Abstract
The aim of the study was to evaluate the androgen (AR) and estrogen receptors’ (ER) expression in epididymis of polychlorinated biphenyls (PCBs)-exposed rats. The rats were assigned to groups. Group I controls were treated with corn oil 80 µL/d intraperitoneally (ip), group II were treated with 2 mg/kg/d of A1254 ip; and group III were treated with 2 mg/kg/d of A1254 ip along with simultaneous oral supplementation of 4 mg/kg/d lycopene . The treatment was given daily for 30 days. After 24 hours of treatment, the rats were killed, and the epididymal regions (caput, corpus, and cauda) were dissected out, weighed, and prepared to estimate the levels of sialic acid, glyceryl phosphoryl choline (GPC), hydrogen peroxide (H2O2), and lipid peroxidation (LPO). The messenger RNA (mRNA) expressions of AR, ERα, and ERβ were analyzed by reverse transcriptase–polymerase chain reaction, and ERα and ERβ protein expressions were analyzed by immunoblotting. The toxicity of PCBs was also confirmed by histology. There was a marked decrease in epididymal weight, sialic acid, and GPC levels, while oxidative stress markers H2O2 and LPO were increased in PCBs-treated rats. The mRNA and protein expression of AR, ERα, and ERβ were decreased in PCBs-treated groups, and the histology confirms the cytoplasmic damage in the regions of caput, corpus, and cauda in PCBs-treated rats. Simultaneous supplementation of lycopene to PCBs-exposed rats resulted in significant decrease in the oxidative stress markers as that of control, while the AR, ERα, and ERβ gene expressions were near to control. The results suggest that lycopene has ameliorative effect against PCBs-induced toxicity in epididymis.
Keywords: polychlorinated biphenyl, androgen receptor, estrogen receptor, epididymis, lycopene
Introduction
Polychlorinated biphenyls (PCBS) are ubiquitous and persistent environmental pollutants. They are widely used in electrical industries and capacitors1 and are mediated by aryl hydrocarbon receptors (AhRs).2 Aroclor 1254 is commercial mixture of PCBs. One of the mechanisms by which PCB exerts its toxic effects is by increasing free radicals, thereby inducing testicular Sertoli and Leydig cellular oxidative stress.3,4
Lycopene, an aliphatic hydrocarbon that is richly found in tomato, is 1 of the 600 known naturally occurring carotenoids.5 Lycopene is a lipid-soluble hydrocarbon that is synthesized by many plants and microorganisms but not by animals and human.6 Lycopene has attracted special attention due to its ability to efficiently scavenge free radicals and its contribution in preventing lipid peroxidation.5 It is a powerful antioxidant with a singlet-oxygen-quenching capacity, 47 and 100 times greater than that of β-carotene and vitamin E, respectively.7,8
Lycopene also exerts direct anticarcinogenic effects including upregulation of gap-junction communication9 and modulation of growth factors such as insulin-like growth factor 1 (IGF-1) or cytokines such as interleukin 6 (IL-6).10 High intake of tomato juice prevents low-density lipoprotein (LDL) oxidation and thiobarbituric reactive species (TBARS) formation in healthy men.11 Epidemiological studies have indicated that elevated consumption of tomato significantly reduces mortality from several cancer sites, especially prostate cancer.12,13 The beneficial effects of tomato-rich diets are generally attributed to lycopene.14,15
The PCBs are considered potential endocrine disruptors, among many other effects, due to their ability to act as estrogen, antiestrogen, and goitrogen.16 Andric et al17 and Kim et al18 have indicated that PCBs can have adverse effects on various aspects of male reproduction in rats. Previous studies from our laboratory also proved that PCBs have adverse effects on male reproductive system in rats.19–21 These effects have heightened concern about the possible effects of PCBs on human reproduction and some evidence correlates PCBs’ exposure with decreased sperm motility in humans.14,22 Aroclor 1254 decreased the epididymal sperm count, sialic acid, and glyceryl phosphoryl choline (GPC) levels in rats. However, estradiol (E2) levels increased significantly. Aroclor 1254 affected the structural integrity of epididymis.23
The major functions carried out by the epididymis are the transport, maturation (acquisition of fertilizing potential), and storage of spermatozoa. The maturation occurs while the sperm passes through the epididymis between the distal caput and the proximal cauda epididymis. Cauda epididymis is the major site for storage of spermatozoa.24,25 An endogenous balance of estrogens and androgens is essential for the maintenance of normal epididymal growth and function.26,27 The secretion of sialic acid28 and GPC29 by the epididymis is an androgen-dependent event. Differential growth and function of epididymis is regulated by estrogen.30
In relation with the above literature, the role of PCB (Aroclor 1254) on androgen (AR) and estrogen (ER) receptor levels in 3 different regions of epididymis of experimental animals and the correlation between reactive oxygen species (ROS) and ARs and ERs have not been extensively studied. Lycopene is a potent singlet oxygen scavenger and has the effect of preventing injuries due to free radicals. Therefore, attention has been focused on the effect of lycopene on PCB-induced changes in the association of ROS and ARs and ERs in rat epididymis.
Materials and Methods
Chemicals
Aroclor 1254 was purchased from Chem Service (West Chester, Pennsylvania). Lycopene was obtained as a gift from Phytoremedies Biolabs Pvt Ltd (Pune, India). The 1-step reverse-transcriptase polymerase chain reaction (RT-PCR) Kit was obtained from Invitrogen (Eugene, Oregon). Primers for AR, ER-α, ERs, and β-actin were purchased from Sigma Aldrich Pvt Ltd, India, and rabbit polyclonal antiestrogen receptor-α and goat polyclonal antiestrogen receptor-β antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, California). Horseradish peroxidase (HRP)-conjugated goat anti-rabbit Immunoglobulin G and rabbit anti-goat antibodies were purchased from GENEI, Bangalore, India. Bovine serum albumin (BSA), acrylamide, bisacrylamide, ammonium persulfate, and N, N, N’, N tetramethyl ethylene diamine (TEMED) solution were purchased from Sigma-Aldrich Pvt Ltd (St Louis, Missouri). All other chemicals were purchased from SRL, India.
