Abstract
A published meta-analysis pooled individual studies by using the study-specific odds ratio (OR) or relative risk (RR) for the highest versus lowest category of soy or isoflavone intake from each study, but it should be problematic to make comparison between studies/populations for lung cancer risk as the quantiles are so different from different studies/populations. Therefore, we conducted a meta-analysis to explore the association between exposure of estimated daily soy protein intake in grams and lung cancer risk. We extracted ORs or RRs and 95% confidence intervals (CIs), converted them to the estimated ones for daily soy protein intake and pooled them using fixed or random effects models from 11 epidemiologic studies. Overall, the inverse association between daily grams of soy protein intake and risk of lung cancer was borderline statistically significant (OR=0.98, 95% CI=0.96 to 1.00); the inverse association was statistically significant in nonsmokers (0.96; 0.93–0.99) and stronger than in smokers (P for difference<0.05). No statistical significance for the associations was observed between genders, the origin of the participants, study design and types of soy intake. This study suggests a borderline reduction in risk of lung cancer with daily soy protein intake in grams, and a significant inverse association in nonsmokers.
Keywords: soy intake, lung cancer, meta-analysis
INTRODUCTION
Animal and population studies have suggested that soy intake may reduce the risk of lung cancer (1–3), but the methods and results from epidemiological studies, especially the measurements of soy food across studies/populations, have been variable (3–7). The measurements of soy food varied from gram per day of soybean products consumption in Chinese (4), gram per day of tofu products consumption in Singaporean (8), to μg per day of isoflavone intake in US populations (3). Furthermore, there was great variability among the studies in the definitions of high and low exposure. For example, in one study conducted in USA, high and low exposure were defined as consumption of isoflavone 1087.9 and 404.8 μg per day (3), whereas in another study conducted in China those were defined as consumption of soybean products > 8 and < 2 kg per year (4). One meta-analysis assessing the association between soy food intake and lung cancer risk indicated that the consumption of soy food is associated with lower lung cancer risk (9), but it did not use a standardized common measure to compare the exposures across studies, just used the study-specific OR or RR for the highest versus lowest category of soy or isoflavone intake which should be problematic to make comparison between studies as the quantiles are so different from different studies and the measurements are variable from different populations. Most meta-analysis on dietary intake and disease risk did not make the dietary measurements units consistent. To improve comparability of these different exposure levels and investigate the association between soy food intake and lung cancer risk, we conducted a meta-analysis to explore in detail the association of consumption of soy foods, using estimated daily grams of soy protein consumption, with risk of lung cancer.
MATERIALS AND METHODS
Search strategy, inclusion criteria, and data abstraction
We performed the following literature search from 1950 to November 2012 using PubMed (MEDLINE), EMBASE and Cochrane Library, without restrictions: soy intake (genistein, daidzein, soy, tofu, miso, natto, soybeans, diet, isoflavones, or phytoestrogens) and lung cancer (neoplasms / cancers / carcinomas / tumors). In addition, each reference that was obtained was reviewed for citations to articles that may have been missed in the search of the publication databases. An attempt was made to obtain unpublished data, additional references, and information through an internet search of soy, and phytoestrogens or isoflavones. Reports were included in the analysis if they were independent original articles excluding ecological and prevalence studies, estimating soy intake and reported data on the endpoints as lung cancer incidence. The reports contained the minimum information necessary to estimate the odds ratio (OR) or relative risk (RR) associated with soy intake and a corresponding measure of uncertainty (ie, 95% confidence interval [CI], standard error, variance, or P value of the significance of the estimate).
All data were extracted independently using a standardized form to assess the eligibility for inclusion by two investigators. Information were tabulated according to title, article’s first author’s name, year of publication, years of the study, origin of the participants, study characteristics (sample size, age range or mean age, sex), the measurement of soy intake, crude or adjusted odds ratio or relative risk of lung cancer risk and the corresponding confidence interval and confounding factors controlled for. When available, we used the adjusted risk estimates.
Classification of soy intake
Analyses were based on two related classifications of soy intake. First, the original measure of soy intake from each study (tofu, soy protein, soy foods or dietary isoflavone intake) was used and the risk associated with the largest difference in exposure between case patients and control subjects was extracted. However, both the measures used to quantify soy intake and the levels of soy intake varied considerably across studies. To permit comparison of exposure across studies using a standardized common measure, soy, tofu or isoflavone exposure in each study was converted to an estimate of grams of soy protein consumed daily. For some studies, the amount of soy protein (g/d) was estimated by multiplying the frequency of consumption (serving/d) by the corresponding portion size (g/serving). If the serving size were not provided, we used the information from the same or similar studies or national surveys. Soy food generally contains 7.02 g of soy protein in Singapore (10), 7.60 g in China (11) and 9.73 g (12) in Japan. Soy beans (per 100 g) contain 35 g of soy protein in China (13) and Japan (14). Regular tofu (100 g) contains 6.8 g or 8.1 g or 3.53 g of soy protein in Japan (14) or in China (13) or in Singapore (10), respectively.
