Abstract
Exocrine pancreatic insufficiency (EPI) is a disease wherein pancreatic acinar cells fail to synthesize and secrete sufficient amounts of digestive enzymes for normal digestion of food. EPI affects many dog breeds, with a dramatically higher prevalence in the German shepherd dog (GSD) population. In this breed and perhaps others, EPI most often results from degeneration of the acinar cells of the pancreas, a hereditary disorder termed pancreatic acinar atrophy (PAA). Evidence of lymphocytic infiltration indicates that PAA is an autoimmune disease, but the genetic etiology remains unclear. Data from global gene expression and single nucleotide polymorphism profiles in the GSD suggest the involvement of the major histocompatibility complex [MHC; dog leukocyte antigen (DLA)]. To determine if alleles of the MHC influence development of EPI, genotyping of polymorphic class I (DLA-88) and II loci (DLA-DRB1, DLA-DQA1, and DLA- DQB1) was carried out for 70 affected and 63 control GSDs, and four-locus haplotypes were determined. One haplotype containing a novel allele of DLA-88 is very highly associated with EPI (OR>17; P=0.000125), while two haplotypes were found to confer protection from EPI (P=0.00087 and 0.0115). Described herein is the genotyping of MHC class I and II loci in a GSD cohort, establishment of four-locus haplotypes, and association of alleles/haplotypes with EPI.
Keywords: Autoimmune, Canine, Exocrine pancreatic insufficiency, Pancreatic acinar atrophy, Major histocompatibility complex
Introduction
Exocrine pancreatic insufficiency (EPI) is the condition resulting from failure of the acinar cells of the pancreas to synthesize and secrete sufficient amounts of digestive enzymes into the duodenum for digestion of food. Weight loss, increased appetite, and diarrhea are common manifestations of EPI, but are not evident until approximately 90 % of the exocrine pancreatic functional reserve has been lost (Dimagno et al. 1973). The primary treatment of patients with EPI is supplementation with pancreatic enzymes. The most common cause of EPI in dogs is idiopathic pancreatic acinar atrophy (PAA), a complex disease wherein pancreatic acinar cells are selectively and progressively degraded (Westermarck and Wiberg 2003).
Definitive diagnosis of PAA requires histological examination of the pancreas. During the initial stage of PAA, pancreatic tissues have been shown to display lymphocytic infiltration, dilation of the rough endoplasmic reticulum, fusion of zymogen granules, swollen mitochondria, and pyknotic nuclei (Westermarck et al. 1993). Pancreatic biopsy specimens from dogs with end-stage PAA show atrophy, scattering, and disorganization of acinar cells (Rogers et al. 1983; Westermarck et al. 1993). Because a definitive diagnosis of PAA as a cause of EPI requires the collection of a biopsy of the pancreas, which is invasive, veterinarians and owners most often elect to clinically screen for EPI through measurement of canine trypsin-like immunoreactivity (cTLI) concentration in serum.
EPI has been reported in many breeds, but approximately 60 % of all cases occur in German shepherd dogs (GSDs; German 2012). Although previous inheritance analyses suggested an autosomal recessive mode of inheritance, a test breeding of two affected GSDs failed to produce an entirely affected litter, contradicting predicted Mendelian ratios and suggesting a more complex mode of inheritance (Moeller et al. 2002; Westermarck et al. 2010). Classical linkage studies using multigenerational families of GSDs segregating EPI did not detect significant linkage with microsatellite markers across 39 canine chromosomes (Clark et al. 2005).
Recently, a canine oligonucleotide array was used to generate single nucleotide polymorphism (SNP) profiles for a genome-wide association study using unrelated dogs (100 GSDs affected with EPI and 79 control GSDs) (Tsai et al. 2012). The data revealed associations on chromosomes 7 and 12 that were supported by multiple SNPs (Tsai et al. 2012). Among the associated SNPs on chromosome 12 were several proximal to the dog leukocyte antigen (DLA) locus, which is part of the canine major histocompatibility complex (MHC). Specifically, an allele of a SNP located approximately 100 kb upstream of the DLA region was found in 22 % of EPI cases but only 1.3 % of controls. A survey of global pancreatic RNA transcript levels in two PAA-affected and three healthy dogs also implicated the MHC (Clark et al. 2005). Among the 244 genes that showed differential expression was DLA-88, which was upregulated 4.6-fold in affected tissues.
The canine MHC is a superlocus comprised of genes and gene families central to immune recognition and regulation. In dogs, two classes of genes analogous to the human leukocyte antigen complex have been characterized: class I and class II, each containing four complete genes (Wagner et al. 1999). Class I and II genes produce cell surface glycoproteins that bind self and non-self antigens for presentation to T cells (Wagner et al. 1999). DLA-88, a highly polymorphic member of class I, is responsible for peptide antigen presentation to CD8+ T cells. DLA-DRB1, DLA-DQA1, and DLA-DQB1 are the most polymorphic class II loci and interact with peptide ligands of CD4+ T cells.
Class I loci in the dog are not well studied and, to date, alleles of DLA-88 have not been associated with disease. Conversely, much attention has been given to alleles of the polymorphic class II genes and multiple associations with autoimmune diseases have been reported (Catchpole et al. 2008; Dyggve et al. 2011; Greer et al. 2010; Hughes et al. 2010; Kennedy et al. 2006a, b; Ollier et al. 2001; Wilbe et al. 2010). Our association and expression data suggesting the involvement of the MHC, taken together with histological evidence for an autoimmune component of PAA, compelled us to investigate DLA genes as a risk factor for EPI. Four polymorphic loci of DLA classes I and II were genotyped in affected and healthy dogs and haplotypes were evaluated for an association with disease status.
