Abstract
Aims/hypothesis
We have previously reported that local activation of β2-adrenergic receptors (B2ARs) in the ventromedial hypothalamus (VMH) enhances hypoglycaemic counter-regulation. This study examines whether peripheral delivery of a selective B2AR agonist could also promote counter-regulatory responses and thereby has potential therapeutic value to limit hypoglycaemia risk.
Methods
Conscious male Sprague–Dawley rats received an intra-arterial injection of the B2AR specific agonist, formoterol, or a control solution either before a hyperinsulinaemic–hypoglycaemic clamp study or immediately before recovery from insulin-induced hypoglycaemia. In addition, the capacity of a VMH-targeted microinjection of a B2AR antagonist to limit the anti-insulin effect of the B2AR agonist was assessed.
Results
Systemic delivery of B2AR agonist markedly reduced the exogenous glucose infusion rate (GIR) required during the hypoglycaemic clamp study. This effect was mediated by blockade of insulin’s inhibitory effect on endogenous glucose production. Local blockade of B2ARs within the VMH using a specific antagonist partially diminished the effect of systemic activation of B2ARs during hypoglycaemia at least in part by diminishing the adrenaline (epinephrine) response to hypoglycaemia. Peripheral B2AR agonist injection also enhanced glucose recovery from insulin-induced hypoglycaemia.
Conclusions/interpretation
Systemic B2AR agonist administration acts to limit insulin-induced hypoglycaemia by offsetting insulin’s inhibitory effect on hepatic glucose production. This effect appears to be predominately mediated via a direct effect on liver B2ARs, but a small stimulatory effect on B2ARs within the VMH cannot be excluded. Our data suggest that formoterol may have therapeutic value to limit the risk of hypoglycaemia in patients with diabetes.
Keywords: B2AR agonist, Counter-regulatory responses, Diabetes, Hypoglycaemia
Introduction
Hypoglycaemia is the major factor limiting the use of intensive insulin treatment in individuals with type 1 diabetes [1, 2]. Rapid compensatory responses designed to defend against hypoglycaemia include the stimulation of counter-regulatory hormone secretion (e.g. glucagon and adrenaline [epinephrine]) as well as the activation of the sympathetic nervous system. Together these responses result in increased endogenous glucose production, reduced tissue glucose use and reduced generation of hypoglycaemia-related symptoms [3, 4]. These protective responses are commonly disrupted in individuals with type 1 diabetes and long-lasting type 2 diabetes [5, 6]. As a result, such individuals are particularly vulnerable to severe hypoglycaemia, leading to the disruption of cognitive function. This vulnerability often occurs without warning symptoms or during sleep [7]. Therapeutic interventions in the outpatient setting are currently limited to consumption of carbohydrate-containing foods by the patient or the injection of glucagon, often by a family member [8].
There is growing evidence that the activation of protective responses against hypoglycaemia are triggered by specialised glucose-sensing neurons within the periphal [9] and central nervous system, in particular within the ventromedial hypothalamus (VMH) [10]. Previous studies have shown that the activation of VMH neurons during hypoglycaemia is in part mediated by the local synaptic release of noradrenaline (norepinephrine) [11], an effect that appears to be specifically mediated via β2-adrenergic receptors (B2ARs), which act to modulate the magnitude of the adrenaline and to lesser extent the glucagon responses to insulin-induce hypoglycaemia [12]. The current study was undertaken to assess the whether the systemic delivery of the widely available long-acting specific B2AR agonist, formoterol, could promote glucose counter-regulation, thereby diminishing hypoglycaemia risk. We also investigated whether the systemic effects of formoterol were mediated at the peripheral or central level.
Methods
Animals
Male Sprague–Dawley rats (Charles River, Wilmington, MA, USA) weighing 275–320 g were individually housed in the Yale Animal Resource Center in temperature- (22–23°C) and humidity-controlled rooms. The rats were fed rat chow, given free access to water and were acclimatised to a 12 h light–dark cycle. The Yale University Institutional Animal Care and Use Committee approved the experimental protocols.
Surgery
Seven to 10 days before each study, all rats were anaesthetised with 4% isoflurane solution before undergoing surgery for the implantation of vascular catheters into a carotid artery and jugular vein. Specifically, the left carotid artery was used for blood sampling and the right jugular vein for the infusions, as previously described [13]. In addition, a subgroup of rats (study 2) also underwent surgery to implant brain guide cannulae into the VMH (coordinates from bregma: anterior-posterior −2.6 mm, medio-lateral ±0.8 mm and dorso-ventral −8.0 mm) for targeted drug delivery to the ventromedial nucleus.
