Abstract
Neuroreceptor imaging in the nonhuman primate (NHP) is valuable for translational research approaches in humans. However, the majority of NHP studies are conducted under anesthesia, which affects the interpretability of receptor binding measures. The aims of this study are to develop awake NHP imaging with minimal head restraint and to compare in vivo binding of GABAA-benzodiazepine radiotracer [11C]flumazenil under anesthetized and awake conditions. We hypothesized that [11C]flumazenil binding potential (BPND) would be higher in isoflurane-anesthetized monkeys.
Methods
The Focus-220 small animal PET scanner was fitted to a mechanical device that raised and tilted the scanner 45° while the awake NHP was tilted back 35° in a custom chair for optimal brain positioning. This required acclimation of the animals to the chair, touch-screen tasks, i.v. catheter insertion, and tilting. For PET studies, the bolus plus constant infusion (B/I) method was used for [11C]flumazenil administration. Two rhesus monkeys were scanned under the awake (n=6 scans) and isoflurane-anesthetized (n=4 scans) conditions. The Vicra infrared camera was used to track head motion during PET scans. Under the awake condition, emission and head motion-tracking data were acquired for 40-75 min post-injection. Anesthetized monkeys were scanned for 90 min. Cortisol measurements were acquired during awake and anesthetized scans. Equilibrium analysis was used for both the anesthetized (n=4) and awake (n=5) datasets to compute mean BPND images in NHP template space, using the pons as a reference region. Percent change per min (%Δ/min) in radioactivity concentration was calculated in high and low binding regions to assess the quality of equilibrium.
Results
The monkeys acclimated to procedures in the NHP chair necessary to perform awake PET imaging. Image quality was comparable between awake and anesthetized conditions. The relationship between awake and anesthetized values was BPND(awake)=0.94BPND(anesthetized)+0.36, r2=0.95. Cortisol levels were significantly higher under the awake condition (p<0.05).
Conclusions
We successfully performed awake NHP imaging with minimal head restraint. There was close agreement in [11C]flumazenil BPND values between awake and anesthetized conditions.
Keywords: PET, monkey, conscious, flumazenil, isoflurane, GABA shift, cortisol
Introduction
Neuroreceptor imaging in nonhuman primates (NHP) with positron emission tomography (PET) has been a pivotal translational approach to understanding pharmacological effects on neurotransmitter systems related to neuropsychiatric illness such as Parkinson's disease, dementia, and substance abuse (1-3). However, a limitation of this approach is that human PET studies are performed in the conscious state, whereas the vast majority of NHP PET studies are conducted under anesthesia. While anesthesia helps control head motion, agents such as isoflurane have been shown to affect neuroreceptor outcome measures by increasing endogenous neurotransmitters or altering receptor availability (4).
PET imaging has been performed successfully in conscious NHPs with the use of an acrylic cap surgically implanted onto the skull, as a means of head restraint, by Tsukada and others. This approach limits the applicability of awake NHP imaging by requiring an invasive surgical procedure, and potentially increasing animal stress during imaging. For each scan, the implant is fixed to the monkey chair after temporary sedation, a process that can potentially affect receptor binding measures.
We propose a novel awake imaging approach using minimal head restraint to permit a wide-range of head movement during the scan. The approach is non-invasive, less restrictive, and completely eliminates the use of anesthesia. To correct for the rapid NHP head motion, we have adapted the Vicra motion-correction technology used in human studies in the high-resolution research tomograph (HRRT) (5) to awake NHP imaging in the Focus-220 small animal PET scanner. The animals are trained in a customized NHP chair for acclimation to the PET scan environment, prior to scanning, thus obviating the need for anesthesia.
The aim of this study was to develop an appropriate methodology to execute awake NHP PET brain imaging with minimal head restraint. The Focus-220 scanner was fitted to a mechanical device that raised and tilted the scanner while the awake NHP was tilted back in the chair for optimal brain positioning. This required acclimation of the animals to the chair, touch-screen tasks, i.v. catheter insertion, and tilting. The Vicra infrared camera tracked its reflective tool that was non-invasively mounted atop the animal's head. During the scan, emission and motion data were acquired simultaneously. We intended the methods we developed to be minimally stressful to the subject, and hence took cortisol measurements during the scans to index the levels of stress.
We tested the utility of this novel imaging paradigm with the GABAA-benzodiazepine (BDZ) receptor antagonist, [11C]flumazenil. A bolus plus constant infusion (B/I) injection schedule for [11C]flumazenil was optimized (6) for awake scanning during the equilibrium phase of the tracer; this reduced scan time and eliminated the need for arterial input data. Equilibrium analysis was used to determine non-displaceable binding potential (BPND), a neuroreceptor binding measure of the ratio of specific to free plus non-specific receptor radioligand binding at equilibrium (7). In this evaluation, we hypothesized that [11C]flumazenil BPND would be higher under isoflurane anesthesia compared with the awake condition. This was based primarily on a human study that showed that isoflurane produced a dose-dependent increase in [11C]flumazenil distribution volume in comparison to awake controls (8). One potential mechanism for this effect is anesthesia-induced increases in GABA; increased GABA levels have been shown to enhance the affinity for ligands to the BDZ site, coined the ‘GABA shift’ (9). Evidence that the GABA shift is detectable with [11C]flumazenil PET, has been provided by a recent human study using tiagabine, a GABA membrane transporter blocker (10). Thus, an increased affinity for GABAA-BDZ receptors due to isoflurane should produce an increase in [11C]flumazenil BPND compared with the awake condition.
