Abstract
The BODE index was developed as a prognostic mortality risk tool for persons with chronic obstructive pulmonary disease (COPD). It incorporates 4 measures: body mass index, lung obstruction, dyspnea, and exercise capacity. The intent of this study was to examine how well a BODE-like index constructed using a simpler lung function measure, peak expiratory flow, in combination with physical functioning and symptom information more readily found in survey data (a quasi-BODE index), performs in identifying persons at higher risk of mortality and whether it may be extended as an assessment of mortality risk to persons without diagnosed COPD. Using US national survey data from the Health Retirement Study for 2006–2010, each unit increase in the quasi-BODE index score was associated with a multiplicative 50% increase in mortality risk (odds ratio = 1.50, 95% confidence interval: 1.41, 1.59). The quasi-BODE index is a multidimensional health status instrument based on the BODE index, which is a good predictor of mortality. The quasi-BODE index was compiled using simple measures of physical and respiratory function. It is a potentially useful prognostic instrument for older adult populations with or without COPD, including those with severe physical limitations, particularly when combined with demographic factors and comorbid conditions.
Keywords: chronic disease; dyspnea; frail elderly; health status indicators; lung diseases, obstructive; mortality; respiratory function tests; survival
Heart disease, stroke, cancer, chronic obstructive pulmonary disease (COPD), and diabetes were leading causes of death in the United States for persons aged 45 years or older in 2006 (1). These chronic illnesses are known to pose increased risks for mortality, but health status is multidimensional and is influenced through diverse processes. There is broad interest in measurable factors beyond chronic disease that provide information about mortality risk, recognizing that measures of risk are population-based and may not be applicable on an individual basis. Limitations in physical functioning, which tend to precede disability, could provide additional information about future health status (2–4).
Disease assessments and screening instruments that consider multiple factors may be particularly useful for identifying persons at risk of adverse outcomes (5–10). For example, systemic consequences of comorbid conditions associated with COPD have been recognized as affecting health status, morbidity, and mortality (11–15). Another recognized dynamic in older adults is frailty, which has been found to be independently predictive of adverse outcomes (9). Frail individuals, who are defined as having 3 or more frailty criteria—unintentional weight loss (>10 pounds (>4.5 kg) in the past year), self-reported exhaustion, weakness (low grip strength), slow walking speed, and low physical activity—are at increased risk of falls, worsening morbidity, Activities of Daily Living disability, hospitalization, and death (9).
One aspect of physical health not often considered in populations without respiratory disease is lung function. Limitations in lung function are among those physiological measures that have been associated with decreased physical capacity, poorer health status, and reduced survival (16–22). Poor lung function has been shown to be both an indicator of impaired health and a predictor of mortality in the elderly, not only for those with respiratory disease but also for those with cardiovascular disease and diabetes (23–26). Dyspnea (difficulty breathing or shortness of breath) is associated not only with asthma and COPD but also with cardiovascular diseases such as coronary artery disease and congestive heart failure. Intuitively, dyspnea results in reduced ability to exercise, and persons with poor lung function may adopt sedentary lifestyles (27). Physical inactivity, in turn, is known to be associated with increased risk of mortality and morbidity (28, 29). Finally, cachexia, which results in loss of muscle mass and diminished imunological response, is frequently associated with increased risk of mortality (30, 31). The BODE index incorporates these 4 characteristics into 1 scored value to assess mortality risk using the following measures: body mass index (BMI), lung obstruction, dyspnea, and exercise capacity (7).
The BODE index was originally developed as a prognostic tool for persons with COPD; its usefulness for persons without COPD has not been examined to date. The BODE index requires BMI (weight (kg)/height (m)2), degree of airflow obstruction measured by means of spirometry (percentage of predicted forced expiratory volume in 1 second (FEV1)), dyspnea as assessed by the modified Medical Research Council dyspnea scale, and exercise capacity as determined by a 6-minute walk test (7). However, only BMI and dyspnea measures are easily obtained. Formal spirometry requires subjects to exhale with maximum effort for at least 6 seconds, which can be very difficult for many elderly or debilitated persons. In community surveys of older adults, the proportion of individuals unable to perform spirometry has ranged from 12% to 17% (32, 33). The 6-minute walk test requires a 100-foot (30.5-m) hallway and measures the distance a person can walk quickly in 6 minutes on a hard, flat surface (34). The BODE index is useful as an assessment of health status and risk for poor outcomes but is impractical for debilitated persons. In one prospective study of survival, 26% of the original cohort of 327 COPD participants either could not complete the 6-minute walk test (64 participants) or could not perform adequate spirometry (20 participants) (35).
Our objective was to construct a quasi-BODE index using a simpler lung function measure, peak expiratory flow (PEF), as well as information more readily found in survey data such as standardized assessments of physical functioning and symptoms. PEF, although not as commonly used as FEV1 as a health status measure, can be a more practical physiological measure to obtain from elderly persons (36–38). Percentage of predicted PEF has been shown to be a predictor of survival among older adults in the general population (10, 18, 24) and in adults with diabetes (25). Others have found significant correlations between percentage of predicted PEF and measures of physical and cognitive function in the elderly (39).
The intent of this study was to examine how well the quasi-BODE index, constructed using proxy measures, performs in identifying persons at higher risk of mortality and in measuring mortality risk not only among persons with COPD but also among those without COPD. We also examined the value of adding other prognostic measures to the quasi-BODE index, including mental health, demographic factors, and parental longevity.
MATERIALS AND METHODS
Cohort and data collection
Source data came from the Health Retirement Study (HRS), in which a biennial survey is administered to stratified samples of US residents over the age of 50 years. The HRS, initiated in 1992, is an ongoing study sponsored by the National Institute on Aging and conducted by investigators at the University of Michigan. Surveys are conducted on a 2-year cycle. Significant survey changes were made in 2002. Physical measurements (e.g., lung function) were added in 2004. Data for this project consist of the publicly available deidentified HRS data.
The HRS data collected for each participant include mortality information, measurements of BMI, physical functioning (e.g., ability to exercise), mobility, strength, and fine motor skills, and questionnaire items about coughing or wheezing and shortness of breath (details are provided in the Web Appendix, available at http://aje.oxfordjournals.org/). A PEF test was added in 2004 to measure lung function for a small sample of participants and was expanded in 2006 and 2008. No HRS physical measures are exactly the same as the 6-minute walk test used to calculate the BODE index, but available measures can reasonably be expected to serve as self-reported equivalents to a physical function domain for a quasi-BODE mortality risk measure. The HRS includes a battery of questionnaires for participants about diagnosed chronic diseases and associated symptoms; family history; self-assessment of general health status and expectations about longevity; physical, cognitive, and affective functioning measures; demographic characteristics; living arrangements; and economic resources. HRS information on mortality comes from the National Death Index, plus interviews with family members.
