Abstract
Background:
A subset of patients treated with initial anti-vascular endothelial growth factor (VEGF) therapy exhibit progressive disease (PD) as the best response per RECIST criteria.
Methods:
Data from patients with metastatic renal cell carcinoma (mRCC) treated with anti-VEGF therapy were collected through the International mRCC Database Consortium from 12 centers.
Results:
One thousand and fifty-six assessable patients received initial VEGF inhibitors and 272 (26%) of these patients had PD as best response. Initial treatment included sunitinib (n = 203), sorafenib (n = 51), or bevacizumab (n = 18). Six percent of patients were at favorable risk, 55% at intermediate risk, and 39% at poor risk. On multivariable analysis, predictors of PD were Karnofsky performance status < 80% [odds ratio (OR) = 2.3, P < 0.0001], diagnosis to treatment < 1 year (OR = 2.1, P < 0.0001), neutrophilia (OR = 1.9, P = 0.0021), thrombocytosis (OR = 1.7, P = 0.0068), and anemia (OR = 1.6, P = 0.0058). Median progression-free survival (PFS) in patients with PD versus without PD was 2.4 versus 11 months (P < 0.0001) and overall survival (OS) was 6.8 versus 29 months (P < 0.0001), respectively. One hundred and eight (40%) VEGF-refractory patients proceeded to receive further systemic therapies. Response rate, PFS, and OS for subsequent therapy were 9%, 2.5 months, and 7.4 months, respectively, with no statistical differences between patients who received VEGF versus mammalian target of rapamycin (mTOR) inhibitors.
Conclusions:
Primary anti-VEGF-refractory mRCC patients have a dismal prognosis. Second-line anti-mTOR and anti-VEGF agents produce similar outcomes.
Keywords: primary refractory, renal cell carcinoma, VEGF, mTOR
introduction
Agents targeting the vascular endothelial growth factor (VEGF) pathway have revolutionized the treatment of metastatic renal cell carcinoma (mRCC). Although response rates and survival have improved since the introduction of targeted therapy, there are still a proportion of patients who are unresponsive to their initial targeted therapy [1–3]. The majority of patients treated with VEGF-targeted therapy exhibit stable disease (SD) or a partial response (PR) by RECIST as their best response. However, ∼9% to 21% of patients treated with VEGF-targeted therapy in phase III randomized controlled trials had progressive disease (PD) as their best response and thus, these patients are deemed primary VEGF refractory [4–8].
This patient population has not been fully characterized in terms of associated risk factors and outcomes. Additionally, the subsequent choice of second-line treatment remains controversial and thus treatment patterns and outcomes need to be documented. In practice, if a patient has primary refractory disease to initial VEGF therapy, clinicians switch to another targeted therapy. Some clinicians may switch patients to an agent targeting the mammalian target of rapamycin (mTOR) inhibitor, such as everolimus, as it was the only drug with level 1 evidence to produce a progression-free survival (PFS) benefit [9] during the time period of this study. Additionally, it may make intuitive sense to target another mechanism of action such as the mTOR pathway, as the first VEGF-targeting approach did not result in any benefit, even in the short term [10, 11]. However, some clinicians may switch to another anti-VEGF therapy due to drug availability, familiarity with the agent, toxicity profile, or as part of a clinical trial with a novel agent [12, 13]. There are also studies that suggest that there is activity of another anti-VEGF agent after failure of the initial one with agents such as sunitinib or sorafenib which suggests incomplete cross-resistance [14, 15]. Certainly, there is no evidence to suggest that either strategy is superior.
This international, multicenter population-based study aims to characterize the primary VEGF-refractory population and to examine practice patterns and outcomes of subsequent second-line therapy.
