Abstract
Release of hypocretins (orexins) by neurons in the lateral hypothalamus is an important contributor to arousal state, thermoregulation, feeding behavior, and has recently been proposed to play a role in breathing and central chemosensitivity. Using the in situ arterially perfused juvenile rat preparation, we determined the effect of hypocretin-1 (hcrt-1) and SB-408124 (antagonist for hypocretin receptor subtype 1, hcrt-r1) on phrenic nerve activity, a neural correlate of breathing (neuroventilation), and the neuroventilatory sensitivity to CO2. Application of hcrt-1 through the perfusate had little effect on baseline firing. Blocking hcrt-r1, however, prevented the phrenic burst frequency response normally associated with hypercapnia. These data suggest that endogenous hypocretinergic modulation enhances neuroventilatory chemosensitivity. Further studies using the in vitro medullary slice preparation explored the effect of hcrt-1 on hypoglossal nerve activity, a correlate of ventilation in vitro. Application of exogenous hcrt-1 failed to significantly alter hypoglossal burst output in neonatal rat slices, indicating that this portion of the neuroventilatory circuit is insensitive to hcrt-1. Taken together, these data suggest that hcrt-1 is a modulator of central chemosensitivity.
1 Introduction
Hypothalamic neuropeptides hypocretin-1 (orexin A) and -2 (orexin B) are synthesized in cells of the lateral hypothalamus that project widely throughout the brain (Peyron et al. 1998). Their extensive homeostatic roles include maintenance of arousal state, thermoregulation and contributions to feeding behavior. Additionally, both anatomical and functional evidence suggests that endogenous hypocretins modulate breathing. Axons of orexin-containing neurons project to respiratory-related nuclei including the nucleus tractus solitarius, pre-Botzinger Complex, hypoglossal and phrenic nuclei, as well as central chemoreceptor areas such as the raphé nuclei (Nakamura et al. 2007; Young et al. 2005; Fung et al. 2001; Peyron et al. 1998). Also, the preBotzinger Complex and the phrenic motor nuclei are immunoreactive for hcrt-r1 (Young et al. 2005), while hcrt-r2 is expressed in hypoglossal motoneurons (Volgin et al. 2002). Physiological evidence also indicates that hypocretins affect ventilation. Injection of hcrt-1 into the lateral ventricle of urethane-anesthetized mice results in a small increase in breathing frequency (∼11%) and a larger increase in tidal volume (∼75%) (Zhang et al. 2005). Administration of hcrt-1 into the preBotzinger Complex in vivo increases diaphragmatic electromyogram activity but does not affect breathing frequency (Young et al. 2005). These results suggest that hypocretin stimulates respiration primarily through increases in tidal volume. Nakamura et al. (2007) looked at the respiratory phenotype of a prepro-orexin knock-out mouse (which lacks both hcrt-1 and -2). Frequency and tidal volume showed sleep-wake dependency in both wildtype and knockout mice, however no significant differences in minute ventilation were observed between the two phenotypes.
Additionally, several studies have shown correlations between the hypocretinergic system and the hypercapnic chemoreflex. Firstly, hypocretin neurons themselves are chemosensitive in vitro (Williams et al. 2007; Sunanaga et al. 2009). Also, Nakamura et al. (2007) established a relationship between the effect of hypocretins on the response to hypercapnia and arousal state. No difference in responses to hypercapnia was observed between wildtype and prepro-orexin knock-out mice during sleep states, however the CO2 response was blunted in knock-outs when awake (Nakamura et al. 2007). Dias et al. (2009) also found a state-dependent effect of hypocretins on the ventilatory hypercapnic response; antagonism of hcrt-r1 in the retrotrapezoid nucleus blunted the response to hypercapnia predominantly in wakefulness.
The aim of the current study was to further examine the link between hypocretins and the modulation of breathing and the ventilatory hypercapnic response.
2 Methods
2.1 Arterially-Perfused In Situ Preparation
Experiments were performed on arterially-perfused in situ preparations derived from juvenile (P26-30; 60–100g) male albino rats. Details of this procedure have been published previously (Paton 1996; Toppin et al. 2007). After one hour of baseline recording at 5% CO2 balanced with 95% O2, CO2 in the perfusate was increased to 10% for 5 min. Comparing the phrenic burst parameters recorded at 10% CO2 relative to 5% CO2 gave us the normal neuroventilatory response to hypercapnia. The perfusate was then re-equilibrated with 5% CO2. Either hcrt-1 (3.0 and 30 nM final concentration; targets both hcrt-1r and hcrt-2r) or SB-408124 (3 μM, hcrt-r1 antagonist) was then dissolved in the artificial cerebrospinal fluid (aCSF) and allowed to circulate for 5 min. The preparation then underwent a second hypercapnic challenge in the presence of the pharmacological agent, or a sham treatment. Differences in the response to CO2 were compared between the drug and control aCSF. Statistical significance was determined using Student's paired t-tests.