Development of quasi-BODE index
In constructing the quasi-BODE index, we strove to approximate the original BODE index as closely as possible (Table 1). HRS data included BMI information. The original BODE index utilized FEV1 categories similar to American Thoracic Society definitions (7). We substituted percentage of predicted PEF using a regression equation for predicted PEF developed from a population of persons with “normal” lung function (i.e., no COPD, no history of smoking, and no coughing/wheezing/shortness-of-breath symptoms reported) within HRS respondents in the 2006 and 2008 surveys (40, 41). Full details of that analysis are provided elsewhere (42). Predicted PEF values were calculated for men and women separately and factored in age (years; continuous variable), height, and race/ethnicity (42, 43).
Table 1.
Elements of the BODE Indexa as Originally Constructed
| Measure | Scale Points |
|---|---|
| Body mass indexb | |
| >21 | 0 |
| ≤21 | 1 |
| FEV1, %c | |
| ≥65 | 0 |
| 50–64 | 1 |
| 36–49 | 2 |
| ≤35 | 3 |
| mMRC dyspnea scale | |
| 0–1 | 0 |
| 2 | 1 |
| 3 | 2 |
| 4 | 3 |
| 6-minute walking distance, m | |
| ≥350 | 0 |
| 250–349 | 1 |
| 150–249 | 2 |
| ≤149 | 3 |
Abbreviations: FEV1, forced expiratory volume in 1 second; mMRC, modified Medical Research Council.
a The BODE index incorporates 4 characteristics into 1 scored value to assess mortality risk using the following measures: body mass index, lung obstruction, dyspnea, and exercise capacity (7).
b Weight (kg)/height (m)2.
c Percentage of predicted FEV1 value.
The dyspnea scale used in the BODE index captured a dyspnea range from “not troubled with breathlessness except with strenuous exercise” to “too breathless to leave the house or breathless when dressing or undressing” (44). We evaluated shortness of breath and wheezing/cough as proxy measures. While the BODE index included 6-minute walk test distance, we substituted difficulties in mobility and strength with walking 1 block and lifting more than 10 pounds (>4.5 kg).
Elements included in the original BODE index had first been chosen because each was independently associated with poorer health status, adverse outcomes, and increased risk of death. The elements included had demonstrated the strongest association with mortality using logistic regression. Each of the potential quasi-BODE index elements from the HRS (BMI ≤21, categories of percentage of predicted PEF, shortness of breath, wheezing/cough, and mobility and strength difficulties) was similarly evaluated independently as a predictor of mortality using logistic regression models and receiver operating characteristic curves. Points were associated with the quasi-BODE elements based on the magnitude of the odds ratio for mortality (1 point: odds ratio (OR) = 1.0–2.4; 2 points: OR = 2.5–5.0; 3 points: OR >5.0).
After constructing the quasi-BODE index, its use as a prognostic factor was tested in logistic regression models for mortality in the 2-year postsurvey period, adjusting for patient demographic and morbidity characteristics. Additional models, in which various comorbid conditions were added to the quasi-BODE index, were constructed to determine whether they contributed additional value to the area under the receiver operating characteristic curve (C statistic). The study population consisted of persons aged 50 years or older with sensitivity analyses conducted for the subpopulation aged 65 years or older. Stratified analyses were conducted for populations with and without COPD because the BODE index was originally constructed using a COPD population. Odds ratios and associated 95% confidence intervals were estimated for risk factors, and the C statistic was used to gauge the predictive power of the models.
SAS statistical software (SAS Institute, Inc., Cary, North Carolina) was used to perform the analyses. All P values are 2-sided with a significance level of 0.05.
RESULTS
Complete information on demographic factors, comorbid conditions, BMI, respiratory symptoms, percentage of predicted PEF, physical functioning limitations, and depression symptoms was available for 12,501 participants (Table 2). Of these, 673 (5.4%) died during the 2 years following the HRS survey. Smoking history was incomplete for persons who were current smokers or who had smoked in the past, and missing values were imputed using mean study population values for age at starting smoking, age at stopping smoking, and packs per day (details given in Web Appendix).
Table 2.
Characteristics of Health Retirement Study National Survey Respondents Over Age 50 Years by Occurrence of Death Within 2 Years Postsurvey, United States, 2006 and 2008
| % of Total (n = 12,501) | % Who Died in Next 2 Years (n = 673) | % Who Remained Alive in Next 2 Years (n = 11,828) | P Value | |
|---|---|---|---|---|
| Age category, years | ||||
| 51–64 | 33.6 | 9.5 | 35.0 | <0.0001 |
| 65–74 | 37.3 | 29.3 | 37.8 | <0.0001 |
| 75–84 | 22.0 | 34.0 | 21.3 | <0.0001 |
| ≥85 | 7.1 | 27.2 | 6.0 | <0.0001 |
| Male sex | 42.1 | 49.6 | 41.7 | <0.0001 |
| Parental longevitya | ||||
| Mother lived to age ≥70 years | 69.5 | 64.6 | 69.7 | 0.008 |
| Father lived to age ≥70 years | 56.5 | 55.6 | 56.5 | 0.15 |
| Mother lived to age ≥75 years | 60.6 | 55.3 | 60.9 | <0.0001 |
| Father lived to age ≥75 years | 44.3 | 42.1 | 44.5 | <0.0001 |
| Race/ethnicity | ||||
| Non-Hispanic white | 76.8 | 78.0 | 76.8 | 0.45 |
| Hispanic | 8.5 | 7.1 | 8.5 | 0.20 |
| Black | 13.3 | 14.1 | 13.2 | 0.50 |
| Other | 1.5 | 0.7 | 1.5 | 0.11 |
| Body mass indexb <21 | 5.3 | 12.8 | 4.8 | <0.0001 |
| Comorbid conditions | ||||
| Diabetes | 21.5 | 35.4 | 20.7 | <0.0001 |
| Cancer | 15.6 | 27.8 | 14.9 | <0.0001 |
| COPD | 10.6 | 22.4 | 10.0 | <0.0001 |
| Heart disease | 26.5 | 50.1 | 25.1 | <0.0001 |
| Stroke | 6.8 | 15.3 | 6.3 | <0.0001 |
| No diabetes/cancer/COPD/ heart disease/stroke | 46.1 | 18.9 | 47.6 | <0.0001 |
| High blood pressure | 59.5 | 68.4 | 59.0 | <0.0001 |
| Arthritis | 63.2 | 69.4 | 62.8 | 0.0006 |
| Respiratory function | ||||
| Persistent cough/wheeze | 18.0 | 26.4 | 17.5 | <0.0001 |
| Shortness of breath | 19.8 | 34.2 | 19.0 | <0.0001 |
| Persistent cough or shortness of breath | 28.8 | 44.1 | 27.9 | <0.0001 |
| Oxygen use | 1.9 | 8.8 | 1.5 | <0.0001 |
| % of predicted PEF | ||||
| ≥65 | 85.9 | 67.3 | 87.0 | <0.0001 |
| 50–64 | 7.9 | 13.5 | 7.6 | <0.0001 |
| 36–49 | 4.4 | 12.3 | 4.0 | <0.0001 |
| 0–35 | 1.8 | 6.8 | 1.5 | <0.0001 |
| Limitations with physical functions | ||||
| Walking 1 block | 14.6 | 40.3 | 13.1 | <0.0001 |
| Lifting or carrying weights >10 pounds (>4.5 kg) | 24.1 | 49.2 | 22.6 | <0.0001 |
| Reaching/extending arms above shoulder level | 16.0 | 26.2 | 15.5 | <0.0001 |
| Use of health-care services | ||||
| Overnight stay in hospital in last 2 years | 27.3 | 51.3 | 25.9 | <0.0001 |
| Visit to a physician in last 2 years | 90.9 | 88.0 | 91.0 | 0.06 |
| Third quartile of number of physician visits in last 2 years | 24.3 | 37.6 | 23.5 | <0.0001 |
| Evidence of depressionc | 13.8 | 22.6 | 13.3 | <0.0001 |
| Smoking status | ||||
| Previous smoker | 56.7 | 66.3 | 56.2 | <0.0001 |
| Current smoker | 12.6 | 16.0 | 12.4 | 0.007 |
| Pack-years of smoking | ||||
| Persons with information | 56.5 | 66.3 | 56.0 | <0.0001 |
| <11 | 14.7 | 11.6 | 14.9 | 0.02 |
| 11–<26 | 13.5 | 13.1 | 13.6 | 0.70 |
| 26–≤40 | 14.6 | 19.0 | 14.4 | 0.001 |
| >40 | 13.7 | 22.6 | 13.2 | <0.0001 |
Abbreviations: COPD, chronic obstructive pulmonary disease; PEF, peak expiratory flow.
