Abstract
Background and Aims
Because of its rarity, achalasia remains a difficult disease to study. The aim of the present analysis was to describe the epidemiology of achalasia and practice patterns in its endoscopic management, utilizing patient records from a large national database of endoscopic procedures.
Methods
The Clinical Outcomes Research Initiative (CORI) maintains a database of endoscopic procedures in diverse clinical practices. The data from 89 endoscopy practices distributed throughout the US during 2000–2008 were used to analyze the characteristics and therapy of patients with achalasia.
Results
Among 521,497 upper endoscopies during the study period, we identified 896 patients with achalasia. Compared with the entirety of all other endoscopic diagnoses, achalasia was more common in men than women (OR=1.39, CI 1.22–1.59), but similar among non-whites and whites (OR=0.87, CI 0.74–1.03). Relatively more achalasia patients were treated at university than community practices (OR=1.52, CI 1.30–1.78). Botox injection was most frequently used as first choice of endoscopic therapy in 41%, followed by balloon dilation in 21%, Savary dilation in 20%, Maloney dilation in 10%, Rigiflex in 4%, and other modalities in 4% of patients. One quarter of achalasia patients treated endoscopically underwent a repeat therapy about every 14 months.
Conclusions
Botox has become the primary choice of initial endoscopic therapy in achalasia. Despite their partial deviation from guidelines and recommendations, these endoscopic patterns reflect the current clinical practice in the United States.
Keywords: Achalasia, Botox injection, epidemiology, pneumatic dilation, practice patterns
Introduction
Achalasia is a primary esophageal motility disorder of unknown etiology. It is characterized by failure of the lower esophageal sphincter to relax and abnormal peristalsis of the esophageal body during swallowing1. Despite its recognition as a clinical entity for several centuries, it has remained one of the least understood gastroenterological diseases, at least in part due to its low prevalence of approximately 0.01%2. While several suggestions have been made as to its underlying etiology, such as exposure to noxious environmental influences, infection, genetic abnormality, or autoimmune disease, none of these theories have survived rigorous study or gained general acceptance. Epidemiological studies could serve as means to identify potential risk factors that play a role in the development of this rare disease. Large centers dedicated to the research of achalasia have followed fewer than 300 patients3–5. Most clinical studies of achalasia have struggled in providing reliable epidemiologic data, as they were rarely able to recruit more than 50–100 patients into a single study1,2,5–9. Similarly, given these relatively small patient groups from individual tertiary care centers, the current practice patterns in the general management of achalasia have not been previously described in a representative population of achalasia patients.
The Clinical Outcomes Research Initiative database (CORI) is a national multi-center consortium of gastroenterology practices distributed throughout the United States. CORI includes community, Department of Veterans Affairs (VA) and academic practices. The CORI database is uniquely suited for the study of achalasia because of its large and geographically varied sample size. The aims of the present study were to utilize the CORI database to describe the demographic characteristics and practice patterns in the endoscopic management of patients with achalasia between 2000 and 2008.
Methods
The CORI database was used to extract the data of all patients undergoing esophago-gastro-duodenoscopy (EGD) between 2000 and 2008. CORI was established in 1995 as a means to study outcomes and utilization of endoscopic procedures among diverse practice settings in the United States10. In addition to strictly endoscopic procedures, the database also contains the records other procedures performed by gastroenterologists in their practice, such as the use of Maloney or pneumatic dilations of the esophagus. Practice sites include community practices, VA medical centers, and academic centers. Endoscopic reports from the participating centers are sent to CORI after the patient records have been de-identified of any personal information. Because of the use of de-identified data only, the study was granted a waiver of consent by the institutional review board at the Oregon Health & Science University. Multiple previous studies have utilized CORI data for peer-reviewed publications11–19.
The CORI database from 2000 until 2008 covered 89 practice sites. The database was queried for all patients undergoing EGD for any type of diagnosis, including the ICD9-code 530.0 representing achalasia. If an achalasia patient had an EGD during the study time frame but the initial endoscopy occurred before the start of the study in 2000, their prior electronic records (since 1995) were also retrieved. Patients under the age of 2 years were excluded from the study. The entirety of patients who underwent EGD, less those with a diagnosis of achalasia, served as the control group. The retrieved data included age, gender, race, type of practice site, type of endoscopic therapy, number of repeat endoscopic therapies, lengths of time interval between repeat endoscopies, and immediate complications following endoscopy. The types of endoscopic therapy were categorized by their use of injection of botulinum toxin (Botox), Maloney dilator, Savary dilator, Rigiflex balloon, other balloon types, and other treatments (Eder-Puestow or unnamed dilation types).
