Abstract
Estradiol 17β (E2β) and ascorbic acid (AA) have been implicated in cancer progression. However, little is known about the actions of biologically active metabolites of E2β, 2-hydroxyestradiol (2OHE2), 4-hydroxyestradiol (4OHE2), 2-methoxyestradiol (2ME2), and 4-methoxyestradiol (4ME2) synthesized sequentially by cytochrome P450, family 1, subfamily A (CYP1A1) and B (CYP1B1), polypeptide 1, and catechol-O-methyltransferase (COMT) on ovarian cancer. Herein, we examined the expression of CYP1A1, CYP1B1, COMT, and estrogen receptor α (ERα) and β (ERβ) in human ovarian surface epithelial (IOSE-385) and cancer cell lines (OVCAR-3, SKOV-3, and OVCA-432). We also investigated the roles of E2β, 2OHE2, 4OHE2, 2ME2, and 4ME2 in cell proliferation, and their interactive effects with AA on ovarian cells. We found the expression of CYP1A1, CYP1B1, COMT, ERα, and ERβ in most cell lines tested. Treating cells with physiological concentrations of E2β and its metabolites promoted (13%-42% of the control) IOSE-385 and OVCAR-3 proliferation. The ER blockade inhibited IOSE-385 (∼76%) and OVCAR-3 (∼87%) proliferative response to E2β but not to its metabolites. The ERα blockade inhibited (∼85%) E2β-stimulated OVCAR-3 proliferation, whereas ERβ blockade attenuated (∼83%) E2β-stimulated IOSE-385 proliferation. The AA at ≥250 μmol/L completely inhibited serum-stimulated cell proliferation in all cell lines tested; however, such inhibition in IOSE-385, OVCAR-3, and OVCA-432 was partially (∼10%-20%) countered by E2β and its metabolites. Thus, our findings indicate that E2β and its metabolites promote cell proliferation and antagonize the AA-suppressed cell proliferation in a subset of ovarian cancer cells, suggesting that blocking the actions of E2β and its metabolites may enhance AA’s antiovarian cancer activity.
Keywords: E2β, E2β metabolites, ascorbic acid, ovarian cancer cells, growth
Introduction
Ovarian cancer is the most lethal gynecological malignancy, largely because cancer cells acquire a chemoresistant phenotype after initial cytoreductive surgery and chemotherapy in the majority of cases.1 In addition, another major challenge of the current cancer treatment is that ovarian cancer cells are highly heterogeneous at the cellular and molecular levels.1 Thus, understanding individual subtypes of ovarian cancer at these levels is critical for developing an efficacious individual therapy.1
Estrogen has been considered as a major risk factor for ovarian cancer, particularly since recent epidemiological studies have demonstrated an elevation of ovarian cancer incidence with postmenopausal usage of estrogen.1 Nonetheless, the exact role of estrogen in ovarian cancer is still controversial, since estrogen may have stimulatory, inhibitory, or no effect on ovarian cancer cell growth, possibly depending on the individual subtypes of ovarian cancer, doses of estrogen used, and patients’ ages (eg, pre- vs postmenopause).1,2 Additionally, although the majority of ovarian cancer cells express estrogen receptor α (ERα) and β (ERβ), these receptors may exert opposite actions on ovarian cancer as ERα promotes ovarian cancer progression, whereas ERβ may antagonize ERα’s actions.1–3 Moreover, recent evidence has also indicated that estrogen-induced growth of ovarian cancer could be independent of ER.1,3 To further complicate the situation and possibly explain this latter observation, estrogen can be converted into a number of biologically active metabolites. For example, as catalyzed by cytochrome P450, family 1, subfamily A (CYP1A1) polypeptide 1 and subfamily B (CYP1B1) polypeptide 1, estradiol 17β (E2β) can be further hydroxylated to form the catecholestradiols 2-hydroxyestradiol (2OHE2) and 4-hydroxyestradiol (4OHE2), which can be subsequently methylated into the methoxyestradiols 2-methoxestradiol (2ME2) and 4-methoxyestradiol (4ME2) by catechol-O-methyltransferase (COMT). 4 Apart from CYP450- and COMT-mediated hydroxylation and methylation, E2β can also be conjugated to glucuronides and sulfates.5,6 These conjugated E2β themselves have no estrogen activity but may restore such an activity after the release of glucuronides and sulfates.7
The physiological plasma concentrations of E2β and its metabolites range from 0.01 to 2.2 nmol/L in premenopausal, nonpregnant women, increase dramatically in pregnant women, and decrease sharply in postmenopausal women.8–11 These E2β metabolites with their biological activities independent of ER4 are either pro- or antiovarian cancer.12–14 Indeed, 4OHE2 at 0.01 to 0.1 nmol/L has been show to stimulate ovarian cancer (OVCAR-3) cell proliferation in vitro,15 whereas 2ME2 at supraphysiological concentrations (inhibitory concentration 50 [IC50] > 200 nmol/L) inhibits cell proliferation of many ovarian cancer cell lines including OVCAR-313,16 and primary ovarian cancer cells.17
Ascorbic acid (AA; vitamin C) has long been implicated in preventing and treating cancers.18 However, no conclusive clinic results have been obtained so far. This is thought to be largely attributed to the fact that AA levels in vivo cannot reach concentrations sufficient to induce anticancer actions even with oral supplementation approaching maximally tolerated doses.19,20 For instance, with the daily oral supplement of AA up to 2500 mg in human, circulating AA levels are likely to be maintained approximately at 80 μmol/L similar to those by the consumption of 5 servings of fruits and vegetables.19,20 At these physiological levels (<100 μmol/L19,20), acute (1-2 hours) treatment of AA does not have any significant impact on the growth of cancer cells.21,22 Conversely, circulating AA levels can be easily elevated to nontoxic, pharmacologic levels (up to 20-30 mmol/L by intravenous injection19,20). At these pharmacologic levels, AA can specifically kill ovarian cancer cells in vitro (with half maximal effective concentration [EC50] < 10 mmol/L) and suppress the progression of ovarian cancer in mice, possibly via locally generating high levels of H2O2.21–23
To date, little is known regarding the actions of E2β and its metabolites and prolonged AA treatment on the growth of ovarian cancer cells. Particularly, it is unknown whether E2β and its metabolites within their physiological ranges can affect anticancer actions of AA, which may be part of reason for the failure of AA at physiological concentrations to prevent and treat ovarian cancer. Therefore, we examined the expression of CYP1A1, CYP1B1, and COMT in ovarian cancer cell lines. To test the hypotheses that E2β and its metabolites stimulate ovarian cancer cell proliferation and migration via an ER or ER-independent manner and attenuate the effects of AA on ovarian cancer cell proliferation, we also investigated cell proliferation and/or migration in response to estrogen and its metabolites as well as AA in the presence of ER antagonists using 1 human ovarian surface epithelial (IOSE-385) and 3 ovarian cancer cell lines (OVCAR-3, SKOV-3, and OVCA-432).
