Abstract
A novel approach for the design of responsive paramagnetic chemical exchange saturation transfer (PARACEST) magnetic resonance imaging (MRI) agents has been developed where the signal is “turned on” by altering the longitudinal relaxation time (T1) of bulk water protons. To demonstrate this approach, a model Eu(DOTA-tetraamide) complex (DOTA = 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid) containing two nitroxide free radical units was synthesized. The nitroxide groups substantially shortened the T1 of the bulk water protons which, in turn, resulted in quenching of the CEST signal. Reduction of paramagnetic nitroxide moieties to a diamagnetic species resulted in the appearance of CEST. The modulation of CEST by T1 relaxation provides a new platform for designing biologically responsive MRI agents.
Magnetic resonance imaging (MRI) is a powerful non-invasive clinical diagnostic tool for anatomical imaging. MR image contrast originates from differences in tissue water and fat content plus inherent differences in tissue water relaxation rates. Any process that alters the net magnetization of the water protons will alter image contrast. Conventional MRI contrast agents (Gd3+ and Mn2+ complexes) work by shortening the longitudinal and transversal relaxation times (T1 and T2) of water protons. A conceptually different class of MRI contrast agents is based on chemical exchange saturation transfer, a process in which net spin magnetization is decreased by transfer of saturated 1H spins from one proton pool into another, typically tissue water. A strict requirement for CEST contrast is that the exchange rate between the two pools (kex) must be slower than the chemical shift difference between the two pools (Δω). Certain paramagnetic lanthanide (Ln3+) complexes in which the central metal ion is coordinated by an octadentate DOTA-tetra(amide) ligand (DOTA = 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid, Figure 1) and a single inner sphere water molecule display large chemical shift difference (Δω) between the two exchanging pools (bound and bulk water) and sufficiently slow water exchange rate to satisfy the CEST requirement, kex < Δω These systems offer the advantage of saturating the bound water pool without affecting the bulk water pool.1–3 The CEST signal is commonly represented as a percent decrease in total bulk water intensity and, assuming complete saturation of the bound water signal, the net magnetization of water protons at steady-state (Mz/M0) is given by:1, 2
Figure 1.
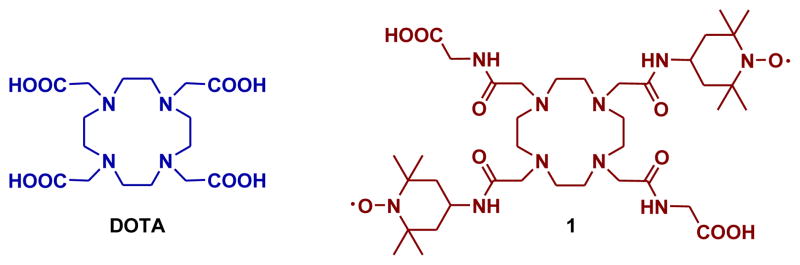
Structure of DOTA and ligand 1 discussed in this work.
| (1) |
where c is the concentration of CEST agent, q, the number of exchanging protons, 55.5 represents the molar concentration of bulk water, T1, is the longitudinal (spin lattice) relaxation time of bulk water, and τM, is the exchange lifetime of the exchanging proton (1/τM = kex). It is relatively easy to design responsive PARACEST agents for MRI because one can incorporate a variety of exchanging species (-NH, -OH groups) that vary in both chemical shift and exchange kinetics. Furthermore, for those PARACEST agents containing a slowly exchanging water molecule, the lifetime of the bound water molecule has been found to be quite sensitive to electronic effects of the ligand pendant arms and alterations in the coordination geometry.4–7 However, as shown by equation 1, Mz/M0 also depends on longitudinal relaxation time (T1) of bulk water protons, a factor not yet considered in the design of responsive PARACEST agents.
