Abstract
Aims
The aim of this study was to assess the association between resting heart rate (HR), chronotropic index (CI), and clinical outcomes in optimally treated chronic heart failure (HF) patients on β-blocker therapy.
Methods and results
We performed a sub-study in 1118 patients with HF and reduced ejection fraction (EF < 35%) included in the HF-ACTION trial. Patients in sinus rhythm who received a β-blocker and who performed with maximal effort on the exercise test were included. Chronotropic index was calculated as an index of HR reserve achieved, by using the equation (220-age) for estimating maximum HR. A sensitivity analysis using an equation developed for HF patients on β-blockers was also performed. Cox proportional hazards models were fit to assess the association between CI and clinical outcomes. Median (25th, 75th percentiles) follow-up was 32 (21, 44) months. In a multivariable model including resting HR and CI as continuous variables, neither was associated with the primary outcome of all-cause mortality or hospitalization. However, each 0.1 unit decrease in CI <0.6 was associated with 17% increased risk of all-cause mortality (hazard ratio 1.17, 95% confidence interval 1.01–1.36; P = 0.036), and 13% increased risk of cardiovascular mortality or HF hospitalization (hazard ratio 1.13, 1.02–1.26; P = 0.025). Overall, 666 of 1118 (60%) patients had a CI <0.6. Chronotropic index did not retain statistical significance when dichotomized at a value of ≤0.62.
Conclusion
In HF patients receiving optimal medical therapy, a decrease in CI <0.6 was associated with adverse clinical outcomes. Obtaining an optimal HR response to exercise, even in patients receiving optimal β-blocker therapy, may be a therapeutic target in the HF population.
Keywords: Heart rate, Heart rate reserve, Chronotropic incompetence, Chronotropic index, Chronic heart failure
Introduction
An increased resting heart rate (HR) is associated with increased cardiovascular (CV) risk and mortality in patients with chronic heart failure (HF).1 Thus, reducing resting HR, usually with β-blocker therapy and additionally with ivabradine, has become an important therapeutic goal.2,3
In addition to resting HR, HR reserve during exercise or chronotropic index (CI) is used to assess the risk of ischaemia and to prescribe exercise training intensity. An impaired chronotropic response is believed to reflect, in part, an underlying abnormality of autonomic nervous system function.4 The concept of impaired chronotropic response was initially developed for apparently healthy subjects not taking β-blockers, with a lower CI serving as a predictor of coronary artery disease (CAD) and mortality.5–7 Chronotropic index is defined as the index of HR reserve used. The latest studies defined chronotropic incompetence as a value of ≤0.62 in patients on β-blockers.8 Indeed, in patients with CAD taking a β-blocker agent, chronotropic incompetence was found to be a predictor of mortality, independent of resting HR.8
In contrast, in studies of chronic HF patients taking β-blocker agents, chronotropic incompetence was not found to be a clinical predictor of mortality, but these studies were of limited size, were performed in observational settings, and only two-thirds of patients were on β-blockers.9,10 Thus, the prognostic value of chronotropic incompetence in optimally treated HF patients on β-blocker therapy is not clear.
The Heart Failure and A Controlled Trial Investigating Outcomes of Exercise TraiNing (HF-ACTION) study was a prospective trial conducted in patients with chronic HF where a cardiopulmonary exercise (CPX) test was performed.11,12 Patients were on stable guideline-based pharmacotherapy for at least 6 weeks prior to randomization. In this study, we aimed to assess the association between resting HR, CI, and clinical outcomes in patients with chronic HF enrolled in the HF-ACTION trial.
Methods
The design and subject eligibility criteria of the HF-ACTION trial have been published elsewhere.11,12 HF-ACTION was a prospective multi-centre, randomized, controlled trial of exercise training vs. usual care in patients with left ventricular ejection fraction (LVEF) ≤35% and New York Heart Association (NYHA) class II–IV symptoms. Overall, 2331 patients from 82 clinical sites were enrolled in the study. Patients were on stable guideline-based pharmacotherapy for at least 6 weeks prior to randomization. If patients were not treated with optimal HF pharmacological treatment as defined by guidelines, the personnel documented the underlying reason (e.g. drug intolerance or patient preference). Overall, 2329 of 2331 patients performed a CPX test at baseline.
Exercise testing
Subjects were scheduled to complete a symptom-limited maximum exercise test, in accordance with American College of Cardiology and the American Heart Association guidelines. Subjects were instructed to take their medications as prescribed on the day of testing, with a specific request that they take their β-blocker agent between 3 and 10 h before the test.
The primary mode of testing was a treadmill. However, a stationary cycle ergometer was used for 108 patients unable to walk on the treadmill or tested at sites that routinely used this modality for exercise testing. Site testing personnel were instructed to exercise patients to symptom-limited maximum. Resting HR was measured prior to testing, after 2 min of seated rest. Peak HR represented the highest HR achieved during the last minute of exercise. To help control for the potentially different effects of various β-blocker agents on peak HR, β-blocker doses were converted to equivalent doses of carvedilol based on dosing from clinical trial data and published studies evaluating the conversion of β-blocker agents.13,14
For this study, we included patients in sinus rhythm who received a β-blocker and who demonstrated maximal effort on the CPX test. Patients with a history of pacemaker or bi-ventricular pacemaker were excluded.
