Abstract
Perchlorate, thiocyanate, and low iodine intake can all decrease iodide intake into the thyroid gland. This can reduce thyroid hormone production since iodide is a key component of thyroid hormone. Previous research has suggested that each of these factors alone may decrease thyroid hormone levels, but effect sizes are small. We hypothesized that people who have all three factors at the same time have substantially lower thyroid hormone levels than people who do not, and the effect of this combined exposure is substantially larger than the effects seen in analyses focused on only one factor at a time. Using data from the 2007-2008 National Health and Nutrition Examination Survey, subjects were categorized into exposure groups based on their urinary perchlorate, iodine, and thiocyanate concentrations, and mean serum thyroxine concentrations were compared between groups. Subjects with high perchlorate (n=1939) had thyroxine concentrations that were 5.0% lower (mean difference = 0.40 µg/dl, 95% confidence interval=0.14-0.65) than subjects with low perchlorate (n=2084). The individual effects of iodine and thiocyanate were even smaller. Subjects with high perchlorate, high thiocyanate, and low iodine combined (n=62) had thyroxine concentrations 12.9% lower (mean difference = 1.07 µg/dl, 95% confidence interval=0.55-1.59) than subjects with low perchlorate, low thiocyanate, and adequate iodine (n=376). Potential confounders had little impact on results. Overall, these results suggest that concomitant exposure to perchlorate, thiocyanate, and low iodine markedly reduces thyroxine production. This highlights the potential importance of examining the combined effects of multiple agents when evaluating the toxicity of thyroid-disrupting agents.
Keywords: perchlorate, iodine, thiocyanate, thyroid hormone, National Health and Nutrition Examination Survey
Introduction
Perchlorate has been used industrially as an oxidizer in solid rocket propellant, slurry explosives, road flares, and air bag inflation systems. In recent analyses from the National Health and Nutrition Examination Survey, perchlorate was detected in every urine sample tested (Blount et al. 2006). Human environmental exposure can occur through food or water following industrial contamination or from perchlorate that is naturally occurring (OEHHA, 2004). High doses of perchlorate have been shown to competitively inhibit iodide uptake by the sodium iodide symporter in the thyroid gland, and in the past perchlorate was used therapeutically for this effect (Stanbury and Wyngaarden, 1952; Wyngaarden et al., 1952). This effect is important since iodide is a key component of thyroid hormone, and blocking iodide uptake into the thyroid can decrease thyroid hormone production. Thyroid hormone plays a key role in many physiologic functions, and in the fetus and child, it is critical for normal brain and neurological development. These functions highlight the public health importance of any widespread environmental agent like perchlorate that potentially affects normal thyroid function.
In an analyses of the 2001-2 National Health and Nutrition Examination Survey, evidence of an association was identified between increasing urinary concentrations of perchlorate and decreasing levels of thyroxine (Blount et al., 2006), with the largest effects found in analyses restricted to women with urinary iodine levels < 100 µg/L and urinary thiocyanate levels in the upper tertile (> 1800 µg/L) (Blount et al., 2006; Steinmaus et al., 2007). Thiocyanate is a metabolite of cyanide found in foods or tobacco smoke and also blocks iodide uptake into the thyroid by the same mechanism as perchlorate (Braverman et al., 2005; Tonacchera et al., 2004; Wyngaarden et al., 1953). These findings suggest that people exposed to perchlorate who are also exposed to thiocyanate and have a low iodine intake will have substantially lower thyroid hormone levels than people who do not have any of these factors or people who are just exposed to perchlorate alone. These effects could have public health implications since they suggest that people exposed to thiocyanate and who have a low iodine intake may have the greatest risk from any adverse effects caused by perchlorate exposure, and thus should be given specific consideration when establishing or revising policies or regulations aimed at reducing perchlorate toxicity.
The National Health and Nutrition Examination Survey 2007-8, which is the first the National Health and Nutrition Examination Survey since 2001-2 with data on both thyroid hormones and perchlorate, provides an opportunity to help confirm the 2001-2 findings. One advantage of the National Health and Nutrition Examination Survey 2007-8 over the 2001-2 survey is that data were collected on several additional outcome measures and potential confounders including free thyroxine, triiodothyronine, free triiodothyronine, thyroid antibodies, and urinary specific gravity. Perhaps an even more important advantage is that perchlorate, iodine, thiocyanate, and thyroid hormones were collected in a larger number of subjects than in 2001-2 (5921 versus 2268). This larger sample size provides greater statistical power to identify associations, which is especially important when examining smaller subgroups.
A statistically significant association between increasing urinary perchlorate concentrations and decreasing serum thyroid hormone concentrations has already been reported in a recent study using data from the 2007-8 survey (Mendez and Eftim, 2012). However, the combined effects of perchlorate with iodine and thiocyanate were not assessed The goal of our study is to use this same data set to investigate whether people exposed to a combination of these three important thyroid disrupting factors (perchlorate, and thiocynate, and low iodine) have lower thyroid hormone levels than people who do not have any of these three factors or people who are exposed to just one. The combined effects of multiple agents on thyroid hormone production has been seen in animal and cellular studies (De Groef et al., 2006). The purpose of this investigation however, is to help confirm whether this same effect can also be seen in a human study.
Materials and Methods
The National Health and Nutrition Examination Survey is a national survey of health, nutrition, and sociodemographic information involving a complex multistage probability sampling design conducted by the Centers for Disease Control and Prevention (CDC, 2007). In the 2007-8 Survey, urinary levels of perchlorate, iodine, nitrate, thiocyanate, creatinine, and specific gravity, and serum levels of thyroxine, thyroid stimulating hormone, free thyroxine, triiodothyronine, free triiodothyronine, and two thyroid antibodies (thyroglobulin antibody and thyroperoxidase antibody) were analyzed in a majority sub-sample of all subjects. Information was also collected on other variables linked to thyroid hormone or antibody levels or to urinary iodine, perchlorate, thiocyanate, or creatinine concentrations in other studies, including gender, race (Mexican American, Other Hispanic, Non-Hispanic White, Non-Hispanic Black, or Other), age (20 year age groups), education (less than high school graduate, high school graduate, college, or other), income (<$20k, 20-60k, 60K+ per year) (Steinmaus 2010), smoking status (Steinmaus 2007), serum albumin (gm/dl), body mass index (kg/m2), 24 hour caloric intake (kcal/day from 24 hour dietary recall data), hours of fasting before sample collection (self-report) (Blount 2006), pregnancy status (based on urinary pregnancy test results and self-reports), post-menopausal status (based on self-report, time since last period, and presence or absence of other reasons for missed menstruation), premenarche status (self-report), current lactation (self-report), past or current thyroid disease (self-report), and use of medications known to affect thyroid hormone levels (e.g., levothyroxine, methimazole, propylthiouracil, beta blockers, lithium, amiodarone, and estrogens) (self-report) (Aoki et al., 2007).
