Abstract
Background
Atrial fibrillation (AF) is a strong risk factor for heart failure (HF); HF onset in patients with AF is associated with increased morbidity and mortality. Risk factors that predict HF in individuals with AF in the community are not well established.
Methods and results
We examined clinical variables related to the 10-year incidence of HF in 725 individuals (mean 73.3 years, 45% women) with documented AF in the Framingham Heart Study. Event rates for incident HF (n = 161, 48% in women) were comparable in women (4.30 per 100 person-years) and men (3.34 per 100 person-years). Age, body mass index, ECG LV hypertrophy, diabetes, significant murmur, and history of myocardial infarction were positively associated with incident HF in multivariable models (C-statistic 0.71; 95% confidence interval 0.67–0.75). We developed a risk algorithm for estimating absolute risk of HF in AF patients with good model fit and calibration (adjusted calibration χ2 statistic 7.29; Pχ2 = 0.61). Applying the algorithm, 47.6% of HF events occurred in the top tertile in men compared with 13.1% in the bottom tertile, and 58.4% in women in the upper tertile compared with 18.2% in the lowest category. For HF type, women had a non-significantly higher incidence of HF with preserved EF compared with men.
Conclusions
We describe advancing age, LV hypertrophy, body mass index, diabetes, significant heart murmur, and history of myocardial infarction as clinical predictors of incident HF in individuals with AF. A risk algorithm may help identify individuals with AF at high risk of developing HF.
Keywords: Atrial fibrillation, Risk score, Epidemiology, Heart failure
Introduction
With the increasing prevalence of AF,1 preventing its sequelae has important public health and economic implications.2 Whereas most efforts at preventing adverse outcomes in AF have successfully targeted stroke,3 little focus has been placed on heart failure (HF). The comparative lack of attention to HF as a complication of AF is of note because, similar to stroke, HF is associated with functional limitations, decreased quality of life, and excess mortality. In addition, HF is a more frequent complication of AF than stroke. Up to 40% of individuals with AF and HF have been shown to develop HF following AF onset.4
Acute AF may be accompanied by overt HF, the symptoms of which often improve after AF termination.5 However, not all patients with AF develop symptomatic HF.4 Since patients who suffer from both AF and HF have a significantly increased risk of cardiovascular and all-cause mortality,4,6 understanding the risk factors for developing HF in patients with AF is an important goal.
To date, our knowledge of AF and HF complicating each other's course largely has been derived from clinical trials7,8 and epidemiological investigations.4 In hospitalized patients with both conditions, AF seems more frequently to have developed first.9 Risk factors such as age, diabetes,10,11 hypertension, valvular disease, and cardiovascular disease12 predispose to AF. The same risk factors also predispose to HF, though possibly with different frequency and magnitude.12 The extent to which these clinical conditions contribute to the development of HF in patients with AF is uncertain.
Establishing an accurate way to stratify an individual's risk of HF may have important implications for AF monitoring and management. First attempts at more accurately defining risk of incident HF in individuals with AF in hospital-based and registry data have reported clinical variables in relation to incident HF.13,14 Discerning the risk factors for developing HF might identify individuals at high risk and help to stratify screening and early interventions. Therefore, we systematically examined clinical factors predisposing to the onset of HF in individuals diagnosed with AF in the community.
Methods
Setting and participants
The current study was performed in two cohorts of the Framingham Heart Study: the Original cohort enrolled in 194815 and Offspring cohort enrolled in the early 1970s.16 Individuals were eligible if they had AF diagnosed after 1960 and before 31 December 1999 (n = 1236). We excluded individuals for the following reasons: HF onset prior to AF (including first diagnosis of HF and AF on the same day, n = 58) or lack of follow-up (n = 295), HF onset within 30 days after first diagnosis of AF (n = 9), death within 30 days after AF onset (n = 99), or missing risk factor data (n = 108). We determined the incidence of HF over 10 years, up to 31 December 2009. The Boston University Medical Center Institutional Review Board approved all study protocols, and participants signed consent at every examination. All authors have read and agree to the manuscript as written.
