Abstract
A rapidly increasing number of studies are quantifying the system-level network architecture of the human brain based on structural-to-structural and functional-to-functional relationships. However, a largely unexplored area is the nature and existence of “cross-modal” structural–functional relationships, in which, for example, the volume (or other morphological property) of one brain region is related to the functional response to a given task either in that same brain region, or another brain region. The present study investigated whether the gray matter volume of a selected group of structures (superior, middle, and inferior frontal gyri, thalamus, and hippocampus) was correlated with the fMRI response to a working memory task, within a mask of regions previously identified as involved with working memory. The subjects included individuals with schizophrenia, their siblings, and healthy controls (n = 154 total). Using rigorous permutation testing to define the null distribution, we found that the volume of the superior and middle frontal gyri was correlated with working memory activity within clusters in the intraparietal sulcus (i.e., dorsal parietal cortex) and that the volume of the hippocampus was correlated with working memory activity within clusters in the dorsal anterior cingulate cortex and left inferior frontal gyrus. However, we did not find evidence that the identified structure–function relationships differed between subject groups. These results show that long-distance structural–functional relationships exist within the human brain. The study of such cross-modal relationships represents an additional approach for studying systems-level interregional brain networks.
Keywords: Structural MRI, Functional MRI, Working memory, Hippocampus, Prefrontal cortex, Connectivity
Introduction
The human brain is connected on a variety of different spatial scales, from synaptic signaling within a cortical column to systems level, interregional communication across physically distant brain regions. At the systems level, recent approaches for studying the human “connectome” include the use of diffusion tractography to map networks of anatomically connected brain regions (Hagmann et al. 2008; Gong et al. 2009; Johansen-Berg and Rushworth 2009) and functional magnetic resonance imaging (fMRI) and magnetoencephalography to study the manner in which different regions are functionally related, either in the presence of an explicit task or in an unconstrained “resting” state (Bassett et al. 2006; Fox and Raichle 2007; Smith et al. 2009; Biswal et al. 2010). Other studies have explored structural relationships by mapping the covariance patterns of volume or cortical thickness across brain regions (Mechelli et al. 2005; Lerch et al. 2006; He et al. 2007; Xu et al. 2009). With the exception of diffusion tractography, which attempts to infer actual macroscopic structural linkage, these studies define connectivity based on statistical dependence (correlation or coherence) across brain regions of the measured variable.
Most studies to date that have defined connectivity based on statistical correlation have focused on understanding the relationships across brain regions of a single type of structural or functional characteristic of the brain, thus yielding connectivity maps based on structural-to-structural, or functional-to-functional correlations. This “unimodal” approach is a natural and logical starting point to begin mapping the patterns of systems-level interregional brain relationships. However, it is also possible to define “cross-modal” brain relationships between disparate measures. For example, one can investigate the correlation of fractional anisotropy, mean diffusivity, or structural volume/area/thickness with functional (fMRI) responses. Most such studies to date have been limited to investigating whether structural variables are related to functional task activity within the same localized region of brain (Siegle et al. 2003; Andrews et al. 2006; Oakes et al. 2007; DaSilva et al. 2008; Siok et al. 2008; Wang et al. 2010). However, only a few studies have looked for possible “long-distance” structural–functional relationships between different brain regions (Calhoun et al. 2006; Schlosser et al. 2007; Correa et al. 2008; Michael et al. 2010, 2011). We suggest that such cross-modal structural–functional studies could potentially yield new insights into whether brain structure in one region influences function at other cortical or subcortical locations.
A question for both unimodal and cross-modal investigations of connectivity is whether the pattern or strength of connectivity is altered in various disease conditions (Zhang and Raichle 2010). In the case of schizophrenia, studies have shown altered structural-to-structural (Mitelman et al. 2005; Bassett et al. 2008) and functional-to-functional (Whitfield-Gabrieli et al. 2009; Repovs et al. 2011; Skudlarski et al. 2010) relationships in individuals with schizophrenia. There is also mixed evidence for differential relationships between structure and neurocognitive function in individuals with schizophrenia compared with controls (for review see Antonova et al. 2004). In the present study, we used a mapping approach to identify locations in the brain where the voxel-level functional response to a working memory task (assessed with blood oxygen level dependent fMRI) was correlated with the regional volume of a selected group of structures, in a cohort of subjects that included healthy controls, individuals with schizophrenia, and their siblings. Our goals were twofold: (1) to identify possible regions that exhibit a structure–function correlation independent of subject group, using the broadest cohort of subjects possible and (2) to investigate whether there was any evidence for differential structure–function relationships between subject groups. Accordingly, we used two different models to identify relevant regions and investigate the possibility of group differences. In one approach (“main effect” model), we first identified clusters where working memory brain activity was correlated with the selected structural volumes across the entire group of subjects. Following the identification of such regions, we then checked whether the structural–functional correlation at that location differed across subject groups. In the second approach (“separate slopes” model), we attempted to directly map locations where the structure–function relationship differed between individuals with schizophrenia and healthy controls.
One challenge in any connectivity study is defining the size and number of regions across which the interregional correlations are to be computed. In order to avoid an excessive number of comparisons, we chose a focused approach involving a select number of structural regions. Also, we limited the choice of structural regions in this particular study to those regions for which volumes in this cohort of subjects were already available from other previous analyses. The search for correlations of those structural volumes with working memory activity was itself constrained to a predefined mask of voxels that was based on meta-analyses of PET and fMRI studies of working memory activity. Overall, both the structural regions and functional mask had a strong connection to regions of the brain involved with working memory. The specific structural volumes involved were the cortical gray matter volume of the superior, middle, and inferior frontal gryi (SFG, MFG, and IFG, respectively), and the volume of the thalamus and hippocampus. Volumes of the MFG and IFG were included because dorso- and ventro-lateral prefrontal cortex is a critical component of the working memory system. The thalamus is also an important component of the working memory system (Miller and Cohen 2001). The volume of SFG was included because the supplementary motor area and dorsal anterior cingulate are frequently implicated in working memory as well, and SFG was the available structural measure that encompassed these two subregions of superior frontal cortex. Unfortunately, regional structure volumes were not available for superior parietal cortex, which is another important brain region in the working memory system. While the hippocampus is not traditionally considered involved with working memory, numerous recent studies indicate that the hippocampus may indeed be involved with aspects of short-term memory (Ranganath and D’Esposito 2001; Axmacher et al. 2007). Further, a prominent model of schizophrenia involves disturbed hippocampal–prefrontal functional connectivity (Meyer-Lindenberg et al. 2005; Ragland et al. 2007). Thus, we speculated that hippocampal–prefrontal structural–functional connectivity might be a relationship that was more likely to differ between subject groups. This was an additional reason for including the hippocampus as one of the structural volumes that was examined.
Methods
Subjects
The subjects included in this study were drawn from a population of subjects who volunteered for studies of brain structure and function at the Conte Center for the Neuro-science of Mental Disorders at Washington University School of Medicine in St. Louis. Subjects consisted of 32 individuals with DSM-IV schizophrenia (28 male), 29 non-psychotic siblings of individuals with schizophrenia (14 male), and 93 healthy participants (39 male) comprised of controls and their siblings. Average age across all subjects was 21.3 years (SD = 3.4 years; range 14.1–30.7 years), and average duration of illness for the individuals with schizophrenia was 4.2 years (SD = 4.4 years). Siblings were full siblings, based on self-report. This cohort of subjects represents all subjects for whom we had usable structural and functional data acquired on the same 1.5-T magnet. Compared with our recent report examining structural integrity in prefrontal cortex (Harms et al. 2010), the subjects for the present study were not restricted to complete sibling pairs, and thus included some individuals who had usable data themselves but whose siblings lacked usable data (34 subjects lacked a sibling in the present study). Further details of subject recruitment, assessment, antipsychotic medication, and lifetime co-morbidities in a substantially similar cohort are available elsewhere (Harms et al. 2007; Mamah et al. 2008). Further details regarding these factors are not critical here because we did not find evidence for differential structure–function relationships across groups (see “Results”). All subjects gave written informed consent for participation following a complete description of the risks and benefits of the study.
