Abstract
Given that hepatic glucose 6-phosphatase (G6Pase, involved in gluconeogenesis) has been demonstrated to be altered long term in animal models of intrauterine growth restriction (IUGR), we hypothesized that hypoxia in utero may regulate G6Pase expression via epigenetic mechanisms. To address this further, a rat model of maternal hypoxia leading to IUGR and impaired liver growth was utilized. In the 12-month-old male offspring of pregnant rat dams exposed to 11.5% atmospheric oxygen from gestational day (gd) 15 to gd 21, nonfasting glucose was lower in association with decreased hepatic G6Pase messenger RNA and protein levels. This was concomitant with enhanced methylation of histone H3 [K9] surrounding the promoter of G6Pase. Moreover, when McA-RH7777 hepatoma cells were exposed to various concentrations of oxygen for 48 hours, we observed an oxygen-dependent decrease in G6Pase expression associated with enhanced histone H3 [K9] methylation. Collectively, these results indicate that hypoxia directly and indirectly impairs G6Pase expression through enhanced methylation of histone H3 [K9].
Keywords: hypoxia, glucose 6-phosphatase, fetal programming, posttranslational histone modifications, liver
Introduction
Impaired glucose tolerance and insulin resistance characterize the metabolic syndrome (MetS) and contribute to the clinical risk of cardiovascular disease in adults.1 The major site for the regulation of glucose is in the liver, which is involved in both glucose and glycogen production.2 Emerging epidemiological evidence suggests that intrauterine growth restriction due to placental insufficiency (PI-IUGR) can permanently alter physiological processes and contribute to the development of the MetS.3,4 Given that PI-IUGR can lead to a decrease in oxygen delivery, elucidating the long-term role of hypoxia in utero in these metabolic diseases would help in developing perinatal strategies to prevent these diseases in adulthood. Utilizing a rat model of hypoxia-induced IUGR allows for the study of oxygen-dependent developmental outcomes in offspring. Previous investigations with this model have demonstrated that offspring from hypoxic (HYP) rat dams are born growth restricted5–8 and that the male offspring have exclusively increased signs and symptoms of cardiovascular disease in 7 months of age.5,6 A decreased liver–body weight ratio in newborn pups are characteristic in this model9; however, effect of maternal hypoxia on long-term circulating glucose is unknown.
Although transcriptional changes may mediate the expression of target genes involved in fetal programming, there is limited knowledge of the links between epigenetic mechanisms on the long-term expression of hepatic target genes. Our laboratory has demonstrated that increases in circulating cholesterol in male IUGR offspring derived from maternal protein restriction were due to long-term silencing of the Cyp7a1 promoter as a result of increased methylation of histone H3 [K9],10 a hallmark of chromatin silencing.11 Moreover, elegant studies by Park et al have demonstrated that uterine ligation-induced IUGR rat offspring developed type 2 diabetes as a result of epigenetic silencing of pancreatic and duodenal homeobox 1 (Pdx1), a critical transcription factor regulating β cell differentiation.12 This included hypermethylation of histone H3.12 It is noteworthy that decreases in oxygen tension have also been demonstrated to impair the expression of surfactant protein A (SP-A) in human type 2 lung cells due to increases in methylation of histone H3 [K9] surrounding the promoter of SP-A. 13
In the present study, a rat model of maternal hypoxia was used to investigate the long-term effects of this in utero insult on circulating glucose along with phosphoenolpyruvate carboxykinase (PEPCK) and glucose 6-phosphatase (G6Pase), which are involved in the rate-limiting, final catalytic steps of gluconeogenesis.35 In addition, we also examined how any hypoxia-induced changes in G6Pase might be influenced by enhanced histone H3 trimethylation [K9] using both in vitro and in vivo models of hypoxia.
Materials and Methods
Animals and Maternal Hypoxia Regime
To initiate pregnancy, female Sprague-Dawley rats (Charles River, Quebec, Canada) were mated at 3 months of age. During pregnancy, dams were allowed ad libitum access to food and water and were housed in a 12:12 light–dark cycle. On day 15 of pregnancy, pregnant dams were randomly assigned to either the (1) control (CTRL) or (2) maternal hypoxia (HYP) group. Rats assigned to the maternal hypoxic group were housed in Plexiglass chambers continuously infused with nitrogen and compressed air, maintaining an oxygen concentration of 11.5% from day 15 of pregnancy until day 21. The oxygen concentration chosen was based upon previous studies whereby mothers exposed to oxygen concentrations between 9% and 14% during late gestation would result in growth-restricted offspring with altered organ proportions but without any severe anomalies in comparison to control.14–16
Hypoxic dams were transferred to atmospheric air on day 21 prior to delivery of offspring (day 22), while dams assigned to the control condition were housed in atmospheric air (21% oxygen concentration) during all the stages of pregnancy. This model has been used extensively, and exposure to a hypoxic environment has been shown to reduce maternal weight gain during pregnancy and pup birth weight but has no effect on litter size, proportion of stillborn pups, or sex distribution. Using this model, considerable catch up growth is observed in the offspring such that a few days after birth, there are no differences in body weights between the CTRL and the HYP groups. At both 4 and 12 months of age, however, male but not female HYP offspring have lower body weights than their respective controls.8 After birth, litters were reduced to 8 pups/litter in a random fashion in order to standardize the postnatal nutritional environments. Offspring were subsequently weaned at 3 weeks of age and allowed ad libitum access to food and water until killing. At 4 and 12 months of age, animals were killed by exsanguination at 9:00 am in the morning while under isoflurane-induced anesthesia. We did not examine the female offspring in this study to prevent confounding factors related to their estrous cycle. More importantly, the maternal hypoxia model has been demonstrated to exhibit early life programming effects in a sexually dimorphic manner, which was not the focus of this investigation.5,9 Plasma samples were collected following cutting of the vena cava. Collected liver samples were immediately snap frozen in liquid nitrogen prior to shipping and storage at −80°C for ex vivo analysis.
McA-RH777 Cell Culture and Hypoxia Experiments
The female Buffalo rat adult hepatoma cell line McA-RH7777 (ATCC, Manassas, Virginia) was utilized in order to establish preliminary relationships regarding hypoxic effects on G6Pase, a liver X receptor α (LXRα) target gene,17 as it has been utilized previously to study the regulation of LXRα.18 The cells were cultured in Dulbecco modified eagle’s medium supplemented with 10% fetal bovine serum and penicillin/streptomycin at 50% to 60% confluence for a period of 48 hours in 20%, 5%, or 1% oxygen in sealed chambers in order to reflect the in vivo differences between adult arterial PO2 values (∼80-100 mm Hg) and fetal arterial PO2 (∼20-30 mm Hg).19 To examine whether outcomes due to decreased oxygen were reversible, some cells were cultured for 24 hours in 1% or 5% oxygen and then transferred to room air for an additional 24 hours. After 24 hours, the cells were scraped and pelleted for RNA analysis.