Outcomes and follow-up
Participants undergo standardized cardiovascular disease risk factor assessment at regular Framingham Heart Study clinic visits (biennial clinic visits for the Original cohort and every 4–8 years for the Offspring participants) including physician physical examinations, ECGs, questionnaires, and laboratory tests. During routine follow-up, records and ECGs from outpatient visits or hospitalizations for cardiovascular disease were obtained. The diagnosis of AF was based on the presence of atrial flutter or AF on ECGs from Framingham clinic visits, outside physician or hospital charts, or Holter reports adjudicated by at least two Framingham cardiologists. We used Framingham clinical variables collected during the examination at or antecedent but closest to AF diagnosis.
Cardiovascular events were adjudicated by a panel of three Framingham investigators reviewing Framingham clinic visits, and outside physician or hospital records. The outcome HF was diagnosed based on major (paroxysmal nocturnal dyspnoea or orthopnoea, auscultatory rales, third heart sound, distended neck veins, hepatojugular reflux, significant weight loss on diuretic therapy, and radiographic pulmonary oedema or increasing cardiomegaly) and minor (night cough, ankle oedema, dyspnoea on exertion, pleural effusion, hepatomegaly, radiographic pulmonary vascular redistribution, tachycardia, and decrease in vital capacity) criteria.17
In secondary analyses, we further differentiated incident HF into reduced systolic function (LVEF <50% or fractional shortening <29%) and preserved EF (LVEF ≥50% or fractional shortening ≥29%). HF was unknown or unclassified if information was insufficient for classification into the above-mentioned categories.
Myocardial infarction was ascertained and classified as described previously.18 The Presence of increased voltage and a strain pattern were interpreted as ECG signs of LV hypertrophy. Prevalent cardiovascular disease comprised myocardial infarction, coronary insufficiency, angina, stroke, transient ischaemic attack, and intermittent claudication.
The definition of cardiovascular disease risk factors included fasting glucose ≥126 mg/dL, non-fasting blood glucose ≥200 mg/dL, or use of hypoglycaemic medications for diabetes; systolic blood pressure ≥140 or diastolic blood pressure ≥90 mmHg, or treatment for hypertension. Framingham clinic physician auscultated grade ≥3 out of 6 systolic murmur or any diastolic murmur was considered a significant cardiac murmur. Current smoking was self-reported regular use of one or more cigarettes/day within the year prior to the Heart Study examination.
Statistical analysis
Incidence rates for the number of HF cases/100 person-years of observation and 95% confidence intervals (CIs) were calculated based on the Haenszel method. We estimated sex-pooled multivariable Cox regression models to assess risk factors for HF incidence over a 10-year follow-up period; after 10 years, follow-up was censored. Death was considered a censoring event except for competing risks models. Hazard ratios for risk factors were assessed for 1 unit deviation increase for continuous variables and the condition present (vs. absent) for dichotomous risk factors. The proportionality assumption of the Cox regression models was not violated. We selected risk factors for analysis based on previous reports and availability in clinical practice.11,12 Besides age and sex, we examined Framingham clinic systolic and diastolic blood pressure, pulse pressure, hypertension, antihypertensive medication, height, weight, body mass index, current cigarette smoking, diabetes mellitus, significant murmur, ECG LV hypertrophy, heart rate, and prevalent myocardial infarction or cardiovascular disease at or prior to the onset of AF.
Biologically plausible potential interactions for age and sex among risk factors were examined and added to the final model if they improved discrimination and calibration. To account for possible secular trends in HF risk factors, we assessed the calendar period of AF onset and forced the year of AF onset into the regression models. Further, we checked for statistical interactions between decade and risk factors for HF onset, and provide characteristics of individuals developing HF by decade. We used the final Cox model for the development of a risk algorithm for incident HF that retained variables significant at the 0.05 level in multivariable models.19 We applied the C-statistic approach to assess discrimination, and estimated the consistency between expected 10-year HF events from the risk algorithm and actually observed events through the Hosmer–Lemeshow statistic modified for survival data.20 We assessed model fit and calibration in younger ( ≤70 years) and older (>70 years) individuals.
