Abstract
Background
Few studies have explored the effects of bisphosphonates on bony healing in patients undergoing spinal fusion surgery. Most previous studies used animal models and found that bisphosphonate shows negative effects on spinal fusion consolidation. We intended to evaluate the effect of a single-dose of zoledronic acid on the volume of the fusion-mass in lumbar spinal fusion.
Methods
A retrospective review was carried out on 44 patients with symptomatic degenerative lumbar spinal stenosis who underwent one or two-level posterolateral fusion from January 2008 and January 2011. They were divided into 4 groups: group 1, autograft and zoledronic acid; group 2, allograft and zoledronic acid; group 3, autograft alone; and group 4, allograft alone. Functional radiography and three-dimensional computed tomography scans were used to evaluate and quantify the volume of the fusion-mass. The visual analog scale (VAS), the Oswestry disability index (ODI), and the short form 36 (SF-36) were used to evaluate the clinical outcomes.
Results
The mean volume of the fusion-mass per level was 8,814 mm3, 8,035 mm3, 8,383 mm3, and 7,550 mm3 in groups 1, 2, 3, and 4, respectively, but there were no significant differences between the groups (p = 0.829). There were no significant decreases in the volume of the fusion-mass (p = 0.533) in the zoledronic acid groups (groups 1 and 2). The VAS, the ODI, and the SF-36 at the 6-month follow-up after surgery were not significantly different (p > 0.05) among the 4 groups. The VAS, the ODI, and the SF-36 were not correlated with the volume of the fusion-mass (p = 0.120, 0.609, 0.642).
Conclusions
A single dose of zoledronic acid does not decrease the volume of the fusion-mass in patients undergoing spinal fusion with osteoporosis. Therefore, we recommend that zoledronic acid may be used after spinal fusion in osteoporotic patients.
Keywords: Osteoporosis, Spinal fusion, Fusion-mass, Bisphosphonate, Zoledronic acid
Spinal lumbar fusion surgery, a relatively common surgical procedure, frequently utilizes bone grafts. Since the aging population is rapidly growing, there is an increasing demand for spinal fusion in elderly patients.1,2) These elderly patients are more likely to have osteoporosis than other patients,3) which is becoming an increasing burden on public health.4) Bisphosphonates, which are widely used to treat osteoporosis, are anti-catabolic drugs that are acidused to treat bone conditions such as stress-shielding or disuse osteoporosis that are the result of catabolism. Since bisphosphonates inhibit osteoclasts and thereby affect bone remodeling,5,6) they may have an effect on bone graft healing and cause deterioration of spinal fusion.
Few studies have explored the effects of bisphosphonates on bony healing in patients undergoing spinal fusion surgery. Most previous studies used animal models and found that bisphosphonate shows negative effects on spinal fusion consolidation, with dose-dependent effects on fusion rates and graft incorporation,7-10) However, studies of distraction osteogenesis, which uses bolus dosing of nitrogen-containing bisphosphonates, have shown significant increases in callus volume, mineral content, and strength in treated animals, even in the presence of stress shielding.11)
We investigated the effect of single-dose zoledronic acid (a third generation nitrogen-containing bisphosphonates) on lumbar spinal fusion. We hypothesized that single doses of zoledronic acid would not decrease the volume of the fusion-mass.
METHODS
In this retrospective study, we reviewed the records of 73 consecutive patients with symptomatic degenerative lumbar spinal stenosis who underwent single or two-level instrumented posterolateral lumbar fusion at our hospital between January 2008 and January 2011. Complete data were available for 44 cases (60.1%; 14 male and 30 female). This study was approved by the Institutional Review Board of the hospital, and informed consent was waived.
All of the patients included in this study had severe low back pain for at least 6 months, sciatica and/or neurogenic claudication. The indications for surgery were symptomatic, one or two level degenerative spinal stenosis at L3-L4 or L4-L5 as confirmed by plain radiography and supplementary magnetic resonance imaging. All of the patients had failed to respond to conservative therapies, such as bed rest, bracing, anti-inflammatory medications, and physical therapy. None of the patients had undergone previous spinal surgery involving decompression and/or fusion.
