Abstract
Multidisciplinary tumor board conferences foster collaboration among health care providers from a variety of specialties and help to facilitate optimal patient care. Typical cases from thoracic tumor board conferences include patients with known or suspected bronchogenic and esophageal carcinomas, as well as less common diseases such as thymomas and mesotheliomas. In most instances, the clinical questions revolve around the best options for establishing a diagnosis, staging the disease and directing treatment. This article describes and illustrates the clinical scenarios of three patients who were presented at our tumor board, focusing on management issues and the role of imaging. These patients had non-small cell lung cancer and mediastinal lymph node metastases; a small, growing ground glass nodule; and oligometastatic non-small cell lung cancer, respectively.
Keywords: Lung cancer, tumor board, multidisciplinary care, stage IIIA, ground glass nodule
Background
Multidisciplinary tumor board conferences foster collaboration among health care providers from a variety of specialties and help to facilitate optimal patient care. The weekly Thoracic Tumor Board conference at our institution includes representatives from Thoracic Radiology, Nuclear Medicine, Radiation Oncology, Medical Oncology, Thoracic Surgery, Pulmonary Medicine, and Pathology. Typical cases include patients with known or suspected bronchogenic and esophageal carcinomas, as well as less common diseases such as thymomas and mesotheliomas. In most instances, the clinical questions revolve around the best options for establishing a diagnosis, staging the disease and directing treatment. This article (Part 1) describes and illustrates the clinical scenarios of three patients who were presented at our tumor board, focusing on management issues and the role of imaging. These patients (cases 1–3) had non-small cell lung cancer (NSCLC) and mediastinal lymph node metastases; a small, growing ground glass nodule; and oligometastatic NSCLC, respectively. Part 2 describes three additional patients.
Case 1: NSCLC and N2 lymph node disease
History and imaging findings
A 52-year-old man who presented with chest pressure was found to have a possible lung lesion on chest radiography; a subsequent chest computed tomography (CT) scan confirmed the presence of a right upper lobe mass (Fig. 1a) and severe underlying emphysema (Fig. 1b). Mildly enlarged right hilar lymph nodes were noted; right paratracheal and subcarinal lymph nodes were at the upper limits of normal in size (Figs. 1c,d). The lung mass was intensely fluorodeoxyglucose (FDG) avid on positron emission tomography (PET) (maximum standardized uptake (SUVmax) 7.9), and right hilar lymph nodes were also abnormal (SUVmax 3.3) (Fig. 1e). A subcarinal lymph node was questionably hypermetabolic (Fig. 1e). The patient’s past medical history included emphysema, hypertension, and coronary artery disease, with a myocardial infarction at age 37 years. He had a 40 pack-year history of smoking, although he recently quit. Pulmonary function tests showed a forced vital capacity (FVC) of 3.39 L (76% of predicted) and forced expiratory volume in first second of expiration (FEV1) of 1.51 L (46% of predicted). A ventilation perfusion scan revealed perfusion of the upper, middle, and lower lung fields of 5.9%, 25.9%, and 19.2%, respectively, on the right and 10.6%, 23.5%, and 14.9%, respectively, on the left.
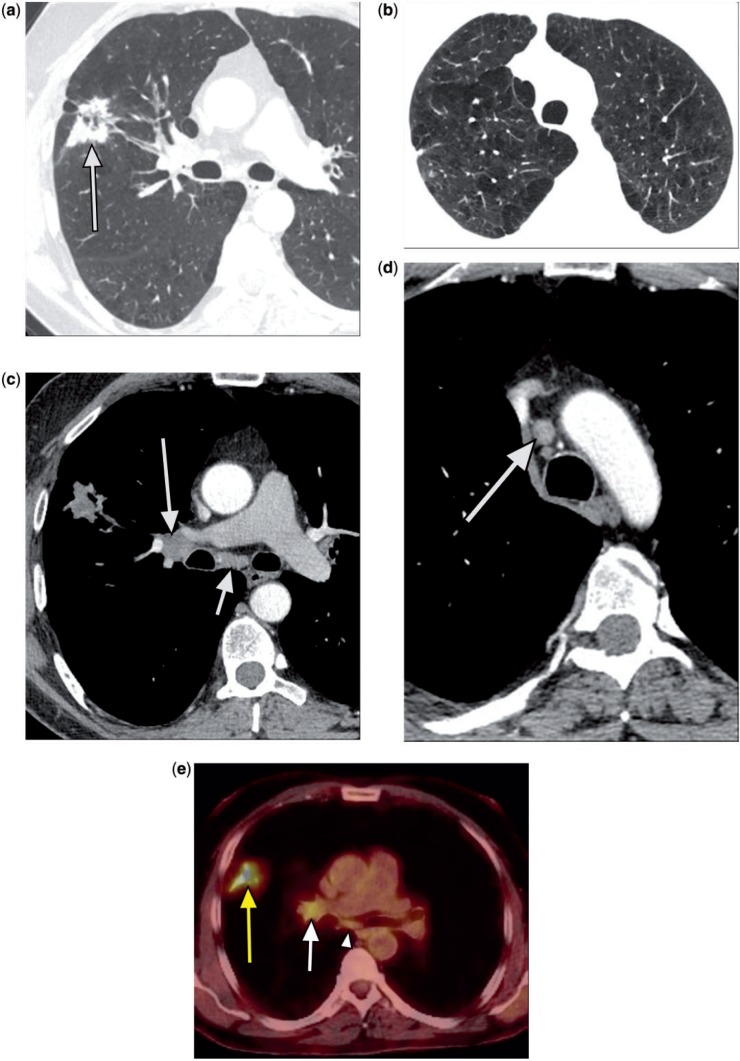
Figure 1.