Effect of B2AR agonist systemic administration on insulin action (Study 1)
In the morning after rats were fasted overnight, a primed-continuous infusion of [3H]glucose was initiated for 90 min to assess basal glucose turnover and then continued for an additional 90 min throughout a hyperinsulinaemic–hypoglycaemic clamp study. Immediately before the hyperinsulinaemic–hypoglycaemic clamp was started either formoterol (1.6 μg/kg) or normal saline (154 mmol/l NaCl) was injected intra-arterially. Thereafter, an intravascular infusion of regular human insulin (10 mU kg−1 min−1) was begun and a variable infusion of 20% glucose adjusted according to 5–10 min measurements of plasma glucose (Analox Instruments, Lunenburg, MA, USA) to achieve a target value of 3 mmol/l. Blood was drawn for assessment of glucose kinetics and measurement of plasma insulin, glucagon, adrenaline and noradrenaline. Glucose kinetics were calculated, as previously described [14–16].
Effect of blockade of VMH B2AR receptors on the response to systemic delivery of B2AR agonist (Study 2)
In the morning 90 min after opening the vascular catheters, 22-gauge microinjection needles designed to extend 1 mm beyond the tip of the guide cannula (Plastics One, Roanoke, VA, USA) were inserted through the guide cannula bilaterally into each VMH. The rats then received microinjections of either artificial aCSF (control) or 100 μmol/l of the B2AR antagonist ICI-118,551 into the VMH over 10 min (0.1 μl/min, total volume 1 μl) using CMA-102 infusion pump (CMA Microdialysis, North Chelmsford, MA, USA). At the same time, the B2AR agonist formoterol (1.6 μg/kg) was injected intra-arterially immediately before initiating a hyperinsulinaemic–hypoglycaemic clamp using a 20 mU kg−1 min−1 dose of insulin. At the end of experiments, the rats were killed with an overdose of sodium pentobarbital (Sleepaway; Fort Dogde Animal Health, Fort Dogde, IO, USA). Brains were removed, then frozen and stored at −80°C until analysis. The accuracy of microinjection needle placement was verified histologically as previously described [17].
Effect of B2AR agonist on recovery from acute hypoglycaemia (Study 3)
A primed-continuous intravascular infusion of regular insulin (10 mU kg−1 min−1) and a variable infusion of 20% glucose was adjusted (based on 5 min measurements of plasma glucose) to achieve a target value of 2.75 mmol/l within 30 min. Thereafter, formoterol (1.6 μg/kg) or normal saline were injected intra-arterially and, simultaneously, the insulin and glucose infusions were discontinued to allow spontaneous recovery of plasma glucose. Thereafter, glucose levels were measured during a 120 min recovery period. Throughout, blood was obtained for measurement of plasma insulin, glucagon, adrenaline and noradrenaline concentrations.
Effect of B2AR agonist on the development of insulin-induced hypoglycaemia (Study 4)
A continuous intravascular infusion of regular insulin (5 mU kg−1 min−1) was started and simultaneously either formoterol (1.6 μg/kg) or normal saline were injected intra-arterially. Thereafter, the insulin infusion was continued for 120 min, and plasma glucose was monitored at 10 min intervals.
Analytical procedures and data analyses
Plasma catecholamine analysis was performed by HPLC using electrochemical detection (ESA, Acton, MA, USA); plasma insulin and glucagon were measured by radioimmunoassay (Linco, St Charles, MO, USA). All data are expressed as means ± SEM. Statistical analysis was performed by mix model two-way ANOVA for repeated measures, as appropriate, followed by post hoc Bonferoni analysis to localise effects of treatment over time using Graph Prism (Prism 4.0; GraphPad Software, San Diego, CA, USA); p<0.05 was considered as statistically significant.
Results
Baseline characteristics
As shown in Table 1, body weight as well as plasma glucose, insulin, glucagon, adrenaline and noradrenaline concentrations did not differ between rats assigned to the control and the experimental groups at baseline in each of the studies.
Table 1.