Materials and methods
Sample collection
Whole blood and fasting serum samples were collected in accordance with protocols approved by the Clinical Research Review Committee (CRRC No. 07–04) at Texas A&M University and the Clemson University Institutional Review Board (IBC2008-17). Serum samples were submitted to the Gastrointestinal Laboratory at Texas A&M University for measurement of serum cTLI concentration to verify the clinical status of each dog. cTLI concentrations ≤2.5 µg/L were considered diagnostic for EPI; dogs having cTLI concentrations ≥5.7 µg/L were considered normal. Control dogs were ≥6 years of age and to the owners’ knowledge had no immediate family members that were diagnosed with EPI. Tissue samples were not available to differentiate EPI due to PAA from those due to other causes. Whole-genome SNP data available from previous studies were used for analysis of population stratification, as described in Tsai et al. (2012).
Genomic DNA was isolated using the Gentra Puregene Blood Kit (Qiagen, Valencia, CA, USA). DNA concentration was quantified using a NanoDrop spectrophotometer (Fisher Scientific, Pittsburgh, PA), and samples were adjusted to a concentration of 50 ng/µl.
DLA-88 genotyping
DLA-88 typing was accomplished by modification of a method previously described (Venkataraman et al. 2007). The locus specific primers used for polymerase chain reaction (PCR) were DLA88F: 5′-GCGGCGACGGCCAG TGTCCCCGGAG-3′ and DLA88R17 5′-CCCTAGTG GAGGCGAGATCGGGGAG-3′. Amplification reactions (typically 30 µl) contained 100 ng of genomic DNA, 200 nM each of the primers and 200 µM each of dNTPs. Velocity DNA polymerase (0.5 µl) was used with the 5× GC buffer provided by the manufacturer (Bioline, Tauton, MA). All reactions were run in a Biorad C1000 Cycler (Bio-Rad, Hercules, CA) using the following amplification conditions: 96 °C for 2 min; 30 cycles of 96 °C for 30 s, 65 °C for 15 s, and 72 °C for 60 s; and 68 °C for 5 min. Alternatively, the products were amplified using the same primers and conditions but with 2× Immomix or MyTaq HSRed mix (both from Bioline). PCR products analyzed by electrophoresis on a 1 % agarose gel produced a sharp band of about 1.1 kb.
Purified products were directly sequenced with a set of three to six primers, and the combinations of alleles were resolved using an in-house custom built software (developed by Dr. D.E. Geraghty, Fred Hutchinson Cancer Research Center). The primers used for sequencing were DLA88F, DLA88R17, DLA88I1F: 5′-CCCGGGCATCTCCCCCTG-3′, DLA88I2F: 5′-GAACCCGCGGGAACTCCCGGGAGG-3′, and DLA88I2R: 5′-GTGACGCCCGGACCCGGACCCTC-3′. The intron–exon boundaries were identified by alignment with DLA-88 genomic sequence (Burnett et al. 1997). The derived amino acid sequence of the new allele was aligned with closely related known alleles and the reference allele DLA88*001:01.
DLA-DRB1, DLA-DQA1, and DLA-DQB1 genotyping
Primers used to amplify the polymorphic regions of DLA-DRB1, DLA-DQA1, and DLA-DQB1 were previously described (Kennedy et al. 2006b). PCR was performed using 50 ng of DNA in a 25-µl reaction containing 2× Reddy Mix Master Mix (1.5 mM MgCl2) (AbGene, Rochester, NY), 0.4 µM of each primer, and sterile water. Thermal cycling parameters were previously described (Kennedy et al. 2006b). Products were run on a 1.5 % agarose gel for visual confirmation of product and size. Purified products were directly sequenced.
Assignment of genotypes was based on the sequence similarity of samples with published nucleotide sequences for DLA alleles (Robinson et al. 2013). Allele assignment was confirmed using SBTengine (GenDX, The Netherlands). Haplotype assignment for all GSDs was performed as described in Kennedy et al. (2006b).
Establishing four-locus haplotypes
Three-locus haplotypes were extended through the incorporation of DLA-88 alleles using the principles established by Kennedy et al. (2006b). Specifically, DLA-88 alleles were first assigned to haplotypes in individuals that were homozygous at all four loci. Alleles that were never observed in homozygosity were assigned to haplotypes by a process of elimination in heterozygotes having one copy of an established four-locus haplotype. This method was also used to create a five-locus haplotype using data for SNP 12.3781476 from the Affymetrix v2 canine SNP array. SNP genotypes were available for 94 affected and 76 control dogs.
Statistical methods
Affected and control dogs were assessed for the presence or absence of each haplotype and the number of individuals in each group was determined. Statistical analyses were completed using VassarStats (Web Site for Statistical Computation, Vassar College, Poughkeepsie, NY). Two-way contingency tables were used to calculate two-tailed Fisher’s exact probability statistic, 95 % confidence intervals, and odds ratios (OR) for association of each allele and haplotype with disease status.
Results
Cohort
Samples from a total of 175 GSDs were collected from the USA and Canada: 97 EPI-affected dogs (cases) and 78 healthy dogs (controls). Average age of the control GSDs at time of collection was 9 years. There was no significant difference in sex distribution between cases and controls (P=0.354). Whole-genome SNP data were available from 169 of the 175 GSDs included in our cohort. Principal component analysis did not show any evidence of population stratification (data not shown).