methods
study population
All patients with mRCC treated with contemporary VEGF-targeted therapy were included in this study. They were derived from consecutive population-based patient samples from 2005 to 2009 at 12 international Cancer Centers from Canada (Alberta Health Services Cancer Care, Sunnybrook Odette Cancer Center, Princess Margaret Hospital, London Health Sciences Center, Queen Elizabeth II Health Sciences Center, British Columbia Cancer Agency), USA (Dana-Farber Cancer Institute, Cleveland Clinic, Karmanos Cancer Center, Beth Israel Deaconess Medical Center), Singapore (National Cancer Center), and Denmark (Aarhus University Hospital). Patients may have been treated on clinical trial or off protocol and may have been treated at major academic centers or community oncology centers. Baseline patient characteristics and outcome data were collected using uniform data collection templates. To improve generalizability, all laboratory values were examined in the context of the institutional upper and lower limits of normal (ULN and LLN). Non-clear-cell carcinoma was ascertained when clear-cell histology was not the predominant subtype. Regulatory approval from local institutional review boards or research ethics boards was obtained for each center.
primary refractory versus non-primary refractory patients
In total, 1056 patients had evaluable disease and available data on their best response. The radiology films or reports at each participating center for each patient were reviewed retrospectively by investigators to determine the best response achieved by RECIST 1.0 [16]. Response data were collected separately from outcome and prognostic factor data collection to prevent investigator measurement bias.
The focus of this study was on primary refractory patients in whom PD was the best response the patient achieved during treatment with a VEGF inhibitor. PD was defined by a >20% increase in the sum of the longest diameter of the target lesions as the best response or an unequivocal clinical progression of disease as determined by the discretion of the treating oncologist. Stopping therapy due to treatment toxicity was not counted as primary refractory disease. Other patients who had SD, PRs, or complete responses (CR) were included in the non-primary refractory category.
statistics
factors associated with primary refractory disease.
Logistic regression analyses were carried out to identify independent factors associated with primary VEGF-refractory disease versus non-primary refractory disease. The case deletion method was used when there were missing data during regression analyses; however, the missing data rate was low (<2% of all data elements for the 1056 patients). All variables examined on univariable analysis are listed in Table 1. The multivariable analysis was limited to the a priori component variables of the Heng et al. criteria [3] and any variables on univariable analysis with a P-value < 0.05.
Table 1.
Baseline characteristics of patients with primary PD versus non-PD (i.e. SD + PR + CR)
| Characteristic | Primary PD (n = 272) | Non-primary PD (n = 784) | P-value |
| Age (mean, years) | 60.3 | 60.9 | 0.4487 |
| Gender (male, %) | 74 | 74 | 0.8600 |
| Heng prognostic group (%) | |||
| Favorable | 6 | 11 | <0.0001 |
| Intermediate | 55 | 70 | |
| Poor | 39 | 19 | |
| Median KPS (%) | 77 | 85 | <0.0001 |
| Diagnosis to treatment interval (years) | 2.08 | 3.33 | <0.0001 |
| Anemia (%) (<LLN) | 68 | 50 | <0.0001 |
| Neutrophil count (mean) | 6.2 | 5.1 | <0.0001 |
| Platelet count (mean) | 351 | 284 | <0.0001 |
| Hypercalcemia (%) (>ULN) | 15 | 8 | 0.0017 |
| LDH elevated (%) (>ULN) | 40 | 29 | 0.0029 |
| Brain metastases present (%) | 7 | 9 | 0.2871 |
| Prior nephrectomy (%) | 75 | 83 | 0.0028 |
| Non-clear-cell histology (%) | 13 | 8 | 0.0176 |
| Initial first-line targeted therapy (%) | |||
| Sunitinib | 75 | 70 | 0.5160 |
| Sorafenib | 19 | 23.3 | |
| Bevacizumab | 6 | 6.5 | |
| Axitinib | 0 | 0.1 | |
| Pazopanib | 0 | 0.1 |
CR, complete response; KPS, Karnofsky performance status; LDH, lactate dehydrogenase; LLN, lower limit of normal; PD, progressive disease; PR, partial response; SD, stable disease; ULN, upper limit of normal.
outcomes.