Structural MR collection and preparation
Magnetic resonance (MR) scans of the whole brain were collected using a Siemens Magnetom Vision 1.5-Tesla imaging system using a standard head coil. We collected (1) multiple (2–4) high-resolution, 3D T1-weighted MPRAGE volumes (voxel size: 1 × 1 × 1.25 mm3, TR: 1,765 ms, TI: 640 ms, TE: 4.0 ms, flip angle: 10°, scan time: 6.5 min per acquisition) that were subsequently aligned and averaged prior to further analysis and (2) a single high-resolution 3D T1-weighted FLASH sequence (voxel size: 1 × 1 × 1 mm3, TR: 20 ms, TE: 5.4 ms, flip angle: 30°, scanning time: 13.5 min). The gray-matter volumes of three sulcal-defined regions of the prefrontal cortex were calculated from the average MPRAGE volume of each subject as detailed in Harms et al. (2010). Briefly, the sulcal boundaries of the superior, middle, and inferior frontal gyri (SFG, MFG, IFG) were manually delineated on a model of the white matter surface, and the gray matter volume of each prefrontal subregion was calculated from labeled cortical distance maps (Miller et al. 2003). Simplistically, the borders of the SFG were the cingulate sulcus medially and the superior frontal sulcus laterally; the borders of the MFG were the superior and inferior frontal sulci; and the IFG was the gray matter inferior to the inferior frontal sulcus that was part of pars opercularis and pars triangularis. The posterior border of these regions was the precentral sulcus and the anterior border was defined using a frontal pole plane that passed through the anterior termination of the olfactory sulcus. The boundaries of the SFG, MFG, and IFG could be consistently and reliably identified, as indicated by average surface overlap exceeding 94% for both inter- and intrarater comparisons (Harms et al. 2010). The volume of the thalamus and hippocampus was obtained from the FLASH scan of each subject by propagating a template surface (carefully outlined in a template scan) to each subject’s scan using large deformation high-dimensional brain mapping (Haller et al. 1997; Csernansky et al. 2004). Volumes from the left and right hemispheres of each structure were summed.
Functional MR collection and preparation
Functional images were collected on the same 1.5 T scanner while subjects performed a working memory (WM) task, namely the 2-back version of the N-back task. In this task, subjects pressed a target button each time a visual stimulus was identical to the stimulus presented two trials previously and a nontarget button otherwise. One run of this working memory task used words as stimuli, and a second run used non-famous faces as stimuli (Brahmbhatt et al. 2006). Each run consisted of four task blocks (40 s each, 16 trials per block) interleaved with three fixation blocks (25 s each). Nineteen slices parallel to the anterior–posterior commissure plane were acquired using an asymmetric spin-echo echo-planar imaging (EPI) sequence sensitive to blood oxygenation level-dependent contrast (TR = 2,500 ms, TE = 50 ms, flip = 90°, thickness = 7 mm, 3.75 × 3.75 mm in-plane resolution). FMRI preprocessing included resampling the functional data to 3 mm cubic voxels in atlas space using an age-appropriate anatomical template constructed at Washington University (“711-2Y”, which is similar but not identical to Talairach atlas space) (Buckner et al. 2004; Van Essen and Dierker 2007). Note that all reported coordinates in this manuscript were converted to MNI152 space using an appropriate affine transformation. The resampling of the functional data to atlas space was accomplished using a single composite transformation matrix that included motion correction and atlas registration (which itself involved a sequence of affine transforms: first frame EPI to T2-weighted turbo spin echo to MPRAGE to age-appropriate atlas representative target). Data were then spatially smoothed with a 6-mm full-width-half-maximum Gaussian kernel. For each subject, we estimated the magnitude of task-related activation in each voxel (relative to fixation) with a general linear model (GLM) using a box-car function convolved with a canonical hemodynamic response, with separate estimates (i.e., beta-weights) for the word and face working memory tasks. The GLM also included terms for signal drift within runs and mean signal shifts across runs. These separate beta estimates for word and face stimuli were then averaged to yield a single estimate of working memory activity, because we were interested in general working memory processes, independent of stimulus type.1
Additionally, encoding and recognition tasks were performed during the same scanning session (Bonner-Jackson et al. 2005), which were used to assess the specificity of the results to the working memory task. The encoding task consisted of two scanning runs, one involving deep encoding of words (abstract/concrete judgment) and the other deep encoding of faces (gender identification). The recognition task also consisted of two scanning runs during which subjects indicated whether they had seen the current stimulus during the previous encoding phase of the experiment. These data were processed in a manner analogous to the working memory task runs, including averaging of the activity estimates (beta-weights) for the word and face runs.
Structural–functional correlations
Two different statistical models were used in conjunction with permutation-based non-parametric inference: (1) a “main effect” model that identified regions that exhibited a correlation between structural volume and WM activity across the entire group of subjects while controlling for group status so as to avoid the possibility that the correlation was actually driven by differences in the mean values across groups and (2) a “separate slopes” model in which we looked for locations where the structure–function relationship differed between individuals with schizophrenia and healthy controls. This is analogous to the common statistical approach of assessing a dataset for a “main” effect of a variable that applies across groups while also checking for a differential (“interaction”) effect between groups. Including all subjects in our main effects analysis allowed us to maintain a parsimonious model with that used for the separate slopes analysis. Additionally, using a homogeneity of variance test, we established that there were no significant variance differences between individuals with schizophrenia and controls for the volumes of the structural regions and the working memory functional activity within the five clusters identified by the main effects analysis (Table 1) (all P > 0.06 according to Levene’s test)2
Table 1.
Locations with a significant correlation between working memory task-related BOLD activity at that location and volume of the indicated structure (“main effect” model, controlling for group and gender)
| Structural volume used as independent variable | Location (mm) of significant correlation between structural volume and WM BOLD activitya | Volume of cluster (mm3) | P value of cluster extentb | t statistic at peak within clusterc | |
|---|---|---|---|---|---|
| 1 | SFG | −40, −41, 40 (IPS, L) | 486 | 0.038 | −4.26 |
| 2 | SFG | 35, −46, 40 (IPS, R) | 432 | 0.047 | −3.93 |
| 3 | MFG | −38, −50, 43 (IPS, L) | 864 | 0.013 | −4.48 |
| 4 | Hippocampus | −56, 12, 1 (IFG, L) | 945 | 0.009 | 4.19 |
| 5 | Hippocampus | 1, 16, 43 (dACC) | 2403 | 0.001 | 5.33 |
df = 145 for SFG and MFG as the structural variable, and 143 for hippocampus
IPS intraparietal sulcus, IFG inferior frontal gyrus, dACC dorsal anterior cingulate cortex, L left, R right
Coordinates are the center of the cluster after conversion to MNI152 space
P values for cluster extent were computed using permutation testing and one-tailed tests. The search volume for clusters was restricted to a set of regions independently identified in previous studies as regions involved in working memory task performance (Fig. 1)
Sign indicates the direction of the relationship (i.e., negative values indicate an inverse correlation)
In the case of assessing a simple regression, the null hypothesis of exchangeability (i.e., no relationship) between independent and dependent variables is straightforwardly assessed by computing the distribution of the regression coefficient in the presence of many permutations (i.e., “shufflings”) of the two variables. However, the present study employed more complicated general linear models that also controlled for the potential “nuisance” variables of group status and gender. Specifically, in the “main effect” model for each structural–functional correlation analysis, we constructed a design matrix that coded group membership, gender, and structural volume (centered at zero) as five explanatory variables (EVs; a “dummy variable” EV for each separate group comprised of 1’s for members of that group and 0’s otherwise, yielding three EVs that modeled an offset for each group, thereby controlling for group status; a fourth EV to account for a main effect of gender; and a fifth EV to model a main effect of structural volume). In the “separate slopes” models, the EV for structural volume was split into three separate EVs coding group-specific structural volumes (yielding EVs containing structural volumes for members of that group, and 0’s for members of the other two groups). This allowed estimation of a separate slope relating structural volume to WM activity for each of the three groups. In all models WM activity was the response variable. Contrasts of interest were the positive and negative relationship of the structural volume regressor to WM activity for the “main effect” models, and the difference (both directions) between the slope (beta) estimates of the schizophrenia and control groups in the “separate slopes” models. We chose to focus on the contrast between the schizophrenia and control groups in the “separate slopes” analyses since we hypothesized that those were the two groups most likely to exhibit slope differences, with the intent that we would then compare those two groups with the siblings of individuals with schizophrenia within any regions that exhibited a significant schizophrenia versus controls slope difference.