The patients were divided into 4 groups. Group 1 underwent bilateral posterolateral lumbar fusion with autogenous iliac and local bone grafting and systemic administration of zoledronic acid 5 mg. Group 2 underwent bone grafting with allogenous and autogenous local bone and systemic administration of zoledronic acid 5 mg. Group 3 underwent bone grafting with autogenous iliac and local bone grafting. Group 4 underwent allogenous and autogenous local bone grafting. There were 11 patients in each group.
Systemic zoledronic acid 5 mg was administered 2 weeks after surgery as a single IV infusion over 20 minutes to patients who were postmenopausal women or men 60 years or older. These patients presented with hip or vertebral (clinical or morphometric) fractures or T-scores ≤ -2.5 at the femoral neck or spine after evaluations to exclude secondary causes. Graft types were not randomized and were selected by surgeons who considered each patient's bone density (T-score). Allografts were chosen when the T-score, which evaluates bone density, was less than -2.0, whereas autogenous iliac bone grafts were chosen when the T-score was equal to or more than -2.0.
Surgical Procedure
The senior author (YSP) performed all procedures with decompression and posterolateral instrumented fusion at 1 or 2 levels in all 44 cases.
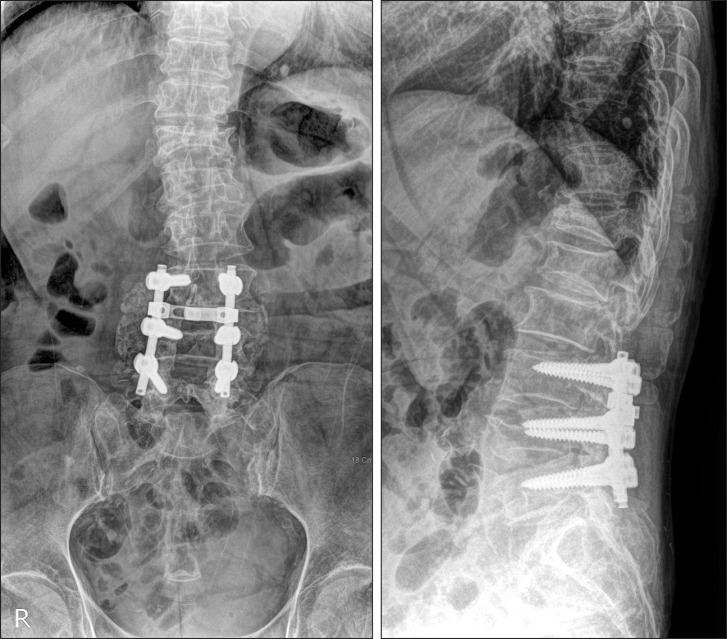
The bone graft material was a mixture of local bone harvested from the spinous process, small bits of bone removed by undercutting the hypertrophied lamina and facets, and autografts or allografts. Autografts were obtained from the posterior iliac crest. Allografts were fresh frozen, vacuum-sealed, nonradiated grafts supplied by a single bone bank and were defrozen in saline for 10 minutes before use. All of the attached soft tissues were removed. A bone mill was used to morselize the grafts and further separate the soft tissue from the bone. The graft volume used, as measured with an electronic scale, was 5 g per each side of the level. Finally, rods were applied using 6.0 or 6.5 mm cannulated titanium alloy pedicle screws and the screws were tightened to apply the compressive load to the bone graft (Fig. 1).
Fig. 1.
An 80-year-old woman received posterolateral fusion and pedicle screw fixation at L3-L5 for degenerative spinal stenosis.
Radiological Assessment
Functional radiography and three-dimensional (3D) computed tomography (CT) scans were used to evaluate the fusion status and the volume of the fusion-mass. An independent musculoradiologist who was blinded to the treatment groups evaluated the results of both procedures. The images were presented randomly on two occasions, 1 month apart, to assess intraobserver agreement. Serial radiological X-rays were performed at 1, 3, and 6 months postoperatively. Successful fusion was identified when the musculoradiologist found radiographic evidence of fusion and solid fusion was defined as continuous intertransverse bony bridging at the target level on the follow-up radiographs and CT scans.