Patient data were stratified by age, gender, race, and site type. Case and control subjects were compared using the Mantel-Haenszel procedure to adjust the odds ratio (OR) and their 95% confidence intervals (CI) to the confounding interactions of age, gender, race, and site type. Other comparisons were based on Chi-square analysis or Student’s t-test.
Results
Between 2000 and 2008, 521,497 upper endoscopies were performed. We identified 896 unique patients with achalasia (0.17%). The mean age of achalasia subjects was 62 ± 19 years compared with 54 ± 20 years in the control population (p < 0.0001). Figure 1 demonstrates the age distribution of male and female achalasia patients expressed as a relative rate. There was an age related rise in the occurrence of achalasia with the majority of cases occurring in patients older than 50 years. This trend was similar in male and female achalasia patients.
Figure 1.
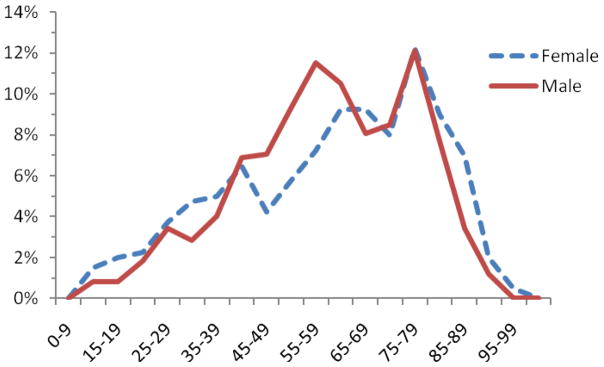
Age distribution of male and female achalasia patients
Table 1 contains the demographic data of achalasia patients and controls. Achalasia was more common in men than women. There was a slight but not significant trend towards achalasia being more common among white than non-white subjects. Of all patients undergoing EGDs, relatively more achalasia was treated at university practices as compared to community or VA practices.
Table 1.
Demographic data
| Achalasia | Control | OR | 95% CI | |
|---|---|---|---|---|
| Total | 896 (100%) | 520,601 (100%) | ||
| Gender | ||||
| Female | 401 (45%) | 250,045 (48%) | 1.00 | |
| Male | 495 (55%) | 270,556 (52%) | 1.39 | (1.22–1.59) |
| Race | ||||
| White | 739 (83%) | 413,329 (79%) | 1.00 | |
| Non-white | 157 (17%) | 107,272 (21%) | 0.87 | (0.74–1.03) |
| Site type | ||||
| Community | 618 (69%) | 379,113 (73%) | 1.00 | |
| University | 195 (22%) | 82,036 (16%) | 1.51 | (1.33–1.78) |
| VA | 83 (9%) | 59,452 (11%) | 0.69 | (0.54–0.88) |
The type of first therapy utilized in the endoscopic management of patients with achalasia is described in Table 2. Of all 896 patients with achalasia, 536 (60%) underwent endoscopic therapy. Botox injection was the most frequently used as first endoscopic therapy, followed by balloon dilation, Savary dilation, Maloney dilation, and pneumatic dilation using the Rigiflex balloon. This distribution was largely independent of gender or race. Compared with other sites, university practices used more Botox and Rigiflex, whereas the use of Savary or Maloney dilators was particularly common among community practices (p < 0.0001). Patients treated with Botox were generally older than those treated with Rigiflex (p=0.0002). As expected, the mean first diameter of dilation was highest for the Rigiflex balloon. About one quarter of patients with achalasia underwent repeat endoscopic therapy within 14 months. The fraction of patients with two or more repetitive treatments during the study period was lower for Savary, Rigiflex or other balloon dilation than in the Botox or Maloney group (p < 0.001). Immediate complications following endoscopic therapy were very rare, only one serious immediate perforation occurred in the Rigiflex group.
Table 2.