Permutation testing (Nichols and Holmes 2002) for both the “main effect” and “separate slopes” models was performed using the ‘randomise’ tool (version 2.5) of FSL version 4.1.4, which uses the method of Freedman and Lane (1983) (see also Anderson and Robinson 2001) to create approximate realizations of the data under the null hypothesis specified by the contrast (see http://www.fmrib.ox.ac.uk/fsl/randomise for further details). This process is repeated to build a distribution of test statistics equivalent under the null hypothesis of exchangeability (i.e., in this case, the null hypothesis of no relationship between structural volume and WM activity). Correction for multiple comparisons across voxels was accomplished using a cluster-based extent (i.e., size) threshold. For each permutation, clusters were formed from voxels satisfying a t-statistic threshold equivalent to a per-voxel alpha of P < 0.001. Based on 10,000 permutations for each contrast analyzed, clusters in the non-permuted original data whose extent satisfied P < 0.05 (corrected) according to the permutation distribution were identified. Nearly identical P values for permutation-based cluster significance were obtained using a cluster mass statistic (Bullmore et al. 1999).
In assessing the cluster extent distribution of both the “main effect” and “separate slopes” models, our analyses limited the search region to an a priori set of regions, independently identified in previous studies as regions involved in working memory task performance. This was done to focus our search for structure–function correlations to the functional regions most consistently implicated in working memory task performance across multiple studies. This working memory mask was created by placing 20 mm diameter spheres around the coordinates from a meta-analysis of N-back neuroimaging results (verbal stimuli portion of Table 2 of Owen et al. 2005), as well as the coordinates from a meta-analysis of working memory tasks more generally (Table 4 of Wager and Smith 2003). While other studies implicate the hippocampus in some aspects of short-term memory (see “Discussion”), we note that this particular WM mask did not itself include the hippocampus. The resulting working memory mask based on the coordinates from these meta-analyses (yellow regions in Fig. 1) was well contained within the regions that displayed a significant functional response to the 2-back task in this particular group of subjects (white borders in surface representation in Fig. 1). As a supplemental analysis, we also repeated the “main effect” analysis using a whole brain mask, the results of which can be found in the Online Resource associated with this article.
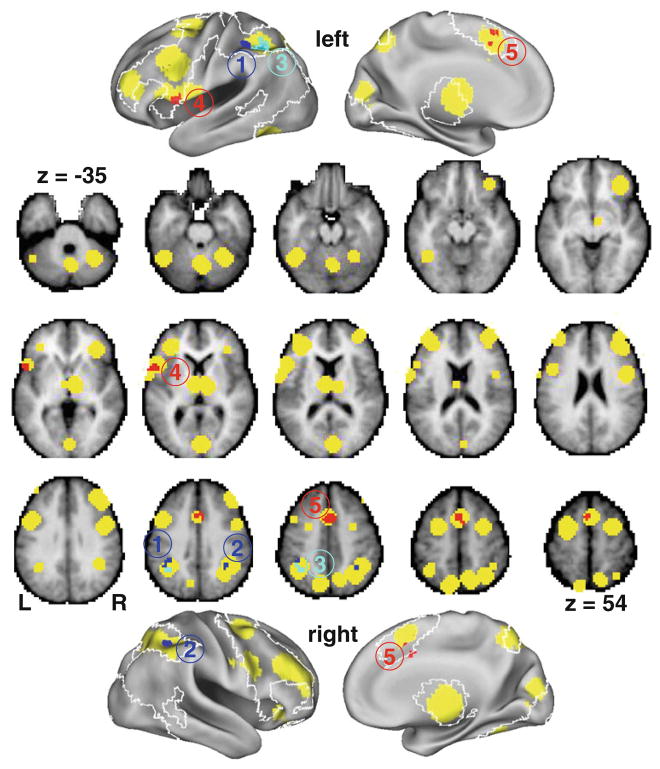
Fig. 1.
Regions where the volume of the SFG (blue, clusters numbered 1 and 2), MFG (cyan, cluster number 3), and hippocampus (red, clusters numbered 4 and 5) were correlated with working memory (2-back) task activity at that location across the entire group of subjects (“main effect” model). The ordering of cluster numbers in the figure corresponds to successive rows in Table 1. The analyses were restricted to a working memory mask (based on published meta-analyses), which is shown in yellow. Analyses were conducted in volume space (middle portion of figure showing axial slices spaced by the equivalent of 6.3 mm in MNI152 space). However, for visualization purposes, the intersections of the regions and mask with an average surface (PALS 12 subject average) (Van Essen 2005) in the same stereotaxic space are also shown on the inflated PALS cortical surfaces using Caret (top and bottom). Because of the nature of this volume to surface mapping, portions of cluster number 5 appear disjointed on the medial surface of both the left and right hemispheres, and the extent of cluster number 5 on those surfaces does not accurately reflect the size of that cluster in the volume (greater than 2.5 times larger in volume than clusters 3 and 4, which were the next two largest clusters). Also shown on the surfaces is an outline (white) of the regions (across the whole brain) where there was a statistically significant positive response to the 2-back task versus fixation (assessed using permutation testing with all 154 subjects, without regard to group status). The medial wall of the surfaces includes a representation of the thalamic portion of the analysis mask and activation to the 2-back task. However, the cerebellar portions of the analysis mask are only visible in the volume space and the cerebellar activation to the 2-back task (which was extensive) is not visualized
For each identified cluster from the “main effect” analyses, the working memory, encoding, or recognition task activity within that cluster was averaged for each subject, and those values were used as the dependent variable in further statistical analyses to probe the specificity of the structure–function relationships regarding subject group, whole brain volume, task specificity, and hemisphere (see “Results”). All these models used a mixed model that estimated the covariance in the residuals due to the familial relationships (using PROC MIXED of SAS 9.2). Degrees of freedom in the mixed model were estimated using the method of Kenward and Roger (1997) (i.e., DDFM = KR option in SAS). For presentation of effect sizes, t-statistics from the mixed model were converted to a partial correlation (controlling for the other effects in the model) according to the equation r = sqrt[t2/(t2+df)]. For this conversion, we assumed the degrees of freedom (df) that would apply given independent residuals [i.e., 145 for results involving the structural volume of the SFG, MFG, or IFG (reflecting 150 subjects with usable MR scans for those structures), and 143 for results involving thalamic and hippocampal volume (reflecting 148 subjects with usable MR scans for those structures)]. Thus, the effect sizes are slightly biased in a conservative direction, since the actual Kenward–Roger estimated degrees of freedom ranged from 120 to 145. Hypothesis testing used Type III tests (invariant to the ordering of effects in the model).
Results
Five clusters were identified within the working memory mask in our “main effect” analyses where there was a significant correlation between working memory task-related BOLD activity and the volume of the SFG, MFG, or hippocampus across the entire group of subjects (Table 1; Fig. 1). There was a correlation between volume of the SFG and WM activity within bilateral regions of the intraparietal sulcus (IPS, i.e., dorsal parietal cortex), and a correlation between volume of the MFG and WM activity within a similarly situated region of left IPS. These three clusters all exhibited negative correlations, such that increasing volume of the indicated structure was associated with decreasing WM task activity. Two clusters exhibited positive correlation between the volume of the hippocampus and WM activity—one in the left IFG and the other in dorsal anterior cingulate cortex (dACC). No regions within the working memory mask exhibited a significant correlation between WM activity and volume of the thalamus or IFG. Also, no regions within the working memory mask exhibited a significantly different relationship of structural volume and WM activity between individuals with schizophrenia and controls in our “separate slopes” models, for any of the five structural volumes.
We then extracted the average response to the WM task for each subject within each cluster identified in the “main effect” analyses, and used that as the dependent variable in further statistical analyses designed to address key questions regarding the identified structure–function correlation. We first examined whether there was any evidence for heterogeneous structure–function relationships across subject groups within those particular clusters, using a mixed model that included gender, group, relevant structural volume, and group-by-volume as fixed effects. Neither the group-by-volume interaction term (F-test involving all three groups) nor the specific contrast of the structure–function slope estimates of individuals with schizophrenia versus controls was significant for any of the five clusters identified in the “main effect” analyses (P > 0.14, and P > 0.07, respectively). This indicates that the structure–function relationships in Table 1 were common to all three subject groups. Together with the null finding from our “separate slopes” permutation analysis (in which we explicitly searched the working memory mask for regions where the structure–function relationships differed between individuals with schizophrenia and controls), these results indicate that there was no statistical evidence for differential slopes across groups.