A 52-year-old man. Chest CT showed a spiculated right upper lobe mass with central areas of lucency (a, arrow) and moderately severe centrilobular and paraseptal emphysema (Fig. 1b). There were mildly enlarged right hilar lymph nodes (c, long arrow); right paratracheal (d, arrow) and subcarinal (c, short arrow) lymph nodes were at the upper limits of normal in size. PET revealed an intensely FDG-avid lung mass (SUVmax 7.9) (e, yellow arrow), abnormal right hilar lymph nodes (SUVmax 3.3) (e, white arrow), and a questionably hypermetabolic subcarinal lymph node (e, arrowhead).
Tumor Board input and clinical course
At the recommendation of the Tumor Board, the patient was scheduled for a mediastinoscopy, to be followed by a thoracoscopic wedge resection of the right upper lobe mass, if the mediastinoscopy were negative for metastasis, and a possible lobectomy, if the wedge resection showed malignancy on frozen section. Unfortunately, frozen section of a subcarinal lymph node obtained at mediastinoscopy revealed metastatic poorly differentiated adenocarcinoma consistent with stage IIIA lung cancer. Further surgery was postponed until after administration of neoadjuvant chemoradiotherapy.
Management issues
When should a lung mass be biopsied preoperatively?
A lung mass should be biopsied when it is likely to change the patient’s management, and the benefits outweigh the risks. At our institution, a biopsy in a patient who appears to be a surgical candidate is not generally performed if the mass is suspicious on CT, hypermetabolic on PET, or has grown on serial imaging. If the suspicion is high, resection would be recommended regardless of the biopsy results. A biopsy is more useful when there are multiple nodules suspicious for metastases, if the diagnosis is questionable by CT and PET criteria, or if the patient is not a surgical candidate and a tissue diagnosis is required before initiation of chemotherapy or radiation therapy. A preoperative diagnosis may also be preferable if the patient has significant comorbidities increasing the risks of resection, or for a central lesion requiring a more extensive resection. Patient preference is also taken into account.
Why was mediastinoscopy performed in this case rather than proceeding directly to resection?
The mediastinal lymph nodes are pathologically staged before lung resection when there is a high suspicion for lymph node metastases on PET or CT. The majority are approached initially with endobronchial ultrasonography (EBUS), targeting any hypermetabolic or enlarged lymph nodes. For T1 lesions with negative mediastinal lymph nodes on PET and/or CT, pulmonary resection with a concurrent mediastinal lymph node dissection is performed without further preoperative mediastinal staging. However, for lesions greater than T1 (as in this patient), the risk of lymph node metastases increases from 6% to 30–40% for tumors 2–4 cm in size, especially for central tumors; therefore mediastinal lymph node sampling is warranted before lung resection in these patients[1,2]. On the other hand, mediastinal lymph nodes may be FDG avid due to granulomatous disease and not metastatic cancer, and therefore a tissue diagnosis of FDG-avid lymph nodes is warranted before excluding a patient from a potentially curative resection. In this patient, a mediastinoscopy was recommended due to the size of the primary tumor, hypermetabolic hilar (N1) lymph nodes, and questionable subcarinal activity seen on PET.
Why was mediastinoscopy performed in this patient rather than an EBUS-guided lymph node biopsy?
Although our initial approach to highly suspicious mediastinal lymph nodes is an EBUS-guided biopsy, false-negatives have been reported with normal-sized lymph nodes or those with minimal FDG avidity, likely due to sampling error, and mediastinoscopy remains the gold standard[3]. In this patient, the subcarinal lymph node was believed to be equivocal by PET and CT criteria. However, due to the mild PET activity and increased risk of mediastinal disease associated with a 4-cm tumor (and likely hilar (N1) disease), a mediastinoscopy was performed.
Why not resect the mass and then give postoperative adjuvant chemoradiation for N2 disease?
For stage IIIA disease, treatment options include definitive chemoradiation, neoadjuvant chemoradiation followed by resection, and resection followed by adjuvant chemotherapy with consideration of adjuvant radiation therapy. Patients with bulky multistation N2 disease are treated with definitive chemoradiation. Those with preoperatively diagnosed single-station N2 disease are treated with neoadjuvant chemoradiation, as in this case. Occasionally, patients thought to have stage I or II disease are found to have occult, microscopic N2 disease, diagnosed after mediastinal lymph node dissection; these patients are treated with adjuvant chemotherapy, with consideration of adjuvant radiation therapy[4].
Did the subcarinal location of the questionable node on PET influence treatment? Is subcarinal disease worse than N2 elsewhere?
Although subcarinal lymph node metastases are considered ipsilateral N2 disease, several series have identified subcarinal involvement as a predictor of poorer survival[5]. Patients with subcarinal lymph node involvement can be treated with neoadjuvant chemoradiation as long as they are single-station non-bulky disease. However, most patients with positive N2 lymph nodes do not have isolated disease and are treated with definitive chemoradiotherapy.
What role did marginal lung function play in this patient?
