Abstract
Objective. The aim of this study was to investigate how the paradoxical response of GH secretion to TRH changes according to tumor volumes. Methods. Patients with newly diagnosed acromegaly were classified as either TRH responders or nonresponders according to the results of a TRH stimulation test (TST), and their clinical characteristics were compared according to responsiveness to TRH and tumor volumes. Results. A total of 41 acromegalic patients who underwent the TST were included in this study. Between TRH responders and nonresponders, basal GH, IGF-I levels, peak GH levels, and tumor volume were not significantly different, but the between-group difference of GH levels remained near significant over the entire TST time. ΔGHmax-min during the TST were significantly different according to the responsiveness to TRH. Peak GH levels and ΔGHmax-min during the TST showed significantly positive correlations with tumor volume with higher levels in macroadenomas than in microadenomas. GH levels over the entire TST time also remained significantly higher in macroadenomas than in microadenomas. Conclusion. Our data demonstrated that the paradoxical response of GH secretion to TRH in GH-producing pituitary adenomas was not inversely correlated with tumor volumes.
1. Introduction
Abnormal responsiveness of growth hormone- (GH-) producing pituitary adenomas to hypothalamic hormones has been previously described [1]. This paradoxical response of GH to thyrotropin-releasing hormone (TRH) in GH-producing pituitary adenomas was first reported in 1972 [2, 3] and observed in 50~75% of untreated acromegalic patients [4]. However, this response is not specific to acromegaly and was also found in various pathologic conditions such as severe hepatic failure [2], chronic renal failure [5], diabetes mellitus [6], and anorexia nervosa [7].
There have been many studies of the predictive value of the paradoxical response for treatment outcome and prognosis in acromegaly [4, 8–10]. However, the detailed mechanism of the paradoxical response of GH to TRH in acromegaly remains unknown despite a number of possible hypotheses: local production of TRH by adenoma cells [11, 12], TRH-induced release of GH [13], TRH production by anterior pituitary gland [14], and inappropriate expression of TRH receptors at tumor cells [15]. Moreover, only a few studies have attempted to observe how the pattern of paradoxical response changes according to tumor volume [10, 16]. The aim of this study was to investigate how the paradoxical response of GH secretion to TRH changes according to tumor volume in acromegalic patients.
2. Methods
2.1. Patients
A total of 65 patients newly diagnosed with acromegaly at Kyung Hee University Hospital between 2005 and 2012 were initially screened. All of them were diagnosed with acromegaly when an oral glucose tolerance test (OGTT) failed to suppress GH levels below 1.0 μg/L, and their insulin-like growth factor I (IGF-I) level was above the upper normal range for age. Among these patients, 41 patients (25 men and 16 women) who underwent the TRH stimulation test (TST) during the diagnostic work-up procedure for acromegaly were enrolled. Their mean age was 41 ± 11 yr. None of these patients received any medications for acromegaly such as somatostatin analogues before the surgical treatment.
2.2. Oral Glucose Tolerance Test
The OGTT was performed after an overnight fast. Patients had blood samples taken at baseline (0 minutes) and then at 30, 60, 90, and 120 minutes after drinking 75 g of a glucose solution. Blood was allowed to clot at room temperature for 15 minutes and then was centrifuged; the serum was frozen at −80°C in multiple aliquots. Blood samples from all time points were assayed for GH levels, and baseline samples were also assayed for IGF-I.
2.3. TRH Stimulation Test
Patients had blood samples taken at baseline (0 minute) and then at 30, 60, 90, and 120 minutes after intravenous administration of 200 μg TRH [10]. TRH-induced GH responsiveness was evaluated by calculating the TRH ratio (the peak/basal ratio of GH during the TST): those with a TRH ratio higher than 2 was defined as TRH responders. This definition had been used in previous reports [16–19]. The assays for GH during the TST were performed in the same way as during the OGTT.
2.4. Octreotide Suppression Test
After an overnight fast, all patients had baseline blood samples taken for GH, and 100 μg of octreotide (Sandostatin, Novartis) was then administered intravenously. Blood sampling for GH testing was continued every hour for four hours. Responders to the OST were defined as those whose nadir GH level was less than 2.5 μg/L during the test [20]. The assays for GH during the TST were performed in the same way as during the OGTT.
2.5. Image Work-Up
Pituitary adenomas were identified in all patients by sellar MRI. Microadenoma was defined as an intrasellar tumor with a diameter less than 10 mm and macroadenoma as a tumor having a diameter greater than 10 mm and impinging upon adjacent sellar structures. The tumor volume was estimated by the equation which had been developed by Di Chiro and Nelson (volume = 0.5 × length × height × width) [21].