Secondary analyses
In pre-specified secondary analyses, we examined subtypes of HF with reduced systolic function and preserved EF. Considering the relatively high age of the participants at baseline, we performed additional models using the Fine and Gray method21 to adjust the risk estimates for the potential bias of the competing risk of death. All analyses were performed using SAS software, version 9.2 (SAS Institute, Cary, NC, USA) and the R 2.11.1 software package.22 A two-tailed P value of <0.05 was considered statistically significant.
Role of the funding source
This work received no defined internal or external funding with direct impact on the study's design, conduct, and reporting.
Results
Participant characteristics
We provide the characteristics of the 725 eligible individuals at the time of AF onset in Table 1. The participants had a mean age of 73.3 years (range 39–96 years) and 45% were women. Cardiovascular conditions had a high prevalence. Sex-specific data are presented in the Supplementary material, Table S1A. Baseline characteristics of the participants with both conditions, AF and HF, first diagnosed on the same day are provided in Supplementary material, Table S1B.
Table 1.
Baseline characteristics of the sample at or prior to atrial fibrillation onset (n = 725)
| Clinical characteristics | Total sample |
|---|---|
| Age, years | 73.3 (10.5) |
| Women, % | 45 |
| Current smoking, % | 17.8 |
| Body mass index, kg/m2 | 27.3 (4.9) |
| Height, cm | 165 (11) |
| Weight, kg | 75 (17) |
| Systolic blood pressure, mmHg | 143 (23) |
| Diastolic blood pressure, mmHg | 77 (12) |
| Pulse pressure, mmHg | 66 (20) |
| Heart rate, b.p.m. | 73 (18) |
| Diabetes, % | 14.5 |
| Hypertension, % | 71.7 |
| Hypertension treatment, % | 45.8 |
| ECG left ventricular hypertrophy, % | 9.7 |
| Significant murmur, % | 11.4 |
| Prevalent myocardial infarction, % | 20.1 |
| Prevalent cardiovascular disease, % | 47.6 |
Values are mean (SD), or percentage.
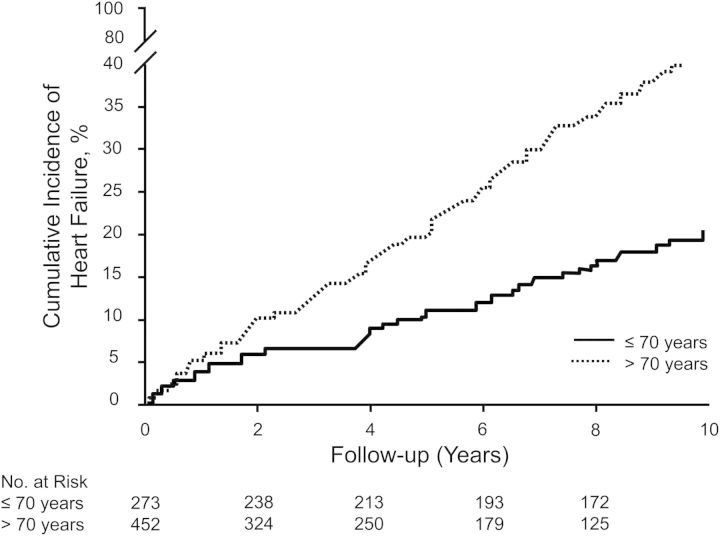
Over the follow-up period of 10 years, 161 participants developed HF; 45% were women. Cumulative incidence plots for total HF stratified by age of AF onset revealed higher incidence rates of HF in older individuals, Plog rank <0.0001 (Figure 1). HF incidence over 10 years accumulated in 20% of individuals ≤70 years of age and 41% in older individuals. Men and women had a comparable cumulative incidence of HF (data not shown).
Figure 1.
Cumulative incidence of heart failure stratified by age of atrial fibrillation onset (≤70 and >70 years) in both sexes (Plog rank < 0.001).
