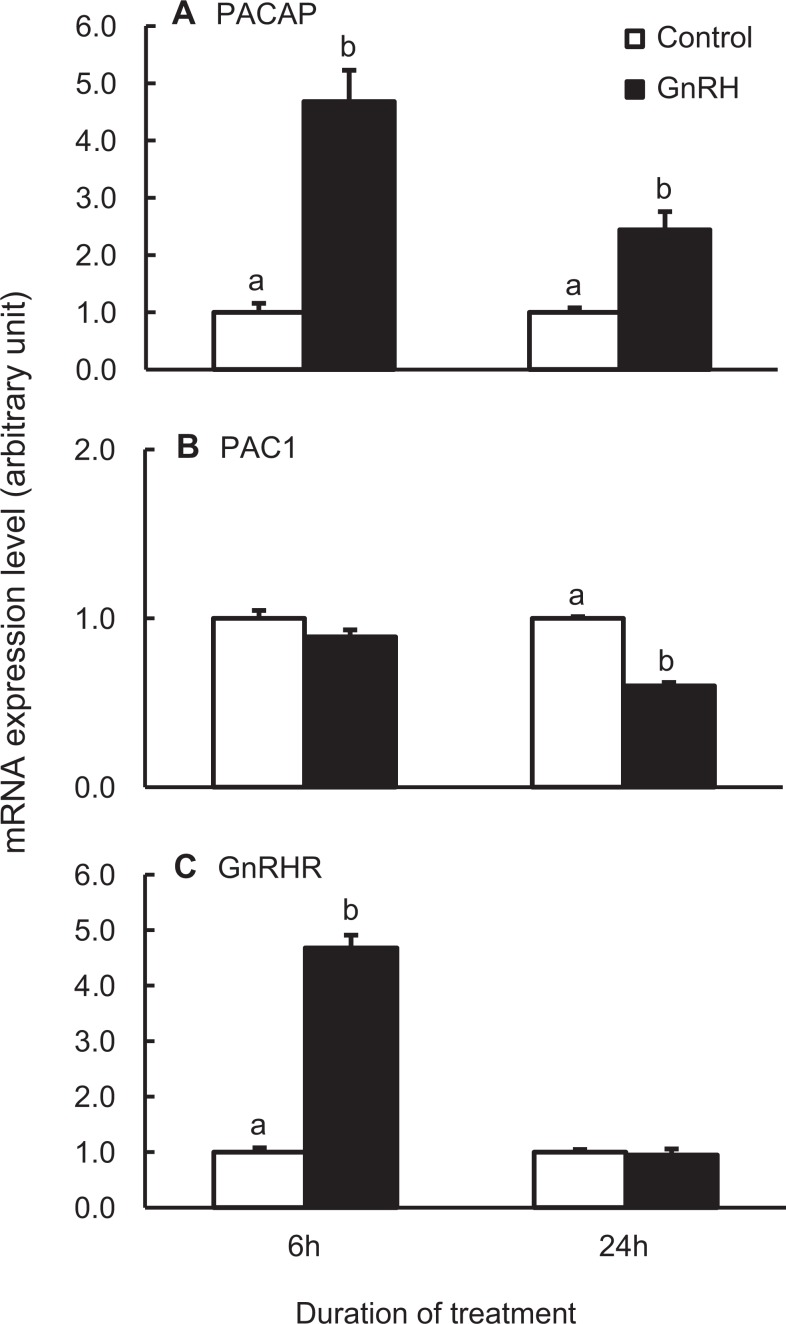
The GnRH treatment of dispersed adult male rat pituitary cells for 6 hours increased PACAP mRNA expression by approximately 5-fold (P < .05; Figure 1A). The GnRH treatment for 24 hours also significantly increased PACAP mRNA expression but to a lesser degree. This temporal response is in accordance with observation in the LβT2 gonadotrope cell line.26 PAC1 mRNA level was not affected by GnRH treatment for 6 hours, but it was significantly decreased after 24 hours of GnRH treatment (Figure 1B).
Figure 1.
The GnRH regulates the mRNA levels of PACAP (A), PACAP receptor (PAC1; B), and GnRHR (C) in dispersed adult male rat pituitary cells. Cells were treated with 100 nmol/L GnRH analog for 6 or 24 hours and harvested for total RNA isolation. The mRNA expression levels were determined by real-time PCR using Taqman gene expression assays with those of the control samples set as 1.0 at each time point, respectively. Bars represent the mean ± standard error of the mean of each treatment group (n ≥ 3). Bars at the same time point with different letters vary significantly (P < .05). GnRH indicates gonadotropin-releasing hormone; mRNA, messenger RNA; PACAP, pituitary adenylate cyclase-activating polypeptide.
As it is known that GnRH induces GnRHR expression, GnRHR mRNA levels were determined as an internal control for the experimental protocol.41,42 Consistent with the previous reports, GnRHR mRNA expression increased approximately 5-fold following 6 hours of GnRH treatment with a return to control levels following 24 hours treatment (Figure 1C). Based on these results, in subsequent experiments the cells were treated with GnRH for only the last 6 hours before sample collection, as detailed in each figure legend.
Dihydrotestosterone Augments GnRH-Mediated Increases in PACAP mRNA Levels
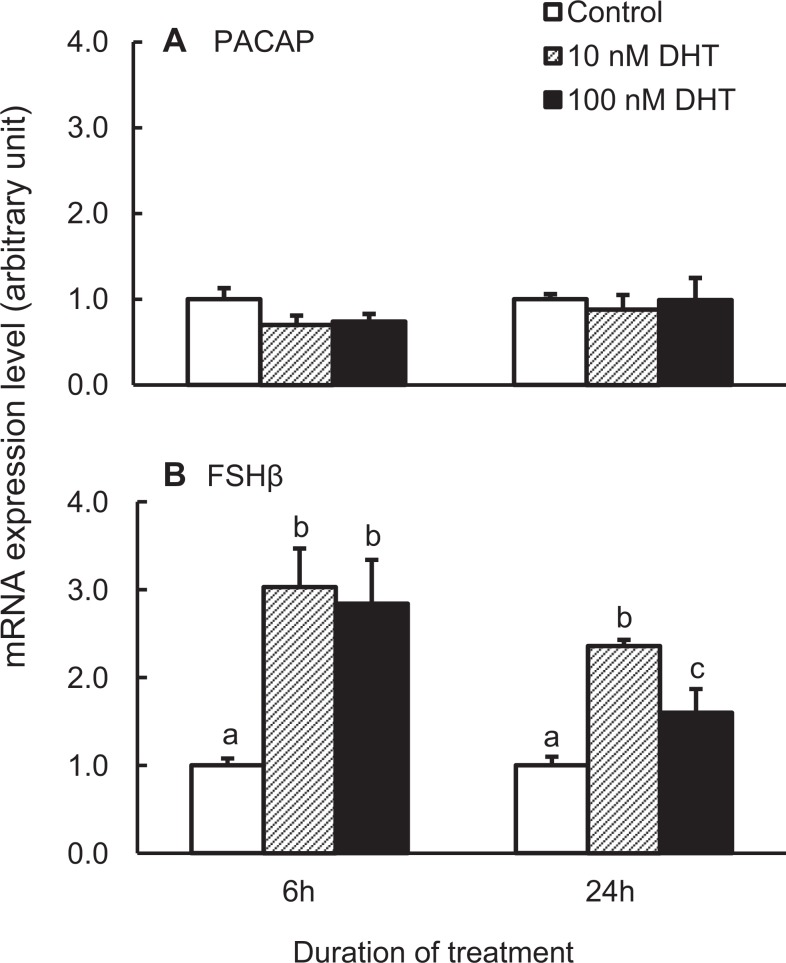
We next investigated the effect of DHT alone on PACAP mRNA expression. As presented in Figure 2A, while 6 hours DHT treatment decreased PACAP mRNA expression by about 25%, this response was not statistically significant. To confirm our experimental procedure, we also measured the expression of FSHβ mRNA, because it has been reported previously that DHT increases pituitary FSHβ mRNA and serum FSH levels in rats.23,43,44 As expected, DHT significantly increased FSHβ mRNA in dispersed pituitary cells at both the 6- and the 24-hourtime points (Figure 2B).
