Abstract
Neurons of the lateral hypothalamic area (LHA) control motivated behaviors such as feeding and ambulatory activity, in part by modulating mesolimbic dopamine (DA) circuits. The hormone, leptin, acts via the long form of the leptin receptor (LepRb) in the brain to signal the repletion of body energy stores, thereby decreasing feeding and promoting activity. LHA LepRb neurons, most of which contain neurotensin (Nts; LepRbNts neurons) link leptin action to the control of mesolimbic DA function and energy balance. To understand potential roles for Nts in these processes, we examined mice null for Nts receptor 1 (NtsR1KO). While NtsR1KO mice consume less food than controls on a chow diet, they eat more and become obese when fed a high-fat, high-sucrose palatable diet; NtsR1KO mice also exhibit augmented sucrose preference, consistent with increased hedonic feeding in these animals. We thus sought to understand potential roles for NtsR1 in the control of the mesolimbic DA system and LHA leptin action. LHA Nts cells project to DA-containing midbrain areas, including the ventral tegmental area (VTA) and the substantia nigra (SN), where many DA neurons express NtsR1. Furthermore, in contrast to wild-type mice, intra-LHA leptin treatment increased feeding and decreased VTA Th expression in NtsR1KO mice, consistent with a role for NtsR1 signaling from LHA LepRb neurons in the suppression of food intake and control of mesolimbic DA function. Additionally, these data suggest that other leptin-regulated LHA neurotransmitters normally oppose aspects of Nts action to promote balanced responses to leptin.
Abbreviations: LHA, lateral hypothalamic area; DA, dopamine; LepRb, long form of the leptin receptor; Nts, neurotensin; NtsR1, neurotensin receptor-1; NtsR2, neurotensin receptor-2; NtsR1KO, neurotensin receptor-1 knock out; VTA, ventral tegmental area; SN, substantia nigra; TH, tyrosine hydroxylase; NAc, nucleus accumbens; OX, Orexin/hypocretin; MCH, melanin concentrating hormone; pSTAT3, phosphorylation of signal transducer and activator of transcription 3; PD, palatable diet
Keywords: Neurotensin, Obesity, Orexin, Dopamine
1. Introduction
The lateral hypothalamic area (LHA) receives and integrates signals, including metabolic stimuli, to modulate motivated behaviors (such as feeding, drinking and locomotor activity) as appropriate for current environmental conditions [1]. Part of this function is mediated by the projection of some LHA neurons to midbrain regions (the ventral tegmental area (VTA) and substantia nigra (SN)), which contain dopamine (DA) neurons [2] that regulate the incentive salience of food and locomotor activity, among other things [3].
The LHA contains several groups of neurons that contribute to energy balance, including glutamatergic orexin/hypocrectin (OX)-containing cells that are activated by signals of energy deficit, and which promote food-seeking and increased vigilance [4,5]. Other LHA neurons containing MCH promote feeding as well [6]. The LHA also contains a distinct population of neurons that express the neuropeptide neurotensin (Nts) [7]. Approximately 30% of LHA Nts neurons co-express the inhibitory neurotransmitter GABA and the leptin receptor (LepRb) and are referred to as LepRbNts neurons; leptin action via these LepRbNts neurons is crucial for the control of DA signaling, locomotor activity, and energy balance [8,9]. Some other LHA Nts neurons (non LepRbNts) may be glutamatergic, since activation of LHA Nts neurons promotes NMDA-mediated EPSCs in DA neurons of the VTA [10]. While the contributions of OX and MCH neuropeptide signaling in energy balance and motivated behaviors are beginning to be understood, the role of Nts signaling via LHA Nts neurons has remained unclear.
Acute Nts administration activates midbrain DA neurons [11–13], promotes DA release to the NAc [12,14,15] and limits feeding [16–18]. Central Nts also site-specifically regulates locomotor activity: NAc Nts treatment attenuates locomotor activity, including blunting the locomotor response to psychostimulants [19–21] but Nts in the VTA promotes spontaneous locomotor activity [22–24]. These reports suggest that endogenous Nts released into the VTA might regulate energy balance (e.g. limit feeding and promote locomotor activity) by regulating midbrain DA signaling. Given that LHA Nts neurons project to the midbrain [9] we reasoned that these neurons might contribute to Nts-mediated regulation of VTA DA signaling and feeding. Additionally, we hypothesized that the population of LepRbNts neurons within the LHA might link leptin signaling to the modulation of midbrain-regulated motivated behaviors that are relevant to energy balance [18,25,26]. Most central actions of Nts are mediated via neurotensin receptor-1 (NtsR1) and neurotensin-receptor-2 (NtsR2), which are 7-transmembrane G-protein coupled receptors expressed broadly throughout the brain [27]. NtsR2 mainly modulates analgesia [28–30]. NtsR1 signaling is required for VTA-stimulated locomotor activity [21] and regulation of feeding, including the suppression of food intake by leptin [16,17]. These findings suggest that NtsR1 might play a role in the control of motivated behaviors by LHA Nts neurons and leptin action. We therefore employed NtsR1-null (NtsR1KO) mice to define roles for NtsR1 signaling in the control of hedonic feeding, as well as the control of the mesolimbic DA system by LHA leptin action. Our data reveal an anatomic circuit via which LHA Nts neurons, including LepRbNts neurons, modulate midbrain DA neurons to regulate energy balance, and disruption of Nts/NtsR1 signaling via this circuit may promote hedonic intake and the development of obesity.
2. Materials and methods
2.1. Materials
Recombinant mouse leptin was the generous gift of Amylin Pharmaceuticals (La Jolla, CA).
2.2. Animals
Wild-type (WT) and NtsR1KO (Ntsr1tm1Dgen; Stock ♯ 005826) mice on the C57BL/6 background were purchased from Jackson Laboratories (Bar Harbor, ME) and bred in our colony to produce study animals. Ntscre mice (Ntstm1(cre)Mgmj/J; Jackson stock ♯ 017525) used for tract tracing studies were generated as described previously [9]. NtsR1cre animals (Tg(Ntsr1-cre)GN209Gsat/Mmucd, Stock ♯ 030780-UCD) were purchased from the Mutant Mouse Regional Resource Center (Davis, CA) and interbred with ROSA26-tdTomato reporter mice (Gt(ROSA)26Sortm9(CAG-tdTomato)Hze/J; Jackson Stock ♯ 006148) to facilitate expression of a red fluorescent protein variant (tdTomato) within cre-expressing neurons. All animals were housed and bred within our colony according to guidelines approved by the University of Michigan Committee on the Care and Use of Animals and the Michigan State University Institutional Animal Care and Use Committee. Mice were given ad libitum access to food and water unless otherwise noted in experimental methods. Male mice were used for all studies.
2.3. Metabolic profiling of NtsR1KO mice and controls
WT and NtsR1KO mice were weaned and single housed at 3 weeks of age. Mice were subsequently maintained for 12 weeks on either a standard rodent chow or a palatable diet that was high in fat and sucrose (PD, Research Diets ♯12451: 45 kCal% fat, 35 kCal% carbohydrate and 20 kCal protein). Animals were then analyzed via the Comprehensive Laboratory Monitoring System (CLAMS, Columbus Instruments), an integrated open-circuit calorimeter equipped with an optical beam activity-monitoring device. Mice were weighed before being individually placed into the sealed chambers (7.9″×4″×5″) with free access to food and water. The study was carried out in an experimentation room set at 20–23 °C with 12–12 h (6:00PM–6:00AM) dark–light cycles. The measurements were carried out continuously for 48–72 h, and data were used from the last 24 h, at which point animals are sufficiently acclimated to the chambers. The amount of food of each animal was monitored through a precision balance attached below the chamber. The system was routinely calibrated each time before the experiment using a standard gas (20.5% O2 and 0.5% CO2 in N2). VO2 and VCO2 in each chamber were sampled sequentially for 5 s in a 10 min interval and the motor activity was recorded every second in X and Z dimensions. The air-flow rate through the chambers was adjusted to keep the oxygen differential around 0.3% at resting conditions. After CLAMS analysis, animals were returned to their home cages and allowed to recover for 1 week prior to euthanasia and dissection of tissues, which occurred after mice had been on chow or PD for 16–17 weeks.
2.4. Sucrose preference testing
To measure sensitivity to reward we analyzed WT and NtsR1KO animals (14–16 weeks of age) via a two-bottle sucrose preference testing paradigm using a Volumetric Drinking Monitor (Columbus Instruments, Columbus, OH). We utilized a 0.5% sucrose/water solution for these tests, which has been shown to induce sucrose preference in C57BL/6 mice [31]. Mice were single housed for at least one week prior to being put into testing cages, which consisted of home cages fitted with two lixits located opposite and equidistant from the food hopper. Mice remained in testing cages during the 6 day-long experiment. For the first 4 days, mice were trained to use the dual lixit system with water in both source bottles. Following training, one source bottle was switched from water to a 0.5% sucrose solution and testing was continued for 2 additional days. Each day, at 07:00, liquid consumption data was collected and lixit positions were switched in order to determine if there was baseline preference for either lixit or for either side that liquid was delivered. If there was lixit preference at baseline we paired the sucrose solution with the less preferred lixit. While we were unable to correct for side bias in this paradigm, only 1 animal showed a side bias during testing. The percentage of sucrose consumed was expressed as a percentage of the total liquid consumption when reporting sucrose preference. Graphed data represent the percent change in preference for sucrose compared to water (Δ preference). Total water consumption during baseline testing days as well as during preference testing days was also calculated but no differences in water intake were observed between genotypes.