Type of first therapy in the endoscopic treatment of achalasia
| Botox | Maloney | Savary | Rigiflex | Other Balloon | Other | Total | |
|---|---|---|---|---|---|---|---|
| Total patients | 218 (41%) | 55 (10%) | 108 (20%) | 23 (4%) | 113 (21%) | 20 (4%) | 536 (100%) |
| Mean age ± sd (years) | 64 ± 20 | 59 ± 18 | 62 ± 18 | 46 ± 21 | 62 ± 17 | 66 ± 23 | 62 ± 19 |
| Gender | |||||||
| Male | 118 (39%) | 30 (10%) | 58 (19%) | 13 (4%) | 72 (24%) | 13 (4%) | 304 (100%) |
| Female | 100 (43%) | 24 (10%) | 50 (22%) | 10 (4%) | 41 (18%) | 7 (3%) | 232 (100%) |
| Race | |||||||
| White | 174 (39%) | 46 (10%) | 93 (21%) | 18 (4%) | 98 (2%) | 18 (4%) | 447 (100%) |
| Non-white | 44 (49%) | 8 (9%) | 15 (17%) | 5 (6%) | 15 (17%) | 2 (2%) | 89 (100%) |
| Site type | |||||||
| University practice | 88 (75%) | 0 (0%) | 4 (3%) | 14 (12%) | 11 (9%) | 0 (0%) | 117 (100%) |
| Community practice | 113 (31%) | 52 (14%) | 86 (23%) | 7 (2%) | 90 (25%) | 18 (5%) | 366 (100%) |
| VA practice | 17 (32%) | 2 (4%) | 18 (34%) | 2 (4%) | 12 (23%) | 2 (4%) | 53 (100%) |
| Therapy details | |||||||
| Mean first diameter ± sd (mm) | 18 ± 5* | 18 ± 1 | 18 ± 1 | 34 ± 4 | 17 ± 3 | 17 ± 1 | 19 ± 3 |
| Patients with multiple endoscopic sessions | 69 (32%) | 16 (30%) | 21 (19%) | 5 (22%) | 28 (25%) | 4 (20%) | 143 (27%) |
| Mean number of repeat therapies ± sd | 0.6 ± 1.2 | 0.4 ± 0.7 | 0.3 ± 1.0 | 0.3 ± 0.7 | 0.6 ± 1.5 | 0.6 ± 1.3 | 0.5 ± 1.2 |
| Mean interval between repeat therapies ± sd (months) | 12 ± 11 | 18 ± 17 | 8 ± 8 | 11 ± 11 | 21 ± 23 | 9 ± 11 | 14 ± 15 |
| Complications | |||||||
| Immediate perforation | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| Immediate bleeding | 2 | 0 | 0 | 0 | 1 | 0 | 3 |
Mean diameter refers to subsequent therapies after the initial Botox injection.
Discussion
Even in the CORI database, dedicated specifically to the outcomes and utilization of endoscopy, achalasia remained a relatively rare disease, involving only 0.17% of patients undergoing an EGD. The data demonstrated a clear age-dependent rise in the disease occurrence. In our patient population, achalasia was slightly more common in men than women. It was most frequently treated in the setting of academic practices. In all practices alike, Botox injection has emerged as the most commonly used endoscopic therapy for achalasia, especially in the elderly. One quarter of achalasia patients treated endoscopically underwent a repeat therapy about every 14 months.
While achalasia can occur at any age, it is generally a disease of older age and rarely occurs before the 2nd decade of life. The mean age of patients with achalasia in this study was comparable to previous reports20–23. The incidence of achalasia increases with age with the highest rates occurring beyond the seventh decade8,20,21,24. In our analysis, the drop in the relative fraction of patients after the age of 85 years was probably a reflection of the small overall number of patients within the oldest age groups. Our study did not allow us to calculate population-based prevalence rates, because the size of the catchment populations, from which patients were recruited into the database, remained unknown. However, two previous epidemiologic studies using nationwide hospital discharge data suggested a continuous age-related rise that affects even the oldest age groups21,24. The reasons for this age-dependent behavior of achalasia remain unknown. A general age-dependent loss in neuronal function could contribute to unmasking the occurrence of achalasia in old age. The loss of ganglion cells in the area of the lower esophageal sphincter could also result from repeated exposure to noxious environmental risk factors whose damaging influences accumulate over a prolonged lifetime.