Kaplan–Meier curves were constructed to compare PFS and overall survival (OS) in patients with PD versus non-PD (CR + PR + SD). PFS and OS were also determined for patients who went on to receive subsequent targeted therapy and an exploratory analysis of PFS and OS for VEGF versus mTOR inhibitors in the second-line targeted therapy setting was carried out. Second-line VEGF inhibitors included sunitinib, sorafenib, bevacizumab, pazopanib, and axitinib. Second-line mTOR inhibitors included temsirolimus and everolimus. Second-line investigational protocols with drugs such as lenalidomide and capecitabine were excluded from the second-line analysis because of the unknown efficacy of these drugs (n = 2). Proportional hazards regression was carried out to adjust the PFS and OS hazard ratio estimates by patient prognostic groups [3]. All analyses were carried out on SAS 9.2 (SAS Institute Inc., Cary, NC).
results
One thousand three hundred and eighty-one patients treated with VEGF inhibitors as their first-line anti-angiogenic therapy were included in this study for which 1056 patients had evaluable disease (Figure 1). Of those, 272 (26%) patients had PD as the best response defined by the RECIST criteria. Their initial treatment was with sunitinib (n = 203), sorafenib (n = 51), or bevacizumab (n = 18). Six percent of patients were favorable risk, 55% intermediate risk, and 39% poor risk as per Heng et al. [3] prognostic factors. The median follow-up of all patients was 29.6 months (range 0–56 months). The median PFS and OS in patients with primary refractory disease versus patients without (i.e. objective response or SD) were 2.4 versus 11 months (P < 0.0001) and 6.8 versus 29 months (P < 0.0001), respectively (Figure 2).
Figure 1.
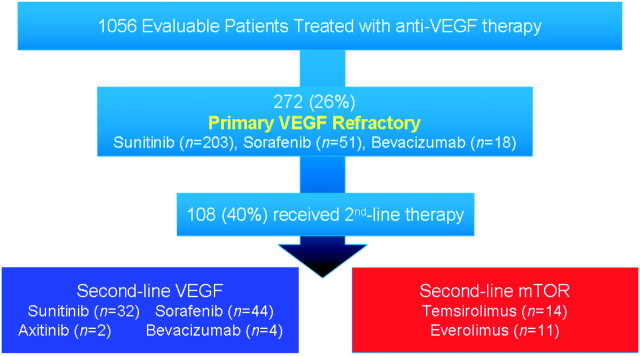
Diagram of assessable patients included in this study and their subsequent treatment. mTOR, mammalian target of rapamycin; VEGF, vascular endothelial growth factor.
Figure 2.
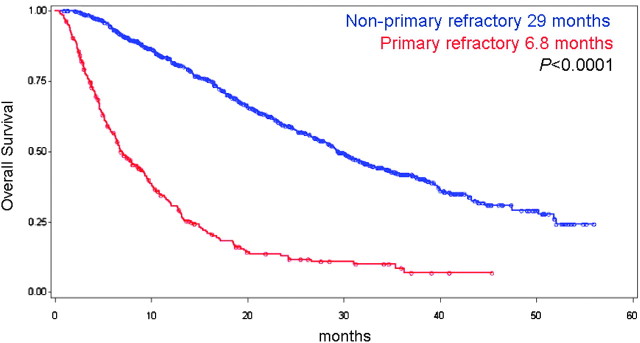
Overall survival of patients with primary refractory disease versus those without.
factors associated with primary refractory disease
A comparison of baseline characteristics of patients that had primary refractory disease versus those that did not is found in Table 1. These groups did not differ by age, gender, type of first-line VEGF-targeted therapy, or presence of brain metastases. However, the primary refractory group had significantly more patients in the poor prognostic group [3], more patients with elevated lactate dehydrogenase (LDH), fewer patients with prior nephrectomy, and more patients with non-clear-cell histology. On multivariable analysis, the variables that were independently associated with primary refractory disease at first restaging were Karnofsky performance status (KPS) < 80% [odds ratio (OR) = 2.3, 95% confidence interval (CI) 1.6–3.1, P < 0.0001], diagnosis to treatment < 1 year (OR = 2.1, 95% CI 1.5–2.9, P < 0.0001), neutrophilia (>ULN; OR = 1.9, 95% CI 1.3–2.9, P = 0.0021), thrombocytosis (>ULN; OR = 1.7, 95% CI 1.2–2.4, P = 0.0068), and anemia (<LLN; OR = 1.6, 95% CI 1.1–2.2, P = 0.0058). Of note, hypercalcemia, elevated LDH, and non-clear-cell histology were not statistically significant on multivariable analysis.