2.5. Cloning of adenoviral synaptophysin-mCherry vector (Ad-Syn-mCherry)
The synaptophysin-mCherry fusion protein (kindly provided by Andreas Jeromin) was cloned into the base adenoviral transfer vector pShuttle CMV-iN [32] using NotI and KpnI restriction sites. The vector was maxi-prepped and adenoviral stocks were generated as described previously [32].
2.6. Stereotaxic injection for tract tracing
NtsCre mice were administered presurgical analgesic, anesthetized using isofluorane and placed in a stereotaxic frame. After exposing the skull, a guide cannula with a stylet (Plastics One, Roanoke, VA) was lowered into the target regions. Coordinates to the LHA (from bregma) were A/P: −1.34, M/L −1.13 and D/V −5.20 in accordance with the atlas of Paxinos and Franklin [33]. The stylet was removed and replaced by an injector, and 250 nl of Ad-Syn-mCherry was injected to the LHA using a 500 nl Hamilton syringe at a rate of 50 nl/30 s. After 5 min the injector and cannula were removed from the skull, and the incision was sutured. Mice were then housed individually for 5–7 days to allow viral infection and transcription/transport of new peptides before euthanasia and tissue collection. Mice were only included for study if Syn-mCherry expressing cell bodies were confined to the LHA (n=5).
2.7. Central leptin administration
WT and NtsR1KO mice underwent surgery essentially as described above to implant an indwelling 26-gauge stainless steel cannula with a removable dummy injector (Plastics One, Roanoke, VA) into the LHA. Mice were allowed to recover for 1 week during which daily food intake and weight were monitored. For treatment, the dummy was replaced with an injector with a 5.2 mm projection used to deliver either 250 nL of sterile PBS or leptin (0.001 ng/nL, thus each dose=0.25 ng) every 12 h for 24 h, during which food intake and weight were monitored. Mice were then treated once more and 2 h later were anesthetized and removed to a rodent coronal brain matrix (1 mm divisions) for microdissection. The ipsilateral and contralateral VTA were microdissected, frozen on dry ice and stored at −80 °C until analysis. The hypothalamus was placed in 4% paraformaldehyde for fixation at 4 °C for 72 h, followed by cryoprotection in 30% sucrose and microtome sectioning (30 µm coronal sections). Hypothalamic sections were immunostained for OX to verify the cannulation site (since LHA OX and LepRbNts neurons reside in the same perifornical region of the LHA) as well as for phosphorylated STAT3 (pSTAT3) to verify that leptin-induced pSTAT3 activation was confined to the LHA. Mice were excluded from analysis if cannulae were misplaced, if they failed to gain weight and eat normally during the surgical recovery period prior to the study, or if mice exhibited significant pSTAT3 outside of the LHA. After exclusion by these criteria, there were n=13 WT mice treated with PBS, n=13 WT mice treated with leptin, n=9 NtsR1KO mice treated with PBS and n=11 NtsR1KO mice treated with leptin for the feeding and weight measures.
RNA was prepared from microdissected VTA hemispheres using Trizol (Invitrogen) and 1 µg samples were converted to cDNA using the Superscript First Strand Synthesis System for RT-PCR (Invitrogen). Sample cDNAs were analyzed in triplicate via quantitative RT-PCR for GAPDH and tyrosine hydroxylase (Th) (Applied Biosystems) using an Applied Biosystems 7500. Relative mRNA expression values are calculated by the 2−DDCt method, with normalization of each sample to the average ΔCt value of the contralateral side. One sample from each treatment group was lost during processing or did not yield sufficient RNA for analysis, thus there were ipsilateral (injected) and contralateral (non-injected control hemisphere) VTA samples from n=12 WT mice treated with PBS, n=12 WT mice treated with leptin, n=8 NtsR1KO mice treated with PBS and n=12 NtsR1KO mice treated with leptin for expression analysis.
2.8. Immunohistochemistry and immunofluorescence
Mice were anesthetized with a lethal dose of intraperitoneal pentobarbital and transcardially perfused with PBS followed by 10% neutral buffered formalin. Brains were removed, post-fixed overnight and then dehydrated in 30% sucrose before coronal sectioning (30 µm) using a freezing microtome (Leica). Immunostaining was performed as previously described [8] using primary antibodies for dsRed/Tomato (Clonetech, rabbit, 1:1000), Orexin-A (Santa Cruz, goat, 1:1000), TH (Millipore; mouse; 1:2000–5000) and pSTAT3 (Cell Signaling, rabbit, 1:250). All antibodies were reacted with species specific Alexa Fluor-488 or -568 conjugated (Invitrogen, 1:200) or FITC-conjugated (Jackson ImmunoResearch, 1:200) secondary antibodies or processed via avidin-biotin/diaminobenzidine (DAB) method (ABC kit, Vector Labs; DAB reagents, Sigma).
2.9. Data analysis
Paired t-tests (to compare two groups) or one-way ANOVA with Bonferroni post-testing (for comparisons between multiple groups) were calculated using Graph Pad Prism software. Differences were considered significant for p<0.05.
3. Results
3.1. Loss of Nts/NtsR1 signaling perturbs locomotor activity and feeding
To determine the potential role for Nts/NtsR1 signaling in energy balance and leptin action, we examined mice with a targeted deletion of Ntsr1 (NtsR1KO mice) and wild-type (WT) controls (Figure 1). NtsR1KO mice fed normal chow consumed slightly less food than controls during the dark cycle, which diminished their total feeding (Figure 1A, Dark cycle: WT=3.2 g±0.1, NtsR1KO=2.5±0.2 g. p<0.01; 24 h: WT=4.4±0.1 g, NtsR1KO=3.6 g±0.3, p<0.001). NtsR1KO mice also exhibited a modest increase in activity compared to control mice (Figure 1B, Dark cycle: WT=1131.1±95.3 counts/h, NtsR1KO=1679.4±268.2 counts/h, p<0.01) consistent with previous findings [16]. Despite these minor changes in feeding and activity, oxygen consumption (VO2) was not changed (Figure 1C), nor was body weight and fat content different than WT animals (Figure 1D and E), suggesting that other energy balance systems compensate for loss of NtsR1 signaling. Thus, NtsR1 signaling contributes to the control of feeding and locomotor activity (motivated behaviors controlled, in part, by the mesolimbic DA system), but does not compromise overall weight gain in the face of a normal chow diet.
Figure 1.
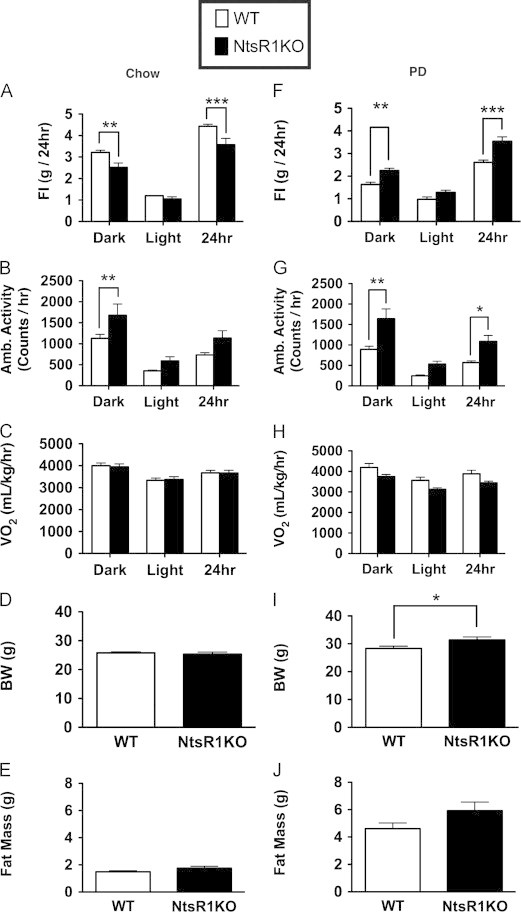
Altered energy balance in response to palatable diet in male NtsR1KO mice. Mice fed ad libitum standard rodent chow exhibit (A) a small decrease in food intake (FI) along with (B) increased ambulatory activity, but NtsR1KO mice do not differ from wild-type (WT) mice in (C) VO2, (D) body weight (BW) or (E) fat mass. In contrast to the hypophagia of NtsR1KO mice maintained on standard chow, (F) NtsR1KO animals eat more of a palatable diet (PD) that is high in fat and sugar compared to WT mice, suggesting increased preference for palatable, calorie-dense food. PD-fed NtsR1KO mice (G) maintain increased ambulatory activity and (H) unaltered VO2 compared to WT mice. The increased PD consumption in NtsR1KO mice promotes (I) a minor, but significant, increase in body weight and (J) a trend for increased fat mass compared to WT animals. Graphs represent average values±SEM. Chow Fed animals: WT n=17, NtsR1KO=10. PD fed animals: WT=10, NtsR1KO=12. Significance was determined via one way ANOVA or student's t-test. *p<0.05, **p<0.01, ***p<0.001.
