Abstract
The prevalence of celiac disease (CD) has increased in recent decades without a clear explanation. The “hygiene hypothesis” theorizes that decreased exposure to bacterial antigens may trigger autoimmunity. We aimed to determine whether Helicobacter pylori infection and CD were associated among patients undergoing upper gastrointestinal endoscopy. We performed a cross-sectional study of patients who underwent esophagogastroduodenoscopy with submission of gastric and duodenal biopsies to Miraca Life Sciences, Inc. (Irving, Texas), a US commercial pathology laboratory, during a 4.5-year period (January 2008–June 2012). We compared the prevalence of H. pylori in CD patients with that in persons without CD. We performed multiple logistic regression analysis, adjusting odds ratios for patient age, gender, and racial, ethnic, and socioeconomic factors. Among 136,179 patients, a total of 2,689 (2.0%) had CD. H. pylori prevalence was significantly lower in patients with CD (4.4%) than in those without CD (8.8%; P < 0.0001). After adjustment for the above covariates, this inverse relationship remained strong (adjusted odds ratio (OR) = 0.48, 95% confidence interval (CI): 0.40, 0.58). The relationships were similar in men (unadjusted OR = 0.51, 95% CI: 0.38, 0.69) and women (unadjusted OR = 0.46, 95% CI: 0.36, 0.58) and in all age groups. We conclude that H. pylori presence and CD are inversely associated, a relationship that persists after adjustment for socioeconomic factors. Future studies should address whether H. pylori modulates immune responses to ingested gluten.
Keywords: celiac disease, gluten, Helicobacter pylori
Celiac disease (CD) is a common autoimmune condition, affecting 0.8%–1.0% of Western populations. The prevalence of CD in the United States has increased sharply in recent decades, with up to a 4-fold increase in the past 50 years (1). Studies of stored serum have shown that this is a true rise in disease incidence, rather than a by-product of increased detection (1–3). Although the cause of this rise is not known, given its rapidity, a number of environmental risk factors have been proposed. The timing of gluten introduction and other infant feeding practices may play a role (4), as may rotavirus infection (5). One recent population-based study linked CD to elective, but not emergent, cesarian section, suggesting that differential perinatal exposure to bacterial microbiota could modulate CD risk (6).
Considering a role for microbial exposure in affecting CD risk, we sought to test for an association between Helicobacter pylori infection and CD. Several lines of evidence suggest that an inverse relationship between H. pylori and CD risk may exist. CD is triggered by the ingestion of gluten, digestion of which may be based on the pH and status of the gastric mucosa. Gastric H. pylori colonization appears to confer protection against asthma and other atopic diseases (7–10), and increased CD prevalence in the United States coincides temporally with declining H. pylori prevalence (11).
We sought to examine whether there is an association between histologically confirmed H. pylori and CD. Using a large pathology database, we hypothesized that the presence of H. pylori is independently associated with decreased risk of CD.
MATERIALS AND METHODS
Miraca Life Sciences, Inc. (Irving, Texas) is a commercial pathology laboratory that receives specimens submitted by approximately 1,500 gastroenterologists from 43 states, the District of Columbia, and Puerto Rico. A prospectively maintained database of pathology specimens contains more than 2,000 patients with CD spanning the years 2008–2012; this database was the source for a recent report on the epidemiology of H. pylori in the United States (12).
We performed a cross-sectional study of all patients undergoing upper gastrointestinal endoscopy with submission of gastric and duodenal biopsies in this database during a 4.5-year period from January 1, 2008, to June 30, 2012. Patients with only gastric or only duodenal biopsies submitted were excluded from this analysis. Among patients with multiple endoscopies, only the first chronological examination with both gastric and duodenal biopsies in the database was included. Patients with a histopathological diagnosis of any upper gastrointestinal malignancy (carcinoma or lymphoma) or gastric or duodenal ulcers were excluded.
In the primary analysis, we studied patients who had concurrent gastric and duodenal biopsies in order to compare H. pylori prevalences in patients with CD and those without CD. In this analysis, we considered any patient with villous atrophy to have CD, and we compared the H. pylori prevalence with that in a reference group comprised of patients with a normal concentration of intraepithelial lymphocytes (i.e., ≤25 lymphocytes per 100 enterocytes) (13) and normal duodenal villous architecture. This group included patients with focal foveolar metaplasia (“peptic duodenitis”) or foci of active inflammation (“duodenitis”) (14). As a secondary outcome, we also compared the prevalence of H. pylori in patients who had intraepithelial lymphocytosis (IEL) but normal villous architecture (Marsh stage 1) with that in the reference group.
Histological definitions
As in prior studies, CD was defined as duodenal histology featuring blunt villi (equivalent to Marsh stage 3A) or flat villi (Marsh stage 3B or 3C) (15). Patients with a documented history of CD in the “clinical indication” field but with normal villous architecture (n = 263) were excluded from this analysis.
Gastric biopsy specimens were evaluated according to the updated Sydney System (16). Specifically, H. pylori gastritis was diagnosed when H. pylori organisms were demonstrated by a specific polyclonal immunochemical stain (Cell Marque Corporation, Rocklin, California), routinely performed on all gastric biopsy specimens. H. pylori-negative chronic active gastritis was defined by focal or diffuse chronic inflammation (lymphocytes and plasma cells) with neutrophils infiltrating surface or foveolar epithelium in the absence of H. pylori organisms. Chronic inactive gastritis was characterized by focal or diffuse chronic inflammation without neutrophilic granulocytes or H. pylori organisms. Criteria for reactive gastropathy were based on the 2005 definition, which includes various combinations of foveolar hyperplasia, regenerative changes in the surface epithelium, edema or hyperemia of the lamina propria, erosions, and smooth muscle proliferation (17). Lymphocytic gastritis was diagnosed when the gastric surface or foveolar epithelium contained more than 25 intraepithelial lymphocytes per 100 epithelial cells; in equivocal cases, an immunohistochemical stain for CD3 lymphocytes (Cell Marque Corporation) was performed (18).