Baseline characteristics of rats in the morning before study
| Characteristic | Study 1
|
Study 2
|
Study 3
|
|||
|---|---|---|---|---|---|---|
| Controls | B2AR agonist peripherally | B2AR agonist peripherally, aCSF to the VMH | B2AR agonist peripherally, B2AR antagonist to the VMH | Controls | B2AR agonist peripherally | |
| n | 5 | 5 | 8 | 8 | 4 | 4 |
| Body weight (g) | 290±15 | 315±7 | 310±20 | 313±7.4 | 301±6 | 304±16 |
| Plasma glucose (mmol/l) | 5.3±0.3 | 5.3±0.1 | 6.2±0.2 | 5.9±0.2 | 6.3±0.1 | 6.4±0.1 |
| Insulin (pmol/l) | 62.5±7.6 | 38.9±8.3 | 132±46 | 111±47 | 60±4.8 | 61±4.8 |
| Glucagon (ng/l) | 44±12 | 42±12 | 53±7.2 | 47±3.5 | 64±6 | 63±4 |
| Adrenaline (pmol/l) | 890±284 | 933±213 | 912±393 | 884±218 | 442±55 | 371±180 |
| Noradrenaline (pmol/l) | 1,868±360 | 1,690±337 | 2,477±449 | 2,554±662 | 851±154 | 904±65 |
Data are presented as means ± SEM
Effect of B2AR agonist systemic administration (Study 1)
Plasma glucose levels before and during the hyperinsulinaemic–hypoglycaemic clamp did not significantly differ between the B2AR agonist and saline control group (Fig. 1a). In addition, plasma insulin concentrations during the clamp (5098±299 vs 6063±694 pmol/l for B2AR agonist and controls, respectively) were indistinguishable between the groups.
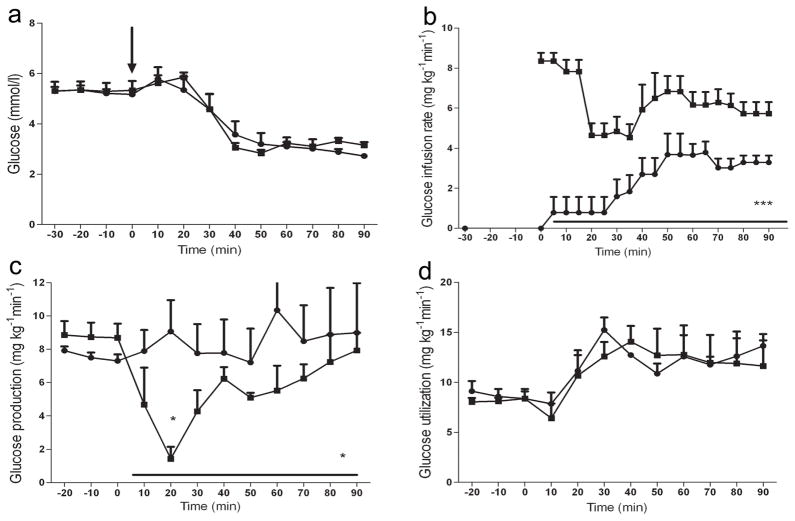
Fig. 1.
Effect of B2AR agonist systemic administration on glucose metabolism during hypoglycaemic clamp. Plasma glucose concentration (a), GIR (b), glucose production (c) and glucose uptake (d) for rats receiving injection of the vehicle (control; n=5) and the B2AR agonist formoterol (n=5) during the hyperinsulinaemic–hypoglycaemic glucose clamp. The arrow indicates the time at which the B2AR agonist was delivered. Circles, B2AR agonist; squares, controls. Results are presented as mean±SEM. *p<0.05 and ***p<0.001 vs control
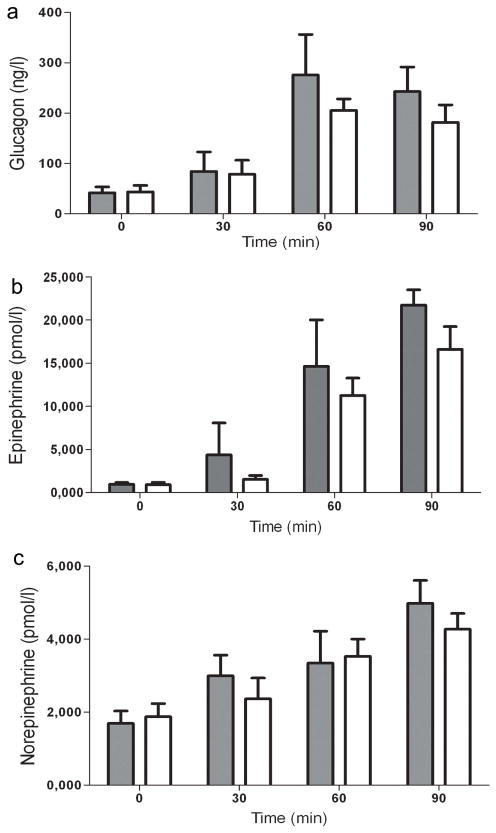
As shown in Fig. 1b systemic delivery of the B2AR agonist rapidly reduced by 60% the exogenous glucose infusion rate (GIR) required to maintain identical target glucose concentrations throughout the entire hypoglycaemic clamp study (2.5±0.3 vs 6.1±0.3 mg kg−1 min−1 in controls, p<0.001). These B2AR-induced changes were accompanied by alternations in endogenous glucose production. The B2AR agonist prevented the initial insulin-induced decrease in hepatic glucose production (HGP) during the first 30 min of the hypoglycaemic clamp (8.2±1.5 vs 3.4±0.1 mg/kg/min in controls, p<0.05). Moreover, HGP during the entire hypoglycaemic clamp was increased by the B2AR agonist (8.5±1.9 vs 5.4±0.6 mg kg−1 min−1 in controls, p<0.05) (Fig. 1c). The difference in GIR during the clamp was almost completely accounted for by HGP; no significant difference in glucose use was detected between the B2AR agonist and control group (Fig. 1d). The delivery of the B2AR agonist had no statistically significant effect on the glucagon (Fig. 2a), adrenaline (Fig. 2b) or noradrenaline (Fig. 2c) responses during hypoglycaemia.