To convert estimated dietary intake of total isoflavones (3, 5, 7, 8, 15) to estimates of soy protein intake, we used data from studies of healthy subjects in the United States (16, 17), in Japan (18) and in Singapore (10) to derive a ratio of 346 mg of soy protein per mg of isoflavone (United States) or 301 mg of soy protein per mg of isoflavone (Japan) or 312 mg (Singapore). For all studies in which the highest and lowest soy exposure categories included were open-ended, the midpoint of the lowest and highest quantiles was estimated if actual distribution data was not available in the report. The lowest quantile was defined as zero to the low quantile cutpoint reported in the study. For the highest quantile, its range was defined to be the same width as that of the second highest quantile reported in the study. Midpoints of these low and high ranges were used to convert differences in soy food intake to soy protein.
Data analysis
The ORs or RRs and 95% CIs for the comparison of the highest versus lowest soy exposure groups were extracted for individual studies. Before data were pooled, relative risks and corresponding standard errors from individual studies were transformed to their natural logarithms to stabilize the variances and to normalize the distributions. In an attempt to normalize the variability among the studies in the definitions of high and low exposure, we converted each comparison of high versus low soy exposure into an estimate of the corresponding grams of soy protein per day and then derived the odds ratio per gram of soy protein per day (19).
To estimate summary coefficients (i.e., per gram per day ORs) from each study we used the methods proposed by Greenland et al (20). The standard errors were derived from the confidence intervals provided in each study. Estimates of the average risk of soy intake on lung cancer and 95% CIs were calculated by using both fixed-effect (21) and DerSimonian and Laird random-effect (22) models. If a significant heterogeneity was present, we reported the pooled estimate from the random-effect models. Heterogeneity among studies was evaluated by the Cochran Q test (21, 23) and the I2 parameter (24).
Sensitivity analyses were performed by varying the assumptions used to calculate the exposure levels or by deleting studies based on factors associated with study quality or weight and then evaluating the impact of the changes on the pooled odds ratios. In addition, we further conducted pre-specified subgroup analyses by sex (men vs. women), smoking (yes vs. no), study design (case-control vs. cohort), origins of participants (Asian vs. American), the type of soy intake (soy/tofu vs. isoflavones), results adjusted for sex or energy or other dietary factors (yes vs. no) respectively, and compared the difference of effect sizes within each subgroup using the method described by Deeks (25). Publication bias was also assessed by the formal test: the Begg-adjusted rank correlation test (26), the Egger’s regression test (27). Statistical analyses were performed using MIX version 1.7 (28, 29) and STATA 11.0 (STATA Corp, College Station, Tex).
RESULTS
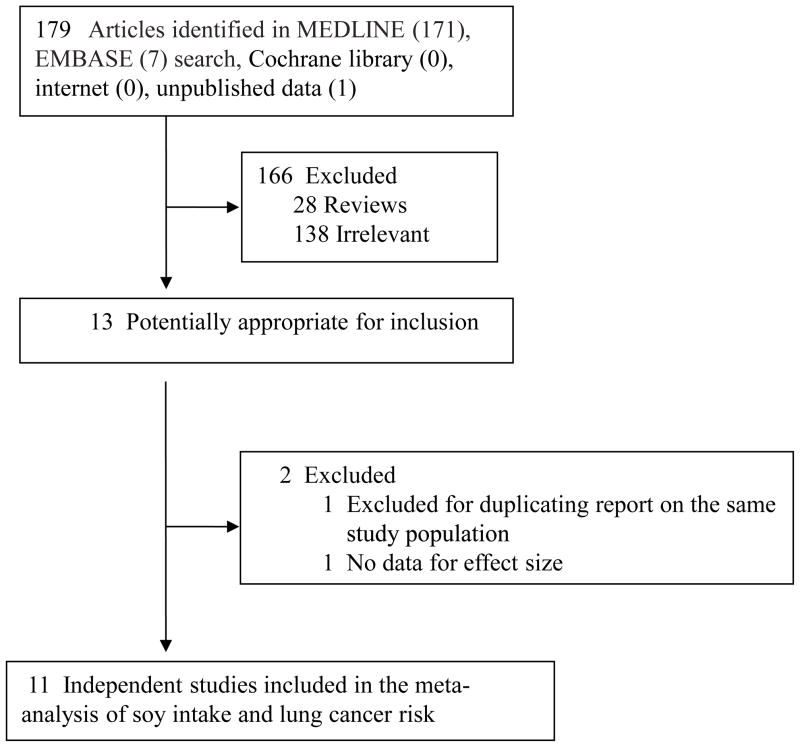
Detailed search steps are described in Figure 1. Briefly, from the initial literature search we identified and screened 179 articles. One hundred sixty-eight of these 179 articles were subsequently excluded from the meta-analysis, and 166 did not satisfy the inclusion criteria. One study (30) was excluded because it did not present the data for its CI of the effect size; the other study (31) was excluded because it was based on the same study populations as the included study (32) but with less cases and controls than the latter.
Figure 1.
Flowchart of selection of studies for inclusion in meta-analysis
Eleven studies (3–8, 15, 32–34) met the inclusion criteria and were included in the final analysis of lung cancer (Supplemental Table 1): 7 case-control studies (3, 4, 6, 15, 32–34) and 4 cohort studies (5, 7, 8, 35). Four studies used frequency of tofu (or soybean curd) consumption as the measure of soy intake (6, 8, 32, 34), 7 used estimated soy intake (4, 6, 8, 15, 33–35), and 6 used estimated total or individual isoflavone intakes (3, 5, 7, 8, 15, 35). The final dataset for our meta-analysis on soy intake and lung cancer included 231,494 participants with a total of 6,811 lung cancer cases.