a Age if alive at the time of the survey or age at death.
b Weight (kg)/height (m)2.
c Score >3 on the 8-item Center for Epidemiologic Studies Depression Scale.
As expected, persons who died during the next 2 years were older than those who did not die (61.2% vs. 27.3% were aged ≥75 years; P < 0.0001) and were more likely to have chronic diseases (80% of those who died had at least 1 of the chronic conditions as compared with slightly more than 50% of survivors). Specifically, they were more likely to have heart disease (50.1% vs. 25.1%; P < 0.0001), diabetes (35.4% vs. 20.7%; P < 0.0001), cancer (27.8% vs. 14.9%; P < 0.0001), or COPD (22.4% vs. 10.0%; P < 0.0001) or to have had a stroke (15.3% vs. 6.3%; P < 0.0001). Among those aged ≥65 years, there was a reduced percentage difference for persons with cancer (27.4% vs. 18.4%; P < 0.0001), but percentage differences were similar for the remaining chronic conditions (data not shown). Persons who died in the next 2 years were also much more likely to have a BMI less than 21 (12.8% vs. 4.8%; P < 0.0001), a percentage of predicted PEF of 49% or less (19.2% vs. 5.5%; P < 0.0001), shortness of breath (34.2% vs. 19.0%; P < 0.0001), and difficulty with walking 1 block (40.3% vs. 13.1%; P < 0.0001) or lifting/carrying more than 10 pounds (>4.5 kg) (49.2% vs. 22.6%; P < 0.0001). Persons who died were more likely to have been hospitalized in the 2 years prior to the survey (51.3% vs. 25.9%; P < 0.0001) or to have had evidence of depression (22.6% vs. 13.3%; P < 0.0001). Race/ethnicity characteristics were not associated with mortality in this cohort, but maternal longevity was significantly associated. Persons whose mothers lived to be aged 75 years or older also had better survival (60.9% vs. 55.3%; P < 0.01).
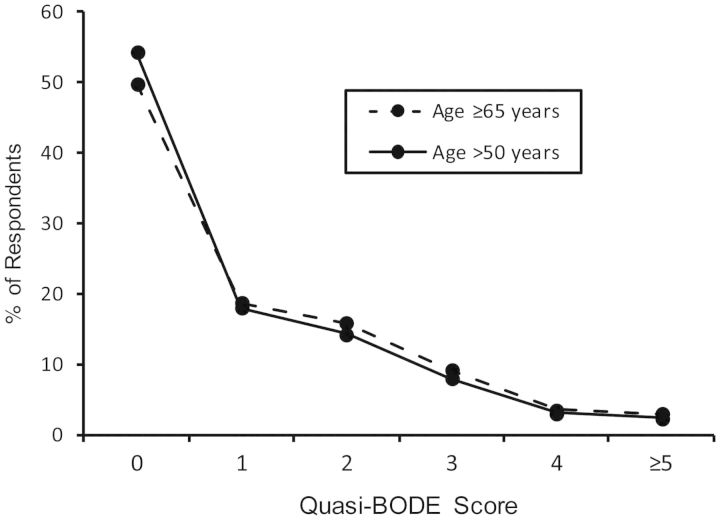
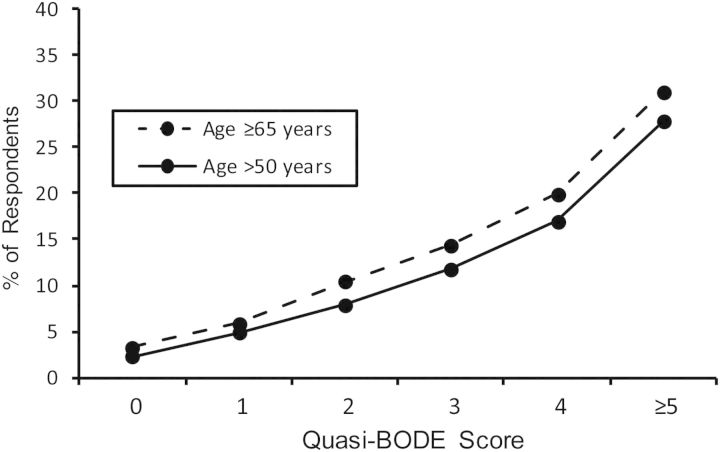
Table 3 shows specific quasi-BODE index elements and how they compare with the original BODE index. Unadjusted odds ratios for mortality risk are also shown in Table 3. The maximum possible value for the original BODE index is 10, and that for the quasi-BODE index is 8. Quasi-BODE index scores greater than 5 applied to only 1% of the population; these were truncated to 5 in our analyses. Figure 1 shows the quasi-BODE index scores for the study population and the subpopulation aged 65 years or older. Figure 2 provides a summary of the percentage within each quasi-BODE index score who died during the 2 years postsurvey. Each 1-unit increase in the quasi-BODE index was associated with a multiplicative 50% increase in mortality risk (OR = 1.50, 95% CI: 1.41, 1.59).
Table 3.