2.2 In Vitro Medullary Slice Preparation
Transverse slices of the medulla (550–650 μm thick) were prepared from neonatal rats (P0-4) using a vibratome (see Smith et al. 1991 for details). Slices containing the pre-Botzinger Complex, the hypoglossal motor nuclei, and the most rostral hypoglossal nerve rootlets. were placed in a recording chamber and superfused with aCSF (composed of, in mM: 124 NaCl, 25 NaHCO3, 8 KCl, 1.5 CaCl2, 1.0 MgSO4, 0.5 NaH2PO4, and 30 D-glucose, equilibrated with 95% O2 and 5% CO2) warmed to 27°C (pH ∼7.4). Inspiratory-related motor discharge of the hypoglossal rootlets was recorded by glass suction electrodes. After 30 minutes of baseline recording, hcrt-1 (30 and 300 nM final concentration) was dissolved in the aCSF and allowed to superfuse the preparation for 10 minutes. The last 2 minutes of hcrt-1 application were used to determine the effect of the drug relative to baseline.
3 Results and Discussion
In the current study, neither dose of hcrt-1 significantly altered baseline neuroventilatory burst frequency or amplitude in the arterially perfused in situ preparation. The only effect observed was an increase in burst duration (Ti) following administration of 30 nM hcrt-1 (14 ± 4% increase). This is consistent with previous findings establishing the primary modulation of respiration by hypocretins is via changes in tidal volume. Moreover, exogenous hypocretin did not affect burst amplitude, a correlate to tidal volume in this preparation, although, the increase in burst duration at the higher dose could indicate a larger breath. Either hypocretin has little to no impact on baseline neuroventilation, there is a restriction to access of hypocretin across the blood-brain barrier, or sufficient endogenous hypocretin remained in the preparation such that addition of exogenous hypocretin produced no additional response. The hcrt-r1 antagonist (SB-408124) did not alter baseline phrenic burst amplitude, however it did slightly increase frequency (8 ± 2% increase from baseline). These data suggest that endogenous hcrt-r1 activation has no impact on neuroventilation other than a potential dampening of burst frequency.
Increasing CO2 to 10% in the perfusate produced a significant increase in burst frequency. It was hypothesized that since hypocretin appears to contribute to the hypercapnic ventilatory response, addition of hcrt-1 to the perfusate could enhance the response seen in situ. However, exogenous hypocretin did not alter this response (Fig. 1). Again, this suggests either that hypocretin is not involved with in situ chemoresponsiveness, or that endogenous hypocretin is sufficient. Adding the hrct-r1 antagonist effectively blunted the phrenic burst frequency response to CO2, indicating that endogenous activation of the hypocretinergic system enhances the ventilatory response to hypercapnia (Fig. 1).
Fig. 1.
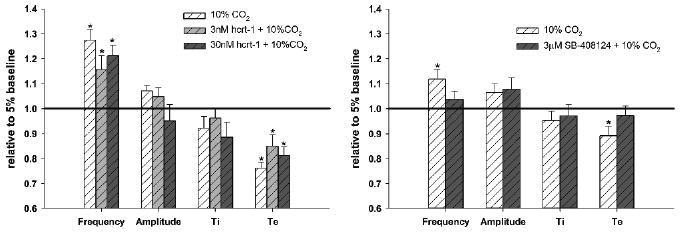
Effect of hcrt-1 and SB-408124 on the fictive ventilatory response to 10% CO2 in the perfused in situ rat preparation. Values are expressed relative to values at normocapnia (5% CO2) before (white hatched bars) and after administration of pharmacological agents (grey hatched bars). Differences from normocapnic baseline are denoted by * (paired t-test, P < 0.05)
We also recorded from neonatal rat rhythmic medullary slices to examine changes in hypoglossal burst activity in response to exogenous hcrt-1. While there existed much inter-slice variability, mean changes were not significant (Fig. 2). Although hypocretin levels in the cerebrospinal fluid prior to dissection exhibit a circadian rhythm (Desarnaud et al. 2004), the response did not depend on time of experiment.
Fig. 2.