Materials and Methods
Animals
Studies were performed in two rhesus monkeys (Macaca mulatta) (1 female, 1 male, mean weight: 10.4±4.4 kg; mean age: 10.2±4.2 y.o.). Each monkey had 3 awake scans and 2 anesthetized scans. The animals were fasted on all scan days. The study protocol was approved by the Yale University Institutional Animal Care and Use Committee. Animals were housed and maintained in accordance with federal guidelines for the care and use of nonhuman primates and provided with enrichment on a regular basis.
Awake NHP Training and Acclimation to Scan Environment
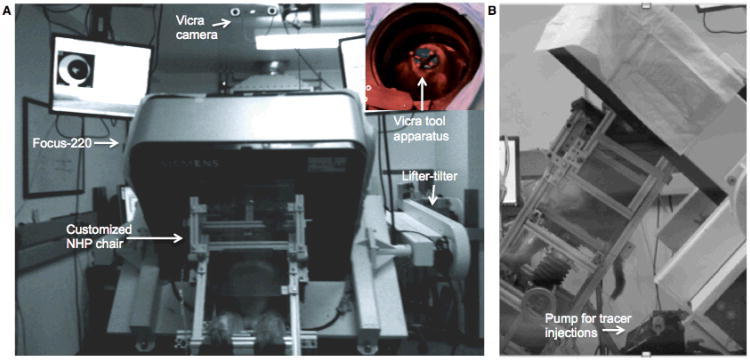
An overview of the awake study set-up with the Focus-220 small animal PET scanner is shown in Fig. 1. The scanner was fitted with a mechanical lifter-tilter designed to raise and rotate the scanner up to an operational limit of 45°. For optimal positioning of the brain in the field-of-view (FOV), the animals learned to tilt back 35° in the custom NHP chair while the Focus-220 was tilted forward. The Vicra motion-tracking camera (NDI, Waterloo, Canada) tracked a remote tool at 20 Hz (Fig. 1A inset) during the PET acquisition. This device was attached to a silicone rubber plug and adhered to the NHP's shaved scalp prior to each scan, and was easily removed after the scan. The computer-controlled pump for tracer administration is shown in Fig. 1B.
Figure 1.
Overview of the awake study set-up in the Focus-220 suite. A) Front view and the Vicra camera view of the tool apparatus on the animal's head from behind the Focus-220 (inset). B) Side view of the monkey sitting in the NHP chair tilted in the scanner. See text for details.
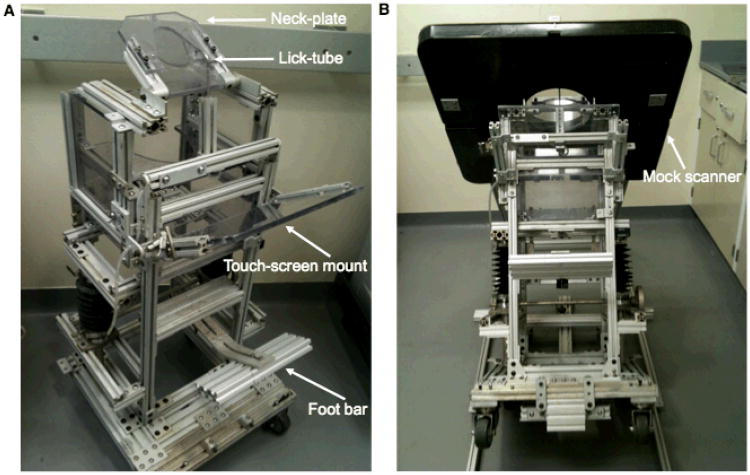
The following describes the training and acclimation steps in preparation for the awake PET scan procedure. Monkeys were first trained in the custom NHP chair (Fig. 2A). Animals were initially trained to transfer into the chair from their home cage for food reward. Next, NHPs were brought into a training room to learn a series of procedures. A touch-screen was mounted to the chair in order to enable the NHP to learn to touch the screen for food and juice reward from the lick-tube. Next, they were trained to accept a neck-plate, which permitted a wide range of head movement. The neck-plate, made of polycarbonate, was slid behind the neck and fastened to the chair with screws. To allow for i.v. catheter insertion necessary for tracer injection or blood sampling, the monkeys were acclimated to having their feet secured to the foot bar with vet-wrap. For preparation for the Vicra tracking tool placement, the animal's head was shaved and a rigid, silicone rubber plug was adhered to the scalp with actor's skin glue. Training for these procedures took a minimum of several weeks with ∼3 sessions per week. Several animals were screened for eligibility to perform awake imaging, where the animals excluded were typically not juice or food motivated and/or required more than 2 months to acclimate to an awake procedure (e.g., chair transfer from cage or neck-plating).
Figure 2.
Custom NHP chair and the Focus-220 mock scanner. A) The NHPs were acclimated to a custom chair designed for scanning in the Focus-220. B) The Focus-220 mock scanner was utilized for training the NHPs to tilt backwards while the mock scanner was slowly lowered over the head. See text for details.
Next, the animals were trained in the mock Focus-220 scanner to tilt backwards as the chair was attached to a mini-ramp (Fig. 2B). The mock scanner was slowly lowered as the monkey continued to perform a task on the touch-screen. After a few tilting sessions in the mock scanner, the animals were brought into the Focus-220 scan room to practice the steps of the bolus plus constant infusion (B/I) awake scan procedure, where scan acquisition occurs during the equilibrium phase of the tracer. For each NHP, there were several practice sessions of the awake scan procedure, with at least two performed with saline infusions. Once the animal was familiar with all procedures and was able to stay tilted in the scanner for a sufficient period of time, the awake [11C]flumazenil scans were performed.