There was great variability among the studies in the definitions of high and low exposure (Supplemental Table 1). For example, in one study (3), high exposure was defined as consumption of isoflavone 1087.9 μg per day, whereas in another study (4) the difference intake between the high and low exposure categories was 26 g of soy protein / day. In an attempt to normalize this variability, we converted each comparison of high versus low soy exposure into an estimate of the corresponding grams of soy protein per day and then derived the odds ratio per gram of soy protein per day (Table 1). Most of the estimated ORs (95% CIs) per gram soy protein per day were a little weak than the original ORs (95% CIs). It is notable that the lowest odds ratio estimate came from the study by Schabath et al (3).
Table 1.
Odds ratios (ORs) and 95% confidence intervals (CIs) for lung cancer risk of daily soy protein intake for individual studies
| Author, year | No. of case patients | Original measure of soy intake | High vs. low exposure difference
|
Original OR (95% CI) (high vs. low exposure) | OR (95% CI) per gram soy protein per day | |
|---|---|---|---|---|---|---|
| Original soy measure a | Grams of soy protein per day | |||||
| Koo 1988 (6) | 88 | Tofu/soy, per month | 0.5, 12.5 | 0.07, 1.69 | 0.28 (0.07, 1.16) | 0.46 (0.19, 1.10) |
| Wu-Williams 1990 (33) | 965 | Soy bean product, per year | 76.5, 544.5 | 0.07, 0.52 | 1.00 (0.80, 1.30) | 1.00 (0.61, 1.79) |
| Hu 1997 (4) | 227 | Soy bean product, kg/year | 1.0, 9.5 | 0.96, 9.10 | 0.60 (0.40, 1.10) | 0.94 (0.89, 1.01) |
| Wakai 1999 (men) (34) | 245 | Soybeans, per day | 0.1, 1.9 | 0.29, 4.69 | 0.63 (0.40, 0.98) | 0.59 (0.35, 0.98) |
| Wakai 1999 (men) (34) | 245 | Tofu, per day | 0.3, 1.0 | 0.85, 3.40 | 0.72 (0.50, 1.04) | 0.88 (0.76, 1.02) |
| Wakai 1999 (women) (34) | 88 | Soybeans, per day | 0.1, 1.9 | 0.29, 4.69 | 1.09 (0.49, 2.39) | 1.10 (0.44, 2.69) |
| Wakai 1999 (women) (34) | 88 | Tofu, per day | 0.3, 1.0 | 0.85, 3.40 | 1.00 (0.54, 1.86) | 1.00 (0.79, 1.28) |
| Takezaki 2001 (adenocardinomas, men) (32) | 367 | Tofu, per week | 0.5, 8.5 | 0.24, 4.13 | 1.24 (0.83, 1.85) | 1.06 (0.95, 1.17) |
| Takezaki 2001 (adenocardinomas, women) (32) | 240 | Tofu, per week | 0.5, 8.5 | 0.24, 4.13 | 0.52 (0.30, 0.91) | 0.85 (0.73, 0.98) |
| Takezaki 2001 (squamous cell and small cell carcinomas, men) (32) | 381 | Tofu, per week | 0.5, 8.5 | 0.24, 4.13 | 1.23 (0.84, 1.81) | 1.06 (0.96, 1.17) |
| Takezaki 2001 (squamous cell and small cell carcinomas, women) (32) | 57 | Tofu, per week | 0.5, 8.5 | 0.24, 4.13 | 3.00 (0.72, 12.60) | 1.33 (0.92, 1.92) |
| Seow 2002 (smokers) (15) | 127 | Soy foods, per week | 55, 350 | 0.55, 2.96 | 1.53 (0.76, 3.11) | 1.19 (0.89, 1.60) |
| Seow 2002 (nonsmokers) (15) | 176 | Soy foods, per week | 55, 350 | 0.55, 2.96 | 0.47 (0.29, 0.76) | 0.73 (0.60, 0.89) |
| Schabath 2005 (overall) (3) | 1674 | Isoflavones, μg/d | 153.3, 793.05 | 0.05, 0.27 | 0.68 (0.54, 0.85) | 0.18 (0.06, 0.48) |
| Schabath 2005 (men) (3) | 900 | Isoflavones, μg/d | 202.4, 1299.5 | 0.07, 0.45 | 0.56 (0.41, 0.76) | 0.22 (0.10, 0.49) |
| Schabath 2005 (women) (3) | 774 | Isoflavones, μg/d | 131.9, 1055.2 | 0.05, 0.45 | 0.78 (0.57, 1.06) | 0.46 (0.17, 1.20) |
| Schabath 2005 (nonsmokers) (3) | 266 | Isoflavones, μg/d | 153.3, 793.05 | 0.05, 0.27 | 0.53 (0.30, 0.93) | 0.06 (0.004, 0.72) |
| Schabath 2005 (former smokers) (3) | 730 | Isoflavones, μg/d | 153.3, 793.05 | 0.05, 0.27 | 0.87 (0.63, 1.20) | 0.53 (0.12, 2.28) |
| Schabath 2005 (current smokers) (3) | 678 | Isoflavones, μg/d | 153.3, 793.05 | 0.05, 0.27 | 0.57 (0.39, 0.82) | 0.08 (0.01, 0.41) |
| Cutler 2008 (nonsmokers) (7) | 113 | Isoflavones, mg/d | 0.1, 54.2 | 0.02, 18.74 | 0.80 (0.41, 1.58) | 0.99 (0.95, 1.03) |