EGDs for any reason were more commonly performed in men than women. Superimposed on this underlying general trend, the present study demonstrated an additional male predominance among EGDs done specifically to diagnose and treat achalasia. The adjusted odds ratio associated with male gender was slightly yet significantly raised above unity. This result runs contrary to other prior studies, which have not supported any gender predominance2,5,6,8,25,26. The reason for a male predominance in our study population is presently unclear. Our finding would need to be confirmed by other studies before it can be assigned any true clinical or epidemiologic relevance. Prior studies have also been unable to identify any predominant race associated with achalasia. Similarly, while there was a slight trend towards increased occurrence of achalasia among white as compared to non-white subjects, this trend was not statistically significant.
In the endoscopic management of achalasia, the university setting had proportionately higher rates of Rigiflex dilation and Botox injection compared to other sites. Gennaro et al27 have documented a similar trend. This trend may reflect on the complexity and risks associated with the endoscopic management of achalasia. As Botox injection has been demonstrated to be safe, effective, inexpensive, and easy to perform, it has become an appealing strategy for the management of achalasia28–30. It is associated with markedly fewer complications than pneumatic dilation30. The present study revealed an older average age among patients treated with Botox than those treated with Rigiflex pneumatic dilation. It is possible that physicians were more likely to utilize Botox injection in patients with advanced age or severe co-morbidities, because of their concerns about the greater risks associated with pneumatic dilation or surgical myotomy25,31. Unexpectedly, we also observed a substantial fraction of patients treated with Savary and Maloney dilators for achalasia. These means of dilation are typically considered less effective in fracturing the muscularis propria of the lower esophageal sphincter, which is the aim of dilation in patients with achalasia. The widespread use of Savary or Maloney dilators among diverse practice sites may again reflect on physician attempts at avoiding risky complications and resorting to relatively safe, albeit less effective means of therapy. The data could also indicate that many gastroenterologists make a diagnosis of achalasia not according to the textbook, but approach it empirically and partly based on the response to their therapeutic efforts.
Our study has several potential limitations. Data on therapy were only available for 60% of all achalasia patients. After endoscopy, a large proportion of patients with the diagnosis of achalasia may have been referred to surgery for myotomy. We may have missed some pneumatic dilations that were not listed in the CORI database or were done outside the GI laboratory. The clinical follow-up of such patients was not possible within the confinements of the present study, because the clinical information contained in the CORI database was limited to the endoscopic or procedural report itself. Similarly, CORI may have underestimated the true fraction of complications. Individual complications are not entered into the electronic endoscopy report, unless they become detected during or shortly after the endoscopic procedure. Lastly, our analysis may have also underestimated the true number of repeat endoscopic treatment sessions in those instances when an individual patient underwent a repeat therapy at an endoscopy center outside the CORI consortium.
These potential shortcomings of the CORI database need to be contrasted with its obvious strengths. The database was specifically designed to capture practice patterns in the management of a gastrointestinal disease among a wide variety of practice settings distributed throughout the United States. For these reasons, its data are uniquely suited to study the epidemiology of rare diseases in a large patient population. In comparison with other national databases, CORI has been shown to provide a representative picture of endoscopic practice in the United States32. Even if some of the patterns in management of achalasia revealed by the present analysis may be unexpected or deviate from published guidelines, they are still likely to represent the true status of current endoscopic practice of managing achalasia in the United States. Because this is an outcome study, we had to use the available data without being able to control for the diagnostic or therapeutic skills of individual gastroenterologists. Unlike a clinical trial, an outcome study cannot rely on an ideal set of prospectively established criteria but needs to utilize existing records and accept their contents at face value.
In conclusion, this large multi-center database reveals that Botox injection has emerged as the preferential first line of endoscopic therapy in achalasia, especially among older patients. Dilation with Rigiflex balloon appears to be utilized more among younger achalasia patients. Some of the treatment patterns may reflect hesitancy or caution on the physicians’ side given the relatively high complication rate associated with pneumatic dilation. Despite their partial deviation from recommendations according to guidelines and the literature, these endoscopic patterns reflect the current clinical practice in the United States.
Footnotes
Statement of Interests
There are no conflicts of interests to declare. The study was supported with funding from NIDDK UO1 CA 89389-01, NIDDK U01 DK057132 and R33-DK61778-01. In addition, the practice network (CORI) has received support from the following entities to support the infrastructure of the practice based network: Astra-Zeneca, Bard International, Pentax USA, ProVation, Endosoft, GIVEN Imaging, and Ethicon. The commercial entities had no involvement in this research.