The methods used to collect and perform laboratory analyses of perchlorate, iodine, thiocyanate, thyroxine, thyroid stimulating hormone, and other agents, and the quality control procedures involved in the 2001-2 and 2007-8 surveys are described in detail elsewhere (CDC, 2007). Briefly, 2007-8 serum samples were assayed for thyroid stimulating hormone using the Access HYPERsensitive human thyroid-stimulating hormone assay, a 3rd generation, two-site immunoenzymatic (“sandwich”) assay; for thyroxine using the Access Total Thyroxine assay, which is a competitive binding immunoenzymatic assay; and for free thyroxine using a two-step enzyme immunoassay. These assays were done using a Beckman Access or Access II Immunoassay System (Beckman Coulter, Fullerton, CA). Laboratory reference ranges for total thyroxine, free thyroxine, and thyroid stimulating hormone were 6.1–12.2 ug/dL, 0.6–1.6 ng/dL, and 0.34-5.60 IU/L, respectively. Similar methods were used for 2001-2 samples, although performed in a different laboratory. Urine samples were analyzed for perchlorate, thiocyanate, and nitrate using ion chromatography coupled with electrospray tandem mass spectrometry. Urine iodine concentrations were determined by inductively coupled plasma dynamic reaction cell mass spectroscopy.
In order to evaluate the combined effects of perchlorate, thiocyanate, and low iodine on thyroid hormone levels, subjects were categorized into one of three groups based on their urinary concentrations of these analytes. Group A, the “low perchlorate, low thiocyanate, and adequate iodine” group was considered the “low exposure” reference group and included subjects who had a combination of urinary perchlorate concentrations in the lower tertile of all urinary perchlorate concentrations, urinary thiocyanate concentrations in the lower tertile of all urinary thiocyanate concentrations, and urinary iodine concentrations ≥100 ug/L. Group B, the “moderate perchlorate, moderate thiocyanate, and adequate iodine” group, was considered the “medium exposure” group and included subjects who had a combination of urinary perchlorate concentrations in the middle tertile, urinary thiocyanate concentrations in the middle tertile, and urinary iodine concentrations ≥100 µg/L. Group C, the “high perchlorate, high thiocyanate, and low iodine” group, was considered the “high exposure” group and included subjects who had a combination of urinary perchlorate concentrations in the upper tertile, urinary thiocyanate concentrations in the upper tertile, and urinary iodine concentrations <100 µg/L. The mean levels of each thyroid hormone (our main dependent variables) in Groups C and B were then compared to those in Group A, with the a priori hypothesis that Groups C would have lower serum thyroxine or higher thyroid stimulating hormone levels than Group A, and Group B would have a mean thyroxine level between that in Groups A and C. The 100 ug/L cut-off for iodine was used because it was the same stratification level used in several previous similar studies (Blount 2006, Cao 2010, Steinmaus 2007) and because 100 µg/L is the cut-off point used for population medians by the World Health Organization (WHO) to define iodine deficiency in a population (WHO, 1994). Iodine tertiles were not used since some data suggests that very high iodine intakes in susceptible individuals can paradoxically decrease thyroid hormone production (Wolff and Chaikoff, 1948). Sample sizes were too small to perform the same categorical analyses on the 2001-2 data, although analyses were done with 2001-2 and 2007-8 data combined.
Previous investigations of thyroid-disrupting agents have commonly examined only one exposure agent at a time (Braverman et al., 2006; Brechner et al., 2000; Steinmaus et al., 2010). In order to compare the effects we found for perchlorate, thiocyanate, and iodine combined with the effects that would be seen from only examining one factor at a time, we performed separate analyses in which thyroid hormone levels in subjects in the upper tertile of perchlorate were compared to those in subjects in the lower tertile of perchlorate (independent of thiocyanate and iodine). Similar analyses were done for thiocyanate and iodine. Using quartiles instead of quartiles had no impact on conclusions.
All statistical analyses were done with SAS version 9.2 (SAS Institute Inc., Cary NC, USA) using Surveyreg with the domain function to analyze subgroups. The Surveyreg procedure allows the National Health and Nutrition Examination Survey complex sample design and weighting scheme to be incorporated with arguably more accurate estimates of variance. Analyses were also done without the use of these complex weighting schemes and these produced almost identical results. Inspection of the thyroid hormone residuals showed them to be normally distributed and the Durbin-Watson statistics for independence were near 2.0.
All categorical analyses were adjusted for age (in 20 year age groups), gender (except in gender stratified analyses), and urine specific gravity (to help adjust for urine dilution). Separate analyses were done in which exposure groups were based on tertiles of specific gravity-adjusted perchlorate, thiocyanate, and iodine concentrations (Sorahan 2008). Entering age in narrower age groups or as a continuous variable or using urine creatinine instead of specific gravity had little impact on results. Indicator variables for pregnancy status, premenarche, menopause, lactation status, obesity, thyroid-related medication use, history of or current thyroid disease, thyroglobulin and thyroid peroxidase antibodies (defined as positive if >4 IU/ml and >9 IU/ml, respectively), kcals, fasting time, albumin, race/ethnicity, highest education level achieved, and income were entered into each model one at a time, and factors that changed the perchlorate-thyroid hormone regression coefficients or mean differences between the exposure categories being assessed by more than 10% were retained in the model. Since nitrate can potentially inhibit iodide uptake and decrease thyroid hormone production by the same mechanism as perchlorate and thiocyanate, and it was evaluated in separate subgroup analyses. We also performed analyses where people on medications known to effect thyroid hormone levels or people with past or current thyroid disease were excluded. Initial univariate and multivariate analyses showed no clear evidence of combined effects of iodine, thiocyanate and urinary perchlorate on serum thyroid stimulating hormone, triiodothyronine, or free triiodothyronine, so the major focus of this paper is on thyroxine and free thyroxine. In addition to the categorical analyses described above, linear regression analyses using the urinary concentration of perchlorate as the independent variable and each thyroid hormone as the dependent variable (with both perchlorate and the thyroid hormone being assessed entered as continuous variables) were also performed, with stratifications by gender, iodine, and thiocyanate levels. These stratified linear regression analyses used the same methods that were used in the earlier analyses of the National Health and Nutrition Examination Survey 2001-2 data and were done for comparison purposes (Blount et al., 2006; Steinmaus et al., 2007).
Results
Mean and median levels of perchlorate, thyroid hormones and other factors in both the 2001-2 and 2007-8 surveys are shown in Table 1. Median levels of thiocyanate and total thyroxine were somewhat lower (12.9 and 5.0% lower, respectively), and median levels of thyroid stimulating hormone were somewhat higher (12.1%) in 2007-8 than in 2001-2. Tables 2 and 3 show the medians and proportions of various demographic and other variables across the three exposure groups we assessed. Urine creatinine and nitrate were lower in Group A than in the other groups (Table 2). Subjects in Group C were more likely to be male (odds ratio in Group C versus Group A = 2.64 (95% confidence interval = 1.53-4.53)), somewhat less likely to be White (odds ratio Group C versus Group A = 0.86 (95% confidence interval, 0.50-1.49)), and younger (the average age in Groups C and A were 37.9 and 48.8 years, respectively; mean difference = 10.9 years (95% confidence interval, 5.1-16.8) (Table 3). The numbers of subjects in Group C is lower than the other groups due to the fairly strong correlation between urinary iodine and perchlorate (Spearman correlation coefficient = 0.53, 95% confidence intervals=0.52-0.55). Most of this correlation remained after adjustment for urine specific gravity (adjusted Spearman correlation coefficient = 0.34, 95% confidence interval=0.32-0.36).