Experimental Design
Adult male albino rats of Wistar strain Rattus norvegicus weighing about 180 to 200 g were used in the present study. All animal procedures were approved by our institute’s animal ethical committee (Ref No. IAEC No: 01/01/11- Second Batch). The animals were housed in clean polypropylene cages, maintained in air-conditioned animal house with constant photoperiod of 12-hour light/dark cycle. The animals were fed with pellet diet (Gold Mohur Ltd, Mumbai, India) and drinking water ad libitum. Rats were assigned into 3 groups, with 6 animals in each group, and the treatment was given regularly for 30 days. Body weight of the animals was monitored throughout the experimental period. Group I rats or the control rats were injected with corn oil intraperitoneally (ip) once daily as a vehicle. Group II rats received ip injection of A1254 at a dose of 2 mg/kg once daily for 30 days. The treatment period was selected per the published studies.31 Group III rats received simultaneous treatment of Aroclor 1254 (ip) and lycopene at a dose of 4 mg/kg through gavage treatment. The dosage of lycopene was selected per the previous study.32
Tissue Collection
After 24 hours of the last treatment, the animals were killed by cervical decapitation. Epididymal regions were excised immediately. Regions were separated according to the method of Hamilton.26
Body and Organ Weight
The body weight of individual rats were monitored throughout the experimental period and recorded. After the treatment period of 30 days, the rats were killed. Epididymis was dissected, washed with ice-cold saline, blot dried, and weighed to obtain relative epididymal weight.
Preparation of the Homogenate
The separated epididymal regions, caput, corpus, and cauda, were homogenized with Tris HCl buffer (0.1 mol/L, pH 7.4), centrifuged at 12 000 rpm for 10 minutes, and the supernatant was used for biochemical analyses described subsequently. Protein concentration of the tissue homogenate was determined by the standard method of Bradford using BSA as a standard.
Estimation of H2O2 and LPO
The H2O2 generation was assessed by the spectrophotometric method of Pick and Keisari.33 The H2O2 content of the samples was expressed as m moles of H2O2 generated/mg protein. The LPO) was measured by the method of Devasagayam and Tarachand.34 The malondialdehyde (MDA) content of the samples was expressed as nanomoles of MDA formed/mg protein.
Estimation of GPC and Sialic Acid
The GPC was estimated by method of White.35 The GPC content of the samples was expressed as mg/100 mg tissue. Sialic acid was estimated per the method of Warren.36 The neuraminic acid content of the samples was expressed as μmol/g tissue.
Reverse Transcriptase-PCR
Total RNA was purified from epididymal tissue using 1 mL of the TRI reagent by the method of Chomczynski and Sacchi.37 The RNA purity and concentration were determined spectrophotometrically at A260/A280 nm. The purity of RNA obtained was 1.8 to 1.91 µg of total RNA reverse transcribed by the RT-PCR kit (Invitrogen) according to the manufacturer’s instructions and further amplified by PCR. The details of the primers used, number of cycles, and size of the PCR-amplified products are listed in Table 1. The PCR products were resolved by electrophoresis through a 2% agarose gel and stained with ethidium bromide. The densities of PCR products in the agarose gel were scanned with a Gel Doc image scanner, Bio-Rad (Hercules, California), and quantified by Quantity One Software, Bio-Rad (Hercules, California).
Table 1.
Details of Primers Utilized, Number of Cycles, and Expected Size of PCR-Amplified cDNA.
| Parameter | Sequence of the Primer | Number of Cycles | Amplified Product Size (bp) | Gene Accession No |
|---|---|---|---|---|
| Androgen receptor | ||||
| Sense | 5′-CCCATCGACTATTACTTCCCACC-3′ | 35 | 291 | M20133 |
| Antisense | 5′-TTCTCCTTCTTCCTGTAGTTTGA-3′ | |||
| Estrogen receptor α | ||||
| Sense | 5′-AATTCTGACAATCGACGCCAG-3′ | 35 | 344 | Y00102 |
| Antisense | 5′-GTGCTTCAACATTCTCCCTCCTC-3′ | |||
| Estrogen receptor β | ||||
| Sense | 5′-TTCCCGGCAGCACCAGTAACC-3′ | 35 | 262 | MN012754 |
| Antisense | 5′-TCCCTCTTTGCGTTTGGACTA-3′ | |||
| β-actin | ||||
| Sense | 5′-GAGATTACTGCCCTGGCTCC-3′ | 35 | 200 | NM_031144.3 |
| Antisense | 5′-GCTCAGTAACAGTCCGCCTA-3′ |
Abbreviations: bp, base pair; cDNA, complementary DNA; PCR, polymerase chain reaction.
Immunoblotting
Tissue lysate was prepared with radioimmuno assay buffer (RIPA) and protease inhibitor. Equal amounts of protein (60 µg) were electrophoresed on 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). Following electrophoresis, separated proteins on SDS-PAGE gels were transferred on polyvinylidene fluoride membrane (Millipore, Bangalore, India). To block the nonspecific binding, the membranes were incubated with 5% skimmed milk for 2 hours. Membranes were probed with primary rabbit polyclonal anti-ER-α (1:2000) and goat polyclonal anti-ER-β (1:500) antibodies for overnight. Then, the blots were incubated with HRP-conjugated secondary antibodies (1:10 000). The bands were developed using ECL kit (Millipore, Bangalore, India) in Chemi Doc image scanner from Bio-Rad (Hercules, California). The band intensity was quantified by Quantity One software (Bio-Rad). The membranes were stripped and reprobed for β-actin (1:5000) as an internal control.