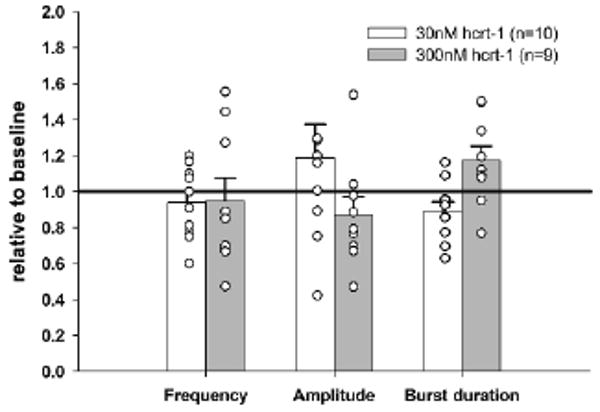
Mean changes in hypoglossal burst parameters in neonatal rat medullary slice preparations exposed to bath application of 30 and 300 nM hcrt-1. Circles represent individual data points
In summary, our data indicate that exogenous hypocretin has no impact on neuroventilation in situ or in vitro. Although hypothalamic hcrt producing neurons may be chemosensitive in vitro (Williams et al. 2007), our data suggest that hcrt is not acting directly as a neurotransmitter, transducing a chemosensory signal and activating respiratory control nuclei. Neuroventilatory sensitivity to CO2 is not enhanced by exogenous hcrt, but this sensitivity in situ is enhanced by endogenous hcrt. We postulate that the sensitivity and/or integration of central chemoreceptors is modulated by hcrt; that endogenous hcrt is sufficient to facilitate maximal sensitivity and enhances normal sensitivity and/or integration of central chemoreceptors. These data support a model where endogenous hcrt facilitation maximizes central chemosensitivity in wakefulness, and disfacilitation associated with reduced hcrt neuron activation during sleep results in reduction of ventilatory chemosensitivity normally associated with transitions from wakefulness to sleep. Facilitation of chemosensitivity by hcrt may also explain the relatively low sensitivity of such potentially chemosensitive neurons and circuits studied in vitro in the absence of endogenous hcrt facilitation, when compared to the chemosensitivity of more intact systems.
References
- Desarnaud F, Murillo-Rodriguez E, Lin L, Xu M, Gerashchenko D, Shiromani SN, Nishino S, Migno E, Shiromani PJ. The diurnal rhythm of hypocretin in young and old F344 rats. Sleep. 2004;27:851–856. doi: 10.1093/sleep/27.5.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias MB, Li A, Nattie EE. Antagonism of orexin receptor-1 in the retrotrapezoid nucleus inhibits the ventilatory response to hypercapnia predominantly in wakefulness. J Physiol. 2009;587(9):2059–2067. doi: 10.1113/jphysiol.2008.168260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung SJ, Yamuy J, Sampogna S, Morales FR, Chase MH. Hypocretin (orexin) input to trigeminal and hypoglossal motoneurons in the cat: A double-labeling immunohistochemical study. Brain Res. 2001;903:257–262. doi: 10.1016/s0006-8993(01)02318-6. [DOI] [PubMed] [Google Scholar]
- Nakamura A, Zhang W, Yanagisawa M, Fukuda Y, Kuwaki T. Vigilance state-dependent attenuation of hypercapnic chemoreflex and exaggerated sleep apnea in orexin knockout mice. J Appl Physiol. 2007;102:241–248. doi: 10.1152/japplphysiol.00679.2006. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Botzinger complex: A brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunanaga J, Deng BD, Zhang W, Kanmura Y, Kuwaki T. CO2 activates orexin-containing neurons in mice. Respir Physiol Neurobiol. 2009;166:184–186. doi: 10.1016/j.resp.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Toppin VA, Harris MB, Kober AM, Leiter JC, St-John WM. Persistence of eupnea and gasping following blockade of both serotonin type 1 and 2 receptors in the in situ juvenile rat preparation. J Appl Physiol. 2007;103:220–227. doi: 10.1152/japplphysiol.00071.2007. [DOI] [PubMed] [Google Scholar]
- Volgin DV, Saghir M, Kubin L. Developmental changes in the orexin 2 receptor mRNA in hypoglossal motoneurons. NeuroReport. 2002;13:433–436. doi: 10.1097/00001756-200203250-00014. [DOI] [PubMed] [Google Scholar]
- Williams RH, Jensen LT, Verkhratsky A, Fugger L, Burdakov D. Control of hypothalamic orexin neurons by acid and CO2. PNAS. 2007;104:10685–10690. doi: 10.1073/pnas.0702676104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JK, Wu M, Manaye KF, Kc P, Allard JS, Mack SO, Haxhiu MA. Orexin stimulates breathing via medullary and spinal pathways. J Appl Physiol. 2005;98:1387–1395. doi: 10.1152/japplphysiol.00914.2004. [DOI] [PubMed] [Google Scholar]
- Zhang W, Fukuda Y, Kuwaki T. Respiratory and cardiovascular actions of orexin-A in mice. Neurosci Lett. 2005;385:131–136. doi: 10.1016/j.neulet.2005.05.032. [DOI] [PubMed] [Google Scholar]