Fig. 2.
Effect of B2AR agonist systemic administration on counter-regulatory hormones during the hypoglycaemic clamp. Plasma glucagon (a), adrenaline (b) and noradrenaline (c) concentrations. Grey bars, B2AR agonist; white bars, controls. Results are presented as means ± SEM
Effect of blockade of VMH B2AR receptors on the B2AR agonist response (Study 2)
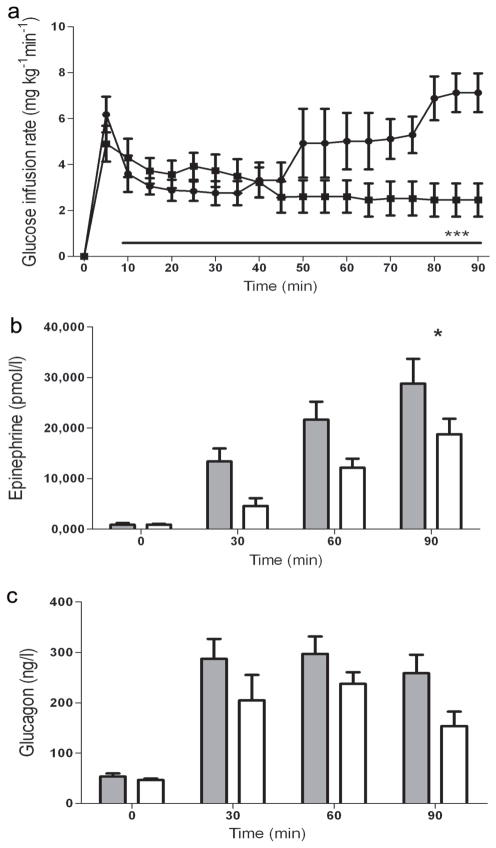
To assess the potential contribution of VMH B2AR activation to the anti-insulin effect of systemic B2AR delivery, a B2AR antagonist or artificial cerebrospinal fluid (aCSF; control) was delivered to the VMH immediately before the agonist was administered systemically. The VMH delivery of the B2AR antagonist did not significantly alter plasma glucose (2.98±0.1 vs 3.17±0.1 mmol/l in controls) during the final 60 min of the hyperinsulinaemic–hypoglycaemic clamp. Similarly, plasma insulin concentrations were not altered in rats receiving the B2AR antagonist (8007±2180 vs 8250±1250 pmol/l in controls). As shown in Fig. 3a, targeted VMH B2AR blockade initially had no significant effect on the exogenous glucose required to maintain target glucose concentrations. However, during the last 30 min of the hypoglycaemic clamp the GIR in the group that received VMH-targeted B2AR blockade was more than twofold higher compared with the controls (5.9±1.0 vs 2.5±0.8 mg kg−1 min−1 in controls, p<0.001). These changes were accompanied by a significant reduction in the adrenaline response during last 30 min of hypoglycaemia (Fig. 3b). Delivery of B2AR antagonist to the VMH did not significantly affect glucagon (Fig. 3c) or noradrenaline (data not presented) responses during hypoglycaemia.
Fig. 3.
