Abstract
Cerebrovascular disease and vascular risk factors are associated with Alzheimer’s disease, but the evidence for their association with other neurodegenerative disorders is limited. Therefore, we compared the prevalence of cerebrovascular disease, vascular pathology and vascular risk factors in a wide range of neurodegenerative diseases and correlate them with dementia severity. Presence of cerebrovascular disease, vascular pathology and vascular risk factors was studied in 5715 cases of the National Alzheimer’s Coordinating Centre database with a single neurodegenerative disease diagnosis (Alzheimer’s disease, frontotemporal lobar degeneration due to tau, and TAR DNA-binding protein 43 immunoreactive deposits, α-synucleinopathies, hippocampal sclerosis and prion disease) based on a neuropathological examination with or without cerebrovascular disease, defined neuropathologically. In addition, 210 ‘unremarkable brain’ cases without cognitive impairment, and 280 cases with pure cerebrovascular disease were included for comparison. Cases with cerebrovascular disease were older than those without cerebrovascular disease in all the groups except for those with hippocampal sclerosis. After controlling for age and gender as fixed effects and centre as a random effect, we observed that α-synucleinopathies, frontotemporal lobar degeneration due to tau and TAR DNA-binding protein 43, and prion disease showed a lower prevalence of coincident cerebrovascular disease than patients with Alzheimer’s disease, and this was more significant in younger subjects. When cerebrovascular disease was also present, patients with Alzheimer’s disease and patients with α-synucleinopathy showed relatively lower burdens of their respective lesions than those without cerebrovascular disease in the context of comparable severity of dementia at time of death. Concurrent cerebrovascular disease is a common neuropathological finding in aged subjects with dementia, is more common in Alzheimer’s disease than in other neurodegenerative disorders, especially in younger subjects, and lowers the threshold for dementia due to Alzheimer’s disease and α-synucleinopathies, which suggests that these disorders should be targeted by treatments for cerebrovascular disease.
Keywords: Alzheimer’s disease, frontotemporal lobar degeneration, vascular disease, dementia, epidemiology, neuropathology
Introduction
Alzheimer’s disease is the most common cause of dementia in the general population, followed by vascular dementia, α-synucleinopathies (including dementia with Lewy bodies, Parkinson’s disease dementia) and frontotemporal lobar degeneration (FTLD) due to tau immunoreactive inclusions (FTLD-Tau) and TAR DNA binding protein 43 immunoreactive inclusions (FTLD-TDP). With age there is an increasing prevalence of coincident Alzheimer’s disease and cerebrovascular disease that is well-recognized. Alzheimer’s disease has been reported to present frequently together with microscopic cerebrovascular lesions (Jellinger and Attems, 2010). Cerebrovascular disease has been previously associated with worse cognitive performance in Alzheimer’s disease and neuropathological studies report that cerebrovascular disease lowers the threshold for dementia in subjects with a neuropathological diagnosis of Alzheimer’s disease (Snowdon et al., 1997; Chui et al., 2006; De Reuck et al., 2012; Bennett et al., 2013). In addition, epidemiological studies have shown that Alzheimer’s disease and cerebrovascular disease not only share age as a risk factor, but also vascular risk factors have been linked to Alzheimer’s disease and are among the most important modifiable risk factors for Alzheimer’s disease (Kling et al., 2013). Cerebrovascular disease has been suggested to contribute to Alzheimer’s disease neuropathological changes including selective brain atrophy and accumulation of abnormal proteins such as amyloid-β (Zlokovic, 2011; Kalaria et al., 2012; Toledo et al., 2012a). Indeed, atherosclerosis in the circle of Willis has been specifically linked to Alzheimer’s disease, but not to a diverse range of other common or rare neurodegenerative diseases (Roher et al., 2011; Yarchoan et al., 2012).
Few studies have investigated the association between cerebrovascular disease and other neurodegenerative diseases such as α-synucleinopathies (Jellinger, 2003; Jellinger and Attems, 2008, 2011; Ghebremedhin et al., 2010; Schwartz et al., 2012) or FTLD (De Reuck et al., 2012) and findings have been inconsistent. No study has compared the presence of cerebrovascular disease across the whole spectrum of neurodegenerative diseases. The degree to which comorbid cerebrovascular disease modifies or otherwise affects the correlation of neurodegenerative disease pathology with a disease’s clinical diagnosis and features is less studied in these neurodegenerative diseases.
To begin to address the question of the differential contribution of cerebrovascular disease to Alzheimer’s disease and other cerebrovascular diseases, we interrogated the National Alzheimer’s Coordinating Centre (NACC) database cases with autopsy-based neuropathological diagnosis (Beekly et al., 2004). Specifically, we: (i) ascertained the concurrence of cerebrovascular disease diagnosis (established based on the neuropathological examination) or the presence of vascular pathology not meeting the threshold for a diagnosis of cerebrovascular disease in the different neurodegenerative disease groups using adjusted multivariable models in the whole sample; (ii) compared the presence of vascular risk factors in the different neurodegenerative disease groups in the whole sample; and (iii) correlated the presence of cerebrovascular disease in the different neurodegenerative disease with clinical data in their last visit.
Materials and methods
Study subjects
The NACC was established by the National Institute on Ageing (U01 AG016976) in 1999 to facilitate collaborative research. The NACC collects data from 35 past and present National Institute of Ageing funded Alzheimer’s disease Centres across the USA. For this study neuropathological data were gathered from the NACC Neuropathology Data Set (Beekly et al., 2004) and clinical data associated with these cases were gathered from both the NACC Minimum Data Set (Weintraub et al., 2009) and the NACC Uniform Data Set (Beekly et al., 2007) in collaboration with NACC personnel (S.E.M., W.A.K.). The Minimum Data Set was implemented in 1999 and contains information on demographics, selected clinical manifestations, clinical diagnoses, and neuropathological diagnoses. The Uniform Data Set superseded the Minimum Data Set in 2005, continuing to follow still living and active cases in the Minimum Data Set, recruiting new cases, and accruing more extensive information than the Minimum Data Set, including neurological examination findings, functional status, neuropsychological test results and genetic information. Our analysis was performed using data from the September 2012 freeze of these data sets. More detailed information on the NACC database can be found online (http://www.alz.washington.edu/).