subsequent second-line therapy and outcomes
One hundred and eight (40%) VEGF-refractory patients proceeded to receive second-line therapies versus 43% in non-primary refractory patients (P = 0.38). These included VEGF inhibitors [sunitinib (n = 32), sorafenib (n = 44), axitinib (n = 2), bevacizumab (n = 4)], mTOR inhibitors [temsirolimus (n = 14), everolimus (n = 11)], or interferon alpha (n = 1). A comparison of patients that did receive second-line therapy versus those that did not is detailed in Table 2. Patients with better KPS and fewer poor prognostic factors were more likely to receive second-line targeted therapy. Patients with primary refractory disease had a poor outcome despite second-line targeted therapy. The response rate, PFS, and OS of second-line therapy were 9%, 2.5 months, and 7.4 months, respectively.
Table 2.
Baseline characteristics of primary PD patients receiving second-line therapy versus not receiving second-line therapy
| Characteristic | Second-line therapy (n = 108) | No second-line therapy (n = 164) | P-value |
| Age (mean, years) | 58.7 | 61.5 | 0.0572 |
| Gender (male, %) | 81 | 70 | 0.0541 |
| Heng prognostic group (%) | |||
| Favorable | 14 | 2 | 0.0002 |
| Intermediate | 59 | 52 | |
| Poor | 27 | 46 | |
| Median KPS (%) | 81 | 75 | 0.0005 |
| Diagnosis to treatment interval (years) | 1.70 | 2.33 | 0.1989 |
| Anemia (%) (<LLN) | 64 | 70 | 0.3185 |
| Neutrophils (mean) | 5.7 | 6.5 | 0.0615 |
| Platelet count (mean) | 316 | 372 | 0.0037 |
| Hypercalcemia (%) (>ULN) | 10 | 18 | 0.0732 |
| Elevated LDH (%) (>ULN) | 30 | 47 | 0.0185 |
| Brain metastases present (%) | 4 | 9 | 0.0849 |
| Prior nephrectomy (%) | 81 | 71 | 0.0685 |
| Non-clear-cell histology (%) | 14 | 13 | 0.8385 |
KPS, Karnofsky performance status; LDH, lactate dehydrogenase; LLN, lower limit of normal; PD, progressive disease; ULN, upper limit of normal.
Patients with primary refractory disease who received second-line VEGF inhibitors or mTOR inhibitors are compared in Table 3. Patients treated with mTOR inhibitors did not have statistically significant differences in prognostic categories (P = 0.4307) compared with those treated with VEGF inhibitors. However, the mTOR-treated patients had worse KPS, more patients with non-clear-cell histologies, and a trend toward fewer nephrectomies. The response rate, PFS, and OS of those receiving second-line VEGF versus mTOR inhibitors were 10% versus 6% (P = not significant), 2.8 versus 2.0 months (P = 0.069), and 7.9 versus 4.7 months (P = 0.40), respectively (Figure 3). The hazard ratio for PFS and OS of patients receiving second-line mTOR inhibitors versus VEGF inhibitors when adjusted for patient prognostic groups [3] was 1.584 (95% CI 0.915–2.741, P = 0.1001) and 1.453 (95% CI 0.774–2.725, P = 0.2447), respectively. These hazard ratios were not substantially changed when adjusted for non-clear-cell histologies.
Table 3.
Baseline characteristics of primary PD patients receiving second-line VEGF versus mTOR drugs
| Characteristic | Second-line VEGF (n = 82) | Second-line mTOR (n = 25) | P-value |
| Age (mean, years) | 58.7 | 58.4 | 0.9031 |
| Gender (male, %) | 82 | 76 | 0.5294 |
| Heng prognostic group (%) | |||
| Favorable | 14 | 14 | 0.4307 |
| Intermediate | 62 | 48 | |
| Poor | 24 | 38 | |
| Median KPS (%) | 83 | 76 | 0.0318 |
| Diagnosis to treatment interval (years) | 1.9 | 1.1 | 0.3215 |
| Anemia (%) (<LLN) | 67 | 55 | 0.3004 |
| Neutrophils (mean) | 5.4 | 6.5 | 0.1276 |
| Platelet count (mean) | 310 | 339 | 0.3804 |
| Hypercalcemia (%) (>ULN) | 8.7 | 14 | 0.4547 |
| Elevated LDH (%) (>ULN) | 28 | 35 | 0.5799 |
| Brain metastases (%) | 3.7 | 4 | 0.9372 |
| Prior nephrectomy (%) | 85 | 68 | 0.0512 |
| Non-clear-cell histology (%) | 9 | 29 | 0.0129 |
KPS, Karnofsky performance status; LDH, lactate dehydrogenase; LLN, lower limit of normal; mTOR, mammalian target of rapamycin; PD, progressive disease; ULN, upper limit of normal; VEGF, vascular endothelial growth factor.