Although an FEV1 of 60% of predicted is preferable for patients undergoing a lobectomy, the patient’s initial FEV1 was marginal at 46% of predicted. However, further evaluation with a quantitative ventilation perfusion scan showed only 5.9% of perfusion to the right upper lung field, giving a postoperative predicted FEV1 of 43%, which is greater than the minimum desired postoperative value of 40% of predicted. His marginal but adequate, pulmonary function did not change the overall treatment plan, but significant comorbidities, symptoms at baseline, and marginal pulmonary function tests lowered the threshold for obtaining a preoperative tissue diagnosis of any potentially positive mediastinal lymph nodes.
Role of imaging
The identification of abnormal hilar lymph nodes at CT and PET, and the finding of a questionably abnormal subcarinal lymph node at PET, contributed to the decision to perform mediastinoscopy before attempted lung resection.
Case 2: small, growing, ground glass nodule
History and imaging findings
A 70-year-old woman had a history of a stage I adenocarcinoma of the right upper lobe treated with an open lobectomy in 2004. Follow-up surveillance CT studies demonstrated a growing left lower lobe ground glass nodule measuring 4 mm in May 2006 (Fig. 2a), 7 mm in November 2007 (Fig. 2b), and 8 mm in May 2008 (Fig. 2c). A CT-guided core needle biopsy in May 2008 (Fig. 2d) showed alveolar parenchyma without significant abnormality; no neoplasm or granulomas were identified. A follow-up CT in November 2008 showed growth of the lesion to approximately 9 mm (Fig. 2e).
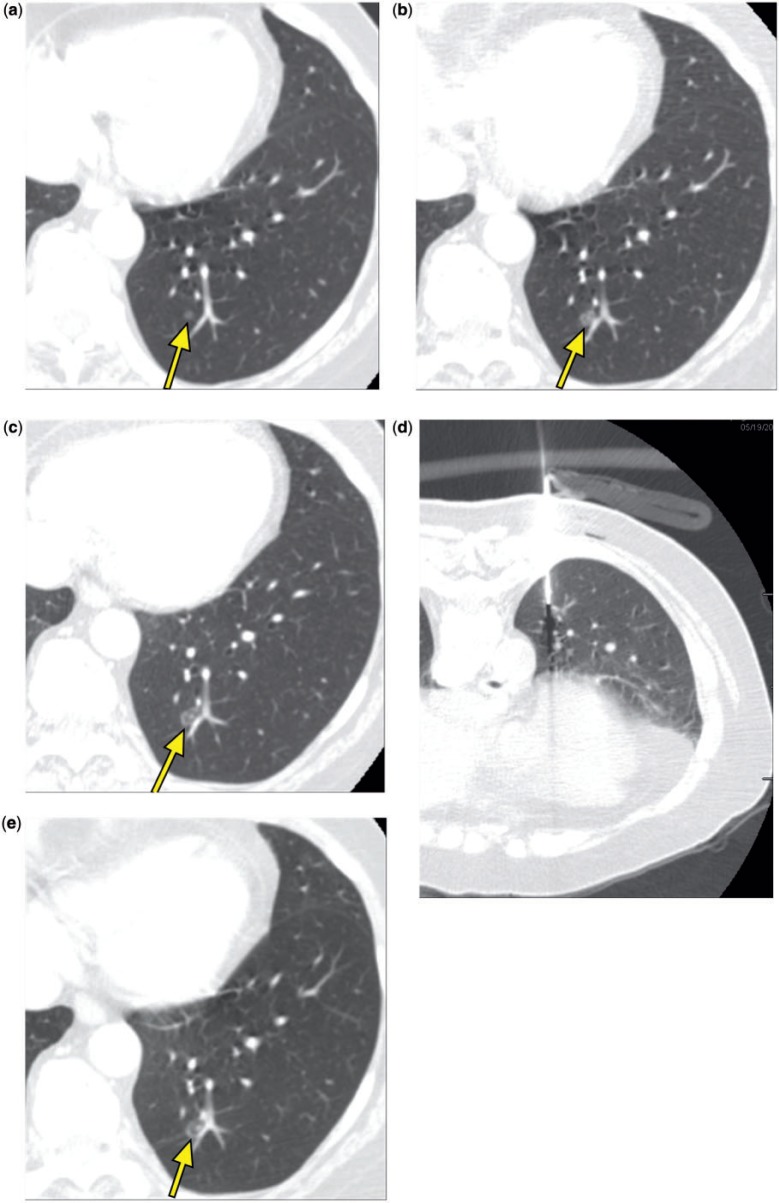
Figure 2.
