Abstract
Emerging evidence indicates that maternal medical risk during pregnancy, such as gestational diabetes mellitus (GDM), preeclampsia, and obesity, predisposes the offspring to suboptimal development. However, the underlying biological/epigenetic mechanism in utero is still unknown. The current pilot study (N = 50) compared the levels of global methylation in the placenta and umbilical cord blood among women with and without each risk condition (GDM, preeclampsia, and obesity) and explored whether the levels of global methylation were associated with fetal/infant growth. Results show that global methylation levels in the placenta were lower in patients with gestational diabetes (P = .003) and preeclampsia (P = .05) but higher with obesity (P = .01). Suggestive negative associations were found between global methylation level in the placenta and infant body length and head circumference. While preliminary, it is possible that the placenta tissue, but not umbilical cord blood, may be epigenetically programmed by maternal GDM, preeclampsia, and obesity to carry out its own specific functions that influence fetal growth.
Keywords: global methylation, fetal programming, placenta, umbilical cord blood, gestational diabetes mellitus, preeclampsia, obesity, birth outcomes
Introduction
An epidemic of metabolic disorders, particularly obesity, diabetes, and cardiovascular disease, in both adults and children represent a growing public health concern. The prevalence of gestational diabetes mellitus (GDM) among pregnant women is also on the rise, especially in ethnic minority, which has doubled over the last 20 years.1,2 The increasing prevalence of metabolic conditions such as GDM has been attributed to characteristics of modern living such as advanced maternal age at first pregnancy, obesity, sedentary working environments, and dietary changes such as greater consumption of saturated fats, sugar, and processed foods.
In utero exposure to suboptimal maternal conditions, such as GDM, preeclampsia, and obesity, has been identified as risk factors for future diseases in the infants. For example, findings from recent studies demonstrate an impaired ability in infants to respond to metabolic challenges3–9 and to develop neurobehavioral impairments in childhood.10,11 These findings lend support to the “Barker hypothesis” or the theory of developmental origins of health and disease, which hypothesizes that antenatal environmental factors lead to alterations in fetal programming that can induce permanent biological and physiologic changes later in life.12–19
It has been hypothesized that critical windows of susceptibility exist during pregnancy,20,21 and many of the acquired adverse effects related to the intrauterine environment result in epigenetic alterations such as changes in DNA methylation.22 Although recent evidence has begun to show a long-term association between maternal GDM and/or preeclampsia and offspring development at birth and in childhood,23–25 the underlying biological mechanism is not yet understood. In one of our previous studies,10 we reported a 14-fold increased risk of Attention Deficit Hyperactivity Disorder (ADHD) among young children who were exposed to both maternal GDM and low family socioeconomic status in childhood. However, although that study indicated an association between GDM and ADHD, it did not examine potential biological mechanisms that may have contributed to the increased risk or possible mediating phenotypes at birth such as low birth weight and small head circumference.
Capitalizing on a newly established birth cohort at an urban, inner city low-income prenatal clinic, we used the Luminometric Methylation Assay (LUMA) with 50 matched samples of placenta and umbilical cord blood to study global methylation in relation to maternal GDM, preeclampsia, and obesity during pregnancy and newborn growth and development, that is, weight, body length, and head circumference. While preliminary due to a relatively small sample size, the current study has identified candidate epigenetic changes associated with suboptimal fetal programming.
Material and Methods
Study Population
Study participants (pregnant women) were recruited from the prenatal obstetrics and gynecological (OB/GYN) clinic at Mount Sinai Medical Center, which draws patients from East Harlem and the South Bronx in New York City, where the majority of the residents are low-income ethnic minority. The pregnant women were followed from the second trimester of their pregnancy to delivery.
