Abstract
Aims
We sought to obtain insights into the efficacy of two websites, www.QTdrugs.org and www.BrugadaDrugs.org, that have the intention to prevent fatal arrhythmias due to unsafe drug use in Long QT syndrome and Brugada syndrome.
Methods and results
Prospective web-use statistical analysis combined with online surveys were employed. Our main outcome measure was the percentage of Long QT syndrome patients and Brugada syndrome patients reporting refraining or discontinuation of possible unsafe drugs. QTdrugs.org has received >3 100 000 visitors from 180 countries. Most visitors originated from the Americas (87%), as compared with Europe (7%), Asia (3%), Oceania (2%), and Africa (1%). The QTdrugs.org survey yielded 340 respondents: 34% were patients and 50% medical professionals. Of the patients, 79% reported that they refrained from, and 61% reported discontinuing drugs due to the website. The website was very much appreciated by 65% of the respondents and 30% found it rather helpful. The BrugadaDrugs.org received >48 000 visitors from 154 countries. Most visitors originated from Europe (46%) and the Americas (39%), but less from Asia (10%), Oceania (4%), and Africa (<1%). The BrugadaDrugs.org survey yielded 178 respondents: 68% were patients and 21% medical professionals. Of the patients, 72% reported refraining from, and 48% discontinuing drugs due to the website. The website was very much appreciated by 72% of the respondents and 25% found it rather helpful.
Conclusion
These websites are extensively used, they promote drug awareness, and they help patients to avoid possible pro-arrhythmic drugs. Visitors find the websites valuable but should note their limitations.
Keywords: Long QT syndrome, Brugada syndrome, Cardiac arrest, Drugs, Side effects, website use, website statistics
What's new?
The websites www.QTdrugs.org and www.BrugadaDrugs.org, which provide up-to-date expert information on safe drug use in Long QT syndrome and Brugada syndrome, are heavily used all over the globe.
Importantly, these websites indeed appear to promote drug awareness and by using these websites the large majority of Long QT syndrome patients and Brugada syndrome patients indeed refrains from or discontinues drugs that may be unsafe for them.
Over 95% of both patients and medical professionals using these websites, find them helpful for determining drug safety in Long QT syndrome and Brugada syndrome.
However, it should be clear to the users of these websites that there are important limitations of these websites due to different risk profiles of the patients and (sometimes) scarce data. These limitations should be acknowledged and used for individual decision-making regarding drug prescription for co-morbidities.
Introduction
When individuals under age 40 experience a cardiac arrest, arrhythmia syndromes cause most of these arrests.1 The arrhythmia syndromes include the cardiomyopathies, which are caused by mutations in the genes which encode for the sarcomeric proteins, and the channelopathies, which are caused by mutations in the genes that encode for the cardiac ion channels.2 Importantly, when patients are affected with either of these syndromes, premature death may also occur after 40 years of age. The channelopathies include the Long QT syndrome and the Brugada syndrome, in which life-threatening arrhythmias are often triggered by the use of certain cardiovascular and non-cardiovascular drugs.3–6 This is caused by the ability of these drugs to influence cardiac ion channel function. In patients with Long QT syndrome or Brugada syndrome these drugs may have fatal consequences because these patients already have impaired cardiac ion channel functioning. Importantly, there are also examples of patients with an acquired form of these syndromes as opposed to the congenital or inherited form. These acquired syndromes include patients who use these same particular drugs often in combination with certain co-morbidities such as electrolyte imbalance, extreme bradycardia, or ischaemia.7,8 It is most likely that these patients carry genetic polymorphisms, as opposed to pathogenic mutations, which only critically decrease their safety of conduction or repolarization upon a certain combination of stressors.9
It is clear that it is very important that patients with either Long QT syndrome or Brugada syndrome avoid potentially pro-arrhythmic drugs.2,6,10 Likewise, it is very important that the family members and medical care providers such as physicians, nurses, and pharmacists are familiar with the potential harm associated with these drugs. To address this need, we developed the website www.QTdrugs.org in 2001, and following this example, we developed the website www.BrugadaDrugs.org in 2009. With the launch of these websites we have since provided free and worldwide availability of information on drug safety in these syndromes. Importantly, drug safety in this context relates to the ability of the drug to cause sudden cardiac death or life-threatening arrhythmias, or to cause the intermediate indicator of increased risk for arrhythmias (i.e. excessive QTc prolongation or development of a type-1 Brugada pattern on the electrocardiogram). To be able to provide consensus recommendations from experts in the field, we formed an advisory board for each of the sites. These boards include many highly respected clinical and basic scientists (Tables 1 and 2) to provide consultation and to aid in making decisions regarding the drug lists based on their experience and their interpretation of summaries of the latest evidence acquired and presented by the website teams. In this way we are able to obtain consensus recommendations and make changes to the websites within a few weeks of receiving new data such as after publication of new evidence in the medical literature.