| Cutler 2008 (smokers) (7) | 647 | Isoflavones, mg/d | 0.1, 54.2 | 0.02, 18.74 | 1.03 (0.80, 1.34) | 1.00 (0.99, 1.02) |
| Seow 2009 (8) | 298 | Tofu and soy products, g/d | 15.4, 242.2 | 0.54, 8.55 | 0.75 (0.53, 1.05) | 0.97 (0.92, 1.01) |
| Seow 2009 (smokers) (8) | 109 | Tofu and soy products, g/d | 15.4, 242.2 | 0.54, 8.55 | 0.81 (0.45, 1.46) | 0.97 (0.91, 1.05) |
| Seow 2009 (nonsmokers) (8) | 189 | Tofu and soy products, g/d | 15.4, 242.2 | 0.54, 8.55 | 0.71 (0.47, 1.07) | 0.96 (0.91, 1.01) |
| Shimazu 2010 (men) (5) | 481 | Isoflavones, mg/d | 15, 78 | 4.52, 23.48 | 0.89 (0.67, 1.19) | 0.99 (0.98, 1.01) |
| Shimazu 2010 (women) (5) | 178 | Isoflavones, mg/d | 15, 77 | 4.52, 23.18 | 0.83 (0.54, 1.29) | 0.99 (0.97, 1.01) |
| Shimazu 2010 (male past smokers) (5) | 89 | Isoflavones, mg/d | 15, 78 | 4.52, 23.48 | 0.96 (0.50, 1.82) | 0.998 (0.964, 1.032) |
| Shimazu 2010 (male current smokers) (5) | 318 | Isoflavones, mg/d | 15, 78 | 4.52, 23.48 | 1.03 (0.72, 1.48) | 1.002 (0.983, 1.021) |
| Shimazu 2010 (male nonsmokers) | 74 | Isoflavones, mg/d | 15, 78 | 4.52, 23.48 | 0.43 (0.21, 0.90) | 0.956 (0.921, 0.994) |
| Shimazu 2010 (female nonsmokers) (5) | 158 | Isoflavone, mg/d | 15, 77 | 4.52, 23.18 | 0.67 (0.41, 1.10) | 0.979 (0.953, 1.005) |
| Yang 2012 (women) (35) | 370 | Soy protein, g/d | 5.0, 30.2 | 5.0, 30.2 | 0.85 (0.76, 0.95) | 0.97 (0.95, 0.99) |
| Yang 2012 (female nonsmokers) (35) | 340 | Soy protein, g/d | 5.0, 30.2 | 5.0, 30.2 | 0.85 (0.75, 0.95) | 0.97 (0.94, 0.99) |
| Yang 2012 (female nsmokers) (35) | 30 | Soy protein, g/d | 5.0, 30.2 | 5.0, 30.2 | 0.80 (0.53, 1.21) | 0.96 (0.88, 1.04) |
If the original published soy intake measure included open-ended categories for lowest and/or highest intake, the difference was estimated using the midpoints of these categories. If the actual midpoints were not provided, they were estimated as follows. For the lowest category the midpoint was taken as halfway between zero and the category cutpoint. For the highest category, the midpoint was taken as the category cutpoint plus half the width of the second highest category.
The odds ratio or relative risk of lung cancer associated with the soy intake in the highest category compared with the lowest category in each study and overall is shown in Supplemental Figure 1. Most of the associations were less than 1.0 and four (3, 15, 32, 35) were statistically significant. Two of the studies gave results stratified by smoking status (7, 15), three stratified by gender (5, 32, 34) and one by cancer specific histological type (32), but did not give the combined results, so the stratified results are shown. Overall, the inverse association was borderline statistically significant (OR = 0.98, 95% CI = 0.96 to 1.00). Compared with the pooled results based on the original measure of soy intake from each study (OR = 0.83, 95% CI = 0.72 to 0.96 for highest versus lowest category) (Supplemental Table 1), the overall soy-lung cancer inverse association based on estimated daily per gram soy protein intake was a little weak.
The findings from the sensitivity analyses showed that the overall risk estimates did not change after the exclusion of studies with the largest (32) and smallest (3) odds ratio (both pooled OR = 0.98, 95% CI = 0.96 to 1.00 after excluding the corresponding study). No confidence intervals changed in a way that would affect interpretation. We calculated pooled odds ratios after excluding the study with the largest weight (7) (pooled OR = 0.97, 95% CI = 0.95 to 1.00). The change of the pooled OR was 1% and the confidence interval did not change in a meaningful way. After excluding one study without the adjustment of smoking status, the pooled estimate (OR = 0.98, 95% CI = 0.96 to 1.00) was the same as the original result. Thus, the pooled result appears robust to potential influential observations.