Table 1. Mean and median levels of urinary perchlorate and other factors: comparison of the National Health and Nutrition Examination Surveys 2001-2 and 2007-8a.
| Perchlorate (µg/L) |
Iodine (µg/L) |
Thiocyanate (µg/L) |
Totalthyroxine (µg/dl) |
Thyroidstimulatinghormone (IU/L) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
||||||
| 2001 | 2007 | 2001 | 2007 | 2001 | 2007 | 2001 | 2007 | 2001 | 2007 | |
| N | 2276 | 6044 | 2274 | 5921 | 2273 | 6044 | 2276 | 6044 | 2273 | 6044 |
| Mean | 5.34 | 5.98 | 425 | 447 | 2405 | 2525 | 8.16 | 7.82 | 2.04 | 2.03 |
| Median | 3.85 | 3.90 | 170 | 160 | 1400 | 1220 | 8.00 | 7.60 | 1.38 | 1.57 |
| Standard deviation | 7.07 | 9.15 | 4131 | 10169 | 2862 | 3660 | 1.92 | 1.66 | 8.83 | 2.74 |
| 10th Percentile | 1.20 | 1.14 | 50 | 53 | 360 | 303 | 6.00 | 6.00 | 0.63 | 0.69 |
| 90th Percentile | 10.0 | 12.0 | 488 | 453 | 6000 | 6530 | 10.50 | 9.80 | 2.94 | 3.47 |
Includes all subjects with a urinary perchlorate and serum thyroxine measurement.
Table 2.
Concentrations of thyroid hormones and other factors in exposure Groups A, B, and C.
| Group A (low perchlorate, low thiocyanate, adequate iodine) | Group B (moderate perchlorate, moderate thiocyanate, adequate iodine) | Group C (high perchlorate, high thiocyanate, low iodine) | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Units | N | Mean | Median | Std | Min | Max | N | Mean | Median | Std | Min | Max | N | Mean | Median | Std | Min | Max | ||
| Albumin | g/dl | 390 | 4.2 | 4.2 | 0.4 | 2.8 | 5.3 | 553 | 4.3 | 4.3 | 0.3 | 2.8 | 5.3 | 64 | 4.2 | 4.3 | 0.3 | 3.6 | 5.0 | ||
| Body mass index | kg/m2 | 386 | 27.5 | 26.6 | 6.0 | 13.6 | 63.9 | 546 | 28.3 | 27.5 | 6.4 | 14.6 | 58.0 | 61 | 28.4 | 27.9 | 7.7 | 18.5 | 61.7 | ||
| Cotinine | ng/ml | 389 | 9.7 | 0.0 | 68.1 | 0.0 | 879.0 | 553 | 7.9 | 0.0 | 42.9 | 0.0 | 459.0 | 64 | 134.6 | 56.7 | 161.9 | 0.0 | 597.0 | ||
| Creatinine | mg/dl | 390 | 92.2 | 74.5 | 66.0 | 11.0 | 446.0 | 553 | 139.1 | 126.0 | 69.5 | 20.0 | 490.0 | 64 | 141.3 | 136.5 | 64.3 | 17.0 | 331.0 | ||
| Fasting time | hours | 390 | 6.8 | 5.0 | 5.3 | 0.0 | 20.0 | 553 | 6.6 | 5.0 | 5.1 | 0.0 | 19.0 | 64 | 7.7 | 9.0 | 5.5 | 0.0 | 20.0 | ||
| Freetriio dothyronine | pg/ml | 390 | 3.11 | 3.10 | 0.47 | 2.00 | 6.30 | 553 | 3.24 | 3.20 | 0.45 | 2.30 | 5.20 | 64 | 3.27 | 3.30 | 0.39 | 2.50 | 4.30 | ||
| Free thyroxine | ng/dl | 390 | 0.82 | 0.80 | 0.20 | 0.40 | 2.60 | 553 | 0.79 | 0.80 | 0.14 | 0.30 | 1.60 | 64 | 0.75 | 0.70 | 0.12 | 0.50 | 1.10 | ||
| Iodine | µg/L | 390 | 2694 | 163 | 39283 | 100 | 762010 | 553 | 421 | 206 | 2248 | 101 | 50502 | 64 | 77 | 80 | 15 | 37 | 99 | ||
| Kilocalories | kcal | 375 | 1788 | 1645 | 832 | 280 | 5500 | 532 | 2077 | 1905 | 945 | 345 | 7211 | 63 | 2237 | 1948 | 1072 | 509 | 48 | ||
| Nitrate | µg/L | 390 | 33403 | 25950 | 27318 | 495 | 302000 | 553 | 59380 | 50000 | 55781 | 495 | 1010000 | 64 | 75095 | 64750 | 39232 | 18200 | 190000 | ||
| Perchlorate | µg/L | 390 | 1.80 | 1.86 | 0.59 | 0.36 | 2.74 | 553 | 4.11 | 4.02 | 0.86 | 2.75 | 5.80 | 64 | 11.35 | 9.15 | 7.49 | 5.82 | 47.80 | ||
| Specific gravity | na | 376 | 1.014 | 1.013 | 0.006 | 1.004 | 1.037 | 531 | 1.019 | 1.019 | 0.006 | 1.003 | 1.037 | 62 | 1.019 | 1.019 | 0.006 | 1.006 | 1.031 | ||
| Triiodothyronine | ng/dl | 390 | 111.0 | 106.5 | 27.4 | 50.0 | 311.0 | 553 | 114.1 | 112.0 | 24.4 | 42.0 | 202.0 | 64 | 116.8 | 119.5 | 24.1 | 71.0 | 190.0 | ||
| Total thyroxine | µg/L | 390 | 8.31 | 8.10 | 1.80 | 3.10 | 18.90 | 553 | 7.84 | 7.70 | 1.59 | 2.00 | 14.20 | 64 | 7.19 | 7.00 | 1.35 | 4.10 | 11.00 | ||
| Thiocyanate | µg/L | 390 | 427 | 436 | 190 | 14 | 778 | 553 | 1261 | 1210 | 323 | 779 | 1900 | 64 | 6800 | 4300 | 5894 | 1970 | 32200 | ||
| Thyroglobulin antibodies | IU/ml | 389 | 20.6 | 0.6 | 163.8 | 0.6 | 2306.3 | 553 | 6.2 | 0.6 | 71.5 | 0.6 | 1537.9 | 64 | 7.2 | 0.6 | 36.3 | 0.6 | 286.3 | ||
| Thyroperoxidase antibodies | IU/ml | 388 | 17.0 | 0.7 | 87.8 | 0.1 | 910.4 | 547 | 15.4 | 0.7 | 73.2 | 0.1 | 859.0 | 63 | 26.0 | 0.5 | 94.7 | 0.1 | 590.9 | ||
| Thyroid stimulating hormone | IU/L | 390 | 2.06 | 1.65 | 1.63 | 0.00 | 13.19 | 553 | 2.13 | 1.67 | 3.70 | 0.01 | 80.97 | 64 | 2.31 | 1.87 | 3.25 | 0.45 | 25.95 | ||
Abbreviations: Max, maximum, Min, minimum; N, sample size; Std, standard deviation.