Risk models
Age- and sex-adjusted Cox proportional hazards models revealed that age, body mass index, weight, blood pressure variables, diabetes, significant murmur, ECG LV hypertrophy, prevalent myocardial infarction, and prevalent cardiovascular disease were associated with incident HF (Table 2). The direction and magnitude of risk factor associations for incident HF were similar in men and women (Supplementary material, Table S2). In multivariable models, the following risk factors remained significantly associated with HF: age, body mass index, diabetes, ECG LV hypertrophy, significant murmur, and prevalent myocardial infarction (Table 3). We did not observe relevant statistical interactions for calendar decade and risk factors for new-onset HF.
Table 2.
Age- and sex-adjusted Cox proportional hazards models for predictors of incident heart failure
| Variable | Hazard ratioa | 95% CI | P-value |
|---|---|---|---|
| Age, years | 1.05 | 1.03–1.06 | <0.001 |
| Men (vs. women)b | 0.95 | 0.69–1.31 | 0.75 |
| Current smoking | 1.21 | 0.78–1.87 | 0.39 |
| Body mass index | 1.06 | 1.03–1.09 | <0.001 |
| Height | 0.98 | 0.96–1.01 | 0.13 |
| Weight | 1.02 | 1.00–1.03 | 0.007 |
| Systolic blood pressure | 1.01 | 1.001–1.015 | 0.03 |
| Diastolic blood pressure | 1.00 | 0.98–1.01 | 0.76 |
| Pulse pressure | 1.01 | 1.00–1.02 | 0.003 |
| Heart rate | 0.996 | 0.99–1.004 | 0.31 |
| Diabetes | 2.16 | 1.51–3.10 | <0.001 |
| Hypertension | 1.61 | 1.09–2.37 | 0.02 |
| Hypertension treatment | 1.45 | 1.05–1.99 | 0.02 |
| ECG left ventricular hypertrophy | 2.11 | 1.38–3.26 | <0.001 |
| Significant murmur | 1.77 | 1.15–2.72 | 0.01 |
| Prevalent myocardial infarction | 2.26 | 1.59–3.21 | <0.001 |
| Prevalent cardiovascular disease | 1.99 | 1.44–2.74 | <0.001 |
CI, confidence interval.
aHazard ratios are expressed per 1 unit increase for continuous variables (see Table 1) and for the condition present in dichotomous variables. Hazards ratios below the line were adjusted for age and sex.
bSex-adjusted for age.
Table 3.
Multivariable-adjusted Cox proportional hazards regression coefficients for 10-year risk of heart failure
| Variable | β | SE | P-value |
|---|---|---|---|
| Age | 0.063 | 0.011 | <0.001 |
| Body mass index | 0.062 | 0.016 | <0.001 |
| Left ventricular hypertrophy | 0.708 | 0.227 | 0.002 |
| Diabetes | 0.632 | 0.186 | 0.001 |
| Significant murmur | 0.607 | 0.224 | 0.007 |
| Prevalent myocardial infarction | 3.589 | 1.430 | 0.01 |
| Age × prevalent myocardial infarction | −0.039 | 0.019 | 0.048 |
S0(10) = 0.705 (10-year baseline survival).
Beta values are given for 1 unit increase for continuous variables (see Table 1 pooled sex) and for the condition present in dichotomous variables.
Significant risk indicators in the final model were included in the risk prediction algorithm. Neither year of AF onset nor sex were significantly associated with incident HF; hence, neither entered the analysis. Beta-coefficients are given in Table 3. We observed a statistically significant interaction between age and myocardial infarction; we fitted an interaction term into the risk prediction model. With higher mean age, the hazard ratio for prevalent myocardial infarction decreased. The final model discrimination C-statistic was 0.71 (95% CI 0.67–0.75; adjusted χ2 statistic 7.29; Pχ2 =0.61]. The model was well calibrated, with a good consistency between predicted and observed risks (Supplementary material, Figure S1).