Chronotropic index
Chronotropic index (CI) was defined as an index of maximal predicted HR reserve achieved. Heart rate reserve is the difference between maximum age-predicted HR and HR at rest. We used two different formulas for the maximum age-predicted HR. We used the traditional maximum HR formula defined for healthy populations by Astrand et al.,15 i.e. (220 − age). The CI was accordingly defined as: CI = (HR at peak exercise − resting HR)/[(220 − age) − resting HR)]. A sensitivity analysis was also performed using a new equation developed by Keteyian et al.16, one that predicts maximum HR in patients with HF taking β-blockers, i.e. [119 + (resting HR/2) − (age/2) − (5 × bike)]. In this equation, the ‘bike’ variable would have value ‘0’ if a treadmill test was performed and ‘1’ if a bicycle was used, i.e. the predicted maximum HR is 5 b.p.m. less on the bicycle test. Based on this, we defined CI using the equation from Keteyian et al., as (HR at peak exercise − resting HR)/[119 + (resting HR/2) − (age/2) − (5 × bike) − resting HR]. Previous studies have shown that measures of CI are better in predicting outcomes than the simple measures of percentage of maximum HR achieved.7,8
Outcomes
Consistent with the main HF-ACTION trial, the primary outcome in this analysis was a composite of all-cause mortality or all-cause hospitalization. We also assessed the association between CI and the secondary outcomes of all-cause mortality, CV mortality or CV hospitalization, and CV mortality or HF hospitalization.
Overall, 1118 patients fit the revised inclusion criteria. Models including only baseline variables used the entire sample of 1118 patients and the sample size was reduced for multivariable modelling due to missing values in covariates.
Statistical analysis
Clinical characteristics are summarized using median (25th, 75th percentile) for continuous variables and number of patients for categorical variables. The relationships between baseline resting HR, CI, and continuous variables are displayed using Spearman correlation coefficients.
Cox proportional hazards models were fit to assess the association between baseline resting HR, CI, and clinical outcomes. The linearity assumption of a Cox model was checked for baseline resting HR and the two CI measures, for each of the four endpoints of interest. When deviations were found, linear splines or variable truncations were used. Hazard ratios correspond to a 0.1 unit change in the CI.
Multivariable analyses used adjustment models that were previously developed based on HF-ACTION baseline data.17 The models used in this paper were developed with the same methodology as the originally published predictive models by O'Connor et al.17 The slight difference between the models in the original article and the ones used here is due to the distinction between models for the purpose of prediction and models for the purpose of adjustment. Medication dosing variables and variables like region are appropriate in adjustment models but not in predictive models. Thus the list of potential variables for the adjustment model used in this study was slightly larger than the list of potential variables for the model published by O'Connor et al. The final adjustment models were derived using backward selection of 61 potential covariates in 200 bootstrap samples for each of 5 imputed datasets (variables that were candidates for inclusion are presented in the Appendix). Treatment (exercise) was a candidate for entry in all models, but it was not used in the final adjustment models because it was not a strong predictor of any of the outcomes. Also, β-blocker dose was a candidate for entry in all models, but it remained significant only in the all-cause death model. The same methodology was followed for each endpoint. To confirm that the models are appropriate for the subset of patients used in this paper, we fit the models in our subset of patients and saw that C-indices were about the same as those reported in the entire HF-ACTION population.
The final covariates used for adjustment are presented for each endpoint within the results section (Tables 1–6). Since different covariates were used for final adjustment, when Cox models were fit with the inclusion of the adjustment model covariates, the number of patients with complete data for all covariates ranged from 813 to 933.
Table 1.
Baseline characteristics of patients, and correlations with baseline heart rate and chronotropic index
| Characteristic | n | Median (25th, 75th) | Spearman correlation with baseline resting heart rate | Spearman correlation with baseline chronotropic index |
|---|---|---|---|---|
| Age, years | 1118 | 57 (49–65) | −0.22 | 0.01 |
| BMI, kg/m2 | 1117 | 31 (26–36) | 0.15 | −0.08 |
| LVEF, % | 1115 | 25 (21–31) | −0.13 | 0.06 |
| BUN, mg/dL | 952 | 19 (14–25) | −0.13 | −0.14 |
| Creatinine, mg/dL | 984 | 1.1 (0.9–1.4) | −0.14 | −0.16 |
| ACE-I dose, mg/day | 1094 | 20 (4–40) | −0.01 | −0.01 |
| Beta-Blocker dose, mg/day | 1109 | 50 (25–50) | −0.05 | −0.16 |
| Loop diuretic dose, mg/day | 1101 | 40 (0–80) | 0.11 | −0.22 |
| Peak VO2, mL/kg/min | 1090 | 15.0 (11.8–18.3) | −0.06 | 0.45 |
| Peak respiratory exchange ratio | 1083 | 1.09 (1.02–1.15) | −0.07 | 0.23 |
| Ve/VCO2 slope | 1082 | 31.6 (27.6–36.3) | 0.04 | −0.13 |
| CPX duration, min | 1104 | 10.0 (7.4–12.7) | −0.11 | 0.48 |
| Six-minute walk distance, m | 1093 | 377 (305–440) | −0.08 | 0.30 |
| Resting heart rate (b.p.m.), CPX test | 1118 | 70 (62–79) | – | 0.09 |
| Chronotropic index | 1118 | 0.56 (0.42–0.71) | 0.09 | – |
| Keteyian chronotropic index | 1112 | 0.93 (0.71–1.19) | 0.05 | 0.99 |
BMI, body max index; LVEF, left ventricular ejection fraction; BUN, blood urea nitrogen; ACE-I, angiotensin-converting enzyme-inhibitors.
Table 2.