Duodenitis was diagnosed when the duodenal mucosa showed active (neutrophilic) inflammation in the epithelium, irrespective of erosion or the magnitude of the lymphoplasmacytic infiltrates, and often was accompanied by foci of gastric foveolar metaplasia (the putative “peptic duodenitis”) (12).
Prior procedures
Because some patients undergoing duodenal biopsy may have had prior evidence of gastric H. pylori, we repeated the analysis, now including all patients with a duodenal biopsy and any prior gastric biopsy dating back 5 years to January 1, 2003. For such patients, if any prior gastric biopsy showed H. pylori, for this analysis they were categorized as having H. pylori.
Accounting for socioeconomic status, race, and ethnicity
Rates of CD and H. pylori differ by socioeconomic status, race, and ethnicity. Information on medical insurance was available for all subjects in this analysis, and 3% of these subjects were enrolled in the Medicaid program at the time of specimen submission. Medicaid enrollment status was included as a dichotomous variable in the multivariate model.
For a subset of specimens (comprising 35% of the patients in this study), the residential zip code of the patient was available. We performed an additional sensitivity analysis, incorporating zip-code-level data on area racial and ethnic composition and household income. For each zip code, we obtained data on median household income, percentage of black residents, and percentage of Hispanic residents from the US Census Bureau's American Community Survey, using data collected during the years spanning 2007–2011 (19). Based on their zip codes of residence, patients were then divided into quartiles of zip-code-level household income, percentage of black residents, and percentage of Hispanic residents. We then compared the associations between CD and H. pylori among persons with and without residential zip code information available in the database, so as to measure possible selection bias in this sample. Sociodemographic predictors of the availability of residential zip code data were also assessed. Within the subset of patients for whom residential zip codes were available, the main logistic regression models were refitted with adjustment for percentage of black residents, percentage of Hispanic residents, and median household income in the residential zip code, with the variables coded into quartiles. In additional sensitivity analyses, inverse probability weights for the presence of a residential zip code were calculated; patient age was the only variable that predicted the availability of zip code data (20–22). These sensitivity analyses including inverse probability weights assessed potential bias due to the exclusion of subjects for whom zip code data were not available.
Statistical analyses
The association between CD and H. pylori infection was measured using odds ratios and 95% confidence intervals. We also performed multiple logistic regression analysis adjusting for age, gender, and Medicaid enrollment. To search for an association between the presence of H. pylori and the degree of villous architectural disturbance, we subsequently calculated crude and adjusted odds ratios relating H. pylori infection to 1) blunt villi (Marsh stage 3A) and 2) flat villi (Marsh stages 3B and 3C). We also performed age-stratified analyses using predetermined age groups (≤19, 20–39, 40–59, and ≥60 years) and analyses stratified by gender and Medicaid enrollment status (versus non-Medicaid status). In a subsequent multivariate model limited to those patients whose residential zip codes were available, we employed generalized estimating equations with robust standard error estimation to account for nonindependence of observations arising from clustering of subjects within zip codes (23). These models adjusted for percentage of black residents, percentage of Hispanic residents, and median household income in the residential zip code. The generalized estimating equations models were then refitted incorporating inverse probability weights for the availability of residential zip code data.
All statistical calculations were performed using SAS, version 9.2 (SAS Institute Inc., Cary, North Carolina). This analysis was submitted to the Institutional Review Board of Columbia University and was deemed non-human-subjects research, since all data were deidentified prior to being provided to the investigators.
RESULTS
After the exclusions detailed above, we identified 142,729 patients with duodenal biopsies during the 4.5 years spanning January 1, 2008–June 30, 2012, and any gastric biopsy going back to January 1, 2003. We excluded 263 patients who had a history of CD in the clinical indication field of the endoscopy report but had a normal duodenal biopsy, which left 142,466 patients. When the data set was restricted to those patients whose gastric and duodenal biopsies were performed during the same procedure, 136,179 persons remained.
The characteristics of patients are shown in Table 1. The mean age was 51 (standard deviation, 18) years, and 89,940 patients (66%) were female. The prevalence of CD was stable during the time period of this analysis (1.9% and 2.1% in the years 2008–2010 and 2011–2012, respectively), as was the prevalence of H. pylori (8.8% and 9.0% in the years 2008–2010 and 2011–2012, respectively). Of the 2,689 patients with histological evidence of CD, gastric H. pylori was detected in 117 (4.4%) patients. H. pylori was significantly less common in CD patients than among patients without CD (8.8%; P < 0.0001 (Table 2)). After adjustment for age, gender, and Medicaid enrollment status, the inverse relationship between CD and H. pylori changed little (adjusted odds ratio (aOR) = 0.48, 95% confidence interval (CI): 0.40, 0.58) (see Table 2). In contrast, the prevalence of H. pylori was higher among persons who had IEL and normal villous architecture (13.3%), an association that remained significant after adjustment for the same covariates.
Table 1.