2.6. Measurement of GH and IGF-I
Serum GH concentration was measured using commercial radioimmunoassay (RIA) kits (hGH-RIACT, Cisbio Bioassays, Bedford, MA, USA). The sensitivity of this kit was 0.01 μg/L with an intra-assay CV of 3.8–5.0% and an interassay CV of 1.3–2.1%. Serum IGF-I concentrations were determined by commercial immunoradiometric assay kits (IGF-I NEXT IRMA CT, IDA S.A., Liège, Belgium). The minimum detectable concentration of IGF-I was 1.25 μg/L. The intra- and interassay CVs were 2.6–4.4% and 7.4–9.1%, respectively.
2.7. Data Analysis
Clinical characteristics of patients were compared according to their responsiveness to TRH, which included age, body mass index (BMI), basal GH and IGF-I, peak GH levels and ΔGHmax-min (defined as the difference between the peak and basal GH levels) during the TST, and tumor volume. GH levels during 120 minutes of the entire TST time were also compared between TRH responders and nonresponders. Then, the same analyses were conducted between macroadenomas and microadenomas. Lastly, the correlation coefficients to explore the relationship between responsiveness to TRH and tumor volumes were calculated. In addition, the ratios between the basal and peak GH levels during the OST were calculated and compared according to the responsiveness to TRH in order to investigate how GH suppression rates during the OST would be different between TRH responders and nonresponders.
2.8. Statistical Analysis
All statistical analyses were performed with PASW (version 18.0; SPSS Inc., Chicago, IL, USA), and a P value < 0.05 was considered statistically significant. Baseline characteristics were described as means and standard deviations. The Mann-Whitney test and repeated measure one-way analysis of variance (RMANOVA) were used to compare the data according to the responsiveness to TRH and also to tumor volumes. A Spearman correlation coefficient was calculated between the tumor volumes and ΔGHmax-min as well as peak GH levels during the TST to investigate the relationship between tumor volume and responsiveness to TRH.
2.9. Ethics Statement
This study was approved by the institutional review board (IRB) of Kyung Hee University Hospital (IRB number KMC IRB 1333-06). The informed consents from the patients were waived by the boards due to the retrospective design of this study.
3. Results
3.1. Comparison of TST Results between TRH Responders and Nonresponders
Thirty-two patients (78.0%) were classified as TRH responders and 9 as nonresponders (Table 1). TRH responders were significantly older than nonresponders, but BMI, basal GH, and IGF-I levels did not show significant difference between TRH responders and nonresponders. ΔGHmax-min during the TST were significantly higher in TRH responders than in nonresponders (82.0 ± 94.3 versus 33.9 ± 43.1 μg/L, P = 0.035 for ΔGHmax-min). Tumor volumes did not show statistically significant difference according to the responsiveness to TRH. The between-group difference in GH levels remained nonsignificant during the entire TST time between TRH responders and nonresponders (P = 0.066, Figure 1).
Table 1.
Baseline characteristics of 41 patients with GH-producing pituitary adenomas according to their response patterns to the TRH suppression test.
| TRH responder | TRH nonresponder | P | |
|---|---|---|---|
| Number of patients | 32 | 9 | |
| Age at diagnosis | 45 ± 11 | 34 ± 8 | 0.002* |
| BMI, kg/m2 | 26.3 ± 3.1a | 27.7 ± 3.8b | 0.336 |
| Basal GH, μg/L | 19.4 ± 28.7 | 49.3 ± 56.6 | 0.072 |
| Basal IGF-I, μg/La | 890 ± 312c | 1012 ± 452d | 0.411 |
| Peak GH during TST, μg/L | 99.1 ± 100.4 | 74.6 ± 70.1 | 0.740 |
| ΔGHmax-min during TST, μg/L | 82.0 ± 94.3 | 33.9 ± 43.1 | 0.035* |
| Tumor volume, mm3 | 4886 ± 15854 | 4834 ± 6338 | 0.429 |
Data were expressed as mean ± standard deviation. The Mann-Whitney test was used for the statistical comparison between TRH responders and non-responders.
*P < 0.05.
aData were available in 16 cases.
bData were available in 10 cases.
cData were available in 25 cases.
dData were available in 13 cases.
Abbreviations: TRH: thyrotropin-releasing hormone; BMI: body mass index; GH: growth hormone; IGF-I: insulin-like growth factor-I; TST: TRH stimulation test.
Figure 1.
