Abstract
Metabolic theory and body size dependent constraints on biomass production and decomposition suggest that differences in the intrinsic potential net ecosystem production (NEPPOT) should be small among contrasting C3 grasslands and therefore unable to explain the wide range in the annual apparent net ecosystem production (NEPAPP) reported by previous studies. We estimated NEPPOT for nine C3 grasslands under contrasting climate and management regimes using multi-year eddy covariance data. NEPPOT converged within a narrow range suggesting little difference in the net carbon dioxide uptake capacity across C3 grasslands. Our results indicate a unique feature of C3 grasslands compared to other terrestrial ecosystems and suggest a state of stability in NEPPOT due to tightly coupled production and respiration processes. Consequently, the annual NEPAPP of C3 grasslands is primarily a function of seasonal and short-term environmental and management constraints, and therefore especially susceptible to changes in future climate patterns and associated adaptation of management practices.
Keywords: climate, ecosystem traits, eddy covariance, inter-comparison, grassland ecosystem functioning, management practices, metabolic theory
INTRODUCTION
Grasslands cover approximately 40% of the Earth’s surface and are located across contrasting climatic and management gradients, which results in considerable variation in ecosystem structure, environmental conditions and disturbance regimes (White et al. 2000; Gilmanov et al. 2010). A decade of eddy covariance measurements made over C3 grasslands across the globe has revealed considerable differences in their annual apparent net ecosystem production (NEPAPP), ranging approximately from −300 to 500 g C m2 y−1 APP (following the ecological sign convention, the positive sign indicates net uptake of carbon dioxide (CO2)) (Novick et al. 2004; Ma et al. 2007; Wohlfahrt et al. 2008a, 2008b; Gilmanov et al. 2010). A number of studies have shown that environmental conditions and management practices commonly account for a large proportion of the variability in the annual apparent NEP (NEPAPP) (Wohlfahrt et al. 2008a, 2008b; Gilmanov et al. 2010; Schmitt et al. 2010; Zeeman et al. 2010). However, little is known about the extent to which contrasting C3 grassland ecosystems exhibit different potentials for NEP (NEPPOT; i.e., the maximum net CO2 uptake reached during optimum conditions) due to differences in ecosystems traits, environmental conditions and management practices. However, such understanding is necessary since variations in NEPPOT might regulate the maximum ‘baseline’ capacity for the annual net CO2 uptake, and thus, provide some additional explanatory power (supplementary to the seasonal and short-term effects from environmental and management constraints) for the observed range in annual NEPAPP among global C3 grasslands.
Although NEP is a flux that can be measured by the eddy covariance technique, it is important to realize that NEP itself is not a process per se but rather the net result of the imbalance between the two ecosystem processes of gross ecosystem production (GEP; i.e. the micrometeorological term equivalent to gross primary production, GPP, assuming that reabsorption of respired CO2 within the canopy is negligible) and ecosystem respiration (ER) (i.e., NEP = GEP - ER) (Chapin III et al. 2006). Furthermore, ER is composed of both autotrophic (Ra) and heterotrophic (Rh) respiration. After accounting for the carbon loss via Ra, which is commonly considered to be a conservative fraction of GEP (Gifford 1994), NEP is essentially the difference between the net primary production (NPP; NPP = GEP - Ra) and Rh (i.e., NEP = NPP – Rh). Remarkably, recent studies have indicated that Rh is strongly controlled by the abundance and properties of the dominant plant functional type resident in an ecosystem (Chapin III 2003; Bardgett 2011). Specifically, slow-growing vegetation is known to produce low-quality litter which decomposes more slowly compared with fast-growing vegetation types. The more labile and easily decomposable litter produced by fast-growing vegetation may also stimulate soil microbial activity through the enhanced release of labile carbon forms contained in root exudates (Hobbie 1992; De Deyn et al. 2008). In return, changes in the mineralization rate affect nutrient availability and uptake and thus plant productivity, which suggests a strong correlation and feedback between NPP and Rh (Hobbie 1992; De Deyn et al. 2008; Bardgett 2011). Moreover, metabolic theory suggests that body size constraints on metabolic rates of biomass production and decomposition may further condition the relationship between NPP and Rh (Brown et al. 2004; Allen et al. 2005). This is because any change in body size and/or NPP rates would be counterbalanced by a subsequent adjustment of decomposer communities and/or rates of Rh and vice versa driven by a supply (i.e., amount and quality) - demand mechanism. Given a 10 000-fold increase in carbon turnover rates moving from tree- to phytoplankton-dominated ecosystems (Allen et al. 2005), such adjustment is likely to occur on relatively short time scales in grassland ecosystems, which are characterised by a short canopy (i.e. relatively small body size). Consequently, one might expect that ecosystems dominated by a given plant functional type might converge to a very similar NEPPOT under optimum conditions (i.e., conditions during which the imbalance between NPP and Rh is maximized by stimulation of NPP and/or suppression of Rh). This is also in line with the metabolic theory that predicts that after adjusting metabolic rates for body size and temperature, organisms should have similar rates of production (Brown et al. 2004). In contrast to similar NEPPOT under optimum conditions, NEPAPP as measured by the eddy covariance method in observational studies, should vary for a given ecosystem type in contrasting regions of the world due to the different region- and site-specific constraints on NPP and Rh.
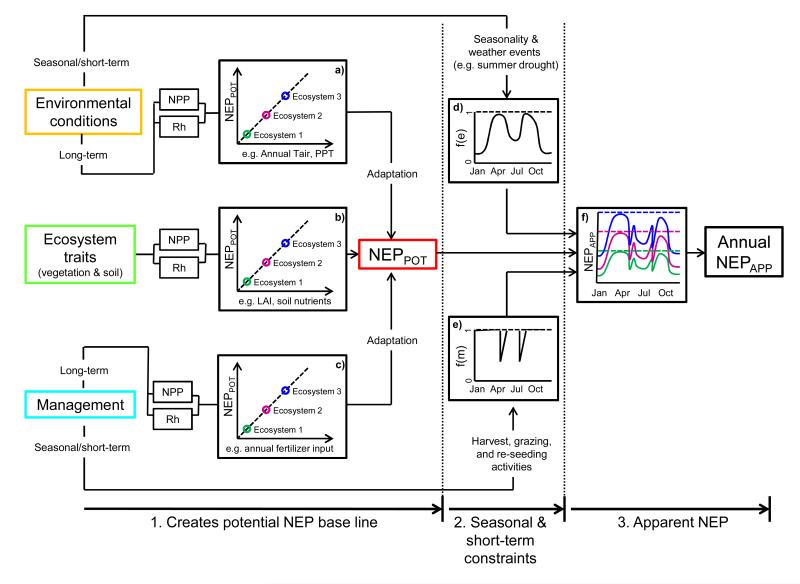
The three main controls on NPP and Rh that subsequently determine NEPPOT include the stationary long-term effects from i) environmental conditions (e.g., adaptation to annual mean air temperature, radiation, total precipitation, etc.), ii) management practices (e.g., adaptation to fertilizer input, grazing, cutting) and iii) ecosystem traits (Figure 1). In this context, ecosystem traits are understood to encompass any plant- or soil-related ecosystem property such as species composition, maximum leaf area index (LAI), maximum leaf photosynthetic capacity, litter quality and soil nutrients. Each of these three controls may affect ecosystem production and respiration processes through modifications of the carbon assimilation, allocation and turnover mechanisms and thus may alter the intrinsic potential for net CO2 uptake.
