Abstract
Background
Both nuclear receptor subfamily 2 group F member 1 (NR2F1) and microRNAs (miRNAs) have been shown to play critical roles in the developing and functional inner ear. Based on previous studies suggesting interplay between NR2F1 and miRNAs, we investigated the coregulation between NR2F1 and miRNAs to better understand the regulatory mechanisms of inner ear development and functional maturation.
Results
Using a bioinformatic approach, we identified 11 potential miRNAs that might coregulate target genes with NR2F1 and analyzed their targets and potential roles in physiology and disease. We selected 6 miRNAs to analyze using quantitative real-time (qRT) -PCR and found that miR-140 is significantly down-regulated by 4.5-fold (P=0.004) in the inner ear of NR2F1 knockout (Nr2f1–/–) mice compared to wild-type littermates but is unchanged in the brain. Based on this, we performed chromatin-immunoprecipitation followed by qRT-PCR and confirmed that NR2F1 directly binds and regulates both miR-140 and Klf9 in vivo. Furthermore, we performed luciferase reporter assay and showed that miR-140 mimic directly regulates KLF9-3’UTR, thereby establishing and validating an example coregulatory network involving NR2F1, miR-140, and Klf9.
Conclusions
We have described and experimentally validated a novel tissue-dependent coregulatory network for NR2F1, miR-140, and Klf9 in the inner ear and we propose the existence of many such coregulatory networks important for both inner ear development and function.
Introduction
Nuclear receptors are a large family of cellular regulators that function as activators, repressors and silencers of transcription when bound by specific ligands or cofactors [1,2]. They are structured with a ligand-binding domain in the C-terminus, activation function domains, and a DNA-binding domain in the N-terminus which binds to specific hormone response DNA elements in the genome [3]. Two major subclasses of nuclear receptors include the nuclear hormone receptors that respond to hormone ligands and the orphan nuclear receptors for which endogenous ligands are not known [4]. Nuclear receptor subfamily 2 group F member 1 (NR2F1) is an orphan nuclear receptor required for the development of the inner ear and cerebral cortex [5–8]. This is illustrated by loss-of-function in knockout mice (Nr2f1 –/–) which impacts neurogenesis, axonal guidance, neocortical patterning, morphogenesis, and patterning of the cochlear sensory epithelium and the inner and outer hair cells in the organ of Corti [7–10]. A recent forward genetics screen in patients with congenital anomalies and cytogenetically balanced chromosomal rearrangements identified a critical paracentric microdeletion of the Nr2f1 locus, implicating haploinsufficiency of NR2F1 as a cause for a 4-year-old child’s deafness, dysmorphism and developmental delay [11].
In an effort to define transcription factor binding sites and target genes in vivo, Montemayor et al. developed a methodology to identify NR2F1 target genes by intersecting gene expression profiling, computational binding site queries, and evolutionary conservation data [12]. This approach is in part based on the concept of ‘phylogenetic footprinting,’ which assumes evolution selects against mutations within DNA regions that have an important regulatory function; thus creating evolutionary cold spots within the critical genomic segments that regulate gene expression [13]. Using this approach we identified and validated several direct target genes of NR2F1, and began to uncover regulatory and feedback networks at the transcriptional and post-translational level that might be necessary in the development and physiology of mammals. To date, relatively few coregulatory networks have been delineated for interactions between nuclear receptors and microRNAs (miRNAs), and even fewer have been experimentally validated [14].
MicroRNAs were discovered as small temporal RNAs that regulate developmental transitions in C. elegans. They have diverse expressive patterns and regulate a variety of areas, including embryologic development, physiology, pathophysiology, and growth, development, progression, and metastasis in cancer [15–17]. Currently, there are 2,578 (human) and 1908 (mouse) mature unique miRNAs identified (mirBase 20, June 2013), and more than half are conserved in vertebrates [18]. In humans, genes encoding miRNAs are located in intergenic (52%), intragenic–intronic (43%), or intragenic-exonic (5%) regions with both sense and antisense orientations [19]. This large family of 21-23 nucleotide single-stranded non-coding RNAs negatively regulate gene expression at the post-transcriptional level by binding the 5’UTR, coding sequences, or 3’UTR. They have the ability to either promote degradation or suppress the translation of their target mRNA, depending on the complementarity with which they bind [15,16]. These small non-coding RNAs are first formed as pri-miRNAs which are capped, adenylated, spliced, and later cleaved by Drosha and Pasha endonucleases (RNase-III type) resulting in precursor miRNAs (pre-miRNA) [16]. Pre-miRNAs are then processed by Dicer to form short duplex RNAs, which are incorporated into a miRNA-induced silencing complex (miRISC), in order to interact with the target mRNA.
MicroRNAs have a specific role in the developing inner ear and hearing, where many miRNA expression profiles change both temporally and spatially during embryogenesis and post-natal maturation and are conserved through evolution [20–22]. The significance of miRNAs in audition is exemplified by mutations in the seed region of human miRNA-96 causing a nonsyndromic progressive hearing loss in humans [21]. Furthermore, Dicer is required for survival of inner ear hair cells in mice, and a loss-of-function results in aberrant hair bundle formation, stereocilia defects, disrupted inner ear morphogenesis, and impaired hearing function [23,24].
Given the apparent roles of NR2F1 and miRNAs in the ontogenesis of the inner ear, and more notably the functional deficits caused when these regulators are mutated or deleted, we sought to discover the genetic coregulatory networks of NR2F1 and miRNAs. We hypothesized that knowing if, how, and when gene regulatory pathways intersect could shed light on the current incomplete knowledge of the regulatory mechanisms of inner ear development and functional maturation [20], and perhaps extend understanding to other organ systems as well. Such mechanistic understanding is a crucial step in discovering innovative ways of protecting, regenerating or rejuvenating hair cells and neurons in patients with partial or complete hearing loss [25,26]. This study discovered coregulatory interactions between NR2F1 and miRNA-140 in their regulation of the gene Krüppel-like factor 9 (Klf9). This is the first description of such a pathway and delineates the regulatory actions of a circuit involving the non-steroidal nuclear receptor transcription factor and miRNAs.
Materials and Methods
Ethics Statement
The Institutional Animal Care and Use Committee of Baylor College of Medicine approved all studies involving the use of animals as mandated by the United States federal government.
Identification of the 11 miRNAs and their putative targets
In order to find the overlap of any miRNA gene with NR2F1 target genes, the human genome (GRCh37/hg19) and mouse genome (NCBI37/mm9) builds were viewed using the UCSC genome browser [27] (http://genome.ucsc.edu) with the NR2F1 binding site custom track supplied by Montemayor et al. [12]. The miRNA track was also viewed to identify every miRNA mentioned by Wang et al. to be expressed in the developing mouse inner ear [22]. Other miRNAs were identified by reviewing the literature involving ontogenesis or NR2F1[21,22,28–30]. To find all possible gene targets for each miRNA, we utilized multiple database prediction tools: miRBase (http://www.mirbase.org) [31], MicroCosm (http://www.ebi.ac.uk/enright-srv/microcosm/htdocs/targets/v5), TargetScan 6.0 (http://www.targetscan.org), microRNA.org (http://www.microrna.org/microrna/home .do), and PITA (http://genie.weizmann.ac.il/pubs/mir07/mir07_dyn_data.html) [32]. The predicted genes from the different databases were combined and redundant genes removed. The final lists were compared with the putative NR2F1 targets identified by Montemayor et al. [12].
miRNA binding site predictions
MicroRNA.org (http://www.microrna.org/microrna/home .do) was used to determine binding sites within the translated regions of the genes while TargetScan 6.2 (http://www.targetscan.org) [33] and miRWalk (http://www.umm.uni-heidelberg.de/apps/zmf/mirwalk/index.html) [34] were used to determine binding sites in the 3’ UTR. The range of the binding sites was manually curated.
Functional annotation and biological significance
The Database for Annotation, Visualization and Integrated Discovery (DAVID) [35,36] querying UniProt and the Genetic Association Database was used to determine the expression pattern and associated neurological diseases of the 28 genes targeted by at least 3 of the 11 miRNAs. Functional clusters were discovered using the Gene Functional Classification function in DAVID. Cancer-related genes targeted by both NR2F1 and the 11 miRNAs were identified by searching the Sanger Institute’s cancer database. To discover genes that affect hearing loss, the hereditary hearing loss databases (hereditaryhearingloss.org), the Corey Lab at Harvard [37,38], and commercial hearing loss databases (SABiosciences, Frederick, MD) were mined and compared with the putative targets of NR2F1 and the 11 miRNAs. In addition, we examined genes involved in circadian rhythm have been shown to be involved in many aspects of embryogenesis [39] and identified 18 genes that are targeted by at least one of the 11 miRNAs. Finally, lists of genes involved in Notch signaling, neurogenesis, and stem cell function were obtained from SABiosciences (Frederick, MD) and filtered against the putative targets of the 11 miRNAs.
RNA extraction and real-time polymerase chain reaction
Inner ears of P0 Nr2f1 –/– (–/–) and WT (+/+) littermates were placed in RNA Later (Ambion) after dissection, stored at 4°C overnight and then at -20°C until the genotype was determined [9]. Total RNA was isolated using the RNeasy Mini Kit (Qiagen) according to the manufacturer’s instructions and quantified using NanoDrop spectrophotometry (Wilmington, DE).