Baseline characteristics of patients
| Characteristic | n | Median (25th, 75th) of baseline resting heart rate | Median (25th, 75th) of baseline chronotropic index |
|---|---|---|---|
| Sex | |||
| Male | 720 | 70 (61–78) | 54 (41–69) |
| Female | 398 | 71 (63–79) | 59 (45–74) |
| Race | |||
| Black or African American | 437 | 72 (63–79) | 55 (42–71) |
| White | 598 | 70 (62–78) | 56 (43–70) |
| Other | 83 | 68 (61–77) | 56 (38–72) |
| Region | |||
| USA | 971 | 71 (63–79) | 56 (42–72) |
| Canada | 94 | 68 (62–77) | 48 (39–61) |
| France | 53 | 70 (59–80) | 55 (47–66) |
| Baseline NYHA class | |||
| II | 771 | 70 (61–78) | 59 (47–73) |
| III/IV | 347 | 72 (64–80) | 48 (34–63) |
| CCS angina class | |||
| No angina | 941 | 70 (62–79) | 56 (43–71) |
| I | 92 | 71 (62–77) | 60 (47–73) |
| II–IV | 85 | 71 (62–77) | 46 (36–60) |
| Aetiology | |||
| Ischaemic | 512 | 68 (61–76) | 53 (39–66) |
| Non-ischaemic | 606 | 72 (64–81) | 57 (46–73) |
| Diabetes | |||
| No | 749 | 69 (61–78) | 58 (45–73) |
| Yes | 369 | 72 (64–80) | 51 (37–65) |
| Mitral regurgitation | |||
| Non-severe/nonea | 918 | 70 (62–78) | 56 (44–71) |
| Severeb | 116 | 72 (61–80) | 54 (37–72) |
| Ventricular conduction prior to CPX | |||
| Normal | 661 | 71 (62–79) | 56 (42–71) |
| LBBB | 233 | 69 (62–77) | 58 (48–72) |
| RBBB | 44 | 73 (64–79) | 57 (36–70) |
| IVCD | 152 | 70 (63–78) | 53 (39–68) |
| ACE inhibitor | |||
| No | 254 | 71 (63–78) | 56 (42–70) |
| Yes | 864 | 70 (62–79) | 56 (42–71) |
| Angiotensin II receptor blocker | |||
| No | 863 | 70 (62–79) | 55 (41–71) |
| Yes | 255 | 71 (63–78) | 57 (44–71) |
| Aldosterone antagonist | |||
| No | 635 | 70 (62–78) | 56 (42–71) |
| Yes | 483 | 71 (63–79) | 55 (42–71) |
| Digoxin | |||
| No | 670 | 70 (62–78) | 57 (43–72) |
| Yes | 448 | 70 (63–79) | 53 (40–68) |
| Loop diuretic | |||
| No | 278 | 69 (61–77) | 60 (47–75) |
| Yes | 840 | 71 (63–79) | 54 (41–69) |
| Non-loop diuretic | |||
| No | 1013 | 70 (62–78) | 56 (43–71) |
| Yes | 105 | 72 (62–79) | 53 (39–70) |
CCS, Canadian Cardiovascular Society; LBBB, left bundle branch block; RBBB, right bundle branch block; IVCD, intraventricular conduction delay.
aA combination of these original categories: none, trivial, mild, mild to moderate, and moderate.
bA combination of the original categories: severe and moderate to severe.
Table 3.
The multivariable Cox association between resting heart rate, chronotropic index and the risk of all-cause mortality or hospitalization
| Model | Variable | Adjusted (N = 834) |
||
|---|---|---|---|---|
| Hazard ratio (95% confidence Interval) | Chi-square | P-value | ||
| 1 | Resting heart rate, hazard ratio for 5 bpm increase | 1.03 (1.00, 1.07) | 3.2 | 0.073 |
| CI, hazard ratio for 0.1 decrease | 1.01 (0.96, 1.06) | 0.1 | 0.73 | |
| 2 | Resting heart rate, hazard ratio for 5 bpm increase | 1.03 (1.00, 1.07) | 3.1 | 0.076 |
| CI, hazard ratio for CI ≤ 0.62 | 1.01 (0.83, 1.23) | <0.1 | 0.93 | |
| 3 | Resting heart rate, hazard ratio for 5 bpm increase | 1.04 (1.00, 1.08) | 3.5 | 0.063 |
| Keteyian CI, hazard ratio for 0.1 decreasea | 1.00 (0.98, 1.03) | 0.1 | 0.76 | |
Each model was adjusted for the following covariates: Weber Class, sex, region (USA vs. non-USA), mitral regurgitation (MR) grade, ventricular conduction prior to CPX test, BUN, LVEF, β-blocker dose, and Kansas City Cardiomyopathy Questionnaire (KCCQ) Symptom Stability Score.
aModels including the Keteyian CI include 829 patients, because 5 patients were missing whether the CPX was a bike or treadmill test.
Table 4.
The multivariable Cox association between resting heart rate, chronotropic index, and the risk of all-cause mortality
| Model | Variable | Adjusted (n = 933) |
||
|---|---|---|---|---|
| Hazard ratio (95% confidence interval) | Chi-square | P-value | ||
| 1 | Resting HR, hazard ratio for 5 b.p.m. increase | 1.03 (0.96, 1.12) | 0.7 | 0.42 |
| CI, hazard ratio for 0.1 decrease below 0.6 | 1.17 (1.01, 1.36) | 4.4 | 0.036 | |
| 2 | Resting HR, hazard ratio for 5 b.p.m. increase | 1.02 (0.94, 1.10) | 0.3 | 0.61 |
| CI, hazard ratio for CI ≤ 0.62 | 1.12 (0.72, 1.76) | 0.3 | 0.62 | |
| 3 | Resting HR, hazard ratio for 5 b.p.m. increase | 1.03 (0.95, 1.11) | 0.4 | 0.53 |
| Keteyian CI, hazard ratio for 0.1 decreasea | 1.06 (0.99, 1.13) | 3.0 | 0.085 | |
Each model was adjusted for the following covariates: CPX test duration, BMI, creatinine, sex, loop diuretic dose, LVEF, the CCS Angina Class, and ventricular conduction prior to the CPX test.
aModels including the Keteyian CI include 928 patients, because 5 patients were missing whether the CPX was a bike or treadmill test.