Histology
The same groups were maintained for histological study. Animals were killed by perfusion. Formaldehyde 10% was used for fixation. The caput, corpus, and cauda were separated from the epididymis and were cleaned and sliced into tissues of 0.5 cubic cm. After further fixation by immersion in 4% formaldehyde in phosphate-buffered saline (PBS, pH 7.4) overnight at room temperature (8-12 hours), washing in PBS, and immersion in 70% ethanol, the tissue was maintained at 37°C until the embedding in paraffin was made. The paraffin blocks were cut into <10-m thickness using rotary microtome. The sections were stained with hematoxylin and eosin.38 The caput, corpus, and cauda morphology were analyzed by Nikon Microscope Eclipse 80i (×100 magnification; M/S Towa Optics, Chennai, India).
Statistical Analysis
Data were analyzed using 1-way analysis of variance. Zar39 followed the post hoc Student Newman-Keul (SNK) test with computer-based software (SPSS version 17). The significance was considered at the level of P < .05. All values were expressed as mean ± standard error of the mean of 6 animals.
Results
Effect of Lycopene on Body Weight and Relative Epididymal Weight of A1254-Exposed Adult Rats
Figure 1 (A) represents the effect of lycopene on body weight of Aroclor 1254–exposed adult rats. The body weight was significantly decreased in Aroclor 1254–exposed rats. Simultaneous supplementation of lycopene increased the body weight but not up to the level of control.
Figure 1.

A. Effect of lycopene on bodyweight of A1254-exposed adult rats. Each bar represents mean ± SEM of 6 animals. Significance at P < .05. (a) Control versus A1254, A1254 + Lyco; (b) A1254 versus A1254 + Lyco. B, Effect of lycopene on relative epididymal weight of A1254-exposed adult rats. Each bar represents mean ± SEM of 6 animals. Significance at P < .05. (a) Control caput versus A1254 caput, A1254 + Lyco caput; (b) A1254 caput versus A1254 + Lyco caput. (a’) Control corpus versus A1254 corpus, A1254+Lyco Corpus; (b’) A1254 corpus versus A1254 + lyco corpus. (a’’) Control cauda versus A1254 cauda, A1254 + Lyco cauda; (b’’) A1254 cauda vs A1254 + Lyco cauda. SEM indicates standard error of the mean; Lyco, lycopene.
Figure 1 (B) represents the effect of Aroclor 1254 and simultaneous supplementation of lycopene on relative epididymal weight of adult rats. Relative weight of caput and caudal epididymis were significantly decreased after Aroclor 1254 exposure. Simultaneous supplementation of lycopene increased the caudal epididymal weight up to the control; the weight of caput also increased but not up to the level of control. Corpus epididymis weight did not show any change in either Aroclor 1254 treatment or supplementation of lycopene.
Effect of Lycopene on H2O2 and LPO in Epididymal Regions of A1254-Exposed Adult Rats
Figure 2 (A and B) represents the effect of Aroclor 1254 and simultaneous supplementation of lycopene on the levels of H2O2 and LPO in rat epididymis. The levels of H2O2 and LPO were significantly increased in rat caput, corpus, and caudal epididymis of Aroclor 1254–exposed rats. However, supplementation of lycopene restored them near to the control levels.
Figure 2.

Effect of lycopene on generation of H2O2 and LPO of A1254-exposed adult rats. Each bar represents mean ± SEM of 6 animals. Significance at P < .05. (a) Control caput versus A1254 caput, A1254 + Lyco caput; (b) A1254 caput versus A1254 + Lyco caput. (a’) Control corpus versus A1254 corpus, A1254 + Lyco corpus; (b’) A1254 corpus versus A1254 + Lyco corpus. (a’’) Control cauda versus A1254 cauda, A1254 + Lyco cauda; (b’’) A1254 cauda versus A1254 + Lyco cauda. SEM indicates standard error of the mean; Lyco, lycopene; H2O2, hydrogen peroxide; LPO, lipid peroxidation.
Effect of Lycopene on Sialic Acid and GPC in Epididymal Region of A1254-Exposed Adult Rats
Figure 3 (A and B) depicts the effect of lycopene on sialic acid and GPC concentration in epididymis of Aroclor 1254–exposed adult rats. The sialic acid and GPC concentrations were significantly decreased in caput, corpus, and cauda regions of epididymis of Aroclor 1254-treated rats. However, simultaneous treatment with lycopene maintained the sialic acid and GPC concentration.
Figure 3.

Effect of lycopene on glyceryl phosphoryl choline (GPC) and sialic acid of A1254-exposed adult rats. Each bar represents mean ± SEM of 6 animals. Significance at P < .05. (a) Control caput versus A1254 Caput, A1254 + Lyco Caput; b: A1254 caput versus A1254 + Lyco Caput. (a’) Control corpus versus A1254 corpus, A1254 + Lyco corpus; (b’) A1254 corpus versus A1254 + Lyco corpus. (a’’) Control cauda versus A1254 cauda, A1254 + Lyco cauda; (b’’) A1254 cauda versus A1254 + Lyco cauda. SEM indicates standard error of the mean; Lyco, lycopene.
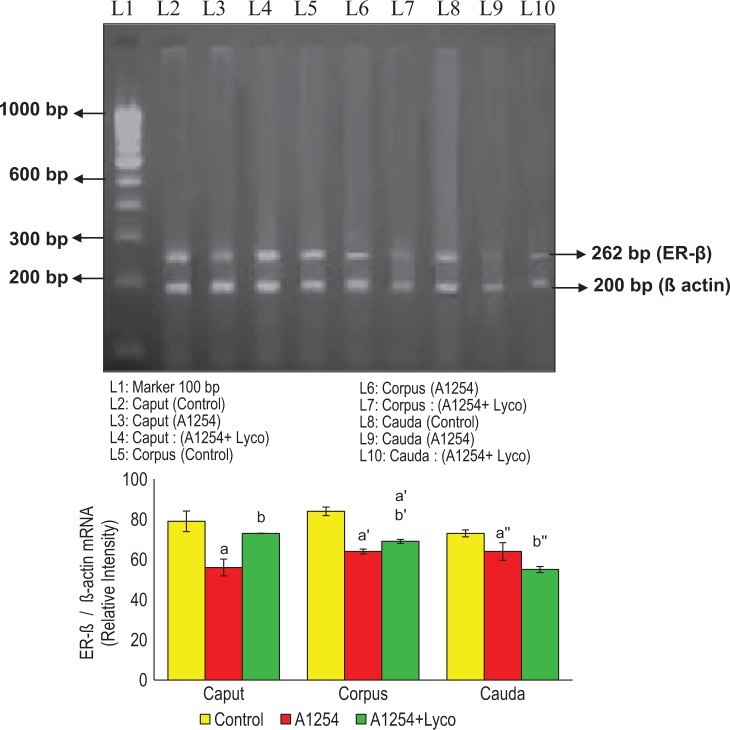
Effect of Lycopene on AR, ER-α, and β mRNA Expression in Epididymal Regions of A1254-Exposed Adult Rats
Figures 4, 5, and 6 show the effect of lycopene on AR, ER-α, and β mRNA expression in caput, corpus, and cauda epididymis of Aroclor 1254–exposed adult rats. The expression of AR, ER-α, and ER-β were significantly decreased in all the epididymal regions of PCBs-treated rats. The AR, ER-α, ER-β expressions were retrieved near to control in caput, corpus, and caudal epididymal regions of the Aroclor 1254 and simultaneous supplementation of lycopene-treated rats when compared with normal rats.