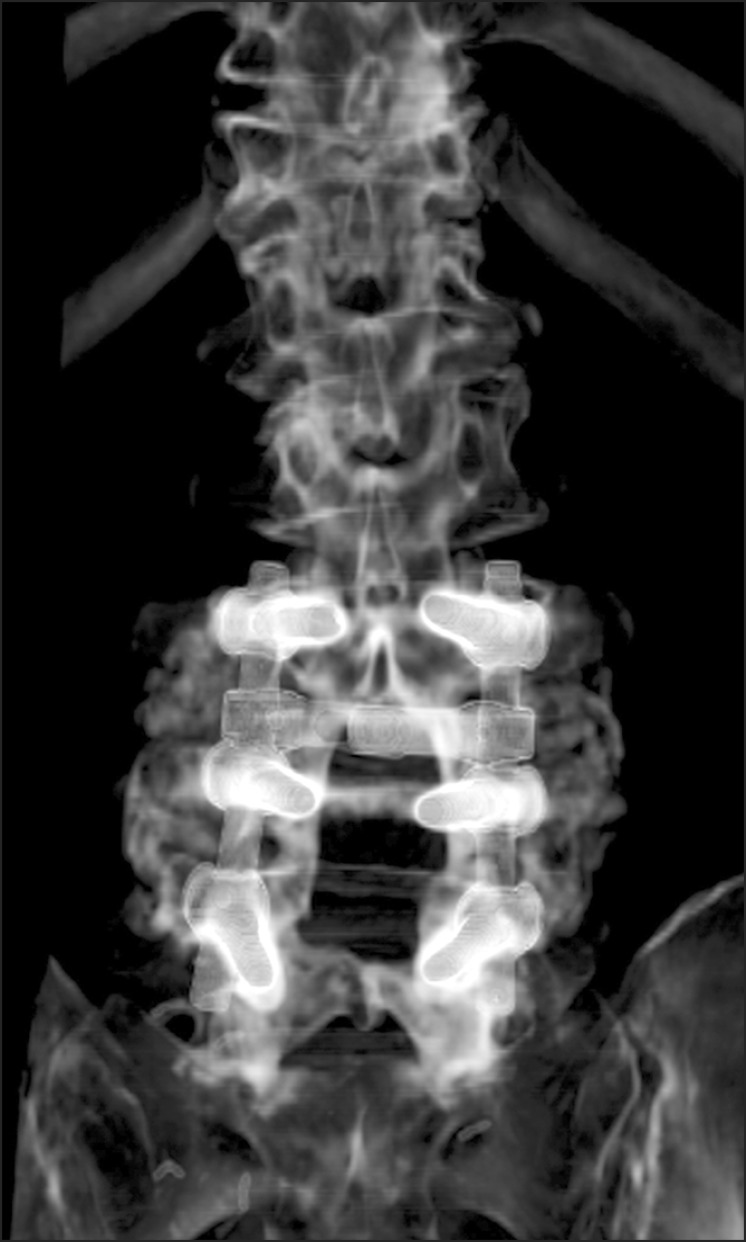
CT scans were performed 6 months postoperatively to quantify the volumes of the posterolateral fusion masses (Fig. 2). A 16-channel multidetector CT (Siemens, Midwest, MI, USA) was used to create axial images in the 1-mm cuts. These axial images were reformatted into sagittal and coronal images and 1 mm slices were made through each spine. The regions of interest were rendered bilaterally around the fusion masses to quantify the fusion bone volume in coronal images at the L3-L4 or L4-L5 level using PiView ver. 5.0.9.80 (Infinitt, Seoul, Korea) digital image viewing software (Fig. 3).
Fig. 2.
Computed tomography reconstruction scan of the lumbosacral spine was checked at 6 months after operation for better identification of successful fusion between the transverse processes.
Fig. 3.