The pooled risk estimates per gram soy protein intake per day did not significantly differ by gender, study design, the origin of participants and types of soy intake (0.96 [0.92–1.01] for soy/tofu intake and 0.99 [0.97–1.01] for isoflavones intake) (Table 2). The inverse associations appeared to be significantly stronger in nonsmokers (0.96 [0.93–0.99]) (P for difference < 0.001), which was consistent with the results based on original RRs (0.59 [0.49–0.71]). The P values for the homogeneity comparison between the same subgroup were similar between the results based on original OR (highest vs. lowest) and estimated OR per gram soy protein intake per day.
Table 2.
Pooled risk estimates of lung cancer risk associated with soy protein intakes (gram/day): subgroup analysis
| Factors Stratified | No. of Studies | Based on estimated RR (per gram soy protein intake per day)
|
Based on original RR (highest vs. lowest)
|
||||
|---|---|---|---|---|---|---|---|
| Odds Ratio (95% CI) | Pa | Pb | Odds Ratio (95% CI) | Pa | Pb | ||
| Soy/tofu/isoflavones | |||||||
| All studies | 11 | 0.98 (0.96, 1.00) | <0.001 | 0.83 (0.72, 0.96) | 0.003 | ||
| Sex | |||||||
| Men | 6 | 0.97 (0.90, 1.04) | 0.0003 | 0.66 | 0.80 (0.61, 1.06) | 0.005 | 0.89 |
| Women | 11 | 0.97 (0.95, 1.00) | 0.001 | 0.79 (0.67, 0.94) | 0.02 | ||
| Ever smokers | |||||||
| Yes | 5 | 1.00 (0.99, 1.01) | 0.10 | <0.001 | 0.91 (0.78, 1.05) | 0.14 | 0.0003 |
| No | 7 | 0.96 (0.93, 0.99) | 0.01 | 0.59 (0.49, 0.71) | 0.71 | ||
| Study design | |||||||
| Case-control | 7 | 0.94 (0.86, 1.04) | <0.001 | 0.11 | 0.83 (0.67, 1.04) | <0.001 | 0.84 |
| Cohort | 4 | 0.99 (0.98, 1.00) | 0.14 | 0.85 (0.74, 0.97) | 0.36 | ||
| Origin of the participants | |||||||
| Asian | 9 | 0.97 (0.95, 1.00) | 0.03 | 0.06 | 0.83 (0.70, 0.99) | 0.004 | 0.71 |
| American | 2 | 0.99 (0.93, 1.05) | 0.03 | 0.83 (0.60, 1.14) | 0.06 | ||
| The type of soy foods intake | |||||||
| Soy/Tofu | 8 | 0.96 (0.92, 1.01) | 0.007 | 0.03 | 0.82 (0.66, 1.01) | 0.002 | 0.54 |
| Isoflavones | 6 d | 0.99 (0.97, 1.01) | 0.02 | 0.80 (0.71, 0.89) | 0.19 | ||
| Sex adjusted | |||||||
| Yes | 9 | 0.98 (0.96, 1.00) | 0.0002 | 0.07 | 0.85 (0.74, 0.99) | 0.004 | 0.08 |
| No | 2 | 0.94 (0.88, 1.00) | 0.11 | 0.55 (0.34, 0.89) | 0.33 | ||
| Energy adjusted | |||||||
| Yes | 3 | 0.99 (0.97, 1.01) | 0.09 | 0.61 | 0.92 (0.79, 1.07) | 0.13 | 0.15 |
| No | 8 | 0.97 (0.94, 1.01) | <0.001 | 0.81 (0.68, 0.98) | 0.005 | ||
| Other dietary factors adjusted | |||||||
| Yes | 4 | 0.98 (0.96, 1.01) | 0.03 | 0.28 | 0.81 (0.69, 0.94) | 0.10 | 0.58 |
| No | 7 | 0.95 (0.90, 1.01) | 0.0002 | 0.85 (0.69, 1.04) | 0.003 | ||
CI: confidence interval.
Test for the homogeneity comparison between studies within subgroups.
Test for the homogeneity comparison between subgroups.
We also explored whether there was any evidence of a dose response by performing a weighted linear regression of log odds ratio versus the estimated soy protein exposure difference. The results provide only weak evidence of a dose response (data not shown).
The publication bias might not affect our results through a funnel plot (Supplemental Figure 2), Egger’s test (b = −0.88 and −2.04, P = 0.10 and 0.01 for overall and nonsmokers) and Begg’s test (z-score = −0.98 and −1.48, P = 0.33 and 0.14 for overall and nonsmokers).
DISCUSSION
The meta-analysis using a standardized common measure found a borderline reduction in risk of lung cancer with soy protein intake in grams per day. The inverse association was statistically significantly observed in nonsmokers and stronger than in smokers. The findings suggested that consumption of regular tofu (100 g) could decrease about 24%, 28% and 13% lung cancer risk among nonsmokers in Japan (14), China (13) and Singapore (10), respectively.