Figure 1. Conceptual diagram outlining the interactions between ecosystems traits, environmental conditions, management, net primary production (NPP), heterotrophic respiration (Rh), potential net ecosystem production (NEPPOT) and apparent NEP (NEPAPP).
It describes NEPAPP as the product of NEPPOT (= NPP – Rh) and functions of seasonal and short-term environmental f(e) and management f(m) constraints. Dotted lines in f) indicate NEPAPP = NEPPOT.
Given such a baseline for potential net CO2 uptake, NEPAPP then deviates from NEPPOT as a function (f) of seasonal and short-term constraints from environmental conditions (e) and management (m) events, each bound between zero and unity, which reduce the imbalance between NPP and Rh and thus determine NEPAPP on a daily (superscript d) and ultimately annually (superscript a) basis (Eq.1, Figure 1):
| (Eq.1) |
Constraints from environmental conditions, i.e., f(e) in Eq. (1), are understood to comprise effects resulting from i) seasonal variation in environmental conditions which determine the length and timing of the growing season period typical for a given climate region (e.g., the occurrence of summer drought in Mediterranean climates or the existence of a winter snow cover at high latitudes/altitudes) as well as from ii) short-term weather and inter-annual deviations from the long-term mean seasonal climate (e.g., a summer drought in a temperate climate).
Previous studies have quantified and compared maxima of daily or annual GEPAPP and NEPAPP for individual years (Falge et al. 2002a, 2002b; Gilmanov et al. 2010). However, daily maxima may be subject to spurious artefacts and data spikes due to unusual events (e.g., short-term effects from weather, disturbance, management, atmospheric phenomena, etc.) and the inherent random variability of eddy covariance flux measurements (Moncrieff et al. 1996; Massman & Lee 2002; Jolly et al. 2005; Stoy et al. 2009). Meanwhile annual estimates are likely constrained by environmental conditions and/or management (Wohlfahrt et al. 2008a; Stoy et al. 2009). Thus, neither daily nor annual maxima might serve as a suitable indicator for NEPPOT. Here, we derive a robust estimate of NEPPOT over a time frame of 30 days, which approximately spans the duration of the peak net production period as evident from the slope of the cumulative NEPAPP. The main underlying assumption is that NEPPOT is not necessarily reached in every year and therefore the true NEPPOT is best approximated from the maximum 30-day NEPAPP observed over multiple years. To our knowledge, NEPPOT by such definition has not yet been explicitly investigated for grasslands or any other terrestrial ecosystem type. In this paper we use the outlined approach to explore multi-year eddy covariance data for the existence of a convergence of NEPPOT for nine different C3 grassland ecosystems from three contrasting climate regions to test the hypothesis that differences in NEPPOT are small among contrasting C3 grasslands.
METHODS
Site selection
The primary criteria for site selection were (i) the availability of four or more complete years of data (with two exceptions, see below) and (ii) their location in one of three generalized and contrasting climate regions:
cold-temperate: region with continuous winter snow cover and/or freezing temperatures,
Mediterranean: region with dry, hot summers and cool, wet winters,
maritime: region with ample water availability and without snow cover and/or freezing temperatures.
The cold-temperate sites included three alpine sites at different elevations as well as one continental prairie site. We also added two sites with less than four years of continuous data (i.e., the cold-temperate alpine site Torgnon and the maritime site Carlow) to determine whether NEPPOT could also be confidently estimated for smaller data sets. Another reason to include the Torgnon site was to obtain an elevation gradient among the cold-temperate alpine sites. The final selected grasslands were the cold-temperate alpine sites Neustift (N), Monte Bondone (MB), Torgnon (TN) and the cold-temperate prairie site Lethbridge (L), the Mediterranean sites Vaira (V) and Tojal (T), and the maritime sites Dripsey (D), Wexford (W) and Carlow (CW). In addition, the site selection contained a gradient of management intensity encompassing natural (abandoned or unmanaged) (L, TN), extensively (V, T, MB) and intensively (N, D, W, CW) managed grasslands. The site-specific details and references are summarized in Table 1.
Table 1. Site characteristics of the nine investigated grassland sites (Neustift, N; Monte Bondone, MB; Torgnon, TN; Lethbridge, L; Vaira, V; Tojal, T; Dripsey, D; Wexford, W; Carlow, CW).
| Site | N | MB | TN | L | V | T | D | W | CW |
|---|---|---|---|---|---|---|---|---|---|
| Country | Austria | Italy | Italy | Canada (AB) | USA (CA) | Portugal | Ireland | Ireland | Ireland |
| Coordinates | 47°07′ N 11°19′ E |
46° 01′ N 11° 2′ E |
45° 50′ N 7° 34′ E |
49° 43′ N 112° 56′ W |
38 °41′ N 120° 95′ W |
38°28′ N 8°01′ W |
51°59′ N 8°45′ W |
52°30′ N 6°40′ W |
52°52′ N 6°54′ W |
| Elevation (m a.s.l.) |
970 | 1550 | 2160 | 951 | 129 | 190 | 195 | 57 | 56 |
| Climate region | Cold-Temperate | Cold-Temperate | Cold-Temperate | Cold-Temperate | Mediterranean | Mediterranean | Maritime | Maritime | Maritime |
| Mean Ta (°C) | 6.5 | 5.5 | 3.1 | 5.4 | 16.5 | 15.5 | 9.4 | 10.1 | 9.4 |
| Mean PPT (mm) | 852 | 1189 | 920 | 402 | 562 | 669 | 1207 | 877 | 824 |
| Snow cover and/or T < 0°C |
Nov - Apr | Nov - Apr | Nov - May | Oct - Apr | none | none | none | none | none |
| Management | intensive meadow |
extensive meadow |
abandoned pasture |
unmanaged prairie |
extensive pasture |
extensive pasture |
intensive meadow/ pasture |
intensive meadow/ pasture |
intensive meadow/ pasture |
| Nitrogen fertilizer application (kg N ha−1 y−1) |
manure | low | none | none | none | none | ~150-250 (inorganic, manure, slurry) |
~200-300 (inorganic, manure, slurry) |
~200 (inorganic, manure, slurry) |
| Soil type | Fluvisol | Typic Hapludalfs |
Cambisol | Orthic dark- brown chernozem |
Lithic haploxerepts |
Luvisol | Gleysol | (Gleyic) Cambisol |
Calcic Luvisol |
| Soil texture | (sandy) loam | loam | loamy sand | clay loam | silt loam | sandy (clay) loam | loam | loam | sandy loam |
| Soil C (kg C m−2) |
8.1 (0-30cm) | 8.7 (0-20cm) | 2.8 (0-20cm) | 3.7 (0-10cm) | 6.0 (0-30cm) | 3.3 (0-30cm) | 9.0 (0-30cm) | 3.9 (0-10cm) | 4.2 (0-10cm) |
| Soil N (kg N m−2) |
n.a. | 0.76 (0-20cm) | 0.22 (0-20cm) | n.a. | 0.60 (0-30cm) | n.a. | 0.76 (0-30cm) | 0.34 (0-10cm) | 0.42 (0-10cm) |
| Max. LAI (m2 m−2) |
5.5 | 4.7 | 2.8 | 1.2 | 2.7 | 2.3 | 2.5 | na | 5.1 |
| Dominant species |
Dactylis
glomerata,
Festuca pratensis, Phleum pratensis, Ranunculus acris, Trifolium spp. |
Festuca rubra,