The initial data pull included 12 738 subjects. Only subjects with a single neurodegenerative disease were selected to be able to compare the coincidence of cerebrovascular disease, and specific vascular lesions in each of the neurodegenerative diseases. From these, 6205 subjects were included and assigned into one of eight neuropathological diagnostic categories for the final analysis: (i) Alzheimer’s disease (n = 4629); (ii) FTLD-Tau (n = 379); (iii) FTLD-TDP43 (n = 207); (iv) α-synucleinopathies (n = 323); (v) hippocampal sclerosis (n = 77); (vi) prion disease (n = 100); (vii) unremarkable brain (n = 210); and (viii) cerebrovascular disease (n = 280). Subjects (n = 6533) were excluded for the following reasons: (i) neuropathological diagnosis could not be assessed accurately (n = 1025); (ii) the underlying disorders were rare diseases in this database (e.g. Huntington’s disease, neuronal intermediate filament inclusion disease) that could not be constituted into a group for statistical analyses (n = 64); (iii) non- neurodegenerative disease conditions (e.g. CNS lymphoma, Wernicke-Korsakoff, n = 43); (iv) cases that were not considered neuropathologically normal, but had insufficient Alzheimer’s disease pathology to establish a diagnosis (n = 160); (v) incidental Lewy bodies (n = 62); (vi) diagnosis was dementia lacking distinct histology (n = 144); (vii) subjects diagnosed with unremarkable (normal) brain but who had cognitive impairment or dementia (n = 54); (viii) had been studied before 1997 [n = 2393, pre-National Institute of Ageing-Reagan criteria (1997) and α-synuclein (Baba et al., 1998) era]; and (ix) multiple co-morbid pathologies, none of which could be assigned unequivocally as the predominant cause of the dementia (n = 2588). The α-synucleinopathy group included cases with dementia with Lewy bodies, Parkinson’s disease dementia, Parkinson’s disease and multiple system atrophy. The FTLD-Tau group included Pick’s disease, corticobasal degeneration, progressive supranuclear palsy, tangle predominant senile dementia, argyrophilic grain disease and unclassifiable FTLD-Tau disorders. The FTLD-TDP group included demented subjects with and without motor neuron disease and amyotrophic lateral sclerosis cases. Finally, cases with unremarkable brain (without cognitive impairment) and cerebrovascular disease were included as negative and positive control groups, respectively, for comparison purposes. The counts/scores of each of the different neuropathological diagnoses within the FTLD-Tau, FTLD-TDP and α-synucleinopathy groups are detailed in Supplementary Table 1.
Two categories for vascular pathology data were available: (i) cerebrovascular disease, in which vascular pathology was classified as a primary or contributing neuropathology (Items 20E1–20E2 in the Neuropathology Data Set); and (ii) vascular pathology (Item 12), in which vascular pathologies were recorded independently of reaching or not a threshold deemed sufficient to contribute to clinical status. Therefore, the vascular pathology category encompasses a wider range of vascular changes and includes the group of cerebrovascular disease cases that represent a more severe stage. Vascular pathologies included large infarcts, multiple microinfarcts, lacunes, subcortical arteriosclerotic leukoencephalopathy and haemorrhages that were coded as present or absent (items 12A–E). Atherosclerosis in the circle of Willis, arteriolosclerosis and cerebral amyloid angiopathy were semi-quantitatively graded as none, mild, moderate and severe (12H–J). For analytical purposes these were collapsed into two categories: none/mild and moderate/severe. For Alzheimer’s disease cases, Braak staging (Braak et al., 2006) and Consortium to Establish A Registry of Alzheimer’s disease (CERAD) amyloid plaque scores for likelihood of Alzheimer’s disease (Mirra, 1997) were available. Brainstem, limbic and neocortical staging was available for α-synucleinopathy cases. All the clinical diagnoses are entered in the database using standardized fields. The criteria that the neuropathologists used to determine the existence of vascular features are described in the Neuropathology Guidebook (https://www.alz.washington.edu/NONMEMBER/NP/npguide9.pdf).
Data on vascular risk factors, which were obtained by the physician, were available only for subjects in the Uniform Data Set (n = 1341) and included hypertension, diabetes, hyperlipidaemia, tobacco use and known histories of cardiovascular disease or clinically defined cerebrovascular disease. These vascular risk factors were coded as unknown, absent, recent/active or remote/inactive. For analytical purposes, active and inactive categories were joined and compared to the absent category. A patient was considered to have coronary heart disease if he or she had a history of any of the following: heart attack, angioplasty/endarterectomy/stent or cardiac bypass procedure.
Statistical analysis
For the comparison of the demographic characteristics of the different neurodegenerative disease groups a Fisher exact test with Monte Carlo simulation was applied (instead of a chi-square test, because in certain analyses the expected cell count was low) (Agresti, 2002), whereas a percentile bootstrap method for comparing trimmed means was applied to assess quantitative variables due to heteroscedasticity associated with varying sample sizes in the different groups (Wilcox, 2012). The association between the different neurodegenerative diseases and the presence of cerebrovascular disease, vascular pathology, vascular risk factors, and dementia were studied in separate age- and gender-adjusted mixed effects logistic regression models that included the different Alzheimer’s disease centres as a random effect to adjust for possible centre variability (Pinheiro and Bates, 2000). These models had Alzheimer’s disease as the reference category so that the other diagnostic neurodegenerative disease groups were compared to Alzheimer’s disease. To assess if the presence of cerebrovascular disease was associated with Braak stage in patients with Alzheimer’s disease and the extent of Lewy body pathology in α-synucleinopathy cases, a binomial logistic regression model adjusted for age at death was applied. The association between the clinical dementia rating sum of boxes and the pathological features was studied using a linear regression model. For the dimensionality reduction of the pathological features a multiple factor analysis was used that allowed us to consider the binary categorical variables and the ordinal variables adequately (Bécue-Bertaut and Pagès, 2008). All analyses were conducted in R 2.15.2.
Results
Demographic differences between groups in the NACC Minimum Data Set database
The neuropathologically diagnosed neurodegenerative disease groups differed with respect to age at death, education, gender, race, APOE genotype, disease duration, age of onset of cognitive symptoms, prevalence of cerebrovascular disease and vascular pathology (Table 1). Cases with coincident cerebrovascular disease or vascular pathology were significantly older in all the neurodegenerative disease groups except the hippocampal sclerosis cases with cerebrovascular disease who showed no age differences when compared to hippocampal sclerosis subjects without cerebrovascular disease (Supplementary Table 2). Subjects with coincident cerebrovascular disease were 4–6 years older in the Alzheimer’s disease and the α-synucleinopathy groups at time of death compared to those without cerebrovascular disease or vascular pathology, but the age difference increased to 10–19 years in the FTLD and prion groups with cerebrovascular disease.
Table 1.
Demographics of the NACC Minimum Data Set sample
| Alzheimer’s disease | FTLD-Tau | FTLD-TDP | α-Synucleinopathy | Hippocampal sclerosis | Prion | Unremarkable brain | Cerebrovascular disease | P-value | |
|---|---|---|---|---|---|---|---|---|---|
| Number of cases | 4629 | 379 | 207 | 323 | 77 | 100 | 210 | 280 | |
| Age at death, years | 81.1 (10.4) | 73.7 (12.0) | 66.8 (10.3) | 77.9 (9.5) | 86.5 (10.8) | 61.8 (11.4) | 83.1 (9.5) | 84.2 (8.2) | 0.0001 |
| Gender, % male | 44.5 | 55.7 | 56.2 | 73.1 | 41.6 | 52.0 | 48.1 | 50.3 | 0.0005 |
| APOE ε4, % | 56.4 | 23.8 | 28.3 | 34.1 | 18.9 | 14.3 | 16.9 | 19.6 | 0.0005 |
| Demented, % | 85.7 | 89.0 | 83.7 | 80.5 | 67.5 | 91.7 | 0 | 44.3 | 0.0005 |
| Cerebrovascular disease, % | 32.3 | 17.3 | 5.2 | 20.2 | 39.2 | 4.8 | – | 100 | <0.0001 |
| Vascular pathology, % | 79.9 | 64.7 | 60.9 | 66.2 | 84.9 | 41.3 | 67.3 | 100 | 0.0005 |
| Large infarcts, % | 12.7 | 5.4 | 3.6 | 8.3 | 17.9 | 1.3 | 10.0 | 28.3 | 0.0005 |
| Lacunes, % | 19.9 | 12.5 | 5.7 | 15.0 | 34.3 | 2.6 | 16.1 | 46.3 | 0.0005 |
| Multiple microinfarcts, % | 20.1 | 8.4 | 6.8 | 12.2 | 32.8 | 3.8 | 17.5 | 39.6 | 0.0005 |
| Arteriosclerotic leukoencephalopathy, % | 9.3 | 11.1 | 11.8 | 7.7 | 13.0 | 1.2 | 2.0 | 18.1 | 0.0005 |
| Haemorrhages, % | 6.8 | 3.0 | 3.0 | 4.8 | 4.4 | 0 | 4.0 | 11.8 | 0.0005 |
| Atherosclerosis, % | 39.8 | 25.2 | 20.5 | 27.0 | 50.7 | 6.3 | 22.6 | 51.5 | 0.0005 |
| Arteriolosclerosis, % | 34.6 | 35.2 | 18.1 | 28.8 | 46.8 | 7.7 | 10.3 | 54.8 | 0.0005 |
| Cerebral amyloid angiopathy, % | 40.8 | 7.2 | 9.2 | 11.9 | 10.5 | 4.1 | 10.7 | 9.1 | 0.0005 |
Data represent percentage or mean (standard deviation).