Table 1.
Advisory board of www.QTdrugs.org
| Directors | Raymond L. Woosley and Klaus Romero, USA |
| Board members | Michael J. Ackerman, USA |
| Jeffrey L. Anderson, USA | |
| Charles Antzelevitch, USA | |
| Jean T. Barbey, USA | |
| Günter E. Breithardt, Germany | |
| Pedro Brugada, Belgium | |
| John A. Camm, England | |
| Nabil E. El-Sherif, USA | |
| Michael R. Franz, USA | |
| Christian J. Funck-Brentano, France | |
| Wilhelm Haverkamp, Germany | |
| Stefan H. Hohnloser, Germany | |
| Craig January, USA | |
| Robert S. Kass, USA | |
| Michael J. Kilborn, Australia | |
| Ralph Lazzara, USA | |
| Samuel Lévy, France | |
| Marek Malik, United Kingdom | |
| Eduardo Marbán, USA | |
| Jay W. Mason, USA | |
| Arthur J. Moss, USA | |
| Silvia Priori, Italy | |
| Eric N. Prystowsky, USA | |
| Dan Roden, USA | |
| Jeremy N. Ruskin, USA | |
| Peter J. Schwartz, Italy | |
| Norman Stockbridge, USA | |
| Robert Temple, USA | |
| Douglas Throckmorton, USA | |
| Jeffrey A. Towbin, USA | |
| Victoria L. Vetter, USA | |
| G. Michael Vincent, USA | |
| Markus Zabel, Germany | |
| Douglas P. Zipes, USA |
Table 2.
Advisory Board of www.BrugadaDrugs.org
| Chair | Pieter G. Postema, Netherlands |
| Board members | Ahmad S. Amin, Netherlands |
| Martin Borggrefe, Germany | |
| Josep Brugada, Spain | |
| Masayasu Hiraoka, Japan | |
| Silvia G. Priori, Italy | |
| Vincent Probst, France | |
| Dan M. Roden, USA | |
| Hanno L. Tan, Netherlands | |
| Arthur A.M. Wilde, Netherlands | |
| Christian Wolpert, Germany |
We have not previously published data on the type of audiences that visit these websites and whether they find it valuable. Nor have we determined whether these websites meet their intended use, i.e. to increase drug awareness and/or prevent the use of possible unsafe drugs. Hence, the goal of the present study was to examine the reasons for visitors to use these websites and to assess their perceived value. We also evaluated which aspects of the websites are most appreciated and where the users recommend improvements. Finally, we discuss several limitations of these websites that are important for their safe use.
Methods
To prospectively evaluate the number and geographic origin of visitors, web-use analysis programs were implemented for both websites. For www.QTdrugs.org, Urchin™ has been used since October 2003, and for www.BrugadaDrugs.org, Google Analytics™ has been used since May 2009. Visitor numbers are presented through the universally used qualification ‘unique visitors’. Unique visitors do not identify people, but are defined from the combination of an IP-address (an essential protocol to connect a computer to the internet) and the used internet browser, irrespective of the number of visits to the website from that address. Since IP-addresses are assigned on a geological basis, the origin of visitors (up to the level of cities) is provided to website owners. Although the use of unique visitors prohibits data on a person level and has specific limitations, a more detailed analysis of visitor numbers is not commonly accepted.
To evaluate the experiences of website visitors and to obtain suggestions for improvement, we launched two identical online surveys in November 2011. To encourage visitors to complete the survey, we offered visitors anonymity. An invitation to complete this survey was sent to all registrants who had requested notification of updates to the websites (www.QTdrugs.org: 674 individuals, www.BrugadaDrugs.org: 544 individuals), and a link to the survey was placed on the home pages of both websites. Data collection was interrupted for analysis at the end of March 2012.
Results
www.QTdrugs.org
Visitor statistics
The www.QTdrugs.org website has received >3 100 000 unique visitors from 180 countries since the start of web-use statistics in October 2003 through March 2012 (Figure 1). In the last year, the site averaged 1416 visitors/day. The majority of visitors (>85%) originated from the USA. When segmented by continent, most visitors originated from the Americas (87%) compared with Europe (7%), Asia (3%), Oceania (2%), and Africa (1%).
Figure 1.