Nardus stricta, Trifolium alpinum |
Nardus stricta,
Arnica montana, Trifolium alpinum, Carex sempervirens |
Agropyron
dasystachyum, Agropyron smithii, Vicia americana, Artemesia frigid, Koeleria cristata, Carex filifolia, Stipa comata, Stipa viridula |
Brachypodium
distachyon, Hypochaeris glabra, Trifolium spp., Dichelostema volubile, Erodium botrys |
Avena barbata,
Lusitanica spp., Vulpia spp., Medicago spp, Trifolium spp., Cynodon dactylon |
Lolium
perenne, Alopecurus pratensis, Holcus lanatus |
Lolium
perenne |
Lolium
perenne, Trifolium. repens |
| Data coverage | 2001 - 2009 | 2003 - 2009 | 2009 - 2010 | 1999 - 2006 | 2001- 2007 | 2005 - 2008 | 2003 - 2006, 2008, 2009 |
2004 - 2006, 2008, 2009 |
2003, 2008 |
| References | Wohlfahrt et al. (2008b) | Marcolla et al. (2011) | Migliavacca et al. (2011a) | Flanagan and Adkinson (2011) | Ma et al. (2007) | Aires et al. (2008) | Peichl et al. (2011) | Peichl et al. (2012) | Flechard et al. (2007) |
Data
We used gap-filled flux and meteorological (precipitation, soil temperature, photosynthetically-active photon flux density, and volumetric soil water content) data supplied by either the principal investigators (PI) at the sites D, W, N, MB, TN, L or retrieved as Level 4 data (quality checked and gap-filled) data from the CarboEurope (T, CW) and Ameriflux (V) databases, both regional networks within FLUXNET (http://fluxnet.ornl.gov/). The procedures for gap-filling and partitioning into the component fluxes GEP and ER followed the methods described by Reichstein et al. (2005) (all but L, D, W) and Barr et al. (2004) (L, D, W). Previous studies have shown consistent estimates of NEP, GEP and ER obtained by the different methods (Moffat et al. 2007; Lasslop et al. 2010). Positive NEP values indicate net CO2 uptake (i.e., GEP > ER). Although the eddy covariance technique provides estimates of the biosphere-atmosphere net ecosystem exchange of CO2 (NEE), we instead use the term NEP (with opposite sign convention to NEE) with the purpose of maintaining an ecological perspective. It is however important to note that due to the underlying measurement methodology, the term NEP as used here does not include (commonly small) lateral losses of respiration-derived dissolved carbon (Chapin III et al. 2006). Additional supporting environmental data including information on LAI and soil properties were either obtained from the site PIs or retrieved from the respective FLUXNET databases.
Potential NEP, GEP and ER
To obtain an estimate of NEPPOT we first determined the mean NEPAPP over 30 consecutive days (i.e., the mean of 30 daily totals of NEPAPP) at each site using a moving window (with 1-day increments starting from January 1) over all available site-years. In a second step, the maximum of all 30-day averages observed across all years at each site was defined as the site-specific NEPPOT. Thus, we assume that the maximum apparent rate occurring under optimum conditions (i.e., conditions resulting in highest productivity with concurrent lowest respiration) within a multi-year time series should approach or ideally equal the potential rate. Clearly, the probability of finding the true NEPPOT increases with the number of years available at a given site. It is therefore possible that NEPPOT was underestimated at the CW and TN sites where only two years of data were available. Furthermore, optimum conditions may have never occurred even within a multi-year data set at a given site. In this case however, the continued constraint on ecosystem functioning is a site characteristic and the period with the least severe constraints was, therefore, considered ‘optimum’ instead. Furthermore, we estimated 30-day means of apparent GEP and ER (GEPAPP and ERAPP) with the same approach used to determine 30-day mean NEPAPP, and denoted GEPAPP and ERAPP at the time of NEPPOT as GEPPOT and ERPOT, respectively.
The averaging period (i.e., window size) of 30 days to determine the apparent mean and potential NEP, GEP and ER was chosen because: i) the cumulative NEP curves for the grasslands commonly showed maximum slopes lasting for about one month during the peak growth period in spring/summer, and ii) this reduced the impact of spurious artefacts and data spikes occurring on shorter time scales (daily to weekly) due to unusual weather (e.g., rain pulses) and management (e.g., soil disturbance from the use of heavy machinery, fertilizer application) events or short-term violation of any of the underlying assumptions in the eddy covariance theory (e.g., occurrence of advection processes, gravity waves, etc.) (Massman & Lee 2002; Jolly et al. 2005; Stoy et al. 2009). Meanwhile, a window size >30 days was not considered in order to avoid interference from periodically re-occurring management practices at longer time scales. Repeating our analysis using 10 and 20-day averaging periods altered the absolute values of NEPPOT which increased with decreasing length of the averaging period, but did not change our overall findings (see Appendix S in Supporting Information, Figure S1). It is noteworthy that given the relationship between absolute potential values and the length of the averaging period, the focus in this approach is on the relative comparison of potential rates for a given averaging period whereas the absolute values are of secondary importance.
Statistical analysis
Significant differences in the potential rates across sites were tested by assessing their respective underlying non-normally distributed (Shapiro-Wilk test, p < 0.05) data points (n = 30 values of daily totals) using the non-parametric Kruskal-Wallis One-Way Analysis-of-Variance (ANOVA) on ranks followed by a Bonferroni type multiple comparison. Using the comparably less and more conservative Tukey-Kramer and Scheffé multiple comparison tests, respectively, did not alter the results. With the same approach, the mean environmental conditions during the period in which NEPPOT occurred were also determined and compared. According to the partial auto-correlation function (PACF) plots, daily NEP replicate samples were independent over the investigated 30-day period at all sites except for one (W). Thus, in addition, we conducted a sign test (which makes very few assumptions about the nature of the underlying distribution) for differences in the medians using a piecewise linear non-parametric empirical cumulative distribution function (ECDF) (Samuels & Witmer 2002). In this approach, NEP POT values originate from the distribution of the annual maximum 30-day mean NEPAPP over all site years. As an additional advantage, this approach also adjusts for the unequal numbers of data-years among sites and its effect on the probability of NEPPOT occurring (i.e., being reached) at a given site within the site-specific available number of data-years.