Reverse transcription and real-time PCR of mRNAs were carried out using 0.25μg of RNA, nuclease-free water, 0.5μl of Oligo(dT), 10μl 5x First-Strand buffer, 4μl of 10mM dNTP, 5μl of 0.1M DTT, 1μl ribonuclease inhibitor (Invitrogen), and 2μl SuperScript II (Invitrogen). Reverse transcription was performed at 42°C for 1h before heating to 70°C for 15 min then returning to 4°C. Real-time PCR was performed in triplicates in 96-well plates in Mastercycler ep realplex (Eppendorf, Hamburg, Germany) using the following program: 1) 50°C for 2min, 2) 95°C for 2min, 3) 45 cycles of 95°C for 15s, 60°C for 1min, and 4) melting curve ramp.
Reverse transcription and real-time PCR of microRNAs were carried out using a protocol modified from Varkonyi-Gasic et al. using stem-loop primers and SYBR Green without any commercial miRNA detection kit [40]. All stem-loop, forward and reverse primers were taken from Tang et al. [41] except for the stem-loop and forward primers for mmu-miR-1192 which have the sequences 5’-ctcaactggtgtcgtggagtcggcaattcagttgagaatttggt-3’ and 5’- acactccagctgggaaacaaacaaaca-3’. Briefly, 0.25μg of RNA for each sample was incubated for 5 minutes at 65°C with nuclease-free water and 0.5μl of 10mM dNTP mix in a final volume of 12.65μl and returned to ice for at least 2 minutes. A mix of 4μl 5X First-Strand buffer, 2μl 0.1M DTT, 0.1μl ribonuclease inhibitor (Invitrogen), 0.25μl SuperScript II RT (Invitrogen) and 1μl of 1μM stem-loop primer was added for each reaction. Reverse transcription was performed using the following program: 1) 16°C for 30min, 2) 45 cycles at 30°C for 30s, 42°C for 30s, and 50°C for 1s, and 3) 85°C for 5min. For real-time PCR, 2μl of the reverse transcribed samples were mixed with 1μl each of the forward and reverse primers (1μM), 4μl of Express SYBR Green (Invitrogen) and 12μl nuclease-free water. The real-time PCR was performed in triplicates in 96-well plates in Mastercycler ep realplex (Eppendorf, Hamburg, Germany) using the following program: 1) 95°C for 5min, 2) 45 cycles of 95°C for 5s, 60°C for 10s, 72°C for 1s, 3) melting curve ramp from 65°C to 95°C at 0.1°C per second.
Expression levels of both mRNA and miRNAs were compared using the relative CT (cycle number) method [42] after normalization to the expression level of cyclophilin A. Primer sequences are included in Table S7 in File S1 .
Chromatin-immunoprecipitation
Cerebral cortex was harvested from C57Bl/6 mice and processed by chromatin crosslinking and immunoprecipitation as described in the Active Motif ChIP-IT Express Kit (Active Motif, Carlsbad, CA). Briefly, 150 mg of cortex was minced and cross-linked with 1% formaldehyde at room temperature with gentle shaking for 12 min. Glycine was added (final concentration of 125 mM) to quench the formaldehyde by incubating for 5 min with gentle shaking. The tissue pieces were then dounce homogenized (loose clearance, 2ml, K885301-002, Kontes, Fisher Scientific, Waltham, MA) in 2 ml of ice-cold PBS supplemented with 10 ul proteinase inhibitor cocktail (PIC, Active Motif, Carlsbad, CA) and 10 ul phenylmethylsulfonyl fluoride (PMSF, 100mM, Active Motif, Carlsbad, CA) to obtain a single-cell slurry which were pelleted at 660xg at 4°C for 5 min. Cells were resuspended in 2 ml Lysis Buffer supplemented with PIC and PMSF, incubated for 30 min on ice before being dounce-homogenized again (tight clearance, 2ml, K885301-002, Kontes, Fisher Scientific, Waltham, MA) to release the nuclei, which were then pelleted with centrifugation for 10 min at 660xg rpm at 4°C. Chromatin fragmentation was performed in shearing buffer using a 450 Branson Sonifier (50 bursts of 30 sec pulse with 30 sec OFF, 30% power output and 90% duty cycle) and the sheared chromatin was collected at 20,000xg for 15 min at 4°C, after which the supernatant was stored at -80°C.
Chromatin-immunoprecipitation (ChIP) was performed at 4°C overnight with antibodies against NR2F1 (Perseus proteomics Inc., PP-H8132, Tokyo, Japan) and histone H3K9-Ac (Cell signaling, C5B11, Danvers MA) while control immunoprecipitations (IPs) were performed with mouse IgG (Active Motif, Carlsbad, CA). Twenty microliters of magnetic beads (Active Motif), 2 ug of antibody, and the chromatin samples were rotated overnight at 4°C in 200 ul volumes each. The next day, beads were washed twice with 800 ul ChIP Buffer 1 and four times with 800 ul ChIP Buffer 2. The beads were then resuspended in Elution Buffer and rotated for 15 min at room temperature. Fifty microliters of Reverse-Cross-linking Buffer were added and the supernatant transferred to a fresh tube. ChIP and input samples were denatured at 95°C for 15 min in a BioRad thermocycler before the proteins were digested with 2 μl Proteinase K (0.5ug/ul) for 1 hour at 37°C. Genomic DNA was isolated using Qiagen PCR Kit (Qiagen, Valencia, CA) and used for RT-PCR as described above.
Luciferase reporter assay
A luciferase reporter construct containing a 3304-bp fragment of the KLF9 3’ untranslated region (UTR) and an empty luciferase reporter construct were purchased from SwitchGear Genomics (Menlo Park, CA; #S813897 and #S890005, respectively). Putative miR-140 binding sites were identical with Klf9 3’UTR. miR-140 mimic and a non-targeting scramble miR mimic were purchased from Life Technologies (Grand Island, NY) and used at a final concentration of 30 nM. Transfection and luciferase assay were carried out according to the manufacturer’s published protocol [43], using HEK293 cells and LipoD293 as the transfection reagent (SignaGen Laboratories, Rockville, MD). Twenty-four hours after transfection, cells were harvested and luciferase signal assayed using LightSwitch Luciferase Assay Reagents (SwitchGear Genomics) according to the manufacturer’s instructions. Luciferase signal was normalized to the total protein concentration of the lysates. Three independent transfection experiments were performed. Statistical analysis was performed using two-tailed Student’s t-test with α=0.05.
Statistics
Data are presented as mean ±SEM. Statistical analysis was performed using two-tailed Student’s t-test with α=0.05.
Results
Intersection of NR2F1 targets and miRNAs expressed in the mouse inner ear
A multi-step approach was used to discover miRNAs that might participate to coregulate NR2F1 target genes. A list of putative NR2F1 target genes identified by Montemayor et al. [12] was used to find positional overlap between NR2F1 target genes with gene loci encoding miRNAs using the UCSC genome browser. Of all the NR2F1 target genes inspected, only one miRNA gene intersection was found in the human genome–the Klf9 gene, which contains the gene encoding miR-1192, was previously validated to be up-regulated and inner ear of Nr2f1 –/– mice [12]. Beyond that, the most proximal miRNA genes found to intersect NR2F1 target genes were those for miR-693 and miR-1954, which were approximately 18kbps upstream and downstream from the Yipf3 and Ak1 genes, respectively.
Next, we investigated recent articles that identified miRNAs in the developing embryonic mouse inner ear [22–24,30,44]. Of the miRNAs expressed, miR-17 and miR-341 have NR2F1 binding sites within their coding regions, miR-140, miR-191 and miR-199b have NR2F1 binding sites within the proximal promoter region of their loci, and miR-183 and miR-181b have NR2F1 binding sites 8 and 15 base pairs immediately upstream of the transcription start sites, respectively. The miR-183 family of miRNAs is expressed and required for maintenance and survival of hair cells [45,46]. There were many others that had binding sites more distant from the miRNA genes and were not investigated in this study. Additionally, miR-96, miR-140, and miR-194 were included for the following reasons. miR-96 is the only miRNA demonstrated to cause hearing loss when mutated [21,28]; miR-140 is highly and selectively expressed in hair cells during development and targets Jag1 and RARβ, both important for hair cell and inner ear development [22,30]; miR-194 targets Fgf10, which is essential for the development of the otic vesicle [27,47,48]. Finally, miR-33 was included because it and NR2F1 are involved with cholesterol metabolism which may participate in hair cell development and function [29,49–51]. With these criteria a set of 11 miRNAs (miR-17, miR-33, miR-96, miR-140, miR-181b, miR-183, miR-191, miR-194, miR-199b, miR-341, and miR-1192) were selected that might participate to coordinate with NR2F1 to regulate inner ear gene expression.
Identification of NR2F1 gene targets co-regulated by the 11 miRNAs
To determine the relative potential for coregulation of target genes by NR2F1 and the miRNAs, we generated a list of the total number of genes targeted by the 11 miRNAs, as well as the subset of target genes whose expression was changed in the Nr2f1 –/– mice, as quantified by microarray expression profiling and/or validated by qRT-PCR [12] (Table S1 in File S1 ). Of the 7201 unique miRNA genes targeted by the 11 miRNAs, 107 genes are also targeted by NR2F1 and 16 genes were previously validated to be changed in Nr2f1 –/– [12] (Figure 1 and Table 1 ).
Figure 1. Intersection of genes targeted by both NR2F1 and select miRNAs.
(A) Identification of 107 genes by intersection of the 182 NR2F1 targets genes [12] with targets of the 11 miRNAs and (B) the percentage of these genes targeted by multiple miRNAs.