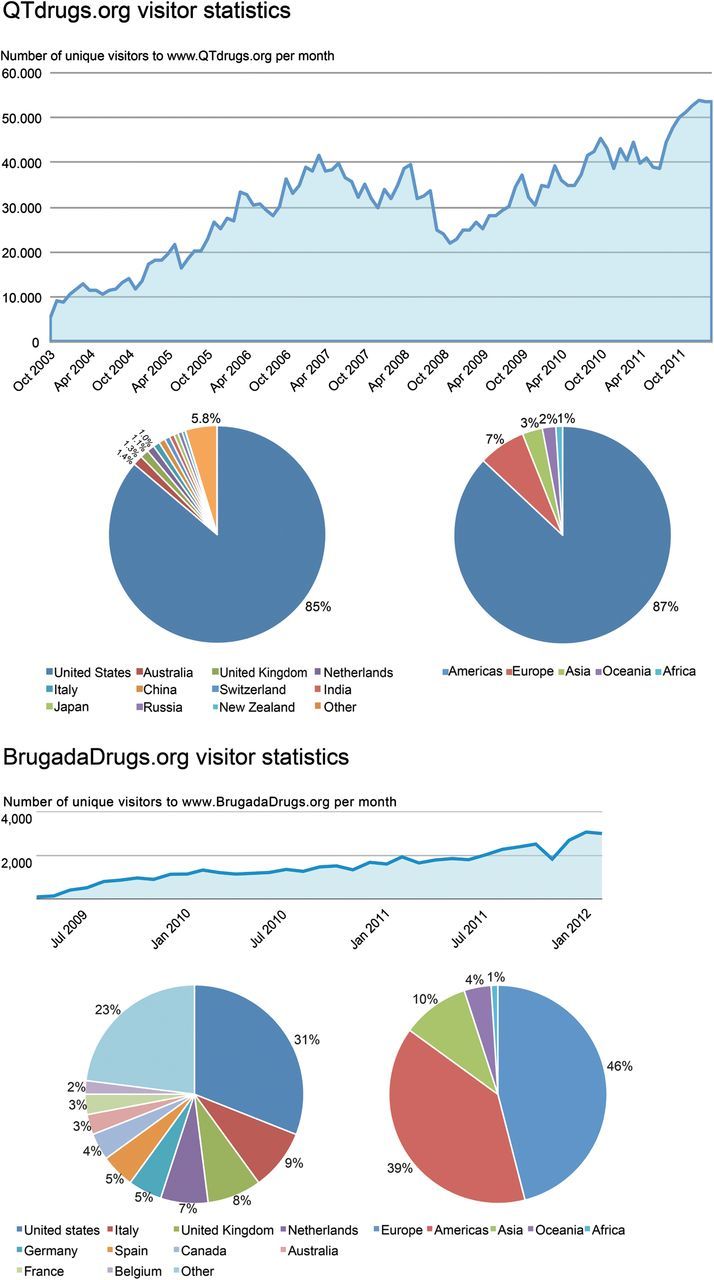
Visitor statistics of www.QTdrugs.org and www.BrugadaDrugs.org. Line graphs: number of unique visitors per month. Pie graphs: origin of these visitors.
Survey
The online survey of visitors to www.QTdrugs.org was completed by 340 respondents (27% of the visitors to the survey page and 0.3% of the visitors in the survey period). Of these respondents, 61% were female, 53% between 40 and 59 years old, 61% originated from the Americas, and 30% from Europe. The majority of respondents (60%) were returning visitors with 22% reported visiting at least every month and 26% visiting every 6 months, while 40% were first time visitors. The largest group of respondents (42%) were medical professionals (physician, pharmacist, or allied health professional), 32% were patients with Long QT syndrome and 10% relatives of someone with Long QT syndrome.
The printable version of the lists with drugs to avoid was used by 56% of the patients with Long QT syndrome who reported giving it to their healthcare providers, while 24% indicated that they knew about the printable lists but did not use them. In addition, 79% of the patients indicated that they have refrained from starting new drugs after visiting the website and 61% indicated that they discontinued drugs after visiting the website. Of note, this probably implies that some patients abandon useful treatments that are innocuous to them. Of the medical professionals, 41% used or referred others to the printable drug lists ((Figure 2).
Figure 2.

Drug awareness for visitors of www.QTdrugs.org and www.BrugadaDrugs.org. Pie graphs: use of the drug lists for medical professionals and patients. Bar graphs: results of drug awareness questions for patients.
Remarks and suggestions for improvement
The website was reported to be very much appreciated by 65% of the respondents, 30% found it rather helpful, while 5% found it not very helpful. In addition, 86% appreciated the separation of the drugs into ‘risk’, ‘possible risk’, and ‘conditional risk’.
One or more positive comments were entered by 43% of the respondents. Some examples of these positive remarks are shown in Table 3. Suggestions for improvement were provided by 22% of the respondents. Most suggestions involved requests for more information on Long QT syndrome (both congenital and acquired Long QT syndrome), greater information on the process used to evaluate evidence supporting the drug lists, shorter format for printed versions, improvements of the website layout, and accessibility on mobile devices. The respondents also encouraged creation of a list of drugs as alternative therapies to the drugs to avoid and to increase awareness among medical professionals.