Figure 3.
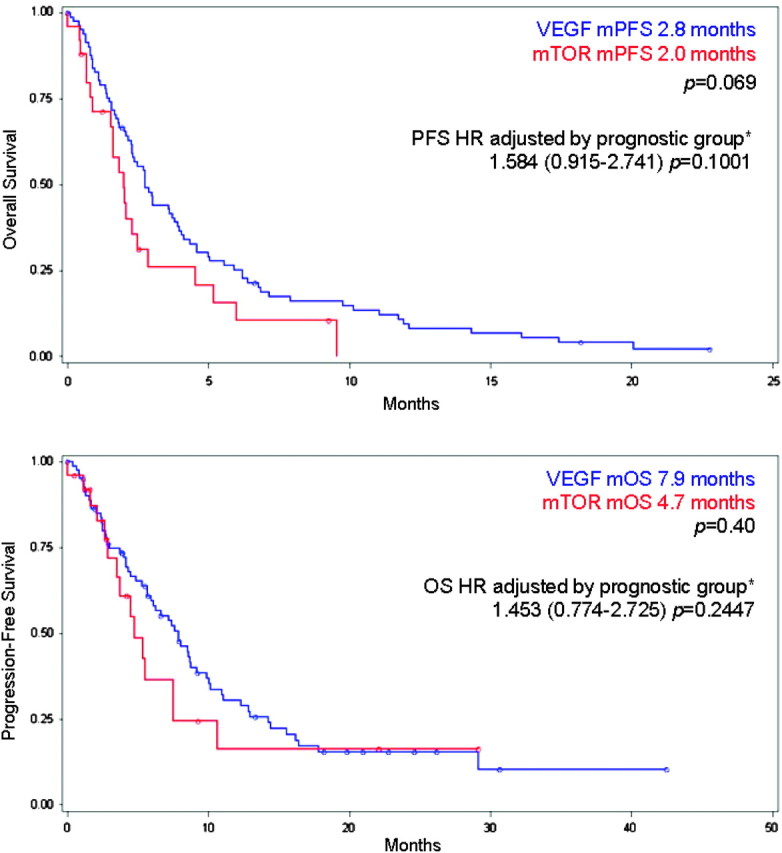
Progression-free survival (PFS) (top) and overall survival (OS) (bottom) of patients treated with second-line vascular endothelial growth factor (VEGF) versus mammalian target of rapamycin (mTOR) agents. Hazard ratio (HR) adjusted by prognostic group [3]. mPFS, median PFS; mOS, median OS.
discussion
Metastatic mRCC patients with progressive disease (PD) as their best response to a VEGF-targeted therapy are deemed primary refractory. This is the first initiative of a large, multicenter international collaboration to characterize and examine the outcomes of primary refractory disease.
The pathogenesis behind primary refractory disease is unknown. Several mechanisms of resistance to VEGF inhibitors have been postulated including angiogenic escape mechanisms in which other pathways including fibroblast growth factor and plasma placental growth factor promote angiogenesis even when the VEGF pathway is inhibited [17]. Tumors may also recruit supporting cells such as pericytes and bone marrow derived pro-angiogenic and inflammatory cells that may potentially override the VEGF blockade [17]. Finally, there may be mechanisms of tumor proliferation outside of angiogenesis in these primary refractory patients that remain to be elucidated. For example, there are case reports of patients with anti-VEGF-targeted therapy refractory disease that respond to cytotoxic chemotherapy regimes [18] and this needs to be prospectively evaluated further as a potential active therapeutic option.