Odds Ratio Associated With Risk of Mortality in the Subsequent 2 Years According to Elements of the Quasi-BODE Index Among Health Retirement Study National Survey Respondents Over Age 50 Years, United States, 2006 and 2008
| Health Retirement Study Measure | Scale Points | Risk of Mortality in Next 2 Years |
|
|---|---|---|---|
| Odds Ratio | 95% Confidence Interval | ||
| Body mass indexa | |||
| >21 | 0 | 1 | Reference |
| ≤21 | 2 | 2.9 | 2.3, 3.7 |
| % of predicted PEF | |||
| ≥65 | 0 | 1 | Reference |
| 50–64 | 1 | 2.2 | 1.8, 2.6 |
| 36–49 | 2 | 4.5 | 3.5, 5.6 |
| ≤35 | 3 | 6.7 | 4.8, 9.5 |
| Shortness of breath | |||
| No shortness of breath | 0 | 1 | Reference |
| Shortness of breath | 1 | 2.2 | 1.9, 2.6 |
| Difficulty with mobility and strengthb | |||
| No difficulty | 0 | 1 | Reference |
| Lifting or carrying >10 pounds (>4.5 kg) | 1 | 2.1 | 1.7, 2.5 |
| Walking 1 block | 2 | 3.1 | 2.6, 3.7 |
Abbreviation: PEF, peak expiratory flow.
a Weight (kg)/height (m)2.
b Points are associated with either difficulty with walking 1 block or difficulty with lifting/carrying >10 pounds (>4.5 kg), whichever has the higher number of points for the individual.
Figure 1.
Percentages of respondents over age 50 years (n = 12,501) and aged 65 years or older (n = 8,301) by quasi-BODE index score, Health Retirement Study national surveys, United States, 2006 and 2008.
Figure 2.
Percentages of respondents over age 50 years (n = 12,501) and aged 65 years or older (n = 8,301) within each quasi-BODE index score who died within 2 years postsurvey, Health Retirement Study national surveys, United States, 2006 and 2008.
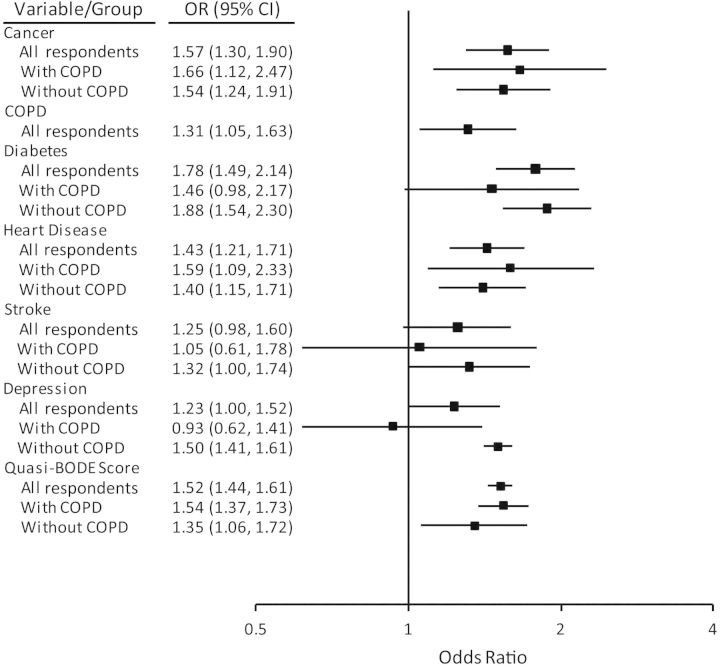
Figure 3 shows the sex- and age-adjusted odds ratio estimates for the quasi-BODE index compared with other morbidity factors considered in the adjusted regression equation. To demonstrate the influence of these factors in populations with COPD (wCOPD) and populations without COPD (woCOPD), stratified estimates for these populations are shown in addition to estimates for the entire study population. Odds ratio estimates for persons aged 65 years or older were similar (data not shown). Regression models were constructed with and without the quasi-BODE index. Inclusion of the quasi-BODE index served to attenuate odds ratios associated with heart disease and stroke in both the wCOPD and woCOPD populations; odds ratios for diabetes were slightly reduced (see Web Tables 1–3).
Figure 3.
Adjusted odds ratio (OR) for risk of mortality within 2 years postsurvey among all respondents over age 50 years (n = 12,501), respondents with chronic obstructive pulmonary disease (COPD) (n = 1,328), and respondents without COPD (n = 11,173), Health Retirement Study national surveys, United States, 2006 and 2008. Odds ratios for a 1-unit increase in quasi-BODE score were estimated separately by group (all respondents, respondents with COPD, and respondents without COPD); odds ratios were also adjusted for sex and age (not shown). Bars, 95% confidence interval (CI).
Possible C statistic values range from 0.0 to 1.0; values of 0.70 or higher are generally considered acceptable, with values of 0.80 or higher considered strong (45). The C statistic values for final models ranged from 0.76 (wCOPD) to 0.82 (woCOPD and all respondents). In a model including only the quasi-BODE index as a factor, the C statistic was 0.72 for all respondents and 0.70 for the wCOPD and woCOPD populations. Adding age and sex to these models increased the C statistic to 0.80 for all respondents and to 0.74 for the wCOPD populations and 0.79 for the woCOPD populations. Further adding morbidity information increased the C statistic by only 0.02 (Web Tables 1–3).
Models of chronic morbidity without the quasi-BODE index, but with age and sex, had C statistics ranging from 0.70 (wCOPD) to 0.80 (woCOPD). Adding the quasi-BODE index increased the value to 0.76 for the wCOPD population model and to 0.82 for the woCOPD and all-respondents models.
Table 4 summarizes characteristics of persons grouped by quasi-BODE index scores. For the total study population, the majority had scores of 0–1 (72.3%), with 22.2% having scores of 2–3 and 5.5% having scores of 4 or higher. The distribution was similar among persons aged 65 years or older (0–1: 68.5%; 2–3: 24.9%; ≥4: 6.5%). Very few persons with COPD (5.9%) or stroke (4.6%) had quasi-BODE index scores of 0 or 1. Persons without any chronic conditions of interest (diabetes, cancer, COPD, heart disease, or stroke) comprised approximately one-half of the 0–1 group, one-third of the 2–3 group, and one-fifth of the ≥4 group. A greater percentage of persons with mothers who lived to age 75 years or over was found in the 0–1 score group (61.8% vs. 53.0% in the ≥4 score group; P < 0.0001).
Table 4.