Table 1. List of 107 predicted miRNAs and NR2F1 coregulated inner ear targets.
| MicroRNA | Putative miRNA/NR2F1 targets |
|---|---|
| mmu-miR17 | Acox1, Ahnak, Ampd3, C1qa, Cidea, Cldn1, Dlk1, Hsd17b11, Kcna5, Klf9, Ldlr, Nr2f1, Pdgfra, Ppia, Sfrs2, Sqstm1, Vps25 |
| mmu-miR-33 | Adora1, Ahnak, Ampd3, Ankrd1, Casq1, Ctbp1, Cxcl12, Ddx3x, Dusp1, Eif4b, Fabp7, Hspb8, Kcna5, Mmp14, Nefl, Nr2f1, Pdgfra, Sat1, Sfrs2, Txnrd1 |
| mmu-miR-96 | 1810015C04Rik, Acan, Ahnak, Ak1, AK162128, Ampd3, Arg1, Cd83, Cldn1, Crym, Ddx3x, Elavl1, Foxk2, Glul, Hmgcs1, Ldlr, Man2a1, Myadm, Pdgfra, Psen1, Sesn1, Slc25a1, Snai2, Stim1, Tbx15, Tectb, Ttr, Tyms, Zrsr1 |
| mmu-miR-140 | Acox1, C1qc, Ddx3x, Dpep1, Eln, Gjb2, Gpx3, H2afy, Klf9, Man2a1, Nr2f1, Pdgfra, Prrx2, Ptgis, Rbp1, Tectb, Timm23, Trip4, Vpreb3, Zbtb16 |
| mmu-miR-181b | Acox1, Ahnak, Cd83, Chordc1, Crym, Cyr61, Ddit4, Ddx3x, Dr1, Eln, Erh, Foxk2, Hist1h2ao, Mmp14, Ms4a6b, Nbr1, Pdgfra, Pros1, Ran, Rbm8a, Sat1, Sfn, Snai2, Sqstm1, Tcn2 |
| mmu-miR-183 | 2810410M20Rik, Acan, Ak1, Cldn1, Ddx3x, Dlk1, Eif4b, Foxk2, Gjb2, Ndufb6, Nefl, Ppia, Psen1, Ran, Sfrs2, Tbx15, Wdhd1 |
| mmu-miR-191 | Dr1, Elavl1, Foxk2, Mgp, Nbr1, Sat1, Satb1, Tsen34 |
| mmu-miR-194 | 2410022L05Rik, Cd83, Cidea, Cldn1, Cxcl12, Cyp51, Dr1, Eln, Erh, Fkbp5, Glul, Hmgcs1, Nbr1, Pdgfra, Ran, Rasa4, Scg2, Sfrs2, Tmem106a, Txnrd1, Vps25, Ywhab |
| mmu-miR-199b | Aaas, C1qa, Ddit4, Dr1, Eln, Fabp7, Ldlr, Ltc4s, Pdgfra, Pros1, Psen1, Relb, Sat1, Sfrs2, Stim1, Tcp11, Tyro3, Vps4a |
| mmu-miR-341 | C1qc, Crabp1, Hsd17b11, Jun, Nfkbia, Ptgis, Rbm8a |
| mmu-miR-1192 | Ampd3, Chordc1, Dbi, Ddit4, Ddx3x, Dhx40, Dr1, Dusp1, Elavl1, Fkbp5, Foxk2, Glul, Hmgcs1, Jun, Klf9, Ldlr, Man2a1, Nedd9, Nefl, Pdgfra, Pros1, Ptgis, Ran, Satb1, Scg2, Stim1, Tbx15, Tek, Tsen34, Wdhd1, Ywhab |
Genes in bold type were previously validated to be changed in Nr2f1 –/– tissues by qRT-PCR [12].
We next selected the genes targeted by at least 3 miRNAs for an in-depth analysis. This group of 28 genes, along with the miRNAs by which they are targeted, and the relative change in expression in the Nr2f1 –/– [12] is shown in Table S2 in File S1 . All 28 genes are targeted by multiple miRNAs: 16 by 3 miRNAs, 8 by 4 miRNAs, 2 by 5 miRNAs, 1 each by 6 and 8 miRNAs. One notable miRNA target is Nr2f1 itself that is targeted by three of these miRNAs (miR-17, -33 and -140), which suggests a feedback regulatory system may be possible. Other interesting observations include that Pdgfrα, a gene known for its role in both development and oncogenesis [52,53], was targeted by 8 of 11 miRNA, more than any of the other genes. Other genes with obvious roles in neurogenesis and neuron maintenance are also featured on this multi-hit list, such as Psen1 and Nefl [54,55]. Analysis of the pathways, tissues, and diseases associated with each gene according to the Database for Annotation, Visualization and Integrated Discovery (DAVID) [35,36] querying the universal protein resource in UniProt [56] and the Genetic Association Database (GAD) [57], revealed 16 of the 28 genes (targeted by at least 3 miRNAs) are expressed in the human brain, and 18 of 24 genes have higher expression in the brain than other mouse tissues. Furthermore, several genes are known to cause diseases related to neurological development and are listed in Table S3 in File S1 .
Multiple binding sites for a miRNA on a target mRNA might indicate greater regulatory control by that miRNA, whereas binding motifs shared by multiple miRNAs may indicate a lack of specificity in regulation [58]. Thus, we closely analyzed the specific miRNA binding site in both the untranslated (UTR) and translated regions of the target mRNA. In the 3’UTR, 14 of the target genes have only one motif per miRNA, 8 have two binding sites, and 6 have three or more binding sites and thus possibly maintain tighter regulatory control (Table S4 in File S1 ). In the translated portion of the mRNA of target genes, there were also some interesting finds as listed in Table S5 in File S1 . While there was no redundancy discovered in the 3’UTR of the target genes, 13 of the genes have binding motifs that are compatible with more than one miRNA.
Regarding the genes that affect hearing loss, we mined databases from the Hereditary Hearing Loss (hereditaryhearingloss.org), the Serial Analysis of Gene Expression (SAGE) and the literature [37,38], and commercial hearing loss databases and found four genes to be targeted by both NR2F1 and miRNAs: Crym and Snai2 were both targeted by miR-96 and miR-181b; Gjb2 was targeted by miR-140 and miR-183 (Table S5 in File S1 ). In addition, 69 other genes implicated in hearing loss were targeted solely by the miRNAs but not NR2F1 (not shown).
Functional clustering of the genes co-targeted by the 11 miRNAs and NR2F1
Using the Gene Ontology (GO) Gene Functional Classification analysis and the DAVID database [35,36], we discovered 9 functional clusters which includes all the 28 co-targeted genes (Table S2 in File S1 ) and contains 66 of the 107 genes targeted by at least 1 of the 11 miRNAs and NR2F1 (Table S6 in File S1 ). Based on GO enrichment score, Cluster 1 genes (Crabp1, Fabp7, Rbp1) involve cellular transport of fatty acids and hormones in metabolic process, including forebrain development, which is defective in Nr2f1 –/–. Cluster 2 genes (Eif4b, Elavl1, Rbm8a, Sfrs2, Zrsr1) involve RNA binding and metabolic processes and post-translational regulation of biological processes. The 10 genes in clusters 3-5 involve steroid, lipid and cholesterol biosynthesis, cation transport and chromatin assembly and organization, which are key processes during development. Cluster 6 has 20 genes that explicitly involve transcription, and include Nr2f1 and Klf9. Clusters 7 through 9 have lower enrichment scores but involves skeletal development, nucleotide binding, cell adhesion, tube development and morphogenesis, the endomembrane system and energy regulation, many functions of which are defective in Nr2f1 –/– [8,9,59].
Validation of miRNA as targets using Nr2f1–/– tissues
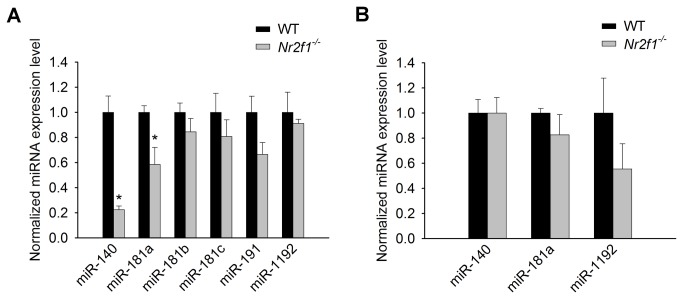
In order to validate this bioinformatics approach, we pursued several miRNAs by determining their levels in the Nr2f1 –/– mice using qRT-PCR. Of note, miR-140, -181b and -191 each have a putative NR2F1 binding site close to the proximal portion of their genes and since miR-181b is found in a family cluster we also included miR-181a and -181c, which may be coregulated and share similar gene targets. MicroRNA-140 has 4 co-targeted genes with NR2F1 amongst the validated expression changes in the Nr2f1 –/– mice and is predicted to target NR2F1 itself (Table 1 & S1 in File S1 ). miR-1192 is the only miR locus that is located within any of the NR2F1 target genes; it is found in the Klf9 gene which is up-regulated in the inner ear of Nr2f1 –/– mouse [12] and is targeted by miR-140 (Table 1 ). By analyzing these 6 miRNAs using RT-PCR, we found that miR-140 and miR-181a are significantly down-regulated in the Nr2f1 –/– inner ear by 4.5-fold (P=0.004) and 1.7-fold (P=0.046) compared with wildtype (WT), respectively, while the other 4 miRNAs were not significantly down-regulated (Figure 2A ). Because Nr2f1 –/– mice have various neural defects [5,6,9–11], we went a step further to quantify the levels of miR-140, -181a, and -1192 in the cerebral cortex of the Nr2f1 –/– but found that their levels were not significantly changed (Figure 2B ). This suggests a tissue-specific role for miR-140 and miR-181a in the Nr2f1 –/– inner ear.
Figure 2. MicroRNA expression analyses in Nr2f1–/– tissues.