Figure 2.
Pituitary adenylate cyclase-activating polypeptide (PACAP; A) and follicle-stimulating hormone β (FSHβ; B) messenger RNA (mRNA) expression in dispersed adult male rat pituitary cells in response to treatment with vehicle, 10, or 100 nmol/L dihydrotestosterone (DHT) for 6 or 24 hours. The samples were processed and analyzed as described in Figure 1. Bars represent the mean ± standard error of the mean of each group (n ≥ 3). Bars at the same time point with different letters vary significantly (P < .05).
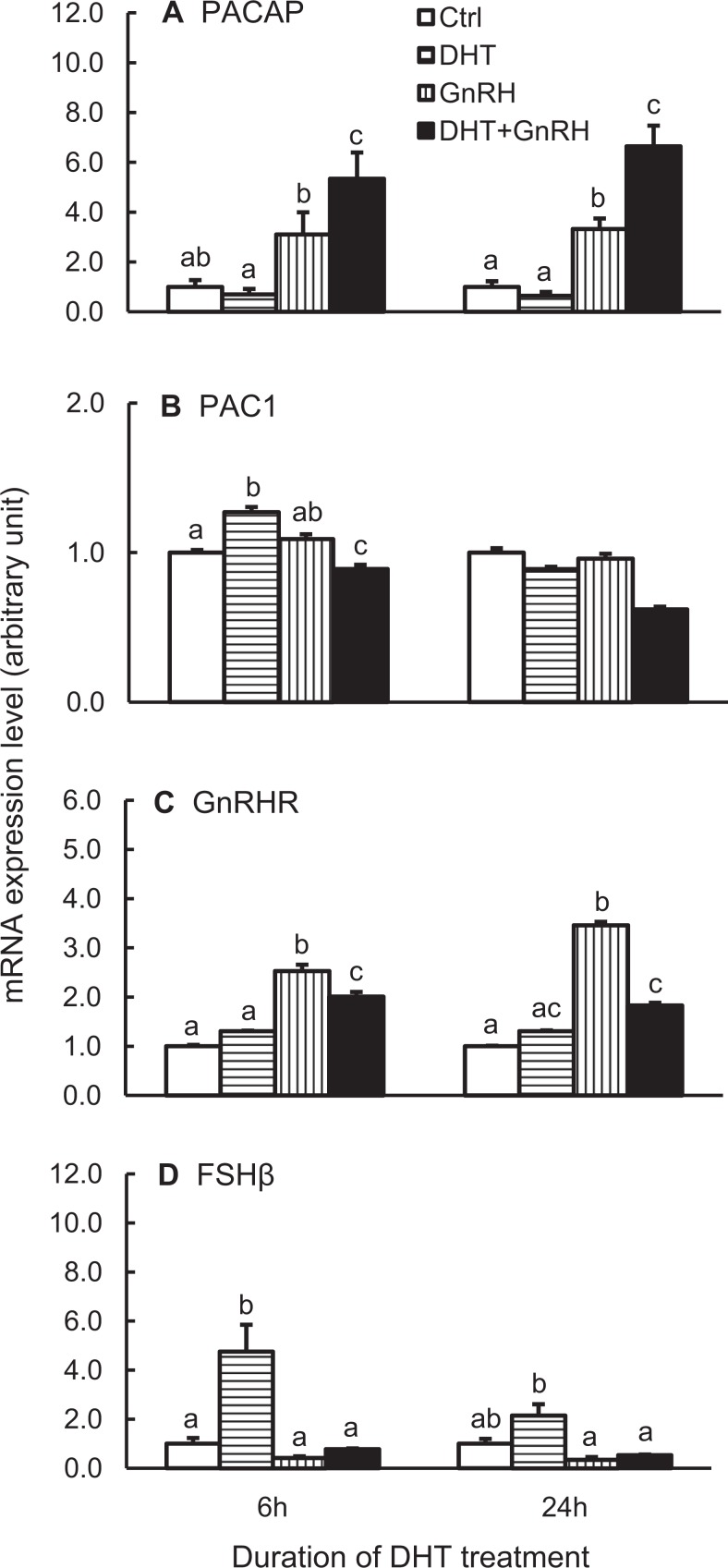
As both DHT and GnRH are present simultaneously in normal physiology, we chose to define the interaction of these 2 hormones on pituitary PACAP mRNA expression. The dispersed pituitary cells were treated with 100 nmol/L DHT for 6 or 24 hours, and GnRH was included in the culture medium during the last 6 hours of treatment. Once again, there was a nonsignificant decrease in PACAP mRNA levels in the presence of DHT with a significant increase in response to GnRH treatment at both time points (Figure 3A). When both DHT and GnRH were included in the culture medium, the expression of PACAP mRNA was significantly greater than that with GnRH treatment alone.
Figure 3.
Interaction between DHT and GnRH on PACAP (A) and PAC1 (B) gene expression. The GnRHR (C) and FSHβ (D) mRNA levels were also analyzed. Dispersed cells were treated with vehicle or 100 nmol/L DHT for 6 or 24 hours, and 100 nmol/L GnRH analog (or equal volume of H2O as control) was included for the last 6 hours of incubation. The samples were then processed and measured as described in Figure 1. Bars represent the mean ± standard error of the mean of each group (n ≥ 3). Bars at the same time point with different letters vary significantly (P < .05). DHT indicates dihydrotestosterone; FSHβ, follicle-stimulating hormone β; GnRH, gonadotropin-releasing hormone; mRNA, messenger RNA; PACAP, pituitary adenylate cyclase-activating polypeptide; PAC1, PACAP receptor.