The goal of the present work was to demonstrate that the CEST signal can be modulated by changes in the longitudinal relaxation time (T1) of bulk water protons. As a continuation of our work on redox sensitive MRI agents,7 we have chosen to incorporate redox-sensitive nitroxide free radicals into the side arms of a DOTA-tetra(amide) ligand (Figure 1).
Nitroxides are stable (persistent) organic free radicals capable of shortening the T1 relaxation time of the bulk water protons due to the paramagnetic effect of the single unpaired electrons. Nitroxides are not terribly efficient as T1-based contrast agents mainly because, unlike Gd3+, a nitroxide has only one unpaired electron.8 They do however, serve as useful in vivo electron spin resonance (ESR) probes of tissue redox chemistry because they can be readily oxidized or reduced to diamagnetic derivatives.9 In vivo deactivation of nitroxides occurs primarily by a one electron reduction to the corresponding hydroxylamine with loss of the ESR signal. Since the rates of these transformations are dependent on tissue redox state, monitoring changes in the ESR signal can be used to map tissue redox status.10,11 In particular, increased reduction rate of nitroxide radicals has been demonstrated under hypoxic conditions such as in tumors or in ischemic heart disease.12 Thus, the rationale for incorporating nitroxide radicals into the chemical structure of a PARACEST agent was that the presence of free electrons would shorten the T1 of nearby water protons and hence quench the CEST signal while conversion of the paramagnetic nitroxide to a diamagnetic derivative by either a chemical or biological means would lengthen the T1 of bulk water protons to a more normal value (approximately 2.6 s) and thereby restore the CEST signal.
The free ligand 1,4,7,10-tetraazacyclododecane-1,7-bis[acetic acid(glycinetert-butylester)amide -4,10-bis[acetic acid - (2′,2′,6′,6,′-tetramethyl-1′-oxyl-4′-piperidyl) amide] (1) was synthesized by alkylating 1,4,7,10-tetraazacyclododecane-1,7-bis[acetic acid (glycine-tert-butyl ester) amide]13 with 4-(2-chloroacetamido)-2,2,6,6-tetramethylpiperidine-1-oxyl radical in the presence of potassium carbonate followed by the acid hydrolysis of the tert-butyl ester groups (Scheme 1). The Eu3+ complex was synthesized by reaction of 1 with EuCl3 in water at pH 6.5 at room temperature. The detailed synthetic procedure is provided in the supporting information.
Scheme 1.
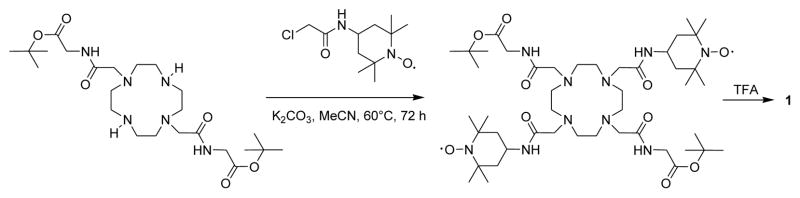
Synthesis of ligand 1 used in this study.