Materials and Methods
Cell Lines
A human ovarian surface epithelial cell line IOSE-385 immortalized by SV40 large-T antigen was kindly provided by Dr Nelly Auersperg of the Canadian Ovarian Tissue Bank. Human ovarian adenocarcinoma cell lines, OVCAR-3 and SKOV-3, were obtained from the American Type Culture Collection (Manassas, Virginia), and OVCA-432 was established as described.24 All these cancer cell lines were isolated from ascites fluid and were classified as cisplatin resistant.25 However, these cancer cells differ in many other aspects. For example, OVCAR-3 and SKOV-3 are p53 mutant and null, respectively, whereas OVCA-432 expresses wild-type p53.25 In addition, OVCAR-3, but not SKOV-3, responds to estrogen, although both express ERα and ERβ.26 Moreover, OVCAR-3 and OVCA-432, but not SKOV-3, express CA125, a major ovarian cancer biomarker.1 Thus, these cancer cell lines may represent cisplatin-resistant cohorts of patients with cancer cells with either positive or negative expression of p53 and CA125.
The OVCAR-3 cells were expanded in RPMI1640 medium (Gibco-BRL, Gaithersburg, Maryland) containing 10% fetal bovine serum (FBS; Gibco-BRL, 1% penicillin/streptomycin [P/S; HyClone], and 10 μg/mL insulin [Sigma, St Louis, Missouri]). The SKOV-3 cells were expanded in RPMI 1640 medium containing 10% FBS and 1% P/S. The OVCA-432 and IOSE385 cells were expanded in medium 199/105 (Sigma) containing 10% FBS and 1% P/S. All cell lines were maintained in a humidified atmosphere containing 5% CO2 at 37°C. The IOSE-385 at passages 13 to 15, OVCAR-3 at passages 32 to 35, SKOV-3 at passages 29 to 31, and OVCA-432 at passages 18 to 20 were used in the current study.
Western Blotting
To examine the expression of CYP1A1, CYP1B1, COMT, ERα, and ERβ in ovarian cells, Western blotting was performed.27–29 The cells were lysed by sonication in buffer. After centrifugation, protein concentrations of the supernatant were determined using bovine serum albumin (fraction V; Sigma) as standards. Protein samples (30 μg) were separated on 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis gels and electrically transferred to polyvinylidene difluoride membranes (100 V, 60 minutes). Nonspecific binding was blocked with 5% fat-free milk in Tris buffer (50 mmol/L Tris-HCl, pH 7.5, 0.15 mol/L NaCl, and 0.05% Tween-20) for 60 minutes. Membranes were incubated with primary antibodies overnight at 4°C. The CYP1A1, CYP1B1, COMT, ERα, and ERβ proteins were probed using anti-CYP1A1 (1:500), anti-CYP1B1 (1:500), anti-COMT (1:1000), anti-ERα (1:500), or anti-ERβ (1:500) antibody, respectively. Glyceraldehyde 3-phosphate dehydrogenase was utilized as a loading control. After washing, membranes were incubated with corresponding peroxidase-conjugated immunoglobulin G for 60 minutes and detected with enhanced chemiluminescence reagents (Amersham Biosciences, Piscataway, New Jersey), followed by exposure to chemiluminescence films.
Cell Proliferation
Cell proliferation assay was performed.28,29 Cells seeded in 96-well plates (1000-5000 cells/well) were cultured in phenol red-free RPMI1640 medium containing 5% charcoal-stripped FBS and 1% P/S (designated as the complete media). After 24 hours, the cells were treated without or with 0.01 to 100 nmol/L of E2β and its metabolites freshly made in the complete media up to 6 days. Additional cells were treated without or with 65 to 2000 μmol/L of AA in media (diluted from the stock solution at 20 mmol/L; pH in final media ≈ 7.4). The medium was replaced with freshly made hormones or AA every 48 hours to ensure stable bioavailability. After 4 to 6 days of culture, the cell number was determined using the crystal violet staining method.28,29 After an optimal dose of E2β, its metabolites, and AA was determined, additional cells were treated with E2β or its metabolites in the presence of 1 μmol/L of ICI (a nonspecific ER antagonist), methyl-piperidino-pyrazole (MPP; a selective ERα antagonist), or pyrazolo [1,5-a] pyrimidine (PHTPP; a selective ERβ antagonist; 1 hour of pretreatment) to verify the role of ER subtypes in ovarian cell proliferation. To examine the effects of E2β and its metabolites on the AA’s actions, additional cells were pretreated (1 hour) with E2β or its metabolites (0.1 nmol/L) in the presence of 1 μmol/L of ICI, MPP, or PHTPP (added 1 hour before the treatment of E2β or its metabolites) and in the absence or presence of 1 μmol/L of ICI, MPP, or PHTPP (added 1 hour before the treatment of E2β or its metabolites), followed by treatment with 80 μmol/L of AA.19,20
Cell Migration
To determine the effects of E2β and its metabolites on ovarian cell migration, the wound healing assay was performed as described.28 Cells were cultured in 12-well plates in complete growth media until reaching confluence, followed by serum starvation for 16 hours. A sterilized 200-μL pipette tip was used to make a straight scratch. The cells were washed once and then treated without or with 0.1 nmol/L of E2β, 2OHE2, 4OHE2, 2ME2, and 4ME2 in the complete media. Two images per scratch were photographed under a 10× objective immediately after scratching and then for every 8 hours up to 40 hours. Sizes of the wound area were calculated using the MetaMorph image analysis software (Molecular Devices, Sunnyvale, California).