We hypothesized that DHT and GnRH could exert cooperative effects via hormone-mediated stimulation of either AR or GnRHR expression. Arguing against this mechanism, AR expression was unchanged by DHT and/or GnRH treatment (data not shown), and DHT actually blunted the ability of GnRH to stimulate expression of its receptor (Figure 3C).
The PAC1 mRNA expression was also analyzed. The DHT treatment caused a biphasic change in PAC1 mRNA expression, with a significant, albeit small, increase at 6 hours but no change by 24 hours of treatment (Figure 3B). As observed previously, GnRH alone did not affect PAC1 expression, because it was only given for 6 hours. Interestingly, when DHT and GnRH were used together, they tended to suppress PAC1 mRNA expression below the other treatment group (Figure 3B).
Similarly, as an internal control, FSHβ mRNA expression was measured. The DHT alone caused a significant increase in FSHβ mRNA expression, and GnRH alone decreased the expression but not to a significant level when compared with the vehicle control (Figure 3D). When both DHT and GnRH were present, the stimulatory effect of DHT on FSHβ mRNA expression was totally suppressed by GnRH.
Estradiol Blunts PACAP Gene Expression When Present Alone
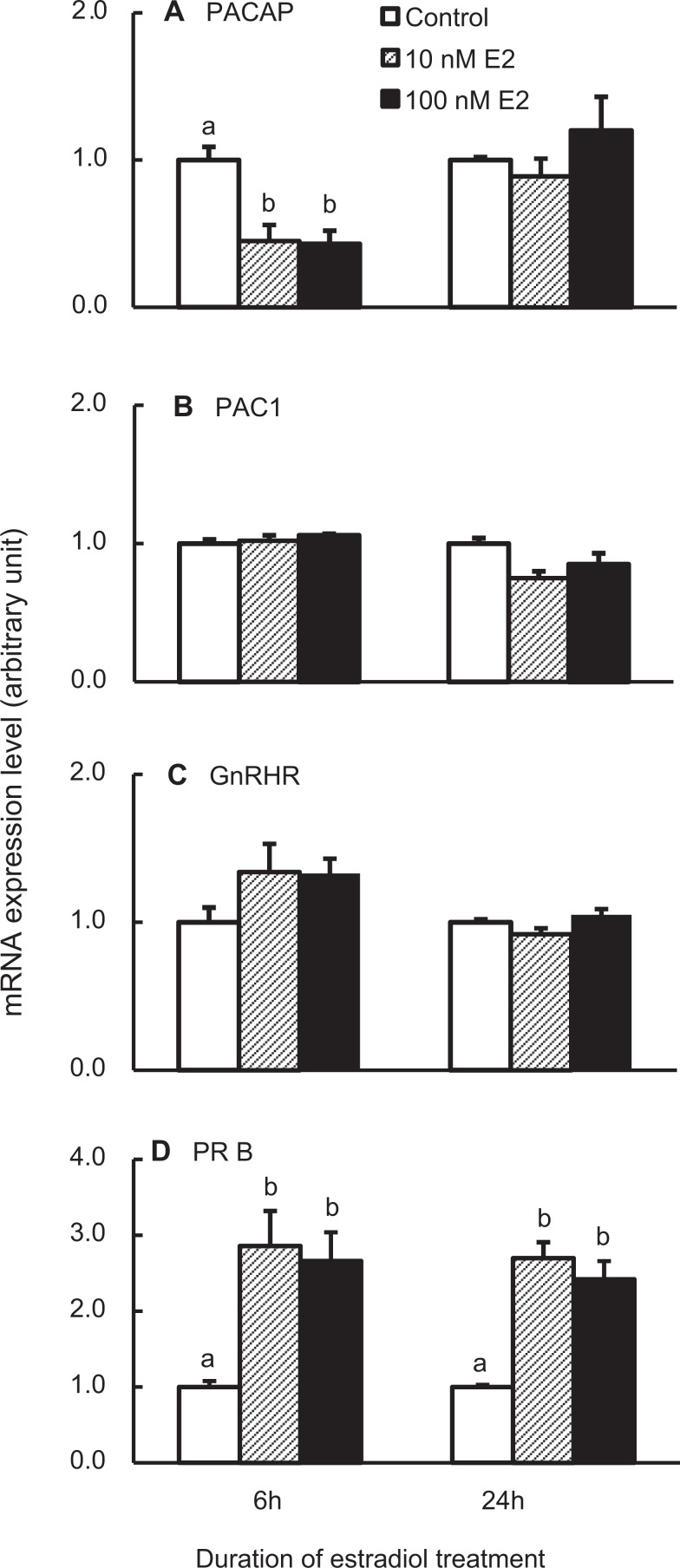
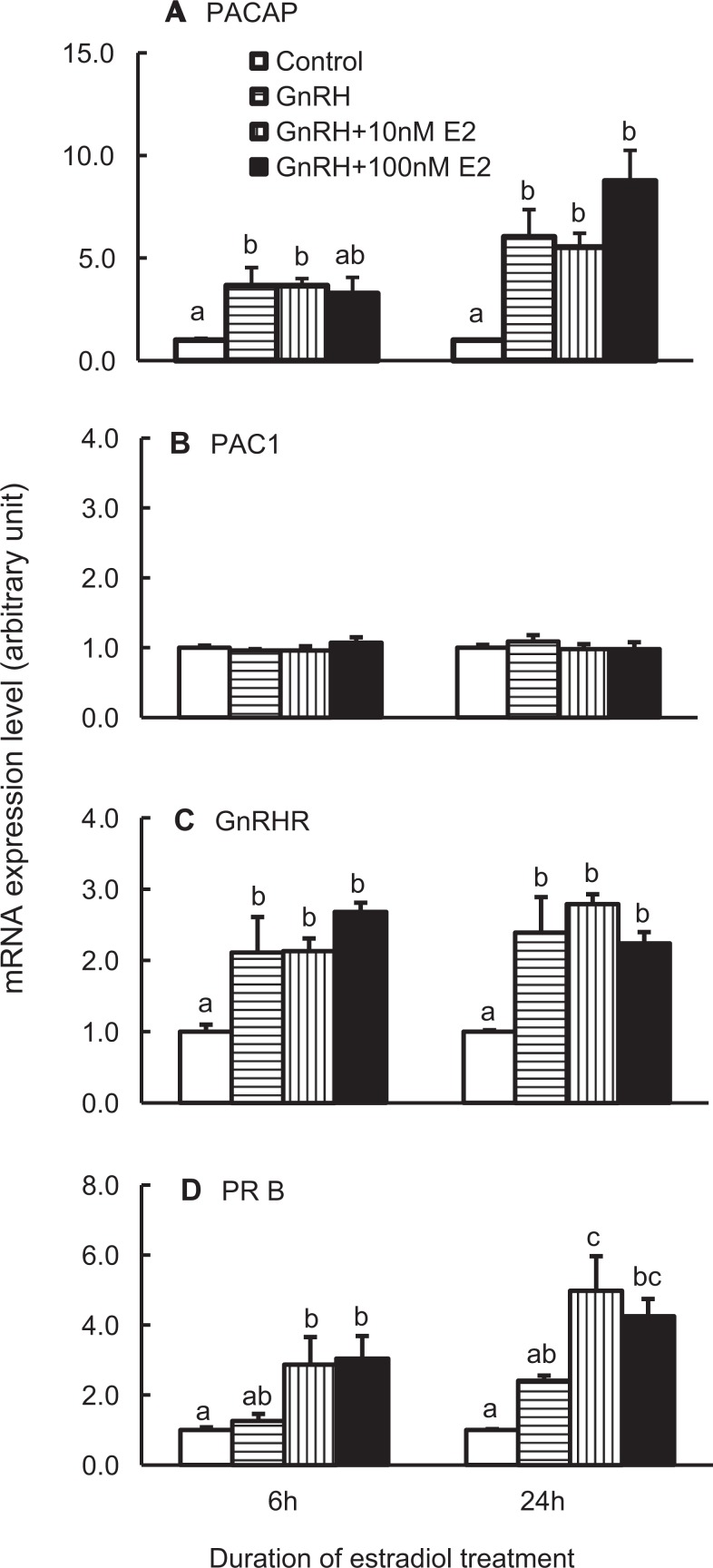
Estradiol controls pituitary function through its feedback directly to the pituitary or indirectly to the hypothalamus. Treatment of dispersed pituitary cells with 10 or 100 nmol/L estradiol for 6 hours decreased PACAP transcript number significantly, a response lost with prolonged treatment of 24 hours (Figure 4A). In contrast, estradiol treatment for 6 or 24 hours did not alter the expression of the PAC1 or GnRHR genes (Figure 4B and C).