(A) The expression of select miRNAs in the Nr2f1–/– inner ear showing miR-140 and miR-181a were significantly down-regulated by 4.5-fold (P=0.004) and 1.7-fold (P=0.046), respectively, compared to the WT. The levels of miR-181b, -181c and -191 were also decreased but did not reach significance (P=0.3, 0.4, 0.1, respectively). (B) The expression of miR-140 and miR-181a were unchanged in the cerebral cortex tissues from the same animals. N=3 in each group. Significance was assessed by a two-tailed t-test for independent comparison between Nr2f1–/– and WT values for each miRNA with α=0.05.
NR2F1 directly regulates miR-140
Since the level of miR-140 was the most down-regulated in the absence of NR2F1 (Figure 2A ), we investigated whether NR2F1 might directly regulate miR-140 expression. We identified putative NR2F1 binding sites immediately upstream of the miR-140 locus [12] (Figure 3A ) and performed chromatin-immunoprecipitation (ChIP) for NR2F1 binding enrichment followed by qRT-PCR analysis. Compared to a non-specific IgG control, there is a significant NR2F1 binding enrichment (P<0.05) at the miR-140 locus (Figure 3B ) coincident with enrichment of the open chromatin marker acetylated H3K9, suggesting NR2F1 might directly regulate miR-140 transcription.
Figure 3. NR2F1 directly regulates miR-140.
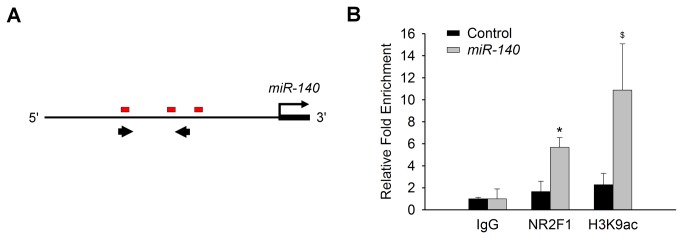
(A) Putative NR2F1 binding sites (red boxes) immediately upstream of the miR-140 locus [12]. Thick arrows define regions of qRT-PCR amplification. (B) Chromatin-immunoprecipitation (ChIP) and qRT-PCR analyses reveal a significant enrichment of the miR-140 locus with NR2F1 pull-down. N=5-6. *P<0.05, $P=0.1 vs IgG control.
Tissue-Dependent Co-regulation of Klf9 by NR2F1 and miR-140
We found it intriguing that miR-1192 expression was not altered whereas the gene Klf9, which harbors the miR-1192 locus, was up-regulated in the Nr2f1 –/– [12], suggesting that Klf9 is regulated independently in the inner ear. Klf9 is one of the 28 genes putatively targeted by both NR2F1 and at least 3 of the 11 miRNAs in this study (Figure 1 & Table S2 in File S1 ). In particular, Klf9 has multiple miR-140 binding sites: 3 sites in its 3’ UTR and 4 sites in its translated region (Tables S4 & S5 in File S1 ). To determine whether miR-140 regulates Klf9 directly, we tested if a miR-140 mimic would target and affect the expression of a luciferase gene construct containing the KLF9 3’UTR sequences (Figure 4A ). We quantified Luciferase activity from the luciferase-KLF9 3'UTR reporter and found that miR-140 mimic reduced the Luciferase signal by more than 90% compared to a scrambled mimic (P<0.001, Figure 4B ). Next, we determined whether NR2F1 regulates Klf9 directly by performing a ChIP followed by qRT-PCR using primers that amplify the Klf9 first exon (Figure 4C ). Impressively, there was a 500-fold enrichment of NR2F1 binding at the Klf9 locus as compared to a non-specific IgG control (P<0.05) (Figure 4D ).
Figure 4. Both miR-140 and NR2F1 directly regulate Klf9 expression.
(A) Putative miR-140 binding sites (red boxes) on KLF9-3’UTR. (B) Luciferase expression from a KLF9 3'UTR reporter is significantly reduced in the presence of the miR-140 mimic while Luc expression from the empty reporter is unchanged. N=3. ***P<0.001. (B) Schematic of the putative NR2F1 binding site (red box) just downstream of the first exon of Klf9 and the relative position of the qRT-PCR primers (Thick arrows). (D) Log-scale presentation of qRT-PCR results following chromatin-immunoprecipitation (ChIP) demonstrates significant enrichment of NR2F1 at the Klf9 locus. N=5. *P<0.05, $P=0.08 vs IgG control.
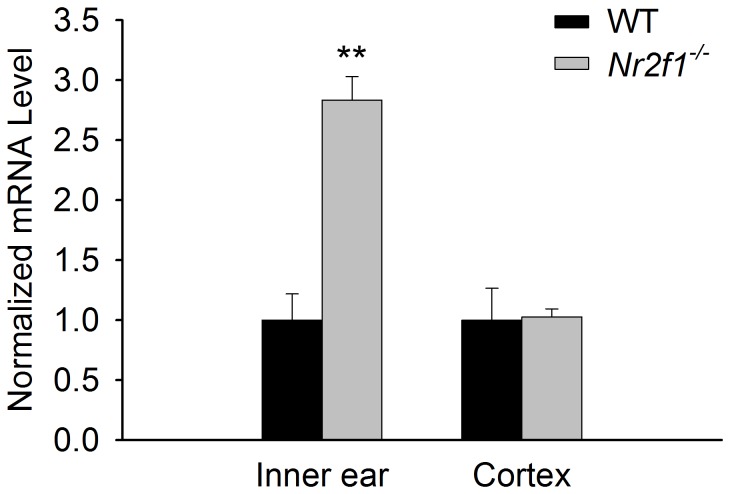
Since miR-140 is down-regulated in the absence of NR2F1 only in the inner ear and not the cerebral cortex (Figure 2 ) and is a direct target of NR2F1 (Figure 3 ), we tested if Klf9 was regulated in a tissue-dependent manner as well. Indeed, using qRT-PCR, we confirmed that Klf9 is up-regulated almost 3-fold in the inner ear (P<0.01 vs WT) but was unchanged in the cerebral cortex of Nr2f1 –/– mice (Figure 5 ). Consistent with the luciferase-Klf9-3’UTR activity that demonstrated a strong repression of Klf9 by the miR-140 mimic (Figure 4B ), these data suggest that miR-140 may be a determining factor in regulating Klf9 expression that is represented in the coregulatory network of NR2F1, miR-140 and Klf9 (Figure 6 ). Together, these experiments provide molecular evidence that Klf9 is directly coregulated by both miR-140 and NR2F1, further validating the bioinformatically predicted coregulatory network involving NR2F1, miR-140 and Klf9.
Figure 5. Klf9 is a downstream inner ear target of NR2F1.
Klf9 expression is significantly up-regulated in Nr2f1–/– (KO) inner ear but not in the cerebral cortex, as determined by qRT-PCR. N=3. **P<0.01.
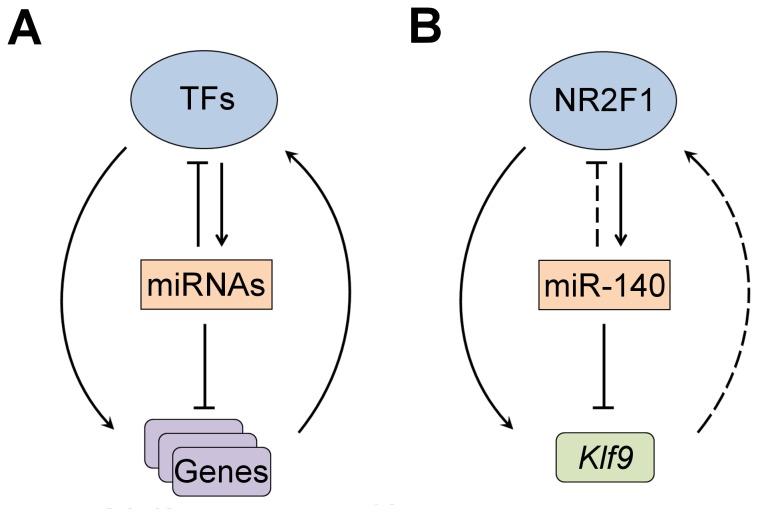
Figure 6. Models of transcription factor-target gene feedback and coregulatory relationships.
(A) Generic model showing feedback loops and coregulation between transcription factors (TFs), miRNAs, and genes predicted to be involved in hearing, cancer, and development based on bioinformatic analyses. (B) Model of a validated coregulatory network involving NR2F1, miR-140, and Klf9. Arrow denotes either positive or negative regulation.
Discussion
Since their discovery approximately 25 years ago [60], there has been great interest in orphan nuclear receptors and their putative ligands. This is based on the knowledge that nuclear receptors and cofactors play crucial roles in many physiological processes during development, reproduction, metabolism and aging [3,61–63]. Orphan nuclear receptors have been discovered to be key components in understanding the regulatory milieu at the genomic scale as they translate cellular, paracrine, neuronal and environmental stimuli into epigenetic and gene expression signals [64–67]. These signals in turn regulate cellular and organismal homeostasis, and dysregulation of this process can lead to diseases including cancer (Tables S3 & S6 in File S1 ) [68].
The orphan nuclear receptor NR2F1, located on chromosome 5q14, and its homolog NR2F2, are the most evolutionarily conserved nuclear receptors across all species [69]. NR2F1 is implicated in neurogenesis, cell fate determination, patterning, and regulation of differentiation in the rodent inner ear and cerebral cortex [7–10]. We report here a proof-of-concept novel coregulatory network involving the orphan nuclear receptor NR2F1 and microRNA-140 with experimental validation of the effects these regulators have on each other as well as a mutual target, the Klf9 gene. This study exemplifies the research approach of using systematic bioinformatic data mining to uncover coregulatory interactions followed by quantitative experimental validations of regulated targets at the transcription, post-transcription and genomic levels. The validation included qRT-PCR, luciferase-3’UTR reporter and ChIP-qRT-PCR assays with tissues from wild type and mutant mice. This approach accomplished several objectives: 1) discovery of a mechanism by which NR2F1 regulates direct target gene expressions; 2) identification of specific miRNAs, such as miR-140, that co-regulate and alter NR2F1 target gene expressions, such as Klf9; 3) delineation of a feedback coregulation that occurs between NR2F1 and miR-140; and 4) identification of a tissue-dependent coregulatory network involving NR2F1, miR-140 and Klf9.