Analysis of class I locus (DLA-88)
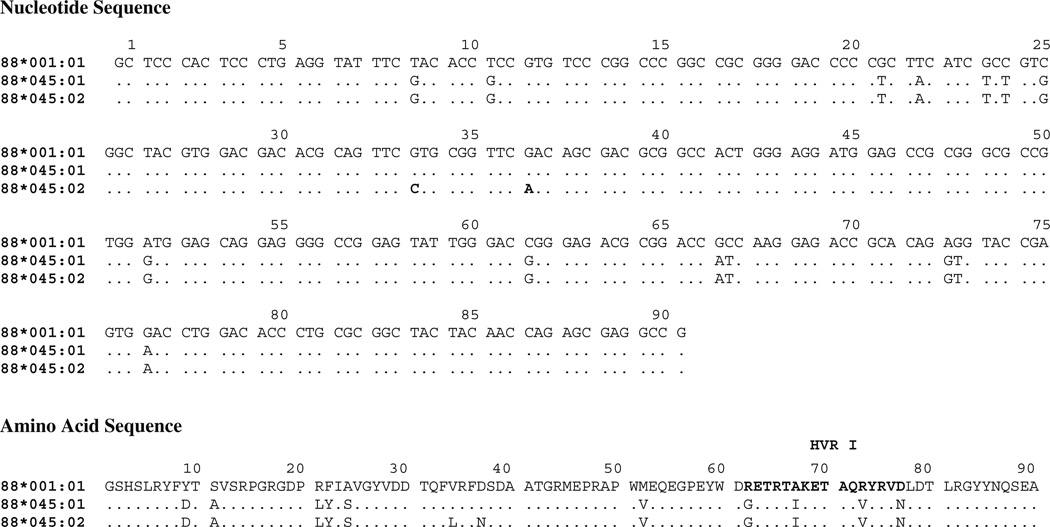
Genotypes for DLA-88 were determined for 133 GSDs, comprised of 70 EPI-affected and 63 controls. Ten alleles of DLA-88 were identified in this cohort (Table 1). One allele could not be amplified and was termed “unknown.” A novel allele was identified and designated DLA-88*045:02 (GenBank accession JX871398) by the curator of the canine immunopolymorphism–MHC database (LJK). Allele DLA-88*045:02 differs from the closest known allele, DLA-88*045:01 (Hardt et al. 2006), at two nucleotide positions (Fig. 1). These changes predict two amino acid substitutions within the alpha 1 domain: leucine for valine at position 34, and asparagine for aspartic acid at position 37.
Table 1.
Alleles of the DLA class I gene, DLA-88, in GSD cases and controls, as well as the total frequency for this cohort, are shown
| DLA-88 | Total |
Cases |
Controls |
|||
|---|---|---|---|---|---|---|
| (2n=266) | (%) | (2n=140) | (%) | (2n=126) | (%) | |
| 002:01 | 125 | 47.0 | 73 | 52.1 | 52 | 41.3 |
| 004:02a | 70 | 26.3 | 28 | 20.0 | 42 | 33.3 |
| 006:01b | 23 | 8.6 | 7 | 5.0 | 16 | 12.7 |
| 045:02c | 18 | 6.8 | 17 | 12.1 | 1 | 0.8 |
| 022:01 | 11 | 4.1 | 6 | 4.3 | 5 | 4.0 |
| Unknown | 8 | 3.0 | 3 | 2.1 | 5 | 4.0 |
| 017:01 | 5 | 1.9 | 1 | 0.7 | 4 | 3.2 |
| 005:01 | 4 | 1.5 | 4 | 2.9 | 0 | 0.0 |
| 007:01 | 1 | 0.4 | 0 | 0.0 | 1 | 0.8 |
| 012:01 | 1 | 0.4 | 1 | 0.7 | 0 | 0.0 |
OR=0.3558, P=0.000176, CI=0.206–0.614
OR=0.3618, P=0.0296, CI=0.143–0.911
OR=17.2764, P=0.000125, CI=2.264–131.824
Fig. 1.
Nucleotide and deduced amino acid sequences are shown for exon 2 of the new class I allele DLA-88*045:02 aligned with the closest known allele, DLA-88*045:01, and the reference al-lele, DLA-88*001:01. Dots indicate identity with the reference allele. Two nucleotide substitutions in allele 88*045:02 are shown in bold. Hypervariable region (HVR) I is shown in bold in the amino acid sequence. There are no changes between DLA-88*045:01 and DLA-88*045:02 in the intron 2 and exon 3 regions. The complete sequence for exon 2, intron 2, and exon 3 has been deposited in GenBank (Accession JX871398)
The most frequently observed allele was DLA-88*002:01, followed by DLA-88*004:02 and DLA-88*006:01. The remaining alleles had frequencies of ≤7 %. Only allele DLA-88*045:02 was significantly more prevalent among EPI cases, while alleles DLA-88*004:02 and DLA-88*006:01 were significantly associated with controls (Table 1).
Analysis of class II three-locus haplotypes (DLA-DRB1, DLA-DQA1, DLA-DQB1)
Genotypes for class II loci were generated for the aforementioned 133 dogs, and an additional 27 EPI-affected and 15 control GSDs. Among all 175 GSDs, there were 12 DLA- DRB1, 8 DLA-DQA1, and 11 DLA-DQB1 alleles (Online resource 1). Fifteen three-locus haplotypes were assigned (Table 2). The most common haplotypes were 1 (42.9%), 2 (27.4%), and 3 (8.9%). The 12 remaining haplotypes occurred at frequencies of ≤6 %. Haplotypes 1 and 4 were more prevalent in cases, while 2 and 3 were found more often among the controls.
Table 2.