The incidence of primary refractory disease in this study was 26%. This was higher than those found in the phase III clinical trials with the highest proportion being 21%. This is likely because clinical trials patients tend to be better selected with fewer patients having poor prognostic criteria, poor performance status, and brain metastases. It is important to recognize that the patients studied here are consecutive series of patients taken from academic and nonacademic centers to reflect the real-world experience of targeted therapy. This indeed may differ from clinical trials patients and may represent the translation from clinical trials efficacy to population-based effectiveness, which is more relevant to the oncologist's daily practice.
It is intuitive that patients with poorer prognostic profiles and patients with the component risk factors (including anemia, thrombocytosis, neutrophilia, poor KPS, and short interval from diagnosis to treatment) were more likely to have primary refractory disease. Hypercalcemia, which is a component of the Heng et al. criteria [3], was not significant in this multivariable analysis, which is limited by low patient numbers and the large number of covariates examined. Additionally, these prognostic factors were not entirely predictive because 6% of patients in the primary refractory group fell into the favorable prognostic group and 55% were deemed to be of intermediate risk. This suggests that clinical factors are not powerful enough to predict patients who have primary refractory disease and that perhaps biomarkers associated with the targeted pathways may need to be investigated to improve prediction.
The striking finding in this study is that second-line therapy in these patients was associated with dismal outcomes. The median PFS from initiation of second-line targeted therapy was only 2.5 months and the median OS was only 7.4 months. This is shorter than the reported 4.9 months PFS in patients who were treated with everolimus after progression on a VEGF inhibitor in the RECORD-1 trial [9] and this is likely because many of these patients initially responded to their first VEGF inhibitor. Additionally, the patients included in this study had more poor-risk criteria compared with those enrolled on to the RECORD-1 clinical trial. The AXIS trial comparing axitinib versus sorafenib in VEGF or immunotherapy-refractory patients revealed a PFS benefit of this new generation VEGF inhibitor after progression on an initial VEGF inhibitor [19]. However, many of the patients enrolled on to this trial were likely not primary refractory.
There was no significant difference between using an mTOR inhibitor or VEGF inhibitor as a subsequent targeted therapy after having primary refractory disease to the initial VEGF inhibitor. In fact, the outcomes numerically slightly favored subsequent VEGF-targeted therapy, although it was not statistically significant. This was somewhat surprising because it may be logical that switching the mechanism of action from a failed VEGF inhibitor to an mTOR inhibitor would have some efficacy as demonstrated in the phase III trial of everolimus [9]. However, these data support a hypothesis that primary refractory RCC may be driven largely by pathways independent of VEGF and/or mTOR and this biology dictates the overall outcome despite subsequent targeted therapy. Notably, however, 9% of patients with primary refractory disease did have a PR to second-line therapy. Thus, although exploration of novel mechanisms and therapeutics in this patient population is needed, the use of existing second-line therapy in this population should not be completely discounted. Further studies elucidating the patient characteristics and biomarkers of second-line responders are needed to better individualize therapy.
The strengths of this study include its multicenter international nature with patients of consecutive series to avoid selection bias. It is the largest study examining this poorly understood population of primary refractory patients. Additionally, this study reflected real-world outcomes that may not necessarily be seen in strict randomized controlled trials with selected patients. Limitations of this study include its retrospective nature, which may predispose the study to selection bias and issues with missing data. Selection bias was minimized by obtaining consecutive series of patients rather than handpicking certain types of patients at each center. Additionally, the missing data rate was low (<2% of all required data elements). The lack of a central radiology review, variable modalities of imaging, and intervals between scans were potential weaknesses; however, it better reflects the real-world experience of clinicians using targeted therapy. Finally, the small number of second-line mTOR-treated patients (n = 25) precludes our ability to determine whether the second-line VEGF or second-line mTOR strategy is superior.
Primary VEGF-refractory disease is an important entity that comprises ∼26% of the advanced RCC population treated with targeted therapy and is associated with a very poor prognosis. Stratification by primary refractory status should be considered in clinical trials evaluating novel therapies in pretreated mRCC. Investigation into the mechanism of primary resistance and alternative therapeutic strategies are urgently needed.
disclosure
DYH, JJK, GAB, CK, SN have received consulting honoraria from Pfizer and Novartis. JK, FD, GB, BR, TC have received research funding from Pfizer and Novartis. JK has received research funding from Bayer. This project received no direct or indirect industry or pharmaceutical company funding. The other authors declare no conflicts of interest.