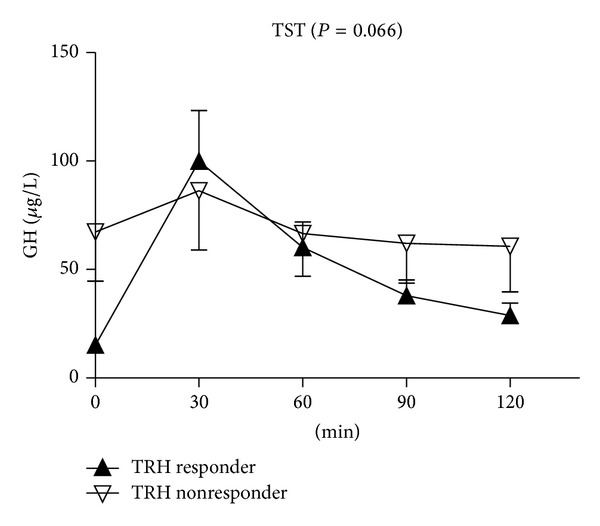
Comparison of GH levels according to the responsiveness to TRH during the entire TST time. Data represent mean ± standard error. RMANOVA was used for the statistical comparison, and Greenhouse-Geisser correction was applied if compound symmetry was not satisfied based upon Mauchly's sphericity test. Abbreviations: GH: growth hormone; TRH: thyrotropin releasing hormone; TST: TRH stimulation test; RMANOVA: repeated measure one-way analysis of variance.
3.2. Comparison of Results of the TST between Macroadenomas and Microadenomas
Thirty-two patients had macroadenomas (mean volume 6187 ± 14583 mm3), and 9 patients had microadenomas (mean volume 175 ± 171 mm3) (Table 2). Twenty-five patients with macroadenomas and 7 with microadenomas were classified as TRH responders. BMI did not show any significant differences when compared according to tumor volume. Basal GH levels were significantly higher in those patients with macroadenomas (P = 0.012), but IGF-I levels did not show any significant difference according to tumor volume. Both peak GH levels during the TST and ΔGHmax-min were higher in macroadenomas than in microadenomas (124.1 ± 129.0 versus 27.5 ± 34.5 μg/L, P = 0.002; 90.3 ± 125.8 versus 12.7 ± 22.0 μg/L, P = 0.026). When only patients with macroadenomas were analyzed, ΔGHmax-min during the TST were significantly higher in TRH responders (105.8 ± 138.1 versus 34.8 ± 30.1 μg/L, P = 0.024: P value not shown in the table), while tumor volumes were not significantly different. GH levels remained significantly higher in macroadenomas than in microadenomas over the entire TST time (P = 0.022, Figure 2).
Table 2.
Baseline characteristics of 41 patients with GH-producing pituitary adenomas according to their tumor sizes.
| Responsiveness to TRH | Macroadenomas | Microadenomas | P ¶ | ||||
|---|---|---|---|---|---|---|---|
| Responders | Non-responders | Total | Responders | Non-responders | Total | ||
| Number of patients | 25 | 7 | 32 | 7 | 2 | 9 | |
| Tumor volume, mm3 | 6937 ± 16399 | 3506 ± 3507 | 6187 ± 14583 | 147 ± 138 | 275 ± 308 | 175 ± 171 | <0.026* |
| Age at diagnosis | 43 ± 12 | 38 ± 8 | 42 ± 12 | 33 ± 9 | 47 ± 14 | 36 ± 11 | 0.181 |
| BMI, kg/ m2 | 25.7 ± 2.9 | 28.9 ± 3.5 | 26.8 ± 3.4a | 27.7 ± 4.1b | N/A | 27.8 ± 4.1b | 0.674 |
| Basal GH, μg/ L | 23.6 ± 29.7 | 79.1 ± 71.4 | 35.8 ± 47.0 | 10.6 ± 13.9 | 13.0 ± 8.3 | 11.2 ± 12.5 | 0.012* |
| Basal IGF-I, μg/ L | 919 ± 309 | 1199 ± 551 | 976 ± 376c | 750 ± 330 | 827 ± 78 | 770 ± 283d | 0.111 |
| §Peak GH during TST, μg/ L | 129.0 ± 139.8 | 105.0 ± 84.4 | 124.1 ± 129.0 | 28.2 ± 39.4 | 24.8 ± 12.9 | 27.5 ± 34.5 | 0.002* |
| §∗ΔGHmax−min during TST, μg/ L | 105.8 ± 138.1 | 34.8 ± 30.1 | 90.3 ± 125.8 | 13.4 ± 25.2 | 10.3 ± 6.5 | 12.7 ± 22.0 | 0.026* |
Data were expressed as mean ± standard deviation.