Because the individual studies we analyzed used different measurements of soy food intake in different populations and different definitions of high and low intake, they varied markedly in the actual exposure effect that they measured. Previous meta-analysis (9) did not use a standardized common measure to compare the exposures across studies, just used the study-specific OR or RR for the highest versus lowest category of soy or isoflavone intake which should be problematic to make comparison between studies as the quantiles are so different from different studies and different populations. In our analysis, the risk estimates based on a standardized common exposure measure were attempted to derive using estimated daily soy protein intake (grams) to improve comparability of these different exposure levels. This analysis found that the resulting risk estimates appeared robust to variation in the assumptions used to derive exposure. Although it is likely that conversion from the original exposure measures to estimate soy protein intake in grams per day entails some error, probably causing the borderline non-significant reduction of the risk in this analysis, it illustrates the large variation across studies in exposure differences. Analyses based on these estimates of soy protein intake showed similar odds ratios across subgroups, ranging from 0.94 to 1.00 per gram of daily soy protein intake.
The apparent reduction in lung cancer risk associated with greater soy intake was stronger in nonsmokers than in smokers with similar level of soy protein intake (g/day). This and the non-significant association in smokers could reflect a greater degree of uncontrolled confounding in the smokers. The subjects in the nonsmokers probably differ in other factors associated with a more health-conscious lifestyle. Such factors may be difficult to separate from the measurements of soy intake. However, the soy-lung cancer associations were evaluated with the adjustment with most of other lifestyle factors among both nonsmokers and smokers in all individual studies. We observed a non-significant protective effect of soy food intake on lung cancer risk for cohort studies. It is possible due to selection bias induced by differential participation rates. For example, control subjects who agree to participate may be more health conscious than the general population without lung cancer.
In our analysis, the study by Schabath et al gave the lowest odds ratio estimate (3), but the specific reasons why the odds ratio was very low were not clear. Therefore, we conducted a sensitivity analysis to estimate the overall relative risk after excluding this study. The findings showed that the overall risk estimate did not change after the exclusion of this study (pooled OR = 0.98, 95% CI = 0.96 to 1.00). In the study by Takezaki et al (32), the odds ratio of soy food intake on lung cancer risk was presented by cancer sites and gender. The combination of odds ratios from different cancer sites relative to a common reference group should use the method described by Hamling et al (36). However, the original paper did not provide the numbers for different levels of soy curds consumption in cases and controls. Thus, we could not get the pooled estimates using suitable combination method. We further conducted a sensitivity analysis to estimate the overall relative risk after excluding this study. The findings from the sensitivity analyses showed that the overall risk estimates did not change after the exclusion of study (pooled OR = 0.98, 95% CI = 0.96 to 1.00). No confidence intervals changed in a way that would affect interpretation. Therefore, the two studies did not materially affect our results.
Thus far, the biologic mechanism that the consumption of genistein or phytoestrogens or soy may be effective in delaying lung cancer progression was still explored. Soy isoflavone supplementation could decrease levels of endogenous oxidative DNA damage (37), and this may be one mechanism by which such compounds could help prevent cancer, since oxidative DNA damage has been associated with the process of carcinogenesis (38). The isoflavone genistein significantly inhibited (53.6%, P < 0.001) lung tumor nodule formation and metastasis in mice (39). Genistein treatment also increased the life span of the tumor-bearing animals (47.7%, P < 0.001), but the isoflavone daidzein had no significant effect on the reduction of lung metastasis induced by B16F-10 melanoma cells (39). Our analysis also showed the inverse association between soy intake and lung cancer risk, especially in nonsmokers.
The present study has some strength. Firstly, this study estimated the pooled results based on an estimated standardized common daily soy protein intake, thus the comparability of the different exposure levels from studies was improved. Secondly, our study included an exhaustive search strategy that would likely capture the most relevant studies. Thirdly, we collected the available studies of the effects of soy intake at different strata on lung cancer risk, thus the information may be comprehensive. Finally, as more data available recently it allowed us to conduct subgroup analysis across individual studies.
In summary, the meta-analysis suggests a borderline reduction in risk of lung cancer with estimated soy protein intake in grams per day, and a significant inverse association in nonsmokers. Given the less study numbers and borderline reduction in lung cancer risk from this analysis, further updated meta-analysis is required to confirm the beneficial effect of soy food intake on lung cancer risk when new individual study available.
Supplementary Material
Supplemental Figure 1. Association between daily soy protein intake and lung cancer risk in all studies in this meta-analysis
Squares represent the effect size for the relative risk odds ratio of lung cancer among subjects with lung cancer vs. those without. Size of the squares is proportional to the size of the cohorts. Error bars represent 95% confidence intervals (CIs). The diamond shape represents the pooled estimates within each analysis. Studies for the analysis were sorted by published year.
Supplemental Figure 2. Begg’s funnel plot with pseudo 95% confidence limits for the pooled estimates of lung cancer risk associated with the daily soy protein intake
The circles represent individual studies, and the horizontal line indicates the summary estimate of odds ratio. OR = logarithm of the odds ratio, SE = standard error of logarithm of the odds ratio. This figure also represents the funnel plot of the observed studies.
Supplemental Table 1. Studies of daily soy protein intakes and lung cancer risk used in meta-analysisa
Acknowledgments
This research was supported by the grants from National Institutes of Health.