Characteristics of 136,179 Patients Who Underwent Gastric and Duodenal Biopsy in the Same Procedure and Whose Biopsy Specimens Were Submitted to Miraca Life Sciences (Irving, Texas) During the Period January 1, 2008–June 30, 2012
| Characteristic | Duodenal Histology |
|||||
|---|---|---|---|---|---|---|
| Normala (n = 127,619) |
Celiac Disease (n = 2,689) |
IEL (n = 5,871) |
||||
| No. | % | No. | % | No. | % | |
| Mean age, years | 51 (18)b | 49 (18) | 44 (17) | |||
| 0–19 | 6,009 | 5 | 160 | 6 | 382 | 7 |
| 20–39 | 26,327 | 21 | 657 | 24 | 1,979 | 34 |
| 40–59 | 51,313 | 40 | 1,046 | 39 | 2,411 | 41 |
| ≥60 | 43,970 | 34 | 826 | 31 | 1,099 | 19 |
| Gender | ||||||
| Male | 43,989 | 34 | 846 | 31 | 1,404 | 24 |
| Female | 83,630 | 66 | 1,843 | 69 | 4,467 | 76 |
| Insurance | ||||||
| Medicaid | 3,846 | 3 | 51 | 2 | 268 | 5 |
| Non-Medicaid | 123,773 | 97 | 2,638 | 98 | 5,603 | 95 |
| Gastric histology | ||||||
| Normal | 32,240 | 25 | 473 | 18 | 1,273 | 22 |
| Active Helicobacter pylori gastritis | 11,207 | 9 | 117 | 4 | 780 | 13 |
| Chronic active (H. pylori- negative) gastritis | 2,081 | 2 | 85 | 3 | 162 | 3 |
| Chronic inactive (H. pylori- negative) gastritis | 11,819 | 9 | 553 | 21 | 742 | 13 |
| Reactive gastropathy | 17,858 | 14 | 215 | 8 | 538 | 9 |
| Lymphocytic gastritis | 172 | 0.1 | 197 | 7 | 55 | 1 |
| Otherc | 52,242 | 41 | 1,049 | 39 | 2,321 | 40 |
Abbreviation: IEL, intraepithelial lymphocytosis.
a Includes normal duodenal mucosa and duodenitis.
b Numbers in parentheses, standard deviation.
c Other diagnoses included mild chronic inactive gastritis, mild reactive gastropathy, atrophic gastritis, collagenous gastritis, granulomatous gastritis, focally enhanced gastritis, and polyps (fundic gland, hyperplastic, and adenomatous).
Table 2.
Association Between Celiac Disease and Helicobacter pylori Infection, by Degree of Villous Atrophy, Among Patients With Biopsy Specimens Submitted to Miraca Life Sciences (Irving, Texas) During the Period January 1, 2008–June 30, 2012
| Characteristic | Total No. |
H. pylori Prevalence |
Unadjusted OR | 95% CI | Adjusteda OR | 95% CI | |
|---|---|---|---|---|---|---|---|
| No. | % | ||||||
| Normal duodenal biopsy | 127,619 | 11,207 | 8.8 | 1.0 | 1.0 | ||
| IEL with normal villi | 5,871 | 780 | 13.3 | 1.61 | 1.49, 1.74 | 1.72 | 1.59, 1.86 |
| All celiac disease patients | 2,689 | 117 | 4.4 | 0.46 | 0.38, 0.56 | 0.48 | 0.40, 0.58 |
| Blunt villi (Marsh stage 3A) | 1,334 | 56 | 4.2 | 0.45 | 0.34, 0.58 | 0.46 | 0.35, 0.60 |
| Flat villi (Marsh stage 3B/C) | 1,355 | 61 | 4.5 | 0.48 | 0.37, 0.62 | 0.50 | 0.39, 0.65 |
Abbreviations: CI, confidence interval; IEL, intraepithelial lymphocytosis; OR, odds ratio.
a Adjusted for age, gender, and Medicaid enrollment status.
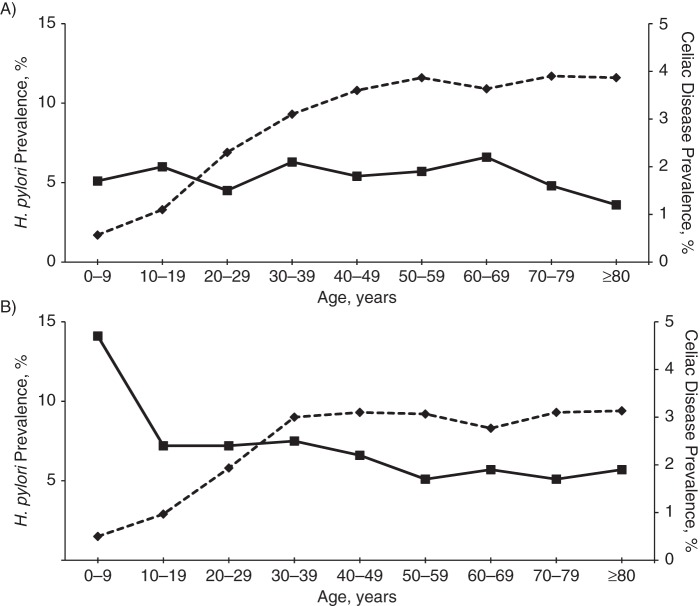
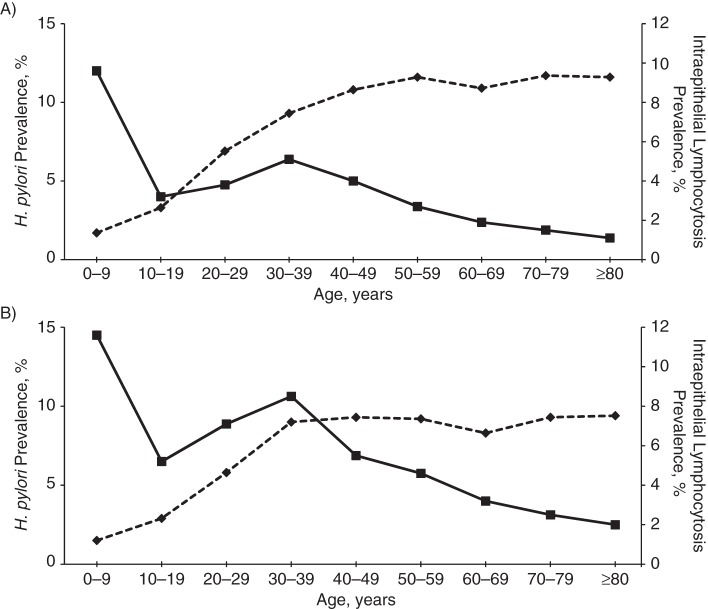
Results of analyses stratified by age group, gender, and Medicaid enrollment are shown in Table 3. The prevalence of H. pylori was greatest in older subjects, men, and Medicaid enrollees. The inverse relationship between H. pylori and CD was similar in most age strata. The relationships were similar in men and women. The association was diminished among patients enrolled in Medicaid, though the confidence intervals were wide. The prevalences of CD and H. pylori by age group are shown in Figure 1. H. pylori prevalence increased with age, while CD prevalence was highest in the younger age strata, although this trend was not apparent among men. Despite the positive correlation between H. pylori and IEL, the prevalence of the latter declined with age (Figure 2). Results of analysis stratified by the most common clinical indications for endoscopy are shown in Table 4. While the prevalences of CD and H. pylori varied by indication, the inverse relationships were present and similar in magnitude regardless of the indication for the procedure.