Table 3. Sociodemographic variables in exposure Groups A, B, and C.
| Group A | Group B | Group C | Total | ||||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| N | % | N | % | N | % | ||
| Age (years old) | |||||||
| < 20 | 32 | 8% | 106 | 19% | 11 | 17% | 149 |
| 20-40 | 92 | 24% | 138 | 25% | 19 | 30% | 249 |
| 40-60 | 79 | 20% | 133 | 24% | 26 | 41% | 238 |
| 60+ | 187 | 48% | 176 | 32% | 8 | 13% | 371 |
| Total | 390 | 100% | 553 | 100% | 64 | 100% | 1007 |
| Gender | |||||||
| Males | 145 | 37% | 307 | 56% | 39 | 61% | 491 |
| Females | 245 | 63% | 246 | 44% | 25 | 39% | 516 |
| Total | 390 | 100% | 553 | 100% | 64 | 100% | 1007 |
| Race | |||||||
| Mexican | |||||||
| American | 83 | 21% | 128 | 23% | 4 | 6% | 215 |
| Other Hispanic | 61 | 16% | 75 | 14% | 8 | 13% | 144 |
| White | 160 | 41% | 249 | 45% | 24 | 38% | 433 |
| Black | 66 | 17% | 88 | 16% | 24 | 38% | 178 |
| Other | 20 | 5% | 13 | 2% | 4 | 6% | 37 |
| Total | 390 | 100% | 553 | 100% | 64 | 100% | 1007 |
| Education | |||||||
| <High School | 123 | 32% | 129 | 23% | 16 | 25% | 268 |
| High School | 89 | 23% | 108 | 20% | 11 | 17% | 208 |
| College | 146 | 37% | 214 | 39% | 27 | 42% | 387 |
| Unknown/Other | 32 | 8% | 102 | 18% | 10 | 16% | 144 |
| Total | 390 | 100% | 553 | 100% | 64 | 100% | 1007 |
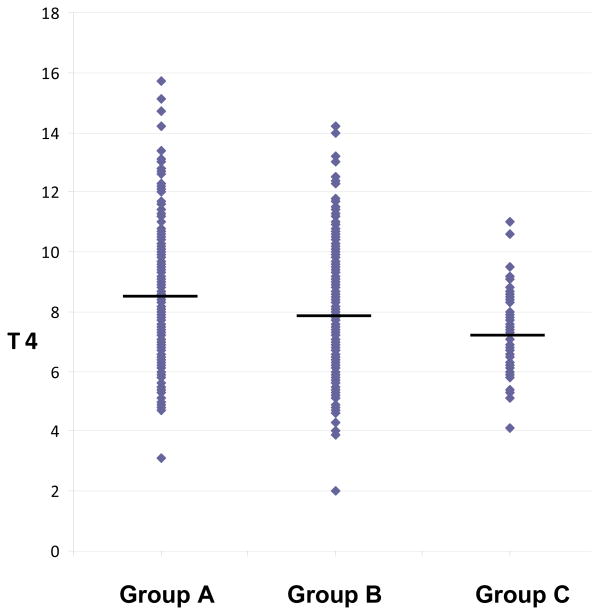
Analyses evaluating each major exposure factor (perchlorate, thiocyanate, and iodine) one at a time are shown in Table 4. As seen, in analyses adjusted for age, gender and specific gravity, differences in iodine (<100 vs. ≥100 µg/L), thiocyanate (upper versus lower tertiles), and perchlorate (upper versus lower tertiles) by themselves were associated with a 0.3%, 2.5%, and 5.0% difference in mean total thyroxine, respectively. Table 5 shows the results of the analyses comparing mean total and free thyroxine levels across exposure Groups A, B, and C. The unadjusted mean total thyroxine values in exposure Groups A, B, and C were 8.31, 7.84, and 7.19 µg/dl, respectively. Subjects in Group C had an age, sex, and specific gravity-adjusted mean total thyroxine value that was 12.9% lower than Group A (mean difference = 1.07 µg/dl, 95% confidence interval=0.55-1.59), while subjects in Group B had an adjusted mean total thyroxine value that was 5.1% lower than subjects in Group A (mean difference = 0.42 µg/dl, 95% confidence interval=0.00-0.85). Similar but somewhat smaller effects were seen for free thyroxine. The individual total thyroxine values used in these analyses are shown in the online Appendix (Figure 1A). Differences in mean total thyroxine levels between Group C and A (mean difference = 0.97 µg/dl, 95% confidence interval=0.59-1.35) and between Groups B and A (mean difference =0.38, 95% confidence interval=-0.07-0.82) remained when 2001-2 and 2007-8 data were combined. And, similar results were seen when exposure groups were based on specific gravity-adjusted perchlorate, thiocyanate, and iodine concentrations (online Appendix Table A1). When urinary nitrate levels were used in place of one of the other thyroid disruptors, the difference in mean total thyroxine levels between the exposure groups was much less. For example, if nitrate is used in place of perchlorate in defining exposure Groups A, B, and C, the age, gender, and specific gravity-adjusted difference in mean total thyroxine levels between Groups C and A decreased from 12.9% to 5.1% (mean difference = 0.40 µg/dl, 95% confidence interval=0.04-0.78).
Table 4. Mean total thyroxine concentrations assessing one exposure factor (perchlorate, thiocyanate, or iodine) at a time.
| Group | N | Total thyroxine | Unadjusted | Adjustedd | |||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Mean | Standard deviation | Differencea | Differencea | ||||||
|
|
|
||||||||
| µg/dl | 95% CI | %b | µg/dl | 95% CI | %b | ||||
| Iodine (µg/L) | |||||||||
| ≥ 100 | 4271 | 7.82 | 1.65 | Refc | -- | -- | Refc | -- | -- |
| < 100 | 1992 | 7.87 | 1.70 | -0.05 | -0.14-0.04 | -0.6% | 0.02 | -0.11-0.15 | 0.3% |
| Thiocyanate | |||||||||
| Lower tertile | 1915 | 8.05 | 1.74 | Refc | -- | -- | Refc | -- | -- |
| Upper tertile | 1952 | 7.63 | 1.65 | 0.42 | 0.31-0.53 | 5.2% | 0.20 | 0.01-0.39 | 2.5% |
| Perchlorate | |||||||||
| Lower tertile | 2084 | 7.99 | 1.75 | Refc | -- | -- | Refc | -- | -- |
| Upper tertile | 1939 | 7.67 | 1.60 | 0.32 | 0.21-0.42 | 4.0% | 0.40 | 0.14-0.65 | 5.0% |
Difference = mean difference compared to the reference group (i.e., total or free thyroxine in the reference group minus total or free thyroxine in the non-reference group).
The percent difference compared to the reference group.
The reference group.
Adjusted for age, sex, and urine specific gravity and weighted using the National Health and Nutrition Examination Survey complex weighting scheme and surveyreg.
Table 5. Mean total and free thyroxine concentrations in exposure Groups A, B, and C.
| Exposure Group | Unadjusted | Adjustedd | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||
| N | Mean | Standard deviation | Differencea | Ne | Differencea | ||||||
|
|
|
||||||||||
| µg/dl | 95% CI | %b | µg/dl | 95% CI | %b | ||||||
| Total thyroxine | |||||||||||
| Group A | 390 | 8.31 | 1.80 | Refc -- | -- | -- | 376 | Refc | -- | -- | |
| Group B | 553 | 7.84 | 1.59 | 0.46 | 0.25-0.68 | 5.5% | 531 | 0.42 | 0.00-0.85 | 5.1% | |
| Group C | 64 | 7.19 | 1.35 | 1.11 | 0.73-1.49 | 13.4% | 62 | 1.07 | 0.55-1.59 | 12.9% | |
| Free thyroxine | |||||||||||
| Group A | 390 | 0.822 | 0.196 | Refc | -- | -- | 376 | Refc | -- | ||
| Group B | 553 | 0.793 | 0.142 | 0.029 | 0.006-0.052 | 3.5% | 531 | 0.023 | -0.009-0.055 | 2.8% | |
| Group C | 64 | 0.750 | 0.121 | 0.072 | 0.036-0.108 | 8.8% | 62 | 0.058 | 0.009-0.055 | 2.8% | |
Difference = mean difference compared to Group A (i.e., total or free thyroxine in Group A minus total or free thyroxine in Groups B or C)
The percent difference compared to Group A.