Figure 4.
Effect of lycopene on androgen receptor (AR) mRNA expression in epididymis of A1254-exposed adult rats. Each bar represents mean ± SEM of 3 independent observations. Significance at P < .05. (a) Control Caput versus A1254 Caput, A1254 + Lyco caput; (b) A1254 caput versus A1254 + Lyco caput. (a’) Control corpus versus A1254 corpus, A1254 + Lyco corpus; (b’) A1254 corpus versus A1254 + Lyco corpus. (a’’) Control cauda versus A1254 cauda, A1254 + Lyco cauda; (b’’) A1254 cauda versus A1254+Lyco cauda. SEM indicates standard error of the mean; Lyco, lycopene; mRNA, messenger RNA.
Figure 5.
Effect of lycopene on estrogen receptor α (ER-α) mRNA expression in epididymis of A1254-exposed adult rats. Each bar represents mean ± SEM of 3 independent observations. Significance at P < .05. (a) Control caput versus A1254 Caput, A1254 + Lyco caput; (b) A1254 caput versus A1254 + Lyco caput. (a’) Control corpus versus A1254 corpus, A1254 + Lyco corpus; (b’) A1254 corpus versus A1254 + Lyco corpus. (a’’) Control cauda versus A1254 cauda, A1254 + Lyco cauda; (b’’) A1254 cauda versus A1254 + Lyco cauda. SEM indicates standard error of the mean; Lyco, lycopene; mRNA, messenger RNA.
Figure 6.
Effect of lycopene on estrogen receptor β (ERβ) mRNA expression in epididymis of A1254-exposed adult rats. Each bar represents mean ± SEM of 3 independent observations. Significance at P < .05. (a) Control caput versus A1254 caput, A1254 + Lyco caput; (b) A1254 caput versus A1254 + Lyco caput. (a’) Control corpus versus A1254 corpus, A1254 + Lyco corpus; (b’) A1254 corpus versus A1254 + Lyco corpus. (a’’) Control cauda versus A1254 cauda, A1254 + Lyco cauda; (b’’) A1254 cauda versus A1254 + Lyco cauda. SEM indicates standard error of the mean; Lyco, lycopene; mRNA, messenger RNA.
Effect of Lycopene on ER-α and ER-β Protein Expression in Epididymis of A1254-Exposed Adult Rats
Figure 7 shows the effect of lycopene on ER-α expression in caput, corpus, and cauda epididymis of Aroclor 1254–exposed adult rats. The expression of ER-α was significantly increased in all the epididymal regions of Aroclor 1254-treated rats. Increased ER-α expression was observed in caput, corpus, and caudal epididymal regions of Aroclor 1254 + lycopene-treated rats but not in rats treated with PCB alone.
Figure 7.
Effect of lycopene on estrogen receptor α protein expression in epididymis of A1254-exposed adult rats. Each bar represents mean ± SEM of 3 independent observations. Significance at P < .05. (a) Control caput versus A1254 Caput, A1254+Lyco caput; (b) A1254 caput versus A1254 + Lyco caput. (a’) Control corpus versus A1254 corpus, A1254 + Lyco corpus; (b’) A1254 corpus versus A1254 + Lyco corpus. (a’’) Control cauda versus A1254 cauda, A1254 + Lyco cauda; (b’’) A1254 cauda versus A1254 + Lyco cauda. SEM indicates standard error of the mean; Lyco, lycopene.
Figure 8 shows the effect of lycopene on ER-β expression in caput, corpus, and cauda epididymis of Aroclor 1254–exposed adult rats. No significant change was observed in all the regions of both Aroclor 1254-alone and Aroclor 1254 + lycopene-treated rats.
Figure 8.
Effect of lycopene on estrogen receptor β protein expression in epididymis of A1254-exposed adult rats. Each bar represents mean ± SEM of 3 independent observations. Significance at P < .05. (a) Control caput versus A1254 caput, A1254 + Lyco caput; (b) A1254 caput versus A1254 + Lyco caput. (a’) Control corpus versus A1254 Corpus, A1254+Lyco corpus; (b’) A1254 corpus versus A1254 + Lyco corpus. (a’’) Control cauda versus A1254 cauda, A1254 + Lyco cauda; (b’’) A1254 cauda versus A1254 + Lyco cauda. SEM indicates standard error of the mean; Lyco, lycopene.
Histology
Figures 9, 10, and 11 show the light micrograph of cross sections of tubules of caput, corpus, and caudal epididymis of control, Aroclor 1254-, Aroclor 1254 + lycopene-treated rats (hematoxylin and eosin, ×20 and ×40).
Figure 9.

Light micrographs of caput epididymis of control and experimental animals (hematoxylin and eosin).
Figure 10.

Light micrographs of corpus epididymis of control and experimental animals (hematoxylin and eosin).
Figure 11.