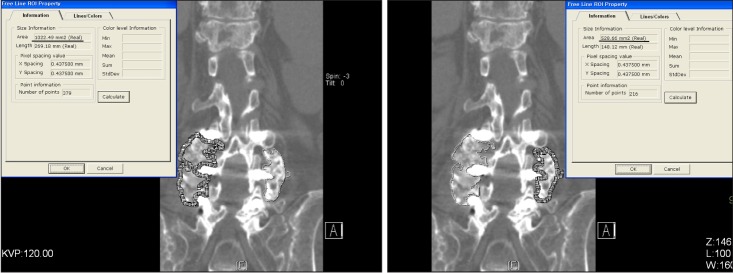
We used the regions of interest (ROI, dotted frame) that had been rendered around the fusion masses bilaterally to quantify the fusion volume in coronal images at the L3-L4 or L4-L5 level.
The fusion bone areas (mm2) of the left and right sides in 1 mm slices were then summed to yield the total volume of the fusion-mass (mm3) for each patient. Each fusion volume (mm3) was determined for the total bilateral fusion-mass. Values were reported as group averages and standard deviations.
Clinical Assessment
To evaluate low back and leg pain before and after surgery, the visual analog scale (VAS) and the Oswestry disability index (ODI) were recorded before and 6 months after surgery. Functional outcomes were assessed using the short form 36 (SF-36).12) SF-36 data were documented for 8 scales before surgery and 6 months after surgery; these scales were physical function, physical role, bodily pain, general health, vitality, social function, emotional role, and mental health.
SPSS ver. 17.0, (SPSS Inc., Chicago, IL, USA) was used for statistical analysis including the Kruskal-Wallis test, intraclass correlation coefficients test, and Pearson's chi-square test. A p-value < 0.05 was considered significant.
RESULTS
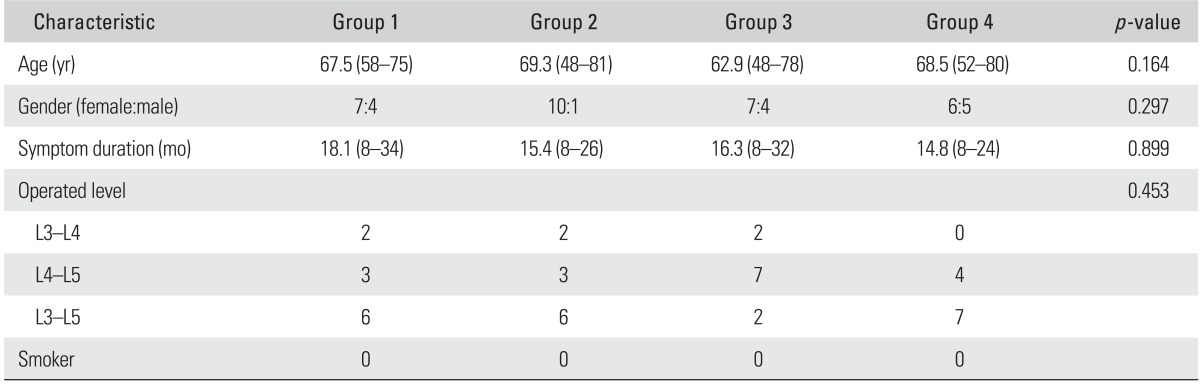
Baseline characteristics are presented in Table 1. The treatment groups were similar with respect to most demographic variables. There were no significant differences in the distributions of age, sex, and number of fused segments among the four groups (p > 0.05).
Table 1.
Demographic Characteristics
Radiologic Outcomes
Most patients showed bone union at 6 months after surgery. Solid fusion was observed in 11 of 11 cases (100%) in group 1, 11 of 11 cases (100%) in group 2, 11 of 11 cases (100%) in group 3, and 9 of 11 cases (82%) in group 4. There were no significant differences in fusion rates among the four groups (p = 0.105). The zoledronic acid groups (groups 1 and 2) did not significantly differ in fusion rates from the non-zoledronic acid groups (groups 3 and 4; p = 0.152).