Since Nts regulates the mesolimbic DA system (which modulates the reward value of food), we reasoned that Nts signaling via NtsR1 might play an important role in the response to a palatable diet. We therefore analyzed energy balance in NtsR1KO mice given ad libitum access to a high fat, high sucrose palatable diet (PD) (Figure 1F–J). In contrast to their mild decrease in food intake on normal chow diet, NtsR1KO mice exhibit increased feeding relative to controls when fed PD (Figure 1F, Dark cycle: WT=1.6 g±0.1, NtsR1KO=2.3±0.1 g, p<0.01; 24 h: WT=2.6±0.1 g, NtsR1KO=3.5 g±0.2, p M 0.001). NtsR1KO animals also demonstrate increased body weight (Figure 1I: WT=28.4±0.8 g, NtsR1KO=31.4±1.1 g, p<0.05) and a trend to increased fat mass (Figure 1J: WT=4.6±0.4 g, NtsR1KO=5.9±0.6 g, p=0.16) relative to controls, despite their sustained increase in overall activity on PD (Figure 1G: Dark cycle: WT=892.4±77.1 counts/h, NtsR1KO=1642.4±228.6 counts/h, p<0.01; 24 h: WT=569.5±42.6 counts/h, NtsR1KO=1088.8±144.9 counts/h, p<0.05). While PD-fed NtsR1KO mice tended toward decreased VO2, this difference was not significant (Figure 1H) nor did we observe any differences in respiratory quotient (Supplemental Figure 1). The disruptions of feeding and locomotor activity in NtsR1KO mice occur specifically during the dark cycle, when rodents are most active and do the majority of their feeding, suggesting that NtsR1 signaling modifies the expression of existing behaviors rather than instigating them. Collectively, these data suggest that global, long-term NtsR1 signaling restrains baseline locomotor activity and the intake of palatable food.
3.2. Increased sucrose preference in NtsR1KO mice
The finding that NtsR1KO animals exhibit increased feeding relative to controls when fed PD, but not standard chow, suggests that NtsR1 may modulate motivated intake, rather than parameters of homeostatic feeding (such as satiation). Thus, we used a two-bottle sucrose preference paradigm to measure the sensitivity of NtsR1KO mice to sucrose reward. This paradigm utilized a mouse home cage with two lixits delivering liquid: one offers normal water under all conditions while the other offered either water or 0.5% sucrose. Consistent with previous findings WT animals exhibited only a modest preference for this low concentration of sucrose relative to water [31]. In contrast, NtsR1KO mice had a much higher preference for sucrose than WT mice (Figure 2, WT=17.3±4.8%, NtsR1KO=36.7±1.7%, p<0.05). The increased sensitivity of NtsR1KO mice to both PD and sucrose suggests that NtsR1 signaling attenuates the consumption of natural rewards.
Figure 2.
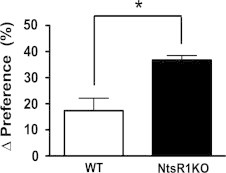
NtsR1KO mice exhibit increased sucrose preference compared to WT animals. Graph represents the percent change in preference for 0.5% sucrose solution compared to water (∆ preference). Error bars depict±SEM. WT n=8, NtsR1KO n=9. Significance determined by student's t-test, *p<0.05.
3.3. Detection of synaptic terminals from LHA Nts neurons in the LHA and midbrain
Motivated intake of natural rewards is regulated via the mesolimbic DA system, and thus Nts/NtsR1 signaling may modify DA-mediated motivated behaviors to blunt the intake of palatable foods. Indeed, many previous studies have demonstrated roles for Nts/NtsR1 in the control of the mesolimbic DA system [34,35]. There are several populations of Nts-containing neurons throughout the brain, each of which presumably possesses unique projection targets and thus sites of action. Indeed, Nts exerts site-specific regulation of the DA system: Nts in the NAc attenuates locomotor response but Nts in the VTA suppresses feeding, reward intake and increases locomotor activity [20–22,36]. Thus, identifying the projection targets of LHA Nts neurons is imperative for understanding their role in regulation of the DA system and motivated behavior. We previously examined projections from LHA Nts neurons via the intra-LHA injection of Ad-iZ/EGFPf (which mediates the expression of a membrane-bound EGFPf in infected cre-expressing neurons) in Ntscre mice, which revealed EGFPf-neurites in midbrain regions [37]. Since EGFPf labels all cell membranes, however, this method cannot distinguish axons of passage from actual synapses. We thus modified our cre-dependent viral tracing system by replacing EGFPf with a synaptophysin-mCherry (Syn-mCherry) fusion protein in Ad-iN/Syn-mCherry (Figure 3A). Synaptophysin is specifically targeted to synaptic terminals, and mCherry enables the detection of synaptophysin/synapses from specific neurons without interference from the large amounts of endogenous synaptophysin in the brain [38].
Figure 3.
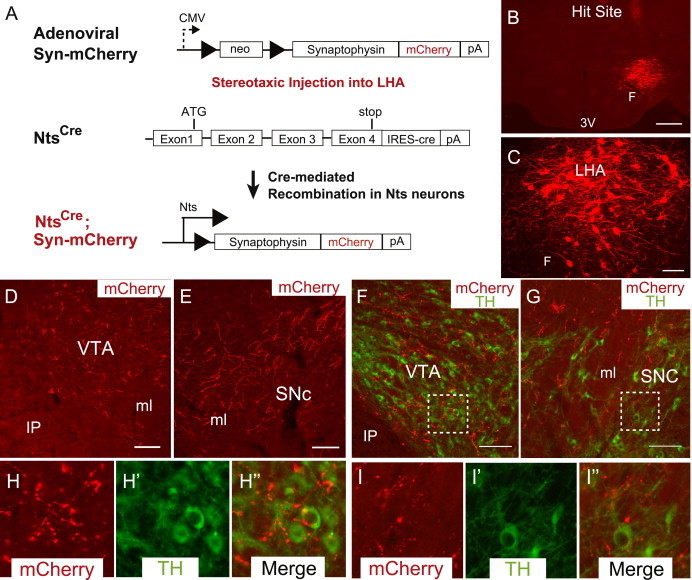
Nts neurons in the LHA project within the LHA and to the midbrain. (A) Adenovirus containing a loxP flanked synaptophysin-mCherry fusion protein (Adenoviral Syn-mCherry) is injected unilaterally into the LHA of mice expressing cre recombinase in Nts neurons (Ntscre mice), permitting expression of synaptophysin mCherry only in LHA Nts neurons. (B) Representative injection site of Adenoviral Syn-mCherry (red) in the LHA of an Ntscre mouse identifies (C) mCherry-expressing cell bodies within the LHA as well as in the (D) VTA and (E) SNc. (F and G) Synaptophysin-mCherry labeled terminals are observed near TH-containing neurons (green) in the VTA (F, H) and the SNc (G, I). Scale bar in B=500 µm, all other scale bars=100 µm. Dashed boxes in F and G identify the regions that are digitally enlarged in H and I, respectively. VTA=ventral tegmental area, SNc=substantia nigra compacta, LHA=lateral hypothalamic area, 3 V=third ventricle, F=fornix, IP=interpeduncular nucleus, ml=medial lemniscus. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
We injected Ad-iN/Syn-mCherry into the LHA of Ntscre mice and examined the distribution of Syn-mCherry in subjects in which the injection was confined to the LHA, where cell bodies positive for Syn-mCherry were restricted to the LHA in the region of the injection site (Figure 3B). Immunofluorescent detection of mCherry in the LHA of injected animals reveals many mCherry-expressing soma and neuronal processes within the LHA (Figure 3C), in a pattern similar to the known distribution of LHA Nts neurons [9,39]. Analysis of the entire brain revealed many punctate mCherry-labeled structures within the VTA (Figure 3D) and SNc (Figure 3E), suggesting that LHA Nts neurons synapse within these DA-rich midbrain regions. Indeed, immunofluorescent detection of tyrosine hydroxylase (TH, which identifies DA neurons) revealed many Syn-mCherry-labeled puncta in close opposition to TH cell bodies in the VTA (Figure 3F and H) as well as the SNc (Figure 3G and I). These data suggest that LHA Nts neurons may synapse upon, and regulate, DA neurons in the VTA and SNc.
3.4. Evidence for NtsR1 expression in midbrain DA neurons
Although we define LHA Nts neurons by their expression of Nts [9], the potential target(s) of Nts signaling specifically by these neurons have yet to be identified. We therefore interrogated the location of NtsR1-expressing neurons within the LHA and the midbrain, as these are potentially important sites of action for Nts released by LHA Nts neurons. The mRNA and protein expression of NtsR1 in the brain is very low, precluding facile and adequate detection for colocalization by in situ hybridization (ISH) or immunohistochemistry, respectively. To identify NtsR1-expressing neurons, we bred NtsR1cre transgenic mice with cre-mediated reporter (Gt(ROSA)26Sortm9(CAG-tdTomato)Hze/J) mice to generate animals in which red fluorescent protein (tdTomato) is expressed specifically in NtsR1cre neurons (NtsR1Tomato mice), permitting visualization of these neurons via the detection of tdTomato (Figure 4). Consistent with previous ISH studies [27] our examination of the brains of NtsR1Tomato mice revealed very few Tomato-labeled neurons within the LHA; none of the few LHA NtsR1Tomato neurons co-labeled with OX-expressing neurons (Figure 4B). In contrast, many Tomato-containing neurons were observed within the VTA and SNc (Figure 4A), consistent with previous reports [27]. Since most midbrain neurons contain DA, and Nts acts via NtsR1 to facilitate DA signaling [40], we examined whether the midbrain NtsR1 neurons are DAergic. Indeed, immunofluorescent detection of Tomato and TH (to identify DA neurons) revealed that 96% of NtsR1Tomato neurons in the VTA contain TH, and 55% of SN NtsR1Tomato neurons contain TH (Figure 4D). Thus, while this analysis revealed no evidence for the expression of NtsR1 in LHA OX neurons, these data suggest that DA neurons in the VTA and SNc contain NtsR1 and thus likely respond to Nts derived from the LHA Nts neurons that project into these regions.