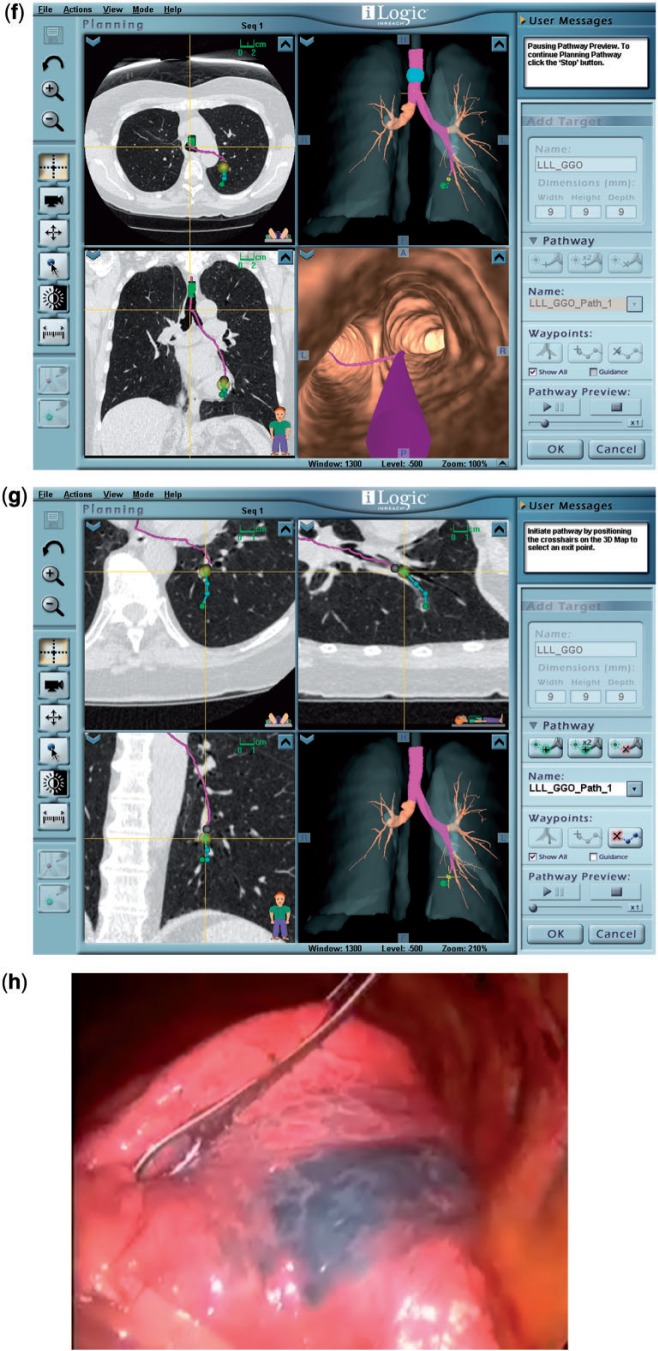
A 70-year-old woman. Chest CT showed a left lower lobe ground glass nodule that measured 4 mm in May 2006 (a, arrow), 7 mm in November 2007 (b, arrow), and 8 mm in May 2008 (c, arrow). A CT-guided core needle biopsy in May 2008 (d) showed alveolar parenchyma without significant abnormality. In November 2008, the lesion measured approximately 9 mm (e, arrow). (f) A view of the planning software module for ENB demonstrating axial (top left) and coronal (bottom left) views of the computer-generated pathway leading to the lesion. The three-dimensional view of the bronchial tree is seen in the top right panel with the pathway projected within it, and the airway tip view is shown on the bottom right. (g) A magnified view that demonstrates editing the pathway in the small, peripheral airways; the blue dots indicate navigation points along the airways leading to the lesion. (h) The area with indigo carmine staining that was visually identified during VATS.
Tumor Board input and clinical course
The patient wished to have the nodule removed, and she was referred to the Tumor Board for consideration of resection of the lesion. Pulmonary function testing showed an FEV1 of 1.68 L (94%) and a carbon monoxide diffusing capacity (Dlco) of 13.20 (68%). The Tumor Board recommended resection; however, due to the location of the nodule and the ground glass appearance, the surgeon believed that intraoperative localization using tactile sensation would be difficult. Therefore, to facilitate intraoperative nodule identification, she was referred for preoperative localization using electromagnetic navigational bronchoscopy (ENB) with pleural dye instillation and metal coil marking.
ENB was planned using CT-based three-dimensional reconstruction of the airways, localization of the target nodule, and plotting of the pathway with simulated airway navigation (InReach, superDimension, Minneapolis, MN) (Fig. 2f,g). During the bronchoscopic procedure, after registration of airway landmarks with a locatable guide-extended working channel, the lesion was located using real-time electromagnetic navigation, and the overlying pleural surface was tattooed with 2.0 mL of indigo carmine dye using a 25G sclerotherapy needle. Two 2-cm long Visicoil linear fiducial markers (IBA Dosimetry, Bartlett, TN) were deployed in close proximity to the nodule. Later that day, during video-assisted thoracoscopic surgery (VATS), the area with indigo carmine staining was visually identified (Fig. 2h), and a wedge resection was carried out to include the tissue surrounding the coils. Gross examination showed a thickened area in the resected specimen. Frozen section was interpreted as consistent with a granuloma. The final pathology report described a 9-mm diameter bronchioloalveolar adenocarcinoma. The surgical margins and visceral pleura were free of tumor. There was also an incidental non-necrotizing granuloma.
Management issues
How likely is a ground glass opacity to be malignant?