The miR-140 gene, located on chromosome 16q22, is most notable for its role in cartilage development and maintenance [70,71]. It also has a suppressive action in liver tumorigenesis [72] and induces pluripotent cells to differentiate into adipocytes [73]. Within the inner ear, miR-140 exhibits differential expression across developmental stages and post-natal maturation [22]. Expression is initially limited to the medial otocyst where cells are proliferating. As development progresses, miR-140 is then expressed in the early sensory epithelium and subsequently in the inner and outer hair cells of the organ of Corti. Lastly, miR-140 is also detected in hair cells of the vestibular apparati by birth [22]. These 3 separate expression patterns suggest miR-140 might have stage-specific roles during inner ear development. Indeed, SOX9 directly activates miR-140 [74] and has a differentially and overlapping expression as SOX2, and is consistent with the miR-140 expression, during cochlear development [75]. This pattern is also highly consistent with that of Nr2f1 expression during inner ear development [7], supporting the possibility of direct regulation of miR-140 by NR2F1. Indeed, we found miR-140 transcripts were significantly down-regulated by 4.5-fold in Nr2f1 –/– newborn inner ears but remained unchanged in the cerebral cortex (Figure 2 ), suggesting NR2F1 acts as a tissue-specific transactivator of miR-140. On the other hand, the Nr2f1 gene itself is a putative target of miR-140 (Table S2 in File S1 ), a relationship that may represent a negative feedback loop between NR2F1 and miR-140 as proposed in Figure 6 . Since this finding purports a regulatory feedback loop, we investigated closely the relationship between NR2F1, miR-140, and a mutual target, Klf9.
Klf9, located on 9q21, acts as a circadian output factor [76], has a role in the regulation of adipogenesis (similar to miR-140) [77], and is up-regulated in a cortisol and differentiation-state-dependent manner. Furthermore, gain- and loss-of-function results in growth arrest and proliferative effects, respectively, in keratinocytes and cancer stem cells [76]. Since all three genes (Nr2f1, miR-140 and Klf9) are expressed in the developing inner ear and adult brain (including the cerebral cortex) during neurogenesis, there are other tissues where miR-140, NR2F1, and Klf9 functionally interact, but the mechanisms of their interactions have not yet been delineated. Indeed, both KLF9 and NR2F1 have a role in lipid processing [76,77], and miR-140 and Klf9 are both regulated in the brain by estrogen-receptor modulation [7,22,78]. In the inner ear, estrogen acts to respond to noise-induced damage, and loss of ERβ causes inner ear defects and hearing loss [79–81]. Klf9 opposes estrogen hormonal action by repressing estrogen receptor (ERα) expression and activity [82–84]. ERα is also known to down-regulate miR-140 in breast cancer stem cells suggesting miR-140 is downstream of estrogen action [83]. The combination of these findings may indicate that the function of mir-140 and Klf9 is important for estrogen action in the inner ear.
Further empiric evidence exists of coregulation between miR-140, NR2F1, and Klf9. Klf9 is regulated by thyroid hormone and its nuclear receptor TRβ, which are essential for normal development and functional maturation of hearing [85–87]. NR2F1, in turn, is a negative regulator of thyroid hormone receptor binding and thereby suppresses the activation of its target genes (e.g. Klf9) [86,88]. Additionally, KLF9 directly suppresses Notch1 expression and its downstream signaling which promotes differentiation and inhibits glioblastoma neurosphere growth [89]. Indeed, NR2F1 inhibits Notch signaling leading to development and differentiation of extra hair cells and supporting cells in the inner ear [8,20]. Cumulatively, these expression patterns and described actions of NR2F1, Klf9 and miR-140 are consistent with the co-regulatory network proposed (Figure 6 ). Our findings indicate that NR2F1 directly regulates Klf9 as well as miR-140, while miR-140 has a direct inhibitory effect on Klf9 transcripts. The indirect regulatory effect of NR2F1 by means of miR-140 might have an additive repressive effect than solely by the direct NR2F1targeting of Klf9.
In addition to this focused regulation of Klf9 by a regulatory network involving miR-140 and NR2F1, our bioinformatics search revealed at least 20 other common targets shared by miR-140 and NR2F1, four of which have been validated to have a change of expression in the Nr2f1 –/– mice [12] (Table S1 in File S1 ). Klf9 is only one of these genes, which according to this model is transactivated at the transcriptional level by NR2F1 but repressed at the post-transcriptional level by miR-140 in order to achieve a balanced expression level. The remaining common targets are also potential miR-140 and NR2F1 co-regulated genes.
Although we did not study them in depth, our bioinformatics search revealed a number of other possible regulatory networks. There were 107 putative gene targets identified that were shared by NR2F1 and the 11 microRNA that we analyzed. Similar to miR-140, miR-17 and miR-33 target NR2F1 and could therefore be involved in regulatory feedback loops with NR2F1. The majority of the genes (59%) were targeted by multiple miRNA, which may indicate a higher level of control over expression (Figure 1B ). Pdgfra, a mutation of which can cause neural tube defects (Table S3 in File S1 ) was targeted by NR2F1 and 8 of the miRNAs. Additionally, the miRNAs themselves may act in concert with each other in regulating genes important for ontogenesis and development in general. Using TargetScan 6.2 and microRNA.org to curate the binding sites on the 28 genes targeted by at least 3 of the 11 miRNAs and NR2F1 (Tables S2, S4 & S5 in File S1 ), we discovered two interesting trends. First, different miRNAs (or even the same miRNA in some cases) may bind to overlapping sites on some of the genes. This implicates coordination between the different miRNAs and may allow for a greater regulation of target genes. Also, depending on how many of the regulatory miRNAs are active, this could also allow fine-tuning of the regulation. Second, the same miRNAs may have multiple binding sites. This suggests a greater or more effective regulatory repression and may allow for fine-tuning depending on the accessibility of the different sites under varying conditions [58]. In all, these findings point to the complexity of the interplay between these miRNAs in achieving a balanced regulation of the genes important for development [15].
Furthermore, these coregulatory networks may have clinical significance. The genes targeted by both NR2F1 and the 11 miRNAs that we identified bioinformatically have previously been implicated in hearing loss (Nr2f1, Crym, Snai2, Gjb2) [11,90–94] and cancer (Pdgfrα, Eln, Jun) [52,95–98], as well as inner ear developmental regulators (Nr2f1, Notch1, Jag 1, Nptx1, Runx1) [7,8,22,99], as listed in Tables S4 & S5 in File S1 . Nr2f1 –/– mice have inner ear developmental defects [7,8,22,99]. Furthermore, miR-140 is selectively expressed in inner ear hair cells during development [22,30] and is demonstrated to regulate targets such as PDGF [100]. It is not surprising that there is a tissue-dependent difference in regulation of Klf9 in the inner ear compared to the cortex of Nr2f1 –/– mice given the site-specific regulation of miR-140 and NR2F1. Nor is it unexpected that defects in the cochlear duct, organ of Corti, and hair cells can occur in animals with impaired NR2F1 or miRNA function [7,8,11,16,21,27]. These defects help to underscore the importance of NR2F1 and microRNAs like miR-140, both individually and as coregulatory partners, and give clinical importance to the networked described here.
In conclusion, we have demonstrated that NR2F1 and the 11-selected miRNA may play an important or indispensable role in ontogenesis and development in general. As a proof-of-principle, we experimentally demonstrated the coregulatory mechanisms and feedback loops between NR2F1, miR-140, and Klf9. We also uncovered several other potential pathways between NR2F1, miRNAs, and genes important for development and disease. These targets and pathways require further studies and validation in order to advance our understanding of ontogenesis and provide novel strategies for protecting or regenerating hair cells and sensory neurons in patients with hearing loss.
Supporting Information
Supporting Information File containing Tables S1 to S7:
Table S1. Number of predicted miRNAs and NR2F1 inner ear targets.
Table S2. Genes targeted by at least 3 of the 11 selected miRNAs.
Table S3. Representative list of genes targeted by multiple miRNAs and their associated neurological and other diseases based on the Genetic Association Database.
Table S4. List of genes targeted by at least 3 of the 11 select miRNAs and the respective miRNA binding sites in their mRNA 3’ untranslated region based on TargetScan 6.2.
Table S5. Genes targeted by at least 3 of 11 selected miRNAs and the respective miRNA binding sites based on miRanda analysis from microRNA.org.
Table S6. Functional classification of the genes targeted by miRNAs and NR2F1.
Table S7. List of primers for real-time RT-PCR.
(DOCX)
Acknowledgments
D.Y.C. is a trainee in the Baylor College of Medicine Medical Scientist Training Program (MSTP).