The 1H NMR spectrum of Eu(1) in D2O shows two highly downfield shifted resonances at + 25.3 and + 24.8 ppm, characteristic of H4 axial protons of the macrocyclic ring confirming that the complex exists largely as a square antiprismatic (SAP) isomer in solution (Figure S1). This was not unexpected as most EuDO-TA-tetra(amide) complexes exist in solution predominantly as SAP coordination isomers. The preference of these complexes for SAP coordination geometry over the closely related twisted square antiprismatic (TSAP) isomer has profound implications for CEST; only the SAP isomers have sufficiently slow inner sphere water exchange rate to satisfy the slow to intermediate condition for CEST. The CEST spectrum of Eu(1) showed a distinct peak for the exchanging Eu3+-bound water molecule at + 51 ppm, but the magnitude of this signal was much lower (Figure 2a, blue line) than normally observed for similar Eu3+ complexes at this same concentration.1 Fitting the experimental CEST data collected at different B1 values to the modified Bloch equations (Figure S2, Table S1) gave an estimated water exchange lifetime of 85 μs, typical of many other EuDOTA-tetra(amide) complexes that exist as SAP isomers.1,2 The spin lattice relaxation time (T1) of bulk water protons in the presence of 10 mM Eu(1) at pH 7, 25°C, 9.4T was 0.2 s as measured using standard inversion recovery methods. This short relaxation time (T1) caused by the paramagnetic effect of the unpaired electrons of nitroxide groups in the ligand scaffold is in agreement with the reported r1 relaxivity of nitroxide radicals.14 Since the water exchange rate of the inner sphere water is slow enough for CEST, the weak CEST signal (blue line) must be due to a reduction in the T1 of bulk water. Upon addition of two equivalent of L-ascorbic acid to a solution of Eu(1) while maintaining the pH at 7, the T1 of bulk water protons increased from 0.2 s to 2.6 s. At the same time, the CEST signal also increased significantly from ~2% to ~20 % as shown by both the CEST spectra (Figure 2a) and the CEST images (Figure 2b). The water exchange rate, τM for the Eu(1) complex in the presence of L-ascorbic acid was found to be 81 μs, as estimated from a fit of the experimental CEST data collected at different B1 values to the Bloch equations (Figure S3 and Table S2).2 Thus, reduction of the nitroxide moieties does not alter the water exchange rate of the complex significantly so this is not the origin of the large increase in CEST. Formation of the hydroxylamine derivative of Eu(1) by addition of L-ascorbic acid was verified by ESI MS where a two mass unit increase was observed for the reduced form of Eu(1) (Figure S4).
Figure 2.
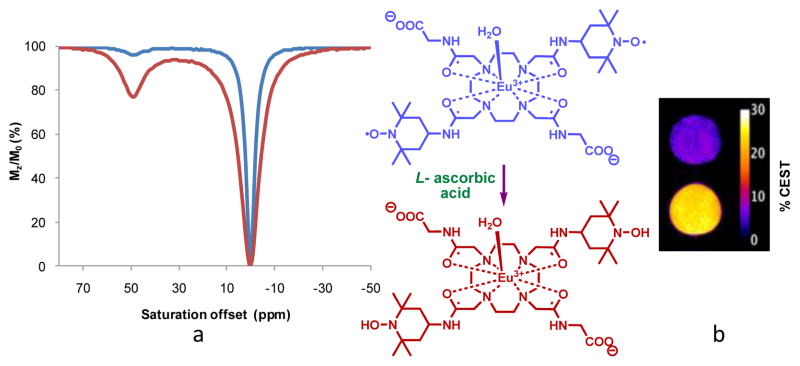
a) CEST spectra of a 10 mM aqueous solution of Eu(1) recorded at 9.4 T, 298 K, pH 7, B1=10 μT, and irradiation time of 5 s before (blue) and after (red) reduction of the complex with L-ascorbic acid. b) The inverted difference CEST images of phantoms containing 10 mM Eu(1) before (blue) and after (red) reduction. The image shown was created by acquiring spin echo CEST images (9.4 T, 298 K, TR =10 s, irradiation time = 5 s, B1 = 10 μT) at −51 ppm (off resonance) and at + 51 ppm (on resonance) and subtracting the on resonance image from the off resonance image.1