Figure 4.
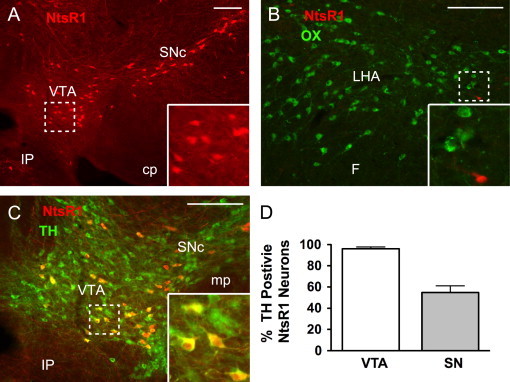
Visualization of NtsR1 neurons in the brain. Transgenic mice expressing cre recombinase in NtsR1 neurons (NtsR1cre mice) were crossed to ROSA26-tdTomato reporter mice to express Tomato in NtsR1 neurons (red). (A) NtsR1 neurons are found within the midbrain, including the VTA and SNc. (B) Few NtsR1 neurons are found in the LHA, none of which co-localize with OX neurons (green). (C) Many of the NtsR1 neurons in the midbrain colocalize with TH (green), a marker of DA neurons. (D) Average percentage of NtsR1 neurons in the VTA and SN that co-express TH. Error bars depict±SEM. Scale bars=100 µm. Dashed boxes identify regions that are digitally enlarged within each panel. VTA=ventral tegmental area, SNc=substantia nigra compacta, IP=interpeduncular nucleus, cp=cerebral peduncle, LHA=lateral hypothalamic area, F=fornix, mp=mammillary peduncle. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.5. Altered responses to LHA leptin action in NtsR1KO mice
Our results reveal that NtsR1 plays an important role in hedonic feeding and activity, and previous studies have demonstrated roles for Nts/NtsR1 signaling in mesolimbic DA function. LHA Nts neurons project to the VTA and SN (Figure 4), both of which contain NtsR1-expressing DA neurons, suggesting a potential role for LHA-derived Nts in the control of mesolimbic DA function and feeding. Similarly, mice lacking LepRb in LHA Nts neurons demonstrate alterations in mesolimbic DA function and activity, suggesting that Nts might contribute to LHA leptin action [9]. To simultaneously test the potential roles for Nts in the control of energy balance and mesolimbic DA function by the leptin-regulated LHA neural circuit, we treated WT and NtsR1KO mice with intra-LHA leptin (Figure 5). Mice were unilaterally cannulated in the LHA (Figure 5A) and treated with 250 pg leptin every 12 h for 26 h. As we showed previously [8], this dose of intra-LHA leptin increased the phosphorylation of signal transducer and activator of transcription 3 (pSTAT3; a well-characterized marker of leptin-stimulated LepRb activation) on the injected side of the LHA. No pSTAT3 (green) was observed in OX neurons at the injection site, confirming that this leptin dose specifically activated LHA LepRb neurons (Figure 5B). No increase in pSTAT3 was detected in other nearby LepRb-containing regions (such as the arcuate nucleus), however, confirming the confinement of the injected leptin to the LHA in this treatment paradigm (Figure 5C).
Figure 5.
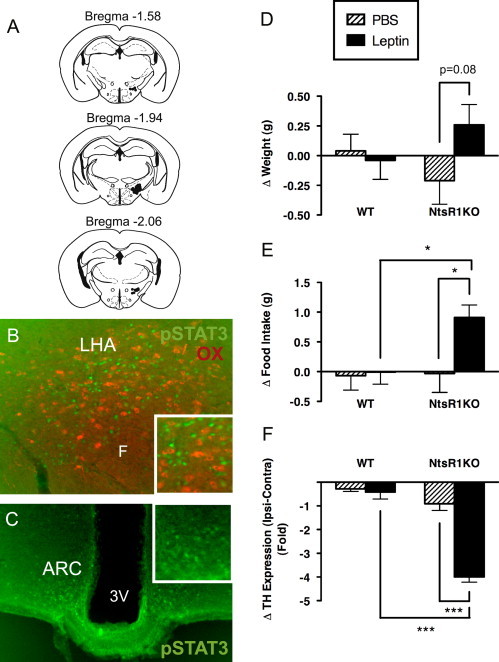
Role of leptin action via LepRbNts neurons in male WT and NtsR1KO mice. (A) Schematic of the cannulation sites within the LHA, through which mice were treated with vehicle (PBS) or 0.25 ng leptin every 12 h for 26 h total. (B) Immunofluorescent detection of phosphorylated STAT3 (pSTAT3, a marker of leptin-activated neurons; green) in the injected LHA of a representative leptin-treated NtsR1KO animal. Note that adjacent OX neurons (red) do not exhibit pSTAT3, and leptin-mediated activation is specific to LepRb neurons (none of which contain OX). (C) Normal distribution of pSTAT3 is observed in the mediobasal hypothalamus, such as the arcuate nucleus (ARC) of leptin-treated animals, representative of endogenous leptin signaling. Further, there is no increase of pSTAT3 on the cannulated side of the ARC (see inset, right) compared to the contralateral (non-injected, left) side of the ARC, indicating that the leptin was confined to the injected LHA and did not spread to the ARC or other LepRb-expressing regions of the brain. (D) Intra-LHA leptin does not alter body weight in WT animals, but promotes a trend toward increased body weight in NtsR1KO mice. (E) Intra-LHA leptin increased food intake in NtsR1KO mice, but not in WT animals. (F) Change in fold expression of mRNA for tyrosine hydroxylase (Th) within the VTA. Values represent the change in Th on the injected side of the LHA compared to the contralateral, non-injected side (=Ipsi-Contra). Intra-LHA leptin treatment reduces Th within the VTA of NtsR1KO mice. Error bars depict±SEM. For D and E: WT+PBS=13, WT+leptin=13, NtsR1KO+PBS=8, NtsR1KO+leptin=10. WT n=8, NtsR1KO n=9. For F, there is one less sample per group due to sample loss during RNA isolation. Significance determined by one way ANOVA, * p<0.05, *** p<0.001. (For interpretation of the references to color in this figure, the reader is referred to the web version of this article.)
Leptin-deficient ob/ob mice are hyperphagic and obese, and we previously demonstrated that intra-LHA leptin treatment decreases feeding and body weight in these animals [8]. Here we examined energy balance in WT and NtsR1KO mice after similar treatment with intra-LHA PBS (vehicle) or leptin. Intra-LHA leptin treatment of WT animals did not significantly decrease body weight (Figure 5D: WT+PBS=0.04±0.14 g, WT+leptin=−0.04±0.16 g), or food intake (Figure 5E: WT+PBS=−0.07±0.24 g, WT+leptin=−0.01±0.20 g) over 26 h, suggesting that the increase in LHA LepRb signaling provoked by this modest unilateral dose of leptin was insufficient to detectably augment the anorectic effects of endogenous leptin. By contrast, intra-LHA leptin treatment tended to promote weight gain over 26 h in NtsR1KO animals (Figure 5D: NtsR1KO+PBS=−0.21±0.20 g, NtsR1KO+leptin=0.26±0.17 g, p=0.08). Furthermore, intra-LHA leptin treatment of NtsR1KO mice increased feeding relative to vehicle-treated controls (Figure 5E: NtsR1KO+PBS=−0.03±0.32 g, NtsR1KO+leptin=0.91±0.21 g, p<0.05). These data suggest that Nts signaling from LHA LepRb neurons contributes to regulation of feeding via NtsR1. Loss of Nts/NtsR1 action mediated via LHA LepRb neurons therefore converts the generally anorectic leptin stimulus to an orexigenic signal.
LHA LepRb neurons that express Nts (LepRbNts neurons) project to the VTA [9], so we hypothesized that Nts from these neurons regulates VTA NtsR1/DA neurons to contribute to the regulation of feeding. Indeed, leptin-deficient ob/ob mice are hyperphagic and exhibit reduced VTA Th expression [41], but intra-LHA leptin treatment blunts feeding and normalizes VTA Th expression to WT levels [8]. To determine whether intra-LHA leptin might also modulate VTA function via NtsR1, we assayed the expression of Th mRNA in the VTA of NtsR1KO and control animals following intra-LHA leptin treatment, revealing that intra-LHA leptin dramatically decreased the expression of Th mRNA in the VTA compared to vehicle-treated NtsR1KO mice and leptin treated controls (Figure 5F: WT+PBS=−0.28±0.11 g, WT+leptin=−0.42±0.29 g, NtsR1KO+PBS=−0.91±0.28 g, NtsR1KO+leptin=−4.00±0.22 g, p<0.001). These observations suggest that leptin signaling in LHA LepRb neurons requires NtsR1 to promote VTA Th expression and suppress feeding, while the loss of NtsR1 signaling in this system unmasks the effects of one or more oppositely-acting leptin-regulated LHA-derived neurotransmitter(s) that suppress VTA Th expression and promote feeding. Presumably, Nts and this/these other neurotransmitters counterbalance each other's effects in the mesolimbic DA system of normal animals (Figure 6).
