Abstract
Elevated alanine aminotransferase (ALT) activity, an important marker of liver injury, has been associated inconsistently with higher mortality. We evaluated whether persons with nonelevated ALT levels are the most appropriate comparison group by examining the relationships of low ALT with mortality and body composition in the US National Health and Nutrition Examination Survey (NHANES). In NHANES 1988–1994, the mortality risk of persons in ALT deciles 1, 2, 3, and 10 was compared with that of persons in deciles 4–9 (mortality was relatively flat across these deciles) over an 18-year period (through 2006) among 14,950 viral-hepatitis-negative adults. In NHANES 1999–2006, low ALT was evaluated in association with dual-energy x-ray absorptiometry body composition measures among 15,028 adults. Multivariate-adjusted mortality was higher for decile 1 (hazard ratio (HR) = 1.42, 95% confidence interval (CI): 1.24, 1.63), decile 2 (HR = 1.27, 95% CI: 1.06, 1.53), and decile 3 (HR = 1.25, 95% CI: 1.04, 1.50) and nonsignificantly higher for decile 10 (HR = 1.21, 95% CI: 0.91, 1.61) than for deciles 4–9. Adjusted appendicular lean mass was decreased among the lowest ALT deciles. In the US population, low ALT was associated with higher mortality risk, possibly attributable to decreased appendicular lean mass. For mortality studies of elevated ALT levels, the most appropriate comparison group is persons in the middle range of ALT rather than all persons with nonelevated ALT.
Keywords: alanine aminotransferase, body composition, mortality, National Health and Nutrition Examination Survey
Elevated serum alanine aminotransferase (ALT) activity is an important diagnostic and prognostic marker of liver disease. However, elevated ALT activity has been associated inconsistently with higher all-cause mortality when compared with normal ALT (1–6). For example, in one study of the US population, elevated ALT was associated with liver disease mortality but not with mortality from all causes, cardiovascular disease, or cancer (6). We evaluated whether persons with nonelevated ALT levels are the most appropriate comparison group by examining the relationship of the lower range of ALT with mortality in a large national population-based prospective study. Additionally, we examined the association of ALT activity with body composition to explore whether low ALT was associated with reduced lean body mass.
MATERIALS AND METHODS
Participants and variables
The Third National Health and Nutrition Examination Survey (NHANES III) was conducted in the United States from 1988 through 1994 by the National Center for Health Statistics of the Centers for Disease Control and Prevention (7). The survey consisted of cross-sectional interview, examination, and laboratory data collected from a complex multistage, stratified, clustered probability sample representative of the civilian, noninstitutionalized population, with oversampling of persons aged 60 years or older, non-Hispanic blacks, and Mexican Americans. The survey was approved by the institutional review board of the Centers for Disease Control and Prevention, and all participants provided written informed consent to participate. The NHANES III public-use linked mortality file was employed for this analysis.
Of 23,258 sampled persons aged 20 years or older, 16,573 (71%) underwent an examination at a mobile examination center. Of these participants, 14,950 were included in the primary analyses after these exclusions: positive for serum hepatitis B surface antigen or hepatitis C antibody (n = 453), missing data on hepatitis B or hepatitis C serology or serum ALT activity (n = 1,159), or unknown vital status (n = 11). A repeat examination on a different day was performed on approximately 5% of the original sample. Repeat ALT activities were in the same range for 72% of participants who had initial values in deciles 1–3 and for 88% of participants who had initial values in deciles 4–10.
Serum samples were shipped weekly at −20°C to laboratories for testing of ALT, aspartate aminotransferase (AST), and γ-glutamyltransferase (GGT) (8). Data on factors known or thought to be related to liver enzyme activity or mortality were collected at baseline. In general, continuous covariates were treated as such to decrease the possibility of residual confounding in regression analyses. However, continuous variables whose distributions were skewed to the right were recoded into deciles and treated as ordinal variables. Nonbinary categorical covariates were coded as indicator variables with the category listed first below serving as the reference group. Because the analytical objective was to examine the relationship of low ALT with mortality after adjustment for possible confounders rather than to generate the most parsimonious model, factors that were previously found to be associated with liver enzyme activity or mortality in the NHANES population were included in all multivariate analyses: age (years), sex, ethnicity (non-Hispanic white, non-Hispanic black, Mexican-American, or other), education (<12, 12, or >12 years), cigarette smoking (never smoker, former smoker, <1 pack/day, or ≥1 pack/day), alcohol consumption (never drinker, former drinker, <1 drink/day, 1–2 drinks/day, or >2 drinks/day), physician-diagnosed diabetes, physical activity (metabolic equivalents/month; deciles), caffeinated beverage consumption (mg/day; deciles), body mass index (weight (kg)/height (m)2), waist:hip circumference ratio, systolic and diastolic blood pressure (mm Hg), estimated glomerular filtration rate (≥60 mL/minute/1.73 m2 or <60 mL/minute/1.73 m2), and serum transferrin saturation (%), hemoglobin A1c concentration (<6.5% or ≥6.5%) (9), total and high-density lipoprotein cholesterol level (mg/dL), and C-reactive protein level (≤0.3 mg/dL or >0.3 mg/dL) (6, 8, 10–17). Participants aged 60 years or over were evaluated with regard to lower-extremity function using a tandem stand, an 8-foot walk, and a repeated chair stand (18, 19). A summary score for these timed tests ranged from 0 (slowest) to 12 (fastest), and scores were dichotomized as ≤8 and >8 (20, 21).
Participants were passively followed for mortality through December 31, 2006, by linking NHANES III participants with National Death Index records through a probabilistic match, a well-established matching method (22). In a validation study of a prior NHANES cohort, the accuracy of the method was high, with 96.1% of decedents and 99.4% of living participants being classified correctly (23). Mortality outcomes were based on death-certificate underlying cause of death coded according to the International Classification of Diseases, Ninth Revision (ICD-9), for deaths occurring between 1988 and 1998 and the International Classification of Diseases, Tenth Revision (ICD-10), for deaths occurring between 1999 and 2006. Deaths occurring prior to 1999 and coded under the ICD-9 were recoded by the National Center for Health Statistics into comparable ICD-10-based underlying-cause-of-death groups. Outcomes for this analysis consisted of all-cause mortality and cause-specific mortality from cardiovascular disease (ICD-10 codes I20–I25, I50, I60–I69, and I70), neoplasms (ICD-10 codes C00–D48), diabetes mellitus (ICD-10 codes E10–E14), and liver disease (ICD-10 codes B15–B19, K70, and K73–K74).