Next, we investigated whether the correlation between structural volume and WM activity in each cluster remained when total cerebral brain volume [defined as in Calabrese et al. (2008)] was included as a covariate, using a mixed model with group, gender, brain volume, and relevant structural volume as fixed effects. The relationship between structural volume and WM activity remained highly significant in each of the five identified clusters (P < 0.003). Table 2 shows that the partial correlations between structural volume and WM activity were either unchanged or slightly decreased by the inclusion of whole brain volume for the locations correlated with SFG and MFG volume, and either unchanged or slightly increased for the locations correlated with hippocampal volume (i.e., compare effect sizes in fourth vs. third columns). Altogether, controlling for brain volume had relatively little impact on the identified structure–function relationships, suggesting some regional specificity to these relationships.
Table 2.
Partial correlations between volume of indicated structure and average working memory, encoding, or recognition task BOLD activity at the indicated location
| Structural volume | Cluster location (mm) of correlated WM activity | Correlation between volume and average WM activity within clustera,c | Correlation additionally controlling for brain volumeb | Correlation between volume and average ENCOD activity within clustera | Correlation between volume and average RECOG activity within clustera | Correlation between volume and WM-ENCOD activity differencea | Correlation between volume and WM-RECOG activity differencea |
|---|---|---|---|---|---|---|---|
| SFG | −40, −41, 40 | −0.33 | −0.33*** | −0.23** | −0.29*** | −0.17* | −0.10 |
| SFG | 35, −46, 40 | −0.30 | −0.25** | −0.20* | −0.25** | −0.22** | −0.10 |
| MFG | −38, −50, 43 | −0.34 | −0.26** | −0.19* | −0.29*** | −0.24** | −0.10 |
| Hippocampus | −56, 12, 1 | 0.35 | 0.34*** | 0.19* | 0.20* | 0.23** | 0.21** |
| Hippocampus | 1, 16, 43 | 0.38 | 0.44*** | 0.17* | 0.33*** | 0.26** | 0.11 |
All partial correlations computed using a t-to-r conversion of t-statistics from mixed model (see “Methods”)
P ≤ 0.05;
P ≤ 0.01;
P ≤ 0.001 (two-sided)
Controlling for group and gender
Controlling for group, gender, and whole brain volume
No P values are indicated for the correlations in this column because the clusters themselves were identified by modeling the same effects (the permutation-based P values for the associated cluster extents are provided in Table 1)
Third, we examined whether the structure–function correlation exhibited specificity to the working memory task. This was necessary because in the current study the working memory (2-back) activation was based on a comparison to fixation, and thus the regions identified were not necessarily related to working memory specific processes, but may be related to more general cognitive processing. We therefore used encoding and recognition tasks collected in the same imaging sessions to investigate whether the BOLD response to those tasks within the identified cluster locations was also correlated with the relevant structural volume. This was assessed using a mixed model with encoding (ENCOD) or recognition (RECOG) task activity (averaged within each cluster for each subject) as the dependent variable, and gender, group, and relevant structural volume as fixed effects. We also repeated the analysis using the difference between WM and either ENCOD or RECOG activity as the dependent variable. All five of the clusters identified in Table 1 exhibited a significant relationship (P < 0.05) between ENCOD or RECOG task activity and the corresponding structural volume. However, the strength of the relationships differed. For the ENCOD task, the effect size of the correlation (r value) was smaller compared with that for the 2-back task (Table 2, compare fifth vs. third columns). As a result, when we used the difference between 2-back and encoding activity as the dependent variable (WM-ENCOD), all five identified regions still exhibited a significant correlation with the relevant structural volume (Table 2, seventh column). For the RECOG task, the effect size of the correlation was more similar to that for the 2-back task in four out of the five regions (Table 2, compare sixth vs. third columns). Consequently, the difference between 2-back and recognition activity (WM-RECOG) was not significantly correlated with the relevant structural volume in those four regions (Table 2, eighth column). An exception to this was that the correlation between hippo-campal volume and the WM-RECOG signal in the left IFG was significant, indicating that the differential response to the WM task (beyond the response to the RECOG task) was itself correlated with hippocampal volume. Altogether, these results indicate that the structure–function correlations identified in Table 1 (using activity to a 2-back task as the initial functional variate) exhibit a mixed degree of task specificity. Comparing the 2-back and encoding tasks, there was evidence that the structure–function relationship was stronger for the 2-back than the encoding task. Comparing 2-back and recognition tasks, the primary outcome was that the identified clusters represent regions supporting cognitive processes common to both the 2-back and recognition tasks, with the notable exception of the relationship between hippocampal volume and 2-back activity in the left IFG, which showed evidence of specificity to the working memory task relative to the recognition task as well.
Fourth, since the structural volumes in the preceding analyses were summed across hemisphere, the question arises whether similar structural–functional correlations would be observed using hemisphere-specific structural volumes. This question of possible hemispheric asymmetry is most relevant for the two identified clusters that lacked bilateral symmetry (MFG volume to WM activity in left IPS, and hippocampal volume to WM activity in left IFG). Therefore, we examined the strength of the correlation between WM activity within these two clusters and hemisphere-specific volumes of the relevant structure (using a mixed model with group, gender and relevant hemisphere-specific volume as fixed effects). Left and right MFG volumes were each significantly related to WM activity within the cluster in the left IPS (P < 0.001 for each; r = 0.33 and 0.28 for left and right MFG volume, respectively). The partial correlation of left and right MFG volume with each other (controlling for group and gender) was r = 0.58. Similarly, left and right hippocampal volumes were each significantly related to WM activity with the cluster in the left IFG (P < 0.001 for each; r = 0.31 and 0.35 for left and right hippocampal volume, respectively). The partial correlation of left and right hippocampal volume with each other (controlling for group and gender) was r = 0.80. These results suggest that the relationships of MFG volume with left IPS activity and hippocampal volume with left IFG activity were not specific to the volumes of the left or right hemispheres, which is consistent with the robust correlations between the volumes of the left and right hemispheres for those structural variables.
Last, as an additional analysis motivated by our hippocampal findings, we examined whether the hippocampal-WM correlation appeared stronger in particular structural regions (“zones”) of the hippocampus. These results suggested that the hippocampal-WM correlations exhibited some degree of specificity along the longitudinal axis of the hippocampus, with WM activity within the left IFG being most strongly related to average surface displacement in the anterior (head) portion of hippocampus, while WM activity within the dACC was approximately equally related to surface displacement in both the anterior (head) and posterior (tail) portions of the hippocampus (see Online Resource for details and further discussion).
Discussion
In the present study, we looked for evidence of relationships between the volumes of selected structures (SFG, MFG, IFG, thalamus and hippocampus) and fMRI activation to a working memory task within a subset of brain regions previously identified as involved with working memory based on meta-analyses (Wager and Smith 2003; Owen et al. 2005). Using rigorous permutation testing to define the null distribution, we found a relationship of SFG and MFG volume with WM activity in the IPS (i.e., dorsal parietal cortex) and between hippocampal volume and WM activity within clusters in the IFG and dACC.
Long-distance structure–function relationships
Most previous studies that have investigated the correlation of structural variables with functional (fMRI) activity have focused on whether these two variables were related within the same localized region of brain (Siegle et al. 2003; Andrews et al. 2006; Oakes et al. 2007; DaSilva et al. 2008; Siok et al. 2008; Wang et al. 2010). To our knowledge, the studies most similar to ours—in which the possibility of “long distance” structure–function relationships were also explored—are those of Michael et al. (2010, 2011), who introduced some methods for quantifying such relationships, using gray matter concentration (assessed using voxel-based morphometry) as their structural variable and fMRI activity to an auditory sensorimotor task (Michael et al. 2010) or Sternberg working memory task (Michael et al. 2011) as their functional variables. In their 2010 paper they proposed three methods that differed in the manner in which the structural–functional cross-correlation matrix was reduced (i.e., collapsed) across voxels, and thus differed in the degree of spatial information that was retained. The approach in our study is conceptually intermediate to their “Method 2” and “Method 3” in that we used regional measures of structure (i.e., volumes of pre-defined regions) but retained a voxel level of spatial specificity for the functional activity. In our study, it was not possible to also compute results with the direction of this asymmetry reversed because the structural measures (volumes) were inherently region-based measures. In a subsequent paper (Michael et al. 2011), these authors extended their “Method 2” using a hierarchical approach that first identified structural, then functional, clusters that maximally captured differential structure–function correlation between individuals with schizophrenia and healthy controls. Broadly, their studies and ours find evidence for “long distance” structural–functional relationships in which the volume of one brain region was associated with functional activation in other brain regions. A similar approach to our study was also employed by Schlosser et al. (2007), who found that a region of white matter change in schizophrenia was related to the BOLD response to a WM task in regions remote from the white matter abnormality. Multivariate approaches that utilize the full set of brain voxels have also been proposed for fusing structural and functional data, including joint independent component analysis (Calhoun et al. 2006) and multimodal canonical correlation analysis (Correa et al. 2008), although the interpretation of the resulting sources or components can be challenging. Altogether, these studies of long-distance cross-modal relationships represent novel approaches to exploring and quantifying the networks of structural–functional relationships that may exist in the brain and suggest that structural characteristics of one region may influence functional activity in other regions that may be part of a common network.