Figure 4.
Expression of pituitary adenylate cyclase-activating polypeptide (PACAP; A), PACAP receptor (PAC1; B), gonadotropin-releasing hormone receptor (GnRHR; C), and progesterone receptors (PRB; D) in rat pituitary cells treated with estradiol (E2). PRB messenger RNA (mRNA) expression was used as positive controls for E2 treatment. The cells were treated with vehicle, 10, or 100 nmol/L E2 for 6 or 24 hours. Samples were analyzed as described in Figure 1. Bars represent the mean ± standard error of the mean of each group (n ≥ 3). Bars at the same time point with different letters vary significantly (P < .05).
To monitor the effectiveness of the estradiol treatment, mRNA levels of the PRB was measured. As presented in Figure 4D, estradiol significantly increased PRB mRNA levels at both 6 and 24 hours, consistent with its reported ability to enhance the PRB expression in gonadotropes.45
We next investigated the responses to treatment with both estradiol and GnRH. As demonstrated in Figure 5, GnRH-mediated increases in PACAP gene expression were not altered by the presence of 10 or 100 nmol/L E2 (Figure 5A). Estradiol did not augment the ability of GnRH to increase the GnRHR transcripts, and, conversely, GnRH treatment did not change the ability of estradiol to stimulate the PRB mRNA levels (Figure 5C and D). The PAC1 gene expression did not change in response to 6 hours of GnRH treatment in the presence of estradiol (Figure 5B).
Figure 5.
Expression of pituitary adenylate cyclase-activating polypeptide (PACAP; A), PACAP receptor (PAC1; B), GnRHR (C), and progesterone receptor (PRB; D) in rat pituitary cells treated with vehicle, 10, or 100 nmol/L E2 for 6 or 24 hours, and 100 nmol/L gonadotropin-releasing hormone (GnRH) analog (or equal volume of H2O as control) was included for the last 6 hours of incubation. The samples were measured as described in Figure 1. Bars represent the mean ± standard error of the mean of each group (n ≥ 3). Bars at the same time point with different letters vary significantly (P < .05).
Progesterone Potentiates GnRH Effects on PACAP mRNA Levels
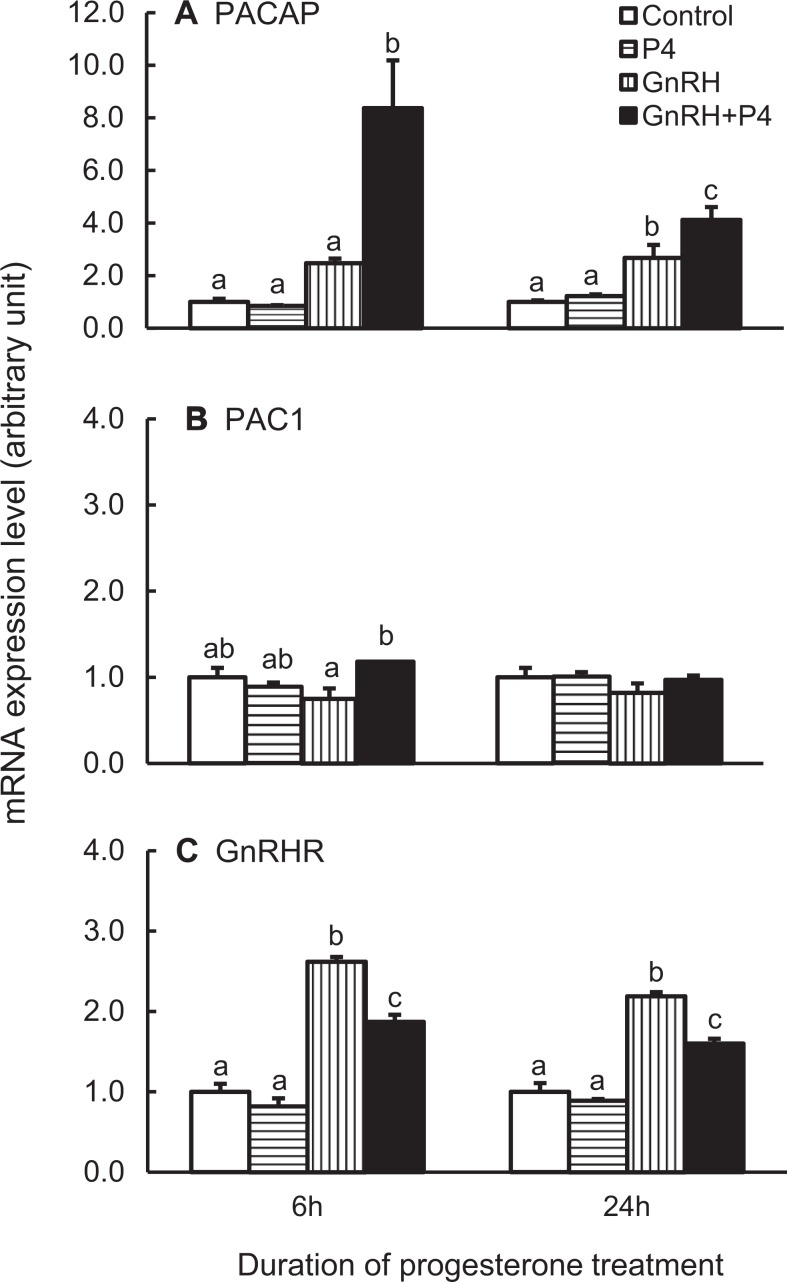
Finally, the effect of progesterone on PACAP mRNA expression was studied. In order to induce PR expression, dispersed anterior pituitary cells were primed with 1 nmol/L estradiol 24 hours prior to and during the progesterone and/or GnRH treatments.