¶ P values between macroadenomas (n = 32) and microadenomas (n = 9) were calculated using the Mann-Whitney test.
*P < 0.05.
§The Mann-Whitney test only between TRH responders and non-responders with macroadenomas demonstrated the significant difference between the two groups.
aData were available in 22 cases.
bData were available in 4 cases.
cData were available in 30 cases.
dData were available in 8 cases.
Figure 2.
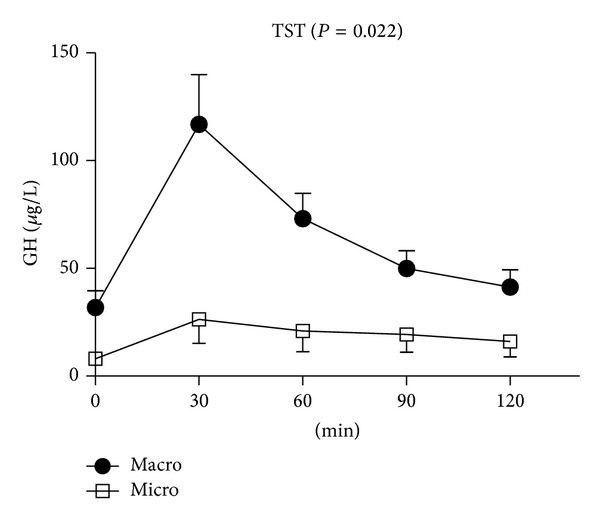
Comparison of GH levels between macroadenomas and microadenomas during the TST. Data represent mean ± standard error. RMANOVA was used for the statistical comparison, and Greenhouse-Geisser correction was applied if compound symmetry was not satisfied based upon Mauchly's sphericity test. Abbreviations: GH: growth hormone; TRH: thyrotropin releasing hormone; TST: TRH stimulation test; macro: macroadenoma; micro: microadenoma; RMANOVA: repeated measure one-way analysis of variance.
3.3. Correlation between Results of the TST and Tumor Volume
The relationship between the peak GH levels during the TST and the tumor volume was investigated by calculating the Spearman correlation coefficient, which revealed a moderate but significant correlation between two parameters (r = 0.498, P = 0.001, Figure 3(a)). The same analysis between ΔGHmax-min during the TST and the tumor volume revealed an analogous result (r = 0.420, P = 0.006, Figure 3(b)).
Figure 3.
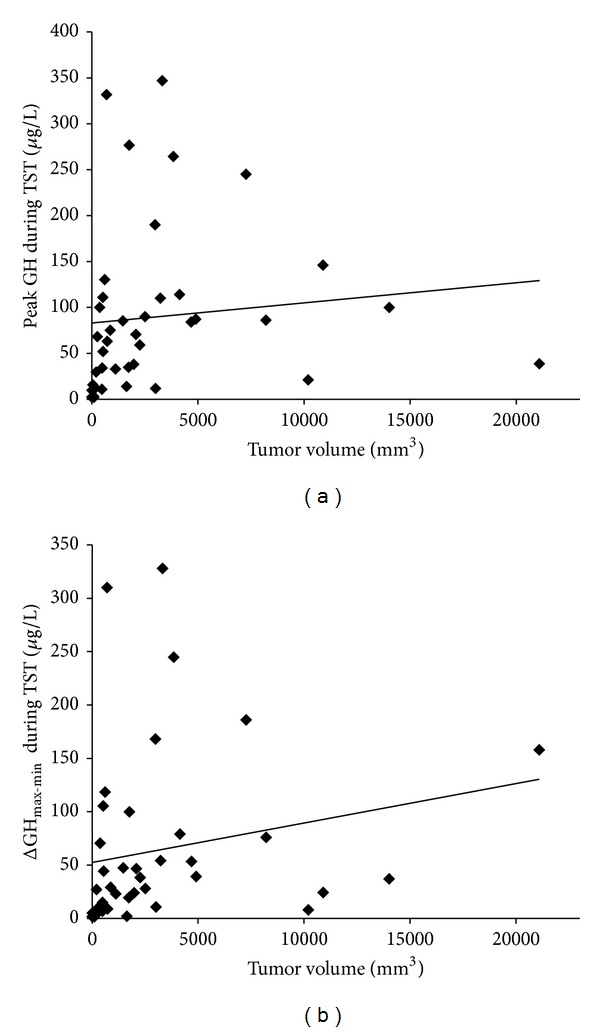
Correlation between the tumor volume and peak GH levels during the TST (r = 0.498, P = 0.001) (a) and ΔGHmax-min during the TST (r = 0.420, P = 0.006) (b).