The reference group.
Adjusted for age, sex, and urine specific gravity and weighted using the National Health and Nutrition Examination Survey complex weighting scheme and surveyreg.
Differences in sample sizes between the adjusted and unadjusted analyses are due to the additional subjects without data on specific gravity in the unadjusted analyses.
Figure 1A. Total thyroxine levels by exposure group.
The horizontal bars represent the mean total thyroxine value in each exposure group. The diamonds represent the unadjusted total thyroxine values for each subject.
Both adjusted and stratified analyses showed that the age and gender differences across the exposure groups had only small effects on results. For example, as shown in Table 5, total thyroxine differences across Groups A, B, and C in the unadjusted analyses were similar to those in the age, gender, and specific gravity adjusted analyses. And, differences in total thyroxine values across the groups remained in analyses stratified by either age or gender (not shown). For example, in analyses stratified into age groups ≤ 20, 21-40, 41-60, and 60+ years, mean total thyroxine values adjusted for sex and specific gravity were 15.2% (mean difference = 1.26 µg/dl), 8.5% (mean difference = 0.69 µg/dl), 10.8% (mean difference = 0.87 µg/dl), and 14.3% (mean difference = 1.22 µg/dl) lower in Group C than in Group A, respectively. For males and females, respectively, mean total thyroxine values adjusted for age and specific gravity were 12.7% (mean difference = 0.98 µg/dl) and 11.1% (mean difference = 0.92 µg/dl) lower in Group C than in Group A. Excluding subjects with a history of thyroid disease, thyroid medication, beta-blocker, lithium, amiodarone, or estrogen use, or excluding pregnant or lactating women involved only a few excluded subjects and had essentially no effect on results. In initial analyses, all models were adjusted for many different potential confounding variables. This includes those factors known to be among the most important determinants of thyroid hormone levels in large population based samples (e.g., age, gender, thyroid disease, medications, and race). None of these factors changed the mean difference by more than 10%.
In linear regression analyses with the logarithm of perchlorate concentration as the main independent variable and each thyroid hormone concentration as the dependent variable, both entered as continuous variables, the fairly strong associations seen in 2001-2 between perchlorate and total thyroxine in women with low iodine, or in women with both low iodine and high thiocyanate are either less strong or not present in the 2007-8 results (not in Tables). For example, the regression coefficient regression between total thyroxine and the logarithm of perchlorate was -0.73 (n=362, p=0.004) in the 2001-2 data and -0.33 (n=907, p=0.16) in the 2007-8 data. Removing outlying values at the upper or lower ranges of total thyroxine or perchlorate, using the 2001-2 upper tertile cut-off point to define an elevated thiocyanate in the 2007-8 analysis, or using urinary creatinine instead of urine specific gravity had no effect on the 2007-8 results.
Discussion
Overall, the results of these analyses provide evidence that subjects who are exposed to a combination of high perchlorate, high thiocyanate, and have low urinary iodine concentrations have markedly lower total thyroxine and free thyroxine levels than people without these three factors. The finding that greater effects are seen when all three factors (high perchlorate and thiocyanate and low iodine) are assessed together than when each was evaluated individually highlights the importance of evaluating the combined effects of multiple agents when assessing the impacts of thyroid disrupting chemicals. Previous data from animal and laboratory studies have shown that perchlorate, thiocyanate, and low iodine may act in combination to lower iodide intake into the thyroid gland (De Groef et al. 2006). However, the relevance of these studies to humans is unknown since in vitro exposures differ from actual human exposures, and rats differ markedly from humans in thyroid hormone half-lives, protein binding, sodium iodide symporter expression, perchlorate sensitivity, and co-exposures (Lewandowski et al., 2004; OEHHA, 2004. The data presented here provides a relatively rare example of these effects in a human epidemiologic study.
Adjustments, stratifications, and exclusions based on age, gender, thyroid disease, medication use, and many other factors provides evidence that confounding is not responsible for the effects identified here. The most likely reason why these factors did not cause important confounding is that while they may be related to thyroid hormone levels, they are not strongly related to iodine, perchlorate or thiocyanate. In order for a variable to cause important confounding, it must not only be associated with the outcome and the exposures of interest, but these associations must be fairly strong (Axelson, 1978). Because many of the variables that can affect thyroid hormone levels are relatively rare (e.g. certain medications or rare medical conditions) or are not strongly associated with urinary perchlorate, iodine, and thiocyanate concentrations (e.g. obesity, thyroid antibodies) it is not surprising that they were not important confounders in our study. It is possible that some other confounding factor which we did not assess was responsible for the effects identified. However, it seems unlikely that a completely unknown factor is prevalent enough, and strongly enough associated with both thyroid hormone and thyroid disruptor levels to cause the 12.9% decrease in mean thyroid hormone levels seen here.
The National Health and Nutrition Examination Survey is a cross-sectional survey so the results presented here are based on only a single assessment of urinary perchlorate, thiocyanate, iodine, and serum thyroid hormones. Previous studies have shown that these factors are all variable within a day and from day to day, so a single measurement might not reflect true long-term levels (Hollowell et al., 1998; Surks et al., 2005). Importantly, though, biologic samples in the National Health and Nutrition Examination Survey are collected and analyzed similarly in all subjects, independent of perchlorate, iodine, thiocyanate, or thyroid hormone status. Thus, any misclassification of these variables is likely to be non-differential, and therefore would bias estimates of association toward the null, not toward the positive effects identified in this study (Rothman and Greenland, 1998).
The major findings presented here are biologically plausible in that they are consistent with the known mechanism by which perchlorate, low iodine, and thiocyanate are all known to affect thyroid function (OEHHA, 2004). Iodide is a key component of thyroid hormone and sufficient suppression of iodide uptake into the thyroid is known to diminish production of thyroid hormone. Perchlorate, thiocyanate, and nitrate competitively inhibit the sodium iodide symporter, the membrane protein that actively transports iodide into the thyroid follicular cell for thyroid hormone production (Stanbury and Wyngaarden, 1952; Wyngaarden et al., 1952). In vitro research involving cells transfected with human sodium iodide symporter have shown that perchlorate, nitrate, and thiocyanate may have additive effects on inhibiting the sodium iodide symporter (Tonacchera et al., 2004). In human studies, thiocyanate has been linked to increased risks of goiter, both in those with moderate to severe iodine deficiency (< 50 µg/g creatinine) and in those within the lower range of adequate iodine intake (100 µg/g creatinine) (Brauer et al., 2006). In a previous study, goiters due to the consumption of thiocyanate in cassava were more likely when iodine levels were low and were reversed with iodine supplementation (Delanghe et al., 1982).
The reason why nitrate showed little effect in our study is unknown. Nitrate is found in highly variable concentrations in many different foods and is a major byproduct of internal metabolism. As such, it is possible that a single urinary nitrate concentration is a poor reflection of long-term nitrate status at the target organ site. Some studies have linked nitrate to goiter and other thyroid effects, but these were mostly ecologic, unblinded, or based on well water or dietary nitrate intake levels rather than urinary concentrations (Gatseva and Argirova, 2008; Ward et al., 2010). A controlled clinical trial found that nitrate doses of three times the acceptable intake level had no effects on thyroid iodide uptake or thyroid hormone levels, supporting our findings that nitrate has weak or no effect on human thyroid function (Hunault et al., 2007).