Progesterone treatment alone did not affect the expression of PACAP mRNA, and GnRH increased the expression as expected (Figure 6A). When both progesterone and GnRH were included in the culture medium, PACAP mRNA expression was increased over 3-fold higher than that of GnRH alone at 6 hours treatment. Progesterone alone did not affect PACAP receptor and GnRHR mRNA expression (Figure 6B and C), but it inhibited the stimulatory effects of GnRH on GnRHR expression (Figure 6C). The GnRH, as observed previously (Figure 1), stimulated GnRHR mRNA expression but did not alter PAC1 expression even in the presence of progesterone (Figure 6B and C).
Figure 6.
Expression of pituitary adenylate cyclase-activating polypeptide (PACAP; A), PACAP receptor (PAC1; B), and GnRHR (C) in rat pituitary cells. The cells were first primed with 1 nmol/L E2 for 24 hours, then with constant E2 priming treated with 100 nmol/L progesterone (P4) for 6 or 24 hours, and 100 nmol/L gonadotropin-releasing hormone (GnRH) analog (or equal volume of H2O as control) was added at 6 hours before sample collection. The samples were processed and measured as described in Figure 1. Bars represent the mean ± standard error of the mean of each group (n ≥ 3). Bars at the same time point with different letters vary significantly (P < .05).
Discussion
Gonadotropin biosynthesis and secretion are tightly regulated through the interaction of hypothalamic-releasing factors, most notably GnRH, gonadal steroid feedback, and intrapituitary regulatory pathways including the activin-follistatin system. More recent investigations have begun to elucidate an important role for both hypothalamic and locally produced PACAP in the regulation of pituitary cell function.
In the experiments reported here, we tested the effects of various hormonal treatments on male rat anterior pituitary cells, which had been dispersed and plated in static culture. Male rats were utilized in order to eliminate any potential confounding effects of estrous cycle stage. As primary end points, we analyzed the expression levels of the transcripts, which encode PACAP, whose expression is limited to the gonadotropes and folliculostellate cells, and the PACAP receptor, PAC1, which is expressed more widely in the pituitary. Gonadotrope cells are unique within this mixed cell population, as they express the GnRHR as well as androgen, estrogen, and progesterone nuclear hormone receptors.46–48 To our knowledge, our data are the first to characterize regulation of PACAP and PAC1 gene expression by GnRH and gonadal steroids in primary pituitary cells. As PACAP itself regulates gonadotropin expression, these data suggest that, in addition to direct action via their receptors, GnRH and gonadal steroids may also modulate gonadotrope function through alterations in a pituitary PACAP autocrine–paracrine system. It will be of interest in the future to define changes in pituitary PACAP protein levels; however, the amount of PACAP present in the anterior pituitary is below the level of detection by standard Western blot in rodents and will likely require the development of a sensitive RIA protocol.49
We have previously reported the ability of GnRH to increase PACAP mRNA expression by over 100-fold in the LβT2 gonadotrope cell line.26 We now confirm a significant GnRH response in primary gonadotropes of 2- to 6-fold. The smaller response in the mixed cell preparation is likely due to the fact that PACAP mRNA produced by the folliculostellate cells is unchanged in response to GnRH.7,50,51 Variations in the fold change across experiments may be the result of minor differences in the treatment protocols, such as differences in the vehicle or the length of incubation times that would have led to differences in paracrine factor involvement.
Although Purwana and colleagues reported a GnRH-mediated increase in PAC1 mRNA levels in the LβT2 cell line after 12 or 24 hours treatment, in our studies of primary pituitary cells, PAC1 expression was unchanged following 6 hours of GnRH treatment and actually decreased with 24 hours of treatment.52 Whether this discrepancy is due to intrinsic differences in primary versus immortalized gonadotrope cells or to other factors will require further investigation. Also of interest, although only the level of transcript expression is analyzed, the fact that GnRH increases PACAP mRNA levels at an early time point and subsequently blunts receptor expression may suggest that the subsequent PACAP effects are temporally self-limited (Figure 1). Of note, the degree and timing of the GnRH effect varied depending on the presence or absence of steroids, adding further complexity to the response.
Although treatment with DHT alone did not alter PACAP expression in primary pituitary cells, our results clearly indicate that GnRH-induced PACAP expression is significantly enhanced in the presence of DHT. The DHT treatment resulted in minimal albeit statistically significant changes in PAC1 transcript number which, as observed in response to GnRH, tended to be the converse of the PACAP response. The functional interaction between DHT and GnRH can be postulated to occur through multiple mechanisms. One of these possibilities, steroid-mediated changes in GnRHR expression, does not appear to be a contributing factor as GnRHR expression drops rather than increases at least at the mRNA level.