Analysis of Plasma Glucose
Concentrations of glucose and insulin serum levels in the 4- and 12-month CTRL and HYP offspring were determined by the Metabolic Phenotyping Laboratory (Robarts Research Institute, London, Ontario, Canada). In order to determine the levels of glucose in serum samples from the nonfasting rats, a glucose assay kit from Roche Diagnostics was run on a Cobas Mira S analyzer (Roche, Mississauga, Ontario, Canada). Concentrations of insulin were determined using an enzyme immunoassay from Alpco Diagnostics (Salem, New Hampshire). The coefficient of variation for the insulin assays was 3.5%, while for glucose it was <0.1%.
Quantitative Real-Time Polymerase Chain Reaction Analysis of Messenger RNA
Total RNA was extracted from both tissues and cells by the modified 1-step method of Chomczynski and Sacchi20 using Trizol reagent (Invitrogen, Burlington, Ontario, Canada ). The RNA yield was determined using a NanoDrop 2000 (ThermoScientific, Nepean, Ontario, Canada), and the RNA quality was measured by means of the A260/A280 ratio. Samples were subsequently run on a 1.2% agarose gel containing ethidium bromide to validate RNA integrity by visualization of the ratio of the 28S:18S bands and to screen for degradation. Once integrity was validated, RNA was treated with deoxyribonuclease to remove any contaminating DNA, and 4 μg of each sample was used to synthesize complementary DNA (cDNA) with a Superscript II Reverse Transcriptase Kit (Invitrogen) with the use of random primers. Samples were incubated for 10 minutes at 25°C, 50 minutes at 50°C, and 15 minutes at 70°C, then stored at −20°C until use for real-time polymerase chain reaction (PCR).
SYBR Green technology (Bio-Rad, Mississauga, Ontario, Canada) was used for the detection of PCR products, as the relative abundance of each transcript was determined utilizing the BioRad CFX384 Real-Time PCR Detection System. A total volume of 12 µL comprised of sample plus primer mix was pipetted in each well, containing 20 ng of complementary DNA (cDNA) per sample along with primer mix to a final concentration of 125 nmol/L. Primer sets directed against rat-specific vascular endothelial growth factor α (VEGFα), β-actin, and G6Pase were generated utilizing the National Center for Biotechnology Information (NCBI) Primer-BLAST tool based on the published sequences as listed in Table 1. Criteria for primer sets used were demonstrated to have linear correlation of slopes between −3.1 and −3.6 and priming efficiency of r 2 > 97% for a range of cDNA concentrations.
Table 1.
Primer Sequences Utilized for Real-Time PCR Analysis.
| Gene | NCBI Accession # | Target Location/Length | Sequence (5′ → 3′) | |
|---|---|---|---|---|
| VEGFα | NM_031836 | 1064-1408 (344 bp) | FOR | ACCTCCACCATGCCAAGT |
| REV | TTGGTCTGCATTCACATCTG | |||
| β-Actin | NM_031144.2 | 248-314 (66 bp) | FOR | ACGAGGCCCAGAGCAAGA |
| REV | TTGGTTACAATGCCGTGTTCA | |||
| G6Pase | NM_013098.2 | 783-919 (136 bp) | FOR | GAAGGCCAAGAGATGGTGTGA |
| REV | TGCAGCTCTTGCGGTACATG |
Abbreviations: FOR, forward; G6Pase, glucose 6-phosphatase; NCBI, National Center for Biotechnology Information; PCR, polymerase chain reaction; REV, reverse; VEGFα, vascular endothelial growth factor α.
After samples and primer mix were loaded, amplification of cDNA occurred using the BioRad CFX384 well system set to the following temperature settings: 50°C for 2 minutes, 95°C for 10 minutes, followed by a 45-cycle loop with incubation at 95°C for 15 seconds, and 1 minute at 60°C. Subsequently, a melt curve was generated for temperatures between 65°C and 95°C after amplification. Relative fold changes were calculated using the comparative cycle times (Ct) method with β-actin as the reference. The relative abundance of each primer set compared to the calibrator (β-actin) was determined by the formula 2ΔΔCt, whereby ΔΔCt is the calibrated Ct value (primer—internal control).
Cytoplasmic and Nuclear Protein Extraction
Tissues were electrically homogenized with NE1 lysis buffer (10 mmol/L 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid [HEPES] pH 7.5, 10 mmol/L MgCl2, 5 mmol/L KCl, 0.1% Triton X, and 0.1 mmol/L EDTA pH 8.0). The homogenate was centrifuged at 2500g in an Eppendorf 5417R Centrifuge (Brinkmann Instruments Canada, Ltd, Mississauga, Ontario) for 10 minutes at 4°C, and the supernatant was collected into a new tube and labeled as the cytoplasmic fraction. The remaining pellet was resuspended in NE1 buffer, vortexed, and centrifuged at 5000 rpm for 5 minutes at 4°C. The supernatant was aspirated, and the remaining pellet resuspended in NE2 buffer (25% glycerol, 20 mmol/L HEPES pH 7.9, 500 mmol/L NaCl, 1.5 mmol/L MgCl2, and 0.2 mmol/L EDTA pH 8.0). The resuspended pellet was incubated on a rocker at 4°C. After centrifugation at 10 000 rpm for 10 minutes at 4°C, the supernatant was retained as the nuclear fraction, and both the fractions were stored at −20°C until further analysis.
Acid Extraction of Histones
Frozen cell pellet or tissue was homogenized with lysis buffer (10 mmol/L HEPES pH 7.9, 1.5 mmol/L MgCl2, 10 mmol/L KCl, and protease inhibitor tablets), and HCl added to a final concentration of 0.2 mol/L. Samples were placed on a rocker in 4°C then centrifuged at 11 000g for 10 minutes in an Eppendorf 5417R Centrifuge at 4°C. The acid-insoluble pellet was discarded and supernatant dialyzed using the Pierce Slide-A-Lyzer MINI dialysis units (VWR International, Mississauga, Ontario) in the following manner at 4°C on a rocker: twice in 0.1 mol/L acetic acid for 1 hour, once in water for 1 hour, once in water for 3 hours, and in water overnight. The samples were then aliquoted into separate tubes and stored at −20°C.