Statistical analysis
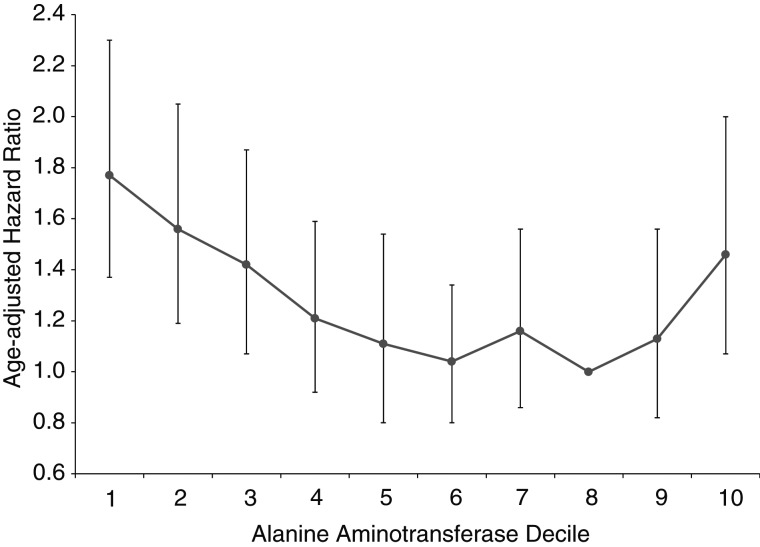
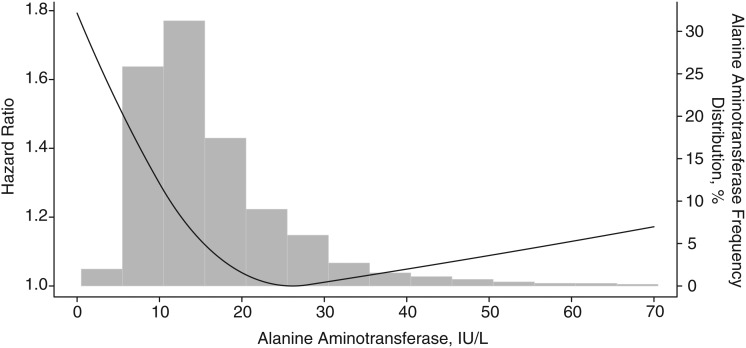
ALT activity was coded using sex-specific cutpoints (Figure 1) because of higher levels in men compared with women, and all-cause mortality was examined initially according to ALT deciles in age-adjusted proportional hazards analysis (Figure 1). Because of relatively flat mortality across deciles 4–9, those deciles were combined and served as the comparison group. Baseline characteristics of participants according to ALT deciles were examined by comparing mean values (and standard deviations) of continuous variables using a t test and percentages of categorical variables using a χ2 test. Cumulative mortality during follow-up was calculated among ALT deciles using Kaplan-Meier analysis. Estimated hazard ratios and 95% confidence intervals for mortality outcomes were calculated using Cox proportional hazards regression analysis (PROC SURVIVAL in SUDAAN, release 10.0; RTI International, Research Triangle Park, North Carolina). Mortality hazard ratios were computed for ALT deciles 1, 2, 3, and 10 relative to deciles 4–9 by categorizing ALT deciles as indicator variables. These relationships were further evaluated using restricted quadratic spline regression (R, release 2.13.0; R Foundation for Statistical Computing, Vienna, Austria). Knots were placed at the 30th (10 IU/L) and 90th (28 IU/L) percentiles for men and women combined, with an ALT of 27 IU/L, the lowest point on the multivariate-adjusted curve, used as the reference group. The consistency of the relationship of low ALT with mortality was evaluated across sex, age, and race/ethnicity strata, and interaction between ALT deciles and demographic subgroups was tested by adding interaction terms individually to regression models. Analyses for sex used common ALT cutpoints for men and women (7, 9, 10, and 28 IU/L). The proportional hazards assumption was met for all Cox regression analyses (24). Multivariate analyses excluded persons with missing values for any factor included in the model. P values were 2-sided, and P < 0.05 was considered to indicate statistical significance. All analyses used sample weights that accounted for unequal selection probabilities and nonresponse. Variance calculations accounted for the design effects of the survey using Taylor series linearization (25).
Figure 1.
Age-adjusted hazard ratios for all-cause mortality by decile of alanine aminotransferase level, United States, 1988–2006. The eighth decile served as the reference group. Alanine aminotransferase cutpoints for the 10th to 90th percentiles were 9, 11, 13, 15, 17, 19, 22, 26, and 33 IU/L, respectively, for men and 7, 8, 9, 10, 11, 13, 14, 17, and 22 IU/L, respectively, for women. Bars, 95% confidence interval.
Analysis of low ALT activity and body composition
ALT activity has been shown to be related to body mass components measured by dual-energy x-ray absorptiometry (DXA) (26), but direct measurements of body composition were not available in NHANES III. However, DXA body composition measurements were performed in NHANES 1999–2006 (26–30). We conducted an analysis of 15,028 adult NHANES 1999–2006 participants without viral hepatitis. ALT was categorized as deciles 1, 2, 3, 4–9, and 10, with decile cutpoints of 16, 18, 20, and 46 IU/L for men and 12, 14, 15, and 31 IU/L for women. Cutpoints were not comparable between NHANES III and NHANES 1999–2006 because of differences in specimen processing. Upper- and lower-extremity lean mass were summed and divided by height squared to generate appendicular lean mass index (19, 31, 32). Appendicular lean mass index values were compared by ALT decile using linear regression analysis (PROC REGRESS in SUDAAN, release 10.0; RTI International) to calculate adjusted (least squares) mean estimates. Multivariate analyses adjusted for age (years), ethnicity (non-Hispanic white, non-Hispanic black, Mexican-American, or other), glucose status (abnormal if physician-diagnosed diabetes or hemoglobin A1c concentration ≥6.5%), serum total cholesterol level (mg/dL), cigarette smoking (never smoker, former smoker, <1 pack/day, or ≥1 pack/day), and alcohol consumption (0, <1, 1–2, or >2 drinks/day). Additional multivariate models were generated that also adjusted for total fat mass (kg).