In the classical steroid hormone receptor genomic pathway, steroids exert their effects via interactions with cognate receptors that bind to DNA directly. Both AR and PR binding sites have in fact been identified in the rat PACAP gene promoter (Grafer et al, unpublished data). Arguing against this mechanism, however, AR mRNA expression was unchanged in our experiments. Steroid receptors and their cofactors have also been shown to be phosphorylated and thereby activated by intracellular signaling pathways such as the MAPK pathways,53 which are known to be the downstream effectors of GnRH action. It is therefore possible that ligand-independent activation of AR subsequently allows for ligand-dependent effects, which are not detectable in the presence of steroid ligand alone.54–58
It has been reported that progesterone induces PACAP and PAC1 mRNA expression in the rat hypothalamus and ovary.35,36 In contrast, progesterone did not affect the expression of either of these genes in primary pituitary cell cultures, demonstrating tissue-specific differences in hormonal responsiveness. Progesterone did significantly augment GnRH induction of PACAP mRNA expression in dispersed pituitary cells. Although the mechanism for this interaction remains unknown, it is interesting to note that progesterone also enhances the ability of GnRH to stimulate the expression of the gonadotropin genes, LHβ, and FSHβ.25,59
The response to estradiol differed from the response to the other 2 gonadal steroids in that PACAP gene expression was altered in the presence of estradiol alone, but there was no apparent functional interaction with GnRH. Of note, estradiol-mediated suppression was observed only at the 6 hours time point and lost by 24 hours of treatment. Tissue-dependent differences in steroid response were again noted as estradiol decreased the PACAP mRNA levels in pituitary cell cultures, although it has been reported to stimulate PACAP gene expression in the hypothalamic ventromedial and arcuate nuclei.34
The effects of estradiol and progesterone treatment on PACAP mRNA levels, which we observed in vitro, are in accordance with changes described previously in pituitary PACAP gene expression across the estrous cycle. Moore et al reported that pituitary expression of PACAP mRNA levels decline moderately on the afternoon of proestrus with a significant increase at 24 hours as determined by qPCR.6 A similar pattern was observed by Heinzlmann et al using in situ hybridization.60 Our data suggest that the late evening peak in pituitary PACAP mRNA levels may be attributable to the loss of inhibitory estradiol input, which occurs during proestrus as well as the stimulatory effects of increasing progesterone levels acting in concert with GnRH.61
The combinatorial effects of GnRH and DHT are gene specific within the gonadotrope. Although GnRH and DHT synergistically increased PACAP mRNA expression (Figure 3A), DHT blocked the increase in GnRHR mRNA induced by GnRH (Figure 3C), and GnRH blocked the DHT-mediated stimulation of FSHβ transcript number (Figure 3D). The PACAP and FSHβ expression are known to be regulated by locally produced activin and its inhibitor follistatin.2 Furthermore, a number of studies have demonstrated the ability of DHT and GnRH to regulate the pituitary follistatin expression, thereby altering the ability of local activin to stimulate FSHβ gene expression.62,63 In fact, it has been reported that androgen-mediated regulation of the FSHβ gene requires protein–protein interactions between the AR and the Smad family of transcription factors, which convey activin signaling.64,65 In addition, androgens have been shown to be correlated with an increase in Smad expression and phosphorylation as well as a decrease in follistatin mRNA levels in primary rat pituitary cells.62 In contrast, GnRH has been shown to increase follistatin promoter activity and mRNA expression in primary pituitary and LβT2 gonadotrope cells. It can be hypothesized that this GnRH-mediated increase in follistatin could blunt the ability of androgens to stimulate the FSHβ mRNA expression. Adding further complexity, GnRH and DHT synergize to stimulate the FSHβ promoter activity in contrast to the inhibition observed at the mRNA level in our studies, perhaps suggesting differential effects on mRNA transcription and stability.65
In summary, the results reported here suggest that the activity of the intrapituitary PACAP-PAC1 system is regulated via the complex interaction of gonadal steroids and hypothalamic GnRH. As PACAP contributes to the regulation of gonadotropin biosynthesis and secretion, these results establish a novel mechanism for the modulation of gonadotrope gene expression and, thereby, normal reproductive function.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work has been supported by NIH R01 HD054782 (LMH).
References
- 1. Conn PM, Marian J, McMillian M, et al. Gonadotropin-releasing hormone action in the pituitary: a three step mechanism. Endocr Rev. 1981;2(2):174–185. [DOI] [PubMed] [Google Scholar]
- 2. Tsujii T, Ishizaka K, Winters SJ. Effects of pituitary adenylate cyclase-activating polypeptide on gonadotropin secretion and subunit messenger ribonucleic acids in perifused rat pituitary cells. Endocrinology. 1994;135(3):826–833. [DOI] [PubMed] [Google Scholar]
- 3. Culler MD, Paschall CS. Pituitary adenylate cyclase-activating polypeptide (PACAP) potentiates the gonadotropin-releasing activity of luteinizing hormone-releasing hormone. Endocrinology. 1991;129(4):2260–2262. [DOI] [PubMed] [Google Scholar]
- 4. Winters SJ, Tsujii T, Attardi B. Effects of GnRH and PACAP on gonadotropin secretion and subunit messenger RNAs. Ann N Y Acad Sci. 1996;805:343–352; discussion 352-344. [DOI] [PubMed] [Google Scholar]
- 5. Osuga Y, Mitsuhashi N, Mizuno M. In vivo effect of pituitary adenylate cyclase activating polypeptide 38 (PACAP 38) on the secretion of luteinizing hormone (LH) in male rats. Endocrinol Jpn. 1992;39(1):153–156. [DOI] [PubMed] [Google Scholar]
- 6. Moore JP, Jr, , Burger LL, Dalkin AC, Winters SJ. Pituitary adenylate cyclase activating polypeptide messenger RNA in the paraventricular nucleus and anterior pituitary during the rat estrous cycle. Biol Reprod. 2005;73(3):491–499. [DOI] [PubMed] [Google Scholar]
- 7. Winters SJ, Moore JP., Jr PACAP, an autocrine/paracrine regulator of gonadotrophs. Biol Reprod. 2011;84(5):844–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Miyata A, Arimura A, Dahl RR, et al. Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochem Biophys Res Commun. 1989;164(1):567–574. [DOI] [PubMed] [Google Scholar]