Prevalence of cerebrovascular disease and vascular pathology in the different neurodegenerative disease groups
Alzheimer’s disease showed a higher coincidence of vascular pathology and cerebrovascular disease (this category represents a subset of the vascular pathology category) than all the other studied disease groups except hippocampal sclerosis in the age, gender (fixed effects) and research centre (random effect) adjusted model (Fig. 1 and Table 2). We then studied if differences in cerebrovascular disease coincidence between Alzheimer’s disease and the other neurodegenerative disease varied with age. We divided the sample in two groups based on a 73 years cut-off (median age of the non-Alzheimer’s disease group) and found a significant interaction between age group and the non-Alzheimer’s disease neurodegenerative disease group [odds ratio (OR) = 0.62, P = 0.013], indicating comparatively lower prevalence of cerebrovascular disease in the younger non-Alzheimer’s disease group, than in the older non-Alzheimer’s disease when compared to the Alzheimer’s disease group. α-Synucleinopathy, FTLD-Tau and FTLD-TDP did not show any difference in vascular pathology or cerebrovascular disease when compared to each other.
Figure 1.
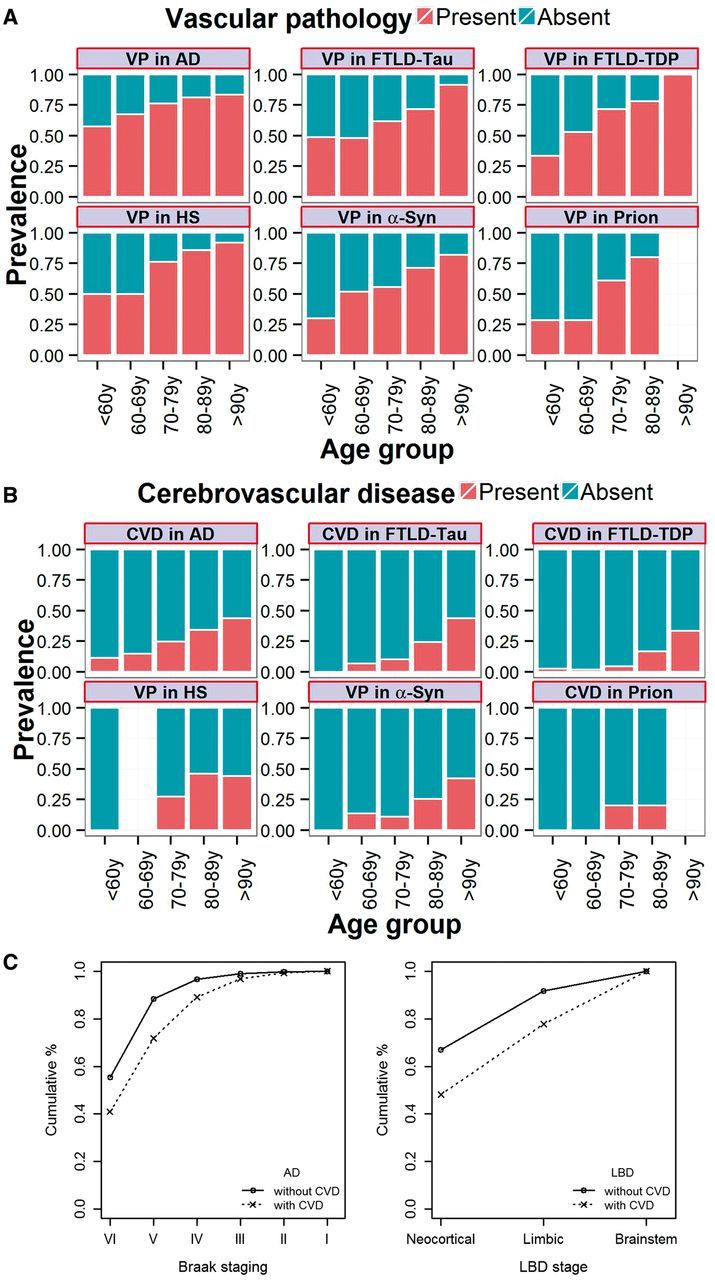
Prevalence of vascular pathology (A) and cerebrovascular disease (B) in the different neuropathologically diagnosed groups, and Braak stage (C left) and Lewy body disease stage (LBD; C right) stratified by the presence of cerebrovascular disease. AD = Alzheimer’s disease; CVD = cerebrovascular disease; HS = hippocampal sclerosis; y = years.
Table 2.
Comparison of vascular pathology and cerebrovascular disease prevalence in the different groups compared to Alzheimer’s disease
| Reference category for analysis | Analysed category | OR (95% confidence interval) for vascular pathology | P-value for vascular pathology | OR (95% confidence interval) for cerebrovascular disease | P-value for cerebrovascular disease |
|---|---|---|---|---|---|
| Alzheimer’s disease | FTLD-Tau | 0.37 (0.28–0.50) | <0.0001 | 0.38 (0.25–0.57) | <0.0001 |
| Alzheimer’s disease | FTLD-TDP | 0.40 (0.28–0.58) | <0.0001 | 0.20 (0.09–0.42) | <0.0001 |
| Alzheimer’s disease | Hippocampal sclerosis | 1.37 (0.64–2.98) | 0.41 | 1.10 (0.59–2.04) | 0.76 |
| Alzheimer’s disease | α-Synucleinopathy | 0.38 (0.28–0.52) | <0.0001 | 0.47 (0.31–0.70) | 0.0002 |
| Alzheimer’s disease | Prion disease | 0.13 (0.08–0.23) | <0.0001 | 0.24 (0.07–0.83) | 0.024 |
| Alzheimer’s disease | Unremarkable Brain | 0.49 (0.39–0.61) | <0.0001 | – | – |
| α-Synucleinopathy | FTLD-TDP | 1.16 (0.71–1.91) | 0.56 | 0.47 (0.19–1.17) | 0.11 |
| α-Synucleinopathy | FTLD-Tau | 0.96 (0.63–1.45) | 0.85 | 0.75 (0.42–1.35) | 0.34 |
| FTLD-TDP | FTLD-Tau | 0.84 (0.53–1.33) | 0.46 | 0.84 (0.53–1.33) | 0.46 |
Finally, we compared the prevalence of the different vascular changes in the different diseases in the adjusted model, comparing Alzheimer’s disease against the other groups finding several statistically significant results (Table 3). When Alzheimer’s disease was compared with the FTLD-Tau and -TDP groups, Alzheimer’s disease showed a higher prevalence of large and multiple microinfarcts than both groups of FTLD. In addition, Alzheimer’s disease showed a higher prevalence of lacunes and moderate to severe arteriolosclerosis than FTLD-TDP and a higher prevalence of arteriosclerotic leukoencephalopathy, haemorrhages and moderate to severe atherosclerosis than FTLD-Tau. When compared to the α-synucleinopathy group the Alzheimer’s disease group showed a higher prevalence of multiple microinfarcts and moderate to severe atherosclerosis and arteriolosclerosis. Prevalence of moderate to severe atherosclerosis and arteriolosclerosis and arteriosclerotic leukoencephalopathy was also higher in patients with Alzheimer’s disease than the unremarkable brain. On the other hand, the hippocampal sclerosis showed a higher prevalence of arteriosclerotic leukoencephalopathy than the Alzheimer’s disease group. The cerebrovascular disease group showed a higher prevalence of all the vascular pathology than Alzheimer’s disease group, except for cerebral amyloid angiopathy, which was higher in the Alzheimer’s disease group. Finally, Alzheimer’s disease showed higher prevalence of moderate to severe cerebral amyloid angiopathy than all of the other groups. The prevalence of vascular changes in the vascular pathology group without cerebrovascular disease and the cerebrovascular disease groups (independently of the presence of a neurodegenerative disease) is summarized in Supplementary Table 3.