RESULTS
Of the 14,950 participants who formed the NHANES III cohort, 4,784 were in the lowest 3 ALT deciles, 8,591 were in deciles 4–9, and 1,575 were in decile 10. Compared with participants with ALT levels in deciles 4–9, those with ALT levels in the lowest 3 deciles at baseline were more likely to be of non-Hispanic black ethnicity and less likely to be Mexican-American; were heavier smokers; had a lower body mass index, waist:hip circumference ratio, total cholesterol level, and diastolic blood pressure; and had a decreased estimated glomerular filtration rate (Table 1). They were also less physically active and more likely to have an elevated C-reactive protein level. In addition, persons in decile 1 were older and less educated and had a lower transferrin saturation and higher caffeine intake and, among those aged 60 years or over, had poorer lower-extremity function than persons in deciles 4–9. Participants in deciles 2 and 3 had a lower prevalence of diabetes, and those in deciles 1 and 3 had higher levels of high-density lipoprotein cholesterol.
Table 1.
Baseline Characteristics of Participants by Decile of Alanine Aminotransferase Level, United States, 1988–1994
| Characteristic | Decile of ALT Levela |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
2 |
3 |
4–9 |
10 |
||||||
| Mean (SD) | % | Mean (SD) | % | Mean (SD) | % | Mean (SD) | % | Mean (SD) | % | |
| ALT level, IU/L | 6.7 (1.4) | 9.4 (1.3) | 10.8 (1.8) | 17.0 (5.4) | 44.1 (23.4) | |||||
| Age, years | ||||||||||
| Mean | 47.8 (20.4)*** | 45.4 (18.8) | 45.4 (18.4) | 44.7 (16.4) | 41.4 (14.6)*** | |||||
| Median | 42.3 (28.6–65.7)b | 40.3 (29.5–60.9) | 41.3 (28.8–59.9) | 41.2 (31.0–56.1) | 37.8 (29.5–50.1) | |||||
| Female sex | 59.7*** | 43.9** | 48.7* | 53.0 | 53.8 | |||||
| Race/ethnicity | ||||||||||
| Non-Hispanic white | 76.6 | 76.6 | 79.5 | 78.1 | 72.6** | |||||
| Non-Hispanic black | 16.0*** | 14.6*** | 10.6** | 8.8 | 7.1** | |||||
| Mexican-American | 2.5*** | 3.6** | 3.5*** | 5.1 | 10.4*** | |||||
| Other | 4.9* | 5.2* | 6.4 | 8.0 | 10.0 | |||||
| Education, years | ||||||||||
| <12 | 30.2*** | 25.6 | 25.8 | 22.8 | 23.6 | |||||
| 12 | 33.4 | 35.5 | 32.1 | 33.7 | 34.4 | |||||
| >12 | 36.4** | 39.0 | 42.1 | 43.5 | 42.0 | |||||
| Body mass indexc | 24.8 (4.8)*** | 24.9 (4.9)*** | 25.3 (5.1)*** | 26.9 (5.6) | 29.5 (6.4)*** | |||||
| Waist:hip circumference ratio | 89.3 (9.4)*** | 90.0 (9.2)* | 89.9 (9.0)*** | 91.1 (9.0) | 93.5 (8.5)*** | |||||
| Abnormal glucose statusd | 6.3 | 4.4** | 5.1* | 6.9 | 12.9*** | |||||
| Total cholesterol, mg/dL | 200 (44)*** | 198 (42)*** | 199 (43)*** | 206 (42) | 213 (46)** | |||||
| HDL cholesterol, mg/dL | 53.2 (16.0)*** | 51.2 (15.0) | 52.3 (15.7)** | 50.4 (15.2) | 46.7 (15.4)*** | |||||
| Systolic blood pressure, mm Hg | 123 (20) | 123 (19) | 122 (18) | 122 (17) | 123 (16) | |||||
| Diastolic blood pressure, mm Hg | 71.8 (10.2)*** | 73.1 (10.0)*** | 73.1 (9.4)*** | 74.4 (9.8) | 77.2 (10.4)*** | |||||
| C-reactive protein, >0.3 mg/dL | 31.0*** | 23.1 | 24.0 | 24.5 | 34.3*** | |||||
| Estimated GFR <60 mL/minute/1.73 m2 | 9.8*** | 6.8** | 6.2** | 4.0 | 2.3** | |||||
| Transferrin saturation, % | 24.2 (11.8)*** | 26.6 (12.8) | 25.9 (11.4) | 26.5 (11.1) | 27.2 (11.4) | |||||
| Cigarette smoking | ||||||||||
| Never smoker | 43.2* | 40.1* | 42.3* | 48.0 | 50.8 | |||||
| Former smoker | 22.2*** | 27.2 | 26.0 | 26.8 | 26.4 | |||||
| Current smoker | ||||||||||
| <1 pack/day | 12.8 | 13.4 | 14.2 | 11.1 | 13.4 | |||||
| ≥1 pack/day | 21.8*** | 19.4* | 17.6* | 14.1 | 9.4** | |||||
| Alcohol drinking | ||||||||||
| Never drinker | 16.2* | 12.6 | 13.1 | 12.5 | 14.0 | |||||
| Former drinker | 34.1 | 33.2 | 31.0 | 32.7 | 31.3 | |||||
| Current drinker | ||||||||||
| <1 drink/day | 35.9** | 40.7 | 40.8 | 40.8 | 36.6* | |||||
| 1–2 drinks/day | 8.5 | 8.4 | 9.3 | 8.3 | 8.8 | |||||
| >2 drinks/day | 5.4 | 5.1 | 5.9 | 5.7 | 9.3** | |||||
| Caffeine intake, mg/day | 255 (305)* | 222 (313) | 249 (293) | 223 (272) | 197 (223)* | |||||
| Physical activity, METs/month | 98 (127)** | 110 (126) | 116 (128) | 113 (135) | 104 (125)* | |||||
| Physical function summary score ≤8e | 35.1** | 31.8 | 27.2 | 26.7 | 31.0 | |||||
| AST level, IU/L | 15.7 (3.9)*** | 16.9 (3.7)*** | 17.6 (4.0)*** | 20.5 (5.9) | 36.3 (23.3)*** | |||||
| GGT level, IU/L | 17.5 (12.7)*** | 19.0 (14.4)*** | 20.2 (16.4)*** | 27.3 (26.9) | 63.7 (77.5)*** | |||||
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; GFR, glomerular filtration rate; GGT, γ-glutamyltransferase; HDL, high-density lipoprotein; MET, metabolic equivalent of task; SD, standard deviation.