RESULTS
Environmental conditions
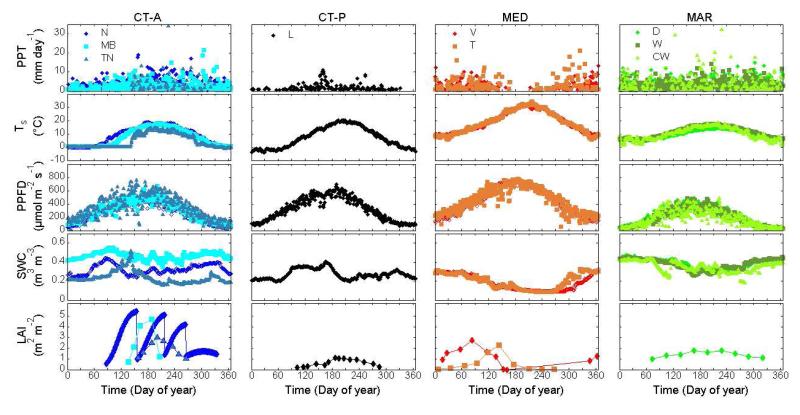
Multi-year averages (n = 2 to 9) of daily environmental variables (as described by f(e) in Eq. 1) showed distinct and characteristic patterns for the three climate regions (Figure 2). Daily precipitation was highest and evenly distributed throughout the year at the maritime sites, and lowest at the cold-temperate-prairie site. In contrast to the summer peak in precipitation at the other sites, the two Mediterranean sites experienced a prolonged period with reduced precipitation during the summer. The peak and amplitude of daily soil temperature were greatest at the Mediterranean sites and smallest at the maritime sites. Soil temperature remained above freezing temperatures at all times at both the Mediterranean and the maritime sites. Among the cold-temperate-alpine sites, a clear elevation gradient was noted for the onset of soil thawing and summer soil temperature maxima. The multi-year average of maximum daily photosynthetically-active photon flux density was lowest at the maritime sites and highest at the Mediterranean sites. Sufficient soil water availability all year round at the maritime sites was in contrast to summer water deficits occurring at the Mediterranean sites. Overall, the maritime sites experienced lower environmental constraints on net CO2 uptake compared to the cold-temperate (extended snow cover) and Mediterranean (pronounced summer water stress) regions.
Figure 2. Multi-year averages of daily precipitation (PPT), soil temperature (Ts), photosynthetically active photon flux density (PPFD), volumetric soil water content (SWC) and leaf area index (LAI; for selected years and sites according to data availability) at the nine grassland sites Neustift (N), Monte Bondone (MB), Torgnon (TN), Lethbridge (L), Vaira (V), Tojal (T), Dripsey (D), Wexford (W) and Carlow (CW) in the three climate regions: cold-temperate (CT; alpine, A, and prairie, P), Mediterranean (MED) and maritime (MAR).
Maximum LAI estimates ranged from 1.2 to 5.5 m2 m−2 (Table 1). Furthermore, the seasonal patterns of LAI development varied among the different grasslands, with maximum LAI occurring in spring at the Mediterranean sites, whereas LAI at the other sites peaked in summer and was occasionally characterized by periodic reduction caused by management practices (i.e., cutting/grazing) (Figure 2).
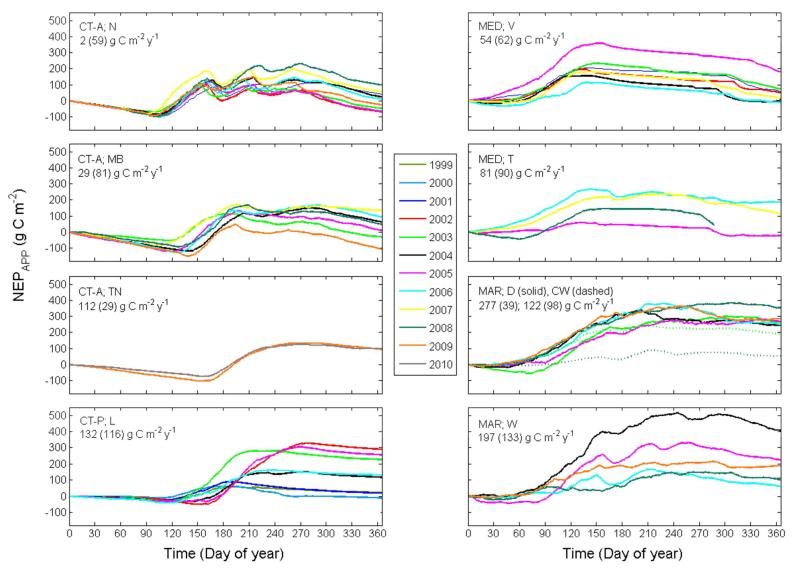
Cumulative apparent NEP
Cumulative NEPAPP showed characteristic temporal patterns for each climate region (e.g., steady net CO2 loss during the dry summer and winter periods at the Mediterranean and cold-temperate sites, respectively) and management regimes (e.g., temporary net CO2 loss following grazing and harvest events) (Figure 3). Annual sums of NEPAPP ranged widely from a net source of −108 g C m−2 y−1 to a net sink of 404 g C m−2 y−1 across all sites and years. Averaged for each climate region, mean (± standard deviation, SD) annual NEPAPP of 222 ± 104 g C m−2 y−1 was higher in the maritime region than in the Mediterranean (64 ± 70 g C m−2 y−1) and cold-temperate regions (60 ± 98 g C m−2 y−1).
Figure 3. Cumulative annual apparent net ecosystem production (NEPAPP) for available site-years at the nine grassland sites Neustift (N), Monte Bondone (MB), Torgnon (TN), Lethbridge (L), Vaira (V), Tojal (T), Dripsey (D), Wexford (W) and Carlow (CW) in the cold temperate (CT; alpine, A, and prairie, P), Mediterranean (MED) and maritime (MAR) climate regions.
Values in upper left corner represent site-specific multi-year average NEP (values in parentheses indicate standard deviation)
Potential NEP, GEP, and ER
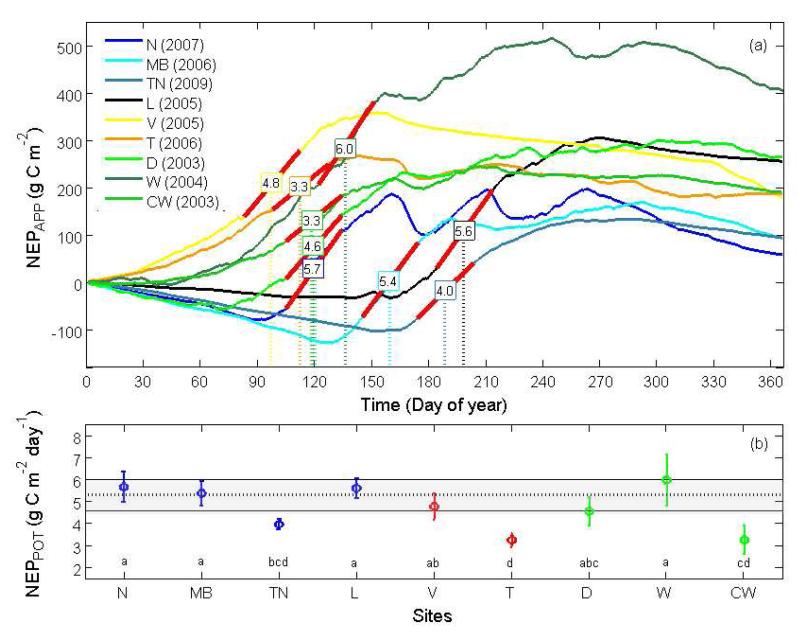
Averaged over 30 days, values for NEPPOT were within a narrow range of 4.6 to 6.0 g C m−2 d−1 and not significantly different (p > 0.05 based on results from both the various multiple comparison tests and the sign test) for six out of nine sites (Figure 4). The remaining three sites (TN, T, and CW) were those at which NEPPOT was estimated from four or fewer site years, with the lowest rate of 3.3 g C m−2 d−1 occurring at T and CW. The mean (± SD) NEPPOT across the six converging sites was 5.3 ± 0.5 g C m−2 d−1.