Table 3.
www.QTdrugs.org—selected responses
| Group | Response |
|---|---|
| Patient | Previously, drugs I was taking induced Long QT, but I have turned my QT level to normal since I refrained from using any drugs on the website. Thank you. |
| Patient | I have had several ER nurses, and a Cardio nurse ask me ‘What is Long QT?’ I take the list to every doctor I visit, since they usually do not know what the condition is or what meds to avoid. Obviously, more education is needed for health care providers. Thank you for allowing me to share your research materials with them. This information might have saved my life! |
| Relative | The website keeps me updated on medications I cannot allow my child to have because of his prolonged QT. |
| Allied professional | I work for a medicines regulatory agency and I use the information on the website to inform some of our regulatory decisions as well as to direct health professionals and consumers to the information it contains. |
| Physician | I'm a cardiologist working in a cancer hospital. Long QT is a side effect of some antineoplastic drugs (in addition to cardiac drugs), and I find it very useful to have links to quick check drug interactions and so on. I've suggested this link to other colleagues in my speeches when I'm requested to talk about cardiovascular effects of anticancer drugs. |
www.BrugadaDrugs.org
Visitor statistics
The BrugadaDrugs.org website has received 60 512 visits from 48 359 unique visitors from 154 countries since its launch in 2009 through March 2012 (Figure 1). In the last year, the website received 25 378 unique visitors, which is an average of 70/day. Most visitors (31%) originated from the USA. When segmented by continent, the majority originated from Europe (46%) followed by the Americas (39%), while the minority originated from Asia (10%), Oceania (4%), and Africa (<1%).
Survey
The online survey of visitors to www.BrugadaDrugs.org was completed by 178 respondents (96% of the visitors to the survey page and 1.4% of the visitors in the survey period). Of these respondents, 44% were female, 62% were between 40 and 59 years old, 65% originated from Europe, and 29% originated from the Americas. The vast majority of respondents were returning visitors with 32% visiting the website at least every month and 49% every 6 months. The largest group of respondents, 68%, were Brugada syndrome patients, while 21% were medical professionals, and 8% were relatives of someone with Brugada syndrome.
A specific feature of the website is the ‘patient letter’. This one-page letter includes all the drugs on the website and is available in 13 languages. It can be downloaded by patients and their relatives and provided to their medical professionals. Of the patients, 73% indicated that they used the patient letter while 19% knew about its existence but had not used it. In addition, 72% of the patients indicated that they refrained from drugs after visiting the website, while 48% indicated that they discontinued drugs after visiting the website. Of the medical professionals, 85% used or referred others to the patient letter (Figure 2).
Remarks and suggestions for improvement
The website was very much appreciated by 72% of the respondents, 25% found it rather helpful and 2% not very helpful. In addition, 100% of the medical professionals appreciated the recommendations provided by the Advisory Board (Class I: convincing evidence/opinion; Class IIa: evidence/opinion less clear; Class IIb: conflicting evidence/opinion; Class III: very little evidence) and the links to the literature underlying this recommendation.
One or more positive remarks were submitted by 46% of the respondents. Some examples are listed in Table 4. Suggestions for improvement were given by 16% of the respondents. Most suggestions were provided by patients or relatives and involved the need for more information on Brugada syndrome, scientific developments, and treatment. Furthermore, respondents encouraged efforts to develop more awareness among medical professionals and asked for the inclusion of a list of safe drugs as alternatives and for a list with brand names of drugs.
Table 4.
www.BrugadaDrugs.org—selected responses
| Group | Response |
|---|---|
| Patient | I was taking an allergy drug daily and had frequent dizzy spells and felt unwell a lot of the time. After reading the information of what to avoid I changed to a different allergy medication and feel much better. No more dizzy spells. My doctors have used your letter in treating me several times and made the necessary changes to avoid complications from Brugada syndrome. |
| Patient | The patient letter has been the most useful for me. I've given it to my doctors and pharmacists. I was first diagnosed because I was taking three of the drugs on the list and running a fever. After a visit to the ER (where I was diagnosed) and a follow up with the cardiologist for confirmation, I'm doing fine. This site has made all the difference for me. It's also been useful for my family to understand my diagnosis. Thank you. |
| Relative | The site allows my family to feel connected and ‘not alone’ with the diagnosis of Brugada syndrome for our 6-year-old son. Also, the site allows me, as a parent, to stay on top of the latest information on contraindicated drugs, and I appreciate the update emails I receive directing me to check the site for updates. |
| Relative | I appreciate all of the information provided regarding medications. I print the patient letter and give it to my son's pediatrician and school nurse every year (unless updated, then I provide it at that time). It has been our experience that many doctors we have worked with are not familiar with Brugada Syndrome. |
| Physician | It's quite reassuring for me to know that I'll be updated about the drugs to avoid. I also appreciate the updating availability for my patients and their relatives affected by Brugada syndrome. |
Discussion
Long QT syndrome and Brugada syndrome are inheritable arrhythmia syndromes associated with sudden cardiac arrest, caused by mutations or variations in the genes encoding for cardiac ion channels and their subunits. The prevalence of both syndromes is estimated to be close to 1 : 2000, but Long QT syndrome may be slightly more prevalent.11,12 The incidence of the acquired forms of these syndromes has not been established. For example, the annual reports of cases of torsades de pointes and cases of QT prolongation received by the Food and Drug Administration are under-estimates and do not include many of the episodes of acquired Long QT syndrome resulting from non-drug causes.