PET Experiment
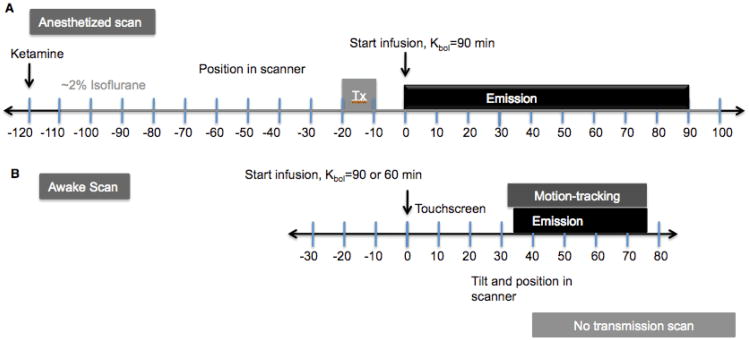
PET scans were performed on the Focus-220 small animal scanner (FWHM ∼1.5 mm) (Siemens/CTI, Knoxville, TN, USA). Study designs are shown in Fig. 3. [11C]Flumazenil was injected as a B/I using a computer-controlled pump (Harvard Apparatus, Inc. Holliston, MA). The appropriate B/I schedule was determined by the parameter, Kbol (min). Specifically, the magnitude of the bolus portion of tracer delivery is equal to the infused amount over Kbol min. Using time-activity curves (TACs) from a bolus study, B/I curves can be estimated and an optimal Kbol can be chosen (6). Under the anesthetized condition, emission data were acquired for 90 min post-injection. Under the awake condition, emission data were acquired for ∼40-75 min post-injection during the equilibrium phase of the tracer. However, [11C]flumazenil was administered as a 3 min bolus for one awake scan where emission data were acquired for 75 min post-injection.
Figure 3.
[11C]Flumazenil bolus plus constant infusion (B/I) study design for A) anesthetized and B) awake scans. See text for details.
The anesthetized scans were repeated on the same day with a 3.5 h interval between the two radiotracer injections. For the anesthetized scans (Fig. 3A), animals were initially immobilized with ketamine hydrochloride (10 mg/kg, IM), then transported to the PET suite for scan preparation 2 h prior to the initial [11C]flumazenil injection. Once intubated, the animals were maintained on oxygen and isoflurane (∼2%) anesthesia throughout both scans. A 9-min transmission scan (TX) preceded each of the emission scans. [11C]Flumazenil was administered as a B/I with Kbol=90 min, and emission data were collected for 90 min.
The timing for the awake scan procedures (Fig. 3B) relative to tracer injection at 0 min, proceeded as follows: Animals were transported in the NHP chair from their cage and brought to the Focus suite prior to [11C]flumazenil injection (-30 min). Once the neck-plate and catheter were placed (-25 to -10 min), the chair was secured to the ramp, and the touch-screen was mounted (-10 to 0 min). [11C]Flumazenil was administered as a B/I (0 min) while the monkey performed a touch-screen task with juice reward (0 min on). For these studies, the initial Kbol values was set to 90 min, the value used in the anesthetized scan. Subsequent examination of the data suggested that a Kbol of 60 min was more appropriate for awake scans, based on analysis of data from the single awake bolus scan, and this value was used in the ensuing awake scans.
The Vicra tool, attached to a silicone rubber plug, was adhered to the head of the animal via actor's skin glue (+20-25 min) (Fig. 1 inset). Then, the NHP chair was tilted back 35°, and the pre-raised scanner was tilted forward 45° (30 min). With the head positioned in the scanner FOV, the Vicra infrared motion-tracking acquisition commenced followed by the emission acquisition (∼35-75 min post-injection). Transmission scans were not acquired for the awake studies.
Radiochemistry
[11C]Flumazenil was prepared from reaction of desmethylflumazenil (∼0.5 mg) with [11C]methyl iodide in anhydrous N,N-dimehtylformamide (DMF) and aqueous KOH (12 N, 8 μL). Synthesis was performed using either the AutoLoop® synthesis module (Bioscan Inc., Washington, D.C.) or the TracerLab® FxC-Pro module (GE Healthcare, Waukesha, WI). The crude reaction mixture was purified by semi-preparative HPLC (Column: Phenomenex LUNA C18, 10 μm, 10 × 250 mm; Mobile phase: 25% acetonitrile and 75% 0.1 M ammonium formate with 0.5% acetic acid, pH 4.2 (v/v); Flow rate: 5 mL/min). The product peak at ∼12.5–13.5 min was collected, diluted with 50 mL of deionized water and loaded onto a Waters C18 Classic SepPak cartridge. The cartridge was washed with 10 mL of 0.001N HCl. The product was eluted off the SepPak with 1 mL of EtOH, followed by 3 mL of saline, into a sterile vial containing 7 mL of saline and 40 μL of 4.2% NaHCO3 solution via a Millipore GV filter (0.22 μm, 33 mm). Radiochemical purity of the final product was >99% with specific activity of 6.71 ± 4.38 mCi/nmol at end of synthesis. Radiochemical yield was 114.0 ± 53.3 mCi.
Cortisol Measurements
Blood samples were taken for measurements of cortisol levels, as an indicator of stress, during one awake and both anesthetized scans for each monkey. In the awake scan, 2 mL venous samples were collected every 10 min until the end of the scan. For the anesthetized scans, 2 mL samples were collected at 10 min intervals beginning ∼30 min before tracer injection. Samples were centrifuged at 2930 g for 5 min, and sent for analysis (ANTECH Diagnostics, Middletown, CT, USA). Within and between animals, statistical analysis between awake and both anesthetized groups was performed with two-tailed, unpaired t-tests with p<0.05.