Although the natural history of ground glass opacities (GGOs) on CT scans remains obscure, persistent lesions are of concern. Given that many GGOs spontaneously resolve, it is difficult to list a comprehensive differential diagnosis, but it is safe to say that the differential should include malignant as well as infectious and inflammatory lesions[6–8]. Studies describing the proportion of malignant nodules among pure GGOs have a relatively short duration of follow-up, and are likely underestimations of the true incidence of malignancy in a GGO, given the slowly growing nature of these lesions. One series examining the natural history of pure GGOs showed that 26 of 69 (38%) pure GGOs less than 10 mm in diameter resolved spontaneously on follow-up imaging, whereas 57 of 117 (49%) mixed density GGOs also resolved[6]. Another study found the prevalence of malignancy to be 43% in GGOs less than 10 mm and 93% in GGOs larger than 20 mm[8]. The pathologic correlate of pure GGO lesions that are malignant is also a confusing topic; the term bronchioloalveolar carcinoma is being phased out by pathologists in favor of the following more up to date terms: atypical adenomatous hyperplasia (AAH) for lesions less than 5 mm in size; adenocarcinoma in situ for lesions 5–30 mm in size with pure lepidic patterns; and minimally invasive adenocarcinoma for lesions up to 30 mm in size with less than 5 mm diameter of an invasive/stromal response[9]. The classification of a GGO lesion on CT is highly dependent on the scan parameters, particularly slice thickness, as thicker sections may lead to an inaccurate estimation of the solid components of a small (<10 mm) nodule.
What is the optimal management of a GGO?
In this patient with a history of lung cancer, there was a non-diagnostic percutaneous biopsy of the nodule. There are no studies specifically addressing the accuracy of ENB for the diagnosis of GGOs, but the overall accuracy of ENB for peripheral nodules is increasing with time and is over 80% in some series; accuracy is greatest for nodules at the end of an identifiable airway[10–12].
An argument can be made for serial observation of ground glass lesions, but in the face of a lung cancer history and nodule growth, and given the preferences of an otherwise healthy patient, resection is a reasonable option. Unfortunately, resection may be very difficult for a surgeon who cannot rely on tactile sensation to locate a small intrapulmonary ground glass lesion. In such cases, the ability to provide localization using ENB can help the surgeon to avoid lengthy exploratory surgery and multiple diagnostic wedge resections. At our institution, we prefer to use both pleural dye and coils in/near the lesion to provide the surgeon with both visual and tactile cues to locate the lesion.
The false-negative percutaneous biopsy in this case underscores the importance of follow-up of suspicious nodules, regardless of histologic (or cytologic) results. We rarely perform biopsies of highly suspicious nodules in patients who are surgical candidates due to the known chance of a false-negative result[13].
Role of imaging
Surveillance CT scans enabled the detection of a small (stage T1a) lung cancer (as well as the recognition of growth) that was potentially curable with a resection. PET scanning was not performed, as this technique is unreliable in characterizing small lesions and nodules with a ground glass pattern at CT. Thin-section CT enabled the planning and use of ENB for preoperative lesion localization.
Case 3: oligometastatic NSCLC
History and imaging findings
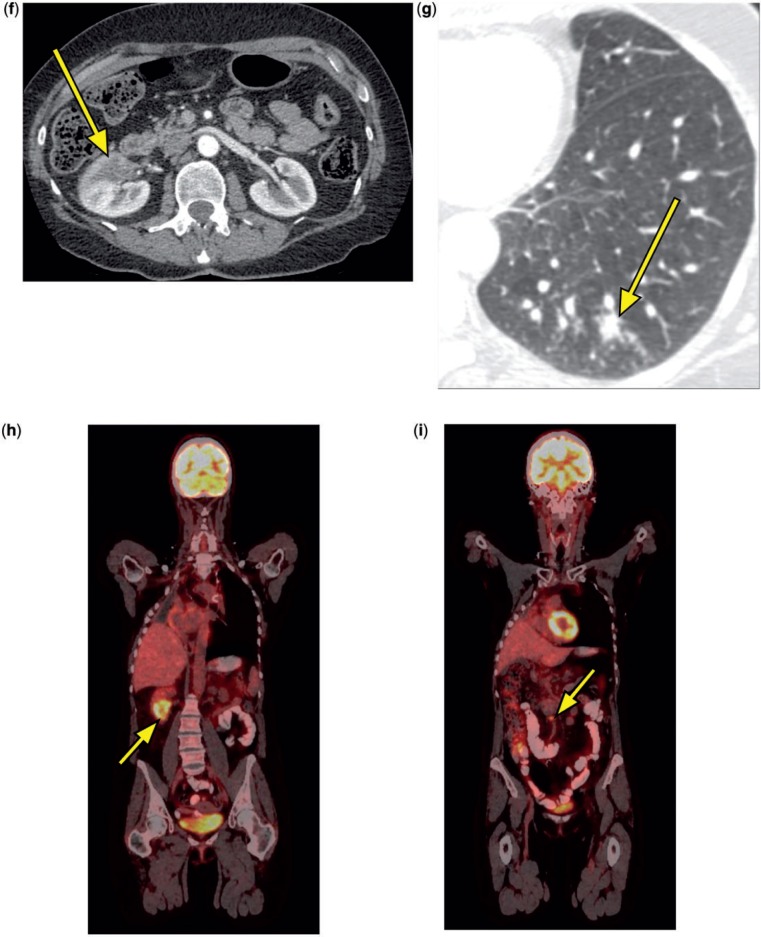
A 53-year-old woman was initially diagnosed with stage III (cT4 N0 M0) adenocarcinoma of the lung in 2003 when she was found to have an obstructive right hilar mass with mediastinal invasion (Fig. 3a). She was treated with neoadjuvant chemoradiotherapy followed by a right pneumonectomy, which revealed extensively necrotic adenocarcinoma with negative margins and lymph nodes (ypT1bN0). In 2007, she developed a new isolated left upper lobe nodule (Fig. 3b). Wedge resection revealed a stage IB (T2N0) adenocarcinoma with involvement of the visceral pleura. In April 2011, she developed progressive dyspnea, and scans revealed a new left upper lobe nodule and a left hilar mass causing narrowing of the left pulmonary artery (Figs. 3c,d). Placement of a pulmonary artery stent (Fig. 3e) improved her dyspnea, and she was treated with concurrent chemoradiotherapy for apparent stage IIA (T1aN1) disease with a good response.