Figure 4. (a) Cumulative apparent net ecosystem production (NEPAPP) for years when potential [=maximum 30-day mean NEPAPP] NEP (NEPPOT) occurred at the grasslands sites Neustift (N), Monte Bondone (MB), Torgnon (TN), Lethbridge (L), Vaira (V), Tojal (T), Dripsey (D), Wexford (W) and Carlow (CW). Red slopes visualize NEPPOT sustained over 30 days; numbers in rectangles show associated slope values; dotted vertical lines visualize the timing of NEPPOT (b) NEPPOT for grasslands in the cold-temperate (blue), Mediterranean (red), and maritime (green) regions; grey band indicates the convergence zone for NEPPOT (dotted horizontal line shows mean NEPPOT); same letters indicate no significant difference; error bars indicate ±2 standard error of site-specific NEPPOT (n = 30).
The timing (defined as the day-of-year, DOY, representing the center point of the NEPPOT period) at which NEPPOT occurred spanned from DOY 97 at the Mediterranean site V to DOY 198 at the cold-temperate prairie site L and correlated with the length of the snow cover (and/or freezing temperatures) period (Figure 4a, see also Table 1 and Figure 2).
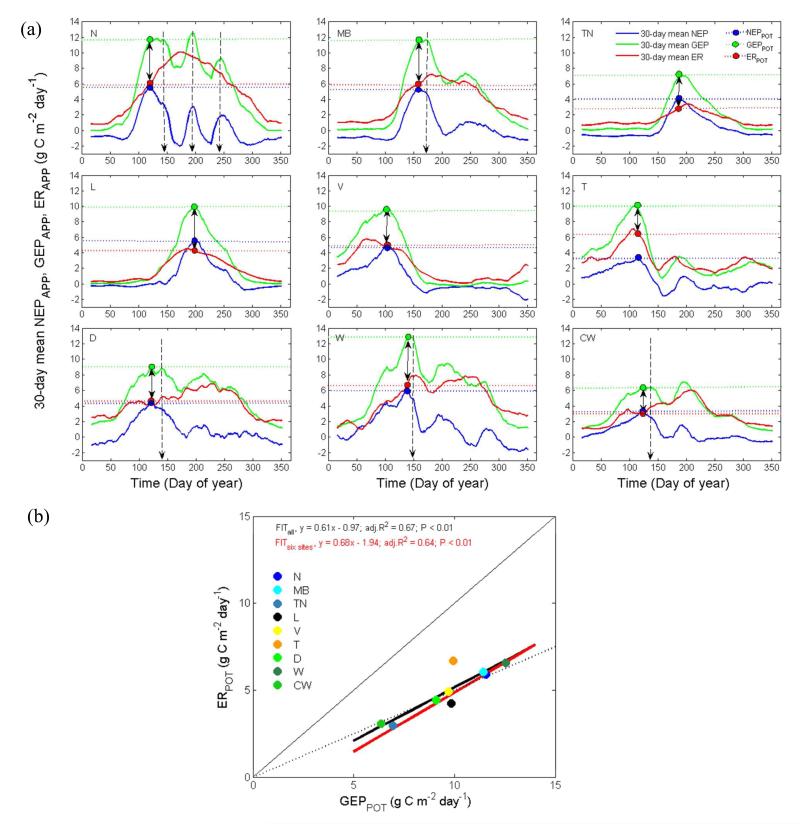
NEPPOT occurred at the time when the 30-day mean GEPAPP was equal (i.e., at MB, TN, L, V, T and D) or close to (i.e., at N, W and CW) its maximum (Figure 5a). NEPPOT occurred close to or lagged (by 17 ± 13 days) the maximum of the 30-day mean ERAPP at the unmanaged sites and extensive pastures (i.e., TN, V, T and L). In contrast, at the meadows and intensively managed pastures (i.e., N, MB, D, W and CW) the timing of NEPPOT preceded the peak of 30-day mean ERAPP by 57 ± 39 days. GEPPOT and ERPOT ranged from 6.4 to 12.6 g C m−2 d−1 and from 3.1 to 6.7 g C m−2 d−1, respectively (Appendix S, Table S1), and showed a strong linear relationship among sites (Figure 5b).
Figure 5. (a) 30-day mean apparent net ecosystem production (NEPAPP), gross ecosystem production (GEPAPP) and ecosystem respiration (ERAPP) at the nine sites (denoted as in Figures 2-4) during the year in which potential NEP (NEPPOT) occurred. Blue, green and red dots and horizontal dotted lines indicate timing and magnitude of NEPPOT and of GEPAPP and ERAPP at the time of NEPPOT (GEPPOT and ERPOT, respectively). Double arrows visualize the difference between GEPPOT and ERPOT. Vertical dotted arrows indicate harvest events. (b) regression between GEPPOT and ERPOT; thick lines indicate linear fits for all sites (black line) and the six conserving sites (red line); thin solid and dashed lines show 1:1 and 2:1 fits, respectively; GEP and ER are shown in absolute values.
No convergence or any consistent pattern was observed among the various environmental parameters within the period in which NEPPOT occurred (Appendix S, Figure S2). At the maritime sites, NEPPOT occurred during periods that were cooler and wetter and received less radiation compared to the other regions.
DISCUSSION
Convergence of potential NEP
While previous synthesis studies of grassland and other ecosystem types commonly compared apparent rates in CO2 exchange (e.g., Falge et al. 2002a, 2002b; Lindroth et al. 2008; Gilmanov et al. 2010; Lund et al. 2010; Moors et al. 2010), we are unaware of any study investigating the potential NEP based on multi-year eddy covariance datasets. Such an analysis has been hampered by the limited number of sites that could provide five or more years of continuous data, which, as suggested by our study, are necessary to estimate NEPPOT. The convergence of NEPPOT observed among the six sites with ≥5 site-years of data available suggests that contrasting C3 grasslands generally hold a similar potential for net CO2 uptake despite vast differences in ecosystem traits, environmental conditions and management practices.
The considerable differences in magnitude and patterns of the environmental variables among the investigated sites indicate that the convergence of NEPPOT was not related to a convergence of the underlying environmental conditions among climate regions (Figure 2, Figure S2). The apparent lack of universal optimum environmental conditions for NEPPOT suggests that the maximum imbalance (i.e., NEPPOT) between production and decomposition rates in C3 grasslands is determined by site-specific optimum combinations of site, climate and management characteristics.