Statistical Analysis
Data were analyzed using 1-way analysis of variance (SigmaStat; Jandel Co, San Rafael, California). When an F test was significant, data were compared with their respective control by the Bonferroni multiple comparison test or Student t test. P ≤ .05 was considered statistically significant.
Results
Expression of CYP1A1, CYP1B1, COMT, ERα, and ERβ
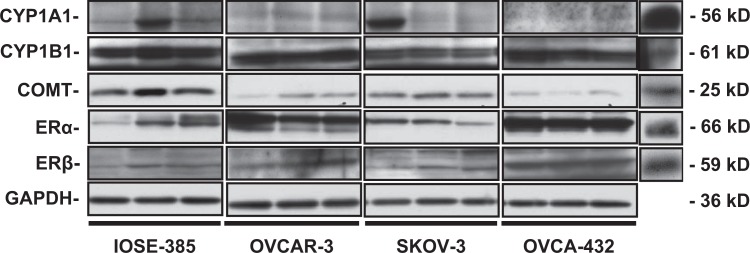
Western blotting revealed the presence of CYP1A1, CYP1B1, COMT, ERα, and ERβ in all cell lines tested except CYP1A1 that was undetectable in OVCA-432 (Figure 1). The levels of CYP1A1 and CYP1B1 were similar among IOSE-385, OVCAR-3, and SKOV-3. The levels of COMT in IOSE-385 cells were at least 2-fold greater (P ≤ .05) than those in other cell lines, and the levels of ERα and ERβ in OVCA-432 cells were higher (P ≤ .05) than those in other cell lines (Table 1). It is also noted that the CYP1B1 levels were at least 2-fold higher (P ≤ .05) than the CYP1A1 levels in IOSE-385, OVCAR-3, and OVCA-432, suggesting that CYP1B1 is a predominant member of CYP1 family in these ovarian cells.
Figure 1.
Western bolt analysis of CYP1A1, CYP1B1, COMT, ERα, and ERβ in ovarian cells. Different lanes in each ovarian cell line represent different passages of cells. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was utilized as a loading control. Positive controls (CTRL) were mouse liver (for CYP1A1), human placenta (for CYP1B1 and COMT), and mouse thyroid extracts (for ERα and ERβ). COMT indicates catechol-O-methyltransferase; CYP1A1, cytochrome P450, family 1, subfamily A; CYP1B1, cytochrome P450, family 1, subfamily B; ERα, estrogen receptor α.
Table 1.
Protein Levels of CYP1A1, CYP1B1, COMT, ERα, and ERβ in IOSE-385, OVCAR-3, SKOV-3, and OVCA-432 Cells.a
| IOSE-385 | OVCAR-3 | SKOV-3 | OVCA-432 | |
|---|---|---|---|---|
| CYP1A1 | 0.36 ± 0.19b | 0.04 ± 0.02b | 0.30 ± 0.30 | ND |
| CYP1B1 | 1.10 ± 0.19b | 1.21 ± 0.09b | 0.75 ± 0.19 | 1.07 ± 0.22 |
| COMT | 1.17 ± 0.57c | 0.18 ± 0.06 | 0.56 ± 0.04 | 0.07 ± 0.02 |
| ERα | 0.35 ± 0.14 | 0.78 ± 0.08 | 0.38 ± 0.14 | 1.96 ± 0.16c |
| ERβ | 0.19 ± 0.07 | 1.13 ± 0.35 | 0.16 ± 0.10 | 1.63 ± 0.12c |
Abbreviations: COMT, catechol-O-methyltransferase; CYP1A1, cytochrome P450, family 1, subfamily A; CYP1B1, cytochrome P450, family 1, subfamily B; ERα, estrogen receptor α; ND, not detectable; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; SEM, standard error of the mean.
a Data normalized to GAPDH are expressed as mean ± SEM (P ≤ .05, n = 3).
b Differs from each other within each individual cell line.
c Differs from other cell lines.
Effects of E2β and Its Metabolites on Cell Proliferation
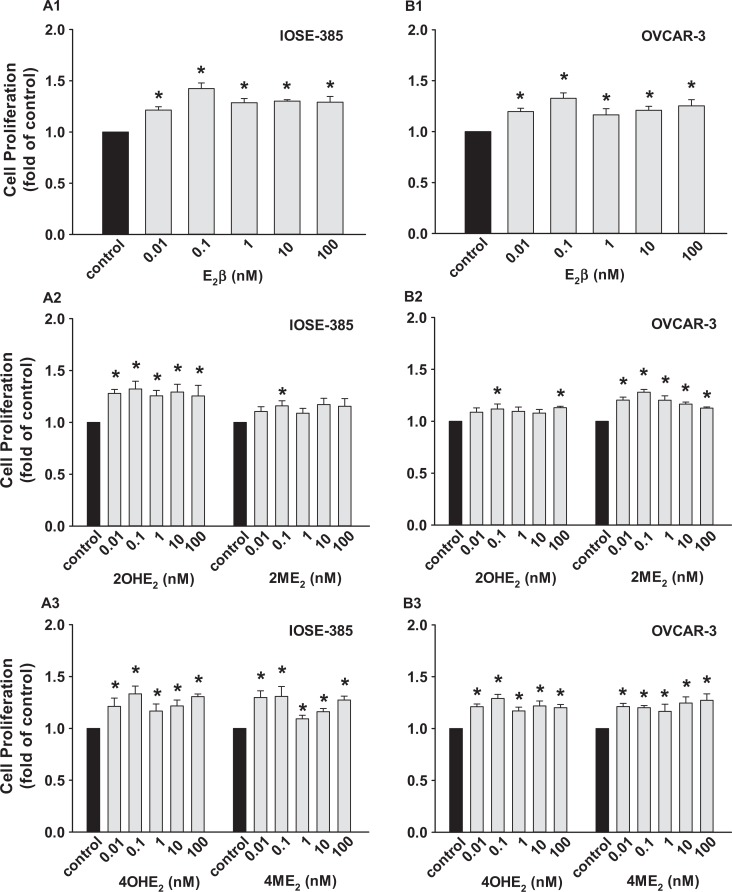
The E2β and its metabolites stimulated (P ≤ .05) IOSE-385 and OVCAR-3 proliferation (Figure 2) but did not affect SKOV-3 and OVCA-432 proliferation (not shown). The E2β stimulated IOSE-385 and OVCAR-3 proliferation with maximum responses observed at 0.1 nmol/L (1.42 ± 0.05 and 1.33 ± 0.05 fold of the control for IOSE-385 and OVCAR-3, respectively; Figure 2A1 and B1). The 2OHE2, 4OHE2, and 4ME2 at all doses studied also similarly promoted (P ≤ .05) IOSE-385 proliferation; however, 2ME2 did so only at 0.1 nmol/L (Figure 2). Moreover, 4OHE2, 2ME2, and 4ME2 at all doses studied also significantly promoted (P ≤ .05) OVCAR-3 proliferation; however, 2OHE2 did so only at 0.1 and 100 nmol/L (Figure 2).