Table 3.
Association Between Celiac Disease and Helicobacter pylori Infection, by Age, Gender, and Medicaid Status, Among Patients With Biopsy Specimens Submitted to Miraca Life Sciences (Irving, Texas) During the Period January 1, 2008–June 30, 2012
| Characteristic | No. of Patients With CD |
H. pylori Prevalence in CD Patients |
H. pylori Prevalence in Non-CD Patients |
Unadjusted Odds Ratio | 95% Confidence Interval | ||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | ||||
| Age, years | |||||||
| 0–19 | 160 | 2 | 1.3 | 161 | 2.7 | 0.46 | 0.11, 1.87 |
| 20–39 | 657 | 21 | 3.2 | 1,975 | 7.5 | 0.41 | 0.26, 0.63 |
| 40–59 | 1,046 | 44 | 4.2 | 4,906 | 9.6 | 0.42 | 0.31, 0.56 |
| ≥60 | 826 | 50 | 6.1 | 4,165 | 9.5 | 0.62 | 0.46, 0.82 |
| Gender | |||||||
| Male | 846 | 45 | 5.3 | 4,379 | 10.0 | 0.51 | 0.38, 0.69 |
| Female | 1,843 | 72 | 3.9 | 6,828 | 8.2 | 0.46 | 0.36, 0.58 |
| Insurance | |||||||
| Medicaid | 51 | 7 | 13.7 | 716 | 18.6 | 0.70 | 0.31, 1.56 |
| Non-Medicaid | 2,638 | 110 | 4.2 | 10,491 | 8.5 | 0.47 | 0.39, 0.57 |
Abbreviation: CD, celiac disease.
Figure 1.
Prevalences of Helicobacter pylori infection and celiac disease, by age and gender, among patients with biopsy specimens submitted to Miraca Life Sciences (Irving, Texas) during the period January 1, 2008–June 30, 2012. A) men; B) women. Solid line, celiac disease; dashed line, H. pylori.
Figure 2.
Prevalences of Helicobacter pylori infection and intraepithelial lymphocytosis, by age and gender, among patients with biopsy specimens submitted to Miraca Life Sciences (Irving, Texas) during the period January 1, 2008–June 30, 2012. A) men; B) women. Solid line, intraepithelial lymphocytosis; dashed line, H. pylori.
Table 4.
Association Between Celiac Disease and Helicobacter pylori Infection, by Clinical Indication for Endoscopy, Among Patients With Biopsy Specimens Submitted to Miraca Life Sciences (Irving, Texas) During the Period January 1, 2008–June 30, 2012
| Indication | Total No. | Celiac Disease Prevalence, % | H. pylori Prevalence, % | Adjusteda Odds Ratio | 95% Confidence Interval | P Value |
|---|---|---|---|---|---|---|
| Dyspepsia | 27,719 | 1.4 | 9.8 | 0.47 | 0.30, 0.75 | 0.0016 |
| Abdominal pain | 62,846 | 1.5 | 8.4 | 0.44 | 0.32, 0.62 | <0.0001 |
| GERD | 47,518 | 1.3 | 8.7 | 0.37 | 0.24, 0.58 | <0.0001 |
| Anemia | 17,698 | 2.2 | 12.6 | 0.41 | 0.26, 0.63 | <0.0001 |
| Diarrhea | 21,297 | 2.5 | 6.4 | 0.59 | 0.38, 0.92 | 0.0191 |
Abbreviation: GERD, gastroesophageal reflux disease.
a Adjusted for age, gender, and Medicaid enrollment status.
Repeating the multivariate analysis, now including all patients with a duodenal biopsy and concurrent or previous gastric biopsy (n = 142,466), the inverse relationship between CD and H. pylori remained essentially unchanged (aOR = 0.48, 95% CI: 0.40, 0.58). The positive relationship between H. pylori and IEL with normal villous architecture was similarly stable in this sensitivity analysis (aOR = 1.71, 95% CI: 1.58, 1.85).
Adjustment for residential zip-code racial, ethnic, and socioeconomic data
Residential zip code was available for 47,440 patients residing in 6,232 zip codes (35% of the cohort). In the subsets for whom zip code data were available, the multivariate analysis was rerun without adjustment for zip-code-level characteristics. The association between CD and H. pylori remained similar in magnitude and direction (aOR = 0.55, 95% CI: 0.41, 0.74) to that observed in the full data set.
Results for quartiles of median household income, percentage of black residents, and percentage of Hispanic residents are shown in Table 5. There was a modest positive association between median income quartile and CD prevalence and an inverse relationship between median income quartile and H. pylori prevalence. Both percentage of black residents and percentage of Hispanic residents were positively associated with H. pylori prevalence and negatively associated with CD prevalence (see Table 5). The correlation between median income and percentage of black residents was modest (r = −0.27), and the correlation between median income and percentage of Hispanic residents was similar (r = −0.24). However, the correlation between percentage of black residents and percentage of Hispanic residents was negligible (r = −0.03). Therefore, all 3 variables (median income quartile, percentage of black residents, and percentage of Hispanic residents) were included in the adjusted model.
Table 5.