Furthermore, it is noteworthy that NEPPOT was identified to occur at different times among the sites spanning from spring at the Mediterranean sites to late summer at the cold-temperate sites. The different timing of NEPPOT and its temporal relationships with maximum GEPAPP and ERAPP reflect the impact of the site-specific seasonal and short-term constraints from environmental conditions and management events on production and decomposition processes. Specifically, the lag of maximum ERAPP compared to NEPPOT (and GEPPOT) in the managed systems was likely a disturbance effect from the harvest events, demonstrating a functional difference to the extensive systems where maximum ERAPP and GEPAPP more or less coincided with NEPPOT. The wide range and climate region-specific timing of the optimum CO2 uptake among the three climate regions entail further implications regarding the impact of future changes in climate and management on the carbon sink strength of C3 grasslands because non-optimum conditions (i.e., droughts, heat waves, cold spells, grazing and harvest events) during these peak productivity periods may considerably reduce the annual NEPAPP (see also Craine et al. 2012).
One possible explanation for the convergence in NEPPOT might be the fast and tightly coupled metabolic rates of the carbon cycle in C3 grassland ecosystems due to the relatively small body size of grasses (Brown et al. 2004; Allen et al. 2005). A close connection among grassland GEP, Ra and Rh through the amount of biomass and litter production has been previously highlighted in other studies (Bahn et al. 2008; Gilmanov et al. 2010; Schmitt et al. 2010; Migliavacca et al. 2011b; Peichl et al. 2011). Moreover, in our study we observed that those sites with higher GEP also showed higher ER rates (with regards to both apparent maxima and at the time when NEPPOT was reached), which demonstrates the tight coupling of these two processes. However, Ra is commonly a conservative fraction of GEP (Gifford 1994) and therefore unlikely to be the process responsible for major differences in the carbon budget among sites. In contrast, Rh is affected by various factors including soil microbial abundance and activity, the supply of decomposable material as well as soil environmental conditions (Chapin 2003; Davidson et al. 2006), and is therefore more likely to vary independently of GEP among different ecosystems. This supports the argument that any differences in NEPPOT would essentially result from a change in the imbalance between NPP and Rh. However, given the strong correlation between NPP and Rh highlighted in previous studies (Hobbie 1992; De Deyn et al. 2008; Bardgett 2011), NEPPOT may remain insensitive to a concurrent increase (or decrease) in NPP and Rh, which, consequently, may explain the convergence of NEPPOT among different sites as a result of a self-regulating supply (i.e. amount and quality) - demand mechanism. Nevertheless, given the current lack of suitable data (i.e., high-resolution time series of NPP and Rh) to further examine underlying mechanisms, additional evidence is required to corroborate this hypothesis in future work.
Another possible explanation that might support the convergence of NEPPOT in C3 grasslands is that, in comparison to other terrestrial ecosystem types, the differences in ecosystem traits are relatively small among contrasting C3 grasslands. The range in vegetation (i.e., living plants) properties such as canopy height, LAI, rooting depth and biomass pool is limited and additional non-vegetation controls (such as woody debris pools affecting Rh in forests or water table position and micro-topography controlling production and decomposition in peatlands) are basically absent. In addition, grasses die back annually because of their life form and provide easily degradable organic matter that, in combination with shorter canopy and rooting systems, allow for fast belowground transport and re-mineralisation of assimilated carbon. Moreover, grasslands are characterized by relatively small differences in body size and associated constraints on production and decomposition (Allen et al. 2005). In contrast, forested ecosystems exhibit a greater span in vegetation properties in relation to stand age and produce tall woody plants with prolonged life times. Furthermore, forested ecosystems build up large amounts of biomass and dead organic material over time which results in a temporal lag and decoupling of production and respiratory processes on longer (seasonal to multi-annual) time scales at both plant and ecosystem levels. Similar scenarios regarding the build up and delay in turnover of dead organic material apply to peatlands (Limpens et al. 2008). For example, maximum monthly NEPAPP data over five years from four temperate pine chronosequence sites (spanning stand ages from 3 to 69 years) (Peichl et al. 2010a) indicated a wide range of 1.6 to 8.3 g C m−2 d−1 as approximation for NEPPOT. Given the same climate and management regimes, this range is solely related to different ecosystem traits (e.g., forest age, LAI, biomass and woody debris pools) among the four forest stands. Thus, ecosystem traits that are critical determinants of NEPPOT vary much less widely in C3 grasslands compared to other biomes, which may facilitate the convergence of NEPPOT. However, while variations in the three controls (i.e., ecosystem traits, environmental conditions, management) did not cause any apparent difference in NEPPOT among the grassland sites in our study, the extent of compensating effects among these three controls and their individual impact on NEPPOT remains unclear.
Implications for the annual CO2 sink-source strength of contrasting global C3 grasslands
The convergence of NEPPOT implies that there are relatively small differences in the net CO2 uptake capacity predetermining the annual CO2 sink-source strength of C3 grasslands and that the range of the latter should be small among global C3 grasslands under optimum conditions. In reality, however, seasonal and short-term constraints from environmental conditions specific to each climate region (e.g., the presence and duration of snow cover, extreme temperatures, summer water deficits, and the length of the growing season, Figure 2) and from management practices (e.g., harvest, grazing, re-seeding) reduce NEPPOT to climate region-specific and site-specific NEPAPP. Thus, given the lack of differences in NEPPOT, it follows that these seasonal and short-term environmental and management constraints may be considered as the major controls of the variations in the annual net CO2 uptake among global C3 grasslands. Moreover, given the interactive effects between weather/climate and associated adaptation in management (i.e., shifts in frequency and timing of management events) (Wohlfahrt et al. 2008a, 2008b), the CO2 exchange of C3 grasslands might be especially sensitive to future climatic changes which modify the occurrence patterns of these seasonal and short-term environmental and management constraints. This finding has also implications with regards to improving process-based models. With a few exceptions (e.g., Hidy et al. 2012), the focus has been on implementing complex processes that describe ecosystem functioning. However, given the convergence of NEPPOT, it follows that i) C3 grasslands may be treated as one functional type and ii) that adequately simulating the impacts from the seasonal and short-term environmental and management constraints (i.e., f(e) and f(m) in Eq.1) should be the primary concern of future model improvement (Groenendijk et al. 2011).
The limited impact of NEPPOT on annual NEPAPP suggests that ecosystem traits, being one of the three main controls on NEPPOT, are also not likely the key determinants of the annual NEPAPP in C3 grasslands. This lack of impact from ecosystem traits on the annual grassland NEPAPP is in contrast to other terrestrial ecosystem types. In forests, for instance, considerable differences in forest stand characteristics (e.g., stand age, plant functional type, biomass pools, LAI) were reported as primary controls on annual NEPAPP over climatic controls (Luyssaert et al. 2007; Lindroth et al. 2008; Peichl et al. 2010a, 2010b; Drake et al. 2011). Similarly, ecosystem traits (i.e., water table depth, species composition, LAI, micro-topography, nutrient status) were noted as important controls on growing season and annual NEPAPP in peatland ecosystems (Humphreys et al. 2006; Lund et al. 2010; Sonnentag et al. 2010). However, Limpens et al. (2008) previously argued that the NEPAPP of undisturbed peatlands (that are similar to grasslands in that they are characterized by a limited range of biomass and LAI) should be less variable than forests, and instead should be primarily controlled by environmental conditions. Species and location were suggested as the primary controls on differences in growing season NEPAPP among croplands (Moors et al. 2010). Thus, the limited effect of ecosystem traits on the cumulative seasonal and annual NEPAPP might be a unique feature of C3 grasslands.