Figure 2.
Effects of E2β, 2OHE2, 4OHE2, 2ME2, and 4ME2 on (A) IOSE-385 and (B) OVCAR-3 proliferation. Cells seeded (1000 and 5000 cells/well for IOSE-385 and OVCAR-3, respectively) were treated with E2β and its metabolites for 6 days. Data are expressed as mean ± SEM fold of the vehicle control (n = 4). *Differs from the vehicle control (P ≤ .05). E2β indicates estradiol 17β; SEM, standard error of the mean; 2ME2, 2-methoxyestradiol; 4ME2, 4-methoxyestradiol; 2OHE2, 2-hydroxyestradiol; 4OHE2, 4-hydroxyestradiol.
Roles of ERα and ERβ in Cell Proliferation
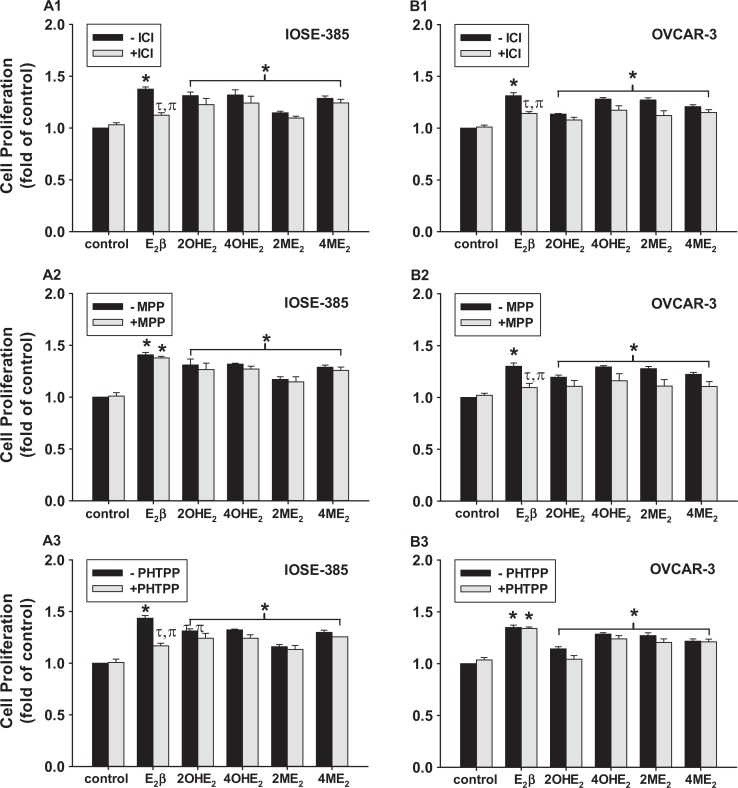
The ICI alone had no effects on IOSE-385 and OVCAR-3 proliferation; however, ICI partially (∼76% and 87% for IOSE-385 and OVCAR-3) inhibited (P ≤ .05) cell proliferative response to E2β but not to its metabolites (Figure 3A1 and B1). The ERα blockade with MPP inhibited (∼80%; P ≤ .05) E2β-stimulated OVCAR-3 but not IOSE-385 proliferation (Figure 3A2 and B2). In contrast, ERβ blockade with PHTPP attenuated (∼80%; P ≤ .05) E2β-stimulated IOSE-385 but not OVCAR-3 proliferation (Figure 3A3 and B3). However, neither MPP nor PHTPP affected IOSE-385 and OVCAR-3 proliferative responses to these E2β metabolites, confirming no participation of ERα and ERβ in these E2β metabolites-stimulated cell proliferations (Figure 3A2 and B2).
Figure 3.
Effects of ICI, MPP, and PHTPP on (A) IOSE-385 and (B) OVCAR-3 proliferative responses to E2β, 2OHE2, 4OHE2, 2ME2, and 4ME2. The cells were treated with 0.1 nmol/L E2β and its metabolites in the presence of 1 μmol/L of ICI, MPP, or PHTPP for 6 days. Data are expressed as mean ± SEM fold of the vehicle control (n = 4). *Differs from the vehicle control (−ICI; P ≤ .05). τDiffer from the E2β treatment (P ≤ .05). πDiffers from the ICI control (P ≤ .05). E2β indicates estradiol 17β; MPP, methyl-piperidino-pyrazole; PHTPP, pyrazolo [1,5-a] pyrimidine; SEM, standard error of the mean; 2ME2, 2-methoxyestradiol; 4ME2, 4-methoxyestradiol; 2OHE2, 2-hydroxyestradiol; 4OHE2, 4-hydroxyestradiol.