Protein Quantification and Immunoblotting
Samples were assayed for protein content using the RC DC Protein Assay Kit II (Bio-Rad). Samples were then prepared using the NuPAGE LDS Sample Buffer NuPAGE Sample Reducing Agent (Invitrogen). Loading amounts were 25 µg for cytoplasmic extracts, 15 to 20 µg for nuclear extracts, and 10 to 15 µg for histone extracts. Samples were run on NuPage 4% to 11.5% Bis-Tris gels (Invitrogen) in MES-SDS running buffer at 150 V for 1 to 2 hours. After transfer to nitrocellulose membranes, the membranes were blocked for 2 hours in 5% nonfat-dried milk in 0.1% Tris-buffered saline and Tween 20 (TBST). Membranes were incubated with antibodies targeted against G6Pase (sc-25804; Santa Cruz Biotechnology, Santa Cruz, California), total nuclear histone H3 (05-499; Millipore, Etobicoke, Ontario, Canada), and trimethylated histone H3 (lysine 9, 07-422; Millipore). After washing in 0.1% TBST, the membranes were incubated for 2 hours with the appropriate secondary antibody (1:10 000 dilution). β-Actin was probed with antibody (Sigma-Aldrich, Oakville, Ontario, Canada) for a 2-hour room temperature incubation. To probe for housekeeping proteins (β-actin for cytoplasmic proteins and total histone H3 for nuclear proteins and modified histones), stripping buffer (200 mmol/L glycine, 0.1% sodium dodecyl sulfate [SDS], and 1% Tween 20) was utilized for 10 minutes, and then detection protocol was implemented as earlier. All bands were detected with a SuperSignal West Dura Extended Duration Substrate (ThermoScientific) and imaged with a VersaDoc Imaging System (Bio-Rad). Densitometric analysis on blots was performed using Quantity One Software (Bio-Rad).
Chromatin Immunoprecipitation
Chromatin immunoprecipitation (ChIP) was performed on snap-frozen medial lobe liver tissue excised from 12-month male and female offspring derived from the CTRL or HYP regime. The ChIP was performed using a modification10 of previously published methods.21 Briefly, a small piece of snap-frozen liver was homogenized and incubated with 1% formaldehyde for 10 minutes at room temperature to cross-link proteins and DNA. Cross-linking was terminated by the addition of glycine (0.125 mol/L, final concentration). The liver tissue was washed once with cold phosphate-buffered saline and placed in 500 µL of SDS lysis buffer (Millipore) with protease inhibitor cocktail (Roche). The lysates were sonicated on ice to produce sheared, soluble chromatin. The lysates were diluted 10 times with the addition of ChIP dilution buffer (Millipore) and aliquoted to 400 µL amounts. Each of the aliquots was precleared with protein A/G plus agarose beads (40 µL; Millipore) at 4°C for 30 minutes. The samples were microfuged at 12 500 rpm to pellet the beads, and the supernatant containing the sheared chromatin was placed in new tubes. The aliquots were incubated with 4 µg of antibodies against trimethylated histone H3 (lysine 9; Millipore) at 4°C overnight. Two aliquots were reserved as “controls”—one incubated without antibody and the other with nonimmune immunoglobulin G (Millipore). Protein A/G plus agarose beads (60 mL) were added to each tube, the mixtures were incubated for 1 hour at 4°C, and the immune complexes collected by centrifugation. The beads containing the immunoprecipitated complexes were washed sequentially for 5 minutes in wash buffer I (20 mmol/L Tris-HCl, pH 8.1, 2 mmol/L EDTA, 0.1% SDS, 1% Triton X-100, and 150 mmol/L NaCl), wash buffer II (same as I, except containing 500 mmol/L NaCl), wash buffer III (10 mmol/L Tris-HCl, pH 8.1, 1 mmol/L EDTA, 1% NP-40, 1% deoxycholate, and 0.25 mol/L LiCl), and 2 ′ Tris-EDTA (TE) buffer. The beads were eluted with 250 µL elution buffer (1% SDS, 0.1 mmol/L NaHCO3 + 20 mg salmon sperm DNA; Sigma-Aldrich) at room temperature. This was repeated once, and the eluates were combined. Cross-linking of the immunoprecipitated chromatin complexes and “input controls” (10% of the total soluble chromatin) was reversed by heating the samples at 65°C for 4 hours. Proteinase K (15 µg; Invitrogen, Carlsbad, California) was added to each sample in buffer (50 mmol/L Tris-HCl, pH 8.5, 1% SDS, and 10 mmol/L EDTA) and incubated for 1 hour at 45°C. The DNA was purified by phenol–chloroform extraction and precipitated in ethanol overnight at 20°C. Samples and “input controls” were diluted in 10 to 100 µL TE buffer just prior to PCR. Real-time PCR was employed using primers designed to target a 5′ LXRE promoter region that is upstream of the transcriptional start site of G6Pase that contains the DR4 element.17 The forward primer (5′-GTCACCCCTTAGCACTGTCAAGCC-3′) and reverse primer (5′-GCAAACAGGCACACAAAAACAGCC-3′) targeted the promoter region −258 to −169 bp upstream of the G6Pase transcriptional start site.
Statistical Analysis
Data are presented as mean ± standard error of the mean. GraphPad Prism 5 software was utilized to conduct 1-way analyses of variances (ANOVAs) to compare differences between oxygen dosage groups of McA-RH7777 cells. Two-way ANOVAs were performed to compare CTRL and HYP rat offspring when offspring were compared by sex and to determine whether any gender-related differences could be detected between the offspring. The ChIP data were analyzed with a Student 2-tailed unpaired t test. Levels of significance were set at a P value of .05 or less. Tukey post hoc test was performed on statistically significant data and analyzed by 1-way ANOVA, and Bonferroni post hoc test was utilized following a 2-way ANOVA.
Results
Circulating Plasma Glucose Is Decreased in 12-Month HYP Offspring
To determine long-term adaptive responses of the fetus due to maternal hypoxia exposure in utero, the plasma levels of insulin and glucose were obtained from CTRL and HYP offspring at 4 and 12 months of age from the animals that were killed. As offspring were allowed ad libitum access to food and water prior to killing and blood sample collection, it should be noted that the values reported are random blood glucose and insulin levels. There was no difference in plasma insulin levels between CTRL and HYP offspring both in early (4 month) and in late (12 month) adult offspring, regardless of gender (Figure 1A). However, differences in levels between genders were observed, as female offspring had significantly lower levels of insulin relative to their male counterparts at both 4 and 12 months of age (Figure 1A). Analysis of random circulating glucose levels revealed that while females did not display any differences in glucose levels, regardless of condition or age, only 12-month HYP male offspring had significantly lower levels of glucose relative to CTRL (P < .05; Figure 1B). Interestingly, there was also a trend for an increase in blood glucose in the CTRL offspring between 4 and 12 months, although this was not significant.
Figure 1.