Effect of blockade of VMH B2AR receptors on the B2AR agonist response. GIR (a) and plasma concentrations of the counter-regulatory hormones adrenaline (b) and glucagon (c) in rats receiving microinjection of the artificial ECF vehicle (control; n=8), or the B2AR antagonist ICI-118,551 (n=8), during the hyperinsulinaemic–hypoglycaemic glucose clamp. The arrow indicates time when B2AR agonist was delivered. Circles and grey bars, B2AR agonist; squares and white bars, control. Results are presented as means ± SEM. *p<0.05 and ***p<0.001 vs control
Effect of systemic B2AR agonist on recovery from hypoglycaemia (Study 3)
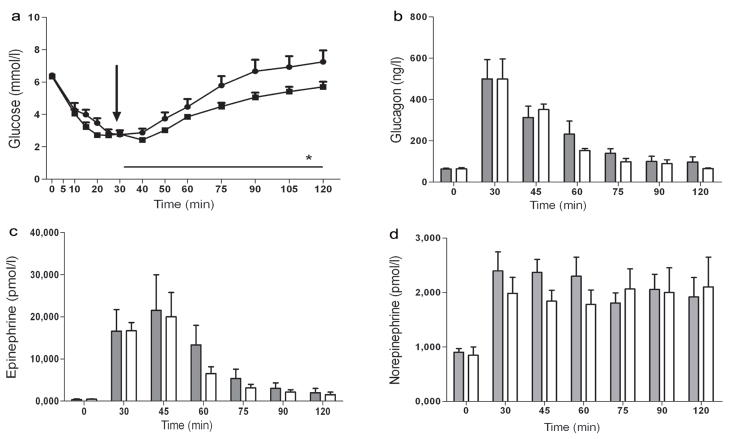
In the initial phase of the study before delivery of the B2AR agonist, plasma glucose (2.80±0.01 vs 2.75±0.22 mmol/l and insulin (3764±166 vs 4326±453 pmol/l in control) levels as well as the GIR required to achieve similar degrees of hypoglycaemia (11.6±0.4 mg kg−1 min−1 for B2AR agonist vs 11.2±0.8 mg kg−1 min−1 for controls) were similar. As shown in Fig. 4a, systemic injection of the B2AR agonist at the time of the glucose nadir more rapidly increased glucose recovery from hypoglycaemia. This became apparent within 30 min after the systemic delivery of the B2AR agonist and persisted throughout the 90 min observation period post injection (p<0.05). Plasma insulin levels were also slightly higher in the B2AR agonist group (173±69 vs 90±69 pmol/l in controls, p=NS) during the final hour of the recovery period. The B2AR agonist had no significant effect on the overall counter-regulatory hormone response during the recovery period. However, the levels of glucagon (Fig. 4b), adrenaline (Fig. 4c) and noradrenaline (Fig. 4d) showed a brief relative increase 30 min after B2AR agonist injection (i.e. at 60 min), the time at which the glucose levels began to diverge.
Fig. 4.
Effect of systemic B2AR agonist on recovery from hypoglycaemia. Plasma concentrations of glucose (a), glucagon (b), adrenaline (c) and noradrenaline (d) in rats receiving peripheral injection of the vehicle (control; n=4) and the B2AR agonist formoterol (n=4) during the recovery from hypoglycaemia. The arrow indicates the time at which the B2AR agonist was delivered. Circles and grey bars, B2AR agonist; squares and white bars, control. Results are presented as means ± SEM. *p<0.05 vs control
Effect of systemic B2AR agonist on the development of insulin-induced hypoglycaemia (Study 4)
The baseline glucose level before delivery of the B2AR agonist was similar between groups (6.65±0.24 vs 6.43±0.14 mmol/l). As shown in Fig. 5, plasma glucose levels declined in the control group, reaching 3.46±0.2 mmol/l at 60 min and 3.1±0.2 mmol/l at 120 min. In contrast, the systemic injection of the B2AR agonist produced an initial increase in glucose level, which reached 9.4±0.2 mmol/l at 40 min; this was followed by a gradual decrease in plasma glucose over an 80 min period. However, the glucose level remained within the normoglycaemic range at the end of the study (4.7±0.6 mmol/l, Fig. 5).
Fig. 5.