Table 3.
Differences in vascular changes in Alzheimer’s disease compared to the different groups in the analysis adjusted for age at death and gender
| Variable | FTLD-Tau | FTLD-TDP | α-Synucleinopathy | Hippocampal sclerosis | Prion | Unremarkable brain | Cerebrovascular disease |
|---|---|---|---|---|---|---|---|
| Large infarcts | 2.0 (0.006) | 2.3 (0.036) | 1.4 (0.10) | 0.9 (0.79) | –a | 1.5 (0.12) | 0.42 (<0.0001) |
| Multiple microinfarcts | 2.4 (<0.0001) | 2.4 (0.004) | 2.0 (0.0006) | 0.6 (0.79) | –a | 1.5 (0.06) | 0.4 (<0.0001) |
| Lacunes | 1.3 (0.17) | 2.3 (0.01) | 1.3 (0.19) | 0.7 (0.19) | –a | 1.5 (0.05) | 0.4 (<0.0001) |
| Arteriosclerotic leukoencephalopathy | 1.6 (0.07) | 1.5 (0.20) | 1.1 (0.74) | 0.3 (0.004) | –a | 5.5 (0.003) | 0.2 (<0.0001) |
| Haemorrhage | 2.1 (0.03) | 1.9 (0.15) | 1.4 (0.26) | 1.9 (0.30) | –a | 1.8 (0.10) | 0.6 (0.022) |
| Atherosclerosis | 1.4 (0.02) | 1.2 (0.38) | 1.4 (0.01) | 1.0 (0.89) | 4.0 (0.004) | 2.8 (<0.0001) | 0.7 (0.006) |
| Arteriolosclerosis | 1.3 (0.13) | 2.4 (0.0001) | 1.6 (0.005) | 0.7 (0.22) | 4.8 (0.0005) | 3.6 (<0.0001) | 0.4 (<0.0001) |
| Cerebral amyloid angiopathy | 12.4 (<0.0001) | 9.2 (<0.0001) | 6.6 (<0.0001) | 9.1 (<0.0001) | 20.0 (<0.0001) | 6.2 (<0.0001) | 7.2 (<0.0001) |
Data are represented as OR (P-values).
aThis disease presented a low prevalence of changes and/or small sample size and could not be studied in the logistic regression model.
Association of cerebrovascular disease with disease burden
Two neurodegenerative disease groups, Alzheimer’s disease and α-synucleinopathy groups, had data regarding their staging. Braak staging was available for Alzheimer’s disease, and α-synucleinopathies were classified as brainstem, limbic/transitional, and neocortical stages. At time of death, demented subjects with a neuropathological diagnosis of Alzheimer’s disease who had coincident cerebrovascular disease had lower Braak stages (I-IV versus V: OR = 0.44, P < 0.0001; I-IV versus VI: OR = 0.41, P < 0.0001) than those without cerebrovascular disease (OR = 0.42, P < 0.0001) (Fig. 1C) in an age-adjusted analysis. In addition, the presence of cerebrovascular disease also was associated with a trend for lower prevalence of Lewy body neocortical pathology in the α-synucleinopathy demented patients with cerebrovascular disease pathology (brainstem versus neocortical: OR = 0.30, P = 0.059) but not for limbic Lewy body disease (brainstem versus limbic: OR = 0.54, P = 0.36).
Association of cerebrovascular disease with dementia status and severity proximal to death
We investigated whether the presence of cerebrovascular disease increased the probability of being demented at the time of death. In the age and gender adjusted model, Braak neurofibrillary tangle staging was the strongest neuropathological predictor for dementia in subjects with a neuropathological diagnosis of Alzheimer’s disease (stage VI versus stage I–IV: OR = 16.9, P < 0.0001; stage V versus stage I–IV: OR = 6.5, P < 0.0001), followed by the CERAD (CERAD C versus CERAD A–B: OR = 2.0, P < 0.0001), and the presence of cerebrovascular disease (OR = 1.90, P = 0.001). Interestingly there was an interaction between cerebrovascular disease and Braak staging indicating that the association between cerebrovascular disease and dementia was lower in cases with higher Braak stage (for Braak VI: OR = 0.44, P = 0.029; for Braak V: OR = 0.48, P = 0.049). In the α-synucleinopathy group, the presence of neocortical Lewy bodies was the strongest neuropathological predictor of dementia (OR = 6.0, P = 0.0001), followed by the presence of cerebrovascular disease (OR = 3.8, P = 0.029), but there was no interaction between the Lewy body stage and the presence of cerebrovascular disease (P = 0.11). Clinical dementia rating sum of boxes scores within 2 years of death were available for 715 subjects with Alzheimer’s disease (median = 8.3, first quartile = 4.7 months, third quartile 13.5 months) and 76 subjects with α-synucleinopathy (median = 8.2 months, first quartile = 4.0 months, third quartile = 12.1 months). Braak stage (P < 0.0001), CERAD score (P < 0.0001), and cerebrovascular disease (P = 0.011) predicted the clinical dementia rating sum of boxes score in the patients with Alzheimer’s disease in the model that was also adjusted for gender and age. Neither the Lewy body disease stage (P = 0.084), nor the presence of cerebrovascular disease (P = 0.58) showed an association with clinical dementia rating sum of boxes score in the α-synucleinopathy group. We further performed a multiple factor analysis and tested the first two components that accounted for 52.7% of the variability of the categorical variables (large infarcts, multiple microinfarcts, lacunes, arteriosclerotic leukoencephalopathy, haemorrhage) and the first component that accounted for 47.0% of the variability of the ordinal variables (atherosclerosis, arteriolosclerosis and cerebral amyloid angiopathy). Only the first component of the ordinal variables showed a significant association with clinical dementia rating sum of boxes (t = 3.7, P = 0.0003) indicating that increasing arteriolosclerosis and atherosclerosis was associated with worse clinical dementia rating sum of boxes.