A natural question is what mechanisms may be responsible for these sort of long-distance structural–functional couplings. One possibility is that the local gray matter volume in one region affects the quantity of functional output from that region, which in turn affects the quantity of synaptic input arriving at a distal cortical location in response to a task. Such a model implies a causal relationship from structure to downstream functional response. Alternatively, the causal direction of the relationship could be reversed, with the amount of functional activity in a region influencing the structural volume of a downstream region, either through excitotoxic or neurotrophic influences. Notably, an excitotoxic downstream effect would be a mechanism by which increased functional activity in one region (if consistently elevated) could lead to decreased structural volume in another region. In all these hypothesized mechanisms, it is possible that the observed structure–function relationship is mediated by intervening regions, i.e., indirect (polysynaptic) connections rather than direct (monosynaptic) connections. Overall then, multiple mechanisms and pathways could lead to a coupling between structural and functional characteristics of the brain. Because these mechanisms are not static, it seems likely that the strength and directionality of structure–function correlations will vary as a function of development, aging, and neurodegenerative disorders.
Prefrontal volume relationship with dorsal parietal working memory activity
We found that the gray matter volumes of the SFG and MFG were inversely related to WM activity in clusters located in the IPS, such that larger SFG and MFG volumes were coupled to reduced WM activity within the identified IPS clusters. The SFG and MFG regions used for the volumetric calculation were defined based on gyral/sulcal borders and in the case of SFG included cortex on both the dorsolateral and dorsomedial portions of prefrontal cortex. Thus, it is difficult to precisely relate our finding to other reports of functional correlations between IPS and more spatially localized prefrontal regions. Nonetheless, it is worth noting that the IPS and dorsolateral prefrontal cortex are components of “task-positive” (Fox et al. 2005) and frontoparietal (Dosenbach et al. 2007) networks identified using resting-state functional connectivity, as well as a similarly constituted frontoparietal network identified in a meta-analysis of regions coactivated in task-based fMRI (Toro et al. 2008).
The direction of the relationship between prefrontal volumes and IPS activity is consistent with our findings of increased activity to the 2-back task in individuals with schizophrenia and their siblings relative to controls in a similar region of left dorsal parietal cortex (Brahmbhatt et al. 2006), along with decreased MFG volume in individuals with schizophrenia relative to controls (Harms et al. 2010) in previous studies from our group that involved substantially overlapping subjects. In a number of other previous studies, increased functional brain activity in individuals with schizophrenia and their siblings has been interpreted as reflecting “inefficient” activity and as evidence of altered function in that region, although this argument has primarily been applied to prefrontal cortex activity (Callicott et al. 2000; Van Snellenberg et al. 2006; Kim et al. 2010). However, if such interpretations are correct, our finding of an inverse relationship between MFG volume and 2-back activity in dorsal parietal cortex would be consistent with the idea that larger prefrontal volumes may contribute to more effective or efficient (i.e., smaller) activation of regions within a dorsal frontoparietal network of regions associated with cognitive control.
Hippocampal volume relationship with prefrontal cortex working memory activity
Our finding of a positive relationship between hippocampal volume and WM activity in two clusters within prefrontal cortex is intriguing for several reasons. The hippocampus and adjacent medial temporal lobe (MTL) have not been traditionally associated with working memory, but rather with long-term memory (Squire et al. 2004). More recently, however, the validity of such a strict dissociation has been questioned. In a review article, Ranganath and Blumenfeld (2005) concluded that short-term memory performance in individuals with MTL lesions may be highly dependent on the particular materials used for testing. FMRI studies in humans (Ranganath and D’Esposito 2001; Karlsgodt et al. 2005; Nichols et al. 2006; Axmacher et al. 2007; Hannula and Ranganath 2008) and lesion studies in rats and monkeys (Zola-Morgan and Squire 1986; Wan et al. 1994) also implicate the MTL in working memory tasks. The volume of the hippocampus has been associated with visual working memory deficits in preterm infants (Beauchamp et al. 2008), and volume reductions in the anterior hippocampus are associated with both immediate and delayed verbal recall in nondemented elderly (Hackert et al. 2002). Thus, a growing body of evidence indicates some degree of involvement of MTL regions with working memory tasks.
Further, the hippocampus is both anatomically and functionally connected to specific regions of prefrontal cortex. Anatomically, tracer studies show that the anterior hippocampus in monkeys projects directly to ventromedial and orbital prefrontal cortices (Rosene and Van Hoesen 1977; Barbas and Blatt 1995; Carmichael and Price 1995). More caudally within the MTL, there are also direct connections of hippocampal transitional cortex (presubiculum and “caudomedial lobule”) and the adjacent retrosplenial cortex with lateral prefrontal cortex (Goldman-Rakic et al. 1984; Selemon and Goldman-Rakic 1988; Parvizi et al. 2006). Functionally, several studies have found evidence of functional connectivity between the hippocampus and dorsolateral, ventrolateral, and ventromedial portions of prefrontal cortex in the context of various memory tasks (Sperling et al. 2003; Rissman et al. 2008; Hannula and Ranganath 2009; van Kesteren et al. 2010). Studies of resting-state functional connectivity find correlated spontaneous fluctuations between the hippocampus and ventromedial, but not lateral, prefrontal cortex (Greicius et al. 2004; Vincent et al. 2006; Kahn et al. 2008). Altogether, these functional connectivity studies provide clear evidence for hippocampal–prefrontal connectivity, but suggest that the specific prefrontal regions involved may be task dependent. Our observation of a relationship between hippocampal volume and activity in the IFG during a WM task is consistent with the observation that functional connectivity between the hippocampus and lateral pre-frontal cortex is itself only observed in the presence of a task.
While anatomical tracer or diffusion tractography studies provide one possible substrate for interpreting observed long-distance structure–function relationships, it is important to note that relationships might be detected even in the absence of direct anatomical connections, due to the influence of other interconnected regions within a network. Further, even though functional connectivity is capable of detecting correlations between regions that are only indirectly connected (via polysynaptic connections) (Honey et al. 2009), it remains the case that the structural–functional correlations of this study represent a fundamentally different type of assessment of network relationships, and thus may identify relationships between brain regions that have not been seen in functional connectivity studies. In this context, we note that none of the aforementioned anatomical and functional studies provide evidence for a relationship between hippocampus and the dorsal anterior cingulate (i.e., dACC). However, the dorsal anterior cingulate is clearly part of a network of brain regions (including dorsal frontal and parietal cortices) involved in working memory and other cognitive control processes (Botvinick et al. 2001; Holroyd and Coles 2002; Dosenbach et al. 2006). As such, the correlation between hippocampal volume and dACC activity may reflect an influence of hippocampal volume on the network as a whole.
Specificity analyses
We showed a mixed degree of specificity to the WM task when comparing the correlations obtained initially using 2-back task activity to those for memory encoding and recognition task activity within the same regions. In all regions, the correlations were stronger for the WM task as compared with encoding. However, several of the correlations were as strong for the recognition task as for the WM task. This result is consistent with prior research showing that many of the same dorsal-frontal and parietal regions are involved in both WM and recognition functions that can both engage cognitive control processes (Buckner et al. 1999; Braver et al. 2001; Hutchinson et al. 2009; Uncapher and Wagner, 2009). Interestingly, the relationship between hippocampal volume and WM activity in the IFG showed evidence of specificity to the WM task relative to the recognition task, while the relationship between hippocampal volume and WM activity in the dACC did not exhibit a similar specificity but rather was common to both the 2-back and recognition tasks. This is noteworthy given the evidence in the literature (discussed above) of functional connectivity between the hippocampus and lateral prefrontal cortex in the context of various memory tasks, but not in the context of resting-state functional connectivity. Thus, the differential task specificity of the two clusters that were correlated with hippocampal volume may be reflective of a similar dichotomy in the functional connectivity literature.