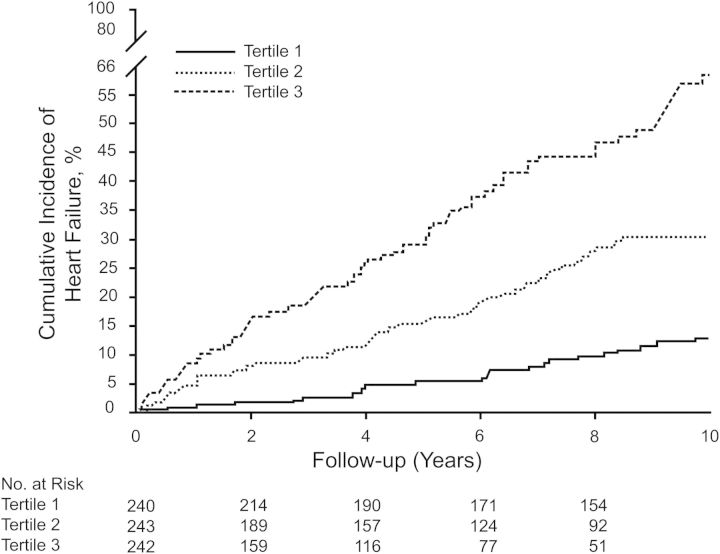
Kaplan–Meier survival curves for tertiles of the risk algorithm for the total sample are presented in Figure 2. Applying the algorithm, 47.6% of HF events occurred in the top tertile in men compared with 13.1% in the lowest tertile, and in women, 58.4% occurred in the top tertile compared with 18.2% in the bottom tertile. The event rate in the highest tertile was 3.6-fold higher compared with the lowest tertile in men, and 3.2 times higher in the upper tertile compared with the lowest category in women. A user-friendly spreadsheet that incorporates the risk function may be downloaded from the Framingham Heart Study website. The range of values that can be entered is limited to that observed in the data set, e.g. 39–96 years of age. We truncated the upper risk estimate at ≥45% to avoid inaccuracies inherent in more extreme observations. For comparison, the risk function calculates the optimal risk for a person of the same age, with a body mass index of 21 kg/m2, and none of the other risk factors.
Figure 2.
Kaplan–Meier 10-year survival curves for tertiles of the risk algorithm in both sexes.
We tested if model performance differed by age group and sex (Supplementary material, Table S3). In younger participants (≤70 years), the C-statistic was 0.73, P = 0.78; in individuals >70 years, it was 0.69, P = 0.03. Model fit was comparable in women with a C-statistic of 0.74, P = 0.17 and men with a C-statistic of 0.70, P = 0.43. Of note, subgroup analyses and their inferences are based on small numbers.
Excluding individuals with atrial flutter did not substantially change the observed associations and the risk algorithm (Supplementary material, Tables S4 and S5).
Secondary analyses
When separated by HF type, women had a non-significant higher incidence of HF with preserved EF compared with men. Similarly, in Kaplan–Meier survival curves, incidence by HF subtype varied, albeit non-significantly (Supplementary material, Figure S2). Women tended to have higher event rates of HF with preserved EF, whereas men tended to have a higher incidence of systolic HF.
During 10 years of follow-up, 45% of people died. To account for the high rate of death in our sample of middle-aged to older adults, we also ran competing risk models21 for multivariable Cox models (Supplementary material, Table S6), which showed results comparable with the main analyses (Table 3).
Discussion
Principal findings
Development of HF in community-based individuals with AF was common in both men and women, with a 22% 10-year incidence of new events. Variables that were associated with incident HF in multivariable-adjusted models, including age, body mass index, diabetes, ECG LV hypertrophy, significant murmur, and prevalent myocardial infarction, were used to create a risk algorithm that may be applied readily in clinical practice. Routinely available risk factors predicted an individual's risk of HF with good discrimination and calibration. The risk score performed similarly in women and men, but better in younger compared with older individuals.
Risk factors for development of heart failure
Our results confirm well-established risk factors for the development of HF, most of which have also been described in relation to AF incidence. Clinical variables such as hypertension, LV hypertrophy, ischaemic heart disease, and valve disease have long been known as risk indicators for both conditions.12 Furthermore, body mass index, in particular obesity,23 and diabetes mellitus10,11 consistently have been related to HF and AF incidence. Thus, some of the HF risk factors may only partially act through AF; rather, the risk factors may represent pathophysiological pathways common to both diseases. However, clear differences can also be demonstrated. Whereas male sex has been reported as a possible risk factor for new-onset HF,12 in individuals with AF, the probability of HF in women approximated the risk of men across all age groups studied. Thus, incident AF seemed to diminish sex-related differences in HF risk in follow-up.