The findings that relatively low levels of perchlorate may impact thyroid function are consistent with several other studies, especially those involving susceptible subgroups like young children or those with low iodine intake. This includes the 2001-2 National Health and Nutrition Examination Survey discussed previously (Blount et al., 2006; Steinmaus et al., 2007). In Arizona, neonatal thyroid stimulating hormone levels were higher in perchlorate-exposed Yuma (water levels of about 6 µg/L) than in unexposed Flagstaff (Brechner et al., 2001; Brechner et al., 2000). Elevations in thyroid stimulating hormone are commonly used to assess decreased total thyroxine production since decreases in total thyroxine stimulate the pituitary to secrete thyroid stimulating hormone, which stimulates the thyroid to produce more total thyroxine. A reanalysis of the Arizona data using a different “unexposed” comparison town found no difference in thyroid stimulating hormone levels compared with Yuma (Lamm, 2003). However, this new comparison town was much smaller and only a few miles from Yuma, and the possibility that mothers from this town either worked, consumed food or water, or gave birth in Yuma, and thus also were also perchlorate-exposed, was not discussed. In an ecologic analysis involving almost 500,000 California mother-offspring pairs, neonates whose mothers had likely residential drinking water perchlorate concentrations > 5 µg/L had an up to 53% higher odds of having an elevated thyroid stimulating hormone (above the 95th percentile) than neonates of mothers with lower drinking water perchlorate concentrations (Steinmaus et al., 2010). In Cao et al., urinary perchlorate (expressed as µg of perchlorate per gram of creatinine) was correlated with urinary thyroid stimulating hormone levels (expressed as mlU of thyroid stimulating hormone per gram of creatinine) in neonates with iodine levels < 100 µg/L but not in neonates with iodine concentrations ≥ 100 µg/L, although adjustments for urinary creatinine for both thyroid stimulating hormone and perchlorate could be responsible for some of this correlation (Cao et al., 2010). Other studies of perchlorate and thyroid hormone levels have not identified clear associations, but most of these have taken place in workers or healthy volunteers who may not be particularly susceptible to perchlorate, and most did not assess the combined effects of perchlorate and other thyroid disruptors (Braverman et al., 2005; Braverman et al., 2006; Greer et al., 2002; Lamm et al., 1999). Studies in Chile and Israel found no association between perchlorate drinking water concentrations near or above 100 µg/L and thyroid hormone levels in neonates (Crump et al., 2000; Tellez Tellez et al., 2005), although in the Chile studies there was a marked overlap in urinary perchlorate concentrations between “exposed” and “unexposed” study areas; 45% of the “exposed” neonates were actually born in the unexposed city; and sample sizes were small in most analyses. Interestingly, the odds for a history of thyroid disease among the Chilean neonate's older family members was almost 5-fold higher in the exposed city than in the unexposed city (OR = 4.97; 95% CI, 1.29 to 19.2) (Crump et al., 2000). In the Israeli study, no perchlorate-total thyroxine associations were found but there were only 31 mothers in the high exposure group. Two cross-sectional studies of urinary perchlorate concentrations and thyroid hormone levels in pregnant women, including subgroups with urinary iodine concentrations < 100 µg/L, have also been negative, although thiocyanate levels were very low in the one study in which they were assessed (e.g. median thiocyanate levels of about 420 vs. 1220 µg/L here) (Pearce et al., 2007; Pearce et al., 2010). In summary, while the current literature includes some evidence that low levels of perchlorate can impact thyroid function, findings are not consistent across all studies. A large prospective cohort study in an important susceptibility group like pregnant women or young children, in a population with a wide range of stable perchlorate exposures would help delineate exact dose-response relationships and the role of important co-exposures like thiocyanate, iodine, and other thyroid disrupting agents.
Although we found evidence that perchlorate, thiocyanate, and low iodine can reduce serum total thyroxine and free thyroxine levels, no clear effect was seen on thyroid stimulating hormone. The reason for this is unknown but several possibilities exist. One relates to the fact that thyroid stimulating hormone levels are generally much more variable than total thyroxine levels. In this dataset, the coefficient of variation for thyroid stimulating hormone was more than 6-times larger than that for thyroxine (135% vs. 21%, respectively). This greater variability will result in less power to identify statistically significant effects. Another possibility is that although suppression of thyroxine is accompanied by a compensatory rise in serum thyroid stimulating hormone in many conditions, this does not necessarily occur in all conditions. For example, iodine deficiency is characterized by decreases in serum total thyroxine with normal or slightly elevated levels of serum triiodothyronine and normal levels of serum thyroid stimulating hormone (Obregon et al., 2005). It has been shown that with iodine deficiency total thyroxine is deiodinated to triiodothyronine (LaFranchi, 2004). Based on this, it has been suggested that although iodine deficiency may cause a decrease in serum total thyroxine levels, an associated increase in triiodothyronine exerts a negative feedback limiting any compensatory rise in thyroid stimulating hormone (Maruta and Greer, 1988). This effect could be particularly deleterious during pregnancy since thyroxine is the predominant maternal form that crosses the placenta (Dussault 1969). This same mechanism could explain why we found clear differences for total thyroxine but not for thyroid stimulating hormone across the three different exposure groups we assessed.
As mentioned, there were fewer subjects in the Group C than in the other groups elevated due to the fairly strong correlation between urinary iodine and perchlorate. The reason for this correlation is unknown but could be caused if both factors occur in the same foods or water or if perchlorate increases the urinary excretion of iodine by inhibiting iodine uptake into other organs.
The results of the linear regression analyses on the associations between perchlorate and total thyroxine in women with low iodine were not consistent between the 2001-2 and 2007-8 surveys. The reason for this is unknown. One possible explanation might be confounding, but as discussed above, a clear and obvious factor is not evident. Another possibility might be chance. Although p-values were low, the fact that multiple comparisons were done in both years increases the possibility that some findings (either positive or negative) could be due to chance. Another explanation could be related to certain potential weaknesses in the linear regression analyses themselves. One potential weakness involves the use of the logarithm of perchlorate, rather than the perchlorate concentration itself, as the main exposure variable. In the previously published 2001-2 analyses (Blount et al., 2006; Steinmaus et al., 2007), the logarithm of perchlorate was used to help normalize the distribution of this variable and its corresponding residuals in order to help meet the assumptions of the linear regression model. It was not done based on a clear a priori hypothesis or on actual mechanistic data. This is important because if the dose-response relationship is not truly logarithm-linear, but rather is some other shape (e.g. linear-linear), this logarithm transformation could lead to true associations being missed. Another potential weakness involves the use of stratified analyses to evaluate potentially susceptible subgroups, and the inability of these stratified analyses to directly evaluate combined effects. In other words, the best way to evaluate whether perchlorate, thiocyanate, and low iodine all act together to decrease total thyroxine levels is to directly compare a group of subjects with high perchlorate, high thiocyanate, and low iodine to another group of subjects with low perchlorate, low thiocyanate, and adequate iodine levels. This is what was done in our categorical analyses (Table 5), but this is not what is done in the stratified linear regression analyses. For example, in the linear regression analyses stratified by iodine levels < 100 µg/L, women with high perchlorate are being compared to women with low perchlorate, but everyone in this analysis has low iodine. If low iodine is truly a susceptibility factor, then we are essentially comparing susceptible people to susceptible people. By not directly comparing people with all three risk factors (i.e. high perchlorate, high thiocyanate, low iodine) to a separate group of people without any of these three risk factors, the true combined impact of these three exposures could be missed.