Based on this first work to quantify and compare NEPPOT using multi-year eddy covariance data, we conclude that NEPPOT converges within a narrow range among contrasting C3 grasslands given long-term observations. This finding indicates a general state of stability in grassland NEPPOT during optimum conditions most likely due to body size and supply-demand related metabolic constraints on production and decomposition processes. Given the wide range in annual NEPAPP compared with the narrow range of NEPPOT reported in this study, we conclude that the control of NEPPOT on the annual NEPAPP is limited. This implies that the variation of the annual sink-source strength for atmospheric CO2 among global C3 grasslands is primarily a function of seasonal and short-term constraints from environmental conditions and site-specific management practices, i.e., the time dependent functions f(e) and f(m) in Eq. (1). Compared to other ecosystems, the CO2 exchange of C3 grasslands might therefore be especially susceptible to the direct and indirect effects from expected future changes in climate and associated (adaptive) changes in management.
Supplementary Material
ACKNOWLEDGMENTS
We thank Bo Ranneby and Kristi Kuljus for helpful discussions during the preparation of the manuscript. We are also grateful to the editor and three anonymous reviewers for insightful and constructive comments on the manuscript.
Footnotes
Statement of authorship: All authors agree to the submission of this manuscript.
MP conceived the study, analyzed the data, wrote the paper
OS conceived the study, assisted with paper writing, provided substantial intellectual input
GW conceived the study, provided data, provided substantial intellectual input
LBF conceived the study, provided data, provided substantial intellectual input
DDB, GK, MG, DG, BM, CP provided data and substantial intellectual input
MBJ provided data
MM and MS provided substantial intellectual input
References
- 1.Aires L, Pio C, Pereira J. Carbon dioxide exchange above a Mediterranean C3/C4 grassland during two climatologically contrasting years. Glob. Change Biol. 2008;14:539–555. [Google Scholar]
- 2.Allen A, Gillooly J, Brown J. Linking the global carbon cycle to individual metabolism. Functional Ecology. 2005;19:202–213. [Google Scholar]
- 3.Bahn M, Rodeghiero M, Anderson-Dunn M, Dore S, Gimeno C, Drösler M, et al. Soil respiration in European grasslands in relation to climate and assimilate supply. Ecosystems. 2008;11:1352–1367. doi: 10.1007/s10021-008-9198-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bardgett RD. Plant-soil interactions in a changing world. F1000 Biol Rep. 2011;3:16. doi: 10.3410/B3-16. doi:10.3410/B3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barr A, Black T, Hogg E, Kljun N, Morgenstern K, Nesic Z. Inter-annual variability in the leaf area index of a boreal aspen-hazelnut forest in relation to net ecosystem production. Agr. Forest Meteorol. 2004;126:237–255. [Google Scholar]
- 6.Brown JH, Gillooly JF, Allen AP, Savage VM, West GB. Toward a metabolic theory of ecology. Ecology. 2004;85:1771–1789. [Google Scholar]
- 7.Chapin FS., III Effects of plant traits on ecosystem and regional processes: a conceptual framework for predicting the consequences of global change. Ann. Bot. 2003;91:455–463. doi: 10.1093/aob/mcg041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chapin FS, III, Woodwell GM, Randerson JT, Rastetter EB, Lovett GM, Baldocchi DD, et al. Reconciling carbon-cycle concepts, terminology, and methods. Ecosystems. 2006;9:1041–1050. [Google Scholar]
- 9.Craine JM, Nippert JB, Elmore AJ, Skibbe AM, Hutchinson SL, Brunsell NA. Timing of climate variability and grassland productivity. P. Natl. Acad. Sci. U.S.A. 2012;109:3401–3405. doi: 10.1073/pnas.1118438109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davidson EA, Janssens IA, Luo Y. On the variability of respiration in terrestrial ecosystems: moving beyond Q10. Glob. Change Biol. 2006;12:154–164. [Google Scholar]
- 11.De Deyn GB, Cornelissen JHC, Bardgett RD. Plant functional traits and soil carbon sequestration in contrasting biomes. Ecol. Lett. 2008;11:516–531. doi: 10.1111/j.1461-0248.2008.01164.x. [DOI] [PubMed] [Google Scholar]
- 12.Drake JE, Davis SC, Raetz LM, DeLucia EH. Mechanisms of age-related changes in forest production: the influence of physiological and successional changes. Glob. Change Biol. 2011;17:1522–1535. [Google Scholar]
- 13.Falge E, Baldocchi D, Tenhunen J, Aubinet M, Bakwin P, Berbigier P, et al. Seasonality of ecosystem respiration and gross primary production as derived from FLUXNET measurements. Agr. Forest Meteorol. 2002a;113:53–74. [Google Scholar]
- 14.Falge E, Tenhunen J, Baldocchi D, Aubinet M, Bakwin P, Berbigier P, et al. Phase and amplitude of ecosystem carbon release and uptake potentials as derived from FLUXNET measurements. Agr. Forest Meteorol. 2002b;113:75–95. [Google Scholar]
- 15.Flanagan LB, Adkinson AC. Interacting controls on productivity in a northern Great Plains grassland and implications for response to ENSO events. Glob. Change Biol. 2011;17:3293–3311. [Google Scholar]
- 16.Flechard CR, Ambus P, Skiba U, Rees RM, Hensen A, Van Amstel A, et al. Effects of climate and management intensity on nitrous oxide emissions in grassland systems across Europe. Agr. Ecosyst. Environ. 2007;121:135–152. [Google Scholar]
- 17.Gifford R. The global carbon cycle: a viewpoint on the missing sink. Aust. J. Plant Physiol. 1994;21:1–15. [Google Scholar]
- 18.Gilmanov TG, Aires L, Barcza Z, Baron VS, Belelli L, Beringer J, et al. Productivity, respiration, and light-response parameters of world grassland and agroecosystems derived from flux-tower measurements. Rangeland Ecol. Manag. 2010;63:16–39. [Google Scholar]
- 19.Groenendijk M, Dolman AJ, Van der Molen MK, Leuning R, Arneth A, Delpierre N, et al. Assessing parameter variability in a photosynthesis model within and between plant functional types using global Fluxnet eddy covariance data. Agr. Forest Meteorol. 2011;151:22–38. [Google Scholar]
- 20.Hidy D, Barcza Z, Haszpra L, Churkina G, Pintér K, Nagy Z. Development of the Biome-BGC model for simulation of managed herbaceous ecosystems. Ecol. Model. 2012;226:99–119. [Google Scholar]
- 21.Hobbie SE. Effects of plant species on nutrient cycling. Trends Ecol. Evol. 1992;7:336–339. doi: 10.1016/0169-5347(92)90126-V. [DOI] [PubMed] [Google Scholar]