Since both cardiovascular and non-cardiovascular drugs may influence cardiac ion channel function, many drugs can result in life-threatening arrhythmias in patients with Long QT syndrome or Brugada syndrome. To increase awareness and provide up-to-date reliable information on drug safety in these syndromes we developed two websites: www.QTdrugs.org and www.BrugadaDrugs.org. In this paper, we describe the population of visitors to these websites, and why they frequently access these resources. Also we have surveyed visitors to assess whether these websites successfully promote safe medication use in patients with Long QT and Brugada syndrome, and sought suggestions for how these websites can better meet the needs of the visitors.
The available data indicate that these websites are indeed frequently and repeatedly visited. The website that was launched first, www.QTdrugs.org, has already attracted over 3.1 million visitors in the past decade, while the more recently developed website, www.BrugadaDrugs.org, also shows a steady increase of its number of visitors. Most of the visitors to www.QTdrugs.org originate from the USA and appear to be medical professionals. In contrast, www.BrugadaDrugs.org has attracted mostly European visitors who appear to be mainly patients with Brugada syndrome. The basis for these differences is uncertain however.
Efficacy and limitations of the websites
It thus seems that these websites are providing an important service to the community with many medical professionals using these websites to support decisions on how to treat their patients. Also, many patients use the information to make personal decisions about their use of drugs that may be unsafe for them. This, however, also directly points towards several important limitations of these websites. Patients with Long QT syndrome or Brugada syndrome have varying underlying levels of risk of developing arrhythmias when taking drugs. This risk profile can be based on many factors including specific mutation(s), the absence or presence of modulating polymorphisms, gender and age, specific electrolyte imbalances, the absence or presence of fever, kidney and liver function, the absence or presence of (cardiac) co-morbidities, appropriate drug dosage or excessive dosing, the use of (other) drugs, electrocardiogram characteristics, and whether they have previously experienced arrhythmias. Because the evidence supporting the association between specific drugs and these arrhythmias consists mainly of case reports, laboratory studies, and cohort analyses, it is difficult to apply these data directly to an individual patient. Hence, none of the drugs listed on these sites will have the same impact for all patients. Moreover, their possible effects might also change over time as a function of treatment (exposure) duration, age, or developing co-morbidities.8 Therefore, it is not feasible for these websites to replace direct clinical judgement and provide patient-specific advice. To partially address this limitation, both websites have created risk subgroups and/or recommendations to further guide healthcare providers and patients. However, as patients age and develop co-morbidities the chance of conflicts between indications for drugs and possible pro-arrhythmic effects will inevitably rise. The potential negative impact of having drugs listed on these websites may thus be that patients could fail to receive potentially effective treatments, either due to reluctance of a physician to prescribe or due to reluctance of patients themselves. Healthcare providers should be aware that these drug lists have limitations and that the pros and cons of each drug should be carefully weighed for individual patients. Such a thought process relies heavily on risk assessment and stratification, which, unfortunately, can be not only extremely difficult in these syndromes, but is still evolving and sometimes disputed among experts.13–16 Some patients might need hospital admission for monitoring when evaluating the response to new drugs. However, this may not be necessary if the patient has an implanted cardioverter as backup. Importantly, there are even several rare sodium channel mutations that results in a mixed phenotype of Long QT syndrome and Brugada syndrome.17,18 Cognizant of the limited safety evidence in this population, we would advise against drugs from both websites for these patients, and it is evident that decision-making about drugs in these individuals is likely to be even more difficult.
A related limitation of the lists is the frequent lack of evidence for identifying therapeutic alternatives to those on the lists. There are almost no reports of investigations on which drugs could be considered safer alternatives in Long QT or Brugada syndrome patients. Meanwhile, the absence of a given drug in the drug lists does not equal the absence of risk with that particular drug. The lack of literature on the IKr and INa blocking properties of many drugs withholds implementation on the websites and may also introduce publication bias in the case of associated arrhythmias. In contrast, to the best of our knowledge there is only one report for Brugada syndrome discussing the safe use of an anti-epileptic drug in a child with both epilepsy and Brugada syndrome.19 However, since we do not know whether this drug is also safe in other patients with both disease entities, this drug is not included as a safe alternative on the website. Likewise, even if a drug were to be reported as ‘safe’ in patients with Long QT syndrome type-1 or type-2 patients, it would not necessarily mean that it is also safe in Long QT syndrome type-3 or others. The usual recommendation is that all drugs should be used with caution and the treating physician is tasked with determining the definition of caution. Unfortunately for patients, it usually means that medicines are not recommended, except perhaps in treating life-threatening situations. Although these limitations are mentioned on the websites, we would like to advocate that medical professionals take the time to explain these limitations to their patients.