Frequencies of class II haplotypes identified in our GSD cohort
| Haplotype No | Class II DRB1*/DQA1*/DQB1* | Total |
Cases |
Controls |
|||
|---|---|---|---|---|---|---|---|
| (2n=350) | (%) | (2n=194) | (%) | (2n=156) | (%) | ||
| 1 | 011:01/002:01/013:02a | 150 | 42.9 | 96 | 49.5 | 54 | 34.6 |
| 2 | 015:01/006:01/003:01b | 96 | 27.4 | 42 | 21.6 | 54 | 34.6 |
| 3 | 001:02/001:01/002:01c | 31 | 8.9 | 11 | 5.7 | 20 | 12.8 |
| 4 | 012:01/004:01/013:03+017:01d | 21 | 6.0 | 20 | 10.3 | 1 | 0.6 |
| 5 | 001:01/001:01/002:01 | 14 | 4.0 | 7 | 3.6 | 7 | 4.5 |
| 6 | 002:01/009:01/001:01 | 11 | 3.1 | 3 | 1.5 | 8 | 5.1 |
| 7 | 015:02/006:01/023:01 | 8 | 2.3 | 3 | 1.5 | 5 | 3.2 |
| 8 | 006:01/005:01:1/007:01 | 5 | 1.4 | 5 | 2.6 | 0 | 0.0 |
| 9 | 020:01/004:01/013:03 | 5 | 1.4 | 4 | 2.1 | 1 | 0.6 |
| 10 | 011:01/002:01/013:03 | 3 | 0.9 | 0 | 0.0 | 3 | 1.9 |
| 11 | 015:01/006:01/023:01 | 2 | 0.6 | 0 | 0.0 | 2 | 1.3 |
| 12 | 015:01/006:01/020:02 | 1 | 0.3 | 1 | 0.5 | 0 | 0.0 |
| 13 | 013:01/003:01/005:02 | 1 | 0.3 | 1 | 0.5 | 0 | 0.0 |
| 14 | 016:01/001:01/002:01 | 1 | 0.3 | 0 | 0.0 | 1 | 0.6 |
| 15 | 040:01/010:01/019:01 | 1 | 0.3 | 1 | 0.5 | 0 | 0.0 |
OR=1.85, P=0.00653, CI= 1.199–2.855
OR=0.52, P=0.00802, CI=0.325–0.839
OR=0.41, P=0.0230, CI=0.190–0.881
OR=17.8, P=0.000123, CI=2.363–134.308
Analysis of four-locus haplotypes (DLA-88, DLA-DRB1, DLA-DQA1, DLA-DQB1)
Fourteen four-locus haplotypes were detected in 133 dogs (Table 3), with ten confirmed in multiple dogs. In the remaining four unique haplotypes, each was paired with an established haplotype confirmed through homozygosity. Two haplotypes (1 and 2) could be subdivided based on DLA-88, although there was clearly one main haplotype for each (1a, 2a). Three haplotypes (13, 14, 15) were not tested for DLA-88 or could not be assigned a DLA-88 allele.
Table 3.
Four-locus haplotypes identified in our GSD cohort
| Haplo No. | DLA-88* | DRB1* | DQA1* | DQB1* | Total |
|
|---|---|---|---|---|---|---|
| (2n=266) | (%) | |||||
| 1a | 002:01 | 011:01 | 002:01 | 013:02 | 117 | 44.0 |
| 1b | 004:02 | 011:01 | 002:01 | 013:02 | 1 | 0.4 |
| 2a | 004:02 | 015:01 | 006:01 | 003:01 | 69 | 25.9 |
| 2b | 002:01 | 015:01 | 006:01 | 003:01 | 3 | 1.1 |
| 3 | 006:01 | 001:02 | 001:01 | 002:01 | 23 | 8.6 |
| 4 | 045:02 | 012:01 | 004:01 | 013:03+017:01 | 18 | 6.8 |
| 5 | 022:01 | 001:01 | 001:01 | 002:01 | 11 | 4.1 |
| 6 | 017:01 | 002:01 | 009:01 | 001:01 | 5 | 1.9 |
| 7 | unknown | 015:02 | 006:01 | 023:01 | 8 | 3.0 |
| 8 | 002:01 | 006:01 | 005:01:1 | 007:01 | 2 | 0.8 |
| 9 | 005:01 | 020:01 | 004:01 | 013:03 | 4 | 1.5 |
| 10 | 002:01 | 011:01 | 002:01 | 013:03 | 3 | 1.1 |
| 11 | 007:01 | 015:01 | 006:01 | 023:01 | 1 | 0.4 |
| 12 | 012:01 | 015:01 | 006:01 | 020:02 | 1 | 0.4 |
No significant difference was observed in overall levels of homozygosity between cases and controls (Table 4). Haplotype 4 was highly associated with EPI (OR=17.276, P=0.000125, CI=2.264–131.824). While 14 affected dogs possessed haplotype 4 (2 homozygous, 12 heterozygous), it was only present in a single control dog. Three of the four alleles (DLA-88*45:02, DLA-DRB1*012:01, and DLA-DQB1*013:03+017:01) are unique to this haplotype. When analyzed in the larger cohort of 175 dogs, the association with alleles DLA-DRB1*012:01 and DLA-DQB1*013:03+017:01 persisted (P=0.000130; Online resource 1). Haplo-type 1a had a slight association with EPI and was also the most common haplotype identified among cases and controls.
Table 4.