Potential sex-related associations were shown for subtypes of HF. In a previous Framingham publication, both individuals with AF and women were observed to have a higher incidence of HF with preserved EF. In comparison, men had a higher incidence of HF with reduced EF.24 In our present study restricted to individuals with AF, we observed similar trends. However, we had limited numbers of individuals with HF and information on preserved or reduced EF. Sex differences did not achieve statistical significance and may be due to chance. A recent investigation also did not show differences by HF subtype.25
Potential clinical utility of risk algorithm
The application of the risk algorithm in individuals with AF may help to distinguish participants who are prone to develop clinically apparent HF from those at low risk for incident HF. For the development of the risk assessment tool, we have identified strong clinical risk factors for HF incidence in individuals with pre-existing AF, which is the first step towards testing successful preventive efforts. Assessment of absolute risk may increase motivation for developing and validating interventions to prevent HF onset.
Our algorithm also demonstrates that we will need additional measures to increase the predictive ability of the risk algorithm for HF. More sophisticated biomarkers or testing may identify subclinical HF or individuals at higher risk for HF onset. Novel risk markers might help to refine the risk scoring system. Cardiac performance in patients with AF is a predictor of cardiovascular outcome,26 and non-invasive echocardiographic measures may help to improve prediction of incident HF.
The community-based nature of the data, the long-term follow-up of individuals, and the scrutiny of outcome ascertainment are study strengths. Some potential limitations merit consideration. The Framingham Heart Study HF definition17 used over the last decades was clinical and not based on echocardiographic or laboratory studies that may have helped refine the diagnosis. Due to the overall small number of individuals at risk of HF (n = 725), we had limited power to perform subgroup analyses in order to detect sex differences for incident HF with reduced vs. preserved EF. We did not distinguish between AF types (e.g. paroxysmal vs. persistent vs. permanent AF vs. atrial flutter) or specific treatment algorithms (e.g. rhythm vs. rate control). Hence, we cannot determine whether different AF types or treatment strategies vary in their propensity for HF. Furthermore, we had limited power to examine individuals with the diagnosis of AF and HF on the same day.
Whereas the risk function showed good calibration and discrimination in the Framingham sample, external validation in an independent sample is necessary. We provide data ascertained since 1960 with the caveat that AF treatment has undergone changes over the decades. We acknowledge that HF incidence and risk factors for new-onset HF in AF may have changed over time. In statistical analyses, we did not observe striking interactions between decade and risk factors for HF onset. However, temporal trends not captured by statistical analyses may be a major limitation of our longitudinal design.
Conclusion
Our HF risk prediction algorithm may become a clinical tool to communicate risk and target modifiable risk markers such as body mass index, hypertension, LV hypertrophy, ischaemic heart disease, and valve disease. The developed risk function can serve as a benchmark against which novel putative risk factors might be evaluated. The identification of high-risk individuals may further help to target individuals for potential HF prevention trials. Once effective preventive therapies are established, the risk algorithm might be useful to identify individuals with AF at highest risk of HF and therefore most likely to benefit from preventive therapies.
Supplementary material
Supplementary material is available at European Journal of Heart Failure online.
Funding
Supported by National Institutes of Health (NIH)/National Heart Lung, and Blood Institute (NHLBI) contract N01-HC-25195; 6R01-NS 17950; NIH grants HL092577-01A1 (P.T.E. and E.J.B.); 1RC1HL101056; 1R01HL102214; 1R01AG028321 (E.J.B.); 5R21DA027021, 1RO1HL104156, 1K24HL105780 (P.T.E.); HL080124, HL077477, and HL71039 (R.S.V.). NIH Research career award 2K24 HL04334 (R.S.V.); Deutsche Forschungsgemeinschaft (German Research Foundation) Research Fellowship SCHN 1149/1-1 and Emmy Noether Program (SCHN 1149/3-1) (R.B.S.); M.R. is supported by a grant from the Netherlands Organization for Scientific Research (Rubicon Grant 825.09.020). This work was partially supported by the Evans Center for Interdisciplinary Biomedical Research ARC on ‘Atrial Fibrillation’ at Boston University (http://www.bumc.bu.edu/evanscenteribr/)
Conflict of interest: none declared.