Association of vascular risk factors and cardiovascular disease with the different disease groups
The NACC Uniform Data Set contained information about vascular risk factors and cardiovascular disease in 1341 subjects. The neurodegenerative disease groups differed in the presence of active/inactive versus absent coronary heart disease, atrial fibrillation and hypertension (Table 4). The only differences when compared with the Alzheimer’s disease group in the age and gender adjusted model were a higher prevalence of active/inactive coronary heart disease in the unremarkable brain group (P = 0.0047), a lower prevalence of active/inactive coronary heart disease in the FTLD-TDP group (P = 0.041), a higher prevalence of active/inactive atrial fibrillation in the α-synucleinopathy (P = 0.046) and cerebrovascular disease (P = 0.022) groups and a higher prevalence of active/inactive hypertension in the cerebrovascular disease group (P = 0.008). For reference, prevalence of active vascular risk factors and cardiovascular disease is summarized in Supplementary Table 4.
Table 4.
Prevalence of vascular risk factors and cardiovascular disease
| Alzheimer’s disease | FTLD- Tau | FTLD- TDP | α-Synucleinopathy | Hippocampal sclerosis | Prion | Unremarkable brain | Cerebrovascular disease | P-value | |
|---|---|---|---|---|---|---|---|---|---|
| Number of cases | 845 | 118 | 86 | 102 | 24 | 44 | 35 | 87 | |
| Coronary heart disease, % | 18.0 | 12.7 | 4.7 | 16.7 | 20.8 | 6.8 | 37.1 | 23.0 | 0.0008 |
| Atrial fibrillation, % | 13.7 | 8.5 | 3.5 | 17.6 | 20.8 | 2.3 | 22.8 | 26.7 | 0.0008 |
| Hypertension, % | 56.2 | 55.1 | 36.5 | 52.0 | 75.0 | 45.5 | 70.6 | 75.6 | 0.0008 |
| Hypercholesterolaemia, % | 47.4 | 45.1 | 35.3 | 45.5 | 45.8 | 43.2 | 51.4 | 46.5 | 0.65 |
| Diabetes, % | 12.2 | 8.5 | 8.1 | 10.8 | 16.7 | 13.6 | 20.0 | 14.9 | 0.61 |
| Smoking history, % | 44.2 | 56.1 | 42.9 | 46.5 | 62.5 | 39.0 | 58.8 | 49.4 | 0.13 |
Discussion
To our knowledge, this study of the association of vascular pathology and cerebrovascular disease with Alzheimer’s disease and other neurodegenerative disease reports on the largest and most diverse group of subjects with a neuropathologically confirmed neurodegenerative disease examined to date, and our analysis of data on uncommon as well as common neurodegenerative disease is unique. There were three novel findings from the age and gender-adjusted models: (i) Alzheimer’s disease has a significantly higher prevalence of vascular pathology than α-synucleinopathy, FTLD-Tau and -TDP, prion disease and unremarkable brain; (ii) Alzheimer’s disease has a significantly higher prevalence of cerebrovascular disease than α-synucleinopathy, FTLD-Tau and -TDP, and prion disease, and this was more prevalent at younger ages (Fig. 1); and (iii) the presence of cerebrovascular disease is associated with an increased risk of dementia in patients with α-synucleinopathy in addition to the increased risk in Alzheimer’s disease.
We found that cerebrovascular disease and vascular pathology increased with age, as expected and described previously (Jellinger and Attems, 2010; Nelson et al., 2011a), and accordingly, all subjects with neurodegenerative disease with cerebrovascular disease were older than those without cerebrovascular disease (except in the hippocampal sclerosis group). This age difference was greater in neurodegenerative disease with earlier ages of onset, like FTLD and prion disease, indicating the importance of age as a risk factor for cerebrovascular disease. Interestingly, this study showed in a single large and comprehensive sample that α-synucleinopathy, FTLD-Tau and FTLD-TDP, and prion disease have a lower prevalence of cerebrovascular disease than Alzheimer’s disease in an age- and gender-adjusted analysis and that the difference in prevalence was even greater in the younger age group.
There is a large body of literature regarding coincidence of cerebrovascular disease and Alzheimer’s disease and its correlation with dementia (Snowdon et al., 1997; Mungas et al., 2001; Petrovitch et al., 2005; Schneider et al., 2007, 2009; Jellinger and Attems, 2010; Deramecourt et al., 2012). It is interesting that we found the association was stronger in lower Braak stages, which was previously described in one study for subcortical vascular pathology (Chui et al., 2006) and for cerebrovascular disease (Petrovitch et al., 2005). Our study confirms the findings of previous reports on the prevalence of cerebrovascular disease in Alzheimer’s disease and the additive or interactive deleterious effect of Alzheimer’s disease pathology and cerebrovascular disease on cognition (Snowdon et al., 1997; Chui et al., 2006; Jellinger and Attems, 2010; Arvanitakis et al., 2011; Bennett et al., 2013), and adds further evidence on the effect of Alzheimer’s disease pathology (mainly plaques and tangles) to produce clinical symptoms (Nelson et al., 2007; Bennett et al., 2013).
In our study we also found that cerebrovascular disease in α-synucleinopathy is associated with dementia and the effect size was greater than in the Alzheimer’s disease sample. Whereas neocortical Lewy body disease, senile plaques and neurofibrillary tangles are recognized as contributors to dementia in Lewy body disease (Irwin et al., 2012), neuropathological studies report conflicting results of lower (Ghebremedhin et al., 2010; Schwartz et al., 2012), no difference (Choi et al., 2010) or higher cerebrovascular disease (Jellinger and Attems, 2008) in patients with Lewy body disease and there is limited neuropathological evidence regarding the impact of cerebrovascular disease on cognitive function in Lewy body disease which, at most, points to a small overall effect (Jellinger, 2012). These conflicting results also extend to the association with cognitive impairment in clinical studies with studies showing a higher burden of white matter hyperintensities in Parkinson’s disease than cognitively normal subjects whereas other studies describe no differences (Bohnen and Albin, 2011; Gonzalez-Redondo et al., 2012). Similar conflicting results have also been described for the association with cognitive outcomes (Beyer et al., 2006; Dalaker et al., 2009; Bohnen and Albin, 2011; Gonzalez-Redondo et al., 2012). In our study we found that the presence of cerebrovascular disease is a predictor of dementia in α-synucleinopathy at time of death. The effect size was larger in the Lewy body disease than in the Alzheimer’s disease group and this association was similar across different degrees of pathology, indicating that the impact of cerebrovascular disease pathology might be stronger in Lewy body disease than in Alzheimer’s disease and that in Lewy body disease the impact is still significant in subjects with diffuse neocortical Lewy bodies. Our results indicate that cerebrovascular disease has an additive effect increasing the risk of dementia in Alzheimer’s disease, although the effect is more prominent in earlier stages. To our knowledge, there are only two publications (Baborie et al., 2011; De Reuck et al., 2012) that have described a low prevalence of vascular pathology in FTLD, but neither of these studies included other neurodegenerative disease groups for comparison. We were not able to study the association between cerebrovascular disease and FTLD because we had no measure of the burden of the disease specific pathologies in this group. In the multiple factor analysis we found that the variables that were significantly associated with the clinical dementia rating sum of boxes 1 year before death in patients with Alzheimer’s disease were the atherosclerosis and arteriolosclerosis grading, two variables that reflect progressive accumulative vascular changes. Two previous studies have described a similar association; intracranial atherosclerosis has been associated with dementia (Dolan et al., 2010; Roher et al., 2011) and in a study by Yarchoan et al. (2012), a significant correlation was reported between large vessel atherosclerosis and neurodegenerative changes in a large cohort of cases with neurodegenerative diseases, suggesting a specific association with plaques and tangles in Alzheimer’s disease. Variables that graded vascular changes in a binary fashion did not show an association with the clinical dementia rating sum of boxes. There might be several explanations such as the absence of a quantification of the changes, the insensitiveness of the clinical dementia rating sum of boxes to the subcortical dementia profile present in cerebrovascular disease, the inclusion of subdural haematomas in the haemorrhage group and some of the vascular changes might have caused the death of the patient and were not present in the last clinical visit. Although we found a significantly higher burden of cerebrovascular disease in Alzheimer’s disease than in other neurodegenerative diseases, some recent clinical studies have challenged the idea of a higher burden of cerebrovascular disease in Alzheimer’s disease (Marchant et al., 2013), although this study mainly consisted of cognitively normal or mildly impaired subjects and was enriched for vascular risk factors.