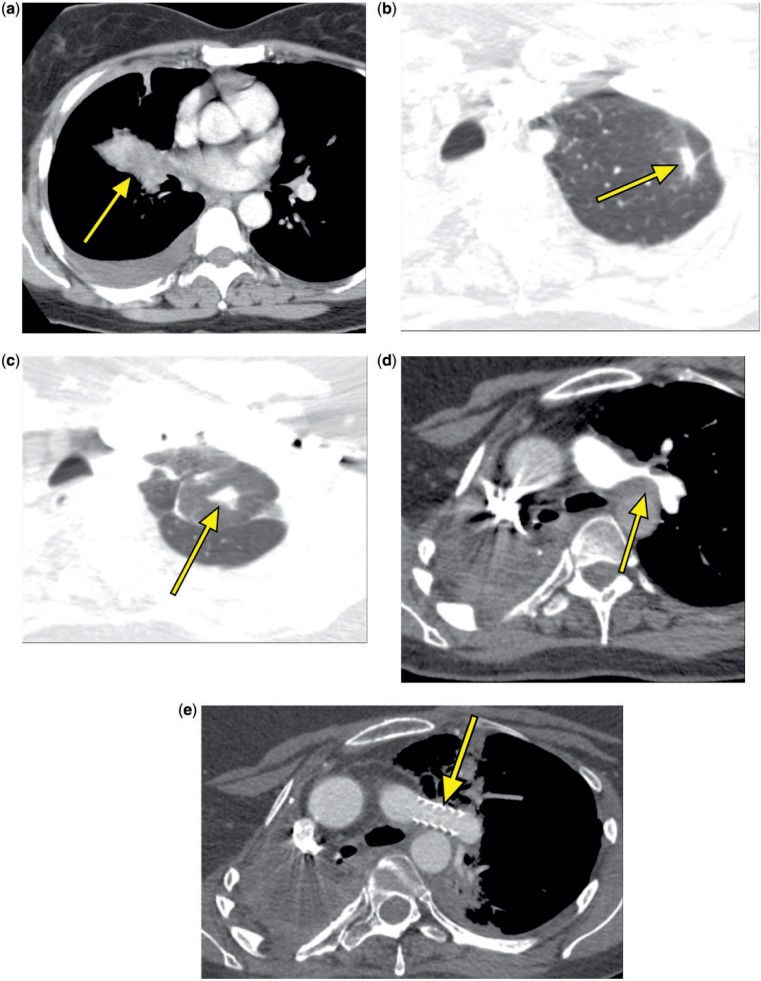
Figure 3.
A 53-year-old woman. CT scan in 2003 showed a right hilar mass (a, arrow). A new left upper lobe nodule was seen at CT in 2007 (b, arrow). A CT scan in April 2011 revealed a new left upper lobe nodule and a left hilar mass causing severe narrowing of the left pulmonary artery (c,d, arrows). A CT scan in November 2011 demonstrated a patent pulmonary artery stent (e, arrow) and a new right renal mass (f, arrow). In January 2012, new lung nodules were visible at CT (g, arrow). PET scan in March 2012 revealed intense FDG avidity in the right renal mass (h, arrow) and two regional retroperitoneal lymph nodes (i, arrow indicates one lymph node), and near resolution of the previously treated left upper lobe nodule and left hilar mass.
A surveillance CT scan in November 2011 revealed a new area of low attenuation in the right kidney (Fig. 3f). Magnetic resonance imaging (MRI) in January 2012 confirmed a right renal mass, and subsequent biopsy showed adenocarcinoma consistent with metastatic lung cancer (TTF1+, Napsin-A+, PAX-8–). While undergoing evaluation for resection of the renal mass, she developed a productive cough. A CT scan obtained at an outside institution in January 2012 revealed 20–30 new lung nodules, each measuring up to 1 cm in diameter (Fig. 3g), and she was informed that she had widely metastatic disease.
Tumor Board input and clinical course
After review of the patient’s clinical situation and imaging studies in January 2012, the Tumor Board believed that the new lung nodules were more likely to represent infection rather than metastases and recommended a short-interval repeat CT scan. CT in February 2012 revealed complete resolution of the lung nodules but further enlargement of the right renal mass. PET scan in March 2012 revealed intense FDG avidity in the right renal mass and two regional retroperitoneal lymph nodes, and near resolution of the previously treated left upper lobe nodule and left hilar mass (Fig. 3h,i). CT scan of the brain was negative. In mid-March 2012, she underwent a right radical nephrectomy that demonstrated metastatic adenocarcinoma invading the perirenal adipose tissue and the renal artery, with negative margins. One retrocaval lymph node and one aortocaval lymph node contained metastatic adenocarcinoma. Follow-up CT scans of the chest and abdomen in June 2012 showed no evidence of recurrent or metastatic disease.