Authors' contributions: R.B.S., E.J.B., and all co-authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, Seward JB, Tsang TS. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–125. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 2.Levy D, Kenchaiah S, Larson MG, Benjamin EJ, Kupka MJ, Ho KK, Murabito JM, Vasan RS. Long-term trends in the incidence of and survival with heart failure. N Engl J Med. 2002;347:1397–1402. doi: 10.1056/NEJMoa020265. [DOI] [PubMed] [Google Scholar]
- 3.Nieuwlaat R, Olsson SB, Lip GY, Camm AJ, Breithardt G, Capucci A, Meeder JG, Prins MH, Levy S, Crijns HJ. Guideline-adherent antithrombotic treatment is associated with improved outcomes compared with undertreatment in high-risk patients with atrial fibrillation. The Euro Heart Survey on Atrial Fibrillation. Am Heart J. 2007;153:1006–1012. doi: 10.1016/j.ahj.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 4.Wang TJ, Larson MG, Levy D, Vasan RS, Leip EP, Wolf PA, D'Agostino RB, Murabito JM, Kannel WB, Benjamin EJ. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation. 2003;107:2920–2925. doi: 10.1161/01.CIR.0000072767.89944.6E. [DOI] [PubMed] [Google Scholar]
- 5.Gosselink AT, Crijns HJ, van den Berg MP, van den Broek SA, Hillege H, Landsman ML, Lie KI. Functional capacity before and after cardioversion of atrial fibrillation: a controlled study. Br Heart J. 1994;72:161–166. doi: 10.1136/hrt.72.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hagens VE, Crijns HJ, van Veldhuisen DJ, van den Berg MP, Rienstra M, Ranchor AV, Bosker HA, Kamp O, Tijssen JG, Veeger NJ, van Gelder IC. Rate control versus rhythm control for patients with persistent atrial fibrillation with mild to moderate heart failure: results from the RAte Control versus Electrical cardioversion (RACE) study. Am Heart J. 2005;149:1106–1111. doi: 10.1016/j.ahj.2004.11.030. [DOI] [PubMed] [Google Scholar]
- 7.Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. The SOLVD Investigators. N Engl J Med. 1991;325:293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 8.Carson PE, Johnson GR, Dunkman WB, Fletcher RD, Farrell L, Cohn JN. The influence of atrial fibrillation on prognosis in mild to moderate heart failure. The V-HeFT Studies. The V-HeFT VA Cooperative Studies Group. Circulation. 1993;87 VI102–VI110. [PubMed] [Google Scholar]
- 9.Smit MD, Moes ML, Maass AH, Achekar ID, Van Geel PP, Hillege HL, van Veldhuisen DJ, van Gelder IC. The importance of whether atrial fibrillation or heart failure develops first. Eur J Heart Fail. 2012;14:1030–1040. doi: 10.1093/eurjhf/hfs097. [DOI] [PubMed] [Google Scholar]
- 10.Aksnes TA, Schmieder RE, Kjeldsen SE, Ghani S, Hua TA, Julius S. Impact of new-onset diabetes mellitus on development of atrial fibrillation and heart failure in high-risk hypertension (from the VALUE Trial) Am J Cardiol. 2008;101:634–638. doi: 10.1016/j.amjcard.2007.10.025. [DOI] [PubMed] [Google Scholar]
- 11.Wang TJ, Parise H, Levy D, D'Agostino RB, Sr, Wolf PA, Vasan RS, Benjamin EJ. Obesity and the risk of new-onset atrial fibrillation. JAMA. 2004;292:2471–2477. doi: 10.1001/jama.292.20.2471. [DOI] [PubMed] [Google Scholar]
- 12.Kannel WB, D'Agostino RB, Silbershatz H, Belanger AJ, Wilson PW, Levy D. Profile for estimating risk of heart failure. Arch Intern Med. 1999;159:1197–1204. doi: 10.1001/archinte.159.11.1197. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki S, Sagara K, Otsuka T, Matsuno S, Funada R, Uejima T, Oikawa Y, Yajima J, Koike A, Nagashima K, Kirigaya H, Sawada H, Aizawa T, Yamashita T. A new scoring system for evaluating the risk of heart failure events in Japanese patients with atrial fibrillation. Am J Cardiol. 2012;110:678–682. doi: 10.1016/j.amjcard.2012.04.049. [DOI] [PubMed] [Google Scholar]