Frequencies for each four-locus haplotype in affected and control dogs
| Haplo no. | Cases (n=70) |
Controls (n=63) |
OR | 95 % CI | P value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hom | Het | No. haplo | (%) | Hom | Het | No. haplo | (%) | ||||
| 1a | 18 | 34 | 70 | 50.0 | 12 | 23 | 47 | 37.3 | 1.681 | 1.030–2.744 | 0.0476 |
| 1b | 0 | 0 | 0 | 0.0 | 0 | 1 | 1 | 0.8 | – | ||
| 2a | 0 | 27 | 27 | 19.3 | 9* | 24 | 42 | 33.3 | 0.478 | 0.273–0.864 | 0.0115 |
| 2b | 0 | 1 | 1 | 0.7 | 1 | 0 | 2 | 1.6 | – | ||
| 3 | 0 | 7 | 7 | 5.0 | 1 | 14 | 16 | 12.7 | 0.362 | 0.144–0.911 | 0.0296 |
| 4 | 2 | 13 | 17 | 12.1 | 0 | 1 | 1 | 0.8 | 17.276 | 2.264–131.82 | 0.000125 |
| 5 | 0 | 6 | 6 | 4.3 | 0 | 5 | 5 | 4.0 | – | ||
| 6 | 0 | 1 | 1 | 0.7 | 0 | 4 | 4 | 3.2 | – | ||
| 7 | 0 | 3 | 3 | 2.1 | 0 | 5 | 5 | 4.0 | – | ||
| 8 | 0 | 2 | 2 | 1.4 | 0 | 0 | 0 | 0.0 | – | ||
| 9 | 0 | 4 | 4 | 2.9 | 0 | 0 | 0 | 0.0 | – | ||
| 10 | 0 | 0 | 0 | 0.0 | 0 | 3 | 3 | 2.4 | – | ||
| 11 | 0 | 1 | 1 | 0.7 | 0 | 0 | 0 | 0.0 | – | ||
| 12 | 0 | 1 | 1 | 0.7 | 0 | 0 | 0 | 0.0 | – | ||
Number of homozygous and heterozygous dogs, as well as the total number of each haplotype observed for both categories, are shown. Statistical values for haplotypes with significant differences between the cases and controls are reported
Hom number of dogs homozygous for that haplotype, Het number of dogs heterozygous for that haplotype, No. haplo total number of haplotype
P=0.000870 for homozygosity; OR could not be calculated
Haplotypes 2a and 3 were significantly more prevalent in the control population. Approximately 14 % of control dogs were homozygous for haplotype 2a, whereas homozygosity was not observed in affected dogs (P=0.00087). The only control dog with haplotype 4 also carried haplotype 3.
Significant associations with other haplotypes were not detected. Allele T of SNP 12.3781476 (CanFam2) is highly associated with EPI in this cohort (P=1.75×10−5). When expanded to a five-locus haplotype, the T allele is in phase with haplotype 4 and is also found with at least two additional haplotypes, although phase could not be determined.
Discussion
Morphologic and immunologic findings in PAA-affected GSDs are consistent with an autoimmune disease, as are inheritance studies that suggest the involvement of multiple genes (Clark and Cox 2012; Westermarck and Wiberg 2012). Linkage and expression studies have suggested that the canine MHC harbors a genetic determinant for PAA susceptibility, but prior to this report, DLA haplotypes had not been examined as a risk factor. We evaluated polymorphic class I and II loci in a large cohort of unrelated GSDs and identified multiple alleles associated with disease status, including a highly correlated risk haplotype. This is also the first report to extend DLA haplotypes to a class I locus and identify an association with DLA-88.
Class I locus (DLA-88)
Genotyping of DLA-88 alleles requires alternative amplification methods and cloning and is more arduous than determining class II alleles (Venkataraman et al. 2007). As a consequence, 44 DLA-88 alleles were initially identified in 1998, but little research has focused on the locus since that time (Graumann et al. 1998; Hardt et al. 2006; Ross et al. 2012; Venkataraman et al. 2007; Wagner et al. 2000). In this study, a total of ten DLA-88 alleles were identified in 133 GSDs, including one novel allele and one “unknown” allele. The observed heterozygosity (66 %) and presence of a predominant allele (47 %) in the GSD were consistent with findings from a 2012 survey of DLA-88 alleles in the golden retriever and boxer breeds (Ross et al. 2012). Together, alleles DLA-88*002:01 and DLA-88*004:01 account for 73 % of alleles in the GSD. This is in contrast to the boxer, in which neither allele was identified and to the golden retriever, in which only a low frequency of DLA-88*002:01 was observed (Ross et al. 2012).
The gene product of the novel allele, DLA-88*045:02, differs in two amino acid positions from the gene product of its most similar allele, 88*045:01. The substitutions are outside the hyper-variable peptide-binding region but within the adjacent alpha-1 domain. Without additional studies, it is unclear what effect, if any, the substitutions may have on protein function. 88*045:02 was a minor allele among our GSDs, accounting for only 6.8 % of alleles, but there was a strong bias towards affected dogs. This highly significant association marks the first identification of a class I risk allele in the dog.
Class II haplotypes (DLA-DRB1, DLA-DQA1, DLA-DQB1)
Of the 15 class II haplotypes identified herein, 12, 13, and 14 were found in single animals only and have not been previously reported in the GSD (Jokinen et al. 2011; Kennedy et al. 2007, 2008). Haplotype 4 was associated with an increased risk of EPI. It is rare among GSDs, accounting for only 5.3 % of haplotypes in the UK (Kennedy et al. 2008) and 2.7 % of haplotypes in Finland (Jokinen et al. 2011). In this study, the overall haplotype frequency was 6 % but is likely higher than would be expected in the general US and Canadian populations because of its association with EPI. This haplotype (4: DLA-DRB1*012:01/DQA1*004:01/DQB1*013:03+017:01) carries two copies of DLA-DQB1: DLA-DQB1*013:03 and DLA-DQB1*017:01 (referred to as DQB1*013:03+017:01). First described in 2007, this combination of duplicated DLA-DQB1 alleles is found only on DLA class II haplotype 4 and is present in at least 15 breeds (Kennedy et al. 2007). Among these are collies, which are reported to have an increased prevalence of EPI (German 2012). A less significant association was identified with a haplotype common to GSDs (1: DLA-DRB1*011:01/DQA1*002:01/DQB1*013:02) indicating that it may also be a risk factor for EPI.