* P < 0.05; **P < 0.01; ***P < 0.001 (compared with 4th–9th deciles).
a ALT cutpoints were 9, 11, 13, and 33 IU/L for men and 7, 8, 9, and 22 IU/L for women.
b Numbers in parentheses, interquartile range.
c Weight (kg)/height (m)2.
d Physician-diagnosed diabetes or hemoglobin A1c concentration of ≥6.5%.
Low ALT level and mortality
The median duration of follow-up was 14.5 years (range, 0–18.1 years), and cumulative mortality from all causes was 21.2% (3,674 deaths) at 18 years of follow-up. The cause-specific cumulative mortality (underlying cause) was 8.0% (1,364 deaths) from cardiovascular disease, 5.7% (818 deaths) from cancer, 0.62% (116 deaths) from diabetes mellitus, 0.20% (37 deaths) from liver disease, and 8.4% (1,339 deaths) from all other causes.
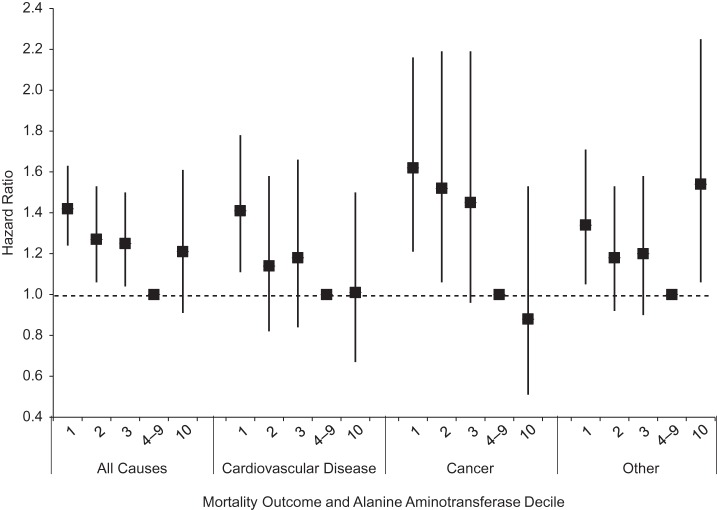
Unadjusted cumulative mortality rates tended to decrease with increasing ALT activity for all outcomes except diabetes and liver disease (Table 2). After age adjustment, all-cause mortality was increased among participants with ALT levels in deciles 1, 2, 3, and 10, as compared with deciles 4–9 (P < 0.05). With adjustment for multiple factors, mortality remained statistically significantly increased for deciles 1, 2, and 3 (Figure 2). Participants in the lowest decile had more than 40% higher mortality from all causes in comparison with deciles 4–9. Among persons aged 60 years or older, adding physical function summary score to the multivariate-adjusted model had little effect on the association of higher mortality with low ALT (data not shown). When all-cause mortality was modeled with continuous ALT as a restricted quadratic spline, the relationship was seen to be nonlinear: higher mortality for the lowest and highest ALT values as compared with the middle of the range (Figure 3). As can be seen from the superimposed histogram (right-hand vertical axis), the ALT distribution at both tails was small: 2% with ALT ≤5 IU/L and 6% with ALT >35 IU/L.
Table 2.
Cumulative Probability of Mortality (Unadjusted) Over an 18-Year Period and Age-adjusted Hazard Ratios for Death (Underlying Cause) by Decile of Alanine Aminotransferase Level (n = 14,950), United States, 1988–2006
| Mortality Outcome and Decile of ALT Levela | No. of Deaths | Unadjusted Cumulative Mortalityb | Age-adjusted Hazard Ratioc | 95% Confidence Interval | P Value |
|---|---|---|---|---|---|
| All causes | |||||
| 1 | 816 | 34.6 | 1.61 | 1.41, 1.83 | <0.001 |
| 2 | 393 | 29.4 | 1.42 | 1.20, 1.68 | <0.001 |
| 3 | 494 | 26.6 | 1.28 | 1.08, 1.53 | 0.006 |
| 4–9 | 1,760 | 17.5 | 1.00 | ||
| 10 | 211 | 17.4 | 1.33 | 1.06, 1.66 | 0.014 |
| Cardiovascular disease | |||||
| 1 | 329 | 16.2 | 1.64 | 1.35, 1.98 | <0.001 |
| 2 | 159 | 9.3 | 1.32 | 1.00, 1.75 | 0.047 |
| 3 | 187 | 11.6 | 1.26 | 0.90, 1.75 | 0.17 |
| 4–9 | 626 | 6.6 | 1.00 | ||
| 10 | 63 | 4.2 | 1.23 | 0.83, 1.81 | 0.30 |
| Cancer | |||||
| 1 | 173 | 8.6 | 1.73 | 1.35, 2.21 | <0.001 |
| 2 | 89 | 11.0 | 1.64 | 1.21, 2.22 | 0.002 |
| 3 | 114 | 7.1 | 1.46 | 1.02, 2.08 | 0.038 |
| 4–9 | 398 | 4.4 | 1.00 | ||
| 10 | 44 | 5.2 | 0.95 | 0.61, 1.48 | 0.82 |
| Diabetes mellitus | |||||
| 1 | 19 | 0.63 | 1.06 | 0.44, 2.55 | 0.89 |
| 2 | 11 | 0.76 | 1.86 | 0.77, 4.52 | 0.16 |
| 3 | 12 | 0.18 | 0.41 | 0.20, 0.85 | 0.017 |
| 4–9 | 63 | 0.54 | 1.00 | ||
| 10 | 11 | 1.7 | 2.74 | 0.97, 7.73 | 0.056 |
| Liver diseased | |||||
| 1 | 2 | 0.004 | 0.02 | 0.003, 0.12 | <0.001 |
| 2 | 1 | 0.046 | 0.25 | 0.036, 1.71 | 0.15 |
| 3 | 3 | 0.028 | 0.15 | 0.031, 0.73 | 0.020 |
| 4–9 | 15 | 0.20 | 1.00 | ||
| 10 | 16 | 0.70 | 5.27 | 1.59, 17.46 | 0.008 |
| Other | |||||
| 1 | 293 | 14.1 | 1.54 | 1.28, 1.85 | <0.001 |
| 2 | 133 | 11.8 | 1.35 | 1.07, 1.69 | 0.011 |
| 3 | 178 | 10.4 | 1.27 | 1.00, 1.62 | 0.049 |
| 4–9 | 658 | 6.9 | 1.00 | ||
| 10 | 77 | 6.8 | 1.47 | 1.07, 2.02 | 0.017 |
Abbreviation: ALT, alanine aminotransferase.
a ALT cutpoints were 9, 11, 13, and 33 IU/L for men and 7, 8, 9, and 22 IU/L for women.
b Estimated using Kaplan-Meier analysis.
c Estimated using Cox proportional hazards regression analysis with age as a continuous variable.
d Nonmalignant liver disease.