At 6 months postoperative follow-up, the mean volume of the fusion-mass per level was 8,814 mm3 (range, 3,118 to 13,079 mm3), 8,035 mm3 (range, 2,592 to 12,658 mm3), 8,383 mm3 (range, 3,438 to 13,890 mm3), and 7,550 mm3 (range, 579 to 15,460 mm3) in groups 1, 2, 3, and 4, respectively. Although group 4 had the lowest volume, there were no significant differences among the four groups (p = 0.829). The zoledronic acid groups (groups 1 and 2) did not significantly differ from the non-zoledronic acid groups (groups 3 and 4) in the volume of the fusion-mass (p = 0.533). The intraobserver agreement was good (intraclass correlation coefficient = 0.908).
Clinical Outcomes
The VAS and the ODI improved significantly after surgery in all four groups (p < 0.05). The VAS and the ODI were not significantly different among the four groups at 6 months follow-up after surgery (p > 0.05). The results of quality of life analysis showed improvement of the SF-36 score as assessed by subscale scores at all follow-up time points after surgery (p < 0.05), but the SF-36 scores were not different between the groups at the 6-month follow-up after surgery (p > 0.05).
The volume of the fusion-mass and clinical outcomes
Relationships between the VAS, the ODI, and the volume of the fusion-mass did not significantly differ depending on the clinical outcome (p = 0.120, p = 0.609). The SF-36 score (used to evaluate functional outcomes) and the volume of the fusion-mass did not significantly differ (p = 0.642).
Complications
Complications were observed in groups 1 and 3 including chronic donor site pain/dysesthesia (1 case) and numbness in the buttock (1 case). However, these were not disabling complications. There were no infections, neurologic deficits, deep vein thromboses, dural tears, pedicle screw malpositions, or instrumentation failures in any of the patients. There were no significant differences in the complication rate among the 4 groups (p = 0.563).
DISCUSSION
Most of the previous studies evaluated the effects of alendronate administered for several weeks before or after spinal fusion in animal models, and showed negative effects on fusion rates.7-10) Pamidronic acid administered continuously for 4 weeks preoperatively and then daily for 4 weeks postoperatively was shown to have negative effects on lumbar fusion in a rabbit model.8)
In contrast, a recent study from Bransford et al.13) observed a 63% fusion rate after a single dose of zoledronic acid, compared to 25% in controls. However, Babat et al.8) found that pamidronic acid administered three times/week for 4 weeks preoperatively and then daily for 4 weeks postoperatively in the same rabbit model led to decreased fusion rates.
We used a single dose of zoledronic acid administered 2 weeks after surgery in the present study, to evaluate its effects on fusion in the light of these previous findings. A single dose of 5 mg of zolendronic acid administered intravenously once yearly was recommended for the treatment of postmenopausal osteoporosis.14) And the efficacy and safety of zolendronic acid (at a dose of 5 mg) after hip fracture was reported.15) A recent study reported that zolendronic acid infusion in the immediate postoperative period of hip fracture had no clinically evident effect on fracture healing.16) So we administered zolendronic acid 5mg at 2 weeks after surgery when the patients were stable, before discharge. We did not observe significant decreases in the volume of the fusion-mass associated with the administration of a single dose of zoledronic acid. Therefore, we suppose that it is not the drug itself, but the dose and the way it is administered, that affects fusion rate. A single dose of zoledronic acid administered 2 weeks after surgery did not affect the volume of the fusion-mass, unlike the results of chronic administration of bisphosphonate. But it is necessary to compare groups that receive continuous administration to groups that receive a single dose of zoledronic acid in future studies.
The recommended dosage of bisphosphonate for humans is 0.5-1.5 mg/kg17) on a monthly or quarterly basis. Zoledronic acid is a potent drug that is currently being tested in trials with once-per-year IV administration to treat osteoporosis, indicating that it is suitable for bolus single dose administration.18) Our purpose in using zoledronic acid in spinal fusion is to prevent osteoporosis and enhance drug adherence without negative effects on an indefinite basis, as must occur with continuous treatment.