Two class II haplotypes (2 and 3) were identified in more controls than cases, suggesting that they are protective for EPI. Haplotype 2 (DLA-DRB1*015:01/DQA1*006:0 1/DQB1*003:01) is one of the most prevalent haplotypes identified in the GSD (Jokinen et al. 2011; Kennedy et al. 2007, 2008). The other, haplotype 3 (DLA-DRB1*00 1:02/DQA1*001:01/DQB1*002:01), is common in some GSD populations but occurs at lower frequencies in others (Jokinen et al. 2011; Kennedy et al. 2007, 2008).
Class II haplotypes have been examined in studies of two other autoimmune disorders of the GSD: chronic superficial keratitis (CSK) and anal furunculosis (AF). Jokinen et al. (2011) identified haplotype 2 as a risk factor for CSK, particularly when homozygous (P=0.017) in a cohort of 55 Finnish GSDs. None of the GSDs in our North American cohort were reported to have CSK. Although it is the second most common haplotype in our study, homozygosity was only observed in 13 dogs. Haplotype 5 (DLA-DRB1*001:01/DQA1*001:01/DQB1*002:01) was associated with AF in a study of over 300 GSDs (Kennedy et al. 2008). The haplotype was present in only 14 dogs in our study; all were heterozygous. None of the four dogs having AF in our cohort possessed this risk haplotype.
Four-locus haplotypes
We established 14 four-locus class I and II haplotypes. The most common DLA-88 alleles, DLA-88*002:01 and DLA-88*004:02, were identified with multiple class II haplotypes, reflecting recombination between DLA-88 and DLA-DRB1, located approximately 1.2 Mb apart. Fewer dogs were used to generate class I genotypes than class II genotypes; therefore, three class II haplotypes (13, 14, and 15) were unable to be expanded to a four-locus haplotype.
The DLA-88 allele in phase with the three-locus haplotype 7 (DLA-DRB1*015:02/DQA1*006:01/DQB1*023:01) could not be determined and was reported herein as “unknown.” Eight dogs were heterozygous for this class II haplotype, yet were homozygous for several different DLA-88 alleles. The homozygous DLA-88 allele corresponded to the second class II haplotype present in each dog. Subsequent reevaluation of the DLA-88 sequencing data confirmed that a second allele was not identifiable. While these findings could support multiple four-locus haplotypes, it is probable that only one four-locus haplotype is present (haplotype 7). Attempts to sequence the unknown allele have so far been unsuccessful. Alternative methods will need to be established to determine the class I sequence on this haplotype.
Haplotype 4 (DLA-88*045:02/DRB1*012:01/DQA1 *004:01/DQB1*013:03+017:01) is very highly correlated with EPI. The observed OR of >17 is one of the highest reported to date in the dog. Only two dogs were homozygous for this haplotype and both were affected. Thirteen of 14 dogs heterozygous for the haplotype were also affected.
Kennedy et al. (2007) reported a frequency of 12.9 % for DLA class II haplotype 4 in 31 UK golden retrievers, a breed that is underrepresented among EPI cases (Batchelor et al. 2007). Interestingly, a recent examination of DLA-88 alleles in 47 US golden retrievers did not identify the 88*045:02 allele (Ross et al. 2012). This finding may indicate that the DLA class II haplotype 4 is present on multiple four-locus haplotypes or reflect different allele frequencies between golden retriever populations (UK vs US).
Haplotype 1a (DLA-88*002:01/DRB1*011:01/DQA1 *002:01/DQB1*013:02) was associated with a small increase in risk of disease (OR<2). This finding could be a consequence of the high frequency (44 %) of the haplotype among all GSDs and the increased prevalence of protective haplotypes in the control population.
Haplotype 3 was overrepresented among control dogs. None of the 12 affected dogs heterozygous for the strong risk haplotype (4) possessed this protective haplotype. The only control dog with risk haplotype 4 was heterozygous for haplotype 3, indicating that the effect of this protective haplotype may outweigh the effect of the risk haplotype.
Homozygosity for haplotype 2a (DLA-88*004:02/DRB1 *015:01/DQA1*006:01/DQB1*003:01) was also protective. Homozygosity was only observed in the control population, while heterozygosity was found at equal frequencies between cases and controls, 38.6 and 38.1 %, respectively. Three affected dogs were heterozygous for haplotypes 2a and 4, suggesting that haplotype 2a is not dominant to the risk haplotypes.
Allele T of SNP 12.3781476 was found with risk haplotype 4, along with other haplotypes present only in affected dogs. In total, the allele was found in 23.4 % of 94 EPI cases, whereas haplotype 4 accounted for only 20 % of 70 EPI cases. The greater correlation of the SNP with disease status suggests that the association detected with the class I and II alleles may be secondary to linkage disequilibrium with a gene farther upstream. While we present substantial evidence for an association of MHC alleles with EPI, further refinement of the locus on CFA 12 is warranted.
Conclusions
In summary, we have identified DLA class I and II alleles and haplotypes that influence the development of PAA in the GSD. GWAS data presented by Tsai et al. (2012) indicated that loci on multiple chromosomes are associated with EPI. The risk haplotype identified herein explains only a subset of the EPI cases in the study, providing further evidence for locus heterogeneity. The presence of dominant protective haplotypes may have hindered the identification of additional risk factors in the GWAS by increasing the incidence of risk alleles among the control dogs. For the identification of other genetic factors, future studies will focus on a subset of dogs that possess no known risk or protective DLA haplotypes.
Supplementary Material
Acknowledgments
This work was funded by grants from the Canine Health Foundation (#1497-A and 934). Parts of the work were also supported by the National Science Foundation-Research Experiences for Undergraduates program (0453360) and a Pepsi Refresh Project award (Health Category, 2010). The authors wish to thank EPI4dogs and the many owners and veterinarians who contributed to this project.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00251-013-0704-y) contains supplementary material, which is available to authorized users.
Conflicts of interest The authors declare that they have no conflict of interest.