3.4. Comparison of GH Suppression during the OST between TRH Responders and Nonresponders
In the OST, TRH responders had a GH suppression rate which was not significantly different from that of TRH nonresponders (89.7 ± 12.4% versus 80.4 ± 19.8%, P = 0.136).
4. Discussion
The present study investigated the relationship between the volume of GH-producing pituitary adenomas and their responsiveness to TRH. Between TRH responders and nonresponders, basal GH, IGF-I levels, and tumor volume were not significantly different but the between-group difference of GH levels remained near significant over the entire TST time. ΔGHmax-min during the TST were significantly different according to the responsiveness to TRH. Peak GH levels and ΔGHmax-min during the TST showed significantly positive correlations with tumor volume with higher levels in macroadenomas than in microadenomas. GH levels over the entire TST time also remained significantly higher in macroadenomas than in microadenomas. Altogether, these results showed that responsiveness to TRH during the TST in GH-producing pituitary adenomas was not inversely correlated with tumor volume. Our additional analysis demonstrated that the GH suppression rate after octreotide injection did not differ regardless of responsiveness to TRH. All of these findings are substantially in conflict with previously reported data [10, 16].
A number of possible mechanisms for the paradoxical response after TRH administration have been suggested. Locally released TRH produced by pituitary adenomas could act as an autocrine and/or paracrine regulator to affect hormone release or tumor growth [11, 12]. Alternatively, it has been hypothesized that TRH may lead to acute GH release by inhibiting somatostatin release [13], or TRH could be synthesized endogenously by the anterior pituitary gland [14]. Yamada et al. have suggested that dedifferentiation of tumor cells as a result of inappropriate expression of TRH receptors may cause the paradoxical response of GH after TRH administration [15]. The level of TRH receptor 1 (THRH-1) mRNA expression was positively correlated with the responsiveness of GH to TRH administration [22]. Thus, the notion of dedifferentiation of tumor cells could be one of the relevant explanations for paradoxical responses, but still its exact mechanism remains largely unclear. Moreover, few studies have explored the relationship between responsiveness to TRH during the TST and tumor volume in GH-producing pituitary adenomas. De Marinis et al. reported that the preoperative GH paradoxical response to TRH was often present in small pituitary adenomas [10]. Arita et al. similarly reported that the tumors of TRH responders were smaller but their levels of serum GH per volume were higher in TRH nonresponders and the TRH-induced GH response was inversely related to the tumor volume [16].
It was reported that patients with GH-producing pituitary adenomas and a tumor-stimulatory G protein (gsp) mutation had small tumor volume with a higher rate of TRH responders [17, 18], which provided a theoretical background for inverse correlation between the GH response to TRH and tumor volume [16]. However, a number of previous studies have also reported that pituitary adenomas with gsp mutation were rather larger than those without mutation [23, 24]. Therefore, the presence of this mutation may not be sufficient to explain the relationship between responsiveness of GH to TRH and tumor volume. We previously reported that as the tumor becomes larger, there exists a portion of the GH secretion which escaped physiologic regulation by somatostatinergic tone (SST) [25]. One of the known hypotheses for the paradoxical response in GH to TRH administration is the inhibition of somatostatin release by TRH [13]. Thus, unlike the previous findings [10, 16], it can be further hypothesized that these two mechanisms may behave synergistically to result in the increase of GH secretion after TRH administration as the tumor volume increases. Interestingly, our data also showed there was no difference in basal IGF-I levels according to the responsiveness to TRH (Table 1). There was no correlation between IGF-I levels and tumor sizes at diagnosis according to the Liege Acromegaly Survey [26], and this may explain the reason for the absence of a significant relationship between responsiveness to TRH and basal IGF-I levels in newly diagnosed acromegalic patients.
A previous study also reported that TRH responders showed a higher GH suppression rate than nonresponders during the OST [16]. This result was seemingly supported by preceding reports that pituitary adenomas with the gsp oncogene mutation were shown to experience stronger GH suppression by octreotide and pituitary adenomas with gsp mutation were more likely to be TRH responders [17, 18]. In contrast, our data failed to demonstrate any significant difference in the GH suppression rate between TRH responders and nonresponders (89.7 ± 12.4% versus 80.4 ± 19.8%, P = 0.136). TRH exerts its action through a phosphatidyl inositol-protein kinase pathway [27]. In contrast, octreotide signaling involves various somatostatin receptors, the inhibition of adenylyl cyclase activity, and modulation of the activity of potassium and calcium channels as well as stimulation of phosphotyrosine phosphatase or mitogen activated protein kinase activity [28–31]. These two signaling pathways have not been shown to interact with each other, suggesting that a significant association between the GH response to TRH and octreotide is unlikely.