Exclusion criteria for participation included HIV infection, maternal psychosis, maternal age <15 years, life-threatening medical complications of the mother, and congenital or chromosomal abnormalities of the fetus. Demographic information, including maternal age, ethnicity, education level, welfare status, marital status, and previous obstetric histories, were obtained through self-administered questionnaires during the second trimester and structured clinical interviews during the third trimester. Data on gestational age at birth, delivery method, infant gender, birth weight, body length, and head circumference were recorded at the time of birth. For this study, the first 50 patients who enrolled and delivered their infants between September 2010 and May 2011 were selected for global methylation measurements. All patients were consented per protocol approved by the institutional review board at Mount Sinai School of Medicine.
Laboratory Procedures
Placental tissue and umbilical cord blood collection
Umbilical cord blood samples for DNA and RNA extraction were collected at birth, prior to the delivery of the placenta. Uncoagulated whole blood was collected in citrated tubes for DNA extraction; PAXgene blood RNA tubes (PreAnalytiX, Hombrechtikon, Switzerland) were used for collecting uncoagulated whole blood for RNA extraction. The citrated tubes were mixed, aliquoted, and stored at −80°C; PAXgene tubes (Qiagen, Valencia, California) were left at room temperature for 2 hours and then stored at −80°C.
The placenta biopsies were collected from the 4 quadrants of chorionic villi of the placenta, midway between the umbilical cord insertion and the placental rim, within 6 hours from the time of delivery. Tissue was extensively washed in cold (4°C), sterile RNase-free phosphate-buffered saline, blotted in sterile gauze, snap frozen in liquid nitrogen, and stored in an ultrafreezer at −80°C.
DNA/RNA extraction and complementary DNA conversion
Frozen placenta tissue was ground to a powder in a liquid nitrogen-cooled mortar (BelArt, Pequannock, New Jersey). Tissue powder was utilized for DNA extraction using the QIAamp DNA Mini Kit and for RNA extraction using the RNeasy Plus Minikit (Qiagen).
DNA from umbilical cord blood was extracted from uncoagulated aliquoted whole blood using the High Pure PCR Template Preparation Kit (Roche Diagnostics, Indianapolis, Indiana). RNA was extracted from whole blood in PAXgene tubes using the PAXgene Blood RNA Kit (Qiagen). RNA was converted into complementary DNA (cDNA) using the AffinityScript cDNA Synthesis Kit (Stratagene, La Jolla, California). Extracted RNA was finally quantified with the Nanodrop spectrophotometer and stored at −80°C. Laboratory protocols followed all manufacturers’ instructions.
Measures
Global DNA methylation
Global methylation of umbilical cord blood and placenta tissue was analyzed using LUMA. The detailed method has been published previously.26 In brief, we followed the modified protocol described by Bjornsson et al.27 5-Aza-dC demethylated and CpG methylated Jurkat genomic DNA (New England Biolabs, lpswich, Massachusetts) were used as unmethylated and methylated controls,respectively, in the assays. The LUMA methylation level was expressed as a percentage obtained from the following equation:
Randomly selected samples were replicated to examine the batch effect or variation between the different runs. The corresponding coefficient of variation was <1%.
Diagnoses
Diagnosis of GDM is based on the results from the glucose challenge test (GCT) during the second trimester (around 24-28 weeks) and subsequent glucose tolerance test (GTT) for those who had the GCT value over a 100 mg/dL. At GTT, those who scored greater than 95 mg/dL fasting and then at or above 180, 155, and 140 mg/dL at 1, 2, and 3 hours, respectively, were given a diagnosis of GDM. Preeclampsia required elevated blood pressure at either 140 systolic or 90 diastolic on a minimum of 2 occasions, which were required to be 6 hours apart as well as exhibit proteinuria of at least 300 mg/24 hours or 2+ on urine dip. Obesity was defined as a body mass index (BMI) of >30, based on the mother’s self-report of the prepregnancy weight and height.
Potential confounders
Maternal educational attainment, welfare (entitlement) status, marital status, race, and sex of the baby were deemed a priority as potential confounders. These confounders were ascertained at the initial face-to-face evaluation provided by a social worker at the clinic.