Ascorbic Acid Suppresses Cell Proliferation But Not Migration
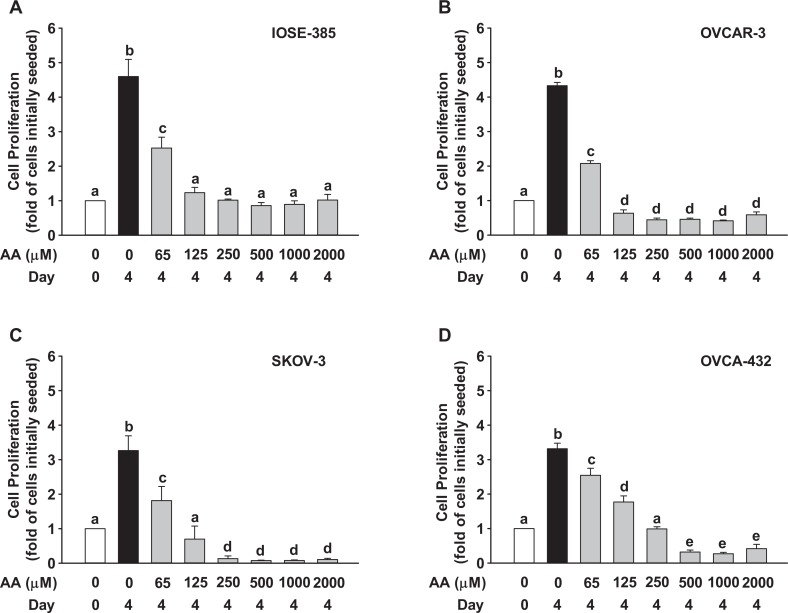
When compared to the day 4 control (without AA), AA decreased (P ≤ .05) cell number in all the doses and in all the cell lines tested (Figure 4). Interestingly, when compared to the cell number initially seeded, AA at any dose studied did not cause cell loss in IOSE-385; however, AA at doses ≥250, 500, and 125 μmol/L, respectively, for SKOV-3, OVCAR-3, and OVCA-432 caused significant (P ≤ .05) cell loss (not shown).
Figure 4.
Effects of AA on (A) IOSE-385, (B) OVCAR-3, (C) SKOV-3, and (D) OVCA-432 proliferation. Cells initially seeded at 3000 cells/well for IOSE-385 and 5000 cells/well for OVCAR-3, SKOV-3, and OVCA-432 were treated with AA in the complete media for 4 days. Data are expressed as mean ± SEM fold of the cells initially seeded (n = 4).a-eDifferent letters differ from each other (P ≤ .05). AA indicates ascorbic acid; SEM, standard error of the mean.
When compared to the corresponding time control, treatments of E2β and its metabolites (not shown) did not alter cell migration in all the cell lines tested even up to 40 hours. However, the pattern of cell migration differed among these cells tested. For example, when compared to the time 0 control, E2β and its metabolites did not cause any change in the scratch gaps in OVCAR-3 and OVCA-432 up to 40 hours (not shown). Nonetheless, the scratch gaps in SKOV-3 and IOSE-385 similarly narrowed sharply at 16 hours and were almost healed at 24 hours between the control versus E2β and its metabolites (not shown).
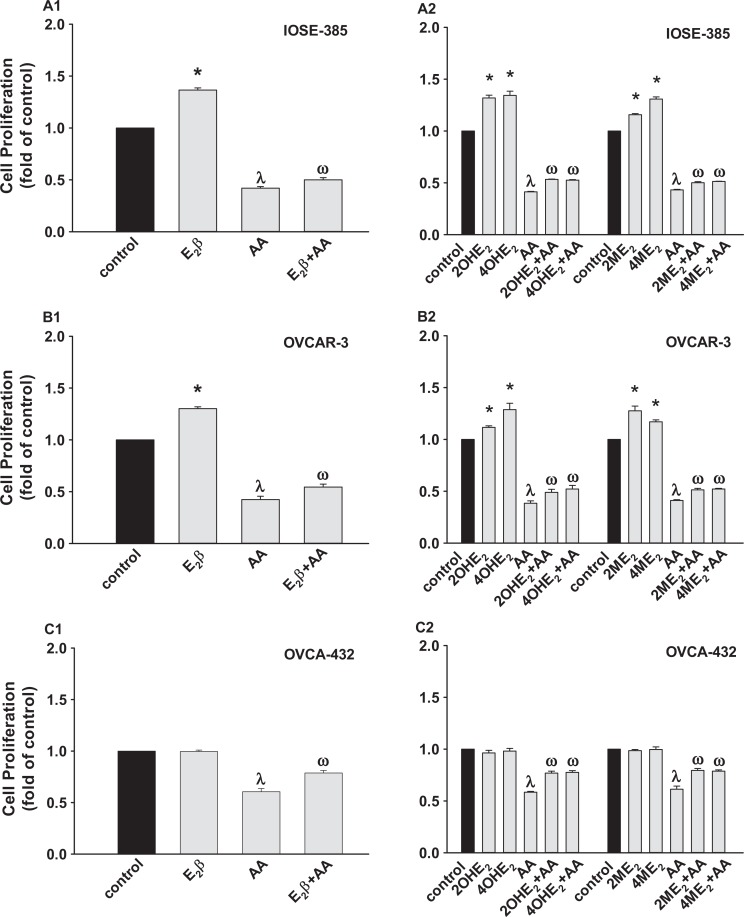
Estradiol2β and Its Metabolites Antagonize AA-Suppressed Cell Proliferation
When compared to the control, AA at 80 μmol/L inhibited (P ≤ .05) cell proliferation in IOSE-385, OVCAR-3, OVCA-432 (Figure 5), and SKOV-3 (not shown). However, pretreatment of E2β and its metabolites at 0.1 nmol/L slightly but significantly (P ≤ .05) attenuated the AA-inhibited cell proliferation in IOSE-385, OVCAR-3, and OVCA-432 (Figure 5) but not in SKOV-3 (not shown). Moreover, neither ICI, MPP, nor PHTPP affected the effects of E2β and its 4 metabolites on AA-inhibited cell proliferation (not shown), suggesting no participation of ER in such stimulatory actions induced by E2β and its 4 metabolites.
Figure 5.
Effects of E2β, 2OHE2, 4OHE2, 2ME2, and 4ME2 on AA-inhibited (A) IOSE-385, (B) OVCAR-3, and (C) OVCA-432 proliferation. Cells seeded at 5000 cells/well were treated with 80 μmol/L of AA in the presence of 0.1 nmol/L of E2β and its metabolites (1 hour pretreatment) for 4 days. Data are expressed as mean ± SEM fold of the vehicle control (n = 4). *,λDiffers from the vehicle control (P ≤ .05). ωDiffers from the AA (P ≤ .05). AA indicates ascorbic acid; E2β, estradiol 17β; SEM, standard error of the mean; 2ME2, 2-methoxyestradiol; 4ME2, 4-methoxyestradiol; 2OHE2, 2-hydroxyestradiol; 4OHE2, 4-hydroxyestradiol.