We were not able to study the association between cerebrovascular disease and FTLD because we had no measure of the burden of the disease-specific pathologies in this group.
Furthermore, unremarkable brain cases, and all subjects with neurodegenerative disease, except for the hippocampal sclerosis cases, showed a lower prevalence of vascular pathology than the Alzheimer’s disease cases. Only pure cerebrovascular disease cases showed a higher prevalence of the different vascular pathology than cases with Alzheimer’s disease. This could be explained by Alzheimer’s disease sharing the same vascular risk factors as cerebrovascular disease or by the interaction of both pathologies, which could favour their common coincident occurrence. These findings lend support to the hypothesis that there is a pathophysiological link between Alzheimer’s disease and cerebrovascular disease (Kalaria et al., 2012), but, more importantly, we show for the first time that an association with cerebrovascular disease is specific for Alzheimer’s disease compared with other neurodegenerative diseases. Interestingly, hippocampal sclerosis showed no differences in the prevalence of vascular pathology and cerebrovascular disease when compared with Alzheimer’s disease, favouring recent studies indicating a strong association with TARDBP deposits rather than cerebrovascular disease (Dickson et al., 1994; Corey-Bloom et al., 1997; Leverenz et al., 2002; Nelson et al., 2011b). Nevertheless, hippocampal sclerosis was the only disorder that showed higher prevalence of several of the studied vascular changes (Table 3), which merits further analysis in studies that specifically study age-matched cohorts of Alzheimer’s disease, hippocampal sclerosis and unremarkable brain cohorts.
This study has several strengths, including the large size of the cohort, the extensive data sets on the subjects we analysed, including different types of vascular pathologies, the diverse range of neurodegenerative diseases examined and the multivariable analysis. Of particular note, the post-mortem neuropathological diagnosis in the FTLD, α-synucleinopathy and cerebrovascular disease groups is of special importance as clinical diagnoses are not as reliably predictive of the neuropathology underlying these disorders (Toledo et al., 2012b). Clinical Alzheimer’s disease, amyotrophic lateral sclerosis and Parkinson’s disease diagnoses generally show a good clinicopathological correlation (Hughes et al., 2001; Toledo et al., 2012b), but clinical FTD syndromes and dementia with Lewy bodies are not as reliably predictive of the neuropathology underlying these disorders (Toledo et al., 2012b). For instance, 40% of patients with clinically diagnosed FTD are found to have a neuropathological diagnosis of Alzheimer’s disease on post-mortem examination (Beach et al., 2012; Nelson et al., 2012; Toledo et al., 2012b). In addition, the clinical criteria for vascular dementia have low diagnostic sensitivity (Gold et al., 1997, 2002; Bacchetta et al., 2007). Therefore, studies based on clinical diagnoses in the absence of a neuropathologically confirmed diagnosis are subject to significant confounds. Recently, a staging system for cerebrovascular disease has been proposed (Deramecourt et al., 2012).
Relative weaknesses of the study are also acknowledged. As noted above, it is unfortunate that vascular risk factors were not recorded in our study subjects earlier in their lifespan at middle age; those recorded in NACC reflect a lifetime history. Although the similar diagnostic neuropathological diagnostic criteria were used, each centre follows different diagnostic procedures; however, we included a random factor in the mixed-effects model analysis to adjust for this factor. Our cases were recruited in tertiary care centres and therefore may differ from community-based studies and the cross-sectional nature of autopsy studies prevents us from establishing when pathological changes may have started. Finally, most of the data available to us were qualitative or categorical and the analyses of the vascular risk factors might have been underpowered for the less represented categories (although this is the largest sample for these cases) and the study of the association between the neuropathological findings and the clinical dementia rating sum of boxes. Additional associations might have been discerned if continuous, quantitative scale data were available.
An implication of this study is that in the absence of any specific disease-modifying treatments for Alzheimer’s disease in the near future, we urge, based on the high prevalence on cerebrovascular disease described in our data here, that aggressive management of vascular risk factors and encouragement of healthy lifestyles in midlife may have benefit for Alzheimer’s disease or α-synucleinopathy individuals at increased risk to become clinically symptomatic, and probably to those with other causes of cognitive impairment. Indeed, even those who already manifest the clinical features of Alzheimer’s disease or α-synucleinopathy may benefit from effective therapies that mitigate vascular risk factors and cerebrovascular disease. Guidelines for treatment and prevention of vascular contributions to dementia are available (Gorelick et al., 2011). Finally, we propose that it is timely to consider inclusion of patients with vascular risk factors, cardiovascular disease and cerebrovascular disease in clinical studies as these cases are often excluded currently, but they account for a large percentage of the subjects with dementia and thereby more accurately embody the challenges we must face in developing disease-modifying therapies for Alzheimer’s disease.
Supplementary Material
Acknowledgements
Thanks to all the Clinical, Neuropathology and Data Management Core and Leaders and their associates for their input and responses to many surveys and questionnaires. S.E.A serves as consultant for Teva and has been a recipient of grants from Penn-Pfizer, Johnson & Johnson, Baxter, Merck, Bristol-Myers Squibb and Eli-Lilly. Other authors had no conflicts of interest. The authors appreciate the ongoing support of Creighton Phelps, PhD, and Marcelle Morrison-Bogorad, PhD, from the National Institute of Ageing in developing the Uniform Data Set and the cooperation of all National Institute of Ageing-supported Alzheimer’s Disease Centres directors and their staff in its implementation.
Glossary
Abbreviations
- CERAD
Consortium to Establish A Registry of Alzheimer’s disease
- FTLD
frontotemporal lobar degeneration
- NACC
National Alzheimer’s Coordinating Centre
Funding
The NACC database is supported by the National Institute of Aging grant UO1 AG016976 and the Penn ADCC by the National Institute of Aging grant AG10124. J.Q.T. is the William Maul Measey-Truman G. Schnabel, Jr., Professor of Geriatric Medicine and Gerontology. J.B.T. is supported by a grant of the Fundación Alfonso Martín Escudero.
Supplementary material
Supplementary material is available at Brain online.