Statistical Analysis
Prior to the analysis, the data were evaluated for normality by examining the univariate indices of skewness. After normality was confirmed, univariate analysis on the level of global methylation in placenta and umbilical cord blood was conducted for each maternal risk condition (ie, GDM, preeclampsia, and obesity). Following these initial univariate comparisons, multivariable analysis was conducted, using a general linear model (GLM), to examine the main effects of the 3 risk conditions (GDM, preeclampsia, and obesity) simultaneously on global methylation in the placenta tissue or umbilical cord blood separately. Multivariable GLMs were conducted to examine the associations between global methylation (in placenta tissue and umbilical cord blood) and birth outcomes (birth weight, gestational age at birth, head circumference, and body length at birth). All analyses were performed first without potential confounders and then with potential confounders for statistical adjustment. An improved Bonferroni procedure for multiple tests of significance28 was applied when related dependent variables were analyzed together.
Results
Global methylation in umbilical cord blood and in placenta tissue was normally distributed with skewness of −0.96 (standard deviation [SD] = .66) and −1.84 (SD = 0.34), respectively. The levels of global methylation in the umbilical cord blood and placenta were not correlated (r = −.11, P = .44). The mean level in umbilical cord blood (69.2%, SD = 7.6) was significantly higher than that of the corresponding placenta (57.1%, SD = 3.4; t = 9.9, P < .0001).
Descriptive statistics for population characteristics and global methylation are presented in Table 1. In brief, 62% of the mothers were Latina, 26% were black, 6% were white, and 6% were Asian. Although there are some mothers with graduate degrees, 28% were high school dropouts, and 8% were still in high school. Approximately three-fourths of the participating women were unmarried. Additionally, 14%, 10%, and 34% of the mothers had GDM, preeclampsia, and obesity, respectively. Of the offsprings, 58% were male and 42% were female. Mean (SD) maternal age was 24.4 (5.7) years.
Table 1.
Global Methylation Level in Specimens (Placenta and Umbilical Cord Blood) and Demographic Characteristics of Mothers.
| Global Methylation Level | Mean (SD) Range |
|---|---|
| Placenta tissue | 57.1 (3.4) 43.1-63.1 |
| Umbilical cord blood | 69.2 (7.6) 48.3-80.2 |
| Demographic Characteristics | N (%) or Mean (SD) Range |
| Mother’s ethnicity | |
| Black | 13 (26) |
| Latina | 31 (62) |
| White | 3 (6) |
| Asian | 3 (6) |
| Mother’s educational attainment | |
| Primary school education | 1 (2) |
| Some high school/dropout | 17 (34) |
| High-school graduate or GED | 10 (20) |
| Some college | 12 (24) |
| Associate degree | 4 (8) |
| BA | 4 (8) |
| Graduate degree | 2 (4) |
| Mother’s marital status at delivery | |
| Married | 13 (26) |
| Single | 36 (72) |
| Widowed | 1 (2) |
| Mother’s problems during pregnancy | |
| Gestational diabetes mellitus (GDM) | 7 (14) |
| Preeclampsia | 5 (10) |
| Obesity | 17 (34) |
| Offspring’s gender | |
| Male | 29 (58) |
| Female | 21 (42) |
| Maternal age | 24.4 (5.7) 15-41 |
Abbreviations: GED, General Educational Development; SD, standard deviation.