Discussion
Herein, we have demonstrated for the first time that physiological concentrations of 2OHE2, 2ME2, and 4ME2 stimulate IOSE-385 and OVCAR-3 proliferation. We have also shown that ERβ and ERα predominantly mediate the E2β-stimulated proliferation of IOSE-385 and OVCAR-3, respectively; however, such E2β metabolite-stimulated cell proliferation is independent of ER. Our current observation that physiological concentrations of AA can inhibit ovarian cancer cell proliferation is important, since it suggests that maintaining stable AA levels at physiological concentrations may have protective benefits against ovarian cancer.2,30 Interestingly, we found that AA at its pharmacological levels easily reached by intravenous injection19,20 can specifically cause loss of ovarian cancer cells but not surface epithelial cells in vitro (Figure 4), supporting the therapeutic effects of AA on ovarian cancer. More importantly, our finding that E2β and its metabolites slightly but significantly inhibit AA-suppressed ovarian cell cancer proliferation implies that E2β and its metabolites can potentially decrease the AA’s antiovarian cancer activity in vivo. These data indicate that in addition to their actions in promoting ovarian cancer cell proliferation, prolonged presence of physiological concentrations of estrogen and its metabolites in vivo may antagonize the AA suppression on ovarian cancer cell growth.
Our current findings that CYP1A1, CYP1B1, and COMT were expressed in human ovarian surface epithelial cells and in most cancer cells studied31,32 suggest that in these cells, E2β derived either from an endogenous or an exogenous source can be potentially converted into 2OHE2, 4OHE2, 2ME2, and 4ME2, acting on these ovarian cells. This is supported by the observation that only ICI partially (∼76% and ∼87%, respectively) inhibited E2β-stimulated cell proliferation in IOSE-385 and OVCAR-3, as the remaining part of proliferation may be stimulated by E2β metabolites via an ER-independent fashion (Figure 3).4
The E2β within its physiological plasma concentrations8 has been shown to stimulate proliferation, metastasis, and/or antiapoptotic activity of ovarian surface epithelial and cancer cells.33–37 Nonetheless, E2β at relatively high concentrations (∼100 μmol/L, the peak concentrations inside ovarian tissues right before ovulation)38,39 inhibits proliferation of these ovarian cells.40–42 In the current study, of 3 cancer cell lines tested, only OVCAR-3 exhibited proliferative response to E2β. The unresponsiveness of SKOV-3 to E2β is not surprising, since ER in SKOV-3 is not functional.26,43 However, it is unclear whether similar mechanisms render unresponsiveness of OVCA-432 to E2β.
Although the underlying mechanisms remain not fully understood, the roles of E2β metabolites in ovarian cancer have been proposed. For example, 2OHE2 and 4OHE2 are carcinogenic in ovarian cancer,16,44 whereas 2ME2, especially at high concentrations, may be anticarcinogenic in many cancers including ovarian cancer.14,45 Our current data confirmed the similar pro-proliferative activity of 2OHE2 and 4OHE2 in ovarian epithelial (IOSE-385) and in an ovarian cancer cell line (OVCAR-3 cells).15 However, in contrast to previous reports on OVCAR-316 and other types of ovarian cancer cells,17 we found that physiological concentrations of 2ME2 also stimulated the OVCAR-3 cell proliferation. This discrepancy may be raised from high supraphysiological dosages (IC50 > 0.2 μmol/L) of 2ME2 used in those previous studies.16,17 Moreover, our current data are the first, as far as we are aware, to report the stimulatory effects of 4ME2 on normal and malignant ovarian epithelial cells.
Our observation that ICI inhibited E2β-stimulated IOSE-385 and OVCAR-3 proliferation clearly indicates a predominant role of ER in these E2β-stimulatory effects. More interestingly, using selective ERα and ERβ antagonists, we further revealed different roles of ERα and ERβ in E2β-mediated cell proliferation between normal and malignant ovarian epithelial cells. These findings support the concept that ERα is the major form of ER responsive for the growth of malignant cancer cells, whereas ERβ is the dominant form in normal epithelial cells or benign tumors.2 Thus, specifically blocking activation of ERα should be considered as a therapeutic approach for a subset of E2β-sensitive ovarian cancer cells as suggested in other cancers. However, blockade of ERα alone may not be sufficient to suppress ovarian cancer growth, since E2β metabolites can similarly stimulate ovarian cancer growth independent of ERα and ERβ (Figure 3). To date, it remains elusive what receptor mediates this E2β metabolites-stimulated cell proliferation; however, 1 candidate might be G protein-coupled ER 30 (GPR30), a potent membrane bound ER, as it is expressed and functional in ovarian cancer cells.46,47
The mechanism underlying the effects of E2β and its metabolites on AA-suppressed ovarian cancer cell proliferation remains unclear. The GPA30, but not classical ER, may mediate such effects. Moreover, given that AA can inhibit cancer growth and progress via elevating local H2O2 levels21–23 and estrogen is a potent scavenger of H2O2,48,49 E2β and its metabolites may decrease local H2O2 levels, attenuating AA- suppressed ovarian cell growth. Thus, although future studies are needed to confirm the in vivo effects of E2β and its metabolites on growth of ovarian cancer, based on the current data, we propose that when using AA as a therapeutic drug for interfering ovarian cancer growth, blocking the actions of E2β and its metabolites should be considered as such blockade may substantially promote AA’s antiovarian cancer activity.
Footnotes
Authors’ Note: This work was done in the University of Wisconsin, Madison, WI, USA.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This study is partially supported by the National Institutes of Health Grants HD38843 (RRM/JZ), HL49210, and HL87144 (RRM).