Characteristics of Health Retirement Study National Survey Respondents Over Age 50 Years by Quasi-BODE Score, United States, 2006 and 2008
| Factor | % With Quasi-BODE Score 0–1 (n = 9,040) | % With Quasi-BODE Score 2–3 (n = 2,776) | P Value (2–3 vs. 0–1)a | % With Quasi-BODE Score ≥4 (n = 685) | P Value (≥4 vs. 0–1)b | P Value (≥4 vs. 2–3)c |
|---|---|---|---|---|---|---|
| Age category, years | ||||||
| 51–64 | 37.1 | 25.4 | <0.0001 | 21.0 | <0.0001 | 0.01 |
| 65–74 | 38.5 | 33.8 | <0.0001 | 35.3 | 0.10 | 0.02 |
| 75–84 | 19.6 | 28.1 | <0.0001 | 29.3 | <0.0001 | 0.45 |
| ≥85 | 4.8 | 12.8 | <0.0001 | 14.3 | <0.0001 | 0.51 |
| Male sex | 45.3 | 32.7 | <0.0001 | 38.4 | 0.0004 | 0.005 |
| Parental longevityd | ||||||
| Mother lived to age ≥70 years | 70.6 | 67.5 | <0.0001 | 62.6 | <0.0001 | 0.04 |
| Father lived to age ≥70 years | 56.9 | 55.3 | 0.005 | 56.5 | 0.53 | 0.03 |
| Mother lived to age ≥75 years | 61.8 | 58.4 | <0.0001 | 53.0 | <0.0001 | 0.03 |
| Father lived to age ≥75 years | 45 | 42.4 | 0.001 | 43.1 | 0.47 | 0.34 |
| Race/ethnicity | ||||||
| Non-Hispanic white | 78.3 | 72.1 | <0.0001 | 76.4 | 0.23 | 0.03 |
| Hispanic | 8.5 | 8.9 | 0.54 | 5.7 | 0.01 | 0.006 |
| Black | 11.8 | 17.2 | <0.0001 | 16.8 | 0.0001 | 0.79 |
| Other | 1.4 | 1.8 | 0.16 | 1.2 | 0.62 | 0.27 |
| Body mass indexe ≤21 | 0.0 | 15.6 | 0.01 | 32.7 | <0.0001 | |
| Comorbid conditions | ||||||
| Diabetes | 19.0 | 28.0 | <0.0001 | 27.3 | <0.0001 | 0.70 |
| Cancer | 14.5 | 18.0 | <0.0001 | 20.6 | <0.0001 | 0.12 |
| COPD | 5.9 | 18.3 | <0.0001 | 41.8 | <0.0001 | <0.0001 |
| Heart disease | 21.4 | 38.5 | <0.0001 | 45.4 | <0.0001 | 0.001 |
| Stroke | 4.6 | 11.4 | <0.0001 | 16.9 | <0.0001 | <0.0001 |
| No diabetes/cancer/COPD/ heart disease/stroke | 52.4 | 32.2 | <0.0001 | 19.1 | <0.0001 | <0.0001 |
| High blood pressure | 56.6 | 67.1 | <0.0001 | 66.7 | <0.0001 | 0.88 |
| Arthritis | 58.6 | 75.4 | <0.0001 | 73.6 | <0.0001 | 0.31 |
| Respiratory function | ||||||
| Persistent cough/wheeze | 12.6 | 27.6 | <0.0001 | 49.5 | <0.0001 | <0.0001 |
| Shortness of breath | 9.4 | 41.9 | <0.0001 | 67.9 | <0.0001 | <0.0001 |
| Persistent cough or shortness of breath | 18.6 | 50.9 | <0.0001 | 74.3 | <0.0001 | <0.0001 |
| Oxygen use | 0.3 | 3.5 | <0.0001 | 16.6 | <0.0001 | <0.0001 |
| % of predicted PEF | ||||||
| ≥65 | 95.4 | 73.3 | <0.0001 | 11.1 | <0.0001 | <0.0001 |
| 50–64 | 4.6 | 14.2 | <0.0001 | 26.4 | <0.0001 | <0.0001 |
| 36–49 | 0.0 | 10.7 | <0.0001 | 37.7 | <0.0001 | <0.0001 |
| 0–35 | 0.0 | 1.8 | <0.0001 | 24.8 | <0.0001 | <0.0001 |
| Limitations with physical functions | ||||||
| Walking 1 block | 0.0 | 48.9 | <0.0001 | 68.2 | <0.0001 | <0.0001 |
| Lifting or carrying weights >10 pounds (>4.5 kg) | 10.9 | 55.2 | <0.0001 | 71.4 | <0.0001 | <0.0001 |
| Reaching/extending arms above shoulder level | 10.0 | 30.0 | <0.0001 | 38.5 | <0.0001 | <0.0001 |
| Use of health-care services | ||||||
| Overnight stay in hospital in last 2 years | 22.0 | 38.2 | <0.0001 | 48.6 | <0.0001 | <0.0001 |
| Visit to a physician in last 2 years | 91.7 | 87.0 | 0.04 | 84.7 | 0.11 | 0.61 |
| Third quartile of number of physician visits in last 2 years | 20.5 | 31.8 | <0.0001 | 38.7 | <0.0001 | <0.0001 |
| Evidence of depressionf | 8.8 | 25.6 | <0.0001 | 32.6 | <0.0001 | 0.0003 |
| Smoking status | ||||||
| Previous smoker | 54.7 | 59.8 | <0.0001 | 71.8 | <0.0001 | <0.0001 |
| Current smoker | 10.4 | 15.6 | <0.0001 | 28.8 | <0.0001 | <0.0001 |
| Pack-years of smoking | ||||||
| Persons with information | 54.5 | 59.5 | <0.0001 | 71.5 | <0.0001 | <0.0001 |
| <11 | 15.6 | 12.9 | 0.0005 | 9.9 | <0.0001 | <0.0001 |
| 11–<26 | 13.8 | 13.3 | 0.48 | 11.2 | <0.0001 | <0.0001 |
| 26–≤40 | 13.7 | 16.1 | 0.0009 | 21.2 | <0.0001 | 0.002 |
| >40 | 11.4 | 17.2 | <0.0001 | 29.2 | <0.0001 | <0.0001 |
Abbreviations: COPD, chronic obstructive pulmonary disease; PEF, peak expiratory flow.
a Quasi-BODE score 2–3 compared with quasi-BODE score 0–1.
b Quasi-BODE score ≥4 compared with quasi-BODE score 0–1.
c Quasi-BODE score ≥4 compared with quasi-BODE score 2–3.
d Age if alive at the time of the survey or age at death.
e Weight (kg)/height (m)2.
f Score >3 on the 8-item Center for Epidemiologic Studies Depression Scale.
Depression was strongly associated with quasi-BODE index scores. Persons who were highly depressed per the 8-item Center for Epidemiologic Studies Depression Scale questionnaire (rating ≥4) comprised 8.8% of those with a quasi-BODE index score of 0–1, 25.6% of those with a score of 2–3, and 32.6% of those with a score of 4 or higher. Findings were similar when the analysis was limited to those aged 65 years or older (0–1: 7.7%; 2–3: 23.1%; ≥4: 31.4%).
Since we had to impute aspects of smoking history, we limited its use to sensitivity analyses. In models that included smoking history as an additional factor, 26–40 pack-years and 40 or more pack-years were significantly associated with an increased risk of mortality, and the estimated odds ratio for the quasi-BODE index was unchanged in these models.
DISCUSSION
We developed a quasi-BODE index that was strongly predictive of mortality using survey items about chronic respiratory symptoms and physical limitations, BMI, and a simple measure of respiratory function. The index was predictive when used alone and also when used in conjunction with sex, age, and other morbidity information. This health status instrument was predictive not only among persons with COPD but also among older persons without COPD. Percentage of predicted PEF was substituted for percentage of predicted FEV1 in this quasi-BODE score, allowing a test that can be more practically administered to older adults, especially those with severe physical limitations.