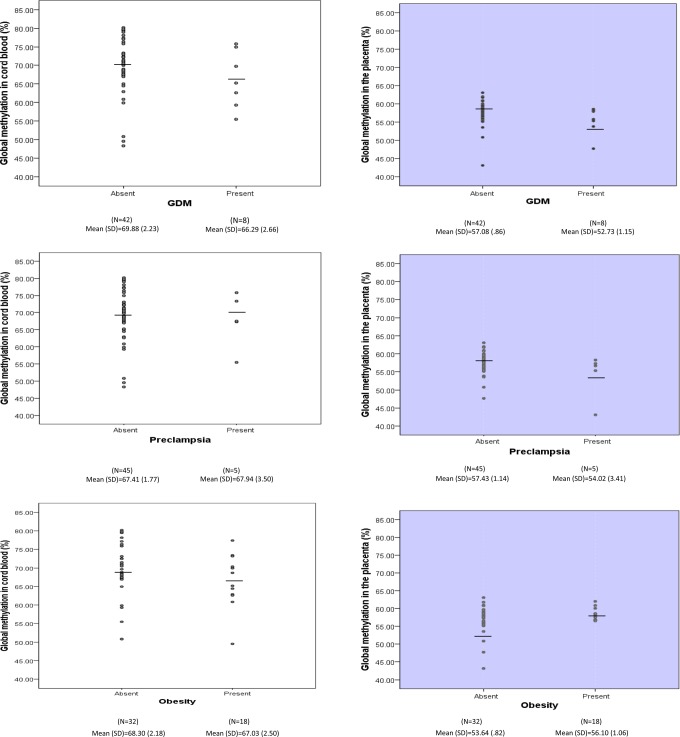
Figure 1 shows the distribution and mean for the levels of global methylation in the umbilical cord blood (gray panels) and in the placenta (white panels). Analysis of umbilical cord blood found no significant differences between the methylation levels for women with each maternal risk condition (GDM, preeclampsia, and obesity) compared to women without each condition. However, analysis of global methylation levels in the placenta tissue showed that women with GDM and women with preeclampsia, compared to their counterparts, had lower levels of global methylation, and women with obesity, compared to their counterparts, had the highest level of global methylation in the placenta. This initial analysis was followed by a multivariable GLM, which analyzed GDM, preeclampsia, and obesity as predictors simultaneously in order to minimize type I error due to multiple testing. First, we analyzed global methylation levels in placenta and umbilical cord blood in relation to maternal GDM, preeclampsia, and obesity without confounders. As seen in Table 2, a lower level of placental methylation was observed in patients with GDM (57.08 vs 52.73, P = .004), and the reversed association was observed for maternal obesity (53.64 vs 56.17, P = .04), compared to mothers without these conditions. The global methylation level in the placenta was lower among women with preeclampsia than women without it (57.43 vs. 54.02), but the difference was not statistically significant. The associations remain significant for the difference between women with and without GDM (P = .004), women with and without obesity (P = .01), and women with and without preeclampsia (P = .05) with the adjustment of potential confounders. With regard to global methylation in umbilical cord blood, no significant associations were detected.
Figure 1.
The level of global methylation (%) in the cord blood and the placenta tissues by maternal gestational diabetes mellitus (GDM), preeclampsia, and obesity.
Table 2.
Main Effects of Maternal GDM, Preeclampsia, and Obesity During Pregnancy on Global Methylation (%) in the Placenta Tissues and Cord Blood in the Multivariable Model.a
| Maternal Characteristics | Global Methylation Placenta Tissue | Global Methylation Umbilical Cord Blood | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Statistics | Statistics | |||||||||||
| Unadjusted Model | Adjusted Model | Unadjusted Model | Adjusted Model | |||||||||
| B | SE | P Value | B | SE | P Value | B | SE | P Value | B | SE | P Value | |
| GDM | 4.34 | 1.25 | .001 | 4.47 | 1.22 | .003 | 3.45 | 3.27 | .30 | 3.72 | 3.44 | .29 |
| Preeclampsia | 2.90 | 1.51 | .06 | 3.02 | 1.48 | .05 | −.29 | 3.96 | .94 | −0.68 | 4.17 | .87 |
| Obesity | −2.4 | 0.89 | .01 | −2.54 | 0.97 | .01 | .33 | 2.32 | .89 | 1.41 | 2.74 | .61 |
Abbreviations: GDM, gestational diabetes mellitus; SE, standard error.
aIn the unadjusted model, no confounder was included. In adjusted models, the effects of sex of the baby, mother’s educational attainment at birth of her child, welfare status, marital status, and ethnicity were statistically controlled for. Maternal GDM, preeclampsia, and obesity during pregnancy were analyzed in the same model simultaneously.