Association of Zip-Code-level Participant Characteristics With Celiac Disease and Helicobacter pylori Infection Among Patients With Biopsy Specimens Submitted to Miraca Life Sciences (Irving, Texas) During the Period January 1, 2008–June 30, 2012
| Zip-Code-level Characteristic and Quartile | Prevalence of Celiac Disease, % | Adjusteda OR | 95% CI | Prevalence of H. pylori, % | Adjusteda OR | 95% CI |
|---|---|---|---|---|---|---|
| Median household incomeb | ||||||
| 1 | 1.7 | 1.0 | 13.4 | 1.0 | ||
| 2 | 2.0 | 1.17 | 0.94, 1.47 | 8.8 | 0.75 | 0.67, 0.84 |
| 3 | 2.2 | 1.25 | 1.00, 1.56 | 7.6 | 0.70 | 0.63, 0.78 |
| 4 | 2.3 | 1.25 | 1.00, 1.58 | 6.4 | 0.66 | 0.59, 0.75 |
| % of black residentsc | ||||||
| 1 | 2.3 | 1.0 | 7.5 | 1.0 | ||
| 2 | 2.3 | 1.00 | 0.81, 1.22 | 7.5 | 0.98 | 0.86, 1.12 |
| 3 | 1.9 | 0.83 | 0.67, 1.03 | 8.8 | 1.07 | 0.94, 1.21 |
| 4 | 1.8 | 0.83 | 0.66, 1.03 | 11.2 | 1.42 | 1.25, 1.60 |
| % of Hispanic residentsd | ||||||
| 1 | 2.3 | 1.0 | 6.8 | 1.0 | ||
| 2 | 2.3 | 1.01 | 0.83, 1.25 | 6.7 | 1.00 | 0.87, 1.14 |
| 3 | 1.9 | 0.85 | 0.67, 1.03 | 7.9 | 1.18 | 1.04, 1.35 |
| 4 | 1.9 | 0.91 | 0.73, 1.13 | 13.4 | 1.86 | 1.65, 2.10 |
Abbreviations: CI, confidence interval; OR, odds ratio.
a Adjusted for age, gender, and Medicaid enrollment status.
b Quartile 1, <$42,104; quartile 2, $42,104–$54,642; quartile 3, $54,643–$74,034; quartile 4, >$74,034.
c Quartile 1, <1%; quartile 2, 1%–3.7%; quartile 3, 3.8%–12.5%; quartile 4, >12.5%.
d Quartile 1, <2.5%; quartile 2, 2.5%–6.4%; quartile 3, 6.5%–16.8%; quartile 4, >16.8%.
Analyses using generalized estimating equations and adjusting for zip-code-level data showed that the inverse relationship between CD and H. pylori remained strong (aOR = 0.59, 95% CI: 0.44, 0.78). Additional analyses that further incorporated inverse probability weights for availability of the zip code data found similar results (aOR = 0.56, 95% CI: 0.47, 0.67).
DISCUSSION
In this analysis of a nationwide pathology database, we found a strong inverse relationship between the presence of H. pylori and CD among patients undergoing gastrointestinal endoscopy for a variety of symptoms. After adjustment for potentially confounding variables, such as age, gender, and socioeconomic status, the inverse relationship between H. pylori and CD remained strong. This association was present in all age groups and both genders, and the relationships were similar regardless of the degree of villous atrophy found in the duodenal biopsy. Because this was a cross-sectional study, the temporality of exposure and outcome could not be ascertained for each individual. However, previous studies have established that H. pylori is usually acquired during the first few years of life (24), while studies of serial serum sampling have shown that CD can develop at any age (2, 25). Therefore, these results raise the possibility that the presence of H. pylori is protective against the development of CD. The epidemiology of these two conditions argues against the reverse—namely, that CD is somehow protective against the acquisition of H. pylori.
The few studies that have investigated the relationship between these two entities have yielded conflicting results. Four studies noted a decreased prevalence of H. pylori among CD patients compared with controls, with the absolute difference in prevalence ranging between 4% and 35% (26–29). Three studies showed no difference in H. pylori prevalence (30–32), while 1 study showed a slightly increased prevalence of H. pylori in CD patients (26% vs. 20%) (33). One other study found that among patients with CD, H. pylori presence is associated with less severe degrees of villous atrophy (34). Variability in these results is probably due to large differences in H. pylori prevalence, small sample sizes (with most studies including fewer than 200 CD patients), lack of histological confirmation of H. pylori in some studies, and lack of adjustment for sociodemographic characteristics. In contrast, the present study comprised a large sample (including 2,689 persons with CD) and included patients from multiple sites throughout the United States. This analysis also adjusted for gender (since H. pylori is more common in men (12) and CD is more often diagnosed in women (35)), age (since H. pylori prevalence increases with age (12)), and socioeconomic status (since H. pylori is more common among patients with lower socioeconomic status (11, 12), whereas CD may be less common among patients with lower socioeconomic status (36)). The fact that a strong inverse relationship between H. pylori and CD persisted after adjustment for these covariates provides evidence that the relationship is robust and independent of confounding influences. We also found a positive association between H. pylori and duodenal IEL, a finding consistent with that of prior investigations (37, 38), providing external validation for the present study. The presence of IEL in the absence of villous atrophy is a finding with a wide differential diagnosis, including H. pylori (39).
The mechanism by which H. pylori might be protective against CD is uncertain. H. pylori is associated with decreased risks of allergic and atopic disease and other inflammatory conditions (7, 9, 40). This may reflect the additional recruitment of both local and systemic regulatory T-regulatory lymphocytes to the gastric mucosa in patients with H. pylori, with both local and systemic effects (41, 42). Experimental murine infections provide strong evidence for the hypothesis that the T-regulatory lymphocytes recruited by H. pylori have systemic effects and specifically protect against allergen-induced asthma (9, 10, 43). T-regulatory lymphocytes also may play a role in the pathogenesis of CD, since the down-regulation of cellular responses mediated by T-regulatory cells in the bowel wall is diminished or lost in CD patients (44). Thus, persons without H. pylori and without the recruited gastric T-regulatory cells may be less likely to down-regulate immune responses to gluten. Alternatively, H. pylori may affect ingested gluten through its modification by gastric pH or through its proteases (24), reducing its immunogenicity.