MRI Scanning and Processing
MR images were acquired on a Siemens Magnetom 3.0T Trio scanner in anesthetized animals (∼2% isoflurane), using an extremity coil. T1-weighted images were acquired in coronal plane with spin echo sequence (TE=3.34, TR=2530, flip angle=7°, section thickness=0.50 mm, FOV=140 mm, image matrix=256×256×176 pixels, matrix size=0.55×55×0.50 mm). The MR images were stripped of skull and muscle so that only the brain remained in the image (FMRIB's Brain Extraction Tool, http://www.fmrib.ox.ac.uk/fsl/bet2/index.html). This procedure was performed once for each monkey prior to co-registration with the PET images.
PET Image Reconstruction and Processing
Listmode data were collected and reconstructed using filtered backprojection with a Shepp filter with cutoff at 30% of the Nyquist frequency and with all corrections (attenuation, normalization, scatter, randoms, and deadtime). Final image dimensions and voxel sizes were 256×256×95 and 0.95×0.95×0.80 mm, respectively. Data from the anesthetized scans were directly reconstructed into a sequence of 27 frames: 6×30 sec; 3×1 min; 2×2 min; 16×5 min.
Awake listmode data were reconstructed with motion correction performed via the multi-acquisition frame-based (MAF) method (11). The listmode and motion data were time-synced, and the motion data were used to divide the listmode data into sub-frames based on an intraframe motion threshold (IFMT) and a minimum sub-frame duration threshold (MFDT) (12). The IFMT defined the maximum motion permitted within a frame and the MFDT defined the minimum frame duration. Thus, periods of large, fast motion were dropped. Motion data consisted of transformation matrices at 20 Hz, and each transformation was applied to eight points chosen as the vertices of a rectangle in the center of the FOV to compute a measure of intraframe motion magnitude (mm). A new sub-frame was started when the IFMT exceeded 2 mm, keeping sub-frames with an MFDT of 3 s.
Each sub-frame image was first reconstructed without attenuation and scatter correction. The sub-frames were then rigidly aligned to a common awake reference space, using the averaged transformation matrix within each sub-frame. For attenuation correction, a transmission image of the same animal from an anesthetized study was used. The transmission image was mapped into the awake reference space by registering the non-attenuation corrected PET summed images of the anesthetized (aligned to the transmission) and the awake reference scans to the MR image and multiplying the two transforms (13). With the transmission image in the reference position, the inverse of the transformation matrices were applied to create a new transmission image for the individual sub-frames to reconstruct each with attenuation and scatter correction. Then, these fully corrected sub-frames were transformed back to the reference position and were summed to produce standard-duration frames (5 × 5 min).
For PET-MR image alignment, summed PET images acquired under awake and anesthetized conditions were registered to each animal's MR via a 6-parameter rigid registration using normalized mutual information in FSL FLIRT (FMRIB's Linear Image Registration Tool, www.fmrib.ox.ac.uk/fsl/flirt/index.html). Summed (50-75 min) PET images from awake scans were registered to the MR, whereas summed PET images from anesthetized scans were chosen with the Multi-transform method and were registered to the MR (14). An affine+nonlinear registration was performed (Bioimage Suite 2.5, http://www.bioimagesuite.org/index.html) for each MR image to a high-resolution NHP MR template where regions-of-interest (ROIs) were defined. PET images were resliced to template space via the two transforms, i.e., PET-to-MR and MR-to-template. The following regions were taken from the template: amygdala (0.10 mL), cerebellum (5.9 mL), cingulate cortex (0.64 mL), frontal cortex (2.5 mL), insula (0.96 mL), occipital cortex (5.2 mL), pons (0.33 mL), temporal cortex (3.2 mL), and thalamus (0.25 mL).
Equilibrium Analysis
To determine the quality of equilibrium, TACs were assessed in the occipital cortex and pons regions, the highest and lowest binding regions, respectively, to measure percent change per min (%Δ/min=slope/mean concentration × 100) for: anesthetized scans (Kbol=90 min, n=4), awake scans (Kbol=90 min, n=3), and awake scans (Kbol=60 min, n=2).
BPND images were computed with the pons as the reference region using equilibrium analysis between 50-75 min as:
| Eq. 1 |
where CROI and CREF are the mean concentrations (Bq/mL) in the ROI and the pons reference region, respectively. BPND was computed as an average of the voxel values in each ROI. For B/I studies, mean BPND images were computed across replicates and monkeys for the anesthetized and awake scans, where a correction factor was applied to the initial awake studies acquired with Kbol=90 min, since these were not quite at equilibrium (see Supplemental Data). The single awake bolus study was analyzed with the simplified reference tissue model (SRTM) (15). Percent change in mean BPND (%Δ BPND) between conditions was computed as
| Eq. 2 |
Statistical analysis of between-group differences was performed with two-tailed, unpaired t-tests with p<0.05, without correction for multiple comparisons, where ROI BPND values were treated independently.
Results
To assess the quality of equilibrium for B/I studies with [11C]flumazenil, TACs in the high (occipital cortex) and low (pons) binding regions were analyzed. Mean %Δ/min were slightly positive for anesthetized scans (n=4) in the occipital cortex (+0.29±0.18) and pons (+0.47±0.48) with Kbol=90 min. Mean %Δ/min were negative for awake scans (n=3) in the occipital cortex (-0.34±0.38) and pons (-1.00±1.96) using the same infusion schedule, Kbol=90 min. Kbol was adjusted to 60 min for the subsequent awake B/I scans (n=2), as determined from the awake bolus scan TACs, and %Δ/min in the TACs improved in the occipital cortex (+0.22±0.91) and pons (+0.51±1.33), which was very similar to the anesthetized values.