Conclusion
In conclusion, the findings from this study provide evidence that fairly common levels of perchlorate and thiocyanate, combined with low iodine intake, act in combination to decrease thyroid hormone production. The public health significance of these findings lies in the widespread nature of exposure to perchlorate, thiocyanate, and the many other thyroid disrupting agents. The significance also lies in the critical nature of thyroid hormone in neurodevelopment and the potential importance that relatively small decreases in thyroid hormones may have in young children and others. Several recent studies have linked small to moderate decreases (e.g. 11% to 32%) in maternal thyroxine during pregnancy and 7% to 16% decreases in IQ or other developmental index scores in children (Haddow et al., 1999; Kooistra et al., 2006; Pop et al., 2003; Pop et al., 1999; Vermiglio et al., 2004). Other studies have identified links between small changes in thyroid hormone and increases in cardiovascular disease risk factors like serum lipids and carotid arterial narrowing in adults, although these involved increases in thyroid stimulating hormone rather than the decreases in thyroxine and free thyroxine we identified here (Asvold et al., 2007; Canaris et al., 2000; Dullaart et al., 2007). The effects reported in some of these studies are seen throughout a wide range of thyroid hormone levels, including in people with thyroxine levels that are within normal reference ranges and in people without any other overt signs of thyroid disease. The important role of thyroid hormone in neurodevelopment and in cardiovascular disease suggests that any common environmental exposure that could result in altered production or utilization of thyroid hornmone should be considered a significant public health issue. Although millions of people in the U.S. have perchlorate in their drinking water (Blount et al., 2010), the U.S. Environmental Protection Agency currently does not have a regulatory standard for perchlorate. Given the results presented here showing impacts at relatively low and common perchlorate exposures, a new regulatory standard might be needed to help protect those people who are exposed to perchlorate in combination with other thyroid disrupting agents.
Supplementary Material
Research Highlights.
Recent data suggest that essentially everyone in the US is exposed to perchlorate.
Perchlorate exposure may be associated with lower thyroid hormone levels.
Some groups may be more susceptible to perchlorate than others.
Acknowledgments
Funding source: Funding for this project was provided by grant 1R01ES020365-01 from the National Institute of Environmental Health Sciences and from the University of California Center for Occupational and Environmental Health.
The views expressed are those of the authors and do not necessarily represent those of the Office of Environmental Health Hazard Assessment, the California Environmental Protection Agency, or the State of California.
Informed consent was obtained from all subjects in the original National Health and Nutrition Examination Survey. The analysis presented here involves only de-identified data that are publically available.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Craig Steinmaus, Email: craigs@berkeley.edu.
Mark D. Miller, Email: ucsfpehsumiller@gmail.com.
Lara Cushing, Email: lara.cushing@berkeley.edu.
Benjamin C. Blount, Email: bkb3@cdc.gov.
Allan H. Smith, Email: ahsmith@berkeley.edu.
References
- Aoki Y, et al. Serum TSH and total T4 in the United States population and their association with participant characteristics: National Health and Nutrition Examination Survey (NHANES 1999-2002) Thyroid. 2007;17:1211–23. doi: 10.1089/thy.2006.0235. [DOI] [PubMed] [Google Scholar]
- Asvold BO, et al. The association between TSH within the reference range and serum lipid concentrations in a population-based study. The HUNT Study. Eur J Endocrinol. 2007;156:181–6. doi: 10.1530/eje.1.02333. [DOI] [PubMed] [Google Scholar]
- Axelson O. Aspects on confounding in occupational health epidemiology. Scand J Work Environ Health. 1978;4:85–89. doi: 10.5271/sjweh.2720. [DOI] [PubMed] [Google Scholar]
- Blount BC, et al. Perchlorate, nitrate, and iodide intake through tap water. Environ Sci Technol. 2010;44:9564–70. doi: 10.1021/es1025195. [DOI] [PubMed] [Google Scholar]
- Blount BC, et al. Urinary perchlorate and thyroid hormone levels in adolescent and adult men and women living in the United States. Environ Health Perspect. 2006:114, 1865–71. doi: 10.1289/ehp.9466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauer VF, et al. The role of thiocyanate in the etiology of goiter in an industrial metropolitan area. Eur J Endocrinol. 2006;154:229–35. doi: 10.1530/eje.1.02076. [DOI] [PubMed] [Google Scholar]
- Braverman LE, et al. The effect of perchlorate, thiocyanate, and nitrate on thyroid function in workers exposed to perchlorate long-term. J Clin Endocrinol Metab. 2005;90:700–6. doi: 10.1210/jc.2004-1821. [DOI] [PubMed] [Google Scholar]
- Braverman LE, et al. Effects of six months of daily low-dose perchlorate exposure on thyroid function in healthy volunteers. J Clin Endocrinol Metab. 2006;91:2721–4. doi: 10.1210/jc.2006-0184. [DOI] [PubMed] [Google Scholar]
- Brechner R, et al. Re: Ammonium perchlorate contamination of Colorado River drinking water is associated with abnormal thyroid function in newborns in Arizona (Letter) J Occup Environ Med. 2001;43:308–9. doi: 10.1097/00043764-200008000-00002. [DOI] [PubMed] [Google Scholar]
- Brechner RJ, et al. Ammonium perchlorate contamination of Colorado River drinking water is associated with abnormal thyroid function in newborns in Arizona. J Occup Environ Med. 2000;42:777–82. doi: 10.1097/00043764-200008000-00002. [DOI] [PubMed] [Google Scholar]
- Canaris GJ, et al. The Colorado thyroid disease prevalence study. Arch Intern Med. 2000;160:526–34. doi: 10.1001/archinte.160.4.526. [DOI] [PubMed] [Google Scholar]
- Cao Y, et al. Goitrogenic anions, thyroid-stimulating hormone, and thyroid hormone in infants. Environ Health Perspect. 2010:118, 1332–7. doi: 10.1289/ehp.0901736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC, Centers for Disease Control and Prevention. National Center for Health Statistics (NCHS) National Health and Nutrition Examination Survey Data 2007-2008. U.S. Department of Health and Human Services. 2007 http://www.cdc.gov/nchs/nhanes.htm.