Figure 2.
Multivariate-adjusted hazard ratios for mortality by decile of alanine aminotransferase level, United States, 1988–2006. Alanine aminotransferase cutpoints were 15, 16, 18, and 30 IU/L for men and 13, 14, 15, and 26 IU/L for women. Bars, 95% confidence interval.
Figure 3.
Multivariate-adjusted relationship of all-cause mortality with serum alanine aminotransferase (ALT) activity, United States, 1988–2006. The relationship is illustrated as a restricted quadratic spline relative to the lowest point (at ALT = 27 IU/L).
We conducted analyses stratified by demographic characteristics. In multivariate-adjusted analyses using common ALT cutpoints for men and women, there was a positive association of higher mortality with low ALT among both men and women, though the relationship was stronger among men (Table 3) (P for interaction = 0.24). Higher mortality with low ALT was found among middle-aged and older adults but not among young adults (P for interaction = 0.065) and among non-Hispanic whites and Mexican Americans but not among non-Hispanic blacks (P for interaction < 0.001).
Table 3.
Multivariate-adjusted Hazard Ratios for the Relationship Between Alanine Aminotransferase Decile and All-cause Mortality, by Sex, Age, and Race/Ethnicity, United States, 1988–2006
| % in First–Third ALT Deciles | Decile of ALT Levela |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
2 |
3 |
4–9 |
10 |
|||||||
| HRb | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | ||
| Sex | |||||||||||
| Male | 14.1 | 1.38 | 1.10, 1.74 | 1.55 | 1.27, 1.89 | 1.39 | 1.09, 1.76 | 1.00 | Reference | 1.04 | 0.73, 1.50 |
| Female | 39.7 | 1.27 | 1.02, 1.58 | 1.21 | 0.98, 1.49 | 1.18 | 0.94, 1.48 | 1.00 | Reference | 1.09 | 0.68, 1.76 |
| Age group, years | |||||||||||
| 20–39 | 28.6 | 0.85 | 0.32, 2.25 | 0.91 | 0.42, 1.96 | 0.99 | 0.37, 2.63 | 1.00 | Reference | 1.45 | 0.64, 3.30 |
| 40–59 | 25.2 | 1.80 | 1.36, 2.37 | 1.44 | 0.84, 2.48 | 1.54 | 1.03, 2.31 | 1.00 | Reference | 1.02 | 0.52, 1.98 |
| ≥60 | 37.6 | 1.66 | 1.40, 1.96 | 1.34 | 1.09, 1.65 | 1.24 | 1.01, 1.52 | 1.00 | Reference | 1.16 | 0.92, 1.46 |
| Race/ethnicity | |||||||||||
| Non-Hispanic white | 29.8 | 1.44 | 1.22, 1.70 | 1.26 | 1.02, 1.56 | 1.25 | 1.03, 1.52 | 1.00 | Reference | 1.33 | 0.96, 1.85 |
| Non-Hispanic black | 39.0 | 1.09 | 0.92, 1.28 | 1.06 | 0.81, 1.39 | 0.91 | 0.70, 1.19 | 1.00 | Reference | 1.12 | 0.82, 1.53 |
| Mexican-American | 18.5 | 1.74 | 1.13, 2.66 | 1.35 | 0.85, 2.13 | 1.28 | 1.00, 1.63 | 1.00 | Reference | 0.78 | 0.54, 1.12 |
Abbreviations: ALT, alanine aminotransferase; CI, confidence interval; HR, hazard ratio.
a ALT cutpoints were 9, 11, 13, and 33 IU/L for men and 7, 8, 9, and 22 IU/L for women. Analyses stratified by sex used common ALT cutpoints for both men and women: 7, 9, 10, and 28 IU/L.
b Estimated using Cox proportional hazards regression analysis and adjusted for age, sex (except for models stratified by sex), race/ethnicity (except for models stratified by race/ethnicity), education, body mass index, waist:hip circumference ratio, glucose status (physician-diagnosed diabetes, hemoglobin A1c concentration of ≥6.5%), serum total cholesterol, high-density lipoprotein cholesterol, systolic blood pressure, diastolic blood pressure, C-reactive protein, estimated glomerular filtration rate, transferrin saturation, cigarette smoking, alcohol drinking, caffeine intake, and physical activity.
For cardiovascular disease mortality, the hazard ratio for persons in ALT deciles 1 and 2 was increased in age-adjusted analysis and remained increased in multivariate-adjusted analysis for decile 1 (Table 2, Figure 2). Similarly, for cancer mortality, the hazard ratio for persons in ALT deciles 1, 2, and 3 was increased in age-adjusted analysis and remained increased for deciles 1 and 2 in multivariate-adjusted analysis. In contrast, diabetes mortality was not statistically significantly or consistently increased among persons in the lower ALT deciles in age-adjusted analysis. For liver disease, mortality was increased among persons in the highest ALT decile, while the numbers of deaths in the lower ALT deciles were very small. Results from multivariate-adjusted analyses of diabetes and liver disease mortality are not presented because of the small numbers of deaths. When mortality from all other causes was examined, hazard ratios for persons in ALT deciles 1, 2, 3, and 10 were increased in age-adjusted analysis and remained increased for deciles 1 and 10 in multivariate-adjusted analysis.
We conducted 2 sensitivity analyses to evaluate the robustness of our findings. Little effect on the results was seen after excluding deaths that occurred during the first 3 years of follow-up (n = 616) (see Web Table 1, available at http://aje.oxfordjournals.org/). We also reran the analysis including participants who were positive for viral hepatitis B or C or were missing data on hepatitis B or C. The results were similar, with the exception of a stronger age-adjusted association of all-cause mortality with the highest ALT decile, which remained statistically significant after multivariate adjustment. This was at least partially explained by substantially increased liver disease mortality among participants in ALT decile 10 (Web Table 2).
We also examined the relationships of low AST and GGT activities with mortality. Participants with an AST level in decile 1, 2, or 3 had higher all-cause mortality in age-adjusted analysis, and this relationship remained statistically significant with multivariate adjustment for decile 1. Higher cancer mortality was found among persons in AST deciles 1 and 2 in age-adjusted and multivariate-adjusted analyses. There was no relationship of low AST with mortality from cardiovascular disease, diabetes, or other causes, while high AST increased the risk of liver disease mortality (Web Table 3). In contrast, low GGT was unrelated to both all-cause mortality and cause-specific mortality (data not shown).