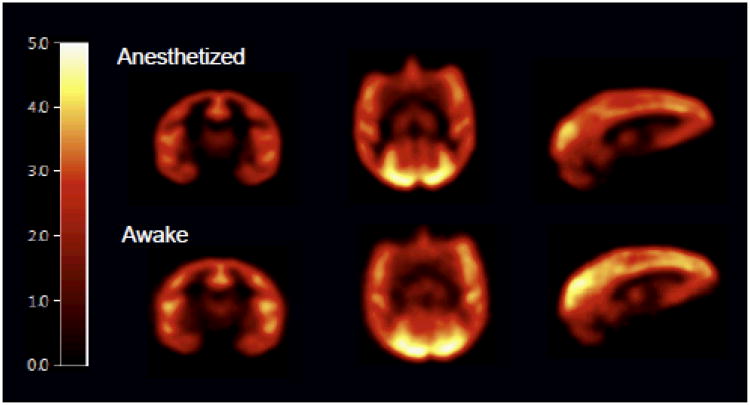
Mean BPND images of [11C]flumazenil show moderately higher binding in awake compared with anesthetized studies (Fig. 4) where
Figure 4.
Comparison of BPND images in the anesthetized and awake studies. Mean BPND images of [11C]flumazenil were computed from B/I studies in anesthetized (n=4) and awake NHPs (n=5). Images are post smoothed with a 3-mm Gaussian and displayed on a common scale after registration to a NHP template.
Mean BPND values in ROIs and %ΔBPND are shown in Table 1. BPND was slightly higher in the awake compared with the anesthetized condition in all regions except for the cerebellum and temporal cortex, but this difference was not significant. The largest %ΔBPND was observed in the amygdala.
Table 1.
Regional [11C]flumazenil BPND values from anesthetized and awake studies.
| ROI | Anesthetized (n=4) | Awake (n=6) | ΔBPND | p |
|---|---|---|---|---|
| Amygdala | 1.08±0.11 | 1.87±0.77 | -42% | 0.08 |
| Cerebellum | 1.78±0.33 | 1.56±0.23 | 14% | 0.26 |
| Cingulate cortex | 3.19±0.46 | 3.73±0.84 | -14% | 0.28 |
| Frontal cortex | 3.29±0.49 | 3.34±0.72 | -2% | 0.90 |
| Insula | 3.08±0.39 | 3.29±0.61 | -6% | 0.56 |
| Occipital cortex | 3.88±0.30 | 4.01±0.77 | -3% | 0.76 |
| Temporal cortex | 2.81±0.33 | 2.77±0.60 | 2% | 0.89 |
| Thalamus | 1.39±0.11 | 1.52±0.39 | -8% | 0.55 |
Values are mean±SD. Significance level was p<0.05 (two-tailed, unpaired t-test) of the difference between the anesthetized and awake scans.
Results for cortisol analysis are shown in Table 2 with statistical comparisons shown within monkey between awake and both anesthetized conditions. Cortisol levels were statistically higher in the awake than in the anesthetized condition. Between animals, monkey 1 had higher cortisol levels than monkey 2 in both conditions. For monkey 1, cortisol levels were significantly higher during the awake scan compared with the two anesthetized scans, which had comparable cortisol levels. For monkey 2, cortisol levels were significantly higher during the awake scan compared to the first anesthetized scan, but not the second anesthetized scan. Each pair of anesthetized scans were performed in one day. As cortisol is a measure of stress which may be linked to GABA function, [11C]flumazenil binding may be altered in awake scans compared with anesthetized scans (see Discussion).
Table 2.
Cortisol levels (μg/dL) from awake and anesthetized studies with [11C]flumazenil.
| Awake scan | Anesthetized scan 1 | Anesthetized scan 2 | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| n | Mean±SD | n | Mean±SD | n | Mean±SD | |
|
|
||||||
| Monkey 1 | 7 | 32.4±5.6*† | 10 | 24.1±5.7 | 10 | 24.4±3.3 |
| Monkey 2 | 8 | 17.1±6.2* | 10 | 7.2±3.4† | 10 | 17.1±1.5 |
Within monkey:
p<0.01 versus anesthetized scan 1,
p<0.01 versus anesthetized scan 2
Discussion
To our knowledge, these studies represent the first PET imaging of awake nonhuman primates with minimal head restraint using extensive acclimation and pre-training of the animals.
The present approach was applied to compare [11C]flumazenil BPND under awake and anesthetized conditions. Our results showed no significant differences, although BPND was slightly higher in the awake condition. These results differ from a human study which showed an isoflurane dose-dependent increase in [11C]flumazenil distribution volume compared with the awake state (8). A subsequent technical note stated that the infusion of phenylephrine, used to maintain mean arterial blood pressure pre-induction, in combination with isoflurane may have increased the free fraction of the tracer (16). Thus, it is uncertain that the distribution volume increase reflected changes in specific binding. Note that the outcome measure used here, BPND, is insensitive to free fraction.
Allowing head movement during the scan posed challenges in terms of image reconstruction. For motion correction, emission list mode data acquired under the awake condition were reconstructed with the MAF method (11) where time-synced motion information were used to divide the data into sub-frames. A disadvantage of this method is data loss due to large/high frequency head motion. As a result, motion-corrected awake scans were noisier, such that images acquired under awake and anesthetized conditions were smoothed for fair visual comparison. Cortical folds in the BPND images of anesthetized studies were better defined than in the awake studies (Fig. 4), since the MAF data had up to 2 mm of intra-frame motion. Better accuracy of motion correction may be achieved with event-by-event motion correction, as used in the Motion-compensation OSEM list-mode algorithm for resolution-recovery reconstruction (MOLAR) for the HRRT (5). MOLAR corrects all lines of response back to their original positions, and all of the data are used. However, at the time of this study, MOLAR implementation was not complete for the Focus-220. For future awake scans, MOLAR may be the optimal reconstruction method.