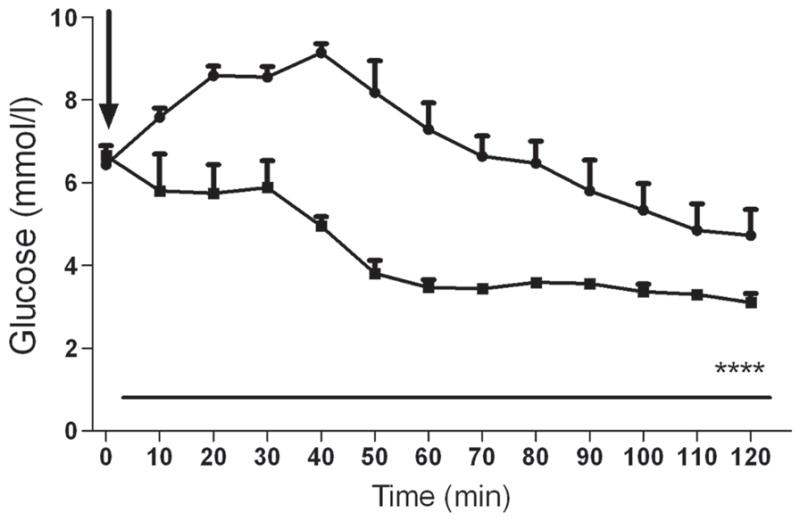
Effect of systemic B2AR agonist on the development of insulin-induced hypoglycaemia. Plasma glucose concentration in rats receiving peripheral injection of the vehicle (control; n=4), and the B2AR agonist formoterol (n=4) during insulin-induced hypoglycaemia. The arrow indicates the time at which the B2AR agonist was delivered. Circles, B2AR agonist; squares, control. ****p<0.0001 vs control
Discussion
Our data demonstrate that peripheral delivery of the specific B2AR agonist formoterol prevents the decline in HGP in response to insulin-induced hypoglycaemia, thereby reducing both the demand for glucose administration and the development of hypoglycaemia. Similarly, peripheral delivery of a B2AR agonist after the production of insulin-induced hypoglycaemia promoted the more rapid recovery of plasma glucose into the normal range. These insulin-antagonistic effects of formoterol appear to be mainly caused by a direct effect on HGP mediated via hepatocyte β2-receptors, as there were negligible changes in the secretion of counter-regulatory hormones or in insulin-stimulated peripheral glucose uptake. In keeping with this possibility, previous studies have shown that B2ARs mediate the stimulatory effects of catecholamines on HGP by initially activating glycogenolysis and then subsequently accelerating gluconeogenesis [18–21]. However, an effect of the peripherally delivered B2AR agonist on the VMH to promote HGP by activating direct neural innervation cannot be entirely excluded, although it is not known whether formoterol crosses the blood–brain barrier. VMH microinjection of a B2AR antagonist induced a delayed reduction in the effect of the peripheral infusion of B2AR agonist on glucose metabolism; a twofold increase in the GIR required to maintain hypoglycaemia was observed. This may be an independent effect as it was associated with a diminution of adrenaline release. The B2AR antagonist might have also acted directly on the hypothalamus to diminish HGP independent of counter-regulatory hormone secretion [22, 23]. The latter possibility is consistent with data suggesting that noradrenaline neurotransmission acts locally to stimulate VMH neurons and in turn counter-regulatory responses during hypoglycaemia [11, 12]. Thus, B2AR agonists may possibly exert multiple effects that could promote recovery from insulin-induced hypoglycaemia.
The current rodent data have potential clinical implications. Formoterol, a long-acting B2AR specific agonist delivered via an inhaler to treat exacerbations of asthma, could offer individuals with diabetes a convenient non-injectable and relatively rapid means of reversing or preventing acute hypoglycaemia. It is intriguing to speculate whether this approach may be particularly useful as a means of diminishing the risk of nocturnal hypoglycaemia and whether it could be used in conjunction with glucose sensors to reverse hypoglycaemia in diabetic patients with hypoglycaemia unawareness. In such a case, the prevention of hypoglycaemia using a B2AR agonist provides a potential means to restore counter-regulatory hormonal and symptomatic responses to hypoglycaemia [24–27]. In this regard it is worth noting that previous studies have shown that administration of the less-specific and shorter-acting B2AR agonist [28] terbutaline reduces nocturnal hypoglycaemia in patients with type 1 diabetes [29, 30]. The more extended duration of action and higher specificity of formoterol may therefore offer greater potential benefit in such individuals. This possibility will of course need to be tested in the clinical setting in individuals with type 1 diabetes. Nevertheless, the current study provides insights into the mechanisms by which B2AR agonists may act in the clinical setting to reduce the risk of insulin-induced hypoglycaemia.
Acknowledgments
The authors are grateful to Aida Groszman, Maria Batsu, Codruta Todeasa and Ralph J. Jacob from the Yale Center for Clinical Investigation Core Laboratory of the Yale Medical School for excellent technical support and assistance and to Yuyan Ding from the Dr Robert Sherwin laboratory for performing animal surgeries.
Funding
This work was supported by a research grants from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (20495). The JDRF and the NIDDK supported the Diabetes Endocrinology Research Center.
Abbreviations
- aCSF
Artificial cerebrospinal fluid
- B2AR
β2-Adrenergic receptor
- GIR
Glucose infusion rate
- HGP
Hepatic glucose production
- VMH
Ventromedial hypothalamus
Footnotes
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Contribution statement
BS designed the study, performed the animal surgery and study, analysed data and wrote the manuscript. WZ performed the animal surgery, acquired data and participated in drafting the article. RSS designed the study, reviewed the data and edited the manuscript. All authors approved the final version.