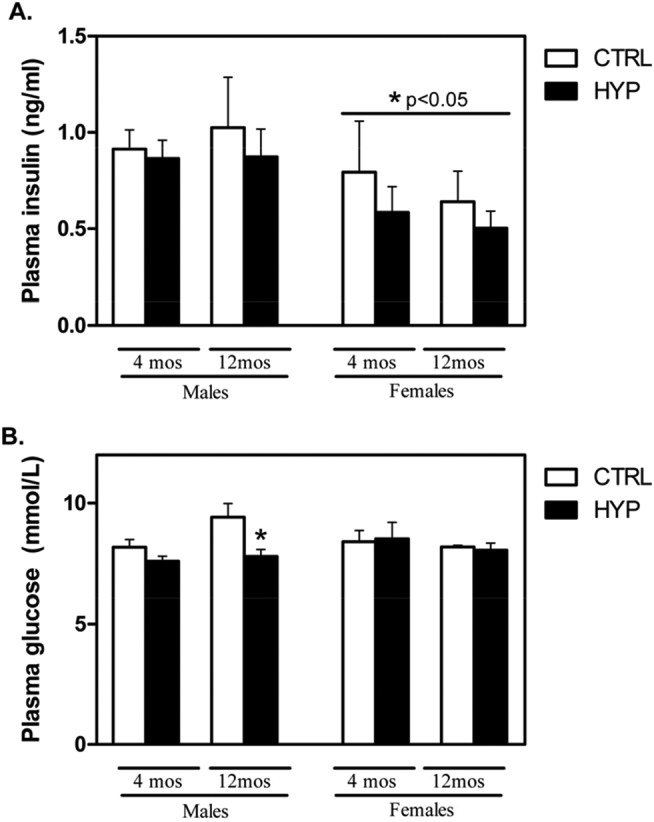
Exposure to hypoxia in utero results in significantly lower circulating glucose levels but not insulin in 12-month male offspring. Random circulating insulin and glucose levels were evaluated in blood plasma of offspring from maternally exposed hypoxic pregnancies (11.5%, HYP) or control (21%, CTRL). A, No differences were detected in circulating insulin levels between CTRL and HYP offspring at 4 or 12 months of age, but females had significantly lower plasma insulin levels at both the ages relative to males utilizing a 2-way ANOVA (n = 8 samples/experimental group). B, Analysis of random circulating glucose found no differences between the groups at 4 months of age, but a significant decrease (P < .05, indicated by *) was observed in 12-month male HYP offspring relative to CTRL. ANOVA indicates analysis of variance.
The Expression of Hepatic G6Pase is Impaired in 12-Month HYP Offspring Circulating Glucose Is Reduced
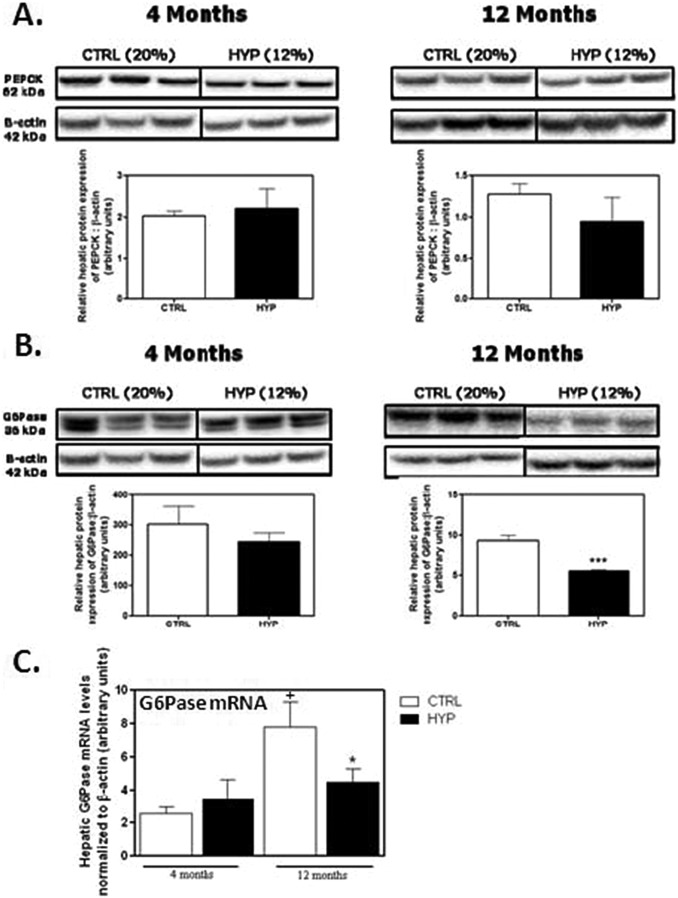
Given that in the 12-month male HYP offspring and that both PEPCK and G6Pase catalyze the final steps of gluconeogenesis, we next determined whether maternal hypoxia in utero influences their hepatic expression in the long term in the offspring. To accomplish this, messenger RNA (mRNA) and protein levels of PEPCK and G6Pase were determined (Figure 2). Western immunoblotting revealed there was no change in the levels of PEPCK protein between CTRL and HYP offspring at either 4 or 12 months. However, a 1.7-fold decrease in G6Pase protein levels was observed only in 12-month HYP males relative to CTRL (P < .001; Figure 2A). To elucidate the underlying mechanisms for a decreased G6Pase protein, the steady state levels of G6Pase mRNA levels were then measured. Quantitative reverse transcriptase PCR (qRT-PCR) analysis revealed a corresponding decrease in 12-month HYP offspring (P < .05; Figure 2B), but this was not evident at 4 months. It should be noted that G6Pase overall (P < .05) increased in the CTRL offspring from 4 to 12 months.
Figure 2.
Glucose 6-phosphatase protein and steady state mRNA levels are significantly lower in hypoxic males at 12 months of age. A, The protein expression of PEPCK and (B) G6Pase was measured through Western blotting using whole-cell extracts from CTRL (21% O2) and HYP (11.5% O2) in 4- and 12-month male hepatic tissue and antibodies specific for PEPCK, G6Pase, and β-actin. Levels of PEPCK and G6Pase were detected via immunoblotting and normalized to β-actin via densitometry and with unpaired 2-tailed t tests. The HYP males at 12 months of age had significantly attenuated levels of G6Pase relative to CTRL (***P < .001). C, Total mRNA extraction from 4- and 12-month hepatic tissue occurred, followed by reverse transcription. Quantitative real-time PCR analysis was performed utilizing SYBR Green technology and the ΔΔCt method. Statistical analysis utilizing a 2-way ANOVA (n = 8 samples/experimental group) revealed that 12–month-old male offspring from HYP pregnancies had significantly decreased mRNA levels of G6Pase (*P < .05) relative to their CTRL counterparts (n = 3-9 samples/experimental group). The expression of G6Pase in the control animals was also significantly (# P < .05) higher in 12-month compared to 4-month normoxic offspring. ANOVA indicates analysis of variance; CTRL, control; G6Pase, glucose 6-phosphatase; HYP, hypoxic; mRNA, messenger RNA; PEPCK, phosphoenolpyruvate carboxykinase; PCR, polymerase chain reaction.