Our study had a number of strengths, including its large sample size, geographic diversity, and uniformity of histological interpretation. The limitations include the fact that the study was restricted to patients who underwent concurrent gastric and duodenal biopsies. Such patients may differ from patients undergoing only gastric biopsy or only duodenal biopsy. To address this issue, we repeated the analysis after including patients with duodenal biopsy and any prior gastric biopsy on record, and found an unchanged inverse relationship. The definition of CD was limited to histological diagnosis, and there are other conditions that can mimic CD histologically (45, 46). However, CD is the most common form of villous atrophy, and we performed separate analyses for Marsh 3A and Marsh 3B/C lesions, the latter being highly specific for CD (47). The gold standard for CD diagnosis is small-bowel biopsy; thus, identification of patients with histological evidence of villous atrophy is likewise a highly sensitive method. Since most patients with CD in the United States are undiagnosed (48, 49), identifying a risk or protective factor for diagnosed disease may not result in the same effect magnitude when considering undiagnosed CD patients as well. As such, the direction of the association between H. pylori and undiagnosed CD cannot be deduced from this analysis. We found an association between H. pylori and increased IEL; because CD3 immunohistochemical staining was not done routinely on small-intestine biopsies in these patients, subtle or borderline cases of IEL may have been missed, yielding an underestimate of the calculated association. We did not have access to information on medication use in this database, and proton pump inhibitors may decrease the sensitivity of gastric biopsy for the diagnosis of H. pylori; however, the fact that the inverse association between CD and H. pylori was present regardless of clinical indication (Table 4) illustrates that such differential use of acid suppression medication is unlikely to have been a confounding variable.
H. pylori may be a marker for another exposure, such as crowded living conditions, dietary habits, or not receiving antibiotics. Adjusting for Medicaid status partially addressed this issue, since Medicaid status has been used previously in analyses of socioeconomic disparities in health-care outcomes (50). To further account for racial, ethnic, and socioeconomic factors that may affect the risk of CD and H. pylori, in a subset of the study population we were able to perform additional analyses taking into account zip-code-level data on these characteristics. This additional analysis uncovered associations between household income, zip-code racial and ethnic composition, and the outcomes of CD and H. pylori in our data set. However, adjustment for these variables did not alter the inverse relationship between CD and H. pylori infection. Our analyses, including incorporation of inverse probability weights, suggest that the subset of patients from whom zip code data were available was representative of the overall studied patient population. Estimates of the association between CD and H. pylori may vary based on the population prevalence of H. pylori, and testing of this association in nations with a higher prevalence of H. pylori may be warranted.
Because this was a cross-sectional study, it is unknown whether gastric colonization with H. pylori preceded the onset of CD or developed subsequent to this condition. While it was impossible to ascertain this information for each individual patient in this analysis, on the whole H. pylori precedes CD development given the known epidemiology of H. pylori (which is usually acquired in childhood) and CD, which can develop de novo at any age.
In conclusion, we found that patients with CD have significantly lower rates of gastric H. pylori infection than patients with normal duodenal mucosa. This association remained unaffected by the confounding influences of age, gender, and socioeconomic status. These results raise the hypothesis that H. pylori confers protection against the development of CD. Additional studies may be warranted for confirmation and to examine whether H. pylori could modulate gluten immunogenicity among genetically susceptible hosts.
ACKNOWLEDGMENTS
Author affiliations: Celiac Disease Center, Department of Medicine, College of Physicians and Surgeons, Columbia University, New York, New York (Benjamin Lebwohl, Peter Green); Department of Epidemiology, Mailman School of Public Health, Columbia University, New York, New York (Benjamin Lebwohl, Andrew Rundle); Department of Medicine, Langone Medical Center, New York University, New York, New York (Martin J. Blaser); Clinical Epidemiology Unit, Department of Medicine, Karolinska University Hospital and Karolinska Institutet, Stockholm, Sweden (Jonas F. Ludvigsson); Department of Pediatrics, Örebro University Hospital, Örebro, Sweden (Jonas F. Ludvigsson); Department of Medicine, Oregon Health & Science University, Portland, Oregon (Amnon Sonnenberg); Department of Pathology, UT Southwestern Medical Center, University of Texas, Dallas, Texas (Robert M. Genta); and Miraca Life Sciences Research Institute, Irving, Texas (Robert M. Genta).
B.L. was supported by the National Center for Advancing Translational Sciences, National Institutes of Health (grant KL2 TR000081). M.J.B. was supported by the National Institutes of Health (grant R01 GM63270) and the Diane Belfer Program in Human Microbial Ecology. J.F.L. was supported by Örebro University Hospital, Karolinska Institutet, the Swedish Society of Medicine, the Swedish Research Council–Medicine (grant 522-2A09-195), and the Swedish Celiac Society.
Selected findings from this study were presented at the Digestive Disease Week conference in Orlando, Florida, on May 18, 2013.
Conflict of interest: none declared.