Methodological issues
A key methodological challenge is in establishing the number and size of the structural and functional elements over which structural–functional relationships are computed. Using elements of small spatial size promotes spatial specificity, but then leads to a daunting multiple comparison problem if a whole brain approach is simultaneously implemented. In our study, to manage this issue, we chose to use a selected number of structural regions that broadly encompassed key regions involved with working memory. We also chose to preserve voxel-level values of the functional data, but limited the search space for correlations to voxels within an a priori working memory mask so as to limit the search space in a justifiable manner. We also note that we did not apply a correction for the multiple structural regions investigated, although the structure–function correlation between hippocampal volume and WM activity in the dACC survives a strict Bonferroni correction for multiple testing. Last, we did not find evidence in our “separate slopes” permutation analysis for locations with differential long-distance structure–function relationships between individuals with schizophrenia and controls, in contrast to the working memory structure–function study of Michael et al. (2011). This may in part reflect methodological differences, since the Michael et al. (2011) study was specifically tailored to identify the structural and functional clusters with the largest such group differences. Alternatively, while our study included a comparable number of total subjects (n = 154 vs. 200), there were only 32 individuals with schizophrenia. Thus, the absence of detectable schizophrenia versus control differences in the present study may in part reflect a sample size issue since differences in slopes between groups are expected to be subtle.
Conclusions
We found evidence for systems-level, long-distance structural–functional relationships involving the volume of the hippocampus and prefrontal cortex with the fMRI response to a working memory task. We propose that such cross-modal investigations of structural–functional relationships can compliment other approaches for studying the human connectome and may yield new insights into the network properties of the brain, particularly regarding the long-standing question of the manner in which brain structure influences function.
Supplementary Material
Acknowledgments
We thank the staff of the Administration/Assessment Core of the CCNMD at Washington University School of Medicine for collection of the clinical and imaging data. This work was supported by the National Institute of Mental Health [MH071616, MH056584] and the National Center for Research Resources [RR015241].
Footnotes
Maps of positive activation computed separately for the word versus fixation and face versus fixation versions of the 2-back task (assessed using permutation testing with all 154 subjects, without regard to group status) were substantially overlapping, with a union overlap (Jaccard coefficient) of 0.72 and a mean overlap (Dice coefficient) of 0.84. This high degree of overlap of regions activated by word- and face-specific working memory stimuli is consistent with meta-analyses of working memory, which find broadly similar activation patterns for different content material (e.g., verbal vs. nonverbal), albeit with support for some limited content-specific dissociations in activation (Wager and Smith 2003; Owen et al. 2005).
Notwithstanding these justifications for including all subjects in the main effects analysis, we conducted a supplementary analysis involving only the 93 healthy participants. In this analysis, the structure–function correlation between hippocampal volume and WM activity remained significant for the clusters in the left IFG and dorsal ACC (yielding clusters analogous to 4 and 5 in Fig. 1; P < 0.003 for the extent of both clusters based on permutation testing). However, no clusters analogous to clusters 1–3 of Fig. 1 survived when using just the 93 healthy participants.
Electronic supplementary material The online version of this article (doi:10.1007/s00429-012-0391-8) contains supplementary material, which is available to authorized users.
Contributor Information
Michael P. Harms, Email: mharms@conte.wustl.edu, Department of Psychiatry, Washington University School of Medicine, Box 8134, 660 S. Euclid Ave., St. Louis, MO 63110, USA
Lei Wang, Department of Psychiatry and Behavioral Sciences, Feinberg School of Medicine, Northwestern University, Chicago, IL, USA.
John G. Csernansky, Department of Psychiatry and Behavioral Sciences, Feinberg School of Medicine, Northwestern University, Chicago, IL, USA
Deanna M. Barch, Department of Psychiatry, Washington University School of Medicine, Box 8134, 660 S. Euclid Ave., St. Louis, MO 63110, USA. Department of Psychology, Washington University, St. Louis, MO, USA
References
- Anderson MJ, Robinson J. Permutation tests for linear models. Aust N Z J Stat. 2001;43:75–88. [Google Scholar]
- Andrews J, Wang L, Csernansky JG, Gado MH, Barch DM. Abnormalities of thalamic activation and cognition in schizophrenia. Am J Psychiatry. 2006;163:463–469. doi: 10.1176/appi.ajp.163.3.463. [DOI] [PubMed] [Google Scholar]
- Antonova E, Sharma T, Morris R, Kumari V. The relationship between brain structure and neurocognition in schizophrenia: a selective review. Schizophr Res. 2004;70:117–145. doi: 10.1016/j.schres.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Axmacher N, Mormann F, Fernandez G, Cohen MX, Elger CE, Fell J. Sustained neural activity patterns during working memory in the human medial temporal lobe. J Neurosci. 2007;27:7807–7816. doi: 10.1523/JNEUROSCI.0962-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas H, Blatt GJ. Topographically specific hippocampal projections target functionally distinct prefrontal areas in the rhesus monkey. Hippocampus. 1995;5:511–533. doi: 10.1002/hipo.450050604. [DOI] [PubMed] [Google Scholar]
- Bassett DS, Meyer-Lindenberg A, Achard S, Duke T, Bullmore E. Adaptive reconfiguration of fractal small-world human brain functional networks. Proc Natl Acad Sci USA. 2006;103:19518–19523. doi: 10.1073/pnas.0606005103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett DS, Bullmore E, Verchinski BA, Mattay VS, Weinberger DR, Meyer-Lindenberg A. Hierarchical organization of human cortical networks in health and schizophrenia. J Neurosci. 2008;28:9239–9248. doi: 10.1523/JNEUROSCI.1929-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp MH, Thompson DK, Howard K, Doyle LW, Egan GF, Inder TE, Anderson PJ. Preterm infant hippocampal volumes correlate with later working memory deficits. Brain. 2008;131:2986–2994. doi: 10.1093/brain/awn227. [DOI] [PubMed] [Google Scholar]
- Biswal BB, Mennes M, Zuo XN, Gohel S, Kelly C, Smith SM, Beckmann CF, Adelstein JS, Buckner RL, Colcombe S, Dogonowski AM, Ernst M, Fair D, Hampson M, Hoptman MJ, Hyde JS, Kiviniemi VJ, Kotter R, Li SJ, Lin CP, Lowe MJ, Mackay C, Madden DJ, Madsen KH, Margulies DS, Mayberg HS, McMahon K, Monk CS, Mostofsky SH, Nagel BJ, Pekar JJ, Peltier SJ, Petersen SE, Riedl V, Rombouts SA, Rypma B, Schlaggar BL, Schmidt S, Seidler RD, Siegle GJ, Sorg C, Teng GJ, Veijola J, Villringer A, Walter M, Wang L, Weng XC, Whitfield-Gabrieli S, Williamson P, Windischberger C, Zang YF, Zhang HY, Castellanos FX, Milham MP. Toward discovery science of human brain function. Proc Natl Acad Sci USA. 2010;107:4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner-Jackson A, Haut K, Csernansky JG, Barch DM. The influence of encoding strategy on episodic memory and cortical activity in schizophrenia. Biol Psychiatry. 2005;58:47–55. doi: 10.1016/j.biopsych.2005.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Brahmbhatt SB, Haut K, Csernansky JG, Barch DM. Neural correlates of verbal and nonverbal working memory deficits in individuals with schizophrenia and their high-risk siblings. Schizophr Res. 2006;87:191–204. doi: 10.1016/j.schres.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Braver TS, Barch DM, Kelley WM, Buckner RL, Cohen NJ, Miezin FM, Snyder AZ, Ollinger JM, Akbudak E, Conturo TE, Petersen SE. Direct comparison of prefrontal cortex regions engaged by working and long-term memory tasks. Neuroimage. 2001;14:48–59. doi: 10.1006/nimg.2001.0791. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Kelley WM, Petersen SE. Frontal cortex contributes to human memory formation. Nat Neurosci. 1999;2:311–314. doi: 10.1038/7221. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Head D, Parker J, Fotenos AF, Marcus D, Morris JC, Snyder AZ. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. Neuroimage. 2004;23:724–738. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Bullmore ET, Suckling J, Overmeyer S, Rabe-Hesketh S, Taylor E, Brammer MJ. Global, voxel, and cluster tests, by theory and permutation, for a difference between two groups of structural MR images of the brain. IEEE Trans Med Imaging. 1999;18:32–42. doi: 10.1109/42.750253. [DOI] [PubMed] [Google Scholar]