References
- 1.Gallo D, Zannoni GF, De Stefano I, Mosca M, Ferlini C, et al. Soy phytochemicals decrease nonsmall cell lung cancer growth in female athymic mice. Journal of Nutrition. 2008;138:1360–1364. doi: 10.1093/jn/138.7.1360. [DOI] [PubMed] [Google Scholar]
- 2.Li DH, Yee JA, McGuire MH, Murphy PA, Yan L. Soybean isoflavones reduce experimental metastasis in mice. Journal of Nutrition. 1999;129:1075–1078. doi: 10.1093/jn/129.5.1075. [DOI] [PubMed] [Google Scholar]
- 3.Schabath MB, Hernandez LM, Wu XF, Pillow PC, Spitz MR. Dietary phytoestrogens and lung cancer risk. Jama-Journal of the American Medical Association. 2005;294:1493–1504. doi: 10.1001/jama.294.12.1493. [DOI] [PubMed] [Google Scholar]
- 4.Hu JF, Johnson KC, Mao Y, Xu T, Lin QS, et al. A case-control study of diet and lung cancer in northeast China. International Journal of Cancer. 1997;71:924–931. doi: 10.1002/(sici)1097-0215(19970611)71:6<924::aid-ijc2>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 5.Shimazu T, Inoue M, Sasazuki S, Iwasaki M, Sawada N, et al. Isoflavone intake and risk of lung cancer: a prospective cohort study in Japan. American Journal of Clinical Nutrition. 2010;91:722–728. doi: 10.3945/ajcn.2009.28161. [DOI] [PubMed] [Google Scholar]
- 6.Koo LC. Dietary Habits and Lung-Cancer Risk Among Chinese Females in Hong-Kong Who Never Smoked. Nutrition and Cancer-An International Journal. 1988;11:155–172. doi: 10.1080/01635588809513983. [DOI] [PubMed] [Google Scholar]
- 7.Cutler GJ, Nettleton JA, Ross JA, Harnack LJ, Jacobs DR, et al. Dietary flavonoid intake and risk of cancer in postmenopausal women: The Iowa Women’s Health Study. International Journal of Cancer. 2008;123:664–671. doi: 10.1002/ijc.23564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seow A, Koh WP, Wang RW, Lee HP, Yu MC. Reproductive Variables, Soy Intake, and Lung Cancer Risk among Nonsmoking Women in the Singapore Chinese Health Study. Cancer Epidemiology Biomarkers & Prevention. 2009;18:821–827. doi: 10.1158/1055-9965.EPI-08-0892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang WS, Va P, Wong MY, Zhang HL, Xiang YB. Soy intake is associated with lower lung cancer risk: results from a meta-analysis of epidemiologic studies. Am J Clin Nutr. 2011;94:1575–1583. doi: 10.3945/ajcn.111.020966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun CL, Yuan JM, Arakawa K, Low SH, Lee HP, et al. Dietary soy and increased risk of bladder cancer: The Singapore Chinese health study. Cancer Epidemiology Biomarkers & Prevention. 2002;11:1674–1677. [PubMed] [Google Scholar]
- 11.Yang G, Shu XO, Jin F, Zhang XL, Li HL, et al. Longitudinal study of soy food intake and blood pressure among middle-aged and elderly Chinese women. American Journal of Clinical Nutrition. 2005;81:1012–1017. doi: 10.1093/ajcn/81.5.1012. [DOI] [PubMed] [Google Scholar]
- 12.Nagata C, Inaba S, Kawakami N, Kakizoe T, Shimizu H. Inverse association of soy product intake with serum androgen and estrogen concentrations in Japanese men. Nutrition and Cancer-An International Journal. 2000;36:14–18. doi: 10.1207/S15327914NC3601_3. [DOI] [PubMed] [Google Scholar]
- 13.China Food Composition 2004. 2. Beijing: Peking University Medical Press; 2009. [Google Scholar]
- 14.Sugiyama Jogakuen University. Report. 2004. Food composition database in Sugiyama University standard tables of food composition in Japan. [Google Scholar]
- 15.Seow A, Poh WT, Teh M, Eng P, Wang YT, et al. Diet, reproductive factors and lung cancer risk among Chinese women in Singapore: Evidence for a protective effect of soy in nonsmokers. International Journal of Cancer. 2002;97:365–371. doi: 10.1002/ijc.1615. [DOI] [PubMed] [Google Scholar]
- 16.Maskarinec G, Singh S, Meng LX, Franke AA. Dietary soy intake and urinary isoflavone excretion among women from a multiethnic population. Cancer Epidemiology Biomarkers & Prevention. 1998;7:613–619. [PubMed] [Google Scholar]
- 17.U.S. Department of Agriculture. USDA National Nutrient Database for Standard Reference, Release 21. Report. Beltsville: USDA; 2008. [Google Scholar]
- 18.Nagata C, Shimizu H, Takami R, Hayashi M, Takeda N, et al. Soy product intake is inversely associated with serum homocysteine level in premenopausal Japanese women. Journal of Nutrition. 2003;133:797–800. doi: 10.1093/jn/133.3.797. [DOI] [PubMed] [Google Scholar]
- 19.Trock BJ, Hilakivi-Clarke L, Clarke R. Meta-analysis of soy intake and breast cancer risk. J Natl Cancer Inst. 2006;98:459–471. doi: 10.1093/jnci/djj102. [DOI] [PubMed] [Google Scholar]
- 20.Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;135:1301–1309. doi: 10.1093/oxfordjournals.aje.a116237. [DOI] [PubMed] [Google Scholar]
- 21.Fleiss JL. Statistical methods for rates and proportions. New York: Hohn Wiley and Sons; 1981. pp. 212–36. [Google Scholar]
- 22.DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled clinical trials; Controlled clinical trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 23.Egger M, Juni P, Bartlett C, Holenstein F, Sterne J. How important are comprehensive literature searches and the assessment of trial quality in systematic reviews? Empirical study. Health technology assessment (Winchester, England) 2003;7:1–76. [PubMed] [Google Scholar]