There is strong appeal for a simple multidimensional assessment tool that correlates well with future prognosis. Since its development in 2004, the BODE index has been demonstrated in many studies to be a more accurate predictor of mortality among patients with COPD than lung function alone and also a predictor of severe exacerbations of COPD resulting in hospitalization (46–50). The BODE index is a stronger predictor of survival among COPD patients than are respiratory health status questionnaires such as the Clinical COPD Questionnaire (51) and the St. George Respiratory Questionnaire (52), which do not include objective physical measurements in their assessments. Other investigators have also examined modified BODE instruments with various proxy measures substituted for the 6-minute walk test, including peak oxygen uptake (53), a shuttle walk test (54), and history of COPD exacerbations (50). The advantages of the quasi-BODE index as compared with the original BODE index and these adapted BODE indices are that 1) it can be administered without any form of exercise challenge, 2) the equipment needed to administer a PEF test is much less expensive and easier to use than spirometry to obtain FEV1 or exercise cycle ergometry to obtain peak oxygen uptake, and 3) it does not require that the patient have a history of COPD. The C statistic for the quasi-BODE index among COPD patients without additional factors for demographic characteristics or comorbid conditions (0.70) was within the range of those reported in prospective COPD cohort studies with mortality as an endpoint (0.67–0.75) (7, 50, 53, 55).
A recent study demonstrated that combining the BODE index with certain prognostically significant comorbid conditions improved the index's predictive accuracy for mortality in persons with COPD (56). Our study also demonstrated that several chronic illnesses and symptoms were associated with increased mortality in this broader population, with depression being notable among them. While prior studies have shown simple correlations between the BODE index and depression scores in COPD (57–60), we have demonstrated that depression is an important prognostic determinant of survival in a broader population even after adjustment for the quasi-BODE index and other comorbidity.
Although we cannot find other studies that have applied the modified or original BODE index to a non-COPD population, it is not surprising that this multidimensional health-status assessment tool works as well in the general adult population as it does in persons who report having been diagnosed with COPD. As in the original BODE index, each element in our quasi-BODE index is independently associated with a higher risk of mortality. Very low BMI has long been recognized as a poor prognostic factor in the general population (61), and the physical status measures in the BODE index are similar to characteristics associated with frailty, which is associated with an increased risk of death (9). Percentage of predicted PEF has been shown to be a predictor of survival in adults with diabetes (25) and in older adults in the general population without COPD (10, 18, 24).
Our study was not without limitations. Chronic condition status was based on self-report, which may have resulted in misclassification errors due to recall bias and underreporting or overreporting, with well-defined diseases (e.g., diabetes) having less error. Physical function and mental health assessments were only recorded at 1 time point and were also subject to self-report bias. Subjective assessment of health, however, cannot be easily separated from objective assessments and is an important factor when assessing future survival (62). Multicenter studies of longer duration have demonstrated wide variability in BODE performance among different populations and with different follow-up times (55). Another multicenter study found that the BODE index calibration (the absolute risk predicted by the score as compared with the observed risk in another population) was poor but could be improved by refitting the BODE scores (63). Validation of the quasi-BODE score requires replication of this work in other older adult populations, and its utility needs to be established in direct comparisons with other prognostic tools that have been used in populations with a high prevalence of chronic disease (7, 55, 56).
The HRS is a valuable research resource for studying the experience of the elderly in the United States A recent study that highlighted characteristics of the “oldest old” HRS participants compared those who lived to age 97 years with those who did not in the cohort born between 1900 and 1911. The study demonstrated that exceptionally long-lived participants tended to have fewer chronic conditions (0.85 morbid conditions as compared with 1.30; P < 0.001), greater mobility, lower scores on the depression scale (1.55 vs. 2.03; P = 0.002), and no history of smoking (72% vs. 56%; P < 0.001), although there was a large portion who were old and in poor health.
Among the exceptional survivors, women were found to have a greater number of functional limitations compared with men (2.6 vs. 1.6; P = 0.005), and a larger percentage were found to have a BMI less than 18.5 (19% compared with 4.6%; P = 0.007). Of interest is that hereditary factors were found to potentially play a role in improved survival: 31.7% of those who were exceptional survivors had a mother who lived to be aged 85 years or older as compared with 23.9% of those who were not (P = 0.01), and 21.9% compared with 16.7% had a father who lived to be aged 85 years or older (P = 0.06). In our analysis, greater maternal longevity was also associated with reduced risk of mortality.
In conclusion, we have developed a multidimensional health-status instrument based on the BODE index that is a good predictor of mortality but is more practical than the original BODE index because it uses much simpler measures of physical and respiratory function. This tool could potentially be useful in older adult populations with or without COPD, including persons with severe physical limitations. We have also identified several demographic factors and comorbid conditions that, when assessed along with the BODE index, can serve as a very strong yet efficient prognostic instrument.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Health Services Research Division, LCF Research, Albuquerque, New Mexico (Melissa H. Roberts, Douglas W. Mapel); Chronic Obstructive Pulmonary Disease Program, Lovelace Respiratory Research Institute, Albuquerque, New Mexico (Shannon Bruse, Hans Petersen, Toru Nyunoya); and Department of Medicine, New Mexico Veterans Administration Health Care System/University of New Mexico, Albuquerque, New Mexico (Toru Nyunoya).
The Health Retirement Study is sponsored by the National Institute on Aging (grant NIA U01AG009740). No financial support was received for the current analysis.
Conflict of interest: none declared.