References
- Consensus recommendations for the postmortem diagnosis of Alzheimer's disease. The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer's Disease. Neurobiol Aging. 1997;18(Suppl 4):S1–S2. [PubMed] [Google Scholar]
- Agresti A. Categorical data analysis. New York: Wiley; 2002. [Google Scholar]
- Arvanitakis Z, Leurgans SE, Barnes LL, Bennett DA, Schneider JA. Microinfarct pathology, dementia, and cognitive systems. Stroke. 2011;42:722–7. doi: 10.1161/STROKEAHA.110.595082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba M, Nakajo S, Tu PH, Tomita T, Nakaya K, Lee VM, et al. Aggregation of alpha-synuclein in Lewy bodies of sporadic Parkinson's disease and dementia with Lewy bodies. Am J Pathol. 1998;152:879–84. [PMC free article] [PubMed] [Google Scholar]
- Baborie A, Griffiths TD, Jaros E, McKeith IG, Burn DJ, Richardson A, et al. Pathological correlates of frontotemporal lobar degeneration in the elderly. Acta Neuropathol. 2011;121:365–71. doi: 10.1007/s00401-010-0765-z. [DOI] [PubMed] [Google Scholar]
- Bacchetta JP, Kovari E, Merlo M, Canuto A, Herrmann FR, Bouras C, et al. Validation of clinical criteria for possible vascular dementia in the oldest-old. Neurobiol Aging. 2007;28:579–85. doi: 10.1016/j.neurobiolaging.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Beach TG, Monsell SE, Phillips LE, Kukull W. Accuracy of the clinical diagnosis of Alzheimer disease at National Institute on Aging Alzheimer Disease Centers, 2005–2010. J Neuropathol Exp Neurol. 2012;71:266–73. doi: 10.1097/NEN.0b013e31824b211b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bécue-Bertaut M, Pagès J. Multiple factor analysis and clustering of a mixture of quantitative, categorical and frequency data. Comput Stat Data Anal. 2008;52:3255–68. [Google Scholar]
- Beekly DL, Ramos EM, Lee WW, Deitrich WD, Jacka ME, Wu J, et al. The National Alzheimer's Coordinating Center (NACC) database: the uniform data set. Alzheimer Dis Assoc Disord. 2007;21:249–58. doi: 10.1097/WAD.0b013e318142774e. [DOI] [PubMed] [Google Scholar]
- Beekly DL, Ramos EM, van Belle G, Deitrich W, Clark AD, Jacka ME, et al. The National Alzheimer's Coordinating Center (NACC) Database: an Alzheimer disease database. Alzheimer Dis Assoc Disord. 2004;18:270–7. [PubMed] [Google Scholar]
- Bennett DA, Wilson RS, Arvanitakis Z, Boyle PA, de Toledo-Morrell L, Schneider JA. Selected findings from the religious orders study and rush memory and aging project. J Alzheimers Dis. 2013;33:S397–403. doi: 10.3233/JAD-2012-129007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer MK, Aarsland D, Greve OJ, Larsen JP. Visual rating of white matter hyperintensities in Parkinson's disease. Mov Disord. 2006;21:223–9. doi: 10.1002/mds.20704. [DOI] [PubMed] [Google Scholar]
- Bohnen NI, Albin RL. White matter lesions in Parkinson disease. Nat Rev Neurol. 2011;7:229–36. doi: 10.1038/nrneurol.2011.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006;112:389–404. doi: 10.1007/s00401-006-0127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SA, Evidente VG, Caviness JN, Shill HA, Sabbagh MN, Connor DJ, et al. Are there differences in cerebral white matter lesion burdens between Parkinson's disease patients with or without dementia? Acta Neuropathol. 2010;119:147–9. doi: 10.1007/s00401-009-0620-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chui HC, Zarow C, Mack WJ, Ellis WG, Zheng L, Jagust WJ, et al. Cognitive impact of subcortical vascular and Alzheimer's disease pathology. Ann Neurol. 2006;60:677–87. doi: 10.1002/ana.21009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey-Bloom J, Sabbagh MN, Bondi MW, Hansen L, Alford MF, Masliah E, et al. Hippocampal sclerosis contributes to dementia in the elderly. Neurology. 1997;48:154–60. doi: 10.1212/wnl.48.1.154. [DOI] [PubMed] [Google Scholar]
- Dalaker TO, Larsen JP, Dwyer MG, Aarsland D, Beyer MK, Alves G, et al. White matter hyperintensities do not impact cognitive function in patients with newly diagnosed Parkinson's disease. Neuroimage. 2009;47:2083–9. doi: 10.1016/j.neuroimage.2009.06.020. [DOI] [PubMed] [Google Scholar]
- De Reuck JL, Deramecourt V, Cordonnier C, Leys D, Pasquier F, Maurage CA. Cerebrovascular lesions in patients with frontotemporal lobar degeneration: a neuropathological study. Neuro-degener Dis. 2012;9:170–5. doi: 10.1159/000335447. [DOI] [PubMed] [Google Scholar]
- Deramecourt V, Slade JY, Oakley AE, Perry RH, Ince PG, Maurage CA, et al. Staging and natural history of cerebrovascular pathology in dementia. Neurology. 2012;78:1043–50. doi: 10.1212/WNL.0b013e31824e8e7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson DW, Davies P, Bevona C, Van Hoeven KH, Factor SM, Grober E, et al. Hippocampal sclerosis: a common pathological feature of dementia in very old ( > or = 80 years of age) humans. Acta neuropathol. 1994;88:212–21. doi: 10.1007/BF00293396. [DOI] [PubMed] [Google Scholar]
- Dolan H, Crain B, Troncoso J, Resnick SM, Zonderman AB, Obrien RJ. Atherosclerosis, dementia, and Alzheimer disease in the Baltimore Longitudinal Study of Aging cohort. Ann Neurol. 2010;68:231–40. doi: 10.1002/ana.22055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghebremedhin E, Rosenberger A, Rub U, Vuksic M, Berhe T, Bickeboller H, et al. Inverse relationship between cerebrovascular lesions and severity of lewy body pathology in patients with lewy body diseases. J Neuropathol Exp Neurol. 2010;69:442–8. doi: 10.1097/NEN.0b013e3181d88e63. [DOI] [PubMed] [Google Scholar]
- Gold G, Bouras C, Canuto A, Bergallo MF, Herrmann FR, Hof PR, et al. Clinicopathological validation study of four sets of clinical criteria for vascular dementia. Am J Psych. 2002;159:82–7. doi: 10.1176/appi.ajp.159.1.82. [DOI] [PubMed] [Google Scholar]
- Gold G, Giannakopoulos P, Montes-Paixao Junior C, Herrmann FR, Mulligan R, Michel JP, et al. Sensitivity and specificity of newly proposed clinical criteria for possible vascular dementia. Neurology. 1997;49:690–4. doi: 10.1212/wnl.49.3.690. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Redondo R, Toledo J, Clavero P, Lamet I, Garcia-Garcia D, Garcia-Eulate R, et al. The impact of silent vascular brain burden in cognitive impairment in Parkinson's disease. Eur J Neurol. 2012;19:1100–7. doi: 10.1111/j.1468-1331.2012.03682.x. [DOI] [PubMed] [Google Scholar]
- Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42:2672–713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes AJ, Daniel SE, Lees AJ. Improved accuracy of clinical diagnosis of Lewy body Parkinson's disease. Neurology. 2001;57:1497–9. doi: 10.1212/wnl.57.8.1497. [DOI] [PubMed] [Google Scholar]