Another challenge was attenuation correction, where acquiring a transmission scan was not feasible for awake, unrestrained NHPs. To circumvent this issue, an algorithm was developed to use transmission datasets acquired under anesthesia for attenuation correction in awake emission scans for the same animal. This algorithm was tested using pairs of emission data with aligned attenuation images from anesthetized scans performed on different days using the procedure described earlier (13). ROI TAC values were compared between emission images corrected for attenuation with a realigned transmission image or the original aligned transmission image for different tracers. Depending on the positioning of the brain in the FOV, the realigned transmission image sometimes had missing values, typically towards the bottom of the FOV, producing underestimations in TACs. This issue was resolved by creating a larger, average transmission image using multiple transmission scans for the same animal in different positions in the FOV. In the event that transmission scans are unavailable for an animal, such data can be easily acquired during a separate scan day while the animal is under anesthesia. Alternatively, transmission data from other animals might be useable, if appropriate interanimal non-rigid registrations are developed and validated. Such algorithms might take advantage of existing monkey MR templates.
The awake imaging protocol was designed to reduce stress to the animal by allowing head motion and decreasing scan time. Both monkeys used in this study acclimated quickly to all training procedures. For this study several animals were evaluated, where the animals chosen were highly food-motivated and readily adapted to awake scan acclimation procedures.
During the awake scan procedure, the animals showed some evidence of stress, particularly during the awake i.v. catheterization required for tracer infusion and cortisol blood sampling. In the current study, the measured cortisol levels under the awake condition were consistent with previous studies involving cortisol measurements as NHPs acclimated to awake procedures (i.e., chair restraint and blood draws) (17-20). Under the anesthetized condition, it is possible that stress levels during the 2nd anesthetized PET study were affected by the long protocol (7 h), however, there are limited data on the effects of long-term anesthesia on cortisol, except in protocols involving a surgical procedure. In a study with female pigs, cortisol levels were monitored under different anesthetics over the course of 4 h with and without (sham condition) surgical intervention (21). Although there were no differences in cortisol levels between the sham condition and surgical intervention with desflurane and propofol, there was a significant increase in cortisol after 4 h with sevoflurane. Additionally, cortisol responses with surgical interventions varied depending on the anesthetic used. Cortisol, in response to stress, has been shown to vary with time of day (i.e., diurnal cycle) and gender in humans (22), in line with the differences in cortisol levels observed between monkeys in the anesthetized scans. Other possible factors contributing to the disparity in cortisol levels between the two animals may be attributed to age and social status (23, 24).
Minimizing stress is important for awake imaging in NHPs as it may influence neuroreceptor measures. PET studies in awake rhesus monkeys using pharmacological stressors have shown an increase in cortisol levels and differences in regional cerebral blood flow (rCBF) and regional cerebral metabolic rate of glucose (rCMRglc) measured with [15O]H2O and [18F]FDG, respectively (25, 26). In addition, microdialysis studies have shown that physically- or pharmacologically-induced stress increases GABA levels in the basolateral amygdala (27, 28). These findings are consistent with our data where the largest difference in [11C]flumazenil BPND was observed in the amygdala in awake monkeys. Taken together, stress may influence PET measures in conscious NHP studies and can be reduced with the appropriate equipment and consistent, regimented training (18, 19)
To improve upon the current awake imaging design, recent structural developments may permit tilting of the scanner to 90° (parallel to the floor). Validation of scanner performance at this configuration is required. Sitting in an upright position during scan acquisition will improve comfort level for the animal. For future awake NHP imaging studies, these modifications are expected to reduce stress and to increase the reliability of PET measures.
The study findings did not support our hypothesis based on the effect of isoflurane on GABA and GABAA-BDZ binding. There is general but not complete agreement in the literature that a primary target for isoflurane anesthetic action is at the GABAA receptor, where GABA is the major inhibitory neurotransmitter in the central nervous system. GABAA is an ionotropic receptor, and contains GABA and BDZ binding sites (29, 30). The BDZ site has been extensively studied in stress and anxiety disorders. BDZ site modulators exert their effects in the presence of GABA, but do not directly activate the GABAA receptor (31). BDZ agonists potentiate the effects of GABA by increasing the conductance of Cl- to intensify hyperpolarization and inhibition of neuronal activity (31, 32). In turn, in vitro homogenate binding studies have demonstrated that GABA increases the affinity for ligands to the BDZ site, termed the ‘GABA shift’ (9).
A recent review reported that volatile anesthetics dose-dependently enhanced extrasynaptic GABA-induced inhibitory postsynaptic currents akin to higher extracellular GABA concentration, in vitro(33). With further evidence of the GABA shift as demonstrated with PET and SPECT studies with tiagabine (10, 34), isoflurane-induced increases in GABA should be detectable with [11C]flumazenil at the GABAA-BDZ receptor site. However, in this study we did not observe statistically significant differences in [11C]flumazenil BPND between awake and anesthetized conditions.