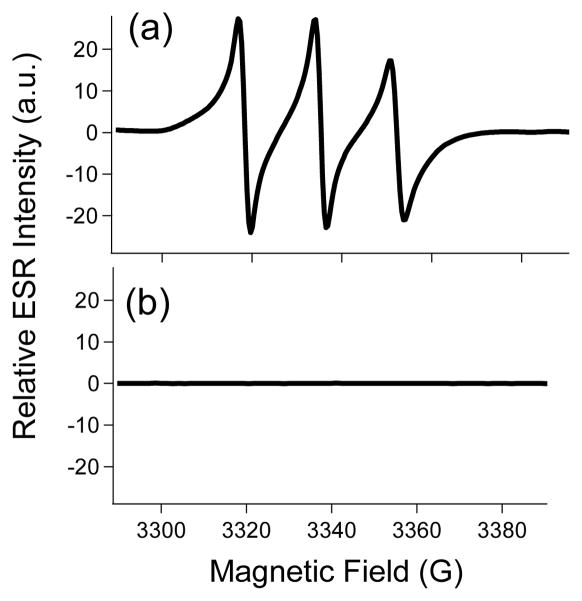
Quenching of the nitroxide radical by L-ascorbate was also confirmed by ESR measurements. As expected, the X-band ESR spectrum of Eu(1) in water consisted of a triplet arising due to the hyperfine coupling of the electron spins of the free radical to the adjacent 14N nuclear spins (Figure S5, red curve). The appearance of a single triplet shows that there are no strong spin exchange and dipolar interactions between the two nitroxide free radicals in the ligand framework. The characteristic signal of the nitroxide radical completely disappeared upon addition of excess L-ascorbic acid. Ex vivo ESR experiments were also performed to study the in vivo fate of the nitroxide radicals present in the ligand framework. Urine samples collected from the bladder of mice with and without the injection of L-ascorbate, as expected, showed the presence and absence of free radicals, respectively (Figure 3). The concentration of the agent in the urine was found to be around 10 mM as determined by ICP-OES. These data demonstrate that the agent accumulates in the bladder in its unreduced form in reasonably high concentration (10 mM) and is completely reduced after injection of L-ascorbate.
Figure 3.
X-band ESR spectra of urine samples collected from the bladder of a) mice injected with Eu(1) (0.1 mmol/kg dose); b) of mice injected with Eu(1) (0.1 mmol/kg dose followed by an injection of L-ascorbic acid (0.1 mmol/kg dose) after a 20 minute delay.
CEST imaging in vivo is often highly compromised by interference from the broad, solids-like magnetization transfer (MT) background signal of tissues that arise from dipolar interactions between water and other small molecules with large biomolecules and membranes.15,16 This is especially problematical for diaCEST agents that have exchangeable proton resonances within a few ppm of the tissue water signal. Although this is less of a problem for PARACEST complexes such as Eu(1) because the water exchange peak in this system is shifted ~50 ppm downfield of water, the MT signal does still interfere because it can be quite intense over a broad range of frequencies, typically ±100 ppm. To avoid this interference problem in testing the potential of Eu(1) to act as a redox sensor, the bladder was chosen because its content is mostly water and other small molecules with no interfering MT signal. Furthermore, Eu(1) (both the oxidized and reduced forms) is a low molecular weight, hydrophilic charged complex that is expected to be rapidly filtered by the kidneys to accumulate in the bladder. Hence, CEST imaging of the bladder should report whether or not the complex was reduced by biological processes as it circulates in the body and eliminates by renal filtration.