Funding Statement
This work was funded by grants T32AG00183 (to FRR) from the National Institutes of Health, 12BGIA12050207 (to NL) and 12PRE11700012 (to DYC) from the American Heart Association, and DC04585 and support from the Huffington Center on Aging and Department of Otolaryngology-HNS (to FAP). DYC was also supported by the Baylor College of Medicine Medical Scientist Training Program Caskey Scholarship. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Rosenfeld MG, Glass CK (2001) Coregulator codes of transcriptional regulation by nuclear receptors. J Biol Chem 276: 36865–36868. doi: 10.1074/jbc.R100041200. PubMed: 11459854. [DOI] [PubMed] [Google Scholar]
- 2. Tsai SY, Tsai MJ (1997) Chick ovalbumin upstream promoter-transcription factors (COUP-TFs): coming of age. Endocr Rev 18: 229–240. doi: 10.1210/er.18.2.229. PubMed: 9101138. [DOI] [PubMed] [Google Scholar]
- 3. Giguère V (1999) Orphan nuclear receptors: from gene to function. Endocr Rev 20: 689–725. doi: 10.1210/er.20.5.689. PubMed: 10529899. [DOI] [PubMed] [Google Scholar]
- 4. Olefsky JM (2001) Nuclear receptor minireview series. J Biol Chem 276: 36863–36864. doi: 10.1074/jbc.R100047200. PubMed: 11459855. [DOI] [PubMed] [Google Scholar]
- 5. Armentano M, Filosa A, Andolfi G, Studer M (2006) COUP-TFI is required for the formation of commissural projections in the forebrain by regulating axonal growth. Development 133: 4151–4162. doi: 10.1242/dev.02600. PubMed: 17021036. [DOI] [PubMed] [Google Scholar]
- 6. Armentano M, Chou S-J, Tomassy GS, Leingärtner A, O’Leary DDM et al. (2007) COUP-TFI regulates the balance of cortical patterning between frontal/motor and sensory areas. Nat Neurosci 10: 1277–1286. doi: 10.1038/nn1958. PubMed: 17828260. [DOI] [PubMed] [Google Scholar]
- 7. Tang LS, Alger HM, Lin F, Pereira F (2005) Dynamic expression of COUP-TFI and COUP-TFII during development and functional maturation of the mouse inner ear. Gene Expr Patterns 5: 587–592. doi: 10.1016/j.modgep.2005.03.012. PubMed: 15907456. [DOI] [PubMed] [Google Scholar]
- 8. Tang LS, Alger HM, Pereira F a (2006) COUP-TFI controls Notch regulation of hair cell and support cell differentiation. Development 133: 3683–3693. doi: 10.1242/dev.02536. PubMed: 16914494. [DOI] [PubMed] [Google Scholar]
- 9. Qiu Y, Pereira Fa, DeMayo FJ, Lydon JP, Tsai SY et al. (1997) Null mutation of mCOUP-TFI results in defects in morphogenesis of the glossopharyngeal ganglion, axonal projection, and arborization. Genes Dev 11: 1925–1937. doi: 10.1101/gad.11.15.1925. PubMed: 9271116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhou C, Qiu Y, Pereira Fa, Crair MC, Tsai SY et al. (1999) The nuclear orphan receptor COUP-TFI is required for differentiation of subplate neurons and guidance of thalamocortical axons. Neuron 24: 847–859. doi: 10.1016/S0896-6273(00)81032-6. PubMed: 10624948. [DOI] [PubMed] [Google Scholar]
- 11. Brown KK, Alkuraya FS, Matos M, Robertson RL, Kimonis VE, et al. (2009) NR2F1 deletion in a patient with a de novo paracentric inversion, inv(5)(q15q33.2), and syndromic deafness. American journal of medical genetics Part A 149A: 931–938. doi: 10.1002/ajmg.a.32764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Montemayor C, Montemayor Oa, Ridgeway A, Lin F, Wheeler et al Da. (2010) Genome-wide analysis of binding sites and direct target genes of the orphan nuclear receptor NR2F1/COUP-TFI. PLOS ONE 5: e8910. doi: 10.1371/journal.pone.0008910. PubMed: 20111703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wasserman WW, Sandelin A (2004) Applied bioinformatics for the identification of regulatory elements. Nat Rev Genet 5: 276–287. doi: 10.1038/nrg1315. PubMed: 15131651. [DOI] [PubMed] [Google Scholar]
- 14. Pandey DP, Picard D (2010) Multidirectional interplay between nuclear receptors and microRNAs. Curr Opin Pharmacol 10: 637–642. doi: 10.1016/j.coph.2010.08.009. PubMed: 20829111. [DOI] [PubMed] [Google Scholar]
- 15. He L, Hannon GJ (2004) MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet 5: 522–531. doi: 10.1038/nrg1379. PubMed: 15211354. [DOI] [PubMed] [Google Scholar]
- 16. Esquela-Kerscher A, Slack FJ (2006) Oncomirs - microRNAs with a role in cancer. Nat Rev Cancer 6: 259–269. doi: 10.1038/nrc1840. PubMed: 16557279. [DOI] [PubMed] [Google Scholar]
- 17. Subramanyam D, Blelloch R (2011) From microRNAs to targets: pathway discovery in cell fate transitions. Curr Opin Genet Dev 21: 498–503. doi: 10.1016/j.gde.2011.04.011. PubMed: 21636265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lagos-Quintana M, Rauhut R, Meyer J, Borkhardt A, Tuschl T (2003) New microRNAs from mouse and human. RNA 9: 175–179. doi: 10.1261/rna.2146903. PubMed: 12554859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hinske LCG, Galante PAF, Kuo WP, Ohno-Machado L (2010) A potential role for intragenic miRNAs on their hosts’ interactome. BMC Genomics 11: 533. doi: 10.1186/1471-2164-11-533. PubMed: 20920310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kopecky B, Fritzsch B (2011) Regeneration of Hair Cells: Making Sense of All the Noise. Pharmaceuticals (Basel) 4: 848–879. doi: 10.3390/ph4060848. PubMed: 21966254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mencía A, Modamio-Høybjør S, Redshaw N, Morín M, Mayo-Merino F et al. (2009) Mutations in the seed region of human miR-96 are responsible for nonsyndromic progressive hearing loss. Nat Genet 41: 609–613. doi: 10.1038/ng.355. PubMed: 19363479. [DOI] [PubMed] [Google Scholar]
- 22. Wang X-R, Zhang X-M, Zhen J, Zhang P-X, Xu G et al. (2010) MicroRNA expression in the embryonic mouse inner ear. Neuroreport 21: 611–617. doi: 10.1097/WNR.0b013e328338864b. PubMed: 20467336. [DOI] [PubMed] [Google Scholar]
- 23. Soukup Ga, Fritzsch B, Pierce ML, Weston MD, Jahan I et al. (2009) Residual microRNA expression dictates the extent of inner ear development in conditional Dicer knockout mice. Dev Biol 328: 328–341. doi: 10.1016/j.ydbio.2009.01.037. PubMed: 19389351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Friedman LM, Dror A, Mor E, Tenne T, Toren G, et al. (2009) MicroRNAs are essential for development and function of inner ear hair cells in vertebrates. Proceedings of the National Academy of Sciences of the United States of America 106: 7915–7920. doi: 10.1073/pnas.0812446106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tang LS, Montemayor C, Pereira FA (2006) Sensorineural hearing loss: potential therapies and gene targets for drug development. IUBMB Life 58: 525–530. doi: 10.1080/15216540600913258. PubMed: 17002980. [DOI] [PubMed] [Google Scholar]
- 26. Rudnicki A, Avraham KB (2012) microRNAs: the art of silencing in the ear. EMBO molecular medicine 4: 849–859. doi: 10.1002/emmm.201100922. [DOI] [PMC free article] [PubMed]
- 27. Kent WJ, Sugnet CW, Furey TS, Roskin KM, Pringle TH et al. (2002) The Human Genome Browser at UCSC. Genome Res 12: 996–1006. doi: 10.1101/gr.229102. PubMed: 12045153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lewis Ma, Quint E, Glazier AM, Fuchs H, De Angelis MH et al. (2009) An ENU-induced mutation of miR-96 associated with progressive hearing loss in mice. Nat Genet 41: 614–618. doi: 10.1038/ng.369. PubMed: 19363478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Najafi-Shoushtari SH, Kristo F, Li Y, Shioda T, Cohen DE et al. (2010) MicroRNA-33 and the SREBP host genes cooperate to control cholesterol homeostasis. Science 328: 1566–1569. doi: 10.1126/science.1189123. PubMed: 20466882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dogini DB, Ribeiro P, Rocha C, Pereira TC, Lopes-Cendes I (2008) MicroRNA expression profile in murine central nervous system development. Journal of molecular neuroscience : MN 35: 331–337. doi: 10.1007/s12031-008-9068-4. [DOI] [PubMed] [Google Scholar]
- 31. Kozomara A, Griffiths-Jones S (2011) miRBase: integrating microRNA annotation and deep-sequencing data. Nucleic Acids Res 39: D152–D157. doi: 10.1093/nar/gkq1027. PubMed: 21037258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kertesz M, Iovino N, Unnerstall U, Gaul U, Segal E (2007) The role of site accessibility in microRNA target recognition. Nat Genet 39: 1278–1284. doi: 10.1038/ng2135. PubMed: 17893677. [DOI] [PubMed] [Google Scholar]
- 33. Friedman RC, Farh KK-H, Burge CB, Bartel DP (2009) Most mammalian mRNAs are conserved targets of microRNAs. Genome Res 19: 92–105. doi: 10.1101/gr.082701.108. PubMed: 18955434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dweep H, Sticht C, Pandey P, Gretz N (2011) miRWalk--database: prediction of possible miRNA binding sites by “walking” the genes of three genomes. J Biomed Inform 44: 839–847. doi: 10.1016/j.jbi.2011.05.002. PubMed: 21605702. [DOI] [PubMed] [Google Scholar]
- 35. Huang DW, Sherman BT, Lempicki R (2009) Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res 37: 1–13. doi: 10.1093/nar/gkn923. PubMed: 19033363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Huang DW, Sherman BT, Lempicki R (2009) Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4: 44–57. doi: 10.1038/nprot.2008.211. PubMed: 19131956. [DOI] [PubMed] [Google Scholar]