Table 5.
The multivariable Cox association between resting heart rate, chronotropic index, and the risk of cardiovascular mortality or cardiovascular hospitalization
| Model | Variable | Adjusted (n = 830) |
||
|---|---|---|---|---|
| Hazard ratio (95% confidence Interval) | Chi-square | P-value | ||
| 1 | Resting HR, hazard ratio for 5 b.p.m. increase | 1.03 (0.99, 1.08) | 2.7 | 0.098 |
| CI, hazard ratio for 0.1 decrease | 0.99 (0.94, 1.04) | 0.1 | 0.70 | |
| 2 | Resting HR, hazard ratio for 5 b.p.m. increase | 1.04 (0.99, 1.08) | 2.9 | 0.088 |
| CI, hazard ratio for CI ≤ 0.62 | 0.90 (0.73, 1.12) | 0.9 | 0.34 | |
| 3 | Resting HR, hazard ratio for 5 b.p.m. increase | 1.04 (1.00, 1.08) | 3.0 | 0.084 |
| Keteyian CI, hazard ratio for 0.1 decreasea | 0.99 (0.96, 1.03) | 0.2 | 0.65 | |
Each model was adjusted for the following covariates: LVEF, MR grade, ventricular conduction prior to the CPX test, KCCQ Symptom Stability Score, BUN, race, sex, nitrate use, Weber class, and KCCQ Total Symptom Score.
aModels including the Keteyian CI include 825 patients, because 5 patients were missing whether the CPX was a bike or treadmill test.
Table 6.
The multivariable Cox association between resting heart rate, chronotropic index, and the risk of cardiovascular mortality or heart failure hospitalization
| Model | Variable | Adjusted (n = 813) |
||
|---|---|---|---|---|
| Hazard Ratio (95% Confidence Interval) | Chi-square | P-value | ||
| 1 | Resting HR, hazard ratio for 5 b.p.m. increase | 1.04 (0.98, 1.11) | 1.9 | 0.17 |
| CI, hazard ratio for 0.1 decrease below 0.6 | 1.13 (1.02, 1.26) | 5.0 | 0.025 | |
| 2 | Resting HR, hazard ratio for 5 b.p.m. increase | 1.04 (0.98, 1.10) | 1.4 | 0.23 |
| CI, hazard ratio for CI ≤ 0.62 | 1.04 (0.76, 1.43) | 0.1 | 0.80 | |
| 3 | Resting HR, hazard ratio for 5 bpm increase | 1.04 (0.98, 1.11) | 1.9 | 0.17 |
| Keteyian CI, hazard ratio for 0.1 decrease below 1.1a | 1.07 (1.01, 1.13) | 4.5 | 0.033 | |
Each model was adjusted for the following covariates: loop diuretic dose, LVEF, MR grade, ventricular conduction prior to the CPX test, KCCQ Symptom Stability Score, BUN, race, sex, age, Weber class, and Ve/VCO2.
aModels including the Keteyian CI include 808 patients, because 5 patients were missing whether the CPX was a bike or treadmill test.
Data were analysed using SAS version 9.2.
Sensitivity analysis
The final adjustment models developed in the HF-ACTION data included among the covariates WEBER class (as a categorized version of peak VO2), creatinine, and β-blocker dose (for the all-cause mortality or hospitalization model). However, as a previous study found (continuous) peak VO2 and not CI as a predictor of outcomes,10 and given the fact that not all the patients used the same exercise test in our study, as well as given the importance of β-blocker dose, we performed a sensitivity analysis in which models were adjusted for peak VO2 instead of Weber class or CPX duration, all models were adjusted for β-blocker dose, and for eGFR instead of creatinine.
Results
Characteristics of patients
The baseline characteristics and correlations with baseline resting HR and CI are presented in Tables 1 and 2. The median age of patients was 57 (49, 65) years, and most patients (69%) were in NYHA II. The median carvedilol equivalent dose of β-blocker was 50 (25, 50) mg/day. Chronotropic index was positively correlated with peak VO2 (r = 0.45) and with the duration of CPX test (r = 0.46), and negatively correlated with Ve/VCO2 slope (r = −0.17). Chronotropic index was lower in patients with angina class II–IV when compared with patients with no angina. The median of CI was 0.56 (0.42, 0.71). The median CI using the formula from Keteyian et al. was 0.93 (0.71, 1.19), and the two indexes were highly correlated (r = 0.99).
Cox univariable and multivariable analysis
All-cause mortality or hospitalization (primary outcome)
The patients were followed up for the primary outcome for a median of 32 (21, 44) months. Overall, 692 (62%) of patients reached the primary endpoint. Both resting HR and CI were linearly related to the primary outcome in the univariable analysis (data not shown).
In the univariable model including resting HR, a 5 b.p.m. increase in resting HR was associated with an increase in the hazard for death or hospitalization of 4% (hazard ratio 1.04; 1.01–1.08; P = 0.007). In the univariable model including CI, a 0.1 decrease in the CI was associated with an increase in the hazard for all-cause death or hospitalization (hazard ratio 1.09; 1.05–1.13; P < 0.001). Similarly, when CI was dichotomized at the previously defined cut-off of 0.62, a CI of ≤0.62 was associated with a 26% increase in the hazard for all-cause mortality or hospitalization (hazard ratio 1.26; 1.08–1.47; P = 0.004).
When only resting HR and CI were included in the model, both were significantly associated with the outcomes. However, when the adjustment model covariates were included in the model, resting HR and CI were not significantly associated with the primary outcome. Results were similar for each definition of CI with resting HR showing a trend towards significance (all P < 0.08) and CI being non-significant (all P > 0.72) in each model (Table 3).