Bisphosphonates may be used in osteoporotic patients who undergo spinal fusion not only to decrease the fracture risk associated with osteoporosis, but also to increase bone-screw fixation. Recent studies have shown that bisphosphonates can improve screw fixation in osteoporotic long bones19) and enhance bone-screw interface fixation in an experimental spine model.20) We observed no cases of osteoporotic fractures, implant failures, or loosening of screws during the follow-up period. Zoledronic acid administered 2 weeks postoperatively did not significantly influence the outcomes of the volume of the fusion-mass. Therefore, we recommend that it may be administered after spinal fusion in osteoporotic patients.
The methods that we used to assess fusion status were reliable. In the current study, we employed thin-slice helical CT for quantitative assessments of the volume of the fusion-mass. Based on these data, we believe that our radiographic assessments were reliable to assess fusion status. We also believe that the use of quantitative data to assess the volume of the fusion-mass was the key to obtaining reliable data in this study.
This study has some limitations. Our sample was small and the results derived were based on short-term follow-up of patients who were monitored for no more than 6 months. However, this study was designed to evaluate the effects of zoledronic acid on fusion, and other studies have provided data of the fusion status at 6 months.21-23) Although this study was statistically underpowered, we did not observe any significant differences in quantitative radiologic fusion status among the four groups, while we were able to show the influence of a single systemic dose of zoledronic acid on spinal fusion.
To our knowledge, there are no previous reports evaluating the effects of zoledronic acid on posterolateral spinal fusion in degenerative spinal stenosis. The present study demonstrated that a single systemic dose of zoledronic acid does not decrease the volume of the fusion-mass in spinal fusion. Goals of future research should include the addition of further anabolic stimuli and the control of premature fusion-mass catabolism without gross remodeling delay.
Our study shows that a single systemic dose of zoledronic acid does not decrease the volume of the fusion-mass in spinal fusion. Our conclusion is that zoledronic acid administered 2 weeks postoperatively does not have a significant influence on the surgical outcome of bone fusion. Therefore, we recommend that it may be administered after spinal fusion in osteoporotic patients.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Deyo RA, Gray DT, Kreuter W, Mirza S, Martin BI. United States trends in lumbar fusion surgery for degenerative conditions. Spine (Phila Pa 1976) 2005;30(12):1441–1445. doi: 10.1097/01.brs.0000166503.37969.8a. [DOI] [PubMed] [Google Scholar]
- 2.Katz JN. Lumbar spinal fusion: surgical rates, costs, and complications. Spine (Phila Pa 1976) 1995;20(24 Suppl):78S–83S. [PubMed] [Google Scholar]
- 3.Greenspan SL, Maitland LA, Myers ER, Krasnow MB, Kido TH. Femoral bone loss progresses with age: a longitudinal study in women over age 65. J Bone Miner Res. 1994;9(12):1959–1965. doi: 10.1002/jbmr.5650091216. [DOI] [PubMed] [Google Scholar]
- 4.Nagahama K, Kanayama M, Togawa D, Hashimoto T, Minami A. Does alendronate disturb the healing process of posterior lumbar interbody fusion? A prospective randomized trial. J Neurosurg Spine. 2011;14(4):500–507. doi: 10.3171/2010.11.SPINE10245. [DOI] [PubMed] [Google Scholar]
- 5.Fisher JE, Rogers MJ, Halasy JM, et al. Alendronate mechanism of action: geranylgeraniol, an intermediate in the mevalonate pathway, prevents inhibition of osteoclast formation, bone resorption, and kinase activation in vitro. Proc Natl Acad Sci U S A. 1999;96(1):133–138. doi: 10.1073/pnas.96.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hughes DE, Wright KR, Uy HL, et al. Bisphosphonates promote apoptosis in murine osteoclasts in vitro and in vivo. J Bone Miner Res. 1995;10(10):1478–1487. doi: 10.1002/jbmr.5650101008. [DOI] [PubMed] [Google Scholar]