Figure 6.
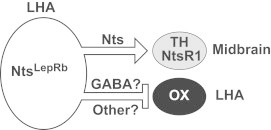
Model of circuit via which LHA Nts neurons signal via NtsR1 to regulate energy balance. Nts neurons in the LHA contain GABA and Nts, including LepRbNts neurons, and project to and regulate OX neurons in the LHA and NtsR1-containing midbrain neurons. The absence of NtsR1 in OX neurons predicts that they are regulated via GABA or other inhibitory signals from upstream LHA Nts neurons, but not via Nts. The presence of NtsR1-expressing neurons in the midbrain, half of which also contain DA, suggests that GABA and Nts released from LHA Nts neurons can act via NtsR1 in the midbrain.
4. Discussion
Acute treatment with Nts decreases food intake while acute blockade of NtsR1 promotes feeding, suggesting a role for Nts/NtsR1 signaling in energy balance [42–44]. These findings predicted that mice null for NtsR1 (NtsR1KO mice) would exhibit hyperphagia and obesity, but surprisingly, NtsR1KO mice exhibit only modest hypophagia and essentially normal energy balance on a chow diet, suggesting preserved homeostatic signaling in these animals [16]. Our present data demonstrate that NtsR1KO mice overeat specifically in response to a palatable diet (PD) and exhibit increased sucrose preference. This modified consumption of pleasurable foods is consistent with potential roles for Nts/NtsR1 action via the mesolimbic DA system, which controls the incentive salience of food and other rewards as well as goal-directed behaviors. Indeed, Nts/NtsR1 signaling activates VTA DA neurons and promotes striatal DA release [12–15]. Neuropeptide-mediated regulation of the mesolimbic DA system, however, is complex and DA cannot be directly ascribed as promoting or inhibiting feeding, per se. For example, both Nts and OX have been characterized as “reward peptides” because they activate VTA DA neurons, promote extracellular DA in the striatum, reinforce reward and rodents will self-administer them into the VTA [45–49]. Nts, however, suppresses the intake of substances that promote mesolimbic DA signaling, including natural rewards (food, sucrose), nicotine and alcohol [18,34,50,51] while OX promotes the intake of these substances [45,46,52]. Thus, while our current data are consistent with a role for Nts/NtsR1 in modulating the intake of natural rewards it remains to be determined how Nts specifically modifies this behavior via the mesolimbic DA system. Nts might, similar to OX, modify the incentive salience of palatable food [45], or influence cue-induced learning and/or goal-directed behaviors (e.g. increased locomotor activity) that indirectly modify feeding.
To identify the circuit by which Nts/NtsR1 might regulate the mesolimbic DA system we examined the localization of NtsR1 neurons and the Nts neurons that project to them. While there are many populations of Nts-containing neurons throughout the brain, we examined Nts neurons from the LHA because of the established role of LHA neurons, including Nts neurons co-expressing LepRb (LepRbNts), in modulating the mesolimbic DA system [9,10]. Indeed, LHA Nts neurons synapse within the LHA and in the midbrain, though NtsR1Tomato-expressing neurons are primarily detected within the midbrain, consistent with previous reports [53–55]. The finding that many NtsR1Tomato neurons within the VTA and SNc co-express TH (a marker of DA neurons) suggests that LHA Nts neurons activate midbrain DA neurons that co-express NtsR1, consistent with previous reports of Nts action on VTA DA neurons [12]. Some LHA Nts synaptic labeling is observed directly on cell soma, an arrangement that suggests these are GABAergic LHA Nts neurons that project to VTA DA neurons. LHA Nts synaptic puncta that do not directly overlap with TH-containing cell bodies may be terminals synapsing with the dendrites of VTA DA neurons. Axonal-dendritic inputs are generally glutamatergic, and some LHA Nts neurons synapsing in the VTA may be glutamatergic, consistent with the report that Nts potentiates glutamate-mediated EPSCs onto VTA DA neurons [10]. Blocking NtsR1 signaling in the VTA suppresses these EPSCs and reward intake (as measured by LHA-self-stimulation) [10]. The most obvious interpretation of these data is that some LHA Nts neurons are glutamatergic. It remains possible, however, that the observed glutamate-mediated effects are due to autaptic (e.g. self) regulation via VTA DA neurons that contain glutamate [56,57]. Going forward it will be important to verify whether there are subpopulations of LHA Nts neurons that differ in classical transmitter content, as these would presumably exert distinct regulatory actions at the midbrain and might underlie the divergent behaviors mediated by Nts.
The adipose-derived hormone, leptin, not only decreases food intake, but also diminishes responding for a variety of drugs of abuse, suggesting that leptin regulates DA-mediated reward signaling [58,59]. Consistently, leptin modulates parameters of mesolimbic DA system function, including the expression of Th (the rate-limiting enzyme in DA production) in the VTA [8,41]. We previously identified LHA-restricted LepRbNts neurons and demonstrated that loss of leptin action via these neurons decreased locomotor activity and DA signaling, suggesting that these Nts-containing neurons were important regulators of the mesolimbic DA system, [9]. Since blockade of NtsR1 signaling interferes with the anorectic action of central leptin [16,44] and Nts action via NtsR1 increases the activity of VTA DA neurons [60,61], we postulated that Nts/NtsR1 signaling might mediate important aspects of LHA leptin action via LepRbNts cells. Indeed, our current finding that LHA Nts cells synapse in the vicinity of midbrain DA cells (many of which express NtsR1), is consistent with a role for these cells in the control of the mesolimbic DA system. We therefore examined the response of NtsR1KO mice to increased leptin action in the LHA- simultaneously parsing the role for leptin-activated LepRbNts neurons and the role of Nts in this isolated circuit. This analysis demonstrated that augmenting LHA leptin action increased feeding and suppressed VTA Th expression in animals null for NtsR1 signaling, in contrast to the neutral response of wild-type, and opposite response of leptin-deficient ob/ob mice [8], to similar treatment. These findings thus reveal that NtsR1 signaling is required for the appropriate regulation of VTA Th expression by LHA LepRbNts neurons, as well as for the appropriate regulation of feeding by leptin action on this circuit. Our data are consistent with reports that Nts upregulates Th expression while suppression of Nts/NtsR1 signaling blunts endogenous Th expression in the VTA [62–64]. Yet, NtsR1KO animals have also been characterized as having hyperactive striatal DA signaling, perhaps due to increased brain expression of NtsR2, and which presumably reflects developmental compensation for the loss of NtsR1 signaling [60,65]. Despite this counterintuitive effect on mesolimbic DA function at baseline in NtsR1KO mice, they remain useful for the examination of potential roles for Nts in mesolimbic DA function, since endogenous and exogenous Nts fail to modulate mesolimbic DA function in these animals [65]. NtsR1KO mice thus permit the analysis of roles for Nts in this system in response to specific stimuli, such as for the study of leptin action via NtsLepRb neurons as we have done here. Indeed, our data suggest important roles for Nts/NtsR1 signaling via LHA LepRbNts cells in the control of mesolimbic DA function and the suppression of feeding by leptin, but also that other leptin-regulated signals/neurotransmitters from this circuit oppose the effects of Nts (Figure 6).
While others have shown that loss of Nts/NtsR1 action blunts the anorectic effect of leptin [16,44] our current results are the first to reveal the site and circuitry by which Nts/NtsR1 signaling contributes to leptin action. Our data demonstrate specifically that LHA LepRbNts neurons signal via NtsR1 neurons in the VTA to modulate food intake and control of the mesolimbic DA system. Given that LepRbNts neurons are restricted to the LHA, the attenuated anorectic action of systemic leptin during NtsR1 blockade presumably reflects the sum of intact leptin regulation of most brain circuits (e.g., the arcuate nucleus and other sites in which LepRb neurons do not contain Nts) combined with the orexigenic signal that results from LHA LepRbNts neurons in the absence of NtsR1 action; the latter may be revealed under circumstances in which the circuit is specifically perturbed (e.g., by LHA leptin injection) or when the mesolimbic DA system predominates for the control of feeding (as in hedonic intake). Indeed, the finding that LHA Nts neurons densely innervate DA-containing midbrain regions raises the possibility that leptin acts via LepRbNts neurons to modulate DA-mediated motivated food intake rather than homeostatic energy balance, which is controlled via non-Nts containing mediobasal and hindbrain LepRb neurons [66]. Thus, the increased sucrose preference and consumption of PD by NtsR1KO mice could reflect altered responses to endogenous leptin via LepRbNts neurons (i.e. loss of ability to suppress motivated feeding). While the GABAergic LHA LepRbNts neurons contribute to regulation of feeding via NtsR1-mediated effects in the VTA, there are also many non-LepRb expressing LHA Nts neurons that may also regulate the midbrain via NtsR1. Indeed, some LHA Nts inputs to the VTA may be glutamatergic and modulate the activity of DA neurons, presynaptically, by increasing EPSC frequency [10,67]. Going forward it will important to examine how these and other neurochemically distinct subsets of LHA Nts neurons regulate the midbrain and energy balance.