All-cause mortality
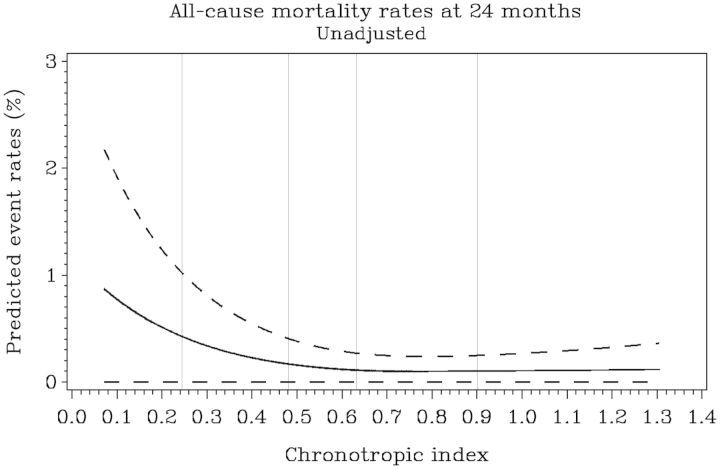
During follow-up, 151 (14%) patients died (all-cause mortality). As opposed to the primary outcome, a non-linear relationship was found between CI and the risk of all-cause mortality (Figure 1). Figure 1 shows that when CI was modelled as a linear variable, the risk of event decreased as the CI increased but only until CI reached ∼0.6. Above 0.6, the risk was roughly constant. As such, the variable was modelled as linear <0.6 and constant >0.6. Overall, 666 of 1118 (60%) patients had a CI of <0.6. From the patients with a CI of <0.6, 427 patients (64%) had a CI of 0.4–0.59, 214 patients (32%) had a CI of 0.2–0.39, and 25 patients (4%) had a CI of <0.2. The linearity assumption was preserved for resting HR and for CI when computed using the equation from Keteyian et al. (data not shown).
Figure 1.
The relationship between chronotropic index and probability of all-cause mortality at 2-year follow-up. The figure shows a significantly non-linear relationship between chronotropic index and all-cause mortality. The risk of event decreases as the chronotropic index goes up, but only until chronotropic index reaches ∼0.6. Above 0.6, the risk is roughly constant, so we assumed a constant hazard above 0.6.
In the univariable model including CI as a linear variable, each 0.1 unit decrease in CI <0.6 was significantly associated with an increased risk of all-cause mortality (hazard ratio 1.48; 1.33–1.65; P < 0.001). In the multivariable model including resting HR, CI, and the adjustment model covariates, each 0.1 unit decrease in CI <0.6 was associated with an increased risk of all-cause mortality (hazard ratio 1.17; 1.01–1.36; P = 0.036) (Table 4). In contrast, when CI was modelled as a dichotomous variable, a CI of ≤0.62 was not significantly associated with the outcome (P = 0.6). However, as shown in Figure 1 and as expected in a linear relationship, the risk of all-cause mortality increased proportionally in patients with a CI of <0.6, and it was particularly elevated in patients with a CI of <0.4.
A decrease in CI Keteyian was not associated with the outcome (P = 0.085).
Cardiovascular mortality or cardiovascular hospitalization
Results for the CV mortality or CV hospitalization endpoint are similar to the results for the primary end-point. The linearity assumption was not rejected for resting HR and for the CI variables. In the multivariable models, none of the CI variables or resting HR were associated with the outcome (Table 5).
Cardiovascular mortality or heart failure hospitalization
In this model, a non-linear relationship was also observed between the CI and the outcome, also in the sense that a decrease in CI <0.6 was associated with a worse outcome. In addition, a similar non-linear relationship was observed for the CI Keteyian, i.e. a decrease in CI <1.1 was associated with a worse outcome (data not shown). Overall, 753 of 1112 (68%) of patients had a CI <1.1.
In adjusted models (Table 6), measures of CI remained significantly associated with the outcome, e.g. for each 0.1 decrease <0.6 in CI, hazard ratio 1.13; 1.02–1.26, P = 0.025. For CI Keteyian, for each 0.1 decrease < 1.1, hazard ratio 1.07; 1.01–1.13; P = 0.033. Similar to previous outcomes, when CI was modelled as a binary variable, a CI of ≤0.62 was not significantly associated with the outcomes.
Sensitivity analysis
Results were similar when the models were adjusted for continuous peak VO2 instead of Weber class or CPX duration, when each model was adjusted for the β-blocker dose, and when all-cause death model was adjusted for eGFR instead of creatinine (data not shown).
Discussion
In this study, we assessed the association between resting HR, CI, and clinical outcomes in patients with systolic HF (LVEF < 35%) receiving optimal β-blocker treatment. In multivariable Cox model including resting HR and CI as continuous variables, neither was associated with the primary outcome of all-cause mortality or hospitalization. In contrast, a lower CI over the range 0–0.6 was associated with a higher risk of all-cause mortality and a higher risk of CV mortality or HF hospitalization. Similar results were shown when CI was determined using the formula by Keteyian et al. When CI was modelled as a dichotomous variable, a CI of ≤0.62 was not significantly associated with clinical outcomes.
An impaired HR response to exercise or so-called chronotropic incompetence in healthy subjects is predictive of CAD and mortality.6,7 In men without a history of myocardial infarction or revascularization, of whom only 24% were taking β-blockers, angiographic CAD was substantially more common in the presence of chronotropic incompetence regardless of whether or not β-blockers were taken.18 In patients with established CAD or at risk for CV disease not taking β-blockers, chronotropic incompetence was also an important predictor of cardiac death and all-cause mortality.7,19,20 In these studies, chronotropic incompetence was defined as either (i) low percentage (<85%) of the age-adjusted maximum HR, e.g. (220 − age) or (ii) low (<80%) CI, a measure that accounts for both peak and resting HR as well as age, e.g. (peak HR − resting HR/(220 − age) − resting HR). However, when using the formula of (220 − age) that was originally developed in apparently healthy populations, it has been pointed-out that the cut-off of impaired chronotropic response may differ according to both the population included and the use of β-blocker agents. Indeed, in patients with CAD taking β-blocker agents, Kahn et al.8 found a non-linear relationship between CI and the risk of all-cause mortality, but with an apparent threshold present ∼70% in continuous analysis. In our continuous analysis, an apparent threshold was present ∼60%, and the risk was substantially increased at a CI of <40%. Our data support the notion that the presence of HF causes a left-shift of the relationship, which would be understandable given the disease characteristics.