Apart from eliminating anesthesia effects on PET measures and brain function, awake neuroreceptor imaging provides the opportunity to simultaneously measure cognitive performance and to correlate various aspects of cognitive function with receptor availability and neuroreceptor binding. However, the current design with minimal head restraint would not permit simultaneous microdialysis measurements, important for relating neurotransmitter release to PET measurements before and after a pharmacological challenge. In the present study, the animals performed touch-screen tasks for juice reward, however, once the scanner was tilted, the animal's view of the touch-screen was limited. For future studies, which aim to correlate PET activity to task performance, careful consideration must be taken to limit over-trained behavior that may affect PET measurements. For behavioral activation studies, where the animal performs a cognitive task during the scan, a baseline scan should be included where the animal performs a control task that does not interfere with the specific neuronal circuitry under study. Henceforth, it will be important to ensure that the animals can perform the tasks effectively throughout the imaging period in order to investigate non-invasive cognitive neurochemistry in the primate.
Conclusion
We developed a novel protocol to image awake NHPs with minimal head restraint using motion-tracking technology. This approach was evaluated with the benzodiazepine radioligand [11C]flumazenil under awake vs. anesthetized conditions. Animals acclimated to procedures in a custom NHP chair and successfully performed repeated awake PET scans. Equilibrium analysis of [11C]flumazenil binding was slightly, but non-significantly higher in unrestrained awake versus anesthetized NHPs. This work provides a foundation for correlating behavioral imaging paradigms with PET radioligand measurements for advancing translational research approaches between humans and nonhuman primates.
Supplementary Material
Acknowledgments
The authors acknowledge the staff at the Yale PET Center, especially Shervin Liddie and Siobhan Ford, and other members of the NHP team, including Brooke Roberts, Amanda Abbott, and David Campbell. Special thanks go to Jodi Scholz, Irina Esterlis, Edward Fung, and Jean-Dominique Gallezot to for helpful scientific discussions. Research support was provided by Glaxo-Smith Kline, and the training grant 1-T90-DK070068. This publication was also made possible by CTSA Grant Number UL1 RR024139 from the National Center for Research Resources (NCRR) and the National Center for Advancing Translational Science (NCATS), components of the National Institutes of Health (NIH), and NIH roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
References
- 1.Ceravolo R, Sgado P, Frosini D, Corsini GU. Assessing neuroprotection in Parkinson's disease: from the animal models to molecular neuroimaging in vivo. J Neural Transm Suppl. 2006:133–141. doi: 10.1007/978-3-211-33328-0_15. [DOI] [PubMed] [Google Scholar]
- 2.Bian SZ, Zhang J, Liu WL, Sun ZH, Gu ZL, Jiang XG. Receptor antagonist of NMDA and animal models of schizophrenia. Fa Yi Xue Za Zhi. 2009;25:443–446. [PubMed] [Google Scholar]
- 3.Howell LL, Murnane KS. Nonhuman primate positron emission tomography neuroimaging in drug abuse research. J Pharmacol Exp Ther. 2011;337:324–334. doi: 10.1124/jpet.108.136689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsukada H, Nishiyama S, Kakiuchi T, et al. Isoflurane anesthesia enhances the inhibitory effects of cocaine and GBR12909 on dopamine transporter: PET studies in combination with microdialysis in the monkey brain. Brain Res. 1999;849:85–96. doi: 10.1016/s0006-8993(99)02018-1. [DOI] [PubMed] [Google Scholar]
- 5.Carson RE, Barker W, Liow JS, Adler S, Johnson C. Design of a motion-compensation OSEM list-mode algorithm for resolution-recovery reconstruction of the HRRT. IEEE Nucl Sci Symp Conf. 2003 Rec. M16-6. [Google Scholar]
- 6.Carson RE, Channing MA, Blasberg RG, et al. Comparison of bolus and infusion methods for receptor quantitation: application to [18F]cyclofoxy and positron emission tomography. J Cereb Blood Flow Metab. 1993;13:24–42. doi: 10.1038/jcbfm.1993.6. [DOI] [PubMed] [Google Scholar]
- 7.Innis RB, Cunningham VJ, Delforge J, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27:1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- 8.Gyulai FE, Mintun MA, Firestone LL. Dose-dependent enhancement of in vivo GABA(A)-benzodiazepine receptor binding by isoflurane. Anesthesiology. 2001;95:585–593. doi: 10.1097/00000542-200109000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Tallman JF, Thomas JW, Gallager DW. GABAergic modulation of benzodiazepine binding site sensitivity. Nature. 1978;274:383–385. doi: 10.1038/274383a0. [DOI] [PubMed] [Google Scholar]
- 10.Frankle WG, Cho RY, Narendran R, et al. Tiagabine increases [11C]flumazenil binding in cortical brain regions in healthy control subjects. Neuropsychopharmacology. 2009;34:624–633. doi: 10.1038/npp.2008.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Picard Y, Thompson CJ. Motion correction of PET images using multiple acquisition frames. IEEE Trans Med Imaging. 1997;16:137–144. doi: 10.1109/42.563659. [DOI] [PubMed] [Google Scholar]
- 12.Jin X, Sandiego CM, Mulnix T, Carson RE. Multiple acquisition frame-based motion correction for awake monkey PET imaging. IEEE Nucl Sci Symp Conf Rec. 2010 [Google Scholar]