In addition to modulating the mesolimbic DA system in response to leptin, LHA LepRbNts cells contribute to the control of LHA OX neurons. Leptin acts via LepRbNts neurons to inhibit the activity of OX neurons and modulate Ox mRNA expression, but leptin and fasting-mediated control of these parameters is disrupted in animals that selectively lack leptin signaling via LepRbNts cells [9]. Since OX neurons contribute to the control of the mesolimbic DA system as well as to the modulation of feeding, it is theoretically possible that dysregulation of OX cells in NtsR1KO mice mediates the NtsR1-dependent effects of LHA leptin. The dearth of NtsR1 expression in the LHA (combined with the robust expression of NtsR1 in midbrain DA neurons) and the lack of detectable NtsR1 in OX neurons argue against this possibility, however. Indeed, neither Ox mRNA expression nor the activity of OX neurons (by c-fos colocalization) is different in NtsR1KO mice than in controls under fed or fasted conditions (data not shown). Thus, the data support that Nts/NtsR1 modulates midbrain DA function but not the LHA OX system. Since LHA LepRbNts cells control OX neuron function, including the response to fasting, these data suggest that additional (non-Nts) neurotransmitters in LHA LepRbNts neurons mediate the effects of these cells on OX neurons.
Our current studies do not rule out a role for Nts actions via NtsR2-expressing neurons in the LHA and midbrain. There are currently no effective immunohistochemical reagents or mouse models to identify NtsR2 neurons, but ISH studies in mouse brain suggest enriched NtsR2 expression in the SNc, but nominal NtsR2 in the LHA and VTA [29,68]. Within the midbrain it is unclear whether NtsR2 is expressed in DA neurons (similar to NtsR1) or GABAergic interneurons, but it has been detected within astrocytes [69,70]. A recent report suggests that OX neurons express NtsR2 as well as Nts [71], but we have not observed significant co-expression of OX and Nts using Ntscre reporter mice or immunofluorescent detection of Nts in colchicine-treated mice. Further, injection of Nts into the LHA has no effect on motivated feeding [72,73], suggesting that Nts actions via the LHA do not contribute to energy balance. In light of these data, and the fact that NtsR2 signaling remains intact in NtsR1KO mice, Nts action via NtsR2 cannot account for the changes in mesolimbic DA function observed in our studies of NtsR1KO mice.
Together, the lack of detectable Nts action on OX neurons by the LHA LepRbNts neurons that control them and the reversal of leptin action on feeding and VTA Th expression by LHA LepRb neurons in NtsR1KO mice suggest that LHA LepRbNts neurons contain neurotransmitters other than Nts. Given that NtsR1 is predominantly a stimulatory receptor (which is opposed in the VTA by the action of other LHA LepRb neurotransmitters) and that LHA LepRb neurons inhibit the activity of OX neurons, these additional neurotransmitters are likely to be inhibitory. Indeed, we previously showed that all LHA LepRb neurons contain Gad1, which synthesizes the inhibitory fast transmitter, GABA [8]. Additionally, a recent publication demonstrates that some LHA LepRb neurons contain the inhibitory peptide galanin (Gal), which also colocalizes extensively with Nts in the LHA [74]. Thus, in one reasonable model, leptin action via LHA LepRb neurons inhibits OX neurons via GABA and/or Gal signaling, while modulating the mesolimbic system by the combined effects of Nts (via NtsR1 neurons) and some combination of these inhibitory neurotransmitters. Testing this dichotomous regulatory model will require a great deal more work on several fronts, and represents an important direction for future research.
Conflict of interest
MGM is an editor in chief at Molecular Metabolism.
Acknowledgments
We thank Amylin Pharmaceuticals (San Diego, CA) for the generous gift of leptin and members of the Myers lab for helpful discussions. Thanks to Andreas Jeromin for providing the Syn-mCherry fusion construct. AG was supported by the NIH-funded Michigan Post-baccalaureate Research Education Program (NIH Grant 5 R25 GM086282). Research support was provided by the Michigan Diabetes Research Center (NIH Grant 5P60 DK020572), the American Diabetes Association, the American Heart Association, the Marilyn H. Vincent Foundation (MGM) and the NIH (MGM: DK78056, GML: DK090101).
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Contributor Information
Martin Myers, Jr, Email: mgmyers@umich.edu.
Gina Leinninger, Email: leinning@msu.edu.
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.molmet.2013.07.008.
Appendix A. Supplementary materials
Supplementary Data
References
- 1.Morrison S.D., Barrnett R.J., Mayer J. Localization of lesions in the lateral hypothalamus of rats with induced adipsia and aphagia. American Journal of Physiology. 1958;193:230–234. doi: 10.1152/ajplegacy.1958.193.1.230. [DOI] [PubMed] [Google Scholar]
- 2.Saper C.B., Swanson L.W., Cowan W.M. An autoradiographic study of the efferent connections of the lateral hypothalamic area in the rat. Journal of Comparative Neurology. 1979;183:689–706. doi: 10.1002/cne.901830402. [DOI] [PubMed] [Google Scholar]
- 3.Kenny P.J. Reward mechanisms in obesity: new insights and future directions. Neuron. 2011;69:664–679. doi: 10.1016/j.neuron.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Toshinai K., Date Y., Murakami N., Shimada M., Mondal M.S., Shimbara T., Guan J.L., Wang Q.P., Funahashi H., Sakurai T., Shioda S., Matsukura S., Kangawa K., Nakazato M. Ghrelin-induced food intake is mediated via the orexin pathway. Endocrinology. 2003;144:1506–1512. doi: 10.1210/en.2002-220788. [DOI] [PubMed] [Google Scholar]
- 5.Yamanaka A., Beuckmann C.T., Willie J.T., Hara J., Tsujino N., Mieda M., Tominaga M., Yagami K., Sugiyama F., Goto K., Yanagisawa M., Sakurai T. Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron. 2003;38:701–713. doi: 10.1016/s0896-6273(03)00331-3. [DOI] [PubMed] [Google Scholar]
- 6.Georgescu D., Sears R.M., Hommel J.D., Barrot M., Bolanos C.A., Marsh D.J., Bednarek M.A., Bibb J.A., Maratos-Flier E., Nestler E.J., DiLeone R.J. The hypothalamic neuropeptide melanin-concentrating hormone acts in the nucleus accumbens to modulate feeding behavior and forced-swim performance. Journal of Neuroscience. 2005;25:2933–2940. doi: 10.1523/JNEUROSCI.1714-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watts A.G., Sanchez-Watts G., Kelly A.B. Distinct patterns of neuropeptide gene expression in the lateral hypothalamic area and arcuate nucleus are associated with dehydration-induced anorexia. Journal of Neuroscience. 1999;19:6111–6121. doi: 10.1523/JNEUROSCI.19-14-06111.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leinninger G.M., Jo Y.H., Leshan R.L., Louis G.W., Yang H., Barrera J.G., Wilson H., Opland D.M., Faouzi M.A., Gong Y., Jones J.C., Rhodes C.J., Chua S., Jr., Diano S., Horvath T.L., Seeley R.J., Becker J.B., Munzberg H., Myers M.G., Jr. Leptin acts via leptin receptor-expressing lateral hypothalamic neurons to modulate the mesolimbic dopamine system and suppress feeding. Cell Metabolism. 2009;10:89–98. doi: 10.1016/j.cmet.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leinninger G.M., Opland D.M., Jo Y.H., Faouzi M., Christensen L., Cappellucci L.A., Rhodes C.J., Gnegy M.E., Becker J.B., Pothos E.N., Seasholtz A.F., Thompson R.C., Myers M.G., Jr. Leptin action via neurotensin neurons controls orexin, the mesolimbic dopamine system and energy balance. Cell Metabolism. 2011;14:313–323. doi: 10.1016/j.cmet.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kempadoo K.A., Tourino C., Cho S.L., Magnani F., Leinninger G.M., Stuber G.D., Zhang F., Myers M.G., Deisseroth K., de Lecea L., Bonci A. Hypothalamic neurotensin projections promote reward by enhancing glutamate transmission in the VTA. Journal of Neuroscience. 2013;33:7618–7626. doi: 10.1523/JNEUROSCI.2588-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Legault M., Congar P., Michel F.J., Trudeau L.E. Presynaptic action of neurotensin on cultured ventral tegmental area dopaminergic neurones. Neuroscience. 2002;111:177–187. doi: 10.1016/s0306-4522(01)00614-5. [DOI] [PubMed] [Google Scholar]
- 12.Sotty F., Souliere F., Brun P., Chouvet G., Steinberg R., Soubrie P., Renaud B., Suaud-Chagny M.F. Differential effects of neurotensin on dopamine release in the caudal and rostral nucleus accumbens: a combined in vivo electrochemical and electrophysiological study. Neuroscience. 1998;85:1173–1182. doi: 10.1016/s0306-4522(97)00691-x. [DOI] [PubMed] [Google Scholar]