In patients with HF receiving optimized β-blocker treatment, Carvalho et al.21 pointed out that a peak HR over 65% of the maximum age-adjusted value could be considered an effort near the maximum. Indeed, in the HF population, HR reserve may depend on chronotropic incompetence, but also on the degree of β- blockade, on patient motivation, as well as on another unidentified factor related to the disease itself. However, in previous studies of chronic HF patients, chronotropic incompetence was not found to be a clinical predictor all-cause mortality, but these studies were limited in size, were performed in observational settings, and not all the patients in these studies were on β-blocker therapy.9,10
Chronotropic incompetence is believed to reflect, at least in part, a desensitization of the β-adrenergic receptors in the failing heart. This may lead to a decrease in β-agonist-stimulated muscle contractility.6,22 Sinus or atrioventricular node dysfunction, probably also a consequence of diminished sympathetic responsiveness and sinus node remodelling, was also mentioned as a possible cause.23,24 However, as the association between chronotropic incompetence and mortality is independent of exercise-induced myocardial perfusion defects and cardiac death, it has been pointed out that autonomous nervous system dysfunction could be the underlying cause of chronotropic incompetence and may lead to adverse outcomes via multiple pathways.16–18 This may also explain why in our study we particularly found an association between impaired chronotropic response and all-cause mortality.
In patients with HF, high sympathetic drive at rest is associated with adverse outcomes and β-blocker therapy has been shown to improve outcomes, coincident with a decrease in resting HR.2 Whether HR reserve would provide additional information about prognosis independent of resting HR in this specific population is therefore clinically relevant. Importantly, our results suggest that, in optimally treated HF patients, a CI decreasing below 0.6 is associated with adverse clinical outcomes, independent of resting HR. This finding is also relevant because a large proportion (60%) of patients in our cohort had a CI of <0.6.
Our results are in contrast with the two published studies on the topic,9,10 but the advantage of our study is the higher sample size and the optimized β-blocker therapy. The β-blocker dose was not specified in the previous HF studies.9,10 The median equivalent dose of β-blocker used in this study was 50 (25–50) mg (carvedilol equivalents). This dose is relatively high and reflects diligent attention to medical therapy. Interestingly, however, resting HR and not CI showed a trend towards a significant association with the primary outcome of all-cause mortality or hospitalization, and with CV mortality or hospitalization. Whether indeed resting HR and chronotropic response at exercise may impact clinical outcomes differently warrants further exploration.
Our results have several clinical and methodological implications. First, the study shows that the assessment of CI is important for risk stratification and possibly a different management strategy in HF patients who present an impaired CI despite optimal therapy. Endurance exercise training may improve chronotropic function in patients with chronic HF, probably by increasing baroreflex sensitivity and reducing sympathetic outflow and plasma levels of neurohormones.25 Rate-adaptive pacing, in conjunction to cardiac resynchronization therapy, has also been shown to increase peak HR and exercise time, particularly in patients with severe chronotropic incompetence, but this therapy requires further invesitigation.26 Secondly, we found that a decrease in CI <0.6 is associated with adverse clinical outcomes in this specific HF population, but this association was not observed when the CI was dichotomized (CI ≤ 0.62). This may be explained by the loss of information when transforming a variable from continuous to categorical. Finally, we presented another formula, one that incorporates work previously published by Keteyian et al. that may be used for assessing CI in patients with HF treated with a β-blocker.
There are several strengths and limitations for our study. The major strength is the use of a database of HF patients on optimized β-blocker therapy who performed a maximal CPX test. To our knowledge, this is among the few studies that explore the prognostic value of chronotropic response in this population, using several clinical outcomes. Both the CPX test and functional assessments were performed within a carefully defined trial protocol. A limitation is that we used post hoc, observational data and, as a result, unmeasured confounding variables may account for some or all of the observed relationships. Another limitation of our study may be the use of a stationary cycle ergometer instead of treadmill in 108 patients unable to walk on the treadmill or tested at sites that use this modality for exercise testing.
In conclusion, we observed that a decrease in CI < 0.6 was associated with all-cause mortality and with CV mortality or HF hospitalization but not with the primary outcome of all-cause mortality or hospitalization in optimally treated HF patients. Obtaining a better HR response to exercise, even in patients receiving optimized β-blocker therapy, may be a therapeutic target in the HF population.
Funding
The HF-ACTION trial was funded by grants from the National Heart, Lung, and Blood Institute.
Conflict of interest: none declared.