Finally, these websites are only of use to those who are aware of their existence. Inclusion of the websites in the guidelines on the prevention of sudden death in patients with Long QT syndrome and Brugada syndrome,2,20 already helps in this respect. However, physicians and allied professionals who do not work in the field of sudden death are very difficult to reach. Therefore, we urge our patients to actively inform all their medical professionals (including their general practitioner, pharmacist, dentist, and allied professionals) about their conditions and the importance of adequate drug choices (see also Tables 3 and 4). Translation of the websites and the drug letters in many different languages is also important and (being) implemented.
Anti-arrhythmic drugs in Long QT and Brugada syndrome
Importantly, there are also anti-arrhythmic drugs for (congenital) Long QT and Brugada syndrome. For Long QT syndrome beta-blockers have a proven high degree of efficacy, especially for type-1 and in lesser extent for Long QT type-2 and type-3.10,21,22 Mexiletine can also be used in type-3 Long QT,23 while Ranolazine is also considered to be a promising drug in type-3.24 In Brugada syndrome, Quinidine is considered the best oral drug of choice.25–27 On www.BrugadaDrugs.org a special page is devoted to potential anti-arrhythmic drugs.
Implementation of suggestions and future plans
For www.QTdrugs.org the lists are now made available in formats viewable on smartphones and tablets. A link to Twitter and a RSS feed are being added so that visitors can quickly be notified of changes to the site. Additional changes include creation of a patient forum, adding a list of drugs currently under review and evaluation by the website team, adding a list of drugs that have been reviewed but not placed on a list, and creating a list of drugs that shorten the QT interval.
On www.BrugadaDrugs.org the suggestions for improvement were translated to a ‘Frequently asked questions’ page on the website. We also included a page linking brand names with generic names to enable searching for brand names. Furthermore, layout issues were adjusted and links were added to increase the accessibility of information sources and discussion forums.
Limitations of this study
There are several limitations to this study that should be acknowledged. First, to encourage participation, the survey entries were anonymous. This feature, together with the high number of dedicated visitors, has probably resulted in relatively high view rates and completion rates (QTdrugs: 1.2 and 27%, respectively, BrugadaDrugs: 1.5 and 96%, respectively) in comparison with other voluntary online surveys which regularly have view rates of 0.1%.28 However, anonymous entries could also enable unjustified use (e.g. multiple entries). Still, as there is no individual benefit of completing the surveys we do not expect this to be a relevant source of bias. Furthermore, inspection of the surveys did not reveal duplicated (i.e. identical) entries. Second, a survey question inquiring whether the visitor was registered on the website was not included, which could imply that predominantly enthusiastic visitors completed the survey. This latter issue is also mirrored in the higher than average view and completion rates. Third, we did not inquire as to which drugs the visitors discontinued as a result of consulting the websites. Fourth, the distribution of the origin of visitors and respondents was different between the websites and not at all evenly distributed around the world. This precludes conclusions about the usefulness of these websites for many regions in the world. Finally, it was not feasible for a survey like the one utilized here to investigate whether the use of these websites actually prevented the development of life-threatening arrhythmias in patients. Instead, this work focused on whether patients reported that they refrained from, or discontinued, possible pro-arrhythmic drugs.
Conclusions
The websites www.QTdrugs.org and www.BrugadaDrugs.org were developed to supply up-to-date and freely available information that would promote safe drug use in patients with Long QT syndrome and Brugada syndrome, based on the latest evidence and on consensus opinions from experts. It appears from the results that these websites are heavily used, that they indeed promote drug awareness and that they appear to prevent patients from being exposed to potentially pro-arrhythmic drugs. Still, there are important limitations to what these websites can accomplish. More effective programmes to promote awareness and continuing improvement of the websites are warranted.
Funding
www.QTdrugs.org: This website is supported by an award from the Agency for Healthcare Research and Quality for the Arizona Center for Education and Research on Therapeutics (AZ CERT) (award number U18 HS010385). www.BrugadaDrugs.org: This website is supported by the Cardionetworks Foundation (www.cardionetworks.org), a non-profit organization that aims to improve access to medical knowledge.