- Irwin DJ, White MT, Toledo JB, Xie SX, Robinson JL, Van Deerlin V, et al. Neuropathologic substrates of Parkinson disease dementia. Ann Neurol. 2012;72:587–98. doi: 10.1002/ana.23659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellinger KA. Prevalence of cerebrovascular lesions in Parkinson's disease. A postmortem study. Acta Neuropathol. 2003;105:415–9. doi: 10.1007/s00401-003-0676-3. [DOI] [PubMed] [Google Scholar]
- Jellinger KA. Neuropathology of sporadic Parkinson's disease: evaluation and changes of concepts. Mov Disord. 2012;27:8–30. doi: 10.1002/mds.23795. [DOI] [PubMed] [Google Scholar]
- Jellinger KA, Attems J. Prevalence and impact of vascular and Alzheimer pathologies in Lewy body disease. Acta Neuropathol. 2008;115:427–36. doi: 10.1007/s00401-008-0347-5. [DOI] [PubMed] [Google Scholar]
- Jellinger KA, Attems J. Prevalence of dementia disorders in the oldest-old: an autopsy study. Acta Neuropathol. 2010;119:421––33. doi: 10.1007/s00401-010-0654-5. [DOI] [PubMed] [Google Scholar]
- Jellinger KA, Attems J. Prevalence and pathology of dementia with Lewy bodies in the oldest old: a comparison with other dementing disorders. Dement Geriatr Cogn Disord. 2011;31:309–16. doi: 10.1159/000327360. [DOI] [PubMed] [Google Scholar]
- Kalaria RN, Akinyemi R, Ihara M. Does vascular pathology contribute to Alzheimer changes? J Neurol Sci. 2012;322:141–7. doi: 10.1016/j.jns.2012.07.032. [DOI] [PubMed] [Google Scholar]
- Kling MA, Trojanowski JQ, Wolk DA, Lee VM, Arnold SE. Vascular disease and dementias: Paradigm shifts to drive research in new directions. Alzheimers Dement. 2013;9:76–92. doi: 10.1016/j.jalz.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leverenz JB, Agustin CM, Tsuang D, Peskind ER, Edland SD, Nochlin D, et al. Clinical and neuropathological characteristics of hippocampal sclerosis: a community-based study. Arch Neurol. 2002;59:1099–106. doi: 10.1001/archneur.59.7.1099. [DOI] [PubMed] [Google Scholar]
- Marchant NL, Reed BR, Sanossian N, Madison CM, Kriger S, Dhada R, et al. The aging brain and cognition: contribution of vascular injury and abeta to mild cognitive dysfunction. JAMA Neurol. 2013;70:488–95. doi: 10.1001/2013.jamaneurol.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirra SS. The CERAD neuropathology protocol and consensus recommendations for the postmortem diagnosis of Alzheimer's disease: a commentary. Neurobiol Aging. 1997;18(Suppl 4):S91–4. doi: 10.1016/s0197-4580(97)00058-4. [DOI] [PubMed] [Google Scholar]
- Mungas D, Reed BR, Ellis WG, Jagust WJ. The effects of age on rate of progression of Alzheimer disease and dementia with associated cerebrovascular disease. Arch Neurol. 2001;58:1243–7. doi: 10.1001/archneur.58.8.1243. [DOI] [PubMed] [Google Scholar]
- Nelson PT, Alafuzoff I, Bigio EH, Bouras C, Braak H, Cairns NJ, et al. Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J Neuropathol Exp Neurol. 2012;71:362–81. doi: 10.1097/NEN.0b013e31825018f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PT, Head E, Schmitt FA, Davis PR, Neltner JH, Jicha GA, et al. Alzheimer's disease is not “brain aging”: neuropathological, genetic, and epidemiological human studies. Acta Neuropathol. 2011a;121:571–87. doi: 10.1007/s00401-011-0826-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PT, Jicha GA, Schmitt FA, Liu H, Davis DG, Mendiondo MS, et al. Clinicopathologic correlations in a large Alzheimer disease center autopsy cohort: neuritic plaques and neurofibrillary tangles “do count” when staging disease severity. J Neuropathol Exp Neurol. 2007;66:1136–46. doi: 10.1097/nen.0b013e31815c5efb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PT, Schmitt FA, Lin Y, Abner EL, Jicha GA, Patel E, et al. Hippocampal sclerosis in advanced age: clinical and pathological features. Brain. 2011b;134(Pt 5):1506–18. doi: 10.1093/brain/awr053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovitch H, Ross GW, Steinhorn SC, Abbott RD, Markesbery W, Davis D, et al. AD lesions and infarcts in demented and non-demented Japanese-American men. Ann Neurol. 2005;57:98–103. doi: 10.1002/ana.20318. [DOI] [PubMed] [Google Scholar]
- Pinheiro JC, Bates DM. Mixed-Effects Models in S and S-PLUS. New York: Springer Verlag; 2000. [Google Scholar]
- Roher AE, Tyas SL, Maarouf CL, Daugs ID, Kokjohn TA, Emmerling MR, et al. Intracranial atherosclerosis as a contributing factor to Alzheimer's disease dementia. Alzheimer Dement. 2011;7:436–44. doi: 10.1016/j.jalz.2010.08.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JA, Arvanitakis Z, Leurgans SE, Bennett DA. The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann Neurol. 2009;66:200–8. doi: 10.1002/ana.21706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JA, Boyle PA, Arvanitakis Z, Bienias JL, Bennett DA. Subcortical infarcts, Alzheimer's disease pathology, and memory function in older persons. Ann Neurol. 2007;62:59–66. doi: 10.1002/ana.21142. [DOI] [PubMed] [Google Scholar]
- Schwartz RS, Halliday GM, Cordato DJ, Kril JJ. Small-vessel disease in patients with Parkinson's disease: a clinicopathological study. Mov Disord. 2012;27:1506–12. doi: 10.1002/mds.25112. [DOI] [PubMed] [Google Scholar]
- Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR. Brain infarction and the clinical expression of Alzheimer disease. The Nun Study. JAMA. 1997;277:813–7. [PubMed] [Google Scholar]
- Toledo JB, Brettschneider J, Grossman M, Arnold SE, Hu WT, Xie SX, et al. CSF biomarkers cutoffs: the importance of coincident neuropathological diseases. Acta Neuropathol. 2012b;124:23–35. doi: 10.1007/s00401-012-0983-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo JB, Toledo E, Weiner MW, Jack CR, Jr, Jagust W, Lee VM, et al. Cardiovascular risk factors, cortisol, and amyloid-beta deposition in Alzheimer's disease neuroimaging initiative. Alzheimer Dement. 2012a;8:483–9. doi: 10.1016/j.jalz.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub S, Salmon D, Mercaldo N, Ferris S, Graff-Radford NR, Chui H, et al. The Alzheimer's Disease Centers' Uniform Data Set (UDS): the neuropsychologic test battery. Alzheimer Dis Assoc Disord. 2009;23:91–101. doi: 10.1097/WAD.0b013e318191c7dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox R. Modern Statistics for the Social and Behavioral Science. Boca Raton: CRC Press; 2012. [Google Scholar]
- Yarchoan M, Xie SX, Kling MA, Toledo JB, Wolk DA, Lee EB, et al. Cerebrovascular atherosclerosis correlates with Alzheimer pathology in neurodegenerative dementias. Brain. 2012;135(Pt 12):3749–56. doi: 10.1093/brain/aws271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. Nat Rev Neurosci. 2011;12:723–38. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