Finally, we explored the association between global methylation in placenta and umbilical cord blood with 4 birth outcomes, birth weight, gestational age, head circumference, and body length of the newborn, by a multivariable GLM after controlling for the effects of confounders (Table 3). Although no associations were observed in the umbilical cord blood global methylation, there was a suggestive association between head circumference (B = −0.24, P = .05) and body length (B = −0.39, P = .05) in the placenta global methylation, suggesting that greater methylation level (%) was associated with reduction in head circumference and body length by 24 and 39 mm, respectively. However, note that P-value of .0125 (0.05/4) is required for the association to be significant with the correction for multiple testing.28
Table 3.
Associations Between Global Methylation (%) and Birth Outcomes in Multivariate Model (N = 50).a
| Birth Outcomes | Placenta Methylation | Cord Blood Methylation | ||
|---|---|---|---|---|
| B (SE) | P Value | B (SE) | P Value | |
| Birth weight | −24.73 (22.27) | .27 | .62 (8.75) | .94 |
| Gestational age | −.15 (.15) | .31 | −.01 (.05) | .82 |
| Head circumference | −.24 (.12) | .05 | .02 (.05) | .76 |
| Body length | −.39 (.19) | .05 | −.02 (.09) | .85 |
Abbreviation: SE, standard error.
aIn the multivariate GLM, 4 birth outcomes were analyzed simultaneously, and the effects of sex of the baby, mother’s educational attainment at birth of her child, welfare status, marital status, and ethnicity were statistically controlled for.
Discussion
This study examines whether maternal GDM, preeclampsia, and obesity have an effect on global methylation levels in placental tissue and umbilical cord blood and whether global methylation levels are predictive of fetal growth and development. Our data provide suggestive evidence that (1) different maternal risk conditions during pregnancy (ie, GDM, preeclampsia, and obesity) were independently associated with global methylation levels in placenta tissue; (2) greater global methylation levels in placenta tissue were associated with smaller head circumference and shorter body length; and (3) the observed associations were tissue specific. These findings reveal a potential biological mechanism through which 3 maternal risk conditions during pregnancy can influence the epigenome in placental tissues and thereby affect fetal programming of development. To our knowledge, this is the first study to examine the relationship between the presence of maternal GDM, preeclampsia, and obesity during pregnancy and the global methylation of different tissues in the human fetus.
This study allowed us to evaluate the utility of peripheral blood as a surrogate tissue for placenta, which has its own function as an exchange organ for all respiratory gases, nutrients, and waste between the fetus and the pregnant woman.29 Our study showed that only global methylation in the placenta tissue, but not in the umbilical cord blood, was significantly associated with any of the 3 maternal risk conditions during pregnancy (ie, GDM, preeclampsia, and obesity) and fetal/infant growth outcomes. These findings, particularly in relation to head circumference, pose the question for future studies, does placenta tissue control not only general fetal growth and development but also specific brain development and function? Although our participants were very young, we were able to monitor the correlations between obstetric outcomes, especially small head circumference, and subsequent neurobehavioral functioning, as they become older in our longitudinal study. It is important to note that the significance levels that we observed in associations between placental global methylation and infant outcomes (head circumference and body length) did not reach the level of significance (ie, 0.0125) after the correction for multiple testing. However, the magnitude of associations between placental methylation and infant outcomes (head circumference and body length) is substantially greater than associations between umbilical cord blood methylation and infant outcomes. While suggestive, it is possible that the associations between placental global methylation, and head circumference and body length were observed due to chance. Future studies with greater sample size should clarify these associations.