- 14.Potpara TS, Polovina MM, Licina MM, Marinkovic JM, Lip GY. Predictors and prognostic implications of incident heart failure following the first diagnosis of atrial fibrillation in patients with structurally normal hearts: the Belgrade Atrial Fibrillation Study. Eur J Heart Fail. 2013 doi: 10.1093/eurjhf/hft004. in press. [DOI] [PubMed] [Google Scholar]
- 15.Dawber T, Meadors G, Moore F., Jr. Epidemiological approaches to heart disease: the Framingham Study. Am J Public Health Nations Health. 1951;41:279–281. doi: 10.2105/ajph.41.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quan SF, Howard BV, Iber C, Kiley JP, Nieto FJ, O'Connor GT, Rapoport DM, Redline S, Robbins J, Samet JM, Wahl PW. The Sleep Heart Health Study: design, rationale, and methods. Sleep. 1997;20:1077–1085. [PubMed] [Google Scholar]
- 17.Ho KK, Anderson KM, Kannel WB, Grossman W, Levy D. Survival after the onset of congestive heart failure in Framingham Heart Study subjects. Circulation. 1993;88:107–115. doi: 10.1161/01.cir.88.1.107. [DOI] [PubMed] [Google Scholar]
- 18.Kannel WB, Wolf PA, Garrison RJ. The Framingham Study: An Epidemiological Investigation of Cardiovascular Disease. Bethesda, MD: National Heart, Lung, and Blood Institute; 1987. [Google Scholar]
- 19.Wang TJ, Massaro JM, Levy D, Vasan RS, Wolf PA, D'Agostino RB, Larson MG, Kannel WB, Benjamin EJ. A risk score for predicting stroke or death in individuals with new-onset atrial fibrillation in the community: the Framingham Heart Study. JAMA. 2003;290:1049–1056. doi: 10.1001/jama.290.8.1049. [DOI] [PubMed] [Google Scholar]
- 20.Nam BH. Discrimination and Calibration in Survival Analysis. Boston, MA: Boston University; 2000. [Google Scholar]
- 21.Fine J, Gray R. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 22.R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2010. [Google Scholar]
- 23.Lee DS, Massaro JM, Wang TJ, Kannel WB, Benjamin EJ, Kenchaiah S, Levy D, D'Agostino RB, Sr, Vasan RS. Antecedent blood pressure, body mass index, and the risk of incident heart failure in later life. Hypertension. 2007;50:869–876. doi: 10.1161/HYPERTENSIONAHA.107.095380. [DOI] [PubMed] [Google Scholar]
- 24.Lee DS, Gona P, Vasan RS, Larson MG, Benjamin EJ, Wang TJ, Tu JV, Levy D. Relation of disease pathogenesis and risk factors to heart failure with preserved or reduced ejection fraction: insights from the Framingham Heart Study of the National Heart, Lung, and Blood Institute. Circulation. 2009;119:3070–3077. doi: 10.1161/CIRCULATIONAHA.108.815944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Linssen GC, Rienstra M, Jaarsma T, Voors AA, van G, I, Hillege HL, van Veldhuisen DJ. Clinical and prognostic effects of atrial fibrillation in heart failure patients with reduced and preserved left ventricular ejection fraction. Eur J Heart Fail. 2011;13:1111–1120. doi: 10.1093/eurjhf/hfr066. [DOI] [PubMed] [Google Scholar]
- 26.Abhayaratna WP, Seward JB, Appleton CP, Douglas PS, Oh JK, Tajik AJ, Tsang TS. Left atrial size: physiologic determinants and clinical applications. J Am Coll Cardiol. 2006;47:2357–2363. doi: 10.1016/j.jacc.2006.02.048. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.