- Calabrese DR, Wang L, Harms MP, Ratnanather JT, Barch DM, Cloninger CR, Thompson PA, Miller MI, Csernansky JG. Cingulate gyrus neuroanatomy in schizophrenia subjects and their non-psychotic siblings. Schizophr Res. 2008;104:61–70. doi: 10.1016/j.schres.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Giuliani NR, Pekar JJ, Kiehl KA, Pearlson GD. Method for multimodal analysis of independent source differences in schizophrenia: combining gray matter structural and auditory oddball functional data. Hum Brain Mapp. 2006;27:47–62. doi: 10.1002/hbm.20166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callicott JH, Bertolino A, Mattay VS, Langheim FJ, Duyn J, Coppola R, Goldberg TE, Weinberger DR. Physiological dysfunction of the dorsolateral prefrontal cortex in schizophrenia revisited. Cereb Cortex. 2000;10:1078–1092. doi: 10.1093/cercor/10.11.1078. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. J Comp Neurol. 1995;363:615–641. doi: 10.1002/cne.903630408. [DOI] [PubMed] [Google Scholar]
- Correa NM, Li YO, Adali T, Calhoun VD. Canonical correlation analysis for feature-based fusion of biomedical imaging modalities and its application to detection of associative networks in schizophrenia. IEEE J Sel Top Signal Process. 2008;2:998–1007. doi: 10.1109/JSTSP.2008.2008265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csernansky JG, Schindler MK, Splinter NR, Wang L, Gado M, Selemon LD, Rastogi-Cruz D, Posener JA, Thompson PA, Miller MI. Abnormalities of thalamic volume and shape in schizophrenia. Am J Psychiatry. 2004;161:896–902. doi: 10.1176/appi.ajp.161.5.896. [DOI] [PubMed] [Google Scholar]
- DaSilva AF, Becerra L, Pendse G, Chizh B, Tully S, Borsook D. Colocalized structural and functional changes in the cortex of patients with trigeminal neuropathic pain. PLoS One. 2008;3:e3396. doi: 10.1371/journal.pone.0003396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, Burgund ED, Grimes AL, Schlaggar BL, Petersen SE. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, Fox MD, Snyder AZ, Vincent JL, Raichle ME, Schlaggar BL, Petersen SE. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci USA. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman D, Lane D. A nonstochastic interpretation of reported significance levels. J Bus Econ Stat. 1983;1:292–298. [Google Scholar]
- Goldman-Rakic PS, Selemon LD, Schwartz ML. Dual pathways connecting the dorsolateral prefrontal cortex with the hippocampal formation and parahippocampal cortex in the rhesus monkey. Neuroscience. 1984;12:719–743. doi: 10.1016/0306-4522(84)90166-0. [DOI] [PubMed] [Google Scholar]
- Gong G, He Y, Concha L, Lebel C, Gross DW, Evans AC, Beaulieu C. Mapping anatomical connectivity patterns of human cerebral cortex using in vivo diffusion tensor imaging tractography. Cereb Cortex. 2009;19:524–536. doi: 10.1093/cercor/bhn102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci USA. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackert VH, den Heijer T, Oudkerk M, Koudstaal PJ, Hofman A, Breteler MM. Hippocampal head size associated with verbal memory performance in nondemented elderly. Neuroimage. 2002;17:1365–1372. doi: 10.1006/nimg.2002.1248. [DOI] [PubMed] [Google Scholar]
- Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey CJ, Wedeen VJ, Sporns O. Mapping the structural core of human cerebral cortex. PLoS Biol. 2008;6:e159. doi: 10.1371/journal.pbio.0060159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller JW, Banerjee A, Christensen GE, Gado M, Joshi S, Miller MI, Sheline Y, Vannier MW, Csernansky JG. Three-dimensional hippocampal MR morphometry with high-dimensional transformation of a neuroanatomic atlas. Radiology. 1997;202:504–510. doi: 10.1148/radiology.202.2.9015081. [DOI] [PubMed] [Google Scholar]
- Hannula DE, Ranganath C. Medial temporal lobe activity predicts successful relational memory binding. J Neurosci. 2008;28:116–124. doi: 10.1523/JNEUROSCI.3086-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannula DE, Ranganath C. The eyes have it: hippocampal activity predicts expression of memory in eye movements. Neuron. 2009;63:592–599. doi: 10.1016/j.neuron.2009.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms MP, Wang L, Campanella C, Aldridge K, Moffitt AJ, Kuelper J, Ratnanather JT, Miller MI, Barch DM, Csernansky JG. Structural abnormalities in gyri of the prefrontal cortex in individuals with schizophrenia and their unaffected siblings. Br J Psychiatry. 2010;196:150–157. doi: 10.1192/bjp.bp.109.067314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms MP, Wang L, Mamah D, Barch DM, Thompson PA, Csernansky JG. Thalamic shape abnormalities in individuals with schizophrenia and their nonpsychotic siblings. J Neurosci. 2007;27:13835–13842. doi: 10.1523/JNEUROSCI.2571-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Chen ZJ, Evans AC. Small-world anatomical networks in the human brain revealed by cortical thickness from MRI. Cereb Cortex. 2007;17:2407–2419. doi: 10.1093/cercor/bhl149. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Coles MG. The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychol Rev. 2002;109:679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Honey CJ, Sporns O, Cammoun L, Gigandet X, Thiran JP, Meuli R, Hagmann P. Predicting human resting-state functional connectivity from structural connectivity. Proc Natl Acad Sci USA. 2009;106:2035–2040. doi: 10.1073/pnas.0811168106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson JB, Uncapher MR, Wagner AD. Posterior parietal cortex and episodic retrieval: convergent and divergent effects of attention and memory. Learn Mem. 2009;16:343–356. doi: 10.1101/lm.919109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen-Berg H, Rushworth MF. Using diffusion imaging to study human connectional anatomy. Annu Rev Neurosci. 2009;32:75–94. doi: 10.1146/annurev.neuro.051508.135735. [DOI] [PubMed] [Google Scholar]
- Kahn I, Andrews-Hanna JR, Vincent JL, Snyder AZ, Buckner RL. Distinct cortical anatomy linked to subregions of the medial temporal lobe revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100:129–139. doi: 10.1152/jn.00077.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsgodt KH, Shirinyan D, van Erp TG, Cohen MS, Cannon TD. Hippocampal activations during encoding and retrieval in a verbal working memory paradigm. Neuroimage. 2005;25:1224–1231. doi: 10.1016/j.neuroimage.2005.01.038. [DOI] [PubMed] [Google Scholar]
- Kenward MG, Roger JH. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics. 1997;53:983–997. [PubMed] [Google Scholar]
- Kim MA, Tura E, Potkin SG, Fallon JH, Manoach DS, Calhoun VD, Turner JA. Working memory circuitry in schizophrenia shows widespread cortical inefficiency and compensation. Schizophr Res. 2010;117:42–51. doi: 10.1016/j.schres.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerch JP, Worsley K, Shaw WP, Greenstein DK, Lenroot RK, Giedd J, Evans AC. Mapping anatomical correlations across cerebral cortex (MACACC) using cortical thickness from MRI. Neuroimage. 2006;31:993–1003. doi: 10.1016/j.neuroimage.2006.01.042. [DOI] [PubMed] [Google Scholar]
- Mamah D, Harms MP, Wang L, Barch D, Thompson P, Kim J, Miller MI, Csernansky JG. Basal ganglia shape abnormalities in the unaffected siblings of schizophrenia patients. Biol Psychiatry. 2008;64:111–120. doi: 10.1016/j.biopsych.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechelli A, Friston KJ, Frackowiak RS, Price CJ. Structural covariance in the human cortex. J Neurosci. 2005;25:8303–8310. doi: 10.1523/JNEUROSCI.0357-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg AS, Olsen RK, Kohn PD, Brown T, Egan MF, Weinberger DR, Berman KF. Regionally specific disturbance of dorsolateral prefrontal–hippocampal functional connectivity in schizophrenia. Arch Gen Psychiatry. 2005;62:379–386. doi: 10.1001/archpsyc.62.4.379. [DOI] [PubMed] [Google Scholar]