Despite these limitations, our findings demonstrate that the global DNA methylation profile of the placenta tissue, but not the umbilical cord blood, is associated with particular maternal, pregnancy, and newborn phenotypes, which deserve further investigation and discussion. The placenta supports and regulates embryonic development by providing the fetal growth environment, coordinating embryogenesis, and supplying the maternal–fetal interface.19 Accordingly, the placenta has been shown to be the least methylated organ of the human body30,31 as is also the case in our study which found that the placenta had a global methylation level about 10% lower than umbilical cord blood. It is plausible that the areas that were found to be differentially methylated in the placenta are critical to the metabolic development of the fetus and are responsive to environmental stimuli. In contrast, these same genomic areas may already be permanently methylated and confined in heterochromatic areas of the already specialized umbilical cord blood cells and are therefore unresponsive to environmental stimuli of metabolic changes. Such programming entails the silencing of genes that are normally functioning in a tissue like the placenta; nonetheless, we should be cautious when using umbilical cord blood as a specialized fetal tissue. However, this conclusion must be considered preliminary until these findings are replicated in a larger scale study with a longer follow-up period.
The association between global methylation and maternal risk conditions during pregnancy under examination (ie, GDM, preeclampsia, and obesity), as well as fetal/infant growth, as measured by birth outcomes, need to be carefully evaluated. For example, GDM and preeclampsia were associated with placental hypomethylation, but placental hypomethylation was then associated with increased head circumference and body length. We have previously reported that LUMA measurement reflects more of a methylation status in the cytosine and guanine (CG)-rich region of the genome such as CpG island or gene promoters.26 Although a useful marker for assessing global methylation status, LUMA methylation cannot tell us whether genes with positive or negative effects have been silenced or have contributed to significant findings. Since a more profound understanding depends on the examination of methylation of specific genes, a comprehensive interpretation should await the completion of whole-genome methylation profiling or gene-specific methylation studies.
Our study has several notable strengths. First, LUMA-analyzed placenta and umbilical cord blood global methylation can show whether surrogate tissue, such as peripheral blood, is comparable with the placenta regarding methylation levels. Second, the mother’s BMI was recorded at the initial prenatal visit, and GDM and preeclampsia were monitored throughout the pregnancy prospectively rather than relying on the mother’s retrospective recollection. Fetal growth and development indices were measured and recorded at birth at the labor and delivery wards by clinicians who were blind to the purpose of the study. Third, we are in the early phase of a prospective follow-up study evaluating the trajectory of the physical, mental, and neurobehavioral development of the children.
While suggestive, our study is nonetheless limited in its statistical power to detect a stronger association by a relatively small sample size (N = 50). Although we did not find any of the maternal risk conditions (GDM, preeclampsia, and obesity) during pregnancy to have significant effects on global methylation in the umbilical cord blood, we should be cautious about dismissing the utility of peripheral blood as a surrogate tissue until future studies with larger sample sizes replicate these findings. Moreover, the significant differences in global methylation in the placenta tissues were relatively small (approximately 3%-5%). However, our group previously reported a similar size of difference between cases and controls (57.3% vs. 52.3%) in a large epidemiologic study of breast cancer.32 Given that LUMA measures global methylation content, 3%-5% reflects a substantial difference in the whole genome at large. This difference in LUMA measures strongly suggests that epigenetics may contribute to disease status. In addition to the small sample size, our data contain potential outliers (see Figure 1). Especially within the risk groups (participants with GDM, preeclampsia, and obesity) the outliers could have influenced the findings. However, without these outliers in groups with GDM and preeclamsia, the mean global methylation level could have been greater, making the difference more distinctive and possibly more significant. There is no outlier in the groups by obesity.
Overall, the current study has generated promising findings which show that a mother’s metabolic problems during pregnancy may influence the epigenome in the offspring. Future studies should continue to test global methylation levels in both placenta and umbilical cord blood. Moreover, studies should prospectively follow the offspring to examine developmental trajectories, especially central nervous system development, by monitoring “trigger events,” such as missing certain developmental milestones, and motor or cognitive performance,33 to see whether epigenetic changes seen in umbilical cord blood and placenta have distinct impacts on human development at different stages across the lifespan.