Acknowledgements
The authors would like to express their thanks to all the contributors to these websites and to the respondents to the surveys. In addition, these websites cannot exist without the continuing support of the members of our Advisory Boards, to whom we are extremely grateful.
Conflict of interest: The authors have no commercial interests in these websites and provide this service without compensation. Both AZCERT and the Cardionetworks Foundation are a non-profit organization created to accept charitable contributions that pay for the costs associated with maintaining the website. J.N., K.R., and R.L.W. contribute their time to the development and maintenance of the QTdrugs.org website while P.G.P., J.S.S.G.de.J. and A.A.M.W. are representatives for the development and maintenance of the BrugadaDrugs.org website.
References
- 1.Ackerman MJ, Priori SG, Willems S, Berul C, Brugada R, Calkins H, et al. HRS/EHRA expert consensus statement on the state of genetic testing for the channelopathies and cardiomyopathies: this document was developed as a partnership between the Heart Rhythm Society (HRS) and the European Heart Rhythm Association (EHRA) Europace. 2011;13:1077–109. doi: 10.1093/europace/eur245. [DOI] [PubMed] [Google Scholar]
- 2.Ackerman MJ, Priori SG, Willems S, Berul C, Brugada R, Calkins H, et al. HRS/EHRA Expert consensus statement on the state of genetic testing for the channelopathies and cardiomyopathies. Heart Rhythm. 2011;8:1308–39. doi: 10.1016/j.hrthm.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 3.De Bruin ML, Langendijk PN, Koopmans RP, Wilde AA, Leufkens HG, Hoes AW. In-hospital cardiac arrest is associated with use of non-antiarrhythmic QTc-prolonging drugs. Br J Clin Pharmacol. 2007;63:216–23. doi: 10.1111/j.1365-2125.2006.02722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sicouri S, Antzelevitch C. Sudden cardiac death secondary to antidepressant and antipsychotic drugs. Expert Opin Drug Saf. 2008;7:181–94. doi: 10.1517/14740338.7.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Straus SM, Sturkenboom MC, Bleumink GS, Dieleman JP, Van der LJ, De Graeff PA, et al. Non-cardiac QTc-prolonging drugs and the risk of sudden cardiac death. Eur Heart J. 2005;26:2007–12. doi: 10.1093/eurheartj/ehi312. [DOI] [PubMed] [Google Scholar]
- 6.Postema PG, Wolpert C, Amin AS, Probst V, Borggrefe M, Roden DM, et al. Drugs and Brugada syndrome patients: review of the literature, recommendations, and an up-to-date. Heart Rhythm. 2009;6:1335–41. doi: 10.1016/j.hrthm.2009.07.002. website (www.brugadadrugs.org. ) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sze E, Moss AJ, Goldenberg I, McNitt S, Jons C, Zareba W, et al. Long QT syndrome in patients over 40 years of age: increased risk for LQTS-related cardiac events in patients with coronary disease. Ann Noninvasive Electrocardiol. 2008;13:327–31. doi: 10.1111/j.1542-474X.2008.00250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Viskin S, Rosso R, Marquez MF, Antzelevitch C. The acquired Brugada syndrome and the paradox of choice. Heart Rhythm. 2009;6:1342–4. doi: 10.1016/j.hrthm.2009.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kolder ICRM, Tanck MWT, Bezzina CR. Common genetic variation modulating cardiac ECG parameters and susceptibility to sudden cardiac death. J Mol Cell Cardiol. 2012;52:620–9. doi: 10.1016/j.yjmcc.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 10.Vincent GM, Schwartz PJ, Denjoy I, Swan H, Bithell C, Spazzolini C, et al. High efficacy of beta-blockers in Long-QT syndrome type 1. Contribution of noncompliance and QT-prolonging drugs to the occurrence of beta-blocker treatment ‘failures. Circulation. 2008;119:215–21. doi: 10.1161/CIRCULATIONAHA.108.772533. [DOI] [PubMed] [Google Scholar]
- 11.Schwartz PJ, Stramba-Badiale M, Crotti L, Pedrazzini M, Besana A, Bosi G, et al. Prevalence of the congenital long-QT syndrome. Circulation. 2009;120:1761–7. doi: 10.1161/CIRCULATIONAHA.109.863209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Postema PG. About Brugada syndrome and its prevalence. Europace. 2012;14:925–8. doi: 10.1093/europace/eus042. [DOI] [PubMed] [Google Scholar]