- 9. Vaudry D, Gonzalez BJ, Basille M, Yon L, Fournier A, Vaudry H. Pituitary adenylate cyclase-activating polypeptide and its receptors: from structure to functions. Pharmacol Rev. 2000;52(2):269–324. [PubMed] [Google Scholar]
- 10. Vaudry D, Falluel-Morel A, Bourgault S, et al. Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol Rev. 2009;61(3):283–357. [DOI] [PubMed] [Google Scholar]
- 11. Dickson L, Finlayson K. VPAC and PAC receptors: from ligands to function. Pharmacol Ther. 2009;121(3):294–316. [DOI] [PubMed] [Google Scholar]
- 12. Thomas RL, Crawford NM, Grafer CM, Halvorson LM. Pituitary adenylate cyclase-activating polypeptide (PACAP) in the hypothalamic–pituitary–gonadal axis: a review of the literature [published online December 10, 2012]. Reprod Sci. 2012. [DOI] [PubMed] [Google Scholar]
- 13. Koh PO, Kwak SD, Kim HJ, et al. Expression patterns of pituitary adenylate cyclase activating polypeptide and its type I receptor mRNAs in the rat placenta. Mol Reprod Develop. 2003;64(1):27–31. [DOI] [PubMed] [Google Scholar]
- 14. Isaac ER, Sherwood NM. Pituitary adenylate cyclase-activating polypeptide (PACAP) is important for embryo implantation in mice. Mol Cell Endocrinol. 2008;280(1-2):13–19. [DOI] [PubMed] [Google Scholar]
- 15. Arimura A, Somogyvari-Vigh A, Miyata A, Mizuno K, Coy DH, Kitada C. Tissue distribution of PACAP as determined by RIA: highly abundant in the rat brain and testes. Endocrinology. 1991;129(5):2787–2789. [DOI] [PubMed] [Google Scholar]
- 16. Koves K, Kantor O, Scammell JG, Arimura A. PACAP colocalizes with luteinizing and follicle-stimulating hormone immunoreactivities in the anterior lobe of the pituitary gland. Peptides. 1998;19(6):1069–1072. [DOI] [PubMed] [Google Scholar]
- 17. Jin L, Tsumanuma I, Ruebel KH, Bayliss JM, Lloyd RV. Analysis of homogeneous populations of anterior pituitary folliculostellate cells by laser capture microdissection and reverse transcription-polymerase chain reaction. Endocrinology. 2001;142(5):1703–1709. [DOI] [PubMed] [Google Scholar]
- 18. Jamen F, Rodriguez-Henche N, Pralong F, et al. PAC1 null females display decreased fertility. Ann N Y Acad Sci. 2000;921:400–404. [DOI] [PubMed] [Google Scholar]
- 19. Reglodi D, Tamas A, Koppan M, Szogyi D, Welke L. Role of PACAP in female fertility and reproduction at gonadal level—recent advances. Front Endocrinol (Lausanne). 2012;3:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shintani N, Mori W, Hashimoto H, et al. Defects in reproductive functions in PACAP-deficient female mice. Regul Pept. 2002;109(1-3):45–48. [DOI] [PubMed] [Google Scholar]
- 21. Vigh S, Arimura A, Gottschall PE, Kitada C, Somogyvari-Vigh A, Childs GV. Cytochemical characterization of anterior pituitary target cells for the neuropeptide, pituitary adenylate cyclase activating polypeptide (PACAP), using biotinylated ligands. Peptides. 1993;14(1):59–65. [DOI] [PubMed] [Google Scholar]
- 22. Rawlings SR, Hezareh M. Pituitary adenylate cyclase-activating polypeptide (PACAP) and PACAP/vasoactive intestinal polypeptide receptors: actions on the anterior pituitary gland. Endocr Rev. 1996;17(1):4–29. [DOI] [PubMed] [Google Scholar]
- 23. Gharib SD, Leung PC, Carroll RS, Chin WW. Androgens positively regulate follicle-stimulating hormone beta-subunit mRNA levels in rat pituitary cells. Mol Endocrinol. 1990;4(11):1620–1626. [DOI] [PubMed] [Google Scholar]
- 24. Kowase T, Walsh HE, Darling DS, Shupnik MA. Estrogen enhances gonadotropin-releasing hormone-stimulated transcription of the luteinizing hormone subunit promoters via altered expression of stimulatory and suppressive transcription factors. Endocrinology. 2007;148(12):6083–6091. [DOI] [PubMed] [Google Scholar]
- 25. Park D, Cheon M, Kim C, Kim K, Ryu K. Progesterone together with estradiol promotes luteinizing hormone beta-subunit mRNA stability in rat pituitary cells cultured in vitro. Eur J Endocrinol. 1996;134(2):236–242. [DOI] [PubMed] [Google Scholar]
- 26. Grafer CM, Thomas R, Lambrakos L, Montoya I, White S, Halvorson LM. GnRH stimulates expression of PACAP in the pituitary gonadotropes via both the PKA and PKC signaling systems. Mol Endocrinol. 2009;23(7):1022–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ando H, Hew CL, Urano A. Signal transduction pathways and transcription factors involved in the gonadotropin-releasing hormone-stimulated gonadotropin subunit gene expression. Comp Biochem Physiol B Biochem Mol Biol. 2001;129(2-3):525–532. [DOI] [PubMed] [Google Scholar]
- 28. Shacham S, Cheifetz MN, Lewy H, et al. Mechanism of GnRH receptor signaling: from the membrane to the nucleus. Ann Endocrinol (Paris). 1999;60(2):79–88. [PubMed] [Google Scholar]
- 29. Cheskis BJ. Regulation of cell signalling cascades by steroid hormones. J Cell Biochem. 2004;93(1):20–27. [DOI] [PubMed] [Google Scholar]
- 30. Thackray VG, Mellon PL, Coss D. Hormones in synergy: regulation of the pituitary gonadotropin genes. Mol Cell Endocrinol. 2010;314(2):192–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Heldring N, Isaacs GD, Diehl AG, et al. Multiple sequence-specific DNA-binding proteins mediate estrogen receptor signaling through a tethering pathway. Mol Endocrinol. 2011;25(4):564–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vasudevan N, Pfaff DW. Membrane-initiated actions of estrogens in neuroendocrinology: emerging principles. Endocr Rev. 2007;28(1):1–19. [DOI] [PubMed] [Google Scholar]
- 33. Vasudevan N, Pfaff DW. Non-genomic actions of estrogens and their interaction with genomic actions in the brain. Front Neuroendocrinol. 2008;29(2):238–257. [DOI] [PubMed] [Google Scholar]
- 34. Apostolakis EM, Lanz R, O'Malley BW. Pituitary adenylate cyclase-activating peptide: a pivotal modulator of steroid-induced reproductive behavior in female rodents. Mol Endocrinol. 2004;18(1):173–183. [DOI] [PubMed] [Google Scholar]
- 35. Ha CM, Kang JH, Choi EJ, et al. Progesterone increases mRNA levels of pituitary adenylate cyclase-activating polypeptide (PACAP) and type I PACAP receptor (PAC(1)) in the rat hypothalamus. Brain Res Mol Brain Res. 2000;78(1-2):59–68. [DOI] [PubMed] [Google Scholar]
- 36. Park JI, Kim WJ, Wang L, et al. Involvement of progesterone in gonadotrophin-induced pituitary adenylate cyclase-activating polypeptide gene expression in pre-ovulatory follicles of rat ovary. Mol Human Reprod. 2000;6(3):238–245. [DOI] [PubMed] [Google Scholar]
- 37. Nemeth J, Tamas A, Jozsa R, et al. Changes in PACAP levels in the central nervous system after ovariectomy and castration. Ann N Y Acad Sci. 2006;1070:468–473. [DOI] [PubMed] [Google Scholar]