- 37. Abe S, Koyama K, Usami S, Nakamura Y (2003) Construction and characterization of a vestibular-specific cDNA library using T7-based RNA amplification. J Hum Genet 48: 142–149. doi: 10.1007/s100380300022. PubMed: 12624726. [DOI] [PubMed] [Google Scholar]
- 38. Chen Z-Y, Corey DP (2002) An inner ear gene expression database. Journal of the Association for Research in Otolaryngology : JARO 3: 140–148. doi: 10.1007/s101620020029. PubMed: 12162364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Seron-Ferre M, Valenzuela GJ, Torres-Farfan C (2007) Circadian clocks during embryonic and fetal development. Birth Defects Res C Embryo Today 81: 204–214. doi: 10.1002/bdrc.20101. PubMed: 17963275. [DOI] [PubMed] [Google Scholar]
- 40. Varkonyi-Gasic E, Wu R, Wood M, Walton EF, Hellens RP (2007) Protocol: a highly sensitive RT-PCR method for detection and quantification of microRNAs. Plant Methods 3: 12. doi: 10.1186/1746-4811-3-12. PubMed: 17931426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tang F, Hajkova P, Barton SC, Lao K, Surani MA (2006) MicroRNA expression profiling of single whole embryonic stem cells. Nucleic Acids Res 34: e9. doi: 10.1093/nar/gnj009. PubMed: 16434699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative CT method. Nat Protoc 3: 1101–1108. doi: 10.1038/nprot.2008.73. PubMed: 18546601. [DOI] [PubMed] [Google Scholar]
- 43. Aldred SF, Collins P, Trinklein N (2011) Identifying targets of human micrornas with the LightSwitch Luciferase Assay System using 3’UTR-reporter constructs and a microRNA mimic in adherent cells. Journal of Visualized Experiments : JoVE: 3–7. doi: 10.3791/3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Xu S, Witmer PD, Lumayag S, Kovacs B, Valle D (2007) MicroRNA (miRNA) transcriptome of mouse retina and identification of a sensory organ-specific miRNA cluster. J Biol Chem 282: 25053–25066. doi: 10.1074/jbc.M700501200. PubMed: 17597072. [DOI] [PubMed] [Google Scholar]
- 45. Pierce ML, Weston MD, Fritzsch B, Gabel HW, Ruvkun G et al. (n.d.) MicroRNA-183 family conservation and ciliated neurosensory organ expression. Evol Dev 10: 106–113. doi: 10.1111/j.1525-142X.2007.00217.x. PubMed: 18184361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Weston MD, Pierce ML, Jensen-Smith HC, Fritzsch B, Rocha-Sanchez S et al. (2011) MicroRNA-183 family expression in hair cell development and requirement of microRNAs for hair cell maintenance and survival. Dev Dyn 240: 808–819. doi: 10.1002/dvdy.22591. PubMed: 21360794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pauley S, Wright TJ, Pirvola U, Ornitz D, Beisel K et al. (2003) Expression and function of FGF10 in mammalian inner ear development. Dev Dyn 227: 203–215. doi: 10.1002/dvdy.10297. PubMed: 12761848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schimmang T (2007) Expression and functions of FGF ligands during early otic development. Int J Dev Biol 51: 473–481. doi: 10.1387/ijdb.072334ts. PubMed: 17891710. [DOI] [PubMed] [Google Scholar]
- 49. Levic S, Yamoah EN (2011) Plasticity in membrane cholesterol contributes toward electrical maturation of hearing. J Biol Chem 286: 5768–5773. doi: 10.1074/jbc.M110.186486. PubMed: 21163952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rajagopalan L, Greeson JN, Xia A, Liu H, Sturm A et al. (2007) Tuning of the outer hair cell motor by membrane cholesterol. J Biol Chem 282: 36659–36670. doi: 10.1074/jbc.M705078200. PubMed: 17933870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Purcell EK, Liu L, Thomas PV, Duncan RK (2011) Cholesterol influences voltage-gated calcium channels and BK-type potassium channels in auditory hair cells. PLOS ONE 6: e26289. doi: 10.1371/journal.pone.0026289. PubMed: 22046269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Andrae J, Gallini R, Betsholtz C (2008) Role of platelet-derived growth factors in physiology and medicine. Genes Dev 22: 1276–1312. doi: 10.1101/gad.1653708. PubMed: 18483217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chen H, Gu X, Liu Y, Wang J, Wirt SE et al. (2011) PDGF signalling controls age-dependent proliferation in pancreatic β-cells. Nature, 478: 349–55. doi: 10.1038/nature10502. PubMed: 21993628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bekris LM, Yu C-E, Bird TD, Tsuang DW (2010) Genetics of Alzheimer disease. J Geriatr Psychiatry Neurol 23: 213–227. doi: 10.1177/0891988710383571. PubMed: 21045163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Houlden H, Reilly MM (2006) Molecular genetics of autosomal-dominant demyelinating Charcot-Marie-Tooth disease. Neuromolecular Med 8: 43–62. doi: 10.1385/NMM:8:1:243. PubMed: 16775366. [DOI] [PubMed] [Google Scholar]
- 56. The UniProt Consortium (2011) Ongoing and future developments at the Universal Protein. Resour - Nucleic Acids Research 39: D214–D219. doi: 10.1093/nar/gkq1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Genetic Association Database (2011). Available: http://geneticassociationdb.nih.gov.
- 58. Valencia-Sanchez MA, Liu J, Hannon GJ, Parker R (2006) Control of translation and mRNA degradation by miRNAs and siRNAs. Genes Dev 20: 515–524. doi: 10.1101/gad.1399806. PubMed: 16510870. [DOI] [PubMed] [Google Scholar]
- 59. Tripodi M, Filosa A, Armentano M, Studer M (2004) The COUP-TF nuclear receptors regulate cell migration in the mammalian basal forebrain. Development 131: 6119–6129. doi: 10.1242/dev.01530. PubMed: 15548577. [DOI] [PubMed] [Google Scholar]
- 60. Giguère V, Yang N, Segui P, Evans RM (1988) Identification of a new class of steroid hormone receptors. Nature 331: 91–94. doi: 10.1038/331091a0. PubMed: 3267207. [DOI] [PubMed] [Google Scholar]
- 61. McKenna NJ, O’Malley BW (2002) Combinatorial control of gene expression by nuclear receptors and coregulators. Cell 108: 465–474. doi: 10.1016/S0092-8674(02)00641-4. PubMed: 11909518. [DOI] [PubMed] [Google Scholar]
- 62. Cho H, Zhao X, Hatori M, Yu RT, Barish GD et al. (2012) Regulation of circadian behaviour and metabolism by REV-ERB-α and REV-ERB-β. Nature 485: 123–127. doi: 10.1038/nature11048. PubMed: 22460952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Foulds CE, Feng Q, Ding C, Bailey S, Hunsaker TL et al. (2013) Proteomic analysis of coregulators bound to ERα on DNA and nucleosomes reveals coregulator dynamics. Mol Cell 51: 185–199. doi: 10.1016/j.molcel.2013.06.007. PubMed: 23850489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yang X, Downes M, Yu RT, Bookout AL, He W et al. (2006) Nuclear receptor expression links the circadian clock to metabolism. Cell 126: 801–810. doi: 10.1016/j.cell.2006.06.050. PubMed: 16923398. [DOI] [PubMed] [Google Scholar]
- 65. Ahmadian M, Suh JM, Hah N, Liddle C, Atkins AR et al. (2013) PPARγ signaling and metabolism: the good, the bad and the future. Nat Med 19: 557–566. doi: 10.1038/nm.3159. PubMed: 23652116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Deblois G, St-Pierre J, Giguère V (2013) The PGC-1/ERR signaling axis in cancer. Oncogene 32: 3483–3490. doi: 10.1038/onc.2012.529. PubMed: 23208510. [DOI] [PubMed] [Google Scholar]
- 67. Rada-Iglesias A, Bajpai R, Prescott S, Brugmann SA, Swigut T et al. (2012) Epigenomic annotation of enhancers predicts transcriptional regulators of human neural crest. Cell Stem Cell 11: 633–648. doi: 10.1016/j.stem.2012.07.006. PubMed: 22981823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Chan CM, Fulton J, Montiel-Duarte C, Collins HM, Bharti N et al. (2013) A signature motif mediating selective interactions of BCL11A with the NR2E/F subfamily of orphan nuclear receptors. Nucleic Acids Research. doi: 10.1093/nar/gkt761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zhang Z, Burch PE, Cooney AJ, Lanz RB, Pereira Fa et al. (2004) Genomic analysis of the nuclear receptor family: new insights into structure, regulation, and evolution from the rat genome. Genome Res 14: 580–590. doi: 10.1101/gr.2160004. PubMed: 15059999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Miyaki S, Sato T, Inoue A, Otsuki S, Ito Y et al. (2010) MicroRNA-140 plays dual roles in both cartilage development and homeostasis. Genes Dev 24: 1173–1185. doi: 10.1101/gad.1915510. PubMed: 20466812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Miyaki S, Nakasa T, Otsuki S, Grogan SP, Higashiyama R et al. (2009) MicroRNA-140 is expressed in differentiated human articular chondrocytes and modulates interleukin-1 responses. Arthritis Rheum 60: 2723–2730. doi: 10.1002/art.24745. PubMed: 19714579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Takata A, Otsuka M, Yoshikawa T, Kishikawa T, Hikiba Y et al. (2013) MicroRNA-140 acts as a liver tumor suppressor by controlling NF-κB activity by directly targeting DNA methyltransferase 1 (Dnmt1) expression. Hepatology 57: 162–170. doi: 10.1002/hep.26011. PubMed: 22898998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Liu Y, Zhang ZC, Qian SW, Zhang YY, Huang HY et al. (2013) MicroRNA-140 promotes adipocyte lineage commitment of C3H10T1/2 pluripotent stem cells via targeting osteopetrosis-associated transmembrane protein 1. J Biol Chem 288: 8222–8230. doi: 10.1074/jbc.M112.426163. PubMed: 23389033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Yang J, Qin S, Yi C, Ma G, Zhu H et al. (2011) MiR-140 is co-expressed with Wwp2-C transcript and activated by Sox9 to target Sp1 in maintaining the chondrocyte proliferation. FEBS Lett 585: 2992–2997. doi: 10.1016/j.febslet.2011.08.013. PubMed: 21872590. [DOI] [PubMed] [Google Scholar]