Light micrographs of cauda epididymis of control and experimental animals (Hematoxylin and Eosin).
Figure 9 (A) shows caput epididymal tall columnar epithelial cells with nuclei situated at the base. The pseudo-stratified epithelium was prominent. More secretory vesicles were seen on both ×20 and ×40 (control).
Figure 9 (B) shows reduction in tubular diameter and degenerated epithelial cells were observed as well (Aroclor 1254 treated).
Figure 9 (C) shows very thin lining of caput epididymal epithelium of Aroclor 1254 + lycopene-treated rats. Interstitial space in between the tubules was filled with stromal cells. Lumen contained spermatozoa.
Figure 10 shows corpus epididymis of control and experimental rats. Cell heights were reduced compared to the other 2 regions (Figure 10A). Aroclor 1254-treated section (Figure 10B) showed reduced number of lipid molecules and less number of sperm in the lumen. Figure 10C showed uniform band of basal cells. Sperm numbers in the lumen were increased in Aroclor 1254 + Lycopene-treated regions.
Figure 11 shows caudal epididymis of control and experimental rats. Figure 11A shows normal highly pseudo-stratified epithelial cells. Lumen was wider than the other 2 regions. Figure 11 (B) shows disorganized cytoplasm, and Figure 11 (C) shows uniform lining of epithelial cells of Aroclor 1254 + lycopene-treated rats.
Discussion
Some of the reproductive deficits from endocrine-disrupting xenobiotics may be attributable to the modulation of estrogen signaling pathway in positive and negative manner, depending on dose, species, and tissue specificity.40 An endogenous balance of estrogens and androgens is essential for the maintenance of normal epididymal growth and function.26 The PCBs, as xenoestrogens, might affect the production of steroidogenic enzymes and alter the rate of synthesis and metabolism of endogenous estrogens.41 Several animal studies have reported that PCB treatment affects the steroid receptor levels as well as steroid metabolism1,42 and circulating androgen concentration.38
The PCBs, especially the higher chlorinated forms, may selectively induce cytochrome P450s43 as a possible source of ROS44 or, alternatively, the oxidation of a broad range of endogenous and exogenous substances (including PCBs). There are possible evidence suggesting that oxidative stress induced by PCBs is due to the interaction of these compounds with arylhydrocarbon receptors (AhRs) and activation of the cytochrome P450 IA subfamily. The cytochrome P450 catalyzed oxidation of lower chlorinated biphenyls that give rise to mono- and di-hydroxy metabolites. Later, this can autooxidize or be enzymatically oxidized to semiquinones and/or quinines.45 Some PCB quinones undergo redox cycling, with the formation of ROS, thus becoming another source of oxidative stress.45 ROS-like HO·, O2 −, and H2O2 are thought to contribute to LPO,46 DNA damage,47 and protein degradation.48
Lycopene is a well-known highly efficient scavenger of singlet oxygen (O2 −) and other excited species. Lycopene has been successfully used to provide protection against oxidative stress in different tissues.15In the present study, A1254 exposure significantly decreased the body weight and relative organ weight of epididymis. This might be due to increased level of ROS, which may induce cell damage. Simultaneous administration of lycopene maintains the body weight and relative organ weight to the control level. It may reduce the cell damage by scavenging ROS, thereby protecting the cells against PCB-induced toxicity.
In the present investigation, ROS (H2O2) and LPO levels were significantly increased in caput, corpus, and cauda of A1254-exposed animals. These ROS and LPO increase may be mediated through AhR and activation of the cytochrome P450 subfamily. Twaroski et al49 indicated that toxic manifestation induced by PCB may be associated with enhanced production of ROS and thereby induced oxidative stress through the initiation of self-propagating LPO reaction. However, simultaneous supplementation of lycopene with PCB treatment significantly reduces the generation of H2O2 and LPO in epididymis when compared to PCB-only treated group. The main reason for reducing the generation of H2O2 is due to O2 −scavenging activity of lycopene, thereby preventing the generation of H2O2 in PCB-treated animals.
The secretion of sialic acid28 and GPC29 by the epididymis is an androgen-dependent event. During epididymal transit, sialic acid residues bind to the sperm surface as terminal sugar of sialoglycoproteins. Yanagimachi et al50 studied that sialic acid moieties on the sperm membrane increased negative surface on sperm during the epididymal transit. Gupta et al51 studied that antiandrogenic treatment decreased the levels of sialic acid in the epididymis and spermatozoa with a loss in the integrity of acrosome of the spermatozoa and their fertilizing ability.
The GPC is a major component of epididymal secretion. It maintains high osmolality of epididymal fluid and sperm membrane stability through inhibition of phospholipase activity.52 Testosterone and thyroid hormone are known to be stimulating factors for the synthesis and secretion of GPC.29 The PCB-induced hypothyroidism might have led to reduced accumulation and degradation of phosphatidyl choline, which in turn might have resulted in reduced levels of GPC. Aroclor 1254 decreased the testosterone synthesis by decreasing steroidogenic enzymes expression and activity in rat Leydig cells.21,53 Interestingly, in the present study, sialic acid and GPC concentrations were significantly decreased in the epididymis of PCB-exposed rats. This observed result correlates the reduction of androgen level in PCB-treated rats21,53 and importance of androgen on sialic acid and GPC concentration. However, simultaneous supplementation of lycopene ameliorated these parameters compared to that of the control. This might be due to the increased level of testosterone in simultaneous supplementation of lycopene in A1254-treated rats.32
Androgens are absolutely essential for the maintenance of spermatogenesis and sperm maturation. Previous studies reported that serum follicle-stimulating hormone (FSH), luteinizing hormone (LH), testosterone (T), and E2 levels were significantly decreased in A1254-treated rats. The PCBs decreased the level of gonadotropin-releasing hormone that was reflected in LH and FSH synthesis.42The present study correlated the reduction in T and its receptor expression. Lycopene supplementation brought back the receptors’ expression to normal, and this might be due to restored level of testosterone. In the present study, AR mRNA expression was decreased in all the epididymal regions after PCB exposure. The AR protein expression study is warranted.