- Michael AM, Baum SA, White T, Demirci O, Andreasen NC, Segall JM, Jung RE, Pearlson G, Clark VP, Gollub RL, Schulz SC, Roffman JL, Lim KO, Ho BC, Bockholt HJ, Calhoun VD. Does function follow form?: methods to fuse structural and functional brain images show decreased linkage in schizophrenia. Neuroimage. 2010;49:2626–2637. doi: 10.1016/j.neuroimage.2009.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael AM, King MD, Ehrlich S, Pearlson G, White T, Holt DJ, Andreasen NC, Sakoglu U, Ho BC, Schulz SC, Calhoun VD. A data-driven investigation of gray matter–function correlations in schizophrenia during a working memory task. Front Hum Neurosci. 2011;5:71. doi: 10.3389/fnhum.2011.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Miller MI, Hosakere M, Barker AR, Priebe CE, Lee N, Ratnanather JT, Wang L, Gado M, Morris JC, Csernansky JG. Labeled cortical mantle distance maps of the cingulate quantify differences between dementia of the Alzheimer type and healthy aging. Proc Natl Acad Sci USA. 2003;100:15172–15177. doi: 10.1073/pnas.2136624100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitelman SA, Buchsbaum MS, Brickman AM, Shihabuddin L. Cortical intercorrelations of frontal area volumes in schizophrenia. Neuroimage. 2005;27:753–770. doi: 10.1016/j.neuroimage.2005.05.024. [DOI] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols EA, Kao YC, Verfaellie M, Gabrieli JD. Working memory and long-term memory for faces: evidence from fMRI and global amnesia for involvement of the medial temporal lobes. Hippocampus. 2006;16:604–616. doi: 10.1002/hipo.20190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes TR, Fox AS, Johnstone T, Chung MK, Kalin N, Davidson RJ. Integrating VBM into the general linear model with voxelwise anatomical covariates. Neuroimage. 2007;34:500–508. doi: 10.1016/j.neuroimage.2006.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvizi J, Van Hoesen GW, Buckwalter J, Damasio A. Neural connections of the posteromedial cortex in the macaque. Proc Natl Acad Sci USA. 2006;103:1563–1568. doi: 10.1073/pnas.0507729103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragland JD, Yoon J, Minzenberg MJ, Carter CS. Neuroimag-ing of cognitive disability in schizophrenia: search for a pathophysiological mechanism. Int Rev Psychiatry. 2007;19:417–427. doi: 10.1080/09540260701486365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C, Blumenfeld RS. Doubts about double dissociations between short- and long-term memory. Trends Cogn Sci. 2005;9:374–380. doi: 10.1016/j.tics.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Ranganath C, D’Esposito M. Medial temporal lobe activity associated with active maintenance of novel information. Neuron. 2001;31:865–873. doi: 10.1016/s0896-6273(01)00411-1. [DOI] [PubMed] [Google Scholar]
- Repovs G, Csernansky JG, Barch DM. Brain network connectivity in individuals with schizophrenia and their siblings. Biol Psychiatry. 2011;69:967–973. doi: 10.1016/j.biopsych.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissman J, Gazzaley A, D’Esposito M. Dynamic adjustments in prefrontal, hippocampal, and inferior temporal interactions with increasing visual working memory load. Cereb Cortex. 2008;18:1618–1629. doi: 10.1093/cercor/bhm195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosene DL, Van Hoesen GW. Hippocampal efferents reach widespread areas of cerebral cortex and amygdala in the rhesus monkey. Science. 1977;198:315–317. doi: 10.1126/science.410102. [DOI] [PubMed] [Google Scholar]
- Schlosser RG, Nenadic I, Wagner G, Gullmar D, von Consbruch K, Kohler S, Schultz CC, Koch K, Fitzek C, Matthews PM, Reichenbach JR, Sauer H. White matter abnormalities and brain activation in schizophrenia: a combined DTI and fMRI study. Schizophr Res. 2007;89:1–11. doi: 10.1016/j.schres.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Goldman-Rakic PS. Common cortical and subcortical targets of the dorsolateral prefrontal and posterior parietal cortices in the rhesus monkey: evidence for a distributed neural network subserving spatially guided behavior. J Neurosci. 1988;8:4049–4068. doi: 10.1523/JNEUROSCI.08-11-04049.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle GJ, Konecky RO, Thase ME, Carter CS. Relationships between amygdala volume and activity during emotional information processing tasks in depressed and never-depressed individuals: an fMRI investigation. Ann N Y Acad Sci. 2003;985:481–484. doi: 10.1111/j.1749-6632.2003.tb07105.x. [DOI] [PubMed] [Google Scholar]
- Siok WT, Niu Z, Jin Z, Perfetti CA, Tan LH. A structural-functional basis for dyslexia in the cortex of Chinese readers. Proc Natl Acad Sci USA. 2008;105:5561–5566. doi: 10.1073/pnas.0801750105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skudlarski P, Jagannathan K, Anderson K, Stevens MC, Calhoun VD, Skudlarska BA, Pearlson G. Brain connectivity is not only lower but different in schizophrenia: a combined anatomical and functional approach. Biol Psychiatry. 2010;68:61–69. doi: 10.1016/j.biopsych.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Watkins KE, Toro R, Laird AR, Beckmann CF. Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci USA. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling R, Chua E, Cocchiarella A, Rand-Giovannetti E, Poldrack R, Schacter DL, Albert M. Putting names to faces: successful encoding of associative memories activates the anterior hippocampal formation. Neuroimage. 2003;20:1400–1410. doi: 10.1016/S1053-8119(03)00391-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- Toro R, Fox PT, Paus T. Functional coactivation map of the human brain. Cereb Cortex. 2008;18:2553–2559. doi: 10.1093/cercor/bhn014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uncapher MR, Wagner AD. Posterior parietal cortex and episodic encoding: insights from fMRI subsequent memory effects and dual-attention theory. Neurobiol Learn Mem. 2009;91:139–154. doi: 10.1016/j.nlm.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC. A population-average, landmark- and surface-based (PALS) atlas of human cerebral cortex. Neuroimage. 2005;28:635–662. doi: 10.1016/j.neuroimage.2005.06.058. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Dierker D. On navigating the human cerebral cortex: response to ‘in praise of tedious anatomy’. Neuroimage. 2007;37:1050–1054. doi: 10.1016/j.neuroimage.2007.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kesteren MT, Fernandez G, Norris DG, Hermans EJ. Persistent schema-dependent hippocampal–neocortical connectivity during memory encoding and postencoding rest in humans. Proc Natl Acad Sci USA. 2010;107:7550–7555. doi: 10.1073/pnas.0914892107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Snellenberg JX, Torres IJ, Thornton AE. Functional neuroimaging of working memory in schizophrenia: task performance as a moderating variable. Neuropsychology. 2006;20:497–510. doi: 10.1037/0894-4105.20.5.497. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Snyder AZ, Fox MD, Shannon BJ, Andrews JR, Raichle ME, Buckner RL. Coherent spontaneous activity identifies a hippocampal–parietal memory network. J Neurophysiol. 2006;96:3517–3531. doi: 10.1152/jn.00048.2006. [DOI] [PubMed] [Google Scholar]
- Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis. Cogn Affect Behav Neurosci. 2003;3:255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- Wan RQ, Pang K, Olton DS. Hippocampal and amygdaloid involvement in nonspatial and spatial working memory in rats: effects of delay and interference. Behav Neurosci. 1994;108:866–882. doi: 10.1037//0735-7044.108.5.866. [DOI] [PubMed] [Google Scholar]
- Wang X, Garfinkel SN, King AP, Angstadt M, Dennis MJ, Xie H, Welsh RC, Tamburrino MB, Liberzon I. A multiple-plane approach to measure the structural properties of functionally active regions in the human cortex. Neuroimage. 2010;49:3075–3085. doi: 10.1016/j.neuroimage.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW, Shenton ME, Green AI, Nieto-Castanon A, LaViolette P, Wojcik J, Gabrieli JD, Seidman LJ. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci USA. 2009;106:1279–1284. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Groth KM, Pearlson G, Schretlen DJ, Calhoun VD. Source-based morphometry: the use of independent component analysis to identify gray matter differences with application to schizophrenia. Hum Brain Mapp. 2009;30:711–724. doi: 10.1002/hbm.20540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Raichle ME. Disease and the brains dark energy. Nat Rev Neurol. 2010;6:15–28. doi: 10.1038/nrneurol.2009.198. [DOI] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire LR. Memory impairment in monkeys following lesions limited to the hippocampus. Behav Neurosci. 1986;100:155–160. doi: 10.1037//0735-7044.100.2.155. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.