Trimethylated Histone H3 3(K9) is Enhanced Surrounding the Proximal Promoter of G6Pase in Offspring Derived From Maternal Hypoxia
In order to determine whether the silencing of hepatic G6Pase expression observed in 12-month male HYP-derived offspring was associated with enhanced trimethylation of histone H3 (K9), ChIP assay was employed in the livers of 12-month offspring using a triMeH3(K9)-specific antibody along with primers targeting the active LXRE-containing site in the promoter region of G6Pase (Figure 3). The QRT-PCR analysis revealed 1.7-fold enhancement of triMeH3(K9) in 12-month HYP males relative to CTRL (Figure 3; P < .05).
Figure 3.
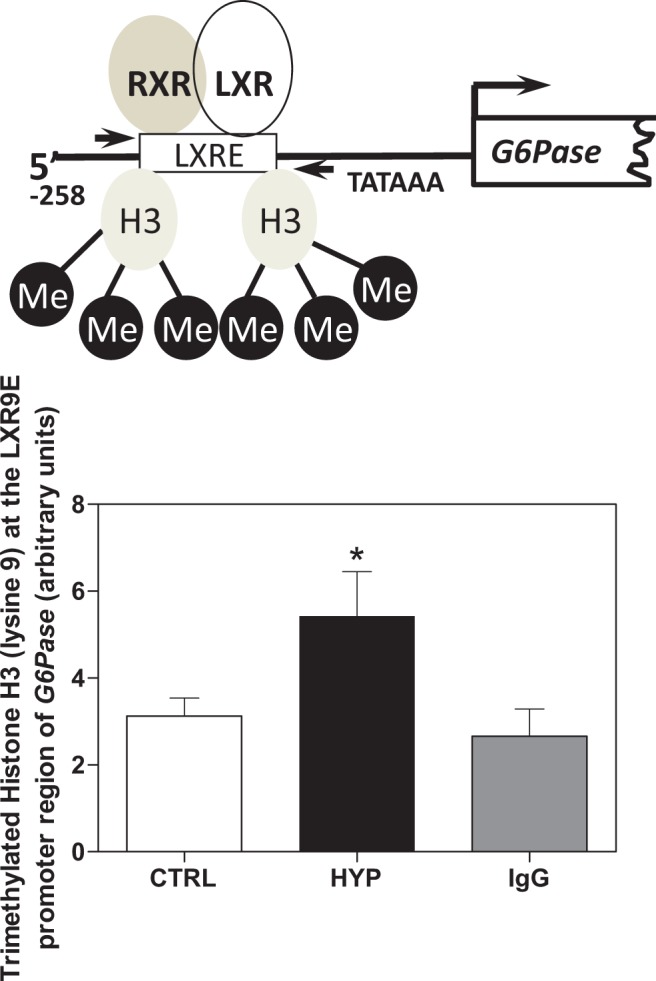
Levels of trimethylated histone H3 (lysine 9) associated with the LXRE-containing region of the G6Pase promoter are increased in 12-month males from hypoxemic pregnancies. Hepatic tissue from 12-month male control (CTRL, 20%) or hypoxic (HYP, 11.5%) offspring was subjected to cross-linking, lysis, and sonication. Solubilized chromatin was immunoprecipitated with a specific antibody for trimethylated histone H3 (lysine 9) or immunoglobulin G control. After immunoprecipitation, DNA was analyzed for the promoter region containing the LXRE region of G6Pase utilizing real-time PCR. After analysis with an unpaired 2-tailed t test, it was determined the 12-month male offspring from hypoxemic dams had significantly increased binding of triMeH3(K9) to the LXRE region of G6Pase relative to CTRL offspring (*P < .05). G6Pase indicates glucose 6-phosphatase; PCR, polymerase chain reaction.
Effect of Decreased Oxygen Tension on Global Hepatic Levels of triMeH3(K9) and G6Pase mRNA Expression in McA-RH7777 Hepatoma Cells
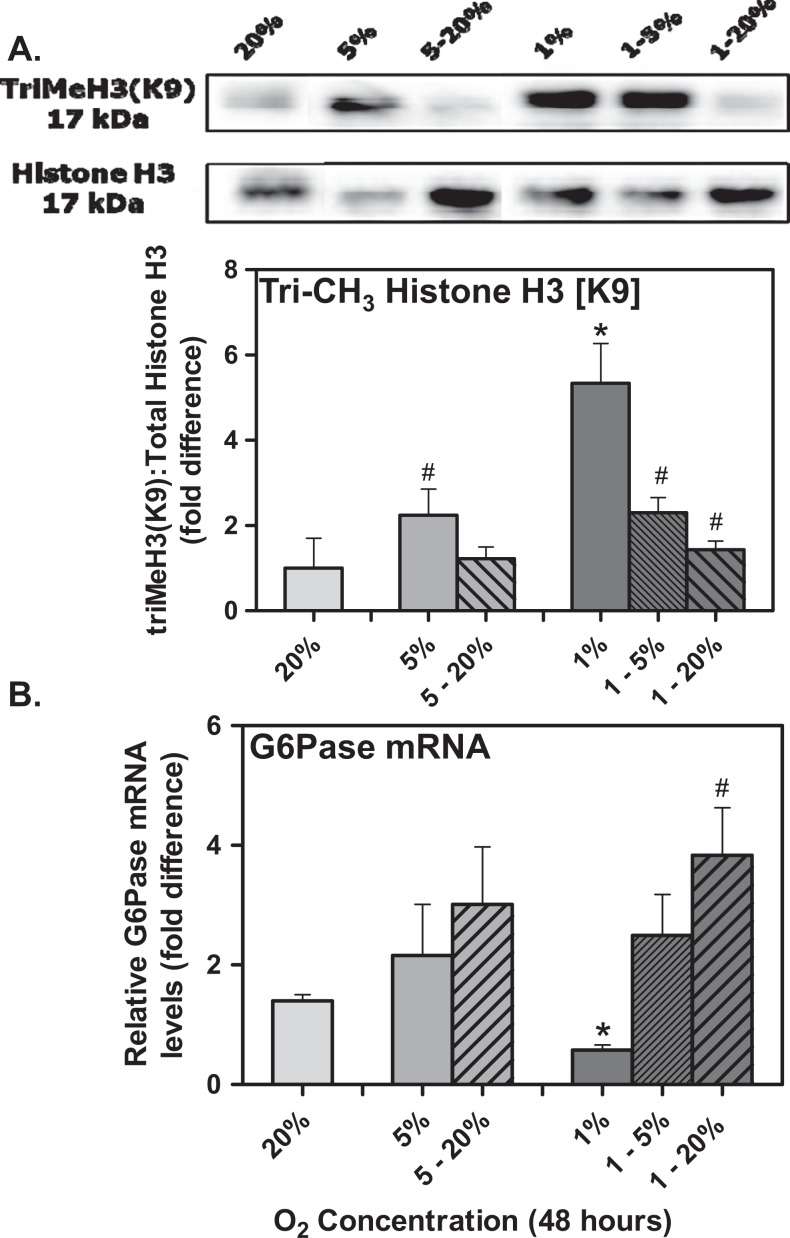
Given that global hepatic methylation of histone 3 (lysine 9) was enhanced surrounding the promoter of G6Pase in 12-month offspring derived from maternal hypoxia concomitant with a decrease in G6Pase expression, we next examined whether decreases in oxygen tension may directly mediate this observation in vitro. Given that McA-RH7777 cells respond to decreases in oxygen tension from 20% to 5% to 1% hypoxia over 48 hours, as indicated by increases in VEGFα mRNA levels (markers of hypoxemia, data not shown), total triMeH3(K9) was subsequently assessed (Figure 4A). After densitometric calculations were normalized to total histone H3 levels, it was determined that exposure to 1% oxygen for 48 hours significantly induced triMeH3(K9) by 5.3-fold relative to levels observed in the 20% conditions (Figure 4A; P < .05). Furthermore, this appeared to be a dose-dependent response, as triMeH3(K9) levels in the 5%, and both the 1% to 5% oxygen and 1% to 20% oxygen were significantly lower than those observed at 1% (Figure 4A; P < .05). When G6Pase mRNA expression was assessed by real-time PCR, there was a significant decrease in 20% and 1% oxygen concentrations after 48 hours in culture (Figure 4B; P < .05). Moreover, a significant increase in G6Pase mRNA levels was observed when the oxygen tension was switched from 1% after 24 hours to 20% treatment for 24 hours (Figure 4B; P < .05).