- 22.Humphreys ER, Lafleur PM, Flanagan LB, Hedstrom N, Syed KH, Glenn AJ, et al. Summer carbon dioxide and water vapor fluxes across a range of northern peatlands. J. Geophys. Res. 2006;111:16. [Google Scholar]
- 23.Jolly WM, Nemani R, Running SW. A generalized, bioclimatic index to predict foliar phenology in response to climate. Glob. Change Biol. 2005;11:619–632. [Google Scholar]
- 24.Lasslop G, Reichstein M, Papale D, Richardson AD, Arneth A, Barr AG, et al. Separation of net ecosystem exchange into assimilation and respiration using a light response curve approach: critical issues and global evaluation. Glob. Change Biol. 2010;16:187–208. [Google Scholar]
- 25.Limpens J, Berendse F, Blodau C, Canadell JG, Freeman C, Holden J, et al. Peatlands and the carbon cycle: from local processes to global implications – a synthesis. Biogeosciences. 2008;5:1475–1491. [Google Scholar]
- 26.Lindroth A, Lagergren F, Aurela M, Bjarnadottir B, Christensen T, Dellwik E, et al. Leaf area index is the principal scaling parameter for both gross photosynthesis and ecosystem respiration of Northern deciduous and coniferous forests. Tellus B. 2008;60:129–142. [Google Scholar]
- 27.Lund M, Lafleur PM, Roulet NT, Lindroth A, Christensen TR, Aurela M, et al. Variability in exchange of CO2 across 12 northern peatland and tundra sites. Glob. Change Biol. 2010;16:2436–2448. [Google Scholar]
- 28.Luyssaert S, Inglima I, Jung M, Richardson a. D., Reichstein M, Papale D, et al. CO2 balance of boreal, temperate, and tropical forests derived from a global database. Glob. Change Biol. 2007;13:2509–2537. [Google Scholar]
- 29.Ma S, Baldocchi DD, Xu L, Hehn T. Inter-annual variability in carbon dioxide exchange of an oak/grass savanna and open grassland in California. Agr. Forest Meteorol. 2007;147:157–171. [Google Scholar]
- 30.Marcolla B, Cescatti A, Manca G, Zorer R, Cavagna M, Fiora A, et al. Climatic controls and ecosystem responses drive the inter-annual variability of the net ecosystem exchange of an alpine meadow. Agr. Forest Meteorol. 2011;151:1233–1243. [Google Scholar]
- 31.Massman W, Lee X. Eddy covariance flux corrections and uncertainties in long-term studies of carbon and energy exchanges. Agr. Forest Meteorol. 2002;113:121–144. [Google Scholar]
- 32.Migliavacca M, Galvagno M, Cremonese E, Rossini M, Meroni M, Sonnentag O, et al. Using digital repeat photography and eddy covariance data to model grassland phenology and photosynthetic CO2 uptake. Agr. Forest Meteorol. 2011a;151:1325–1337. [Google Scholar]
- 33.Migliavacca M, Reichstein M, Richardson AD, Colombo R, Sutton MA, Lasslop G, et al. Semiempirical modeling of abiotic and biotic factors controlling ecosystem respiration across eddy covariance sites. Glob. Change Biol. 2011b;17:390–409. [Google Scholar]
- 34.Moffat A, Papale D, Reichstein M, Hollinger D, Richardson A, Barr A, et al. Comprehensive comparison of gap-filling techniques for eddy covariance net carbon fluxes. Agr. Forest Meteorol. 2007;147:209–232. [Google Scholar]
- 35.Moncrieff J. b., Malhi Y, Leuning R. The propagation of errors in long-term measurements of land-atmosphere fluxes of carbon and water. Glob. Change Biol. 1996;2:231–240. [Google Scholar]
- 36.Moors EJ, Jacobs C, Jans W, Supit I, Kutsch WL, Bernhofer C, et al. Variability in carbon exchange of European croplands. Agr. Ecosyst. Environ. 2010;139:325–335. [Google Scholar]
- 37.Novick KA, Stoy PC, Katul GG, Ellsworth DS, Siqueira MBS, Juang J, et al. Carbon dioxide and water vapor exchange in a warm temperate grassland. Oecologia. 2004;138:259–274. doi: 10.1007/s00442-003-1388-z. [DOI] [PubMed] [Google Scholar]
- 38.Peichl M, Arain MA, Brodeur JJ. Age effects on carbon fluxes in temperate pine forests. Agr. Forest Meteorol. 2010a;150:1090–1101. [Google Scholar]
- 39.Peichl M, Brodeur JJ, Khomik M, Arain MA. Biometric and eddy-covariance based estimates of carbon fluxes in an age-sequence of temperate pine forests. Agr. Forest Meteorol. 2010b;150:952–965. [Google Scholar]
- 40.Peichl M, Carton O, Kiely G. Management and climate effects on carbon dioxide and energy exchanges in a maritime grassland. Agr. Ecosyst. Environ. 2012;158:132–146. [Google Scholar]
- 41.Peichl M, Leahy P, Kiely G. Six-year stable annual uptake of carbon dioxide in intensively managed humid temperate grassland. Ecosystems. 2011;14:112–126. [Google Scholar]
- 42.Reichstein M, Falge E, Baldocchi D, Papale D, Aubinet M, Berbigier P, et al. On the separation of net ecosystem exchange into assimilation and ecosystem respiration: review and improved algorithm. Glob. Change Biol. 2005;11:1424–1439. [Google Scholar]
- 43.Samuels ML, Witmer JA. Statistics for the Life Sciences. 3rd edn Prentice Hall: 2002. [Google Scholar]
- 44.Schmitt M, Bahn M, Wohlfahrt G, Tappeiner U, Cernusca A. Land use affects the net ecosystem CO2 exchange and its components in mountain grasslands. Biogeosciences. 2010;7:2297–2309. doi: 10.5194/bg-7-2297-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sonnentag O, Van der Kamp G, Barr AG, Chen JM. On the relationship between water table depth and water vapor and carbon dioxide fluxes in a minerotrophic fen. Glob. Change Biol. 2010;16:1762–1776. [Google Scholar]
- 46.Stoy PC, Richardson AD, Baldocchi DD, Katul GG, Stanovick J, Mahecha MD, et al. Biosphere-atmosphere exchange of CO2 in relation to climate: a cross-biome analysis across multiple time scales. Biogeosciences. 2009;6:2297–2312. [Google Scholar]
- 47.White R, Murray S, Rohweder M. Pilot analysis of global ecosystems: grassland ecosystems. World Resources Institute; Washington, DC: 2000. [Google Scholar]
- 48.Wohlfahrt G, Anderson-Dunn M, Bahn M, Balzarolo M, Berninger F, Campbell C, et al. Biotic, abiotic, and management controls on the net ecosystem CO2 exchange of European mountain grassland ecosystems. Ecosystems. 2008a;11:1338–1351. [Google Scholar]
- 49.Wohlfahrt G, Hammerle A, Haslwanter A, Bahn M, Tappeiner U, Cernusca A. Seasonal and inter-annual variability of the net ecosystem CO2 exchange of a temperate mountain grassland: Effects of weather and management. J Geophys Res-Atmos. 2008b;113 doi: 10.1029/2007jd009286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zeeman MJ, Hiller R, Gilgen AK, Michna P, Plüss P, Buchmann N, et al. Management and climate impacts on net CO2 fluxes and carbon budgets of three grasslands along an elevational gradient in Switzerland. Agr. Forest Meteorol. 2010;150:519–530. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.