Acknowledgments
We are grateful to the Labor & Delivery and Postpartum Units at the Mount Sinai School of Medicine, OB/GYN Department for assistance in acquisition of extraplacental tissues. We thank Michelle Yoon for her research coordination; Rachel Lifshitz, Michael Tymon, Vanitha Gounder, Kristian Picon, and Eddie Ham for help with tissue collection and processing; Solomon Bienstock and Stephanie Gampel for DNA and RNA extraction; and James Schmeidler, PhD, for statistical analysis related to multiple testing.
Footnotes
Authors’ Contribution: Nomura and Lambertini contributed equally to this work.
Declaration of Conflicting Interests: The author(s) declared no conflicts of interest with respect to the authorship and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research and/or authorship of this article: the National Institutes of Health (NIH ) grants from the NIMH (K01 MH080062), the NCRR (P20 RR018728), Queens College, CUNY Research Enhancement Grant, and the Venture Capital Research Funding Program of the Mount Sinai Children’s Environmental Health Center.
References
- 1. Berkowitz G, Lapinski R, Wein R, Lee D. Race/ethnicity and other risk factors for gestational diabetes. Am J Epidemiol. 1992;135(9):965–973. [DOI] [PubMed] [Google Scholar]
- 2. Ferrara A. Increasing prevalence of gestational diabetes mellitus: a public health perspective. Diabetes Care. 2007;30(suppl 2):S141–S146. [DOI] [PubMed] [Google Scholar]
- 3. Boney C, Verma A, Tucker R, Vohr B. Metabolic syndrome in childhood: association with birth weight, maternal obesity and gestational diabetes mellitus. Pediatrics. 2005;115(3):290–296. [DOI] [PubMed] [Google Scholar]
- 4. Catalano P, Presley L, Minium J, Hauguel-De S. Fetuses of obese mothers develop insulin resistance in utero. Diabetes Care. 2009;32(6):1076–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Catalano P, Farrell K, Thomas A, et al. Perinatal risk factors for childhood obesity and metabolic dysregulation. Am J Clin Nutr. 2009;90(5):1303–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Knight M; UKOSS. Eclampsia in the United Kingdom 2005. BJOG. 2007;114(9):1072–1078. [DOI] [PubMed] [Google Scholar]
- 7. Kral J, Naslund E. Surgical treatment of obesity. Nat Clin Pract Endocrinol Metab. 2007;3(8):574–583. [DOI] [PubMed] [Google Scholar]
- 8. Lawlor D, Timpson N, Harbord R, et al. Exploring the developmental overnutrition hypothesis using parental-offspring associations and FTO as an instrumental variable. PLoS Med. 2008;5(3):84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Taylor P, Poston L. Developmental programming of obesity in mammals. Exp Physiol. 2006;92(2):287–298. [DOI] [PubMed] [Google Scholar]
- 10. Nomura Y, Marks D, Grossman B, et al. Exposure to gestational diabetes mellitus and low socioeconomic status: effects on neurocognitive development and risk of attention-deficit/hyperactivity disorder in offspring. Arch Pediatr Adolesc Med. 2012;166(4):337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Krakowiak P, Walker C, Bremer A, et al. Maternal metabolic conditions and risk for autism and other neurodevelopmental disorders. Pediatrics. 2012;129(5):1121–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Barker DJ. Mothers, Babies, and Health in Later Life. 2nd ed Edinburgh, UK: Churchill Livingstone; 1998. [Google Scholar]
- 13. McMillen IC, Robinson JS. Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol Rev. 2005;85(2):571–633. [DOI] [PubMed] [Google Scholar]
- 14. Thompson C, Syddall H, Rodin I, Osmond C, Barker D. Birth weight and the risk of depressive disorder in late life. Br J Psychiatry. 2001;179:450–455. [DOI] [PubMed] [Google Scholar]