Figure 4.
Reduced oxygen tension enhances global levels of triMeH3(K9)-associated impaired G6Pase in McA-RH7777 hepatoma cells. McA-RH7777 hepatoma cells were cultured for 48 hours at 21%, 5%, or 1% oxygen environments, with some dishes receiving 1% or 5% oxygen for 24 hours followed by recovery in 20% oxygenation for an additional 24 hours. A, Acid extraction of total histones was performed, and samples were immunoblotted with antibodies specific for trimethylated histone H3 (lysine 9) and total histone H3. Relative levels of triMeH3(K9) to total histone H3 were assessed by densitometry followed by a 1-way ANOVA. B, After extraction of total mRNA and reverse transcription, SYBR Green real-time PCR analysis was performed with Ct values normalized to the housekeeping gene β-actin and expressed as fold difference from the 20% tension values. # indicates values significantly different (P < .05) from each other, whereas * indicates different from control as determined by a 1-way ANOVA (n = 3 independent experiments performed in triplicate). ANOVA indicates analysis of variance; G6Pase, glucose 6-phosphatase; mRNA, messenger RNA; PCR, polymerase chain reaction; Ct, cycle time.
Discussion
Clinical studies in humans have demonstrated that adverse PI-IUGR contributes to long-term programming events leading to the MetS, and therefore, cardiovascular disease.22 As the liver is essential to glucose and cholesterol metabolism,2 alterations in hepatic development may impair their homeostatic regulation, leading to the development of the MetS. Therefore, this study investigated how maternal hypoxia, leading to fetal hypoxia and impaired birth weight, influences circulating glucose and insulin as well as hepatic enzyme levels in mature offspring. As histone methylation results in a “histone code” that can determine whether chromatin is in a transcriptionally active state or packaged as silent heterochromatin,23 we examined whether maternal hypoxemia in utero alters hepatic G6Pase gene expression in offspring due to alterations in the methylation of histone H3 [K9], a marker of chromatin silencing. Previously, we have demonstrated in a maternal undernutrition model that IUGR rat offspring have impaired expression of Cyp7a1 due to histone modifications resulting in repressive changes to its promoter.10 In the present study, we demonstrated that maternal hypoxia in utero led to decreased circulating glucose in 12-month male offspring, due to chromatin silencing of the G6Pase promoter, resulting in decreased G6Pase expression.
Examination of plasma insulin and glucose levels determined no significant differences in insulin levels between CTRL and HYP offspring, but there was a significant decrease in glucose levels in male HYP offspring at 12 months of age relative to CTRL. Although there was a trend for an increase in blood glucose in the CTRL offspring between 4 and 12 months, this was not significant. Others have demonstrated that blood glucose levels do not differ between 3- and 15–month rat offspring.24 Although previous studies with this model have demonstrated that body weight is decreased in male HYP offspring at both 4 and 12 months,25 food intake was not assessed in these 4- and 12-month offspring. Therefore, it is conceivable that decreased caloric intake may also play a role leading to the decreased circulating blood glucose. Studies examining the altitude of residency during pregnancy in humans have determined that at high elevations, there is a decrease in blood flow in the uterine artery.26 However, an increase in maternal hemoglobin levels raises arterial oxygen content, concomitant with an increase in fetal hemoglobin to result in similar oxygen delivery to the fetus as observed at sea level.27 Therefore, there is a possibility that resultant IUGR from hypoxic exposure may not result directly from decreased oxygen delivery but rather from secondary pathways. Chronic hypoxia during pregnancy has been demonstrated to decrease fetal glucose consumption, as the high-altitude fetus consumes less glucose/mole of oxygen.28 Decreases in glucose consumption may be a result of an induced hypometabolic state in response to decreased fetal oxygen supply in order to reduce energy demands.29–31 To date, plasma glucose levels in human and animal offspring from hypoxemic pregnancies have only been examined at term, and although offspring are typically classified as hypoglycemic at birth,28 no follow-up studies have yet been conducted, so it was inconclusive whether these findings persist into adulthood.
More recently, Rueda-Clausen et al have demonstrated that maternal hypoxia did not alter circulating glucose in rat pups by 9 weeks of age nor did it alter glucose handling, glucose tolerance, and insulin sensitivity by 4 months of age.8 However, the long-term effects (eg, 12 months) were not examined. Interestingly, if these hypoxic offspring were exposed to a high-fat (45%) diet, they exhibited glucose intolerance and impaired insulin sensitivity by 4 months.8 Studies using primary cultures of hepatocytes have found that they exhibit a 29% decrease in glucose production when isolated from in vivo hypoxia-exposed rats.32 Our data further support the observation of the effects of hypoxia to decrease circulating glucose persisting to 1 year at age.