Xenobiotic response elements are shown to be present in the upstream promoter sequences of many estrogen responsive genes. Ohtake et al54 studied that a direct genomic interaction between xenoestrogens and natural ER is established in vivo, resulting in various estrogenic responses. Both ER-α and ER-β were highly expressed in the epithelial cells of epididymis and have been reported to have a relatively high affinity for endocrine disruptor chemical, and its activation results in the transcriptional responses.55
Hess30 demonstrated that ER-α is dominant on epididymal regions. The PCBs compete with estrogen for binding to the ER,56 and developmental PCB exposure has recently reported to increase estrogen sensitivity57 and to modulate sexual differentiation in rodent brain. The PCBs and their metabolites can exert estrogenic effects by binding to the ER.34 The E2 upregulates ER level.58
Previous studies have shown that the dietary carotenoids including lycopene inhibits the estrogen signaling of 17-β E2.55 Lycopene and other carotenoids inhibited estrogen-induced transactivation of estrogen response element (ERE) that was mediated by both ER-α and ER-β.59 In the present study, ER-α was significantly increased in epididymis of PCB-treated animals. This might be due to the estrogenic activity of PCB by binding to ER present in epididymis, and thereby it may upregulate the expression of ERs. However, simultaneous supplementation of lycopene maintains both ER-α and ER-β. The histoarchitecture of caput, corpus, and caudal epididymis showed disorganized epithelium after PCB exposure. Simultaneous lycopene-treated rats showed decreased cytoplasmic damage and organized lumen on all the epididymal regions.
In summary, the present study in epididymis demonstrates that lycopene possesses prooxidant activity on PCB-exposed animals and the expression of ER, and the functional markers are alleviated by the supplementation of lycopene. The data generated in the present study demonstrated that caput region in rats contains more GPC, caudal region has more sialic acid suggesting that spermatozoa are rapidly processed in caput region, and caudal have more storage. Inside the cell, testosterone can be converted into dehydrotestosterone (DHT) by 5 α-reductase 2. The DHT may also facilitate transformation of the receptor for more efficient transactivation. The study on AR protein expression as well as DHT is warranted. Further study is needed to demonstrate a region-specific gene expression pattern. To conclude, lycopene, a potent antioxidant, preserved the epididymis from PCB-induced damage.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This research was supported by a UGC SAP , UGC RFSMS, and DST, New Delhi, India.
References
- 1. Safe Kamrin MA, Ringer RK. PCB residue in mammals: a review. Toxicol Environ Chem. 1994;41(1-2):63–84. [Google Scholar]
- 2. Tilson HA, Kodavanti PR. The Neurotoxicology of polychlorinated biphenyls. Neurotoxicology. 1998;19(4-5):517–526. [PubMed] [Google Scholar]
- 3. Senthil kumar J, Banudevi S, Sharmila M, et al. Effects of vitamin C and E on PCB (Aroclor 1254) induced oxidative stress, androgen binding protein and lactate in rat Sertoli cells. Reprod Toxicol. 2004;19(2):201–208. [DOI] [PubMed] [Google Scholar]
- 4. Murugesan P, Senthil kumar J, Balasubramanian K, Aruldhas MM, Arunakaran J. Impact of polychlorinated biphenyl Aroclor 1254 on testicular antioxidant system in adult rats. Hum Exp Toxicol. 2005;24(2):61–66. [DOI] [PubMed] [Google Scholar]
- 5. Cohen LA. A review of animal model studies of tomato carotenoids, lycopene, and cancer chemoprevention. Exp Biol Med. 2002;227(10):864–868. [DOI] [PubMed] [Google Scholar]
- 6. Paiva SA, Russell RM. Beta carotene and other carotenoids as antioxidants. J Am Coll Nutr. 1999;18(5):426–433. [DOI] [PubMed] [Google Scholar]
- 7. Di Mascio P, Kaiser S, Sies H. Lycopene as the most efficient biological carotenoid singlet oxygen quencher. Arch Biochem Biophys. 1989;274(2):532–538. [DOI] [PubMed] [Google Scholar]
- 8. Liu CC, Huang CC, Lin WT, et al. Lycopene supplementation attenuated xanthine oxidase and myeloperoxidase activities in skeletal muscle tissues of rats after exhaustive exercise. Br J Nutr. 2005;94(4):595–601. [DOI] [PubMed] [Google Scholar]
- 9. Bhuvaneswari V, Nagini S. Lycopene: a review of its potential as an anticancer agent. Curr Med Chem Anticancer Agents. 2005;5(6):627–635. [DOI] [PubMed] [Google Scholar]
- 10. Siler U, Herzog A, Spitzer V, et al. Lycopene effects on rat normal prostate and prostate tumor tissue. J Nutr. 2005;135(8):2050S–2052S. [DOI] [PubMed] [Google Scholar]
- 11. Bub A, Watzl B, Abrahamse L, et al. Moderate intervention with carotenoid-rich vegetable products reduces lipid peroxidation in men. J Nutr. 2000;130(9):2200–2206. [DOI] [PubMed] [Google Scholar]
- 12. Clinton SK. Lycopene: chemistry, biology, and implications for human health and disease. Nutr Rev. 1998;56(2 pt 1):35–51. [DOI] [PubMed] [Google Scholar]
- 13. Rao AV, Agarwal S. Role of lycopene as an antioxidant carotenoid in the prevention of chronic disease: a review. Nutr Res. 1999;19(2):305–323. [Google Scholar]