- 15. Sallout B, Walker M. The fetal origin of adult diseases. J Obstet Gynecol. 2003;23(5):555–560. [DOI] [PubMed] [Google Scholar]
- 16. Talge N, Neal C, Glover V. Antenatal maternal stress and long-term effects on child neurodevelopment: how and why? J Child Psychol Psychiatry. 2007;48(3-4):245–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Barker D. The developmental origins of adult disease. J Am Coll Nutr. 2004;23(suppl 6):588S–595S. [DOI] [PubMed] [Google Scholar]
- 18. Barker D. Intrauterine programming of adult disease. Mol Med Today. 1995;1(9):418–423. [DOI] [PubMed] [Google Scholar]
- 19. Lambertini L, Lee MJ, Marsit CJ, Chen J. Genomic imprinting in human placenta. In: Zheng J, ed. Recent Advances in Research on the Human Placenta. Rijeka, Croatia: InTech Open Access Publisher; 2012:357–377. [Google Scholar]
- 20. Santos F, Dean W. Epigenetic reprogramming during early development in mammals. Reproduction. 2004;127(6):643–651. [DOI] [PubMed] [Google Scholar]
- 21. Tobi EW, Lumey LH, Talens RP, et al. DNA methylation differences after exposure to prenatal famine are common and timing- and sex-specific. Hum Mol Genet. 2009;18(21):4046–4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cutfield W, Hofman P, Mitchell M, Morison I. Could epigenetics play a role in the developmental origins of health and disease? Pediatr Res. 2007;61(5 pt 2): 68R–75R. [DOI] [PubMed] [Google Scholar]
- 23. Ornoy A, Ratzon N, Greenbaum C, Wolf A, Dulitzky M. School-age children born to diabetic mothers and to mothers with gestational diabetes exhibit a high rate of inattention and fine and gross motor impairment. J Pediatr Endocrinol Metab. 2001;14(suppl 1):681–689. [DOI] [PubMed] [Google Scholar]
- 24. Dionne G, Boivin M, Séguin JR, Pérusse D, Tremblay RE. Gestational diabetes hinders language development in offspring. Pediatrics. 2008;122(5):1073–1079. [DOI] [PubMed] [Google Scholar]
- 25. Veena SR, Krishnaveni GV, Srinivasan K, et al. Childhood cognitive ability: relationship to gestational diabetes mellitus in India. Diabetologia. 2010;53(10):2134–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xu X, Gammon MD, Herandez-Vargas H, et al. DNA methylation in peripheral blood measured by LUMA is associated with breast cancer in population-based study. FASEB J. 2012;26(6):2657–2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bjornsson HT, Sigurdsson MI, Fallin MD, et al. Intra individual change over time in DNA methylation with familial clustering. JAMA. 2008;299(24):2877–2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Simes RJ. An improved Bonferroni procedure for multiple tests of significance. Biometrika. 1986;73(3):751–754. [Google Scholar]
- 29. Ramsey EM. The Placenta: Human and Animal. 1st ed New York, NY: Praeger; 1982. [Google Scholar]
- 30. Ehrlich M, Gama-Sosa MA, Huang LH, et al. Amount and distribution of 5-methylcytosine in human DNA from different types of tissues of cells. Nucleic Acids Res. 1982;10(8):2709–2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gama-Sosa MA, Midgett RM, Slagel VA, et al. Tissue-specific differences in DNA methylation in various mammals. Biochim Biophys Acta. 1983;740(2):212–219. [DOI] [PubMed] [Google Scholar]
- 32. Hu D, Cross JC. Development and function of trophoblast giant cells in the rodent placenta. Int J Dev Biol. 2010;54(2-3):341–354. [DOI] [PubMed] [Google Scholar]
- 33. Williams PL, Seage III GR, van Dyke RB, et al. A trigger-based design for evaluating the safety of in utero antiretroviral exposure in uninfected children of human immunodeficiency virus-infected mothers. Am J Epidemiol. 2012;175 (9):950–961. [DOI] [PMC free article] [PubMed] [Google Scholar]