- Crump C, et al. Does perchlorate in drinking water affect thyroid function in newborns or school-age children? J Occup Environ Med. 2000;42:603–12. doi: 10.1097/00043764-200006000-00009. [DOI] [PubMed] [Google Scholar]
- De Groef B, et al. Perchlorate versus other environmental sodium/iodide symporter inhibitors: potential thyroid-related health effects. Eur J Endocrinol. 2006;155:17–25. doi: 10.1530/eje.1.02190. [DOI] [PubMed] [Google Scholar]
- Delanghe F, et al. Nutritional factors involved in the goitrogenic action of cassava. International Development Research Centre; Ottawa: 1982. pp. 1–25. [Google Scholar]
- Dullaart RP, et al. Carotid artery intima media thickness is inversely related to serum free thyroxine in euthyroid subjects. Clin Endocrinol (Oxf) 2007;67:668–73. doi: 10.1111/j.1365-2265.2007.02943.x. [DOI] [PubMed] [Google Scholar]
- Gatseva PD, Argirova MD. Iodine status and goitre prevalence in nitrate-exposed schoolchildren living in rural Bulgaria. Public health. 2008;122:458–61. doi: 10.1016/j.puhe.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Greer MA, et al. Health effects assessment for environmental perchlorate contamination: the dose response for inhibition of thyroidal radioiodine uptake in humans. Environ Health Perspect. 2002:110, 927–37. doi: 10.1289/ehp.02110927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddow JE, et al. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. NEJM. 1999;341:549–55. doi: 10.1056/NEJM199908193410801. [DOI] [PubMed] [Google Scholar]
- 8.Hollowell JG, et al. Iodine nutrition in the United States. Trends and public health implications: iodine excretion data from National Health and Nutrition Examination Surveys I and III (1971-1974 and 1988-1994) J Clin Endocrinol Metab. 1998;83:3401. doi: 10.1210/jcem.83.10.5168. [DOI] [PubMed] [Google Scholar]
- Hunault CC, et al. Effects of sub-chronic nitrate exposure on the thyroidal function in humans. Toxicol Lett. 2007;175:64–70. doi: 10.1016/j.toxlet.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Kooistra L, et al. Neonatal effects of maternal hypothyroxinemia during early pregnancy. Pediatrics. 2006;117:161–7. doi: 10.1542/peds.2005-0227. [DOI] [PubMed] [Google Scholar]
- LaFranchi S. Endemic Goiter and Cretinism. In: Behrman R, et al., editors. Nelson Textbook of Pediatrics. Saunders; Philadelphia: 2004. [Google Scholar]
- Lamm SH. Perchlorate exposure does not explain differences in neonatal thyroid function between Yuma and Flagstaff. J Occup Environ Med. 2003;45:1131–2. doi: 10.1097/01.jom.0000094991.31330.d3. [DOI] [PubMed] [Google Scholar]
- Lamm SH, et al. Thyroid health status of ammonium perchlorate workers: a cross-sectional occupational health study. J Occup Environ Med. 1999;41:248–60. doi: 10.1097/00043764-199904000-00006. [DOI] [PubMed] [Google Scholar]
- Lewandowski TA, et al. Interspecies differences in susceptibility to perturbation of thyroid homeostasis: a case study with perchlorate. Regul Toxicol Pharmacol. 2004;39:348–62. doi: 10.1016/j.yrtph.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Maruta S, Greer MA. Evidence that thyroxine inhibits either basal or TRH-induced TSH secretion only after conversion to triiodothyronine. Proc Soc Exp Biol Med. 1988;187:391–7. doi: 10.3181/00379727-187-42679. [DOI] [PubMed] [Google Scholar]
- Mendez W, Jr, Eftim SE. Biomarkers of perchlorate exposure are correlated with circulating thyroid hormone levels in the 2007-2008 NHANES. Environmental research. 2012;118:137–44. doi: 10.1016/j.envres.2012.05.010. [DOI] [PubMed] [Google Scholar]
- Obregon MJ, et al. The effects of iodine deficiency on thyroid hormone deiodination. Thyroid. 2005;15:917–29. doi: 10.1089/thy.2005.15.917. [DOI] [PubMed] [Google Scholar]
- OEHHA. Public Health Goals for Chemicals in Drinking Water. Perchlorate Office ofEnvironmental Health Hazard Assessment California Environmental Protection Agency; Oakland, CA: 2004. [Google Scholar]
- Pearce E, et al. Thyroid function is not affected by environmental perchlorate in first trimester pregnant women. Thyroid. 2007;17:S133. [Google Scholar]
- Pearce EN, et al. Perchlorate and thiocyanate exposure and thyroid function in first-trimester pregnant women. J Clin Endocrinol Metab. 2010;95:3207–15. doi: 10.1210/jc.2010-0014. [DOI] [PubMed] [Google Scholar]
- Pop VJ, et al. Maternal hypothyroxinaemia during early pregnancy and subsequent child development: a 3-year follow-up study. Clin Endocrinol (Oxf) 2003;59:282–8. doi: 10.1046/j.1365-2265.2003.01822.x. [DOI] [PubMed] [Google Scholar]
- Pop VJ, et al. Low maternal free thyroxine concentrations during early pregnancy are associated with impaired psychomotor development in infancy. Clin Endocrinol (Oxf) 1999;50:149–55. doi: 10.1046/j.1365-2265.1999.00639.x. [DOI] [PubMed] [Google Scholar]
- Rothman K, Greenland S. Precision and validity of studies. In: Rothman K, Greenland S, editors. Modern Epidemiology. Lippincott Raven; Philadelphia: 1998. pp. 115–134. [Google Scholar]
- Stanbury J, Wyngaarden J. Effect of perchlorate on the human thyroid gland. Metab Clin Exp. 1952;1:533–39. [PubMed] [Google Scholar]
- Steinmaus C, et al. Impact of smoking and thiocyanate on perchlorate and thyroid hormone associations in the 2001-2002 National Health and Nutrition Examination Survey. Environ Health Perspect. 2007;115:1333–8. doi: 10.1289/ehp.10300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmaus C, et al. Perchlorate in drinking water during pregnancy and neonatal thyroid hormone levels in California. J Occup Environ Med. 2010;52:1217–1224. doi: 10.1097/JOM.0b013e3181fd6fa7. [DOI] [PubMed] [Google Scholar]
- Surks MI, et al. The thyrotropin reference range should remain unchanged. J Clin Endocrinol Metab. 2005;90:5489–96. doi: 10.1210/jc.2005-0170. [DOI] [PubMed] [Google Scholar]
- Tellez R. Long-term environmental exposure to perchlorate through drinking water and thyroid function during pregnancy and the neonatal period. Thyroid. 2005;15:963–75. doi: 10.1089/thy.2005.15.963. [DOI] [PubMed] [Google Scholar]
- Tonacchera M, et al. Relative potencies and additivity of perchlorate, thiocyanate, nitrate, and iodide on the inhibition of radioactive iodide uptake by the human sodium iodide symporter. Thyroid. 2004;14:1012–9. doi: 10.1089/thy.2004.14.1012. [DOI] [PubMed] [Google Scholar]
- Vermiglio F, et al. Attention deficit and hyperactivity disorders in the offspring of mothers exposed to mild-moderate iodine deficiency: a possible novel iodine deficiency disorder in developed countries. J Clin Endocrinol Metab. 2004;89:6054–60. doi: 10.1210/jc.2004-0571. [DOI] [PubMed] [Google Scholar]
- Ward MH, et al. Nitrate intake and the risk of thyroid cancer and thyroid disease. Epidemiology. 2010;21:389–95. doi: 10.1097/EDE.0b013e3181d6201d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. Indicators for assessing iodine deficiency disorders and their control through salt iodization. Geneva: World Health Organization (WHO)/International Council for the Control of Iodine Deficiency Disorders; 1994. WHO/NUT/94.6. [Google Scholar]
- Wolff J, Chaikoff I. Plasma inorganic iodide as a homeostatic regulator of thyroid function. Journal of Biological Chemistry. 1948;174:555–564. [PubMed] [Google Scholar]
- Wyngaarden J, et al. The effect of certain anions on the accumulation and retention of iodide by the thyroid gland. Endocrinology. 1952;50:537–49. doi: 10.1210/endo-50-5-537. [DOI] [PubMed] [Google Scholar]
- Wyngaarden JB, et al. The effects of iodine, perchlorate, thiocyanate, and nitrate administration upon the iodide concentrating mechanism of the rat thyroid. Endocrinology. 1953;52:568–74. doi: 10.1210/endo-52-5-568. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.