REFERENCES
- 1.Rubio-Tapia A, Kyle RA, Kaplan EL, et al. Increased prevalence and mortality in undiagnosed celiac disease. Gastroenterology. 2009;137(1):88–93. doi: 10.1053/j.gastro.2009.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Catassi C, Kryszak D, Bhatti B, et al. Natural history of celiac disease autoimmunity in a USA cohort followed since 1974. Ann Med. 2010;42(7):530–538. doi: 10.3109/07853890.2010.514285. [DOI] [PubMed] [Google Scholar]
- 3.Lohi S, Mustalahti K, Kaukinen K, et al. Increasing prevalence of coeliac disease over time. Aliment Pharmacol Ther. 2007;26(9):1217–1225. doi: 10.1111/j.1365-2036.2007.03502.x. [DOI] [PubMed] [Google Scholar]
- 4.Ivarsson A, Persson LA, Nystrom L, et al. Epidemic of coeliac disease in Swedish children. Acta Paediatr. 2000;89(2):165–171. doi: 10.1080/080352500750028771. [DOI] [PubMed] [Google Scholar]
- 5.Stene LC, Honeyman MC, Hoffenberg EJ, et al. Rotavirus infection frequency and risk of celiac disease autoimmunity in early childhood: a longitudinal study. Am J Gastroenterol. 2006;101(10):2333–2340. doi: 10.1111/j.1572-0241.2006.00741.x. [DOI] [PubMed] [Google Scholar]
- 6.Marild K, Stephansson O, Montgomery S, et al. Pregnancy outcome and risk of celiac disease in offspring: a nationwide case-control study. Gastroenterology. 2012;142(1):39–45. doi: 10.1053/j.gastro.2011.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Y, Blaser MJ. Inverse associations of Helicobacter pylori with asthma and allergy. Arch Intern Med. 2007;167(8):821–827. doi: 10.1001/archinte.167.8.821. [DOI] [PubMed] [Google Scholar]
- 8.Chen Y, Blaser MJ. Helicobacter pylori colonization is inversely associated with childhood asthma. J Infect Dis. 2008;198(4):553–560. doi: 10.1086/590158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arnold IC, Dehzad N, Reuter S, et al. Helicobacter pylori infection prevents allergic asthma in mouse models through the induction of regulatory T cells. J Clin Invest. 2011;121(8):3088–3093. doi: 10.1172/JCI45041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oertli M, Sundquist M, Hitzler I, et al. DC-derived IL-18 drives Treg differentiation, murine Helicobacter pylori-specific immune tolerance, and asthma protection. J Clin Invest. 2012;122(3):1082–1096. doi: 10.1172/JCI61029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grad YH, Lipsitch M, Aiello AE. Secular trends in Helicobacter pylori seroprevalence in adults in the United States: evidence for sustained race/ethnic disparities. Am J Epidemiol. 2012;175(1):54–59. doi: 10.1093/aje/kwr288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sonnenberg A, Lash RH, Genta RM. A national study of Helicobacter pylori infection in gastric biopsy specimens. Gastroenterology. 2010;139(6):1894–1901. doi: 10.1053/j.gastro.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 13.Walker MM, Murray JA, Ronkainen J, et al. Detection of celiac disease and lymphocytic enteropathy by parallel serology and histopathology in a population-based study. Gastroenterology. 2010;139(1):112–119. doi: 10.1053/j.gastro.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Genta RM, Kinsey RS, Singhal A, et al. Gastric foveolar metaplasia and gastric heterotopia in the duodenum: no evidence of an etiologic role for Helicobacter pylori. Hum Pathol. 2010;41(11):1593–1600. doi: 10.1016/j.humpath.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 15.Lebwohl B, Kapel RC, Neugut AI, et al. Adherence to biopsy guidelines increases celiac disease diagnosis. Gastrointest Endosc. 2011;74(1):103–109. doi: 10.1016/j.gie.2011.03.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dixon MF, Genta RM, Yardley JH, et al. Classification and grading of gastritis: the updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20(10):1161–1181. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Genta RM. Differential diagnosis of reactive gastropathy. Sem Diag Pathol. 2005;22(4):273–283. doi: 10.1053/j.semdp.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 18.Wu TT, Hamilton SR. Lymphocytic gastritis: association with etiology and topology. Am J Surg Pathol. 1999;23(2):153–158. doi: 10.1097/00000478-199902000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Bureau of the Census, US Department of Commerce. Washington, DC: Bureau of the Census; 2013. American Community Surveyhttp://www.census.gov/acs. (Accessed June 19, 2013) [Google Scholar]
- 20.Hernan MA, Hernandez-Diaz S, Robins JM. A structural approach to selection bias. Epidemiology. 2004;15(5):615–625. doi: 10.1097/01.ede.0000135174.63482.43. [DOI] [PubMed] [Google Scholar]
- 21.Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11(5):550–560. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 22.Curtis LH, Hammill BG, Eisenstein EL, et al. Using inverse probability-weighted estimators in comparative effectiveness analyses with observational databases. Med Care. 2007;45(10 suppl 2):S103–S107. doi: 10.1097/MLR.0b013e31806518ac. [DOI] [PubMed] [Google Scholar]
- 23.Hubbard AE, Ahern J, Fleischer NL, et al. To GEE or not to GEE: comparing population average and mixed models for estimating the associations between neighborhood risk factors and health. Epidemiology. 2010;21(4):467–474. doi: 10.1097/EDE.0b013e3181caeb90. [DOI] [PubMed] [Google Scholar]
- 24.McColl KE. Helicobacter pylori infection. N Engl J Med. 2010;362(17):1597–1604. doi: 10.1056/NEJMcp1001110. [DOI] [PubMed] [Google Scholar]
- 25.Vilppula A, Kaukinen K, Luostarinen L, et al. Increasing prevalence and high incidence of celiac disease in elderly people: a population-based study. BMC Gastroenterol. 2009;9:49. doi: 10.1186/1471-230X-9-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rostami Nejad M, Rostami K, Yamaoka Y, et al. Clinical and histological presentation of Helicobacter pylori and gluten related gastroenteropathy. Arch Iran Med. 2011;14(2):115–118. [PMC free article] [PubMed] [Google Scholar]