During gestation, there is a negligible amount of gluconeogenic activity in the fetus.33 A slight increase in gluconeogenesis has been detected in the sheep and rat toward term, with a sharp increase in gluconeogenic enzymatic activity occurring at birth.34 Given that G6Pase catalyzes the final step in gluconeogenesis to result in the release of free glucose and inorganic phosphate,35 we examined whether its expression was altered in male offspring at 12 months of age derived from hypoxia. In these HYP offspring, which exhibited decreased circulating glucose concentrations, hepatic G6Pase expression levels were significantly decreased relative to CTRL. Moreover, in vitro experiments using cultured rat hepatoma cells indicated that the levels of G6Pase mRNA were significantly lower in cells cultured at 1% oxygen for a full 48-hour duration relative to those that were placed in a 20% oxygen recovery environment for the last 24 hours of the experimental period. Collectively, these experiments suggest that G6Pase is regulated by alterations in oxygen tension, and that in pregnancy, maternal hypoxia may impair G6Pase expression in the long term.
Given that hypoxia may impair the expression of genes in vitro and in vivo via epigenetic mechanisms,12,13 hypoxia may silence G6Pase expression directly or indirectly via increases in methylation of histone H3 [K9]. As the histone status regulates the chromatin state of associated genes, it is possible that epigenetic mechanisms may play a greater role in the silencing of G6Pase both in vivo and in vitro. Changes in the epigenetic profile of offspring as a result of IUGR are one adaptive mechanism that has been suggested to result in the occurrence of metabolic reprogramming.36 Histone modification states can determine whether chromatin is in a transcriptionally active (euchromatin) or inactive (heterochromatin) state.23 Previous studies of IUGR in rodents have reported the occurrence of a change in histone modification states relative to those observed in CTRL offspring.12 Furthermore, oxygen tension has been implicated to regulate histone H3 methylation and coactivator recruitment.13 Therefore, we investigated how histone H3 methylation is altered in vivo and in vitro. In vitro experiments revealed that when placed in decreased oxygen tensions, McA-RH7777 hepatocytes had attenuated G6Pase mRNA levels concomitant with an oxygen dose-dependent decline in overall protein levels of triMeH3(K9). Similar silencing effects of reduced oxygen tension on SP-A gene expression via increased trimethylated histone H3 expression have been previously reported.13 In order to determine promoter-specific histone pattern changes in vivo, ChIP was employed to detect triMeH3(K9) binding to the active LXRE promoter region of G6Pase, as a decrease in G6Pase was apparent in the HYP offspring, leading to decreases in G6Pase protein expression in HYP males. In vivo experiments using ChIP revealed a significant increase in triMeH3(K9) binding to the LXRE region of G6Pase in 12-month male HYP offspring relative to CTRL. Interestingly, in a model of undernutrition (eg, low protein diet) during pregnancy in piglets, the low-protein male offspring had increased hepatic G6Pase expression associated with lower trimethylated histone H3 [K9] in the proximal promoter of porcine G6Pase compared to the controls.37 Collectively, these studies suggest that the expression of hepatic G6Pase is tightly governed by the trimethylation status of histone H3 [K9] in the short and long term. Although previous studies have demonstrated that uterine artery ligation can also lead to the long-term silencing of gene expression (eg, PDX-1) in postnatal life due to increases in histone H3 [K9] methylation,12 this is the first study to demonstrate that the maternal hypoxia in pregnancy can also lead to hypermethylation of gene promoters in the long term. This helps to elucidate why these offspring, derived from maternal hypoxia in pregnancy, have decreased levels of steady state G6Pase mRNA and protein levels in the long term. These findings illustrate the importance of examining direct histone interactions with genes in a promoter-specific manner.
In conclusion, this study has elucidated that maternal hypoxia during pregnancy inhibits the expression of a subset of gluconeogenic enzymes in the long term in offspring, concomitant with a decrease in postnatal nonfasting plasma glucose. This effect may be regulated, indirectly, by epigenetic mechanisms, via modifications in histone methylation patterns. By identifying epigenetic mechanisms involved in the development of impaired glucose homeostasis, further studies are now warranted to examine how postnatal diet or drug interventions early in life may restore circulating glucose in the long term via posttranslational mechanisms promoting a permissive chromatin environment.
Acknowledgment
We would like to thank Dr Lin Zhao for his technical expertise.
Footnotes
Authors’ Note: Dr Sandra T. Davidge is a Canada Research Chair in Women’s Cardiovascular Health.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work is supported by operating grant from the Canadian Institutes of Health Research (MOP 111001 to DBH and MOP 77648 to STD).
References
- 1. Mathieu P, Pibarot P, Despres JP. Metabolic syndrome: the danger signal in atherosclerosis. Vasc Health Risk Manag. 2006;2(3):285–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Spear BT, Jin L, Ramasamy S, Dobierzewska A. Transcriptional control in the mammalian liver: liver development, perinatal repression, and zonal gene regulation. Cell Mol Life Sci. 2006;63(24):2922–2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lamarche B, Lemieux S, Dagenais GR, Despres JP. Visceral obesity and the risk of ischaemic heart disease: insights from the Quebec cardiovascular study. Growth Horm IGF Res. 1998;8(suppl B):1–8. [DOI] [PubMed] [Google Scholar]
- 4. Ross MG, Beall MH. Adult sequelae of intrauterine growth restriction. Semin Perinatol. 2008;32(3):213–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Xu Y, Williams SJ, O'Brien D, Davidge ST. Hypoxia or nutrient restriction during pregnancy in rats leads to progressive cardiac remodeling and impairs postischemic recovery in adult male offspring. FASEB J. 2006;20(8):1251–1253. [DOI] [PubMed] [Google Scholar]
- 6. Hemmings DG, Williams SJ, Davidge ST. Increased myogenic tone in 7-month-old adult male but not female offspring from rat dams exposed to hypoxia during pregnancy. Am J Physiol Heart Circ Physiol. 2005;289(2):H674–H682. [DOI] [PubMed] [Google Scholar]