- 38. Lee J, Park HJ, Choi HS, et al. Gonadotropin stimulation of pituitary adenylate cyclase-activating polypeptide (PACAP) messenger ribonucleic acid in the rat ovary and the role of PACAP as a follicle survival factor. Endocrinology. 1999;140(2):818–826. [DOI] [PubMed] [Google Scholar]
- 39. Morelli MB, Barberi M, Gambardella A, et al. Characterization, expression, and functional activity of pituitary adenylate cyclase-activating polypeptide and its receptors in human granulosa–luteal cells. J Clin Endocrinol Metabol. 2008;93(12):4924–4932. [DOI] [PubMed] [Google Scholar]
- 40. Purwana IN, Kanasaki H, Oride A, et al. GnRH-induced PACAP and PAC1 receptor expression in pituitary gonadotrophs: a possible role in the regulation of gonadotropin subunit gene expression. Peptides. 2010;31(9):1748–1755. [DOI] [PubMed] [Google Scholar]
- 41. Kaiser UB, Jakubowiak A, Steinberger A, Chin WW. Regulation of rat pituitary gonadotropin-releasing hormone receptor mRNA levels in vivo and in vitro. Endocrinology. 1993;133(2):931–934. [DOI] [PubMed] [Google Scholar]
- 42. Bauer-Dantoin AC, Weiss J, Jameson JL. Roles of estrogen, progesterone, and gonadotropin-releasing hormone (GnRH) in the control of pituitary GnRH receptor gene expression at the time of the preovulatory gonadotropin surges. Endocrinology. 1995;136(3):1014–1019. [DOI] [PubMed] [Google Scholar]
- 43. Burger LL, Haisenleder DJ, Aylor KW, Dalkin AC, Prendergast KA, Marshall JC. Regulation of luteinizing hormone-beta and follicle-stimulating hormone (FSH)-beta gene transcription by androgens: testosterone directly stimulates FSH-beta transcription independent from its role on follistatin gene expression. Endocrinology. 2004;145(1):71–78. [DOI] [PubMed] [Google Scholar]
- 44. Wierman ME, Wang C. Androgen selectively stimulates follicle-stimulating hormone-beta mRNA levels after gonadotropin-releasing hormone antagonist administration. Biol Reprod. 1990;42(3):563–571. [DOI] [PubMed] [Google Scholar]
- 45. Scott RE, Wu-Peng XS, Pfaff DW. Regulation and expression of progesterone receptor mRNA isoforms A and B in the male and female rat hypothalamus and pituitary following oestrogen treatment. J Neuroendocrinol. 2002;14(3):175–183. [DOI] [PubMed] [Google Scholar]
- 46. Stefaneanu L. Pituitary sex steroid receptors: localization and function. Endocr Pathol. 1997;8(2):91–108. [DOI] [PubMed] [Google Scholar]
- 47. Pelletier G, Labrie C, Labrie F. Localization of oestrogen receptor alpha, oestrogen receptor beta and androgen receptors in the rat reproductive organs. J Endocrinol. 2000;165(2):359–370. [DOI] [PubMed] [Google Scholar]
- 48. Turgeon JL, Waring DW. Progesterone regulation of the progesterone receptor in rat gonadotropes. Endocrinology. 2000;141(9):3422–3429. [DOI] [PubMed] [Google Scholar]
- 49. Moore JP, Jr, , Yang RQ, Winters SJ. Targeted pituitary overexpression of pituitary adenylate-cyclase activating polypeptide alters postnatal sexual maturation in male mice. Endocrinology. 2012;153(3):1421–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kaiser UB, Conn PM, Chin WW. Studies of gonadotropin-releasing hormone (GnRH) action using GnRH receptor-expressing pituitary cell lines. Endocr Rev. 1997;18(1):46–70. [DOI] [PubMed] [Google Scholar]
- 51. Schang AL, Counis R, Magre S, et al. Reporter transgenic mouse models highlight the dual endocrine and neural facet of GnRH receptor function. Ann N Y Acad Sci. 2011;1220:16–22. [DOI] [PubMed] [Google Scholar]
- 52. Purwana IN, Kanasaki H, Oride A, Mijiddorj T, Miyazaki K. Expression of the pituitary adenylate cyclase-activating polypeptide (PACAP) type 1 receptor (PAC1R) potentiates the effects of GnRH on gonadotropin subunit gene expression. Biochem Biophys Res Commun. 2011;410(2):295–300. [DOI] [PubMed] [Google Scholar]
- 53. Kato S, Masuhiro Y, Watanabe M, et al. Molecular mechanism of a cross-talk between oestrogen and growth factor signalling pathways. Genes Cells. 2000;5(8):593–601. [DOI] [PubMed] [Google Scholar]
- 54. Heinlein CA, Chang C. The roles of androgen receptors and androgen-binding proteins in nongenomic androgen actions. Mol Endocrinol. 2002;16(10):2181–2187. [DOI] [PubMed] [Google Scholar]
- 55. Cheskis BJ, Greger J, Cooch N, et al. MNAR plays an important role in ERa activation of Src/MAPK and PI3K/Akt signaling pathways. Steroids. 2008;73(9-10):901–905. [DOI] [PubMed] [Google Scholar]
- 56. Qiu M, Olsen A, Faivre E, Horwitz KB, Lange CA. Mitogen-activated protein kinase regulates nuclear association of human progesterone receptors. Mol Endocrinol. 2003;17(4):628–642. [DOI] [PubMed] [Google Scholar]
- 57. Qiu M, Lange CA. MAP kinases couple multiple functions of human progesterone receptors: degradation, transcriptional synergy, and nuclear association. J Steroid Biochem Mol Biol. 2003;85(2-5):147–157. [DOI] [PubMed] [Google Scholar]
- 58. Culig Z. Androgen receptor cross-talk with cell signalling pathways. Growth Factors. 2004;22(3):179–184. [DOI] [PubMed] [Google Scholar]
- 59. Kerrigan JR, Dalkin AC, Haisenleder DJ, Yasin M, Marshall JC. Failure of gonadotropin-releasing hormone (GnRH) pulses to increase luteinizing hormone beta messenger ribonucleic acid in GnRH-deficient female rats. Endocrinology. 1993;133(5):2071–2079. [DOI] [PubMed] [Google Scholar]
- 60. Heinzlmann A, Kirilly E, Meltzer K, et al. PACAP is transiently expressed in anterior pituitary gland of rats: in situ hybridization and cell immunoblot assay studies. Peptides. 2008;29(4):571–577. [DOI] [PubMed] [Google Scholar]
- 61. Szabo M, Kilen SM, Nho SJ, Schwartz NB. Progesterone receptor A and B messenger ribonucleic acid levels in the anterior pituitary of rats are regulated by estrogen. Biol Reprod. 2000;62(1):95–102. [DOI] [PubMed] [Google Scholar]
- 62. Burger LL, Haisenleder DJ, Wotton GM, Aylor KW, Dalkin AC, Marshall JC. The regulation of FSHbeta transcription by gonadal steroids: testosterone and estradiol modulation of the activin intracellular signaling pathway. Am J Physiol Endocrinol Metab. 2007;293(1):E277–E285. [DOI] [PubMed] [Google Scholar]
- 63. Kaiser UB, Chin WW. Regulation of follistatin messenger ribonucleic acid levels in the rat pituitary. J Clin Invest. 1993;91(6):2523–2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Thackray VG, Mellon PL. Synergistic induction of follicle-stimulating hormone beta-subunit gene expression by gonadal steroid hormone receptors and Smad proteins. Endocrinology. 2008;149(3):1091–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Spady TJ, Shayya R, Thackray VG, Ehrensberger L, Bailey JS, Mellon PL. Androgen regulates follicle-stimulating hormone beta gene expression in an activin-dependent manner in immortalized gonadotropes. Mol Endocrinol. 2004;18(4):925–940. [DOI] [PMC free article] [PubMed] [Google Scholar]