References
- 1.Park W, Vaezi MF. Etiology and pathogenesis of achalasia: the current understanding. Am J Gastroenterol. 2005;100:1404–1414. doi: 10.1111/j.1572-0241.2005.41775.x. [DOI] [PubMed] [Google Scholar]
- 2.Mayberry JF. Epidemiology and demographics of achalasia. Gastrointest Endosc Clin N Am. 2001;11:235–48. v. [PubMed] [Google Scholar]
- 3.Farhoomand K, Connor JT, Richter JE, Achkar E, Vaezi MF. Predictors of outcome of pneumatic dilation in achalasia. Clin Gastroenterol Hepatol. 2004;2:389–394. doi: 10.1016/s1542-3565(04)00123-5. [DOI] [PubMed] [Google Scholar]
- 4.Pandolfino JE, Kwiatek MA, Nealis T, Bulsiewicz W, Post J, Kahrilas PJ. Achalasia: a new clinically relevant classification by high-resolution manometry. Gastroenterology. 2008;135:1526–1533. doi: 10.1053/j.gastro.2008.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eckardt VF, Hoischen T, Bernhard G. Life expectancy, complications, and causes of death in patients with achalasia: results of a 33-year follow-up investigation. Eur J Gastroenterol Hepatol. 2008;20:956–960. doi: 10.1097/MEG.0b013e3282fbf5e5. [DOI] [PubMed] [Google Scholar]
- 6.Birgisson S, Richter JE. Achalasia in Iceland, 1952–2002: an epidemiologic study. Dig Dis Sci. 2007;52:1855–1860. doi: 10.1007/s10620-006-9286-y. [DOI] [PubMed] [Google Scholar]
- 7.Farrokhi F, Vaezi MF. Idiopathic (primary) achalasia. Orphanet J Rare Dis. 2007;2:38. doi: 10.1186/1750-1172-2-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farrukh A, DeCaestecker J, Mayberry JF. An epidemiological study of achalasia among the South Asian population of Leicester, 1986–2005. Dysphagia. 2008;23:161–164. doi: 10.1007/s00455-007-9116-1. [DOI] [PubMed] [Google Scholar]
- 9.Podas T, Eaden J, Mayberry M, Mayberry J. Achalasia: a critical review of epidemiological studies. Am J Gastroenterol. 1998;93:2345–2347. doi: 10.1111/j.1572-0241.1998.00686.x. [DOI] [PubMed] [Google Scholar]
- 10. [Accessed on November 18, 2010];Clinical Outcomes Research Initiative. Available at www.cori.org.
- 11.Lieberman D, Fennerty MB, Morris CD, Holub J, Eisen G, Sonnenberg A. Endoscopic evaluation of patients with dyspepsia: results from the national endoscopic data repository. Gastroenterology. 2004;127:1067–1075. doi: 10.1053/j.gastro.2004.07.060. [DOI] [PubMed] [Google Scholar]
- 12.Lieberman DA, Holub J, Eisen G, Kraemer D, Morris CD. Utilization of colonoscopy in the United States: results from a national consortium. Gastrointest Endosc. 2005;62:875–883. doi: 10.1016/j.gie.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 13.Lieberman DA, Holub J, Eisen G, Kraemer D, Morris CD. Prevalence of polyps greater than 9 mm in a consortium of diverse clinical practice settings in the United States. Clin Gastroenterol Hepatol. 2005;3:798–805. doi: 10.1016/s1542-3565(05)00405-2. [DOI] [PubMed] [Google Scholar]
- 14.Enestvedt BK, Gralnek IM, Mattek N, Lieberman DA, Eisen GM. Endoscopic therapy for peptic ulcer hemorrhage: practice variations in a multi-center U.S. consortium. Dig Dis Sci. 2010;55:2568–2576. doi: 10.1007/s10620-010-1311-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Enestvedt BK, Gralnek IM, Mattek N, Lieberman DA, Eisen G. An evaluation of endoscopic indications and findings related to nonvariceal upper-GI hemorrhage in a large multicenter consortium. Gastrointest Endosc. 2008;67:422–429. doi: 10.1016/j.gie.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 16.Olson JS, Lieberman DA, Sonnenberg A. Empiric dilation in non-obstructive dysphagia. Dig Dis Sci. 2008;53:1192–1197. doi: 10.1007/s10620-007-0024-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thukkani N, Williams JL, Sonnenberg A. Epidemiologic characteristics of patients with inflammatory bowel disease undergoing colonoscopy. Inflamm Bowel Dis. 2010 doi: 10.1002/ibd.21513. [DOI] [PubMed] [Google Scholar]