Contributor Information
Kate L. Tsai, Department of Genetics and Biochemistry, College of Agriculture, Forestry and Life Sciences, Clemson University, 154 Poole Agricultural Center, 130 McGinty Ct, Clemson, SC 29634, USA
Alison N. Starr-Moss, Department of Genetics and Biochemistry, College of Agriculture, Forestry and Life Sciences, Clemson University, 154 Poole Agricultural Center, 130 McGinty Ct, Clemson, SC 29634, USA
Gopalakrishnan M. Venkataraman, Fred Hutchinson Cancer Research Center, Seattle, WA, USA
Christopher Robinson, Department of Genetics and Biochemistry, College of Agriculture, Forestry and Life Sciences, Clemson University, 154 Poole Agricultural Center, 130 McGinty Ct, Clemson, SC 29634, USA.
Lorna J. Kennedy, Centre for Integrated Genomic Medical Research, School of Medicine, University of Manchester, Manchester, UK
Jörg M. Steiner, Department of Small Animal Clinical Sciences, College of Veterinary Medicine and Biomedical Sciences, Texas A&M University, College Station, TX, USA
Leigh Anne Clark, Department of Genetics and Biochemistry, College of Agriculture, Forestry and Life Sciences, Clemson University, 154 Poole Agricultural Center, 130 McGinty Ct, Clemson, SC 29634, USA, lclark4@clemson.edu.
References
- Batchelor DJ, Noble PJ, Cripps PJ, Taylor RH, McLean L, Leibl MA, German AJ. Breed associations for canine exocrine pancreatic insufficiency. J Vet Intern Med. 2007;21:207–214. doi: 10.1892/0891-6640(2007)21[207:bafcep]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Burnett RC, DeRose SA, Wagner JL, Storb R. Molecular analysis of six dog leukocyte antigen class I sequences including three complete genes, two truncated genes and one full-length processed gene. Tissue Antigens. 1997;49:484–495. doi: 10.1111/j.1399-0039.1997.tb02783.x. [DOI] [PubMed] [Google Scholar]
- Catchpole B, Kennedy LJ, Davison LJ, Ollier WE. Canine diabetes mellitus: from phenotype to genotype. J Small Anim Pract. 2008;49:4–10. doi: 10.1111/j.1748-5827.2007.00398.x. [DOI] [PubMed] [Google Scholar]
- Clark LA, Cox ML. Current status of genetic studies of exocrine pancreatic insufficiency in dogs. Top Companion Anim Med. 2012;27:109–112. doi: 10.1053/j.tcam.2012.04.001. [DOI] [PubMed] [Google Scholar]
- Clark LA, Wahl JM, Steiner JM, Zhou W, Ji W, Famula TR, Williams DA, Murphy KE. Linkage analysis and gene expression profile of pancreatic acinar atrophy in the German Shepherd Dog. Mamm Genome. 2005;16:955–962. doi: 10.1007/s00335-005-0076-1. [DOI] [PubMed] [Google Scholar]
- Dimagno E, Go VL, Summerskill W. Relations between pancreatic enzyme outputs and malabsorption in severe pancreatic insufficiency. N Engl J Med. 1973;288:813–815. doi: 10.1056/NEJM197304192881603. [DOI] [PubMed] [Google Scholar]
- Dyggve H, Kennedy LJ, Meri S, Spillmann T, Lohi H, Speeti M. Association of Doberman hepatitis to canine major histocompatibility complex II. Tissue Antigens. 2011;77:30–35. doi: 10.1111/j.1399-0039.2010.01575.x. [DOI] [PubMed] [Google Scholar]
- German AJ. Exocrine pancreatic insufficiency in the dog: breed associations, nutritional considerations, and long-term outcome. Top Companion Anim Med. 2012;27:104–108. doi: 10.1053/j.tcam.2012.04.004. [DOI] [PubMed] [Google Scholar]
- Graumann MB, DeRose SA, Ostrander EA, Storb R. Polymorphism analysis of four canine MHC class I genes. Tissue Antigens. 1998;51:374–381. doi: 10.1111/j.1399-0039.1998.tb02976.x. [DOI] [PubMed] [Google Scholar]
- Greer KA, Wong AK, Liu H, Famula TR, Pedersen NC, Ruhe A, Wallace M, Neff MW. Necrotizing meningoencephalitis of pug dogs associates with dog leukocyte antigen class II and resembles acute variant forms of multiple sclerosis. Tissue Antigens. 2010;76:110–118. doi: 10.1111/j.1399-0039.2010.01484.x. [DOI] [PubMed] [Google Scholar]
- Hardt C, Ferencik S, Tak R, Hoogerbrugge PM, Wagner V, Grosse-Wilde H. Sequence-based typing reveals a novel DLA-88 allele, DLA-88*04501, in a beagle family. Tissue Antigens. 2006;67:163–165. doi: 10.1111/j.1399-0039.2006.00497.x. [DOI] [PubMed] [Google Scholar]
- Hughes AM, Jokinen P, Bannasch DL, Lohi H, Oberbauer AM. Association of a dog leukocyte antigen class II haplotype with hypoadrenocorticism in Nova Scotia Duck Tolling Retrievers. Tissue Antigens. 2010;75:684–690. doi: 10.1111/j.1399-0039.2010.01440.x. [DOI] [PubMed] [Google Scholar]
- Jokinen P, Rusanen EM, Kennedy LJ, Lohi H. MHC class II risk haplotype associated with canine chronic superficial keratitis in German shepherd dogs. Vet Immunol Immunopathol. 2011;140:37–41. doi: 10.1016/j.vetimm.2010.11.007. [DOI] [PubMed] [Google Scholar]