REFERENCES
- 1.National Heart, Lung, and Blood Institute. Morbidity and Mortality: 2009 Chart Book on Cardiovascular, Lung, and Blood Diseases. Bethesda, MD: National Heart, Lung, and Blood Institute; 2009. http://www.nhlbi.nih.gov/resources/docs/2009_ChartBook.pdf. (Accessed October 17, 2011) [Google Scholar]
- 2.Martin LG, Schoeni RF, Andreski PM. Trends in health of older adults in the United States: past, present, future. Demography. 2010;47(suppl):S17–S40. doi: 10.1353/dem.2010.0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freedman VA, Martin LG. Contribution of chronic conditions to aggregate changes in old-age functioning. Am J Public Health. 2000;90(11):1755–1760. doi: 10.2105/ajph.90.11.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robine JM, Michel JP. Looking forward to a general theory on population aging. J Gerontol A Biol Sci Med Sci. 2004;59(6):590–597. doi: 10.1093/gerona/59.6.m590. [DOI] [PubMed] [Google Scholar]
- 5.Papaioannou AI, Loukides S, Gourgoulianis KI, et al. Global assessment of the COPD patient: time to look beyond FEV1? Respir Med. 2009;103(5):650–660. doi: 10.1016/j.rmed.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Moore WC, Meyers DA, Wenzel SE, et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med. 2010;181(4):315–323. doi: 10.1164/rccm.200906-0896OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(10):1005–1012. doi: 10.1056/NEJMoa021322. [DOI] [PubMed] [Google Scholar]
- 8.Norra C. PW01–71—a common comorbidity: how to deal with depression in chronic heart failure? [abstract] Eur Psychiatry. 2010;25:1487. [Google Scholar]
- 9.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 10.Cook NR, Evans DA, Scherr PA, et al. Peak expiratory flow rate and 5-year mortality in an elderly population. Am J Epidemiol. 1991;133(8):784–794. doi: 10.1093/oxfordjournals.aje.a115957. [DOI] [PubMed] [Google Scholar]
- 11.Maclay JD, McAllister DA, Macnee W. Cardiovascular risk in chronic obstructive pulmonary disease. Respirology. 2007;12(5):634–641. doi: 10.1111/j.1440-1843.2007.01136.x. [DOI] [PubMed] [Google Scholar]
- 12.Decramer M, De Benedetto F, Del Ponte A, et al. Systemic effects of COPD. Respir Med. 2005;99(suppl B):S3–S10. doi: 10.1016/j.rmed.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 13.Decramer M, Rennard S, Troosters T, et al. COPD as a lung disease with systemic consequences—clinical impact, mechanisms, and potential for early intervention. COPD. 2008;5(4):235–256. doi: 10.1080/15412550802237531. [DOI] [PubMed] [Google Scholar]
- 14.Cosio BG, Agusti A. Update in chronic obstructive pulmonary disease 2009. Am J Respir Crit Care Med. 2010;181(7):655–660. doi: 10.1164/rccm.201001-0111UP. [DOI] [PubMed] [Google Scholar]
- 15.McGarvey LP, John M, Anderson JA, et al. Ascertainment of cause-specific mortality in COPD: operations of the TORCH Clinical Endpoint Committee. Thorax. 2007;62(5):411–415. doi: 10.1136/thx.2006.072348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller MR, Pedersen OF, Lange P, et al. Improved survival prediction from lung function data in a large population sample. Respir Med. 2009;103(3):442–448. doi: 10.1016/j.rmed.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 17.Eisner MD, Iribarren C, Yelin EH, et al. Pulmonary function and the risk of functional limitation in chronic obstructive pulmonary disease. Am J Epidemiol. 2008;167(9):1090–1101. doi: 10.1093/aje/kwn025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fragoso CAV, Gahbauer EA, Van Ness PH, et al. Peak expiratory flow as a predictor of subsequent disability and death in community-living older persons. J Am Geriatr Soc. 2008;56(6):1014–1020. doi: 10.1111/j.1532-5415.2008.01687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Young RP, Hopkins R, Eaton TE. Forced expiratory volume in one second: not just a lung function test but a marker of premature death from all causes. Eur Respir J. 2007;30(4):616–622. doi: 10.1183/09031936.00021707. [DOI] [PubMed] [Google Scholar]
- 20.Sin DD, Wu L, Man SF. The relationship between reduced lung function and cardiovascular mortality: a population-based study and a systematic review of the literature. Chest. 2005;127(6):1952–1959. doi: 10.1378/chest.127.6.1952. [DOI] [PubMed] [Google Scholar]
- 21.Coultas DB, Mapel D, Gagnon R, et al. The health impact of undiagnosed airflow obstruction in a national sample of United States adults. Am J Respir Crit Care Med. 2001;164(3):372–377. doi: 10.1164/ajrccm.164.3.2004029. [DOI] [PubMed] [Google Scholar]
- 22.Schunemann HJ, Dorn J, Grant BJ, et al. Pulmonary function is a long-term predictor of mortality in the general population: 29-year follow-up of the Buffalo Health Study. Chest. 2000;118(3):656–664. doi: 10.1378/chest.118.3.656. [DOI] [PubMed] [Google Scholar]
- 23.Hole DJ, Watt GC, Davey-Smith G, et al. Impaired lung function and mortality risk in men and women: findings from the Renfrew and Paisley prospective population study. BMJ. 1996;313(7059):711–715. doi: 10.1136/bmj.313.7059.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tilvis R, Valvanne J, Sairanen S, et al. Peak expiratory flow is a prognostic indicator in elderly people. BMJ. 1997;314(7080):605–606. [PMC free article] [PubMed] [Google Scholar]
- 25.Klein BE, Moss SE, Klein R, et al. Peak expiratory flow rate: relationship to risk variables and mortality: the Wisconsin Epidemiologic Study of Diabetic Retinopathy. Diabetes Care. 2001;24(11):1967–1971. doi: 10.2337/diacare.24.11.1967. [DOI] [PubMed] [Google Scholar]
- 26.Klein OL, Krishnan JA, Glick S, et al. Systematic review of the association between lung function and type 2 diabetes mellitus. Diabet Med. 2010;27(9):977–987. doi: 10.1111/j.1464-5491.2010.03073.x. [DOI] [PubMed] [Google Scholar]
- 27.Gardener EA, Huppert FA, Guralnik JM, et al. Middle-aged and mobility-limited: prevalence of disability and symptom attributions in a national survey. J Gen Intern Med. 2006;21(10):1091–1096. doi: 10.1111/j.1525-1497.2006.00564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brach JS, Simonsick EM, Kritchevsky S, et al. The association between physical function and lifestyle activity and exercise in the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2004;52(4):502–509. doi: 10.1111/j.1532-5415.2004.52154.x. [DOI] [PubMed] [Google Scholar]
- 29.Larson EB, Bruce RA. Exercise and aging [editorial] Ann Intern Med. 1986;105(5):783–785. doi: 10.7326/0003-4819-105-5-783. [DOI] [PubMed] [Google Scholar]
- 30.Wallace JI, Schwartz RS. Epidemiology of weight loss in humans with special reference to wasting in the elderly. Int J Cardiol. 2002;85(1):15–21. doi: 10.1016/s0167-5273(02)00246-2. [DOI] [PubMed] [Google Scholar]
- 31.Lang PO, Michel JP, Zekry D. Frailty syndrome: a transitional state in a dynamic process. Gerontology. 2009;55(5):539–549. doi: 10.1159/000211949. [DOI] [PubMed] [Google Scholar]
- 32.Bellia V, Pistelli R, Catalano F, et al. Quality control of spirometry in the elderly— the SA.R.A. study. Am J Respir Crit Care Med. 2000;161(4):1094–1100. doi: 10.1164/ajrccm.161.4.9810093. [DOI] [PubMed] [Google Scholar]