Appendix
Variables that were candidates for inclusion in the HF-ACTION adjustment models for each outcome.
| Variable | |
|---|---|
| 1 | Region (USA vs. non-USA) |
| 2 | Age |
| 3 | Sex |
| 4 | Race |
| 5 | History of MI |
| 6 | Previous revascularization |
| 7 | Diabetes |
| 8 | History of PVD |
| 9 | Smoking status |
| 10 | History of chronic obstructive pulmonary disease |
| 11 | HF hospitalizations in the last 6 months |
| 12 | Hospitalizations in the last 6 months |
| 13 | Aetiology of HF |
| 14 | NYHA Class (II vs. III/IV) |
| 15 | Canadian Angina Class |
| 16 | On a β-blocker at baseline |
| 17 | β-Blocker baseline daily dose |
| 18 | On a angiotensin-converting enzyme (ACE) inhibitor at baseline |
| 19 | ACE inhibitor baseline daily dose |
| 20 | On a loop diuretic at baseline |
| 21 | Loop diuretic baseline daily dose |
| 22 | On other diuretic |
| 23 | On a nitrate |
| 24 | On insulin |
| 25 | On a HMG-CoA reductase inhibitor |
| 26 | Baseline AICD |
| 27 | Baseline bi-ventricular pacemaker |
| 28 | On two or more diuretics at baseline |
| 29 | On spiro/eplerenone |
| 30 | On a pacer at baseline |
| 31 | Atrial fibrillation/flutter |
| 32 | Mitral regurgitation grade by echo |
| 33 | Systolic blood pressure |
| 34 | Diastolic blood pressure |
| 35 | Baseline heart rate |
| 36 | Body mass index |
| 37 | Left ventricular EF |
| 38 | KCCQ: Total symptom score |
| 39 | KCCQ: Quality-of-life score |
| 40 | KCCQ: Self-efficacy score |
| 41 | KCCQ: Symptom stability score |
| 42 | KCCQ: Physical limitation score |
| 43 | KCCQ: Social limitation score |
| 44 | Beck depression index II |
| 45 | Heart rate reserve on the baseline CPX test |
| 46 | Heart rate at peak exercise (baseline CPX test) |
| 47 | HR at the end of the 2nd stage of the baseline CPX test |
| 48 | Exercise duration on the baseline CPX test |
| 49 | Rest ECG rhythm (baseline CPX test) |
| 50 | Peak VO2 (baseline CPX test) |
| 51 | Weber class (baseline CPX test) |
| 52 | Peak oxygen pulse on the baseline CPX test |
| 53 | Ventricular conduction prior to the baseline CPX test |
| 54 | Peak RER on the baseline CPX test |
| 55 | Ve/VCO2 slope (baseline CPX test) |
| 56 | Six-minute walk distance |
| 57 | Serum creatinine |
| 58 | Sodium |
| 59 | BUN |
| 60 | Haemoglobin |
| 61 | Treatment group (exercise vs. usual care) |
References
- 1.Pocock SJ, Wang D, Pfeffer MA, Yusuf S, McMurray JJ, Swedberg KB, Ostergren J, Michelson EL, Pieper KS, Granger CB. Predictors of mortality and morbidity in patients with chronic heart failure. Eur Heart J. 2006;27:65–75. doi: 10.1093/eurheartj/ehi555. [DOI] [PubMed] [Google Scholar]
- 2.Lechat P, Hulot JS, Escolano S, Mallet A, Leizorovicz A, Werhlen-Grandjean M, Pochmalicki G, Dargie H. Heart rate and cardiac rhythm relationships with bisoprolol benefit in chronic heart failure in CIBIS II Trial. Circulation. 2001;103:1428–1433. doi: 10.1161/01.cir.103.10.1428. [DOI] [PubMed] [Google Scholar]
- 3.Swedberg K, Komajda M, Bohm M, Borer JS, Ford I, Dubost-Brama A, Lerebours G, Tavazzi L. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet. 2010;376:875–885. doi: 10.1016/S0140-6736(10)61259-7. [DOI] [PubMed] [Google Scholar]
- 4.Colucci WS, Ribeiro JP, Rocco MB, Quigg RJ, Creager MA, Marsh JD, Gauthier DF, Hartley LH. Impaired chronotropic response to exercise in patients with congestive heart failure. Role of postsynaptic beta-adrenergic desensitization. Circulation. 1989;80:314–323. doi: 10.1161/01.cir.80.2.314. [DOI] [PubMed] [Google Scholar]
- 5.Lauer MS, Okin PM, Larson MG, Evans JC, Levy D. Impaired heart rate response to graded exercise. Prognostic implications of chronotropic incompetence in the Framingham Heart Study. Circulation. 1996;93:1520–1526. doi: 10.1161/01.cir.93.8.1520. [DOI] [PubMed] [Google Scholar]
- 6.Lauer MS, Pashkow FJ, Larson MG, Levy D. Association of cigarette smoking with chronotropic incompetence and prognosis in the Framingham Heart Study. Circulation. 1997;96:897–903. doi: 10.1161/01.cir.96.3.897. [DOI] [PubMed] [Google Scholar]
- 7.Azarbal B, Hayes SW, Lewin HC, Hachamovitch R, Cohen I, Berman DS. The incremental prognostic value of percentage of heart rate reserve achieved over myocardial perfusion single-photon emission computed tomography in the prediction of cardiac death and all-cause mortality: superiority over 85% of maximal age-predicted heart rate. J Am Coll Cardiol. 2004;44:423–430. doi: 10.1016/j.jacc.2004.02.060. [DOI] [PubMed] [Google Scholar]
- 8.Khan MN, Pothier CE, Lauer MS. Chronotropic incompetence as a predictor of death among patients with normal electrograms taking beta blockers (metoprolol or atenolol) Am J Cardiol. 2005;96:1328–1333. doi: 10.1016/j.amjcard.2005.06.082. [DOI] [PubMed] [Google Scholar]