- 7. Morton JS, Rueda-Clausen CF, Davidge ST. Mechanisms of endothelium-dependent vasodilation in male and female, young and aged offspring born growth restricted. Am J Physiol Regul Integr Comp Physiol. 2010;298(4):R930–R938. [DOI] [PubMed] [Google Scholar]
- 8. Rueda-Clausen CF, Dolinsky VW, et al. Hypoxia-induced intrauterine growth restriction increases the susceptibility of rats to high-fat diet-induced metabolic syndrome. Diabetes. 2011;60(2):507–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Williams SJ, Campbell ME, McMillen IC, Davidge ST. Differential effects of maternal hypoxia or nutrient restriction on carotid and femoral vascular function in neonatal rats. Am J Physiol Regul Integr Comp Physiol. 2005;288(2):R360–R367. [DOI] [PubMed] [Google Scholar]
- 10. Sohi G, Marchand K, Revesz A, Arany E, Hardy DB. Maternal protein restriction elevates cholesterol in adult rat offspring due to repressive changes in histone modifications at the cholesterol 7alpha-hydroxylase promoter. Mol Endocrinol. 2011;25(5):785–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shilatifard A. Chromatin modifications by methylation and ubiquitination: implications in the regulation of gene expression. Annu Rev Biochem. 2006;75:243–269. [DOI] [PubMed] [Google Scholar]
- 12. Park JH, Stoffers DA, Nicholls RD, Simmons RA. Development of type 2 diabetes following intrauterine growth retardation in rats is associated with progressive epigenetic silencing of Pdx1. J Clin Invest. 2008;118(6):2316–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Islam KN, Mendelson CR. Permissive effects of oxygen on cyclic AMP and interleukin-1 stimulation of surfactant protein A gene expression are mediated by epigenetic mechanisms. Mol Cell Biol. 2006;26(8):2901–2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Van Geijn HP, Kaylor WM, Jr , Nicola KR, Zuspan FP. Induction of severe intrauterine growth retardation in the Sprague-Dawley rat. Am J Obstet Gynecol. 1980;137(1):43–47. [DOI] [PubMed] [Google Scholar]
- 15. Gleed RD, Mortola JP. Ventilation in newborn rats after gestation at simulated high altitude. J Appl Physiol. 1991;70(3):1146–1151. [DOI] [PubMed] [Google Scholar]
- 16. de Grauw TJ, Myers RE, Scott WJ. Fetal growth retardation in rats from different levels of hypoxia. Biol Neonate. 1986;49(2):85–89. [DOI] [PubMed] [Google Scholar]
- 17. Mitro N, Mak PA, Vargas L, et al. The nuclear receptor LXR is a glucose sensor. Nature. 2007;445(7124):219–223. [DOI] [PubMed] [Google Scholar]
- 18. Tamehiro N, Shigemoto-Mogami Y, Kakeya T, et al. Sterol regulatory element-binding protein-2- and liver X receptor-driven dual promoter regulation of hepatic ABC transporter A1 gene expression: mechanism underlying the unique response to cellular cholesterol status. J Biol Chem. 2007;282(29):21090–21099. [DOI] [PubMed] [Google Scholar]
- 19. Battaglia FC. Fetal respiratory physiology. In: Battaglia FC, Meschia G, eds. An Introduction of Fetal Physiology. Orlando, Florida, FL: Academic Press, Inc; 1986:154–184. [Google Scholar]
- 20. Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate–phenol–chloroform extraction. Anal Biochem. 1987;162(1):156–159. [DOI] [PubMed] [Google Scholar]
- 21. Chakrabarti SK, James JC, Mirmira RG. Quantitative assessment of gene targeting in vitro and in vivo by the pancreatic transcription factor, Pdx1. importance of chromatin structure in directing promoter binding. J Biol Chem. 2002;277(15):13286–13293. [DOI] [PubMed] [Google Scholar]
- 22. Barker DJ. Fetal origins of cardiovascular disease. Ann Med. 1999;31(suppl 1):3–6. [PubMed] [Google Scholar]
- 23. Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293(5532):1074–1080. [DOI] [PubMed] [Google Scholar]
- 24. Hales CN, Desai M, Ozanne SE, Crowther NJ. Fishing in the stream of diabetes: from measuring insulin to the control of fetal organogenesis. Biochem Soc Trans. 1996;24(2):341–350. [DOI] [PubMed] [Google Scholar]
- 25. Rueda-Clausen CF, Morton JS, Davidge ST. Effects of hypoxia-induced intrauterine growth restriction on cardiopulmonary structure and function during adulthood. Cardiovasc Res. 2009;81(4):713–722. [DOI] [PubMed] [Google Scholar]
- 26. Mayhew TM. Changes in fetal capillaries during preplacental hypoxia: growth, shape remodelling and villous capillarization in placentae from high-altitude pregnancies. Placenta. 2003;24(2-3):191–198. [DOI] [PubMed] [Google Scholar]
- 27. Postigo L, Heredia G, Illsley NP, et al. Where the O2 goes to: preservation of human fetal oxygen delivery and consumption at high altitude. J Physiol. 2009;587(pt 3):693–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zamudio S, Torricos T, Fik E, et al. Hypoglycemia and the origin of hypoxia-induced reduction in human fetal growth. PLoS One. 2010;5(1):e8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Richardson BS, Bocking AD. Metabolic and circulatory adaptations to chronic hypoxia in the fetus. Comp Biochem Physiol A Mol Integr Physiol. 1998;119(3):717–723. [DOI] [PubMed] [Google Scholar]
- 30. Hooper SB, Coulter CL, Deayton JM, Harding R, Thorburn GD. Fetal endocrine responses to prolonged hypoxemia in sheep. Am J Physiol. 1990;259(4 pt 2):R703–R708. [DOI] [PubMed] [Google Scholar]
- 31. Illsley NP, Caniggia I, Zamudio S. Placental metabolic reprogramming: do changes in the mix of energy-generating substrates modulate fetal growth? Int J Dev Biol. 2010;54(2-3):409–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pison CM, Chauvin C, Fontaine E, et al. Mechanism of gluconeogenesis inhibition in rat hepatocytes isolated after in vivo hypoxia. Am J Physiol. 1995;268(5 pt 1):E965–E973. [DOI] [PubMed] [Google Scholar]
- 33. Hay WW, Jr , Sparks JW, Quissell BJ, Battaglia FC, Meschia G. Simultaneous measurements of umbilical uptake, fetal utilization rate, and fetal turnover rate of glucose. Am J Physiol. 1981;240(6):E662–E668. [DOI] [PubMed] [Google Scholar]
- 34. Fowden AL, Li J, Forhead AJ. Glucocorticoids and the preparation for life after birth: are there long-term consequences of the life insurance? Proc Nutr Soc. 1998;57(1):113–122. [DOI] [PubMed] [Google Scholar]
- 35. Stetten MR, Taft HL. Metabolism of inorganic pyrophosphate. ii. the probable identity of microsomal inorganic pyrophosphatase, pyrophosphate phosphotransferase, and glucose 6-phosphatase. J Biol Chem. 1964;239:4041–4046. [PubMed] [Google Scholar]
- 36. Gluckman PD, Hanson MA. Living with the past: evolution, development, and patterns of disease. Science. 2004;305(5691):1733–1736. [DOI] [PubMed] [Google Scholar]
References
- 37. Jia Y, Cong R, Li R, et al. Maternal low-protein diet induces gender-dependent changes in epigenetic regulation of the glucose-6-phosphatase gene in newborn piglet liver. J Nutr. 2012;142(9):1659–1665. [DOI] [PubMed] [Google Scholar]