Low ALT level and body composition
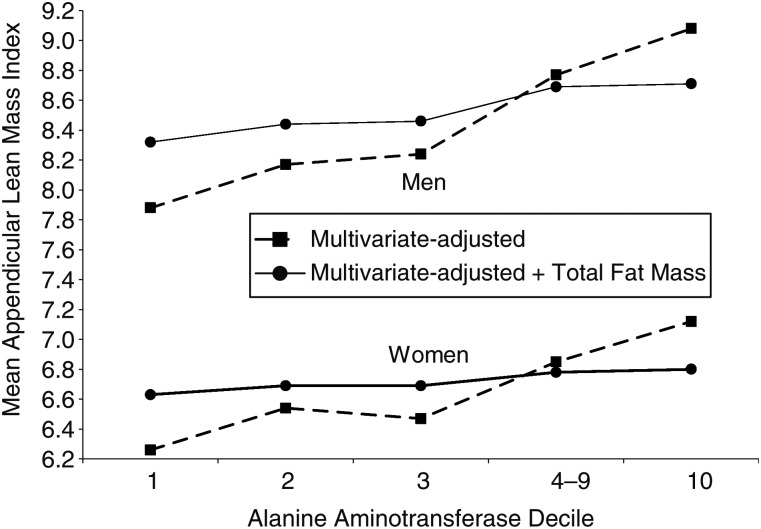
To examine the relationship of low ALT activity with lean body mass using data from NHANES 1999–2006, we first compared appendicular lean masses by ALT decile. Mean appendicular lean mass index (kg/m2) for deciles 1, 2, 3, 4–9, and 10, respectively, was 7.9 (standard deviation (SD), 1.2), 8.2 (SD, 1.2), 8.2 (SD, 1.2), 8.8 (SD, 1.3), and 9.2 (SD, 1.4) for men and 6.4 (SD, 1.2), 6.6 (SD, 1.3), 6.5 (SD, 1.2), 6.8 (SD, 1.4), and 7.1 (SD, 1.4) for women. Compared with deciles 4–9, values were lower for deciles 1, 2, and 3 and higher for decile 10 among both men and women (P < 0.05 for all comparisons). A similar positive association was seen between ALT and total fat mass index (kg/m2) (data not shown). The association of low ALT with appendicular lean body mass was further evaluated in linear regression analyses. Mean appendicular lean mass index was lower in deciles 1, 2, and 3 compared with deciles 4–9 among men and women after adjustment for multiple factors (P < 0.001 for all comparisons; Figure 4). Adding total fat mass (kg) to multivariate models attenuated but did not eliminate the association of low ALT with lower lean body mass (P < 0.05 for all comparisons; Figure 4).
Figure 4.
Multivariate-adjusted mean appendicular lean mass index (upper- and lower-extremity lean mass (kg) divided by height (m) squared) according to decile of alanine aminotransferase level among men and women, United States, 1999–2006. Alanine aminotransferase cutpoints were 16, 18, 20, and 46 IU/L for men and 12, 14, 15, and 31 IU/L for women.
DISCUSSION
In this large national population-based prospective study, participants with ALT activity in the 3 lowest deciles had statistically significant 30%–50% higher all-cause mortality after adjustment for multiple factors. Mortality from cardiovascular disease, cancer, and all other causes was increased among persons in the lowest ALT decile. Participants in the highest ALT decile, compared with deciles 4–9, had non-statistically significantly increased all-cause mortality in multivariate-adjusted analysis. Results varied little when the first 3 years of follow-up were excluded or when participants with viral hepatitis were included, except for higher all-cause mortality in the highest ALT decile with the inclusion of participants with viral hepatitis. A similar U-shaped relationship with mortality was found for AST but not for GGT. The linear relationship with GGT is consistent with the strong association of mortality with high GGT in studies that used the entire range of nonelevated values as the reference group (1, 6).
Results have varied among the few studies that have evaluated the relationship between mortality and ALT within the normal range. Three studies that included the entire range of ALT values did not find a relationship with low ALT (2, 4, 33). In one of these studies, all-cause mortality was increased even for ALT levels within the normal range as compared with an ALT level less than 20 IU/L (2). In contrast, higher all-cause mortality among persons with low ALT was found in 3 studies of older adults (34–36). Two studies compared mortality based on ALT levels above and below the median value (34, 35), and 1 study compared mortality for the lowest quartile of ALT relative to the higher 3 quartiles (36). Our study may have been the first to examine systematically the level at which an association between low ALT and mortality actually occurs.
The increased mortality that we found with lower ALT was confined to persons over age 40 years. Given that ALT activity decreases with age, this is a topic for further investigation. Interestingly, the strongest associations of low ALT activity with mortality were among those with higher ALT levels—for example, men, middle-aged persons, and Mexican Americans. It is possible that among subgroups with lower ALT levels, such as non-Hispanic blacks, the lowest deciles included both persons with physiologically normal low ALT levels and persons at higher risk of death.
The reason for higher mortality among persons with low ALT remains unclear. Low ALT was associated with decreased body mass index (Table 1), and in a recent meta-analysis, normal weight increased the risk of mortality relative to overweight (37). However, the higher mortality that we found with low ALT remained even in analyses that adjusted for body mass index. In a Welsh study of older, community-dwelling men, increased mortality with lower ALT was explained by age and frailty (35). No frailty index was available in NHANES III; however, it is unlikely to have explained the strong relationship of higher mortality with low ALT that we found among not only older adults but also middle-aged adults. It is possible that residual confounding was present as a result of incomplete adjustment for one or more variables. Possible confounding factors were measured only at baseline but may have varied over the course of the study, resulting in misclassification. Alternatively, an as-yet-unidentified characteristic may explain the higher mortality observed among persons with low ALT levels.
Although the majority of serum ALT activity is produced in the liver, a minority is derived from skeletal muscle. Therefore, low ALT could reflect sarcopenia with reduced release of ALT into the blood by muscle. Because sarcopenia has been associated with higher mortality (38, 39), the increased mortality found with low ALT could result from smaller muscle mass. Muscle strength, rather than muscle mass, may be more strongly associated with mortality (40). Impaired lower-extremity function, which is closely related to and has been proposed as a component of sarcopenia (19), was measured in NHANES III among older participants. Although it was associated with low ALT, decreased lower-extremity function did not account for the relationship between low ALT and mortality. Body composition, including lean soft tissue mass, was not measured in NHANES III. However, using DXA body composition data from another US national survey, we found that low ALT was associated with sarcopenia defined as decreased appendicular lean mass (19, 31, 32). Although low ALT was also associated with decreased fat mass, the relationship with appendicular lean mass was independent of total fat mass. Thus, a possible explanation for increased mortality with low ALT may be the association with sarcopenia. Additional studies will be needed to determine whether body composition mediates the higher mortality found with low ALT levels.