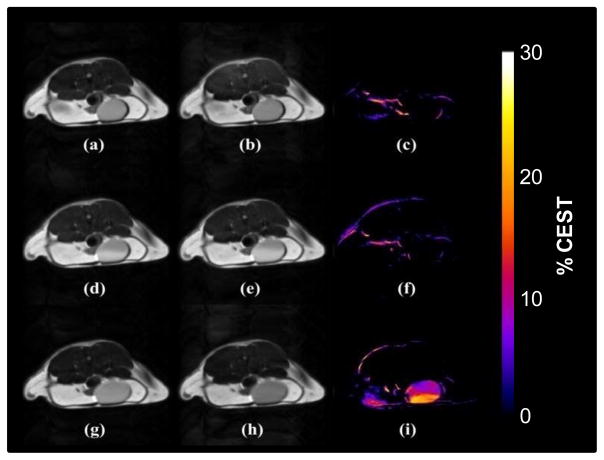
As shown in the CEST images in Figure 4, no CEST signal was detected in the bladder of a mouse prior to injection of the agent (Fig. 4c). The CEST image of this same mouse collected 10 min after injection of Eu(1) also showed no CEST signal (Fig. 4f) even though the slightly brighter images (due to a shortening of water T1) provided evidence for the presence of the agent (oxidized form) in the bladder (Figure S6). Subsequent IV administration of L-ascorbic acid resulted in excretion of this reducing agent into the bladder followed by chemical reduction of the nitroxide species to the diamagnetic CEST-active form of Eu(1). This resulted in strong CEST contrast of the bladder contents (Fig. 4i). Interestingly, a concentration gradient can be seen as the L-ascorbic acid diffused into the bladder and reacted with the complex. The lack of CEST signal in Fig 4(f) indicates that in a healthy animal, Eu(1) is eliminated from the blood basically unchanged, i.e., no significant amount of agent was reduced by biological processes during the short period of time the agent circulated in the animal. However, based on the in vivo fate of nitroxide spin probes, one can anticipate that under certain physiological conditions (e. g. in hypoxic tissues) the transformation of Eu(1) to a diamagnetic derivative would be much faster and a significant portion of the injected complex would be converted to a CEST active species. Thus, we envision applications of Eu(1) or other similar PARACEST reporters as MRI agents for hypoxic or ischemic regions or simply as a quick test of whole-body redox status. In addition, it is worth noting that nitroxides can act as scavengers for reactive radical species such as hydroxide radical or superoxide anion, and thus, other potential applications may involve the detection of these reactive species in vivo.17
Figure 4.
CEST images of the bladder of a female black-6 mouse. Top row: pre-injection spin echo images with saturation at (a) −43 ppm and (b) +43 ppm. Note that the CEST peak shifts from +51 ppm at 25°C to +43 ppm at 37°C. The CEST difference image (c) was obtained by subtracting on-resonance image (b) from the off resonance image (a). Middle row: similar off resonance (d), on resonance (e) and difference (f) image taken 10 minutes after injection of Eu(1) (0.1 mmol/kg dose in 100 μL) into the same mouse via tail vein catheter. Bottom row: off resonance (g), on resonance (h) and difference (i) image taken one hour after an intravenous dose of L-ascorbic acid (0.1 mmol/kg, 100 μL). Some motion and possible susceptibility artifacts are also visible at the fat/muscle boarder regions in the CEST images (c, f, i). The animal experiments were performed according to a protocol approved by Institutional Review Board of the University of Texas Southwestern Medical Center (APN 2010-0245).
In conclusion, we have designed and synthesized a PARACEST agent with two nitroxide moieties incorporated into a EuDOTA-tetra(amide) complex. In vitro MR spectroscopy and imaging experiments demonstrated that the CEST signal originating from a metal ion-bound water molecule can be modulated by T1 relaxation of water protons which, in turn, is controlled by the redox state of the nitroxide groups. Eu(1) has a very weak CEST signal alone due to T1 relaxation of water by the nitroxide unpaired electrons. However, reduction of Eu(1) to the diamagnetic bis(hydroxylamine) derivative restores the T1 relaxation of the bulk water to the range expected for a Eu3+ complex of a diamagnetic ligand (around 2.6 s) and activates transfer of saturated water proton spins between the Eu3+-bound water molecule and bulk water. Proof of concept CEST imaging experiments in vivo demonstrated that Eu(1) can be activated by reduction of the nitroxide groups with L-ascorbic in the bladder. These results imply that modulation of a PARACEST signal by T1 relaxation of water is a viable approach for the design of responsive imaging agents. Since nitroxide radicals can be deactivated under certain biological conditions,12 this preliminary work suggests that LnDOTA-tetra(amide nitroxide) derivatives such as this may serve as redox-sensitive PARACEST probes that respond to changes in tissue redox activity and report the redox status of tissues and cells in vivo.