Acknowledgments
This was presented as an oral presentation at the Genitourinary Cancers Symposium 2011 in Orlando, FL. This is a project of the International mRCC Database Consortium. We wish to thank all contributing institutions and data collectors.
References
- 1.Choueiri TK, Garcia JA, Elson P, et al. Clinical factors associated with outcome in patients with metastatic clear-cell renal cell carcinoma treated with vascular endothelial growth factor-targeted therapy. Cancer. 2007;110:543–550. doi: 10.1002/cncr.22827. [DOI] [PubMed] [Google Scholar]
- 2.Heng DY, Chi KN, Murray N, et al. A population-based study evaluating the impact of sunitinib on overall survival in the treatment of patients with metastatic renal cell cancer. Cancer. 2009;115:776–783. doi: 10.1002/cncr.24051. [DOI] [PubMed] [Google Scholar]
- 3.Heng DY, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol. 2009;27:5794–5799. doi: 10.1200/JCO.2008.21.4809. [DOI] [PubMed] [Google Scholar]
- 4.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 5.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 6.Escudier B, Pluzanska A, Koralewski P, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet. 2007;370:2103–2111. doi: 10.1016/S0140-6736(07)61904-7. [DOI] [PubMed] [Google Scholar]
- 7.Rini BI, Halabi S, Rosenberg JE, et al. Phase III trial of bevacizumab plus interferon alfa versus interferon alfa monotherapy in patients with metastatic renal cell carcinoma: final results of CALGB 90206. J Clin Oncol. 2010;28:2137–2143. doi: 10.1200/JCO.2009.26.5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28:1061–1068. doi: 10.1200/JCO.2009.23.9764. [DOI] [PubMed] [Google Scholar]
- 9.Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449–456. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 10.Molina AM, Ginsberg MS, Motzer RJ. Long-term response with everolimus for metastatic renal cell carcinoma refractory to sunitinib. Med Oncol. doi: 10.1007/s12032-010-9640-y. 2011 [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vickers MM, Choueiri TK, Rogers M, et al. Clinical outcome in metastatic renal cell carcinoma patients after failure of initial vascular endothelial growth factor-targeted therapy. Urology. 2010 doi: 10.1016/j.urology.2009.12.031. [DOI] [PubMed] [Google Scholar]
- 12.Filson CP, Redman BG, Dunn RL, Miller DC. Initial patterns of care with oral targeted therapies for patients with renal cell carcinoma. Urology. 2011 doi: 10.1016/j.urology.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sablin MP, Negrier S, Ravaud A, et al. Sequential sorafenib and sunitinib for renal cell carcinoma. J Urol. 2009;182:29–34. doi: 10.1016/j.juro.2009.02.119. discussion 34. [DOI] [PubMed] [Google Scholar]
- 14.Tamaskar I, Garcia JA, Elson P, et al. Antitumor effects of sunitinib or sorafenib in patients with metastatic renal cell carcinoma who received prior antiangiogenic therapy. J Urol. 2008;179:81–86. doi: 10.1016/j.juro.2007.08.127. [DOI] [PubMed] [Google Scholar]
- 15.Porta C, Procopio G, Cartenì G, et al. Sequential use of sorafenib and sunitinib in advanced renal-cell carcinoma (RCC): an Italian multicentre retrospective analysis of 189 patient cases. BJU Int. doi: 10.1111/j.1464-410X.2011.10186.x. 2011 May 23 [epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 17.Grepin R, Pages G. Molecular mechanisms of resistance to tumour anti-angiogenic strategies. J Oncol. 2010;2010:835680. doi: 10.1155/2010/835680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Richey SL, Ng C, Lim ZD, et al. Durable remission of metastatic renal cell carcinoma with gemcitabine and capecitabine after failure of targeted therapy. J Clin Oncol. 2010 doi: 10.1200/JCO.2010.31.6091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rini BI, Escudier B, Tomczak P, et al. Axitinib versus sorafenib as second-line therapy for metastatic renal cell carcinoma (mRCC): results of phase III AXIS trial. J Clin Oncol. 2011;29(Suppl) (Abstr 4503) [Google Scholar]