Management issues
What is the usefulness of surveillance CT scans in patients with previously treated NSCLC?
The usefulness of surveillance scans in patients with treated NSCLC is controversial. Patients treated with curative intent are at very high risk for both recurrence and second primary lung cancers. At the least, extrapolating from the recent National Lung Screening Trial data[14], it would seem prudent to perform annual low-dose CT scans to screen for early-stage second primary cancers that can be treated for potential cure. Although most recurrences are incurable, patients with local or regional recurrences may sometimes be cured with chemoradiotherapy, and it may be justifiable to obtain surveillance chest CT scans at more frequent intervals for the first 2–3 years after initial treatment[15]. Extensive imaging with CT/MRI of the brain, PET, or bone scans to detect distant metastases remains difficult to justify, because the benefits of early treatment of metastatic disease have not been demonstrated.
Did our patient have a recurrence of her previous lung cancer or a new malignancy in 2011?
In our patient, although it is possible that the lung nodule and associated hilar lymph node that developed in 2011 could have been metastatic from either the original tumor or the second tumor in 2007, the relatively long disease-free intervals and the lack of other sites of metastatic disease on CT and PET favored a de novo malignancy and justified an aggressive treatment approach with definitive chemoradiotherapy. However, the subsequent development of the renal mass in November 2011, with histologic features characteristic of lung adenocarcinoma, was unequivocal evidence of distant metastatic disease (stage M1b).
Should patients with oligometastatic disease from NSCLC receive aggressive treatment?
In recent years, the development of more effective treatments for controlling both locoregional and systemic disease has led to a growing interest in the aggressive management of patients with oligometastatic NSCLC[16]. Because the benefits of aggressive interventions in patients with metastatic disease remain largely unknown, and the potential risks may be substantial, it is imperative that only the most appropriate patients be considered for such approaches. Both clinical evaluation and advanced imaging technologies are important aspects of the selection process. Clinically, patients must have an excellent performance status with limited comorbidities and an expectation for substantial survival if the oligometastatic disease can be controlled. Optimal imaging studies should be used to ensure that all locoregional disease is controlled and that the patient does indeed have limited sites of hematogenous metastases that are amenable to site-directed treatment.
Our patient was still relatively young, with an excellent performance status and no major comorbidities (aside from having only one lung). Radiographically, both CT of the chest and MRI of the abdomen initially revealed the right kidney as the only site of metastatic disease. A subsequent CT performed because of new symptoms demonstrated multiple lung nodules concerning for more widely metastatic disease. However, the rapid appearance of these nodules, and the fact that she had developed a productive cough that improved with antibiotics around the time this scan was obtained, suggested to our Tumor Board that the nodules might represent an infectious process. A short-interval scan demonstrated their complete resolution.
A final PET scan to further ensure the absence of other metastases did demonstrate two FDG-avid, regional retroperitoneal lymph nodes. Although these new findings did raise concerns as to the rationality of an aggressive approach to the renal metastasis, their identification did help to clarify that non-surgical site-directed techniques, such as stereotactic body radiotherapy, would not offer optimal disease control. In addition, the PET scan helped guide the extent of the subsequent resection, allowing for the removal of the involved lymph nodes, which would not have been sought without this vital imaging data.
Although it remains highly likely that this patient will eventually succumb to her disease, the aggressive management of her oligometastases, which was fostered by the directed use of advanced imaging technology and the expert opinion of the Tumor Board, will hopefully improve both her quality-of-life and overall survival.
What are the technical problems when performing lung surgery in a patient with only a single lung after a previous pneumonectomy?
The adequacy of remaining pulmonary function must be carefully assessed to insure sufficient predicted postoperative reserve to allow an additional resection. Spirometry (measurement of FVC and FEV1) and determination of Dlco are basic. A ventilation/perfusion scan demonstrates potential areas of the lung that are not contributing substantially to overall pulmonary function and may therefore be resected with little negative physiologic impact. The right middle lobe normally contributes only 5% of pulmonary function. In a patient with good pulmonary function who has undergone a previous left pneumonectomy and develops a right middle lobe malignancy, a middle lobectomy may be very well tolerated. Large and central tumors requiring resection of a more major lobe may simply not be resectable.
Unlike the patient undergoing an initial video-assisted (VATS) pulmonary resection in which single lung ventilation of the contralateral lung during the operation is routine, the patient with only a single remaining lung will usually not tolerate much deflation. An open thoracotomy is generally required in such patients, working closely with the anesthesiologist so that ventilation can be temporarily halted while a wedge resection of a peripherally located T1 lesion is carried out. The availability of surgical staplers provides a means for more rapidly resecting such lung lesions with an adequate 2-cm margin and less postoperative air leak than was associated with lung suturing techniques.
Epidural anesthesia for postoperative pain control is a vital adjunct in insuring that the one remaining lung will ventilate maximally early after surgery. This, combined with aggressive early ambulation of the patient, allows additional pulmonary resections with greater safety in patients with only a single remaining lung.
Role of imaging
The initial CT interpretation in January 2012 was misleading by suggesting widespread, incurable pulmonary metastases in a patient with multiple previous lung cancers. However, reinterpretation by the Tumor Board, in light of the full clinical picture, gave a more balanced assessment, ultimately resulting in a significantly different treatment approach. PET scanning was helpful in establishing the limited nature of the metastases, in order to plan for surgical resection.