There are some limitations to this study, including a relatively small number of acromegalic patients with TRH nonresponders. However, the percentage of TRH nonresponders was relatively similar between previously published data (17/62, 27.4%) [16] and ours (9/41, 22.0%, Table 1). Also, there were not many patients with microadenomas, and as a result, it could cause a paradox that most of microadenomas (7/9) but only 78% of macroadenomas were classified as TRH responders (25/32, Table 2), possibly leading to misunderstanding that responsiveness might track with smaller size. It is well known that approximately 70% of GH-producing adenomas are macroadenomas at diagnosis [32], and thus there must be larger prospective studies to solve this limitation. In addition, the histologic characteristics of each tumor were not taken into account in this study.
In conclusion, we expanded the number of patients with newly diagnosed GH-producing pituitary adenomas who had been evaluated with TRH to examine the paradoxical response and concluded that, unlike the previously published data [10, 16], the relationship between the GH response to TRH and tumor volume did not demonstrate any evidence for the inverse correlation; ΔGHmax-min during the TST but not basal GH, IGF-I levels, and tumor volume showed significant differences according to responsiveness to TRH and tumor volume. Peak GH during the TST was significantly different according to tumor volume. Both ΔGHmax-min during the TST and peak GH during the TST were positively correlated with tumor volumes. The paradoxical response of GH to TRH appears to result from the unpredicted and still unknown interactions of various factors [16, 33], and this may explain why there have been inconsistent results regarding the relationship between the responsiveness to TRH and tumor volume. Additional studies with a larger number of patients are warranted to provide better understanding of the basic characteristics of responsiveness to TRH during the TST in patients with GH-producing pituitary tumors.
Conflict of Interests
The authors declare that they have no conflict of interests.
Acknowledgment
The authors would like to thank all the members of the Research Institute of Endocrinology at Kyung Hee University Hospital for their help in the management of the study samples.
References
- 1.Hanew K, Kokubun M, Sasaki A. The spectrum of pituitary growth hormone responses to pharmacological stimuli in acromegaly. Journal of Clinical Endocrinology and Metabolism. 1980;51(2):292–297. doi: 10.1210/jcem-51-2-292. [DOI] [PubMed] [Google Scholar]
- 2.Irie M, Tsushima T. Increase of serum growth hormone concentration following thyrotropin-releasing hormone injection in patients with acromegaly or gigantism. Journal of Clinical Endocrinology and Metabolism. 1972;35(1):97–100. doi: 10.1210/jcem-35-1-97. [DOI] [PubMed] [Google Scholar]
- 3.Schalch DS, Gonzalez-Barcena D, Kastin AJ, Schally AV, Lee LA. Abnormalities in the release of TSH in response to thyrotropin-releasing hormone (TRH) in patients with disorders of the pituitary, hypothalamus and basal ganglia. Journal of Clinical Endocrinology and Metabolism. 1972;35(4):609–615. doi: 10.1210/jcem-35-4-609. [DOI] [PubMed] [Google Scholar]
- 4.Biermasz NR, Smit JWA, van Dulken H, Roelfsema F. Postoperative persistent thyrotrophin releasing hormone-induced growth hormone release predicts recurrence in patients with acromegaly. Clinical Endocrinology. 2002;56(3):313–319. doi: 10.1046/j.1365-2265.2002.01465.x. [DOI] [PubMed] [Google Scholar]
- 5.Czernichow P, Dauzet MC, Broyer M, Rappaport R. Abnormal TSH, PRL and GH response to TSH releasing factor in chronic renal failure. Journal of Clinical Endocrinology and Metabolism. 1976;43(3):630–637. doi: 10.1210/jcem-43-3-630. [DOI] [PubMed] [Google Scholar]