- 13.Sandiego CM, Mulnix T, Carson RE. Attenuation correction for awake non-human primate PET using transmission data from anesthetized studies. International Symposium on Functional Neuroreceptor Mapping of the Living Brain (Conference presentation); 2010; p. S182. [Google Scholar]
- 14.Sandiego CM, Weinzimmer D, Carson RE. Optimization of PET-MR registrations for nonhuman primates using mutual information measures: A Multi-transform method (MTM) Neuroimage. 2013;64:571–581. doi: 10.1016/j.neuroimage.2012.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lammertsma AA, Hume SP. Simplified reference tissue model for PET receptor studies. Neuroimage. 1996;4:153–158. doi: 10.1006/nimg.1996.0066. [DOI] [PubMed] [Google Scholar]
- 16.Alkire MT, Krejcie TC, Avram MJ. Anesthesia-induced alterations in plasma tracer concentrations may have relevance for brain imaging studies. Anesthesiology. 2002;97:281–282. doi: 10.1097/00000542-200207000-00048. author reply 282. [DOI] [PubMed] [Google Scholar]
- 17.Morrow-Tesch JL, McGlone JJ, Norman RL. Consequences of restraint stress on natural killer cell activity, behavior, and hormone levels in rhesus macaques (Macaca mulatta) Psychoneuroendocrinology. 1993;18:383–395. doi: 10.1016/0306-4530(93)90013-b. [DOI] [PubMed] [Google Scholar]
- 18.Reinhardt V. Working with rather than against macaques during blood collection. J Appl Anim Welf Sci. 2003;6:189–197. doi: 10.1207/S15327604JAWS0603_04. [DOI] [PubMed] [Google Scholar]
- 19.Ruys JD, Mendoza SP, Capitanio JP, Mason WA. Behavioral and physiological adaptation to repeated chair restraint in rhesus macaques. Physiol Behav. 2004;82:205–213. doi: 10.1016/j.physbeh.2004.02.031. [DOI] [PubMed] [Google Scholar]
- 20.Winterborn AN, Bates WA, Feng C, Wyatt JD. The efficacy of orally dosed ketamine and ketamine/medetomidine compared with intramuscular ketamine in rhesus macaques (Macaca mulatta) and the effects of dosing route on haematological stress markers. J Med Primatol. 2008;37:116–127. doi: 10.1111/j.1600-0684.2007.00234.x. [DOI] [PubMed] [Google Scholar]
- 21.Kostopanagiotou G, Kalimeris K, Christodoulaki K, et al. The differential impact of volatile and intravenous anaesthetics on stress response in the swine. Hormones (Athens) 2010;9:67–75. doi: 10.14310/horm.2002.1255. [DOI] [PubMed] [Google Scholar]
- 22.Lovallo WR, Farag NH, Vincent AS. Use of a resting control day in measuring the cortisol response to mental stress: diurnal patterns, time of day, and gender effects. Psychoneuroendocrinology. 2010;35:1253–1258. doi: 10.1016/j.psyneuen.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goncharova ND, Lapin BA. Effects of aging on hypothalamic-pituitary-adrenal system function in non-human primates. Mech Ageing Dev. 2002;123:1191–1201. doi: 10.1016/s0047-6374(02)00012-x. [DOI] [PubMed] [Google Scholar]
- 24.Abbott DH, Keverne EB, Bercovitch FB, et al. Are subordinates always stressed? A comparative analysis of rank differences in cortisol levels among primates. Horm Behav. 2003;43:67–82. doi: 10.1016/s0018-506x(02)00037-5. [DOI] [PubMed] [Google Scholar]
- 25.Takamatsu H, Noda A, Murakami Y, Tatsumi M, Ichise R, Nishimura S. A PET study after treatment with an anxiety-provoking agent, m-chlorophenyl-piperazine, in conscious rhesus monkeys. J Nucl Med. 2003;44:1516–1521. [PubMed] [Google Scholar]
- 26.Takamatsu H, Noda A, Kurumaji A, et al. A PET study following treatment with a pharmacological stressor, FG7142, in conscious rhesus monkeys. Brain Res. 2003;980:275–280. doi: 10.1016/s0006-8993(03)02987-1. [DOI] [PubMed] [Google Scholar]
- 27.Cook CJ. Stress induces CRF release in the paraventricular nucleus, and both CRF and GABA release in the amygdala. Physiol Behav. 2004;82:751–762. doi: 10.1016/j.physbeh.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 28.Reznikov LR, Reagan LP, Fadel JR. Effects of acute and repeated restraint stress on GABA efflux in the rat basolateral and central amygdala. Brain Res. 2009;1256:61–68. doi: 10.1016/j.brainres.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 29.Bonin RP, Orser BA. GABA(A) receptor subtypes underlying general anesthesia. Pharmacol Biochem Behav. 2008;90:105–112. doi: 10.1016/j.pbb.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 30.Li GD, Chiara DC, Cohen JB, Olsen RW. Numerous classes of general anesthetics inhibit etomidate binding to gamma-aminobutyric acid type A (GABAA) receptors. J Biol Chem. 2010;285:8615–8620. doi: 10.1074/jbc.M109.074708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Atack JR. The benzodiazepine binding site of GABA(A) receptors as a target for the development of novel anxiolytics. Expert Opin Investig Drugs. 2005;14:601–618. doi: 10.1517/13543784.14.5.601. [DOI] [PubMed] [Google Scholar]
- 32.Mandrioli R, Mercolini L, Raggi MA. Benzodiazepine metabolism: an analytical perspective. Curr Drug Metab. 2008;9:827–844. doi: 10.2174/138920008786049258. [DOI] [PubMed] [Google Scholar]
- 33.Kotani N, Akaike N. The effects of volatile anesthetics on synaptic and extrasynaptic GABA-induced neurotransmission. Brain Res Bull. 2012 doi: 10.1016/j.brainresbull.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 34.Sybirska E, Seibyl JP, Bremner JD, et al. [123I]iomazenil SPECT imaging demonstrates significant benzodiazepine receptor reserve in human and nonhuman primate brain. Neuropharmacology. 1993;32:671–680. doi: 10.1016/0028-3908(93)90080-m. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.