- 33.Hegewald MJ, Lefor MJ, Jensen RL, et al. Peak expiratory flow is not a quality indicator for spirometry: peak expiratory flow variability and FEV1 are poorly correlated in an elderly population. Chest. 2007;131(5):1494–1499. doi: 10.1378/chest.06-2707. [DOI] [PubMed] [Google Scholar]
- 34.Crapo RO, Casaburi R, Coates AL, et al. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 35.Ko FW, Tam W, Tung AH, et al. A longitudinal study of serial BODE indices in predicting mortality and readmissions for COPD. Respir Med. 2011;105(2):266–273. doi: 10.1016/j.rmed.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 36.Bellia V, Pistelli F, Giannini D, et al. Questionnaires, spirometry and PEF monitoring in epidemiological studies on elderly respiratory patients. Eur Respir J. 2003;21(suppl 40):21s–27s. doi: 10.1183/09031936.03.00402303. [DOI] [PubMed] [Google Scholar]
- 37.Vaz Fragoso CA, Gahbauer EA, Van Ness PH, et al. Reporting peak expiratory flow in older persons. J Gerontol A Biol Sci Med Sci. 2007;62(10):1147–1151. doi: 10.1093/gerona/62.10.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klein R, Klein BE, Knudtson MD. Frailty and age-related macular degeneration: the Beaver Dam Eye Study. Am J Ophthalmol. 2005;140(1):129–131. doi: 10.1016/j.ajo.2004.12.049. [DOI] [PubMed] [Google Scholar]
- 39.Cook NR, Albert MS, Berkman LF, et al. Interrelationships of peak expiratory flow-rate with physical and cognitive function in the elderly: MacArthur Foundation studies of aging. J Gerontol A Biol Sci Med Sci. 1995;50(6):M317–M323. doi: 10.1093/gerona/50a.6.m317. [DOI] [PubMed] [Google Scholar]
- 40.Nunn AJ, Gregg I. New regression equations for predicting peak expiratory flow in adults. BMJ. 1989;298(6680):1068–1070. doi: 10.1136/bmj.298.6680.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Quanjer PH, Lebowitz MD, Gregg I, et al. Peak expiratory flow: conclusions and recommendations of a Working Party of the European Respiratory Society. Eur Respir J Suppl. 1997;24:2S–8S. [PubMed] [Google Scholar]
- 42.Roberts MH, Mapel DW. Limited lung function: impact of reduced peak expiratory flow on health status, health-care utilization, and expected survival in older adults. Am J Epidemiol. 2012;176(2):127–134. doi: 10.1093/aje/kwr503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gillum RF. Chronic obstructive pulmonary disease in blacks and whites: pulmonary function norms and risk factors. J Natl Med Assoc. 1991;83(5):393–401. [PMC free article] [PubMed] [Google Scholar]
- 44.Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest. 1988;93(3):580–586. doi: 10.1378/chest.93.3.580. [DOI] [PubMed] [Google Scholar]
- 45.Hosmer D, Lemeshow S. Applied Logistic Regression. 2nd ed. New York, NY: John Wiley & Sons, Inc; 2000. [Google Scholar]
- 46.Cote CG, Dordelly LJ, Celli BR. Impact of COPD exacerbations on patient-centered outcomes. Chest. 2007;131(3):696–704. doi: 10.1378/chest.06-1610. [DOI] [PubMed] [Google Scholar]
- 47.Marin JM, Carrizo SJ, Casanova C, et al. Prediction of risk of COPD exacerbations by the BODE index. Respir Med. 2009;103(3):373–378. doi: 10.1016/j.rmed.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 48.McKellar A, Cottrell WN, Whelan A. BODE score is a useful predictor of hospital admission in rural patients with chronic obstructive pulmonary disease. Respirology. 2008;13(3):438–443. doi: 10.1111/j.1440-1843.2007.01169.x. [DOI] [PubMed] [Google Scholar]
- 49.Ong KC, Earnest A, Lu SJ. A multidimensional grading system (BODE index) as predictor of hospitalization for COPD. Chest. 2005;128(6):3810–3816. doi: 10.1378/chest.128.6.3810. [DOI] [PubMed] [Google Scholar]
- 50.Soler-Cataluna JJ, Martinez-Garcia MA, Sanchez LS, et al. Severe exacerbations and BODE index: two independent risk factors for death in male COPD patients. Respir Med. 2009;103(5):692–699. doi: 10.1016/j.rmed.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 51.Sundh J, Janson C, Lisspers K, et al. Clinical COPD Questionnaire score (CCQ) and mortality. Int J Chron Obstruct Pulmon Dis. 2012;7:833–842. doi: 10.2147/COPD.S38119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marin JM, Cote CG, Diaz O, et al. Prognostic assessment in COPD: health related quality of life and the BODE index. Respir Med. 2011;105(6):916–921. doi: 10.1016/j.rmed.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 53.Cote CG, Pinto-Plata VM, Marin JM, et al. The modified BODE index: validation with mortality in COPD. Eur Respir J. 2008;32(5):1269–1274. doi: 10.1183/09031936.00138507. [DOI] [PubMed] [Google Scholar]
- 54.Williams JE, Green RH, Warrington V, et al. Development of the i-BODE: validation of the incremental shuttle walking test within the BODE index. Respir Med. 2012;106(3):390–396. doi: 10.1016/j.rmed.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 55.Marin JM, Alfageme I, Almagro P, et al. Multicomponent indices to predict survival in COPD: The COllaborative COhorts to assess multicomponent indices of COPD in Spain-COCOMICS study. Eur Respir J. [published online ahead of print December 6, 2012]. ( doi:10.1183/09031936.00121012) [Google Scholar]
- 56.Divo M, Cote C, de Torres JP, et al. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186(2):155–161. doi: 10.1164/rccm.201201-0034OC. [DOI] [PubMed] [Google Scholar]
- 57.Al-Shair K, Dockry R, Mallia-Milanes B, et al. Depression and its relationship with poor exercise capacity, BODE index and muscle wasting in COPD. Respir Med. 2009;103(10):1572–1579. doi: 10.1016/j.rmed.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 58.An L, Lin YX, Yang T, et al. Predictive validity of BODE index for anxious and depressive symptoms in patients with chronic obstructive pulmonary disease. Chin Med J (Engl) 2010;123(14):1845–1851. [PubMed] [Google Scholar]
- 59.Farkas J, Kosnik M, Zaletel-Kragelj L, et al. Distribution of self-rated health and association with clinical parameters in patients with chronic obstructive pulmonary disease. Wien Klin Wochenschr. 2009;121(9-10):297–302. doi: 10.1007/s00508-009-1170-2. [DOI] [PubMed] [Google Scholar]
- 60.Funk GC, Kirchheiner K, Burghuber OC, et al. BODE index versus GOLD classification for explaining anxious and depressive symptoms in patients with COPD—a cross-sectional study. Respir Res. 2009;10(1):1. doi: 10.1186/1465-9921-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Simopoulos AP, Van Itallie TB. Body weight, health, and longevity. Ann Intern Med. 1984;100(2):285–295. doi: 10.7326/0003-4819-100-2-285. [DOI] [PubMed] [Google Scholar]
- 62.Hurd MD, McGarry K. The predictive validity of subjective probabilities of survival. Econ J. 2002;112(482):966–985. [Google Scholar]
- 63.Puhan MA, Garcia-Aymerich J, Frey M, et al. Expansion of the prognostic assessment of patients with chronic obstructive pulmonary disease: the updated BODE index and the ADO index. Lancet. 2009;374(9691):704–711. doi: 10.1016/S0140-6736(09)61301-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.