Conclusion
Imaging plays an important role in guiding the care of thoracic oncology patients. However, although imaging is usually helpful, it may be inconclusive or occasionally misleading and must be interpreted in light of the clinical context. Input from the entire multidisciplinary oncology tumor board team helps to integrate imaging findings into an optimal patient care plan.
Conflict of interest
The authors have no conflicts of interest to declare.
Footnotes
This paper is available online at http://www.cancerimaging.org. In the event of a change in the URL address, please use the DOI provided to locate the paper.
References
- 1.Defranchi SA, Cassivi SD, Nichols FC, et al. N2 disease in T1 non-small cell lung cancer. Ann Thorac Surg. 2009;88:924–928. doi: 10.1016/j.athoracsur.2009.05.039. PMid:19699921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fuwa N, Mitsudomi T, Daimon T, et al. Factors involved in lymph node metastasis in clinical stage I non-small cell lung cancer–from studies of 604 surgical cases. Lung Cancer. 2007;57:311–316. doi: 10.1016/j.lungcan.2007.04.003. PMid:17509726. [DOI] [PubMed] [Google Scholar]
- 3.Defranchi SA, Edell ES, Daniels CE, et al. Mediastinoscopy in patients with lung cancer and negative endobronchial ultrasound guided needle aspiration. Ann Thorac Surg. 2010;90:1753–1757. doi: 10.1016/j.athoracsur.2010.06.052. PMid:21095301. [DOI] [PubMed] [Google Scholar]
- 4.Robinson LA, Ruckdeschel JC, Wagner H, Jr, Stevens CW. Treatment of non-small cell lung cancer-stage IIIA: ACCP evidence-based clinical practice guidelines. 2nd ed. Chest. 2007;132:243S–265S. doi: 10.1378/chest.07-1379. PMid:17873172. [DOI] [PubMed] [Google Scholar]
- 5.Iwasaki A, Shirakusa T, Miyoshi T, et al. Prognostic significance of subcarinal station in non-small cell lung cancer with T1–3 N2 disease. Thorac Cardiovasc Surg. 2006;54:42–46. doi: 10.1055/s-2005-865828. PMid:16485188. [DOI] [PubMed] [Google Scholar]
- 6.Oh JY, Kwon SY, Yoon HI, et al. Clinical significance of a solitary ground-glass opacity (GGO) lesion of the lung detected by chest CT. Lung Cancer. 2007;55:67–73. doi: 10.1016/j.lungcan.2006.09.009. PMid:17092604. [DOI] [PubMed] [Google Scholar]
- 7.Park CM, Goo JM, Lee HJ, Lee CH, Chun EJ, Im JG. Nodular ground-glass opacity at thin-section CT: histologic correlation and evaluation of change at follow-up. Radiographics. 2007;27:391–408. doi: 10.1148/rg.272065061. PMid:17374860. [DOI] [PubMed] [Google Scholar]
- 8.Yoon HE, Fukuhara K, Michiura T, et al. Pulmonary nodules 10 mm or less in diameter with ground-glass opacity component detected by high-resolution computed tomography have a high possibility of malignancy. Jpn J Thorac Cardiovasc Surg. 2005;53:22–28. doi: 10.1007/s11748-005-1004-8. PMid:15724498. [DOI] [PubMed] [Google Scholar]
- 9.Travis WD, Brambilla E, Noguchi M, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society International Multidisciplinary Classification of Lung Adenocarcinoma. J Thorac Oncol. 2011;6:244–285. doi: 10.1097/JTO.0b013e318206a221. PMid:21252716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eberhardt R, Morgan RK, Ernst A, Beyer T, Herth FJ. Comparison of suction catheter versus forceps biopsy for sampling of solitary pulmonary nodules guided by electromagnetic navigational bronchoscopy. Respiration. 2010;79:54–60. doi: 10.1159/000232394. PMid:19648733. [DOI] [PubMed] [Google Scholar]
- 11.Makris D, Scherpereel A, Leroy S, et al. Electromagnetic navigation diagnostic bronchoscopy for small peripheral lung lesions. Eur Respir J. 2007;29:1187–1192. doi: 10.1183/09031936.00165306. PMid:17360724. [DOI] [PubMed] [Google Scholar]
- 12.Sethi S, Bansal S, Gildea T, et al. Impact of the airway-nodule relationship on electromagnetic navigational bronchoscopy yield. Chest J. 2007;132:452a. [Google Scholar]
- 13.Quint LE, Kretschmer M, Chang A, Nan B. CT-guided thoracic core biopsies: value of a negative result. Cancer Imaging. 2006;6:163–167. doi: 10.1102/1470-7330.2006.0027. PMid:17098648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. PMid:21714641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ettinger DS, Akerley W, Bepler G, et al. Non-small cell lung cancer. J Natl Compr Canc Netw. 2010;8:740–801. doi: 10.6004/jnccn.2010.0056. PMid:20679538. [DOI] [PubMed] [Google Scholar]
- 16.Oh K, Sundaram B, Krishnamurthy V, et al. Site-directed therapy for lung cancer metastases. In: Keshamouni V, Arenberg D, Kalemkerian G, editors. Lung cancer metastasis: novel biological mechanisms and impact on clinical practice. New York: Springer; 2009. pp. p. 351–381. [Google Scholar]