- 6.Maeda K, Kato Y, Yamaguchi N. Growth hormone release following thyrotrophin releasing hormone injection into patients with anorexia nervosa. Acta Endocrinologica. 1976;81(1):1–8. doi: 10.1530/acta.0.0810001. [DOI] [PubMed] [Google Scholar]
- 7.Salerno F, Cocchi D, Frigerio C. Anomalous growth hormone responses to thyrotropin-releasing hormone and glucose in cirrhotic patients: the effect of metergoline. Journal of Clinical Endocrinology and Metabolism. 1980;51(3):641–646. doi: 10.1210/jcem-51-3-641. [DOI] [PubMed] [Google Scholar]
- 8.Faglia G, Paracchi A, Ferrari C, Beck-Peccoz P. Evaluation of the results of trans-sphenoidal surgery in acromegaly by assessment of growth hormone response to thyrotrophin-releasing hormone. Clinical Endocrinology. 1978;8(5):373–380. doi: 10.1111/j.1365-2265.1978.tb02171.x. [DOI] [PubMed] [Google Scholar]
- 9.Brockmeier SJ, Buchfelder M, Fahlbusch R. TRH/GnRH test in acromegaly. Long-term follow-up experience with successfully treated patients. Hormone and Metabolic Research. 1993;25(5):275–277. doi: 10.1055/s-2007-1002096. [DOI] [PubMed] [Google Scholar]
- 10.De Marinis L, Mancini A, Bianchi A, et al. Preoperative growth hormone response to thyrotropin-releasing hormone and oral glucose tolerance test in acromegaly: a retrospective evaluation of 50 patients. Metabolism. 2002;51(5):616–621. doi: 10.1053/meta.2002.32017. [DOI] [PubMed] [Google Scholar]
- 11.Le Dafniet M, Lefebvre P, Barret A, et al. Normal and adenomatous human pituitaries secrete thyrotropin-releasing hormone in vitro: modulation by dopamine, haloperidol, and somatostatin. Journal of Clinical Endocrinology and Metabolism. 1990;71(2):480–486. doi: 10.1210/jcem-71-2-480. [DOI] [PubMed] [Google Scholar]
- 12.Pagesy P, Croissandeau G, Le Dafniet M, Peillon F, Li JY. Detection of thyrotropin-releasing hormone (TRH) mRNA by the reverse transcription-polymerase chain reaction in the human normal and tumoral anterior pituitary. Biochemical and Biophysical Research Communications. 1992;182(1):182–187. doi: 10.1016/s0006-291x(05)80128-7. [DOI] [PubMed] [Google Scholar]
- 13.Blanco MG, Brion DE. Studies in constitutionally tall adolescents: somatostatin decrease associated with growth hormone increase after TRH injection. Clinical Endocrinology. 1984;21(4):459–463. doi: 10.1111/j.1365-2265.1984.tb03232.x. [DOI] [PubMed] [Google Scholar]
- 14.May V, Wilber JF, U’Prichard DC, Childs GV. Persistence of immunoreactive TRH and GnRH in long-term primary anterior pituitary cultures. Peptides. 1987;8(3):543–558. doi: 10.1016/0196-9781(87)90022-2. [DOI] [PubMed] [Google Scholar]
- 15.Yamada M, Monden T, Satoh T, et al. Pituitary adenomas of patients with acromegaly express thyrotropin-releasing hormone receptor messenger RNA: cloning and functional expression of the human thyrotropin-releasing hormone receptor gene. Biochemical and Biophysical Research Communications. 1993;195(2):737–745. doi: 10.1006/bbrc.1993.2107. [DOI] [PubMed] [Google Scholar]
- 16.Arita H, Kinoshita M, Oshino S, et al. Biological characteristics of growth hormone-producing pituitary adenomas are different according to responsiveness to thyrotropin-releasing hormone. The Journal of Clinical Endocrinology & Metabolism. 2012;97(8):2741–2747. doi: 10.1210/jc.2012-1125. [DOI] [PubMed] [Google Scholar]
- 17.Bakhtiar Y, Hirano H, Arita K, et al. Relationship between cytokeratin staining patterns and clinico-pathological features in somatotropinomae. European Journal of Endocrinology. 2010;163(4):531–539. doi: 10.1530/EJE-10-0586. [DOI] [PubMed] [Google Scholar]
- 18.Yasufuku-Takano J, Takano K, Morita K, Takakura K, Teramoto A, Fujita T. Does the prevalence of gsp mutations in GH-secreting pituitary adenomas differ geographically or racially? Prevalence of gsp mutations in Japanese patients revisited. Clinical Endocrinology. 2006;64(1):91–96. doi: 10.1111/j.1365-2265.2005.02423.x. [DOI] [PubMed] [Google Scholar]