- 18.Urquhart J, Eisen G, Faigel DO, Mattek N, Holub J, Lieberman DA. A closer look at same-day bidirectional endoscopy. Gastrointest Endosc. 2009;69:271–277. doi: 10.1016/j.gie.2008.04.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lieberman DA, Holub JL, Moravec MD, Eisen GM, Peters D, Morris CD. Prevalence of colon polyps detected by colonoscopy screening in asymptomatic black and white patients. JAMA. 2008;300:1417–1422. doi: 10.1001/jama.300.12.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mayberry JF, Atkinson M. Variations in the prevalence of achalasia in Great Britain and Ireland: an epidemiological study based on hospital admissions. Q J Med. 1987;62:67–74. [PubMed] [Google Scholar]
- 21.Sonnenberg A. Hospitalization for achalasia in the United States 1997–2006. Dig Dis Sci. 2009;54:1680–1685. doi: 10.1007/s10620-009-0863-8. [DOI] [PubMed] [Google Scholar]
- 22.Stein DT, Knauer CM. Achalasia in monozygotic twins. Dig Dis Sci. 1982;27:636–640. doi: 10.1007/BF01297220. [DOI] [PubMed] [Google Scholar]
- 23.Sadowski DC, Ackah F, Jiang B, Svenson LW. Achalasia: incidence, prevalence and survival. A population-based study. Neurogastroenterol Motil. 2010;22:e256–61. doi: 10.1111/j.1365-2982.2010.01511.x. [DOI] [PubMed] [Google Scholar]
- 24.Sonnenberg A, Massey BT, McCarty DJ, Jacobsen SJ. Epidemiology of hospitalization for achalasia in the United States. Dig Dis Sci. 1993;38:233–244. doi: 10.1007/BF01307540. [DOI] [PubMed] [Google Scholar]
- 25.Francis DL, Katzka DA. Achalasia: update on the disease and its treatment. Gastroenterology. 2010;139:369–374. doi: 10.1053/j.gastro.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 26.Marlais M, Fishman JR, Fell JM, Haddad MJ, Rawat DJ. UK incidence of achalasia: an 11-year national epidemiological study. Arch Dis Child. 2010 doi: 10.1136/adc.2009.171975. in press. [DOI] [PubMed] [Google Scholar]
- 27.Gennaro N, Portale G, Gallo C, et al. Esophageal Achalasia in the Veneto Region: Epidemiology and Treatment : Epidemiology and Treatment of Achalasia. J Gastrointest Surg. 2010 doi: 10.1007/s11605-010-1392-7. in press. [DOI] [PubMed] [Google Scholar]
- 28.Pasricha PJ, Rai R, Ravich WJ, Hendrix TR, Kalloo AN. Botulinum toxin for achalasia: long-term outcome and predictors of response. Gastroenterology. 1996;110:1410–1415. doi: 10.1053/gast.1996.v110.pm8613045. [DOI] [PubMed] [Google Scholar]
- 29.Pasricha PJ, Ravich WJ, Hendrix TR, Sostre S, Jones B, Kalloo AN. Intrasphincteric botulinum toxin for the treatment of achalasia. N Engl J Med. 1995;332:774–778. doi: 10.1056/NEJM199503233321203. [DOI] [PubMed] [Google Scholar]
- 30.Leyden JE, Moss AC, MacMathuna P. Endoscopic pneumatic dilation versus botulinum toxin injection in the management of primary achalasia. Cochrane Database Syst Rev. 2006;(4):CD005046. doi: 10.1002/14651858.CD005046.pub2. [DOI] [PubMed] [Google Scholar]
- 31.Campos GM, Vittinghoff E, Rabl C, et al. Endoscopic and surgical treatments for achalasia: a systematic review and meta-analysis. Ann Surg. 2009;249:45–57. doi: 10.1097/SLA.0b013e31818e43ab. [DOI] [PubMed] [Google Scholar]
- 32.Sonnenberg A, Amorosi SL, Lacey MJ, Lieberman DA. Patterns of endoscopy in the United States: analysis of data from the Centers for Medicare and Medicaid Services and the National Endoscopic Database. Gastrointest Endosc. 2008;67:489–496. doi: 10.1016/j.gie.2007.08.041. [DOI] [PubMed] [Google Scholar]