- 7.Huang RC, Khan SN, Sandhu HS, et al. Alendronate inhibits spine fusion in a rat model. Spine (Phila Pa 1976) 2005;30(22):2516–2522. doi: 10.1097/01.brs.0000186470.28070.7b. [DOI] [PubMed] [Google Scholar]
- 8.Babat LB, McLain R, Milks R, Ferrara L, Sohn MJ. The effects of the antiresorptive agents calcitonin and pamidronate on spine fusion in a rabbit model. Spine J. 2005;5(5):542–547. doi: 10.1016/j.spinee.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 9.Lehman RA, Jr, Kuklo TR, Freedman BA, Cowart JR, Mense MG, Riew KD. The effect of alendronate sodium on spinal fusion: a rabbit model. Spine J. 2004;4(1):36–43. doi: 10.1016/s1529-9430(03)00427-3. [DOI] [PubMed] [Google Scholar]
- 10.Takahata M, Ito M, Abe Y, Abumi K, Minami A. The effect of anti-resorptive therapies on bone graft healing in an ovariectomized rat spinal arthrodesis model. Bone. 2008;43(6):1057–1066. doi: 10.1016/j.bone.2008.08.124. [DOI] [PubMed] [Google Scholar]
- 11.Little DG, Smith NC, Williams PR, et al. Zoledronic acid prevents osteopenia and increases bone strength in a rabbit model of distraction osteogenesis. J Bone Miner Res. 2003;18(7):1300–1307. doi: 10.1359/jbmr.2003.18.7.1300. [DOI] [PubMed] [Google Scholar]
- 12.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- 13.Bransford R, Goergens E, Briody J, Amanat N, Cree A, Little D. Effect of zoledronic acid in an L6-L7 rabbit spine fusion model. Eur Spine J. 2007;16(4):557–562. doi: 10.1007/s00586-006-0212-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Black DM, Delmas PD, Eastell R, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356(18):1809–1822. doi: 10.1056/NEJMoa067312. [DOI] [PubMed] [Google Scholar]
- 15.Lyles KW, Colon-Emeric CS, Magaziner JS, et al. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007;357(18):1799–1809. doi: 10.1056/NEJMoa074941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colon-Emeric C, Nordsletten L, Olson S, et al. Association between timing of zoledronic acid infusion and hip fracture healing. Osteoporos Int. 2011;22(8):2329–2336. doi: 10.1007/s00198-010-1473-1. [DOI] [PubMed] [Google Scholar]
- 17.Lin JT, Lane JM. Bisphosphonates. J Am Acad Orthop Surg. 2003;11(1):1–4. doi: 10.5435/00124635-200301000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Reid IR, Brown JP, Burckhardt P, et al. Intravenous zoledronic acid in postmenopausal women with low bone mineral density. N Engl J Med. 2002;346(9):653–661. doi: 10.1056/NEJMoa011807. [DOI] [PubMed] [Google Scholar]
- 19.Moroni A, Faldini C, Hoang-Kim A, Pegreffi F, Giannini S. Alendronate improves screw fixation in osteoporotic bone. J Bone Joint Surg Am. 2007;89(1):96–101. doi: 10.2106/JBJS.F.00484. [DOI] [PubMed] [Google Scholar]
- 20.Xue Q, Li H, Zou X, et al. Alendronate treatment improves bone-pedicle screw interface fixation in posterior lateral spine fusion: an experimental study in a porcine model. Int Orthop. 2010;34(3):447–451. doi: 10.1007/s00264-009-0759-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glassman SD, Dimar JR, Carreon LY, Campbell MJ, Puno RM, Johnson JR. Initial fusion rates with recombinant human bone morphogenetic protein-2/compression resistant matrix and a hydroxyapatite and tricalcium phosphate/collagen carrier in posterolateral spinal fusion. Spine (Phila Pa 1976) 2005;30(15):1694–1698. doi: 10.1097/01.brs.0000172157.39513.80. [DOI] [PubMed] [Google Scholar]
- 22.Singh K, Smucker JD, Gill S, Boden SD. Use of recombinant human bone morphogenetic protein-2 as an adjunct in posterolateral lumbar spine fusion: a prospective CT-scan analysis at one and two years. J Spinal Disord Tech. 2006;19(6):416–423. doi: 10.1097/00024720-200608000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Seo WY, Park YS, Cho JL. Does a radiolucent zone surrounding the pedicle screws mean nonunion? J Korean Orthop Assoc. 2009;44(3):344–349. [Google Scholar]