- 24.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (Clinical research ed) 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deeks JJ, Altman DG. statistical methods for examining heterogeneity and combining resuts from several studies in meta-analysis. In: Egger M, Davey Smith G, Altman DG, editors. Systematic Reviews in Health Care: Meta-analysis in Context. 2. London: BMJ Publication Group; 2001. pp. 285–312. [Google Scholar]
- 26.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics; Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 27.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical research ed); BMJ (Clinical research ed) 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bax L, Yu LM, Ikeda N, Tsuruta H, Moons KG. Development and validation of MIX: comprehensive free software for meta-analysis of causal research data. BMC medical research methodology. 2006;6:50. doi: 10.1186/1471-2288-6-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bax L, Yu LM, Ikeda N, Moons KG. A systematic comparison of software dedicated to meta-analysis of causal studies. BMC medical research methodology. 2007;7:40. doi: 10.1186/1471-2288-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Swanson CA, Mao BL, Li JY, Lubin JH, Yao SX, et al. Dietary Determinants of Lung-Cancer Risk - Results from A Case-Control Study in Yunnan Province, China. International Journal of Cancer. 1992;50:876–880. doi: 10.1002/ijc.2910500609. [DOI] [PubMed] [Google Scholar]
- 31.Matsuo K, Hiraki A, Ito H, Kosaka T, Suzuki T, et al. Soy consumption reduces the risk of non-small-cell lung cancers with epidermal growth factor receptor mutations among Japanese. Cancer Science. 2008;99:1202–1208. doi: 10.1111/j.1349-7006.2008.00812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takezaki T, Hirose K, Inoue M, Hamajima N, Yatabe Y, et al. Dietary factors and lung cancer risk in Japanese: with special reference to fish consumption and adenocarcinomas. British journal of cancer. 2001;84:1199–1206. doi: 10.1054/bjoc.2001.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu-williams AH, Dai XD, Blot W, Xu ZY, Sun XW, et al. Lung-Cancer Among Women in North-East China. British journal of cancer. 1990;62:982–987. doi: 10.1038/bjc.1990.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wakai K, Ohno Y, Genka K, Ohmine K, Kawamura T, et al. Risk modification in lung cancer by a dietary intake of preserved foods and soyfoods: findings from a case-control study in Okinawa, Japan. Lung cancer (Amsterdam, Netherlands) 1999;25:147–159. doi: 10.1016/s0169-5002(99)00051-3. [DOI] [PubMed] [Google Scholar]
- 35.Yang G, Shu XO, Chow W, Zhang XL, Li HL, et al. Soy food Intake and risk of lung cancer: evidence from the Shanghai Women’s Health Study and a meta-analysis. American Journal of Epidemiology. 2012 doi: 10.1093/aje/kws168. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hamling J, Lee P, Weitkunat R, Ambuhl M. Facilitating meta-analyses by deriving relative effect and precision estimates for alternative comparisons from a set of estimates presented by exposure level or disease category. Statistics in medicine. 2008;27:954–970. doi: 10.1002/sim.3013. [DOI] [PubMed] [Google Scholar]
- 37.Djuric Z, Chen G, Doerge DR, Heilbrun LK, Kucuk O. Effect of soy isoflavone supplementation on markers of oxidative stress in men and women. Cancer Letters. 2001;172:1–6. doi: 10.1016/s0304-3835(01)00627-9. [DOI] [PubMed] [Google Scholar]
- 38.Marnett LJ. Oxyradicals and DNA damage. Carcinogenesis. 2000;21:361–370. doi: 10.1093/carcin/21.3.361. [DOI] [PubMed] [Google Scholar]
- 39.Menon LG, Kuttan R, Nair MG, Chang YC, Kuttan G. Effect of isoflavones genistein and daidzein in the inhibition of lung metastasis in mice induced by B16F-10 melanoma cells. Nutrition and Cancer-An International Journal. 1998;30:74–77. doi: 10.1080/01635589809514644. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Association between daily soy protein intake and lung cancer risk in all studies in this meta-analysis
Squares represent the effect size for the relative risk odds ratio of lung cancer among subjects with lung cancer vs. those without. Size of the squares is proportional to the size of the cohorts. Error bars represent 95% confidence intervals (CIs). The diamond shape represents the pooled estimates within each analysis. Studies for the analysis were sorted by published year.
Supplemental Figure 2. Begg’s funnel plot with pseudo 95% confidence limits for the pooled estimates of lung cancer risk associated with the daily soy protein intake
The circles represent individual studies, and the horizontal line indicates the summary estimate of odds ratio. OR = logarithm of the odds ratio, SE = standard error of logarithm of the odds ratio. This figure also represents the funnel plot of the observed studies.
Supplemental Table 1. Studies of daily soy protein intakes and lung cancer risk used in meta-analysisa