A limitation of the current study is that a single serum ALT concentration was obtained at baseline in NHANES. ALT levels may have varied over the follow-up period, leading to misclassification. Among participants with repeat ALT measures, the majority of those who had initial values in the lowest 3 deciles also had repeat values within the same low range. In addition, as previously reported, there was a possible loss of ALT activity after the brief storage period and shipping that were used in NHANES III (6). Degradation of ALT activity could have resulted in lower decile cutpoints but would not be expected to affect the relative position of participants within the ALT distribution or relationships of low ALT with mortality. Another limitation of the current study is the lack of validation of cause of death. Although ascertainment of vital status using the National Death Index is very high, assigning cause of death based on death certificate diagnoses is prone to misclassification. Thus, the associations with cause-specific mortality might not be as reliable as the results for all-cause mortality. Finally, despite up to 18 years of follow-up, the number of mortality outcomes was limited for diabetes and liver disease. These limitations are balanced by the benefits of a large national population-based sample, particularly the avoidance of the ascertainment bias that occurs in clinical studies of selected patients and the ability to generalize the results to the US population.
In conclusion, in the US population, a low ALT level was associated with higher mortality from all causes, as well as from cardiovascular disease, cancer, and all other causes. Relatively low appendicular lean mass may be a reason for the higher mortality. For studies of mortality and elevated ALT activity, whether due to fatty liver or to other etiologies, the most appropriate comparison group is persons in the middle range of ALT values rather than all persons with nonelevated ALT levels.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Social and Scientific Systems, Inc., Silver Spring, Maryland (Constance E. Ruhl); and National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, Maryland (James E. Everhart).
This work was supported by a contract (HHSN276201200161U) from the National Institute of Diabetes and Digestive and Kidney Diseases.
The authors thank Dr. Naji Younes for assistance with generation of the spline figure and with programming in R.
This work was presented as a poster at The Liver Meeting 2012 (63rd Annual Meeting of the American Association for the Study of Liver Diseases), Boston, Massachusetts, November 10–13, 2012.
Conflict of interest: none declared.
REFERENCES
- 1.Arndt V, Brenner H, Rothenbacher D, et al. Elevated liver enzyme activity in construction workers: prevalence and impact on early retirement and all-cause mortality. Int Arch Occup Environ Health. 1998;71(6):405–412. doi: 10.1007/s004200050299. [DOI] [PubMed] [Google Scholar]
- 2.Kim HC, Nam CM, Jee SH, et al. Normal serum aminotransferase concentration and risk of mortality from liver diseases: prospective cohort study. BMJ. 2004;328(7446):983. doi: 10.1136/bmj.38050.593634.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakamura K, Okamura T, Kanda H, et al. The value of combining serum alanine aminotransferase levels and body mass index to predict mortality and medical costs: a 10-year follow-up study of National Health Insurance in Shiga, Japan. J Epidemiol. 2006;16(1):15–20. doi: 10.2188/jea.16.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schindhelm RK, Dekker JM, Nijpels G, et al. Alanine aminotransferase predicts coronary heart disease events: a 10-year follow-up of the Hoorn Study. Atherosclerosis. 2007; 191(2):391–396. doi: 10.1016/j.atherosclerosis.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 5.Lee TH, Kim WR, Benson JT, et al. Serum aminotransferase activity and mortality risk in a United States community. Hepatology. 2008;47(3):880–887. doi: 10.1002/hep.22090. [DOI] [PubMed] [Google Scholar]
- 6.Ruhl CE, Everhart JE. Elevated serum alanine aminotransferase and gamma-glutamyltransferase and mortality in the United States population. Gastroenterology. 2009;136(2):477–485. doi: 10.1053/j.gastro.2008.10.052. [DOI] [PubMed] [Google Scholar]
- 7.National Center for Health Statistics. Plan and Operation of the Third National Health and Nutrition Examination Survey, 1988–94. Hyattsville, MD: National Center for Health Statistics; 1994. (Vital and health statistics, series 1: programs and collection procedures, no. 32). (DHHS publication no. (PHS) 94-1308) [PubMed] [Google Scholar]
- 8.Gunter EW, Lewis BG, Koncikowski SM . . Atlanta, GA: National Center for Environmental Health, Centers for Disease Control and Prevention; 1996. Laboratory Procedures Used for the Third National Health and Nutrition Examination Survey (NHANES III), 1988–1994http://www.cdc.gov/nchs/data/nhanes/nhanes3/cdrom/nchs/manuals/labman.pdf. (Accessed July 29, 2013) [Google Scholar]
- 9.American Diabe tes Association. Executive summary: standards of medical care in diabetes—2010. Diabetes Care. 2010;33(suppl 1):4S–10S. doi: 10.2337/dc10-S004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruhl CE, Everhart JE. Determinants of the association of overweight with elevated serum alanine aminotransferase activity in the United States. Gastroenterology. 2003;124(1):71–79. doi: 10.1053/gast.2003.50004. [DOI] [PubMed] [Google Scholar]
- 11.Ruhl CE, Everhart JE. Relation of elevated serum alanine aminotransferase activity with iron and antioxidant levels in the United States. Gastroenterology. 2003;124(7):1821–1829. doi: 10.1016/s0016-5085(03)00395-0. [DOI] [PubMed] [Google Scholar]
- 12.Ruhl CE, Everhart JE. Coffee and caffeine consumption reduce the risk of elevated serum alanine aminotransferase activity in the United States. Gastroenterology. 2005; 128(1):24–32. doi: 10.1053/j.gastro.2004.09.075. [DOI] [PubMed] [Google Scholar]