- 13.St-Gelais F., Legault M., Bourque M.J., Rompre P.P., Trudeau L.E. Role of calcium in neurotensin-evoked enhancement in firing in mesencephalic dopamine neurons. Journal of Neuroscience. 2004;24:2566–2574. doi: 10.1523/JNEUROSCI.5376-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalivas P.W., Duffy P. Effect of acute and daily neurotensin and enkephalin treatments on extracellular dopamine in the nucleus accumbens. Journal of Neuroscience. 1990;10:2940–2949. doi: 10.1523/JNEUROSCI.10-09-02940.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sotty F., Brun P., Leonetti M., Steinberg R., Soubrie P., Renaud B., Suaud-Chagny M.F. Comparative effects of neurotensin, neurotensin(8–13) and [d-Tyr(11)]neurotensin applied into the ventral tegmental area on extracellular dopamine in the rat prefrontal cortex and nucleus accumbens. Neuroscience. 2000;98:485–492. doi: 10.1016/s0306-4522(00)90023-x. [DOI] [PubMed] [Google Scholar]
- 16.Kim E.R., Leckstrom A., Mizuno T.M. Impaired anorectic effect of leptin in neurotensin receptor 1-deficient mice. Behavioural Brain Research. 2008;194:66–71. doi: 10.1016/j.bbr.2008.06.024. [DOI] [PubMed] [Google Scholar]
- 17.Kim E.R., Mizuno T.M. Role of neurotensin receptor 1 in the regulation of food intake by neuromedins and neuromedin-related peptides. Neuroscience Letters. 2010;468:64–67. doi: 10.1016/j.neulet.2009.10.064. [DOI] [PubMed] [Google Scholar]
- 18.Luttinger D., King R.A., Sheppard D., Strupp J., Nemeroff C.B., Prange A.J., Jr. The effect of neurotensin on food consumption in the rat. European Journal of Pharmacology. 1982;81:499–503. doi: 10.1016/0014-2999(82)90116-9. [DOI] [PubMed] [Google Scholar]
- 19.Boules M., McMahon B., Warrington L., Stewart J., Jackson J., Fauq A., McCormick D., Richelson E. Neurotensin analog selective for hypothermia over antinociception and exhibiting atypical neuroleptic-like properties. Brain Research. 2001;919:1–11. doi: 10.1016/s0006-8993(01)02981-x. [DOI] [PubMed] [Google Scholar]
- 20.Ervin G.N., Birkemo L.S., Nemeroff C.B., Prange A.J., Jr. Neurotensin blocks certain amphetamine-induced behaviours. Nature. 1981;291:73–76. doi: 10.1038/291073a0. [DOI] [PubMed] [Google Scholar]
- 21.Steinberg R., Brun P., Fournier M., Souilhac J., Rodier D., Mons G., Terranova J.P., Le Fur G., Soubrie P. SR 48692, a non-peptide neurotensin receptor antagonist differentially affects neurotensin-induced behaviour and changes in dopaminergic transmission. Neuroscience. 1994;59:921–929. doi: 10.1016/0306-4522(94)90295-x. [DOI] [PubMed] [Google Scholar]
- 22.Elliott P.J., Nemeroff C.B. Repeated neurotensin administration in the ventral tegmental area: effects on baseline and d-amphetamine-induced locomotor activity. Neuroscience Letters. 1986;68:239–244. doi: 10.1016/0304-3940(86)90149-7. [DOI] [PubMed] [Google Scholar]
- 23.Feifel D., Reza T.L. Effects of neurotensin administered into the ventral tegmental area on prepulse inhibition of startle. Behavioural Brain Research. 1999;106:189–193. doi: 10.1016/s0166-4328(99)00123-0. [DOI] [PubMed] [Google Scholar]
- 24.Kalivas P.W., Burgess S.K., Nemeroff C.B., Prange A.J., Jr. Behavioral and neurochemical effects of neurotensin microinjection into the ventral tegmental area of the rat. Neuroscience. 1983;8:495–505. doi: 10.1016/0306-4522(83)90195-1. [DOI] [PubMed] [Google Scholar]
- 25.Cador M., Kelley A.E., Le Moal M., Stinus L. Behavioral analysis of the effect of neurotensin injected into the ventral mesencephalon on investigatory and spontaneous motor behavior in the rat. Psychopharmacology (Berlin) 1985;85:187–196. doi: 10.1007/BF00428412. [DOI] [PubMed] [Google Scholar]
- 26.Hawkins M.F., Baker J.D., Baumeister A.A. Neurotensin-induced polydipsia: a structure-activity study. Brain Research. 1989;487:188–191. doi: 10.1016/0006-8993(89)90957-8. [DOI] [PubMed] [Google Scholar]
- 27.Geisler S., Berod A., Zahm D.S., Rostene W. Brain neurotensin, psychostimulants, and stress—emphasis on neuroanatomical substrates. Peptides. 2006;27:2364–2384. doi: 10.1016/j.peptides.2006.03.037. [DOI] [PubMed] [Google Scholar]
- 28.Boules M., Johnston H., Tozy J., Smith K., Li Z., Richelson E. Analgesic synergy of neurotensin receptor subtype 2 agonist NT79 and morphine. Behavioral Pharmacology. 2011;22:573–581. doi: 10.1097/FBP.0b013e3283474a3a. [DOI] [PubMed] [Google Scholar]
- 29.Maeno H., Yamada K., Santo-Yamada Y., Aoki K., Sun Y.J., Sato E., Fukushima T., Ogura H., Araki T., Kamichi S., Kimura I., Yamano M., Maeno-Hikichi Y., Watase K., Aoki S., Kiyama H., Wada E., Wada K. Comparison of mice deficient in the high- or low-affinity neurotensin receptors, Ntsr1 or Ntsr2, reveals a novel function for Ntsr2 in thermal nociception. Brain Research. 2004;998:122–129. doi: 10.1016/j.brainres.2003.11.039. [DOI] [PubMed] [Google Scholar]
- 30.Smith K.E., Boules M., Williams K., Fauq A.H., Richelson E. The role of NTS2 in the development of tolerance to NT69L in mouse models for hypothermia and thermal analgesia. Behavioural Brain Research. 2011;224:344–349. doi: 10.1016/j.bbr.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sclafani A. Enhanced sucrose and Polycose preference in sweet sensitive (C57BL/6 J) and subsensitive (129P3/J) mice after experience with these saccharides. Physiology and Behavior. 2006;87:745–756. doi: 10.1016/j.physbeh.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 32.Leshan R.L., Louis G.W., Jo Y.H., Rhodes C.J., Munzberg H., Myers M.G., Jr. Direct innervation of GnRH neurons by metabolic- and sexual odorant-sensing leptin receptor neurons in the hypothalamic ventral premammillary nucleus. Journal of Neuroscience. 2009;29:3138–3147. doi: 10.1523/JNEUROSCI.0155-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paxinos, G., Franklin, B., 2001. The Mouse Brain in Stereotaxic Coordinates. Second edn., (Academic Press, 2001).
- 34.Boules M., Iversen I., Oliveros A., Shaw A., Williams K., Robinson J., Fredrickson P., Richelson E. The neurotensin receptor agonist NT69L suppresses sucrose-reinforced operant behavior in the rat. Brain Research. 2007;1127:90–98. doi: 10.1016/j.brainres.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 35.Panayi F., Colussi-Mas J., Lambas-Senas L., Renaud B., Scarna H., Berod A. Endogenous neurotensin in the ventral tegmental area contributes to amphetamine behavioral sensitization. Neuropsychopharmacology. 2005;30:871–879. doi: 10.1038/sj.npp.1300638. [DOI] [PubMed] [Google Scholar]
- 36.Kalivas P.W., Taylor S. Behavioral and neurochemical effect of daily injection with neurotensin into the ventral tegmental area. Brain Research. 1985;358:70–76. doi: 10.1016/0006-8993(85)90949-7. [DOI] [PubMed] [Google Scholar]
- 37.Leinninger G.M. Lateral thinking about leptin: a review of leptin action via the lateral hypothalamus. Physiology and Behavior. 2011;104:572–581. doi: 10.1016/j.physbeh.2011.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meyer M.P., Smith S.J. Evidence from in vivo imaging that synaptogenesis guides the growth and branching of axonal arbors by two distinct mechanisms. Journal of Neuroscience. 2006;26:3604–3614. doi: 10.1523/JNEUROSCI.0223-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kahn D., Abrams G.M., Zimmerman E.A., Carraway R., Leeman S.E. Neurotensin neurons in the rat hypothalamus: an immunocytochemical study. Endocrinology. 1980;107:47–54. doi: 10.1210/endo-107-1-47. [DOI] [PubMed] [Google Scholar]
- 40.Seutin V., Massotte L., Dresse A. Electrophysiological effects of neurotensin on dopaminergic neurones of the ventral tegmental area of the rat in vitro. Neuropharmacology. 1989;28:949–954. doi: 10.1016/0028-3908(89)90194-9. [DOI] [PubMed] [Google Scholar]
- 41.Fulton S., Pissios P., Manchon R.P., Stiles L., Frank L., Pothos E.N., Maratos-Flier E., Flier J.S. Leptin regulation of the mesoaccumbens dopamine pathway. Neuron. 2006;51:811–822. doi: 10.1016/j.neuron.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 42.Beck B., Stricker-Krongrad A., Richy S., Burlet C. Evidence that hypothalamic neurotensin signals leptin effects on feeding behavior in normal and fat-preferring rats. Biochemical and Biophysical Research Communications. 1998;252:634–638. doi: 10.1006/bbrc.1998.9712. [DOI] [PubMed] [Google Scholar]
- 43.Hawkins M.F. Central nervous system neurotensin and feeding. Physiology and Behavior. 1986;36:1–8. doi: 10.1016/0031-9384(86)90064-8. [DOI] [PubMed] [Google Scholar]