References
- 1.The Diabetes Control and Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. TN Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 2.The Diabetes Control and Complications Trial Research Group . Hypoglycemia in the Diabetes Control and Complications Trial. Diabetes. 1997;46:271–286. [PubMed] [Google Scholar]
- 3.Sherwin RS, Shamoon H, Hendler R, Sacca L, Eigler N, Walesky M. Epinephrine and the regulation of glucose metabolism: effect of diabetes and hormonal interactions. Metabolism. 1980;29:1146–1154. doi: 10.1016/0026-0495(80)90024-4. [DOI] [PubMed] [Google Scholar]
- 4.Rizza RA, Cryer PE, Gerich JE. Role of glucagon, catecholamines, and growth hormone in human glucose counterregulation. Effects of somatostatin and combined alpha- and beta-adrenergic blockade on plasma glucose recovery and glucose flux rates after insulin-induced hypoglycemia. J Clin Invest. 1979;64:62–71. doi: 10.1172/JCI109464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.UK Hypoglycaemia Study Group . Risk of hypoglycaemia in types 1 and 2 diabetes: effects of treatment modalities and their duration. Diabetologia. 2007;50:1140–1147. doi: 10.1007/s00125-007-0599-y. [DOI] [PubMed] [Google Scholar]
- 6.Amiel SA, Tamborlane WV, Simonson DC, Sherwin RS. Defective glucose counterregulation after strict glycemic control of insulin-dependent diabetes mellitus. N Engl J Med. 1987;316:1376–1383. doi: 10.1056/NEJM198705283162205. [DOI] [PubMed] [Google Scholar]
- 7.Jones TW, Porter P, Sherwin RS, et al. Decreased epinephrine responses to hypoglycemia during sleep. N Engl J Med. 1998;338:1657–1662. doi: 10.1056/NEJM199806043382303. [DOI] [PubMed] [Google Scholar]
- 8.Cryer PE. Elimination of hypoglycemia from the lives of people affected by diabetes. Diabetes. 2011;60:24–27. doi: 10.2337/db10-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donovan CM, Halter JB, Bergman RN. Importance of hepatic glucoreceptors in sympathoadrenal response to hypoglycemia. Diabetes. 1991;40:155–158. doi: 10.2337/diab.40.1.155. [DOI] [PubMed] [Google Scholar]
- 10.Borg MA, Sherwin RS, Borg WP, Tamborlane WV, Shulman GI. Local ventromedial hypothalamus glucose perfusion blocks counterregulation during systemic hypoglycemia in awake rats. J Clin Invest. 1997;99:361–365. doi: 10.1172/JCI119165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beverly JL, de Vries MG, Bouman SD, Arseneau LM. Noradrenergic and GABAergic systems in the medial hypothalamus are activated during hypoglycemia. Am J Physiol Regul Integr Comp Physiol. 2001;280:R563–569. doi: 10.1152/ajpregu.2001.280.2.R563. [DOI] [PubMed] [Google Scholar]
- 12.Szepietowska B, Zhu W, Chan O, Horblitt A, Dziura J, Sherwin RS. Modulation of beta-adrenergic receptors in the ventromedial hypothalamus influences counterregulatory responses to hypoglycemia. Diabetes. 2011;60:3154–3158. doi: 10.2337/db11-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCrimmon RJ, Shaw M, Fan X, et al. Key role for AMP-activated protein kinase in the ventromedial hypothalamus in regulating counterregulatory hormone responses to acute hypoglycemia. Diabetes. 2008;57:444–450. doi: 10.2337/db07-0837. [DOI] [PubMed] [Google Scholar]
- 14.McCrimmon RJ, Fan X, Ding Y, Zhu W, Jacob RJ, Sherwin RS. Potential role for AMP-activated protein kinase in hypoglycemia sensing in the ventromedial hypothalamus. Diabetes. 2004;53:1953–1958. doi: 10.2337/diabetes.53.8.1953. [DOI] [PubMed] [Google Scholar]
- 15.Wall JS, Steele R, de Bodo RC, Altszuler N. Effect of insulin on utilization and production of circulating glucose. Am J Physiol. 1957;189:43–50. doi: 10.1152/ajplegacy.1957.189.1.43. [DOI] [PubMed] [Google Scholar]
- 16.Steele R, Wall JS, de Bodo RC, Altszuler N. Measurement of size and turnover rate of body glucose pool by the isotope dilution method. Am J Physiol. 1956;187:15–24. doi: 10.1152/ajplegacy.1956.187.1.15. [DOI] [PubMed] [Google Scholar]