References
- 1. Bast RC, Jr, Hennessy B, Mills GB. The biology of ovarian cancer: new opportunities for translation. Nat Rev Cancer. 2009;9(6):415–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cunat S, Hoffmann P, Pujol P. Estrogens and epithelial ovarian cancer. Gynecol Oncol. 2004;94(1):25–32. [DOI] [PubMed] [Google Scholar]
- 3. Thomas C, Gustafsson JA. The different roles of ER subtypes in cancer biology and therapy. Nat Rev Cancer. 2011;11(8):597–608. [DOI] [PubMed] [Google Scholar]
- 4. Dubey RK, Tofovic SP, Jackson EK. Cardiovascular pharmacology of estradiol metabolites. J Pharmacol Exp Ther. 2004;308(2):403–409. [DOI] [PubMed] [Google Scholar]
- 5. Santen RJ, Leszczynski D, Tilson-Mallet N, et al. Enzymatic control of estrogen production in human breast cancer: relative significance of aromatase versus sulfatase pathways. Ann N Y Acad Sci. 1986;464:126–137. [DOI] [PubMed] [Google Scholar]
- 6. Hobkirk R. Steroid sulfotransferases and steroid sulfate sulfatases: characteristics and biological roles. Can J Biochem Cell Biol. 1985;63(11):1127–1144. [DOI] [PubMed] [Google Scholar]
- 7. Ball P, Knuppen R. Formation, metabolism, and physiologic importance of catecholestrogens. Am J Obstet Gynecol. 1990;163(6 pt 2):2163–2170. [DOI] [PubMed] [Google Scholar]
- 8. Albrecht ED, Pepe GJ. Placental steroid hormone biosynthesis in primate pregnancy. Endocr Rev. 1990;11(1):124–150. [DOI] [PubMed] [Google Scholar]
- 9. Xu X, Roman JM, Issaq HJ, Keefer LK, Veenstra TD, Ziegler RG. Quantitative measurement of endogenous estrogens and estrogen metabolites in human serum by liquid chromatography-tandem mass spectrometry. Anal Chem. 2007;79(20):7813–7821. [DOI] [PubMed] [Google Scholar]
- 10. Berg D, Thaler F, Kuss E. Concentrations of 2-hydroxyoestrogens in human sera measured by a heterologous immunoassay with an 125I-labelled ligand. Acta Endocrinol (Copenh). 1982;100(1):154–160. [DOI] [PubMed] [Google Scholar]
- 11. Berg D, Sonsalla R, Kuss E. Concentrations of 2-methoxyoestrogens in human serum measured by a heterologous immunoassay with an 125I-labelled ligand. Acta Endocrinol (Copenh). 1983;103(2):282–288. [DOI] [PubMed] [Google Scholar]
- 12. Lakhani NJ, Venitz J, Figg WD, Sparreboom A. Pharmacogenetics of estrogen metabolism and transport in relation to cancer. Curr Drug Metab. 2003;4(6):505–513. [DOI] [PubMed] [Google Scholar]
- 13. Seeger H, Mueck AO. The effect of estradiol metabolites and progestogens on the proliferation of human ovarian cancer cells. Panminerva Med. 2006;48(1):13–17. [PubMed] [Google Scholar]
- 14. Mueck AO, Seeger H. 2-Methoxyestradiol-biology and mechanism of action. Steroids. 2010;75(10):625–631. [DOI] [PubMed] [Google Scholar]
- 15. Seeger H, Wallwiener D, Kraemer E, Mueck AO. Estradiol metabolites are potent mitogenic substances for human ovarian cancer cells. Eur J Gynaecol Oncol. 2005;26(4):383–385. [PubMed] [Google Scholar]
- 16. Cushman M, He HM, Katzenellenbogen JA, Lin CM, Hamel E. Synthesis, antitubulin and antimitotic activity, and cytotoxicity of analogs of 2-methoxyestradiol, anendogenous mammalian metabolite of estradiol that inhibits tubulin polymerization by binding to the colchicine binding site. J Med Chem. 1995;38(12):2041–2049. [DOI] [PubMed] [Google Scholar]
- 17. Kato S, Sadarangani A, Lange S, et al. 2-methoxyestradiol mediates apoptosis through caspase-dependent and independent mechanisms in ovarian cancer cells but not in normal counterparts. Reprod Sci. 2008;15(9):878–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cameron E, Pauling L. Supplemental ascorbate in the supportive treatment of cancer: Prolongation of survival times in terminal human cancer. Proc Natl Acad Sci USA. 1976;73(10):3685–3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Levine M, Padayatty SJ, Espey MG. Vitamin C: a concentration–function approach yields pharmacology and therapeutic discoveries. Adv Nutr. 2011;2(2):78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mamede AC, Tavares SD, Abrantes AM, Trindade J, Maia JM, Botelho MF. The role of vitamins in cancer: a review. Nutr Cancer. 2011;63(4):479–494. [DOI] [PubMed] [Google Scholar]
- 21. Chen Q, Espey MG, Krishna MC, et al. Pharmacologic ascorbic acid concentrations selectively kill cancer cells: action as a pro-drug to deliver hydrogen peroxide to tissues. Proc Natl Acad Sci USA. 2005;102(38):13604–13609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen Q, Espey MG, Sun AY, et al. Pharmacologic doses of ascorbate act as a prooxidant and decrease growth of aggressive tumor xenografts in mice. Proc Natl Acad Sci USA. 2008;105(32):11105–11109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen Q, Espey MG, Sun AY, et al. Ascorbate in pharmacologic concentrations selectively generates ascorbate radical and hydrogen peroxide in extracellular fluid in vivo. Proc Natl Acad Sci USA. 2007;104(21):8749–8754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bast RC, Jr, Feeney M, Lazarus H, Nadler LM, Colvin RB, Knapp RC. Reactivity of a monoclonal antibody with human ovarian carcinoma. J Clin Invest. 1981;68(5):1331–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hagopian GS, Mills GB, Khokhar AR, Bast RC, Jr, Siddik ZH. Expression of p53 in cisplatin-resistant ovarian cancer cell lines: modulation with the novel platinum analogue (1R, 2R-diaminocyclohexane) (trans-diacetato) (dichloro)-platinum (IV). Clin Cancer Res. 1999;5(3):655–663. [PubMed] [Google Scholar]