- 19.Ikuyama S, Natori S, Nawata H, et al. Characterization of growth hormone-releasing hormone receptors in pituitary adenomas from patients with acromegaly. Journal of Clinical Endocrinology and Metabolism. 1988;66(6):1265–1271. doi: 10.1210/jcem-66-6-1265. [DOI] [PubMed] [Google Scholar]
- 20.Gilbert JA, Miell JP, Chambers SM, McGregor AM, Aylwin SJB. The nadir growth hormone after an octreotide test dose predicts the long-term efficacy of somatostatin analogue therapy in acromegaly. Clinical Endocrinology. 2005;62(6):742–747. doi: 10.1111/j.1365-2265.2005.02278.x. [DOI] [PubMed] [Google Scholar]
- 21.Di Chiro G, Nelson KB. The volume of the sella turcica. The American Journal of Roentgenology, Radium Therapy, and Nuclear Medicine. 1962;87:989–1008. [PubMed] [Google Scholar]
- 22.Kim K, Arai K, Sanno N, Teramoto A, Shibasaki T. The expression of thyrotrophin-releasing hormone receptor 1 messenger ribonucleic acid in human pituitary adenomas. Clinical Endocrinology. 2001;54(3):309–316. doi: 10.1046/j.1365-2265.2001.01237.x. [DOI] [PubMed] [Google Scholar]
- 23.Harris PE, Alexander JM, Bikkal HA, et al. Glycoprotein hormone α-subunit production in somatotroph adenomas with and without Gsα mutations. Journal of Clinical Endocrinology and Metabolism. 1992;75(3):918–923. doi: 10.1210/jcem.75.3.1517386. [DOI] [PubMed] [Google Scholar]
- 24.Adams EF, Brockmeier S, Friedmann E, et al. Clinical and biochemical characteristics of acromegalic patients harboring gsp-positive and gsp-negative pituitary tumors. Neurosurgery. 1993;33(2):198–203. doi: 10.1227/00006123-199308000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Chin SO, Rhee SY, Chon S, et al. Change in somatostatinergic tone of acromegalic patients according to the size of growth hormone-producing pituitary tumors. Annual Scientific Meeting of the Korea Endocrine Society (KES) Joint with APDO (Asian Pacific Diabetes and Obesity Study Group), April 2011.
- 26.Petrossians P, Tichomirowa MA, Stevenaert A, Martin D, Daly AF, Beckers A. The Liege Acromegaly Survey (LAS): a new software tool for the study of acromegaly. Ann Endocrinol. 2012;73(3):190–201. doi: 10.1016/j.ando.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 27.Alster DK, Bowers CY, Jaffe CA, Ho PJ, Barkan AL. The growth hormone (GH) response to GH-releasing peptide (His-DTrp-Ala- Trp-DPhe-Lys-NH2), GH-releasing hormone, and thyrotropin-releasing hormone in acromegaly. Journal of Clinical Endocrinology and Metabolism. 1993;77(3):842–845. doi: 10.1210/jcem.77.3.8370708. [DOI] [PubMed] [Google Scholar]
- 28.Bruns C, Weckbecker G, Raulf F, et al. Molecular pharmacology of somatostatin-receptor subtypes. Annals of the New York Academy of Sciences. 1994;733:138–146. doi: 10.1111/j.1749-6632.1994.tb17263.x. [DOI] [PubMed] [Google Scholar]
- 29.Reisine T, Bell GI. Molecular biology of somatostatin receptors. Endocrine Reviews. 1995;16(4):427–442. doi: 10.1210/edrv-16-4-427. [DOI] [PubMed] [Google Scholar]
- 30.Patel YC. Molecular pharmacology of somatastatin receptor subtypes. Journal of Endocrinological Investigation. 1997;20(6):348–367. doi: 10.1007/BF03350317. [DOI] [PubMed] [Google Scholar]
- 31.Patel YC. Somatostatin and its receptor family. Frontiers in Neuroendocrinology. 1999;20(3):157–198. doi: 10.1006/frne.1999.0183. [DOI] [PubMed] [Google Scholar]
- 32.Melmed S. Acromegaly. The New England Journal of Medicine. 2006;355(24):2558–2573. doi: 10.1056/NEJMra062453. [DOI] [PubMed] [Google Scholar]
- 33.Ehrchen J, Peters A, Lüdecke DK, Visser T, Bauer K. Analysis of thyrotropin-releasing hormone-signaling components in pituitary adenomas of patients with acromegaly. Journal of Clinical Endocrinology and Metabolism. 2000;85(8):2709–2713. doi: 10.1210/jcem.85.8.6707. [DOI] [PubMed] [Google Scholar]