- Kennedy LJ, Barnes A, Ollier WER, Day MJ. Association of common dog leukocyte antigen class II haplotype with canine primary immune-mediated haemolytic anemia. Tissue Antigens. 2006a;68:502–508. doi: 10.1111/j.1399-0039.2006.00715.x. [DOI] [PubMed] [Google Scholar]
- Kennedy LJ, Davison LJ, Barnes A, Short AD, Fretwell N, Jones CA, Lee AC, Ollier WE, Catachpole B. Identification of susceptibility and protective major histocompatibility complex haplotypes in canine diabetes mellitus. Tissue Antigens. 2006b;68:467–476. doi: 10.1111/j.1399-0039.2006.00716.x. [DOI] [PubMed] [Google Scholar]
- Kennedy LJ, Barnes A, Short A, Brown JJ, Lester S, Seddon J, Fleeman L, Francino O, Brkljacic M, Knyazev S, Happ GM, Ollier WE. Canine DLA diversity: 1. New alleles and haplotypes. Tissue Antigens. 2007;69(Suppl 1):272–288. doi: 10.1111/j.1399-0039.2006.00779.x. [DOI] [PubMed] [Google Scholar]
- Kennedy LJ, O’Neill T, House A, Barnes A, Kyöstilä, Innes J, Fretwell N, Day MJ, Catchpole B, Lohi H, Ollier WE. Risk of anal furunculosis in German shepherd dogs is associated with the major histocompatibility complex. Tissue Antigens. 2008;71:51–56. doi: 10.1111/j.1399-0039.2007.00964.x. [DOI] [PubMed] [Google Scholar]
- Moeller EM, Steiner JS, Clark LA, Murphy KE, Famula TR, Williams DA, Stankovics ME, Vose AS. Inheritance of pancreatic acinar atrophy in German shepherd dogs. Am J Vet Res. 2002;63:1429–1434. doi: 10.2460/ajvr.2002.63.1429. [DOI] [PubMed] [Google Scholar]
- Ollier WE, Kennedy LJ, Thompson W, Barnes AN, Bell SC, Bennett D, Angles JM, Innes JF, Carter SD. Dog MHC alleles containing the human RA shared epitope confer susceptibility to canine rheumatoid arthritis. Immunogenetics. 2001;53:669–673. doi: 10.1007/s002510100372. [DOI] [PubMed] [Google Scholar]
- Robinson J, Halliwell JA, McWilliam H, Lopez R, Marsh SG. IPD-the immuno polymorphism database. Nucleic Acids Res. 2013;41:D1234–D1240. doi: 10.1093/nar/gks1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers WA, O’Dorisio TM, Johnson SE, Cataland S, Stradley RP, Sherding RG. Postprandial release of gastric inhibitory polypeptide in dogs with pancreatic acinar atrophy. Dig Dis Sci. 1983;28:345–349. doi: 10.1007/BF01324952. [DOI] [PubMed] [Google Scholar]
- Ross P, Buntzman AS, Vincent BG, Grover EN, Gojanovich GS, Collins EJ, Frelinger JA, Hess PR. Allelic diversity at the DLA-88 locus in the golden retriever and boxer breeds is limited. Tissue Antigens. 2012;80:175–183. doi: 10.1111/j.1399-0039.2012.01889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai KL, Noorai RE, Starr-Moss AN, Quignon P, Rinz CJ, Ostrander EA, Steiner JM, Murphy KE, Clark LA. Genome-wide association studies for multiple diseases of the German shepherd dog. Mamm Genome. 2012;23:203–211. doi: 10.1007/s00335-011-9376-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkataraman GM, Stroup P, Graves SS, Storb R. An improved method for dog leukocyte antigen 88 typing and two new major histocompatibility complex class I alleles, DLA88*01101 and DLA88*01201. Tissue Antigens. 2007;70:53–57. doi: 10.1111/j.1399-0039.2007.00839.x. [DOI] [PubMed] [Google Scholar]
- Wagner JL, Burnett RC, Storb R. Organization of the canine major histocompatibility complex: current perspectives. J Hered. 1999;90:35–38. doi: 10.1093/jhered/90.1.35. [DOI] [PubMed] [Google Scholar]
- Wagner JL, Creer SA, Storb R. Dog class I gene DLA-88 histocompatibility typing by PCR-SSCP and sequencing. Tissue Antigens. 2000;55:564–567. doi: 10.1034/j.1399-0039.2000.550607.x. [DOI] [PubMed] [Google Scholar]
- Westermarck E, Wiberg M. Exocrine pancreatic insufficiency in dogs. Vet Clin N Am Small. 2003;33:1165–1179. doi: 10.1016/s0195-5616(03)00057-3. [DOI] [PubMed] [Google Scholar]
- Westermarck E, Wiberg M. Exocrine pancreatic insufficiency in the dog: historical background, diagnosis, and treatment. Top Companion Anim Med. 2012;27:96–103. doi: 10.1053/j.tcam.2012.05.002. [DOI] [PubMed] [Google Scholar]
- Westermarck E, Batt RM, Vaillant C, Wiberg M. Sequential study of pancreatic structure and function during development of pancreatic acinar atrophy in a German shepherd dog. Am J Vet Res. 1993;54:1088–1094. [PubMed] [Google Scholar]
- Westermarck E, Saari SAM, Wiberg ME. Heritability of exocrine pancreatic insufficiency in German shepherd dogs. J Vet Intern Med. 2010;24:450–452. doi: 10.1111/j.1939-1676.2009.0461.x. [DOI] [PubMed] [Google Scholar]
- Wilbe M, Sundberg K, Hansen IR, Standberg E, Nachreiner RF, Hedhammer A, Kennedy LJ, Andersson G, Björnerfeldt S. Increased genetic risk or protection for canine autoimmune lymphocytic thyroiditis in Giant Schnauzers depends on DLA class II genotype. Tissue Antigens. 2010;75:712–719. doi: 10.1111/j.1399-0039.2010.01449.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.