- 27.Rostami-Nejad M, Villanacci V, Mashayakhi R, et al. Celiac disease and Hp infection association in Iran. Rev Esp Enferm Dig. 2009;101(12):850–854. doi: 10.4321/s1130-01082009001200004. [DOI] [PubMed] [Google Scholar]
- 28.Ciacci C, Squillante A, Rendina D, et al. Helicobacter pylori infection and peptic disease in coeliac disease. Eur J Gastroenterol Hepatol. 2000;12(12):1283–1287. doi: 10.1097/00042737-200012120-00004. [DOI] [PubMed] [Google Scholar]
- 29.Diamanti A, Maino C, Niveloni S, et al. Characterization of gastric mucosal lesions in patients with celiac disease: a prospective controlled study. Am J Gastroenterol. 1999;94(5):1313–1319. doi: 10.1111/j.1572-0241.1999.01082.x. [DOI] [PubMed] [Google Scholar]
- 30.Aydogdu S, Cakir M, Yuksekkaya HA, et al. Helicobacter pylori infection in children with celiac disease. Scand J Gastroenterol. 2008;43(9):1088–1093. doi: 10.1080/00365520802101846. [DOI] [PubMed] [Google Scholar]
- 31.Luzza F, Mancuso M, Imeneo M, et al. Helicobacter pylori infection in children with celiac disease: prevalence and clinicopathologic features. J Pediatr Gastroenterol Nutr. 1999;28(2):143–146. doi: 10.1097/00005176-199902000-00009. [DOI] [PubMed] [Google Scholar]
- 32.Crabtree JE, O'Mahony S, Wyatt JI, et al. Helicobacter pylori serology in patients with coeliac disease and dermatitis herpetiformis. J Clin Pathol. 1992;45(7):597–600. doi: 10.1136/jcp.45.7.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Konturek PC, Karczewska E, Dieterich W, et al. Increased prevalence of Helicobacter pylori infection in patients with celiac disease. Am J Gastroenterol. 2000;95(12):3682–3683. doi: 10.1111/j.1572-0241.2000.03421.x. [DOI] [PubMed] [Google Scholar]
- 34.Villanacci V, Bassotti G, Liserre B, et al. Helicobacter pylori infection in patients with celiac disease. Am J Gastroenterol. 2006;101(8):1880–1885. doi: 10.1111/j.1572-0241.2006.00621.x. [DOI] [PubMed] [Google Scholar]
- 35.Lebwohl B, Tennyson CA, Holub JL, et al. Sex and racial disparities in duodenal biopsy to evaluate for celiac disease. Gastrointest Endosc. 2012;76(4):779–785. doi: 10.1016/j.gie.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olen O, Bihagen E, Rasmussen F, et al. Socioeconomic position and education in patients with coeliac disease. Dig Liver Dis. 2012;44(6):471–476. doi: 10.1016/j.dld.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 37.Cabral VL, Patricio FR, Gabbay MA, et al. Intraepithelial lymphocytes in duodenum from Brazilian adolescents with type 1 diabetes. Influence of Helicobacter pylori. Pediatr Diabetes. 2009;10(5):316–320. doi: 10.1111/j.1399-5448.2008.00478.x. [DOI] [PubMed] [Google Scholar]
- 38.Memeo L, Jhang J, Hibshoosh H, et al. Duodenal intraepithelial lymphocytosis with normal villous architecture: common occurrence in H. pylori gastritis. Mod Pathol. 2005;18(8):1134–1144. doi: 10.1038/modpathol.3800404. [DOI] [PubMed] [Google Scholar]
- 39.Brown I, Mino-Kenudson M, Deshpande V, et al. Intraepithelial lymphocytosis in architecturally preserved proximal small intestinal mucosa: an increasing diagnostic problem with a wide differential diagnosis. Arch Pathol Lab Med. 2006;130(7):1020–1025. doi: 10.5858/2006-130-1020-ILIAPP. [DOI] [PubMed] [Google Scholar]
- 40.Chen Y, Segers S, Blaser MJ. Association between Helicobacter pylori and mortality in the NHANES III study. Gut. 2013;62(9):1262–1269. doi: 10.1136/gutjnl-2012-303018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robinson K, Kenefeck R, Pidgeon EL, et al. Helicobacter pylori-induced peptic ulcer disease is associated with inadequate regulatory T cell responses. Gut. 2008;57(10):1375–1385. doi: 10.1136/gut.2007.137539. [DOI] [PubMed] [Google Scholar]
- 42.Wang SK, Zhu HF, He BS, et al. CagA+ H. pylori infection is associated with polarization of T helper cell immune responses in gastric carcinogenesis. World J Gastroenterol. 2007;13(21):2923–2931. doi: 10.3748/wjg.v13.i21.2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arnold IC, Lee JY, Amieva MR, et al. Tolerance rather than immunity protects from Helicobacter pylori-induced gastric preneoplasia. Gastroenterology. 2011;140(1):199–209. doi: 10.1053/j.gastro.2010.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hmida NB, Ben Ahmed M, Moussa A, et al. Impaired control of effector T cells by regulatory T cells: a clue to loss of oral tolerance and autoimmunity in celiac disease? Am J Gastroenterol. 2012;107(4):604–611. doi: 10.1038/ajg.2011.397. [DOI] [PubMed] [Google Scholar]
- 45.Pallav K, Leffler DA, Tariq S, et al. Noncoeliac enteropathy: the differential diagnosis of villous atrophy in contemporary clinical practice. Aliment Pharmacol Ther. 2012;35(3):380–390. doi: 10.1111/j.1365-2036.2011.04938.x. [DOI] [PubMed] [Google Scholar]
- 46.Rubio-Tapia A, Herman ML, Ludvigsson JF, et al. Severe spruelike enteropathy associated with olmesartan. Mayo Clin Proc. 2012;87(8):732–738. doi: 10.1016/j.mayocp.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bao F, Bhagat G. Histopathology of celiac disease. Gastrointest Endosc Clin N Am. 2012;22(4):679–694. doi: 10.1016/j.giec.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 48.Murray JA, Van Dyke C, Plevak MF, et al. Trends in the identification and clinical features of celiac disease in a North American community, 1950–2001. Clin Gastroenterol Hepatol. 2003;1(1):19–27. doi: 10.1053/jcgh.2003.50004. [DOI] [PubMed] [Google Scholar]
- 49.Rubio-Tapia A, Ludvigsson JF, Brantner TL, et al. The prevalence of celiac disease in the United States. Am J Gastroenterol. 2012;107(10):1538–1544. doi: 10.1038/ajg.2012.219. [DOI] [PubMed] [Google Scholar]
- 50.Lebwohl B, Wang TC, Neugut AI. Socioeconomic and other predictors of colonoscopy preparation quality. Dig Dis Sci. 2010;55(7):2014–2020. doi: 10.1007/s10620-009-1079-7. [DOI] [PubMed] [Google Scholar]