- 26. Lau KM, Mok SC, Ho SM. Expression of human estrogen receptor-alpha and -beta, progesterone receptor, and androgen receptor mRNA in normal and malignant ovarian epithelial cells. Proc Natl Acad Sci USA. 1999;96(10):5722–5727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jobe SO, Ramadoss J, Koch JM, Jiang Y, Zheng J, Magness RR. Estradiol-17beta and its cytochrome P450- and catechol-O-methyltransferase-derived metabolites stimulate proliferation in uterine artery endothelial cells: role of estrogen receptor-alpha versus estrogen receptor-beta. Hypertension. 2010;55(4):1005–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dai C, Jiang Y, Li Y, Wang K, Liu P, Patankar MS, Zheng J. Expression and roles of Slit/Robo in human ovarian cancer. Histochem Cell Biol. 2011;135(5):475–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zheng J, Wen Y, Song Y, Wang K, Chen DB, Magness RR. Activation of multiple signaling pathways is critical for fibroblast growth factor 2- and vascular endothelial growth factor-stimulated ovine fetoplacental endothelial cell proliferation. Biol Reprod. 2008;78(1):143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cameron E, Pauling L. Supplemental ascorbate in the supportive treatment of cancer: reevaluation of prolongation of survival times in terminal human cancer. Proc Natl Acad Sci USA. 1978;75(9):4538–4542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Murray GL. The role of cytochrome P450 in tumour development and progression and its potential in therapy. J Pathol. 2000;192(4):419–426. [DOI] [PubMed] [Google Scholar]
- 32. Leung YK, Lau KM, Mobley J, Jiang Z, Ho SM. Overexpression of cytochrome P450 1A1 and its novel spliced variant in ovarian cancer cells: alternative subcellular enzyme compartmentation may contribute to carcinogenesis. Cancer Res. 2005;65(9):3726–3734. [DOI] [PubMed] [Google Scholar]
- 33. Murdoch WJ, Van Kirk EA. Oestradiol inhibits spontaneous and cisplatin-induced apoptosis in epithelial ovarian cancer cells: relationship to DNA repair capacity. Apoptosis. 1997;2(5):478–484. [DOI] [PubMed] [Google Scholar]
- 34. Bai W, Oliveros-Saunders B, Wang Q, Acevedo-Duncan ME, Nicosia SV. Estrogen stimulation of ovarian surface epithelial cell proliferation. In Vitro Cell Dev Biol Anim. 2000;36(10):657–666. [DOI] [PubMed] [Google Scholar]
- 35. Choi KC, Kang SK, Tai CJ, Auersperg N, Leung PC. Estradiol up-regulates antiapoptotic Bcl-2 messenger ribonucleic acid and protein in tumorigenic ovarian surface epithelium cells. Endocrinology. 2001;142(6):2351–2360. [DOI] [PubMed] [Google Scholar]
- 36. Syed V, Ulinski G, Mok SC, Yiu GK, Ho SM. Expression of gonadotropin receptor and growth responses to key reproductive hormones in normal and malignant human ovarian surface epithelial cells. Cancer Res. 2001;61(18):6768–6776. [PubMed] [Google Scholar]
- 37. Song J, Fadiel A, Edusa V, et al. Estradiol-induced ezrin overexpression in ovarian cancer: a new signaling domain for estrogen. Cancer Lett. 2005;220(1):57–65. [DOI] [PubMed] [Google Scholar]
- 38. Clement PB. Histology of the ovary. Am J Surg Pathol. 1987;11(4):277–303. [DOI] [PubMed] [Google Scholar]
- 39. Carr BR. The ovary. In: Bruce R, Carr BR, Blackwell RE, eds. Textbook of Reproductive Medicine. Norwalk, CT: Appleton and Lange; 1993:183–207. [Google Scholar]
- 40. Bechtel MK, Bonavida B. Inhibitory effects of 17beta-estradiol and progesterone on ovarian carcinoma cell proliferation: a potential role for inducible nitric oxide synthase. Gynecol Oncol. 2001;82(1):127–138. [DOI] [PubMed] [Google Scholar]
- 41. Wright JM, Stouffer RL, Rodland KD. Estrogen inhibits cell cycle progression and retinoblastoma phosphorylation in rhesus ovarian surface epithelial cell culture. Mol Cell Endocrinol. 2003;208(1-2):1–10. [DOI] [PubMed] [Google Scholar]
- 42. Wright JW, Stouffer RL, Rodland KD. High-dose estrogen and clinical selective estrogen receptor modulators induce growth arrest, p21, and p53 in primate ovarian surface epithelial cells. J Clin Endocrinol Metab. 2005;90(6):3688–3695. [DOI] [PubMed] [Google Scholar]
- 43. Yap OW, Bhat G, Liu L, Tollefsbol TO. Epigenetic modifications of the estrogen receptor beta gene in epithelial ovarian cancer cells. Anticancer Res. 2009;29(1):139–144. [PMC free article] [PubMed] [Google Scholar]
- 44. Gao N, Nester RA, Sarkar MA. 4-Hydroxy estradiol but not 2-hydroxy estradiol induces expression of hypoxia-inducible factor 1alpha and vascular endothelial growth factor A through phosphatidylinositol 3-kinase/Akt/FRAP pathway in OVCAR-3 and A2780-CP70 human ovarian carcinoma cells. Toxicol Appl Pharmacol. 2004;196(1):124–135. [DOI] [PubMed] [Google Scholar]
- 45. Mooberry SL. Mechanism of action of 2-methoxyestradiol: new developments. Drug Resist Updat. 2003;6(6):355–361. [DOI] [PubMed] [Google Scholar]
- 46. Bologa CG, Revankar CM, Young SM, et al. Virtual and biomolecular screening converge on a selective agonist for GPR30. Nat Chem Biol. 2006;2(4):207–212. [DOI] [PubMed] [Google Scholar]
- 47. Henic E, Noskova V, Høyer-Hansen G, Hansson S, Casslén B. Estradiol attenuates EGF-induced rapid uPAR mobilization and cell migration via the G-protein-coupled receptor 30 in ovarian cancer cells. Int J Gynecol Cancer. 2009;19(2):214–222. [DOI] [PubMed] [Google Scholar]
- 48. Tang M, Subbiah MT. Estrogens protect against hydrogen peroxide and arachidonic acid induced DNA damage. Biochim Biophys Acta. 1996:1299(2):155–159. [DOI] [PubMed] [Google Scholar]
- 49. Bokov AF, Ko D, Richardson A. The effect of gonadectomy and estradiol on sensitivity to oxidative stress. Endocr Res. 2009;34(1-2):43–58. [DOI] [PMC free article] [PubMed] [Google Scholar]