- 13.Ainsworth BE, Haskell WL, Leon AS, et al. Compendium of Physical Activities: classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993;25(1):71–80. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Michels KB, Willett WC, Fuchs CS, et al. Coffee, tea, and caffeine consumption and incidence of colon and rectal cancer. J Natl Cancer Inst. 2005;97(4):282–292. doi: 10.1093/jnci/dji039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Center for Health Statistics. Rockville, MD: Westat, Inc; 1988. National Health and Nutrition Examination Survey III. Body Measurements (Anthropometry)http://www.cdc.gov/nchs/data/nhanes/nhanes3/cdrom/nchs/manuals/anthro.pdf. (Accessed July 29, 2013) [Google Scholar]
- 16.National Center for Health Statistics. Rockville, MD: Westat, Inc; 1989. National Health and Nutrition Examination Survey III Cycle 2: Pulse and Blood Pressure Procedures for Household Interviewershttp://www.cdc.gov/nchs/data/nhanes/nhanes3/cdrom/nchs/manuals/pressure.pdf. (Accessed July 29, 2013) [Google Scholar]
- 17.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009; 150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Center for Health Statistics. Rockville, MD: Westat, Inc; 1993. National Health and Nutrition Examination Survey III. Physical Function Examination Manualhttp://www.cdc.gov/nchs/data/nhanes/nhanes3/cdrom/nchs/manuals/physical.pdf. (Accessed July 29, 2013) [Google Scholar]
- 19.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39(4):412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55(4):M221–M231. doi: 10.1093/gerona/55.4.m221. [DOI] [PubMed] [Google Scholar]
- 21.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 22.National Center for Health Statistics. Hyattsville, MD: National Center for Health Statistics; 2009. The Third National Health and Nutrition Examination Survey (NHANES III) Linked Mortality File: Mortality Follow-up Through 2006. Matching Methodologyhttp://www.cdc.gov/nchs/data/datalinkage/matching_methodology_nhanes3_final.pdf. (Accessed July 29, 2013) [Google Scholar]
- 23.Menke A, Muntner P, Batuman V, et al. Blood lead below 0.48 micromol/L (10 microg/dL) and mortality among US adults. Circulation. 2006;114(13):1388–1394. doi: 10.1161/CIRCULATIONAHA.106.628321. [DOI] [PubMed] [Google Scholar]
- 24.Kleinbaum DG. Survival Analysis: A Self-Learning Text. New York, NY: Springer Publishing Company; 1996. [Google Scholar]
- 25.Breslow NE, Day NE. Statistical Methods in Cancer Research. Vol 2. The Design and Analysis of Cohort Studies. Lyon, France: International Agency for Research on Cancer; 1987. pp. 48–79. (IARC Scientific Publication no. 82) [PubMed] [Google Scholar]
- 26.Ruhl CE, Everhart JE. Trunk fat is associated with increased serum levels of alanine aminotransferase in the United States. Gastroenterology. 2010;138(4):1346–1356. doi: 10.1053/j.gastro.2009.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.National Center for Health Statistics. Hyattsville, MD: National Center for Health Statistics; 2000. National Health and Nutrition Examination Survey. Body Composition Procedures Manualhttp://www.cdc.gov/nchs/data/nhanes/bc.pdf. (Accessed July 29, 2013) [Google Scholar]
- 28.Flegal KM, Shepherd JA, Looker AC, et al. Comparisons of percentage body fat, body mass index, waist circumference, and waist-stature ratio in adults. Am J Clin Nutr. 2009; 89(2):500–508. doi: 10.3945/ajcn.2008.26847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kelly TL, Wilson KE, Heymsfield SB. Dual energy x-ray absorptiometry body composition reference values from NHANES. PLoS One. 2009;4(9):e7038. doi: 10.1371/journal.pone.0007038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.National Center for Health Statistics. Hyattsville, MD: National Center for Health Statistics; 2008. National Health and Nutrition Examination Survey: Technical Documentation for the 1999–2004 Dual Energy X-Ray Absorptiometry (DXA) Multiple Imputation Data Fileshttp://www.cdc.gov/nchs/data/nhanes/dxa/dxa_techdoc.pdf. (Accessed July 29, 2013) [Google Scholar]
- 31.Delmonico MJ, Harris TB, Lee JS, et al. Alternative definitions of sarcopenia, lower extremity performance, and functional impairment with aging in older men and women. J Am Geriatr Soc. 2007;55(5):769–774. doi: 10.1111/j.1532-5415.2007.01140.x. [DOI] [PubMed] [Google Scholar]
- 32.Dufour AB, Hannan MT, Murabito JM, et al. Sarcopenia definitions considering body size and fat mass are associated with mobility limitations: the Framingham Study. J Gerontol A Biol Sci Med Sci. 2013;68(2):168–174. doi: 10.1093/gerona/gls109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yun KE, Shin CY, Yoon YS, et al. Elevated alanine aminotransferase levels predict mortality from cardiovascular disease and diabetes in Koreans. Atherosclerosis. 2009; 205(2):533–537. doi: 10.1016/j.atherosclerosis.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 34.Elinav E, Ackerman Z, Maaravi Y, et al. Low alanine aminotransferase activity in older people is associated with greater long-term mortality. J Am Geriatr Soc. 2006; 54(11):1719–1724. doi: 10.1111/j.1532-5415.2006.00921.x. [DOI] [PubMed] [Google Scholar]
- 35.Le Couteur DG, Blyth FM, Creasey HM, et al. The association of alanine transaminase with aging, frailty, and mortality. J Gerontol A Biol Sci Med Sci. 2010;65(7): 712–717. doi: 10.1093/gerona/glq082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ford I, Mooijaart SP, Lloyd S, et al. The inverse relationship between alanine aminotransferase in the normal range and adverse cardiovascular and non-cardiovascular outcomes. Int J Epidemiol. 2011;40(6):1530–1538. doi: 10.1093/ije/dyr172. [DOI] [PubMed] [Google Scholar]
- 37.Flegal KM, Kit BK, Orpana H, et al. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA. 2013;309(1):71–82. doi: 10.1001/jama.2012.113905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bigaard J, Frederiksen K, Tjonneland A, et al. Body fat and fat-free mass and all-cause mortality. Obes Res. 2004; 12(7):1042–1049. doi: 10.1038/oby.2004.131. [DOI] [PubMed] [Google Scholar]
- 39.Fisher AL. Of worms and women: sarcopenia and its role in disability and mortality. J Am Geriatr Soc. 2004;52(7): 1185–1190. doi: 10.1111/j.1532-5415.2004.52320.x. [DOI] [PubMed] [Google Scholar]
- 40.Newman AB, Kupelian V, Visser M, et al. Strength, but not muscle mass, is associated with mortality in the Health, Aging and Body Composition Study cohort. J Gerontol A Biol Sci Med Sci. 2006;61(1):72–77. doi: 10.1093/gerona/61.1.72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.