- 44.Sahu A., Carraway R.E., Wang Y.P. Evidence that neurotensin mediates the central effect of leptin on food intake in rat. Brain Research. 2001;888:343–347. doi: 10.1016/s0006-8993(00)03107-3. [DOI] [PubMed] [Google Scholar]
- 45.Borgland S.L., Chang S.J., Bowers M.S., Thompson J.L., Vittoz N., Floresco S.B., Chou J., Chen B.T., Bonci A. Orexin A/hypocretin-1 selectively promotes motivation for positive reinforcers. Journal of Neuroscience. 2009;29:11215–11225. doi: 10.1523/JNEUROSCI.6096-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Borgland S.L., Taha S.A., Sarti F., Fields H.L., Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;49:589–601. doi: 10.1016/j.neuron.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 47.Felszeghy K., Espinosa J.M., Scarna H., Berod A., Rostene W., Pelaprat D. Neurotensin receptor antagonist administered during cocaine withdrawal decreases locomotor sensitization and conditioned place preference. Neuropsychopharmacology. 2007;32:2601–2610. doi: 10.1038/sj.npp.1301382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Glimcher P.W., Giovino A.A., Hoebel B.G. Neurotensin self-injection in the ventral tegmental area. Brain Research. 1987;403:147–150. doi: 10.1016/0006-8993(87)90134-x. [DOI] [PubMed] [Google Scholar]
- 49.Glimcher P.W., Margolin D.H., Giovino A.A., Hoebel B.G. Neurotensin: a new ‘reward peptide’. Brain Research. 1984;291:119–124. doi: 10.1016/0006-8993(84)90657-7. [DOI] [PubMed] [Google Scholar]
- 50.Boules M., Oliveros A., Liang Y., Williams K., Shaw A., Robinson J., Fredrickson P., Richelson E. A neurotensin analog, NT69L, attenuates intravenous nicotine self-administration in rats. Neuropeptides. 2011;45:9–16. doi: 10.1016/j.npep.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 51.Li Z., Boules M., Richelson E. NT69L blocks ethanol-induced increase of dopamine and glutamate levels in striatum of mouse. Neuroscience Letters. 2011;487:322–324. doi: 10.1016/j.neulet.2010.10.048. [DOI] [PubMed] [Google Scholar]
- 52.Harris G.C., Wimmer M., Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- 53.Boudin H., Pelaprat D., Rostene W., Beaudet A. Cellular distribution of neurotensin receptors in rat brain: immunohistochemical study using an antipeptide antibody against the cloned high affinity receptor. Journal of Comparative Neurology. 1996;373:76–89. doi: 10.1002/(SICI)1096-9861(19960909)373:1<76::AID-CNE7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 54.Goedert M., Pittaway K., Williams B.J., Emson P.C. Specific binding of tritiated neurotensin to rat brain membranes: characterization and regional distribution. Brain Research. 1984;304:71–81. doi: 10.1016/0006-8993(84)90862-x. [DOI] [PubMed] [Google Scholar]
- 55.Young W.S., 3rd, Kuhar M.J. Neurotensin receptor localization by light microscopic autoradiography in rat brain. Brain Research. 1981;206:273–285. doi: 10.1016/0006-8993(81)90532-1. [DOI] [PubMed] [Google Scholar]
- 56.Birgner C., Nordenankar K., Lundblad M., Mendez J.A., Smith C., le Greves M., Galter D., Olson L., Fredriksson A., Trudeau L.E., Kullander K., Wallen-Mackenzie A. VGLUT2 in dopamine neurons is required for psychostimulant-induced behavioral activation. Proceedings of the National Academy of Sciences of United States of America. 2010;107:389–394. doi: 10.1073/pnas.0910986107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sulzer D., Joyce M.P., Lin L., Geldwert D., Haber S.N., Hattori T., Rayport S. Dopamine neurons make glutamatergic synapses in vitro. Journal of Neuroscience. 1998;18:4588–4602. doi: 10.1523/JNEUROSCI.18-12-04588.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hao J., Cabeza de Vaca S., Carr K.D. Effects of chronic ICV leptin infusion on motor-activating effects of d-amphetamine in food-restricted and ad libitum fed rats. Physiology and Behavior. 2004;83:377–381. doi: 10.1016/j.physbeh.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 59.Shalev U., Yap J., Shaham Y. Leptin attenuates acute food deprivation-induced relapse to heroin seeking. Journal of Neuroscience. 2001;21:RC129. doi: 10.1523/JNEUROSCI.21-04-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Leonetti M., Brun P., Clerget M., Steinberg R., Soubrie P., Renaud B., Suaud-Chagny M.F. Specific involvement of neurotensin type 1 receptor in the neurotensin-mediated in vivo dopamine efflux using knock-out mice. Journal of Neurochemistry. 2004;89:1–6. doi: 10.1046/j.1471-4159.2003.02231.x. [DOI] [PubMed] [Google Scholar]
- 61.Pettibone D.J., Hess J.F., Hey P.J., Jacobson M.A., Leviten M., Lis E.V., Mallorga P.J., Pascarella D.M., Snyder M.A., Williams J.B., Zeng Z. The effects of deleting the mouse neurotensin receptor NTR1 on central and peripheral responses to neurotensin. Journal of Pharmacology and Experimental Therapeutics. 2002;300:305–313. doi: 10.1124/jpet.300.1.305. [DOI] [PubMed] [Google Scholar]
- 62.Azzi M., Betancur C., Sillaber I., Spanagel R., Rostene W., Berod A. Repeated administration of the neurotensin receptor antagonist SR 48692 differentially regulates mesocortical and mesolimbic dopaminergic systems. Journal of Neurochemistry. 1998;71:1158–1167. doi: 10.1046/j.1471-4159.1998.71031158.x. [DOI] [PubMed] [Google Scholar]
- 63.Azzi M., Nicot A., Gully D., Kitabgi P., Berod A., Rostene W. Increase in neurotensin receptor expression in rat brain induced by chronic treatment with the nonpeptide neurotensin receptor antagonist SR 48692. Neuroscience Letters. 1994;172:97–100. doi: 10.1016/0304-3940(94)90671-8. [DOI] [PubMed] [Google Scholar]
- 64.Najimi M., Robert J.J., Mallet J., Rostene W., Forgez P. Neurotensin induces tyrosine hydroxylase gene activation through nitric oxide and protein kinase C signaling pathways. Molecular Pharmacology. 2002;62:647–653. doi: 10.1124/mol.62.3.647. [DOI] [PubMed] [Google Scholar]
- 65.Liang Y., Boules M., Li Z., Williams K., Miura T., Oliveros A., Richelson E. Hyperactivity of the dopaminergic system in NTS1 and NTS2 null mice. Neuropharmacology. 2010;58:1199–1205. doi: 10.1016/j.neuropharm.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grill H.J. Leptin and the systems neuroscience of meal size control. Frontiers in Neuroendocrinology. 2010;31:61–78. doi: 10.1016/j.yfrne.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kortleven C., Bruneau L.C., Trudeau L.E. Neurotensin inhibits glutamate-mediated synaptic inputs onto ventral tegmental area dopamine neurons through the release of the endocannabinoid 2-AG. Neuropharmacology. 2012;63:983–991. doi: 10.1016/j.neuropharm.2012.07.037. [DOI] [PubMed] [Google Scholar]
- 68.Sarret P., Beaudet A., Vincent J.P., Mazella J. Regional and cellular distribution of low affinity neurotensin receptor mRNA in adult and developing mouse brain. Journal of Comparative Neurology. 1998;394:344–356. [PubMed] [Google Scholar]
- 69.Nouel D., Faure M.P., St, Pierre J.A., Alonso R., Quirion R., Beaudet A. Differential binding profile and internalization process of neurotensin via neuronal and glial receptors. Journal of Neuroscience. 1997;17:1795–1803. doi: 10.1523/JNEUROSCI.17-05-01795.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yamauchi R., Wada E., Kamichi S., Yamada D., Maeno H., Delawary M., Nakazawa T., Yamamoto T., Wada K. Neurotensin type 2 receptor is involved in fear memory in mice. Journal of Neurochemistry. 2007;102:1669–1676. doi: 10.1111/j.1471-4159.2007.04805.x. [DOI] [PubMed] [Google Scholar]
- 71.Furutani N., Hondo M., Kageyama H., Tsujino N., Mieda M., Yanagisawa M., Shioda S., Sakurai T. Neurotensin co-expressed in orexin-producing neurons in the lateral hypothalamus plays an important role in regulation of sleep/wakefulness States. PLoS One. 2013;8:e62391. doi: 10.1371/journal.pone.0062391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Alexiou C., Zimmermann J.P., Schick R.R., Schusdziarra V. Xenin—a novel suppressor of food intake in rats. Brain Research. 1998;800:294–299. doi: 10.1016/s0006-8993(98)00535-6. [DOI] [PubMed] [Google Scholar]
- 73.Hawkins M.F., Barkemeyer C.A., Tulley R.T. Synergistic effects of dopamine agonists and centrally administered neurotensin on feeding. Pharmacology, Biochemistry and Behavior. 1986;24:1195–1201. doi: 10.1016/0091-3057(86)90170-x. [DOI] [PubMed] [Google Scholar]
- 74.Laque A., Zhang Y., Gettys S., Nguyen T.A., Bui K., Morrison C.D., Muenzberg-Gruening H. Leptin receptor neurons in the mouse hypothalamus are co-localized with the neuropeptide galanin and mediate anorexigenic leptin action. American Journal of Physiology—Endocrinologyand Metabolism. 2013 doi: 10.1152/ajpendo.00643.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Data