- 75. Mak ACY, Szeto IYY, Fritzsch B, Cheah KSE (2009) Differential and overlapping expression pattern of SOX2 and SOX9 in inner ear development. Gene Expr Patterns 9: 444–453. doi: 10.1016/j.gep.2009.04.003. PubMed: 19427409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Spörl F, Korge S, Jürchott K, Wunderskirchner M, Schellenberg K et al. (2012) Krüppel-like factor 9 is a circadian transcription factor in human epidermis that controls proliferation of keratinocytes. Proc Natl Acad Sci U S A 109: 10903–10908. doi: 10.1073/pnas.1118641109. PubMed: 22711835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Pei H, Yao Y, Yang Y, Liao K, Wu J-R (2011) Krüppel-like factor KLF9 regulates PPARγ transactivation at the middle stage of adipogenesis. Cell Death Differ 18: 315–327. doi: 10.1038/cdd.2010.100. PubMed: 20725087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Dai K, Khatun I, Hussain MM (2010) NR2F1 and IRE1beta suppress microsomal triglyceride transfer protein expression and lipoprotein assembly in undifferentiated intestinal epithelial cells. Arterioscler Thromb Vasc Biol 30: 568–574. doi: 10.1161/ATVBAHA.109.198135. PubMed: 20007910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Charitidi K, Meltser I, Canlon B (2012) Estradiol treatment and hormonal fluctuations during the estrous cycle modulate the expression of estrogen receptors in the auditory system and the prepulse inhibition of acoustic startle response. Endocrinology 153: 4412–4421. doi: 10.1210/en.2012-1416. PubMed: 22778224. [DOI] [PubMed] [Google Scholar]
- 80. Simonoska R, Stenberg AE, Duan M, Yakimchuk K, Fridberger A et al. (2009) Inner ear pathology and loss of hearing in estrogen receptor-beta deficient mice. J Endocrinol 201: 397–406. doi: 10.1677/JOE-09-0060. PubMed: 19293293. [DOI] [PubMed] [Google Scholar]
- 81. Meltser I, Tahera Y, Simpson E, Hultcrantz M, Charitidi K et al. (2008) Estrogen receptor beta protects against acoustic trauma in mice. J Clin Invest 118: 1563–1570. doi: 10.1172/JCI32796. PubMed: 18317592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Simmons CD, Pabona JMP, Zeng Z, Velarde MC, Gaddy D et al. (2010) Response of adult mouse uterus to early disruption of estrogen receptor-alpha signaling is influenced by Krüppel-like factor 9. J Endocrinol 205: 147–157. doi: 10.1677/JOE-09-0474. PubMed: 20164373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Zhang Y, Eades G, Yao Y, Li Q, Zhou Q (2012) Estrogen receptor α signaling regulates breast tumor-initiating cells by down-regulating miR-140 which targets the transcription factor SOX2. J Biol Chem 287: 41514–41522. doi: 10.1074/jbc.M112.404871. PubMed: 23060440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Le Dily F, Métivier R, Guéguen M-M, Le Péron C, Flouriot G et al. (2008) COUP-TFI modulates estrogen signaling and influences proliferation, survival and migration of breast cancer cells. Breast Cancer Res Treat 110: 69–83. doi: 10.1007/s10549-007-9693-6. PubMed: 17674191. [DOI] [PubMed] [Google Scholar]
- 85. Rüsch A, Erway LC, Oliver D, Vennström B, Forrest D (1998) Thyroid hormone receptor beta-dependent expression of a potassium conductance in inner hair cells at the onset of hearing. Proc Natl Acad Sci U S A 95: 15758–15762. doi: 10.1073/pnas.95.26.15758. PubMed: 9861043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Denver RJ, Williamson KE (2009) Identification of a thyroid hormone response element in the mouse Kruppel-like factor 9 gene to explain its postnatal expression in the brain. Endocrinology 150: 3935–3943. doi: 10.1210/en.2009-0050. PubMed: 19359381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Dettling J, Franz C, Zimmermann U, Lee SC, Bress A et al. (2013) Autonomous functions of murine thyroid hormone receptor TRα and TRβ in cochlear hair cells. Molecular and Cellular Endocrinology. doi: 10.1016/j.mce.2013.08.025. [DOI] [PubMed] [Google Scholar]
- 88. Cooney AJ, Tsai SY, O’Malley BW, Tsai MJ (1992) Chicken ovalbumin upstream promoter transcription factor (COUP-TF) dimers bind to different GGTCA response elements, allowing COUP-TF to repress hormonal induction of the vitamin D3, thyroid hormone, and retinoic acid receptors. Mol Cell Biol 12: 4153–4163. PubMed: 1324415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ying M, Sang Y, Li Y, Guerrero-Cazares H, Quinones-Hinojosa A et al. (2011) Krüppel-like family of transcription factor 9, a differentiation-associated transcription factor, suppresses Notch1 signaling and inhibits glioblastoma-initiating stem cells. Stem Cells 29: 20–31. doi: 10.1002/stem.561. PubMed: 21280156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Abe S, Katagiri T, Saito-Hisaminato A, Usami S, Inoue Y et al. (2003) Identification of CRYM as a candidate responsible for nonsyndromic deafness, through cDNA microarray analysis of human cochlear and vestibular tissues. Am J Hum Genet 72: 73–82. doi: 10.1086/345398. PubMed: 12471561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Oshima A, Suzuki S, Takumi Y, Hashizume K, Abe S et al. (2006) CRYM mutations cause deafness through thyroid hormone binding properties in the fibrocytes of the cochlea. J Med Genet 43: e25. doi: 10.1136/jmg.2005.034397. PubMed: 16740909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Pingault V, Ente D, Dastot-Le Moal F, Goossens M, Marlin S et al. (2010) Review and update of mutations causing Waardenburg syndrome. Hum Mutat 31: 391–406. doi: 10.1002/humu.21211. PubMed: 20127975. [DOI] [PubMed] [Google Scholar]
- 93. Brown KK, Rehm HL (2012) Molecular diagnosis of hearing loss. Current protocols in human genetics / editorial board, Jonathan L Haines . [et al.] Chapter 9 Unit9.16. doi: 10.1002/0471142905.hg0916s72. [DOI] [Google Scholar]
- 94. Davarnia B, Babanejad M, Fattahi Z, Nikzat N, Bazazzadegan N et al. (2012) Spectrum of GJB2 (Cx26) gene mutations in Iranian Azeri patients with nonsyndromic autosomal recessive hearing loss. Int J Pediatr Otorhinolaryngol 76: 268–271. doi: 10.1016/j.ijporl.2011.11.019. PubMed: 22172221. [DOI] [PubMed] [Google Scholar]
- 95. Krishnan R, Cleary EG (1990) Elastin gene expression in elastotic human breast cancers and epithelial cell lines. Cancer Res 50: 2164–2171. PubMed: 2156612. [PubMed] [Google Scholar]
- 96. Lapis K, Tímár J (2002) Role of elastin-matrix interactions in tumor progression. Semin Cancer Biol 12: 209–217. doi: 10.1016/S1044-579X(02)00024-X. PubMed: 12083851. [DOI] [PubMed] [Google Scholar]
- 97. Fu L, Balasubramanian M, Shan J, Dudenhausen EE, Kilberg MS (2011) Auto-activation of c-JUN gene by amino acid deprivation of hepatocellular carcinoma cells reveals a novel c-JUN-mediated signaling pathway. J Biol Chem 286: 36724–36738. doi: 10.1074/jbc.M111.277673. PubMed: 21862593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Vogt PK (2001) Jun, the oncoprotein. Oncogene 20: 2365–2377. doi: 10.1038/sj.onc.1204443. PubMed: 11402333. [DOI] [PubMed] [Google Scholar]
- 99. Inoue K, Shiga T, Ito Y (2008) Runx transcription factors in neuronal development. Neural Dev 3: 20. doi: 10.1186/1749-8104-3-20. PubMed: 18727821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Eberhart JK, He X, Swartz ME, Yan Y-L, Song H et al. (2008) MicroRNA Mirn140 modulates Pdgf signaling during palatogenesis. Nat Genet 40: 290–298. doi: 10.1038/ng.82. PubMed: 18264099. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information File containing Tables S1 to S7:
Table S1. Number of predicted miRNAs and NR2F1 inner ear targets.
Table S2. Genes targeted by at least 3 of the 11 selected miRNAs.
Table S3. Representative list of genes targeted by multiple miRNAs and their associated neurological and other diseases based on the Genetic Association Database.
Table S4. List of genes targeted by at least 3 of the 11 select miRNAs and the respective miRNA binding sites in their mRNA 3’ untranslated region based on TargetScan 6.2.
Table S5. Genes targeted by at least 3 of 11 selected miRNAs and the respective miRNA binding sites based on miRanda analysis from microRNA.org.
Table S6. Functional classification of the genes targeted by miRNAs and NR2F1.
Table S7. List of primers for real-time RT-PCR.
(DOCX)