- 9.Witte KK, Cleland JG, Clark AL. Chronic heart failure, chronotropic incompetence, and the effects of beta blockade. Heart. 2006;92:481–486. doi: 10.1136/hrt.2004.058073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Najjar Y, Witte KK, Clark AL. Chronotropic incompetence and survival in chronic heart failure. Int J Cardiol. 2010;157:48–52. doi: 10.1016/j.ijcard.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 11.O'Connor CM, Whellan DJ, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, Leifer ES, Kraus WE, Kitzman DW, Blumenthal JA, Rendall DS, Miller NH, Fleg JL, Schulman KA, McKelvie RS, Zannad F, Pina IL. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301:1439–1450. doi: 10.1001/jama.2009.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whellan DJ, O'Connor CM, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, Leifer ES, Kraus WE, Kitzman DW, Blumenthal JA, Rendall DS, Houston-Miller N, Fleg JL, Schulman KA, Pina IL. Heart failure and a controlled trial investigating outcomes of exercise training (HF-ACTION): design and rationale. Am Heart J. 2007;153:201–211. doi: 10.1016/j.ahj.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 13.Di Lenarda A, Sabbadini G, Salvatore L, Sinagra G, Mestroni L, Pinamonti B, Gregori D, Ciani F, Muzzi A, Klugmann S, Camerini F. Long-term effects of carvedilol in idiopathic dilated cardiomyopathy with persistent left ventricular dysfunction despite chronic metoprolol. The Heart-Muscle Disease Study Group. J Am Coll Cardiol. 1999;33:1926–1934. doi: 10.1016/s0735-1097(99)00134-5. [DOI] [PubMed] [Google Scholar]
- 14.Maack C, Elter T, Nickenig G, LaRosee K, Crivaro M, Stablein A, Wuttke H, Bohm M. Prospective crossover comparison of carvedilol and metoprolol in patients with chronic heart failure. J Am Coll Cardiol. 2001;38:939–946. doi: 10.1016/s0735-1097(01)01471-1. [DOI] [PubMed] [Google Scholar]
- 15.Astrand I. Aerobic work capacity in men and women with special reference to age. Acta Physiol Scand Suppl. 1960;49:1–92. [PubMed] [Google Scholar]
- 16.Keteyian SJ, Kitzman D, Zannad F, Landzberg J, Arnold JM, Brubaker P, Brawner CA, Bensimhon D, Hellkamp AS, Ewald G. Predicting maximal heart rate in heart failure patients on beta-blockade therapy. Med Sci Sports Exerc. 2012;44:371–376. doi: 10.1249/MSS.0b013e318234316f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'Connor CM, Whellan DJ, Wojdyla D, Leifer E, Clare RM, Ellis SJ, Fine LJ, Fleg JL, Zannad F, Keteyian SJ, Kitzman DW, Kraus WE, Rendall D, Pina IL, Cooper LS, Fiuzat M, Lee KL. Factors related to morbidity and mortality in patients with chronic heart failure with systolic dysfunction: the HF-ACTION predictive risk score model. Circ Heart Fail. 2012;5:63–71. doi: 10.1161/CIRCHEARTFAILURE.111.963462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gauri AJ, Raxwal VK, Roux L, Fearon WF, Froelicher VF. Effects of chronotropic incompetence and beta-blocker use on the exercise treadmill test in men. Am Heart J. 2001;142:136–141. doi: 10.1067/mhj.2001.115788. [DOI] [PubMed] [Google Scholar]
- 19.Elhendy A, Mahoney DW, Khandheria BK, Burger K, Pellikka PA. Prognostic significance of impairment of heart rate response to exercise: impact of left ventricular function and myocardial ischemia. J Am Coll Cardiol. 2003;42:823–830. doi: 10.1016/s0735-1097(03)00832-5. [DOI] [PubMed] [Google Scholar]
- 20.Lauer MS, Francis GS, Okin PM, Pashkow FJ, Snader CE, Marwick TH. Impaired chronotropic response to exercise stress testing as a predictor of mortality. JAMA. 1999;281:524–529. doi: 10.1001/jama.281.6.524. [DOI] [PubMed] [Google Scholar]
- 21.Carvalho VO, Guimaraes GV, Ciolac EG, Bocchi EA. Heart rate dynamics during a treadmill cardiopulmonary exercise test in optimized beta-blocked heart failure patients. Clinics (Sao Paulo) 2008;63:479–482. doi: 10.1590/S1807-59322008000400011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brubaker PH, Kitzman DW. Prevalence and management of chronotropic incompetence in heart failure. Curr Cardiol Rep. 2007;9:229–235. doi: 10.1007/BF02938355. [DOI] [PubMed] [Google Scholar]
- 23.Samejima H, Omiya K, Uno M, Inoue K, Tamura M, Itoh K, Suzuki K, Akashi Y, Seki A, Suzuki N, Osada N, Tanabe K, Miyake F, Itoh H. Relationship between impaired chronotropic response, cardiac output during exercise, and exercise tolerance in patients with chronic heart failure. Jpn Heart J. 2003;44:515–525. doi: 10.1536/jhj.44.515. [DOI] [PubMed] [Google Scholar]
- 24.Sanders P, Kistler PM, Morton JB, Spence SJ, Kalman JM. Remodeling of sinus node function in patients with congestive heart failure: reduction in sinus node reserve. Circulation. 2004;110:897–903. doi: 10.1161/01.CIR.0000139336.69955.AB. [DOI] [PubMed] [Google Scholar]
- 25.Gademan MG, Swenne CA, Verwey HF, van der Laarse A, Maan AC, van de Vooren H, van Pelt J, van Exel HJ, Lucas CM, Cleuren GV, Somer S, Schalij MJ, van der Wall EE. Effect of exercise training on autonomic derangement and neurohumoral activation in chronic heart failure. J Card Fail. 2007;13:294–303. doi: 10.1016/j.cardfail.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 26.Tse HF, Siu CW, Lee KL, Fan K, Chan HW, Tang MO, Tsang V, Lee SW, Lau CP. The incremental benefit of rate-adaptive pacing on exercise performance during cardiac resynchronization therapy. J Am Coll Cardiol. 2005;46:2292–2297. doi: 10.1016/j.jacc.2005.02.097. [DOI] [PubMed] [Google Scholar]