Supplementary Material
Acknowledgments
The authors acknowledge partial financial support for this work from the National Institutes of Health (CA-115531, EB-02584, and EB-00482), Robert A. Welch Foundation (AT-584) and the Cancer Prevention Research Institute of Texas (CPRIT, RP100146).
Footnotes
Notes
The authors declare no competing financial interest.
Synthetic procedures, NMR, CEST and ESR spectroscopy and CEST imaging protocols and data. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Viswanathan S, Kovacs Z, Green KN, Ratnakar SJ, Sherry AD. Chem Rev. 2010;110:2960– 3018. doi: 10.1021/cr900284a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woessner DE, Zhang S, Merritt ME, Sherry AD. Magn reson Med. 2005;53:790–799. doi: 10.1002/mrm.20408. [DOI] [PubMed] [Google Scholar]
- 3.Zhang S, Winter P, Wu K, Sherry AD. J Am Chem Soc. 2001;123:1517– 1518. doi: 10.1021/ja005820q. [DOI] [PubMed] [Google Scholar]
- 4.Ratnakar SJ, Woods M, Lubag AJM, Kovacs Z, Sherry AD. J Am Chem Soc. 2008;130:6–7. doi: 10.1021/ja076325y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mani T, Tircso G, Togao O, Zhao P, Soesbe TC, Takahashi M, Sherry AD. Contrast Media Mol Imaging. 2009;4:183– 191. doi: 10.1002/cmmi.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Viswanathan S, Ratnakar SJ, Green KN, Kovacs Z, De Leon-Rodriguez LM, Sherry AD. Angew Chem, Int Ed. 2009;48:9330– 9333. doi: 10.1002/anie.200904649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ratnakar SJ, Viswanathan S, Kovacs Z, Jindal AK, Green KN, Sherry AD. J Am Chem Soc. 2012;134:5798– 5800. doi: 10.1021/ja211601k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vallet P, Vanhaverbeke Y, Bonnet PA, Subra G, Chapat JP, Muller RN. Magn Reson Med. 1994;32:11. doi: 10.1002/mrm.1910320103. [DOI] [PubMed] [Google Scholar]
- 9.Hyodo F, Matsumoto KI, Matsumoto A, Mitchell JB, Krishna MC. Cancer Res. 2006;66:9921– 9928. doi: 10.1158/0008-5472.CAN-06-0879. [DOI] [PubMed] [Google Scholar]
- 10.Hyodo F, Murugesan R, Matsumoto KI, Hyodo E, Subramanian S, Mitchell JB, Krishna MC. J Magn Reson. 2008;190:105– 112. doi: 10.1016/j.jmr.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis RM, Mitchell JB, Krishna MC. Anti-Cancer Agents Med Chem. 2011;11:347– 358. doi: 10.2174/187152011795677526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khramtsov VV. In: Nitroxides-Theory, Experiment and Applications; Kokorin AI, editor. InTech; Rijeka: 2012. [Google Scholar]
- 13.Green KN, Viswanathan S, Rojas-Quijano FA, Kovacs Z, Sherry AD. Inorg Chem. 2011;50:1648– 1655. doi: 10.1021/ic101856d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keana JF, Pou S, Rosen GM. Magn Reson Med. 1987;5:525– 536. doi: 10.1002/mrm.1910050603. [DOI] [PubMed] [Google Scholar]
- 15.Ward KM, Aletras AH, Balaban RS. J Magn Reson. 2000;143:79– 87. doi: 10.1006/jmre.1999.1956. [DOI] [PubMed] [Google Scholar]
- 16.Li AX, Suchy M, Li C, Gati JS, Meakin S, Hudson RHE, Menon RS, Bartha R. Magn Reson Med. 2011;66:67– 72. doi: 10.1002/mrm.22772. [DOI] [PubMed] [Google Scholar]
- 17.Soule BP, Hyodo F, Matsumoto K, Simone NL, Cook JA, Krishna MC, Mitchell JB. Free Radic Biol Med. 2007;42:1632–1650. doi: 10.1016/j.freeradbiomed.2007.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.