- 14. Rozati R, Reddy PP, Reddanna P, Mujtaba R. Role of environmental estrogens in the deterioration of male factor fertility. Fertil Steril. 2002;78(6):1187–1194. [DOI] [PubMed] [Google Scholar]
- 15. Stahl W, Sies H. Lycopene: a biologically important carotenoid for humans? Arch Biochem Biophys. 1996;336(1):1–9. [DOI] [PubMed] [Google Scholar]
- 16. Hansen LG. Stepping backward to improve assessment of PCB congener toxicities. Environ Health Perspect. 1998;106(suppl 1):171–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Andric SA, Kostic TS, Stojilkovic SS, Kovacevic RZ. Inhibition of rat testicular androgenesis by a polychlorinated biphenyl mixture aroclor 1248. Biol Reprod. 2000;62(6):1882–1888. [DOI] [PubMed] [Google Scholar]
- 18. Kim IS, Ariyaratne HB, Chamindrani Mendis-Handagama SM. Effects of continuous and intermittent exposure of lactating mothers to aroclor 1242 on testicular steroidogenic function in the adult male offspring. Tissue Cell. 2001;33(2):169–177. [DOI] [PubMed] [Google Scholar]
- 19. Sridhar M, Venkataraman P, Dhanammal S, et al. Impact of polychlorinated biphenyl (Aroclor 1254) and vitamin C on antioxidant system of rat ventral prostate. Asian J Androl. 2004;6(1):19–22. [PubMed] [Google Scholar]
- 20. Murugesan P, Kanagaraj P, Balasubramanian K, Aruldhas MM, Arunakaran J. The inhibitory effects of polychlorinated biphenyl Aroclor 1254 on Leydig cell LH receptors, steroidogenic enzymes, and antioxidant enzymes in adult rats. Reprod Toxicol. 2005;20(1):117–126. [DOI] [PubMed] [Google Scholar]
- 21. Murugesan P, Muthusamy T, Balasubramanian K, Arunakaran J. Studies on the protective role of Vitamin C and E against polychlorinated biphenyl (Aroclor 1254) induced oxidative damage in Leydig cells. Free Radic Res. 2005;39(11):1259–1272. [DOI] [PubMed] [Google Scholar]
- 22. Rozati R, Reddy PP, Reddanna P, Mujtaba R. Xenoestrogens and male infertility: myth or reality? Asian J Androl. 2000;2(4):263–269. [PubMed] [Google Scholar]
- 23. Anbalagan J, Kanagaraj P, Srinivasan N, Aruldhas MM, Arunakaran J. Effect of polychlorinated biphenyl, Aroclor 1254 on rat epididymis. Indian J Med Res. 2003;118:236–242. [PubMed] [Google Scholar]
- 24. Orlowski J, Clark AF. Androgen 5α-reductase and 3α-hydroxy steroid dehydrogenase activities in ventral prostate epithelial and stromal cells from immature and mature rat. J Endocrinol. 1983;99(1):131–134. [DOI] [PubMed] [Google Scholar]
- 25. Robaire B, Hermo L. Efferent ducts, epididymis, and vas deferens: structure, functions and their regulation. In: Knobil E, Neill JD, eds. The Physiology of Reproduction. Vol 1 New York, NY: Raven Press; 1988:999–1080. [Google Scholar]
- 26. Hamilton DW. Structure and function of the epithelium lining the ductuli efferentes, ductus epididymids and ductus deferens in the rat. In: Hamilton DW, Greep RO, eds. Handbook of Physiology. Vol 5 Washington, DC: American Physiological Society; 1975:259–301. [Google Scholar]
- 27. Mann T, Lutwak-Mann C. Biochemistry of spermatozoa. Chemical and functional correlation in ejaculate semen. In: Mann T, Lutwak-Mann C, eds. Male Reproductive Function and Semen. Themes and Trends in Physiology, Biochemistry and Investigation Andrology. New York, NY: Springer-Verlag; 1981:195–278. [Google Scholar]
- 28. Rajalakshmi M, Prasad MR. Physiology of the epididymis and spermatozoa. J Endocrinol. 1968;41(4):471–476. [DOI] [PubMed] [Google Scholar]
- 29. Brooks DE, Hamilton DW, Mallek AH. Carnitine and glyceryl phosphorylcholine in the reproductive tract of the male rat. J Reprod Fertil. 1974;36(1):141–160. [DOI] [PubMed] [Google Scholar]
- 30. Hess RA. Estrogen in the adult male reproductive tract: a review. Reprod Biol Endocrinol. 2003;1:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Venkataraman P, Muthuvel R, Krishnamoorthy G, et al. PCB (Aroclor 1254) enhances oxidative damage in rat brain regions: protective role of ascorbic acid. Neurotoxicology. 2007;28(3):490–498. [DOI] [PubMed] [Google Scholar]
- 32. Elumalai P, Krishnamoorthy G, Selvakumar K, Arunkumar R, Venkataraman P, Arunakaran J. Studies on the protective role of lycopene against polychlorinated biphenyls (Aroclor 1254)-induced changes in StAR protein and cytochrome P450 scc enzyme expression on Leydig cells of adult rats. Reprod Toxicol. 2009;27(1):41–45. [DOI] [PubMed] [Google Scholar]
- 33. Pick E, Keisari Y. Superoxide anion and hydrogen peroxide production by chemically elicited peritoneal macrophages-induction by multiple nonphagocytic stimuli. Cell Immunol. 1981;59(2):301–318. [DOI] [PubMed] [Google Scholar]
- 34. Devasagayam TP, Tarachand U. Decreased lipid peroxidation in the rat kidney during gestation. Biochem Biophys Res Commun. 1987;145:134–138. [DOI] [PubMed] [Google Scholar]
- 35. White IG. Studies on the estimation of glycerol, fructose and lactic acid with particular reference to semen. Aust J Exp Biol Med Sci. 1959;37:441–450. [DOI] [PubMed] [Google Scholar]
- 36. Warren L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959;234(8): 1971–1975. [PubMed] [Google Scholar]
- 37. Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanatephenol-chloroform extraction. Anal Biochem. 1987;162(1):156–159. [DOI] [PubMed] [Google Scholar]