- 13.Amin AS, Giudicessi JR, Tijsen AJ, Spanjaart AM, Reckman YJ, Klemens CA, et al. Variants in the 3′ untranslated region of the KCNQ1-encoded Kv7.1 potassium channel modify disease severity in patients with type 1 long QT syndrome in an allele-specific manner. Eur Heart J. 2012;33:714–23. doi: 10.1093/eurheartj/ehr473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barsheshet A, Goldenberg I, O-Uchi J, Moss AJ, Jons C, Shimizu W, et al. Mutations in cytoplasmic loops of the KCNQ1 channel and the risk of life-threatening events: implications for mutation-specific response to β-blocker therapy in type 1 Long-QT syndrome. Circulation. 2012;125:1988–96. doi: 10.1161/CIRCULATIONAHA.111.048041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilde AA, Viskin S. EP testing does not predict cardiac events in Brugada syndrome. Heart Rhythm. 2011;8:1598–600. doi: 10.1016/j.hrthm.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 16.Brugada J, Brugada R, Brugada P. Electrophysiologic testing predicts events in Brugada syndrome patients. Heart Rhythm. 2011;8:1595–7. doi: 10.1016/j.hrthm.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 17.Makita N, Behr E, Shimizu W, Horie M, Sunami A, Crotti L, et al. The E1784K mutation in SCN5A is associated with mixed clinical phenotype of type 3 long QT syndrome. J Clin Invest. 2008;118:2219–29. doi: 10.1172/JCI34057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Postema PG, Van den Berg MP, Van Tintelen JP, Van den Heuvel F, Grundeken M, Hofman N, et al. Founder mutations in the Netherlands. SCN5a 1795insD, the first described arrhythmia overlap syndrome and one of the largest and best described characterised families worldwide. Neth Heart J. 2009;17:422–8. doi: 10.1007/BF03086296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Gorp V, Danschutter D, Huyghens L, Hachimi-Idrissi S, Sarkozy A, Chierchia GB, et al. Monitoring the safety of antiepileptic medication in a child with Brugada syndrome. Int J Cardiol. 2010;145:e64–7. doi: 10.1016/j.ijcard.2008.12.156. [DOI] [PubMed] [Google Scholar]
- 20.Zipes DP, Camm AJ, Borggrefe M, Buxton AE, Chaitman B, Fromer M, et al. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death) developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Europace. 2006;8:746–837. doi: 10.1093/europace/eul108. [DOI] [PubMed] [Google Scholar]
- 21.Goldenberg I, Bradley J, Moss A, McNitt S, Polonsky S, Robinson JL, et al. Beta-blocker efficacy in high-risk patients with the congenital Long-QT syndrome types 1 and 2: implications for patient management. J Cardiovasc Electrophysiol. 2010;21:893–901. doi: 10.1111/j.1540-8167.2010.01737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilde AA, Kaufman ES, Shimizu W, Moss AJ, Benhorin J, Lopes CM, et al. Sodium channel mutations, risk of cardiac events, and efficacy of beta-blocker therapy in type 3 Long QT syndrome (abstract) Heart Rhythm. 2012;9:S321. [Google Scholar]
- 23.Valdivia CR, Ackerman MJ, Tester DJ, Wada T, McCormack J, Ye B, et al. A novel SCN5A arrhythmia mutation, M1766L, with expression defect rescued by mexiletine. Cardiovasc Res. 2002;55:279–89. doi: 10.1016/s0008-6363(02)00445-5. [DOI] [PubMed] [Google Scholar]
- 24.Moss AJ, Zareba W, Schwarz KQ, Rosero S, McNitt S, Robinson JL. Ranolazine shortens repolarization in patients with sustained inward sodium current due to type-3 Long-QT syndrome. J Cardiovasc Electrophysiol. 2008;19:1289–93. doi: 10.1111/j.1540-8167.2008.01246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Viskin S, Wilde AA, Tan HL, Antzelevitch C, Shimizu W, Belhassen B. Empiric quinidine therapy for asymptomatic Brugada syndrome: time for a prospective registry. Heart Rhythm. 2009;6:401–4. doi: 10.1016/j.hrthm.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Belhassen B, Glick A, Viskin S. Excellent long-term reproducibility of the electrophysiologic efficacy of quinidine in patients with idiopathic ventricular fibrillation and Brugada syndrome. Pacing Clin Electrophysiol. 2009;32:294–301. doi: 10.1111/j.1540-8159.2008.02235.x. [DOI] [PubMed] [Google Scholar]
- 27.Mizusawa Y, Sakurada H, Nishizaki M, Hiraoka M. Effects of low-dose quinidine on ventricular tachyarrhythmias in patients with Brugada syndrome: low-dose quinidine therapy as an adjunctive treatment. J Cardiovasc Pharmacol. 2006;47:359–64. doi: 10.1097/01.fjc.0000206437.27854.65. [DOI] [PubMed] [Google Scholar]
- 28.Eysenbach G. Improving the quality of Web surveys: the Checklist for Reporting Results of Internet E-Surveys (CHERRIES) J Med Internet Res. 2004;6:e34. doi: 10.2196/jmir.6.3.e34. [DOI] [PMC free article] [PubMed] [Google Scholar]