- 17.McCrimmon RJ, Song Z, Cheng H, et al. Corticotrophin-releasing factor receptors within the ventromedial hypothalamus regulate hypoglycemia-induced hormonal counterregulation. J Clin Invest. 2006;116:1723–1730. doi: 10.1172/JCI27775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chu CA, Sindelar DK, Neal DW, Allen EJ, Donahue EP, Cherrington AD. Effect of a selective rise in sinusoidal norepinephrine on HGP is due to an increase in glycogenolysis. Am J Physiol. 1998;274:E162–E171. doi: 10.1152/ajpendo.1998.274.1.E162. [DOI] [PubMed] [Google Scholar]
- 19.Sacca L, Vigorito C, Cicala M, Corso G, Sherwin RS. Role of gluconeogenesis in epinephrine-stimulated hepatic glucose production in humans. Am J Physiol. 1983;245:E294–E302. doi: 10.1152/ajpendo.1983.245.3.E294. [DOI] [PubMed] [Google Scholar]
- 20.Hendler RG, Sherwin RS. Epinephrine-stimulated glucose production is not diminished by starvation: evidence for an effect on gluconeogenesis. J Clin Endocrinol Metab. 1984;58:1014–1021. doi: 10.1210/jcem-58-6-1014. [DOI] [PubMed] [Google Scholar]
- 21.Erraji-Benchekroun L, Couton D, Postic C, et al. Overexpression of beta2-adrenergic receptors in mouse liver alters the expression of gluconeogenic and glycolytic enzymes. Am J Physiol Endocrinol Metab. 2005;288:E715–E722. doi: 10.1152/ajpendo.00113.2004. [DOI] [PubMed] [Google Scholar]
- 22.Frizzell RT, Hendrick GK, Brown LL, et al. Stimulation of glucose production through hormone secretion and other mechanisms during insulin-induced hypoglycemia. Diabetes. 1988;37:1531–1541. doi: 10.2337/diab.37.11.1531. [DOI] [PubMed] [Google Scholar]
- 23.Connolly CC, Myers SR, Neal DW, Hastings JR, Cherrington AD. In the absence of counterregulatory hormones, the increase in hepatic glucose production during insulin-induced hypoglycemia in the dog is initiated in the liver rather than the brain. Diabetes. 1996;45:1805–1813. doi: 10.2337/diab.45.12.1805. [DOI] [PubMed] [Google Scholar]
- 24.Liu D, McManus RM, Ryan EA. Improved counter-regulatory hormonal and symptomatic responses to hypoglycemia in patients with insulin-dependent diabetes mellitus after 3 months of less strict glycemic control. Clin Invest Med. 1996;19:71–82. [PubMed] [Google Scholar]
- 25.Cranston I, Lomas J, Maran A, Macdonald I, Amiel SA. Restoration of hypoglycaemia awareness in patients with long-duration insulin-dependent diabetes. Lancet. 1994;344:283–287. doi: 10.1016/s0140-6736(94)91336-6. [DOI] [PubMed] [Google Scholar]
- 26.Dagogo-Jack S, Rattarasarn C, Cryer PE. Reversal of hypoglycemia unawareness, but not defective glucose counterregulation, in IDDM. Diabetes. 1994;43:1426–1434. doi: 10.2337/diab.43.12.1426. [DOI] [PubMed] [Google Scholar]
- 27.Fanelli C, Pampanelli S, Epifano L, et al. Long-term recovery from unawareness, deficient counterregulation and lack of cognitive dysfunction during hypoglycaemia, following institution of rational, intensive insulin therapy in IDDM. Diabetologia. 1994;37:1265–1276. doi: 10.1007/BF00399801. [DOI] [PubMed] [Google Scholar]
- 28.Bartow RA, Brogden RN. Formoterol. An update of its pharmacological properties and therapeutic efficacy in the management of asthma. Drugs. 1998;55:303–322. doi: 10.2165/00003495-199855020-00016. [DOI] [PubMed] [Google Scholar]
- 29.Cooperberg BA, Breckenridge SM, Arbelaez AM, Cryer PE. Terbutaline and the prevention of nocturnal hypoglycemia in type 1 diabetes. Diabetes Care. 2008;31:2271–2272. doi: 10.2337/dc08-0520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saleh TY, Cryer PE. Alanine and terbutaline in the prevention of nocturnal hypoglycemia in IDDM. Diabetes Care. 1997;20:1231–1236. doi: 10.2337/diacare.20.8.1231. [DOI] [PubMed] [Google Scholar]