- 38. Hany J, Lilienthal H, Roth-Harer A, Ostendorp G, Heinzow B, Winneke G. Behavioral effects following single and combined maternal exposure to PCB 77.3,4,3',4'-tetrachlorobiphenyl; and PCB 47 (.2,4,2',4'- tetrachlorobiphenyl) in rats. Neurotoxicol Teratol. 1999;21(2):147–156. [DOI] [PubMed] [Google Scholar]
- 39. Zar JH. Multisample hypothesis: The analysis of variance. In: Zar JH, ed. Biostatistical Analysis. New York, NY: Prentice Hall Inc. [Google Scholar]
- 40. Hutz RJ. Reproductive endocrine disruption by environmental xenobiotics that modulate the estrogen signaling pathway, particularly tetrachlorodibenzo-p-dioxin (TCDD). J Reprod Der. 1999;45(1999):1–12. [Google Scholar]
- 41. Korach KS, Sarver P, Chae K, McLachlan JA, McKinney JD. Estrogen receptor-binding activity of polychlorinated hydroxybiphenyls: conformationally restricted structural probes. Mol Pharmacol. 1988;33(1):120–126. [PubMed] [Google Scholar]
- 42. Venkataraman P, Sridhar M, Dhanammal S, et al. Effects of vitamins supplementation on PCB (Aroclor 1254)-induced changes in ventral prostatic androgen and estrogen receptors. Endocr Res. 2004;30(3):469–480. [DOI] [PubMed] [Google Scholar]
- 43. Parkinson A, Safe SH, Robertson LW, et al. Immunochemical quantitation of cytochrome P-450 isozymes and epoxide hydrolase in liver microsomes from polychlorinated or polybrominated biphenyl treated rats. A study of structure–activity relationships. J Biol Chem. 1983;258(9):5967–5976. [PubMed] [Google Scholar]
- 44. Schlezinger JJ, Keller J, Verbrugge LA, Stegeman JJ. 3,3’,4,4’-Tetrachlorobiphenyl oxidation in fish, bird and reptile species: relationship to cytochrome P4501A inactivation and reactive oxygen production. Comp Biochem Physiol C Toxicol Pharmacol. 2000;125(3):273–286. [DOI] [PubMed] [Google Scholar]
- 45. McLean MR, Twaroski TP, Robertson LW. Redox cycling of 2-(.x'-mono, -di,-trichlorophenyl)-1, 4-benzoquinones, oxidation products of polychlorinated biphenyls. Arch Biochem Biophys. 2000;376(2):449–455. [DOI] [PubMed] [Google Scholar]
- 46. Hochstein P, Ernster L. ADP-Activated lipid peroxidation coupled to THE TPNH oxidase system of microsomes. Biochem Biophys Res Commun. 1963;12(5):388–394. [DOI] [PubMed] [Google Scholar]
- 47. Kasai H, Crain PF, Kuchino Y, Nishimura S, Ootsuyama A, Tanooka H. Formation of 8-hydroxyguanine moiety in cellular DNA by agents producing oxygen radicals and evidence for its repair. Carcinogenesis. 1986;7(11):1849–1851. [DOI] [PubMed] [Google Scholar]
- 48. Griffith HR, Unswoth J, Blake DR, Lunec J. Free Radicals in Chemistry . Pathology and Medicine. London, England: Richeliue;1988:439–454. [Google Scholar]
- 49. Twaroski TP, O’Brien ML, Larmonier N, Glauert HP, Rorbertson LW. Polychlorinated biphenyls-induced effects of metabolic enzymes, AP-1 binding, vitamin E, and oxidative stress in the rat liver. Toxicol Appl Pharmacol. 2001;171(2):85–93. [DOI] [PubMed] [Google Scholar]
- 50. Yanagimachi R, Nodar YD, Fujmoto M, Nicolson GL. The distribution of negative surface charges on mammalian spermatozoa. Am J Anat. 1972;135(4):497–519. [DOI] [PubMed] [Google Scholar]
- 51. Gupta G, Rajalakshmi M, Prasad MR, Moudgal NR. Alteration of epididymal function and its relation to maturation of spermatozoa. Andrologia. 1974;6(1):35–44. [DOI] [PubMed] [Google Scholar]
- 52. Scott TW, Wales JC, Wallace JC, White IG. Composition of Ram epididymal and testicular fluid and the biosynthesis of GPC by the rabbit epididymis. J Reprod Fertil. 1963;6:49–59. [DOI] [PubMed] [Google Scholar]
- 53. Murugesan P, Balaganesh M, Balasubramanian K, Arunakaran J. Effects of polychlorinated biphenyl (Aroclor 1254) on steroido-genesis and antioxidant systemin cultured adult rat Leydig cells. J Endocrinol. 2007;192(2):325–338. [DOI] [PubMed] [Google Scholar]
- 54. Ohtake F, Takeyama K, Matsumoto T, et al. Modulation of oestrogen receptor signalling by association with the activated dioxin receptor. Nature. 2003;423(6939):545–550. [DOI] [PubMed] [Google Scholar]
- 55. Hall JC, Hadley J, Doman T. Correlation between changes in rat sperm membrane lipids, protein, and the membrane physical state during epididymal maturation. J Androl. 1991;12(1):76–87. [PubMed] [Google Scholar]
- 56. DeCastro BR, Korrick SA, Spengler JD, Soto AM. Estrogenic activity of polychlorinated biphenyls present in human tissue and the environment. Environ Sci Technol. 2006;40(8):2819–2825. [DOI] [PubMed] [Google Scholar]
- 57. Ceccatelli R, Faass O, Schlumpf M, Lichtensteiger W. Gene expression and estrogen sensitivity in rat uterus after developmental exposure to the polybrominated diphenylether PBDE 99 and PCB. Toxicology. 2006;220(2-3):104–116. [DOI] [PubMed] [Google Scholar]
- 58. Ing NH, Tornesi MB. Estradiol up-regulates estrogen receptor and progesterone receptor gene expression in specific ovine uterine cells. Biol Reprod. 1997;56(5):1205–1215. [DOI] [PubMed] [Google Scholar]
- 59. Hirsch K, Atzmon A, Danilenko M, Levy J, Sharoni Y. Lycopene and other carotenoids inhibit estrogenic activity of 17beta-estradiol and genistein in cancer cells. Breast Cancer Res Treat. 2007;104(2):221–230. [DOI] [PubMed] [Google Scholar]