Abstract
Objectives
Bisphosphonates (BP) are widely used in medicine for inhibiting bone resorption; however bisphosphonate-related osteonecrosis of the jaw (BRONJ) is a major side effect of BP. To date, there have been no specific reports on the incidence of BRONJ among Koreans. This study investigated the preliminary results from a nationwide survey of BRONJ in the Departments of Oral and Maxillofacial Surgery (OMFS) at individual training hospitals.
Materials and Methods
A total of 15 OMFS departments (10 from dental schools, 4 from medical schools, and 1 from a dental hospital) participated in a multi-centric survey. This study assessed every BRONJ case diagnosed between January 2010 and December 2010. The patient age and BP type were evaluated.
Results
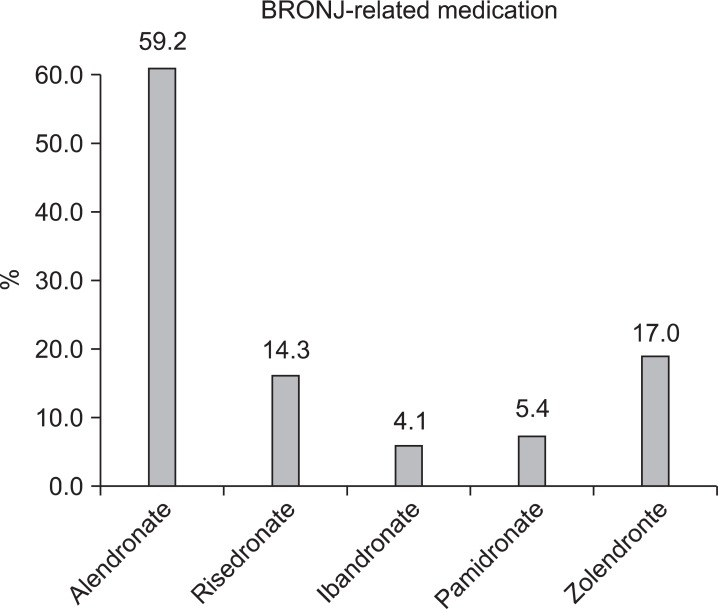
A total of 254 BRONJ cases were collected. The majority of BRONJ cases were associated with oral BP therapy, while 21.8% of the cases were associated with intravenous administration. Alendronate was the drug most frequently related to BRONJ (59.2% of cases), followed by risedronate (14.3%) and zolendronate (17.0%). The average age of BRONJ patients was 70.0±10.1 years, with a range of 38-88 years of age. With the number of BP patients in Korea reported to be around 600,000 in 2008, the estimated incidence of BRONJ is at least 0.04% or 1 per 2,300 BP patients.
Conclusion
The results suggest that the estimated incidence of BRONJ in Korea is higher than the incidence of other countries. Future prospective studies should be carried out to investigate the exact epidemiological characteristics of BRONJ in Korea.
Keywords: Bisphosphonate, Osteonecrosis, Jaw, Data collection
I. Introduction
The injection of relatively high doses of bisphosphonate (BP) into malignant tumor patients is known to increase the incidence of bisphosphonate-related osteonecrosis of the jaw (BRONJ), whereas the injection of BP in as much as the amount used to treat osteoporosis hardly causes BRONJ. Though the results of many multicentric surveys have been reported from Japan1, Germany2, and Italy3, there is no accurate report in Korea. To minimize the various pains and inconvenience of BRONJ patients, both treatment and conditions of the disease are important. Therefore, to provide basic data on BRONJ for many clinicians, Korean Association of Oral and Maxillofacial Surgeons (KAOMS) conducted a multicentric survey in 2010 so as to identify the nationwide incidence of BRONJ.
II. Materials and Methods
In September 2010, the Director's Committee of KAOMS decided to start the nationwide survey in 2010 (the 4th President-elect: Kyung-Wook Kim). In October 2010, the BRONJ chart was sent to the director of each training hospital; in April 2011, the preliminary result was recorded by collecting the basic data of patients. The age and BP medicine type of BRONJ patients who visited and received diagnosis in each training hospital were surveyed first.
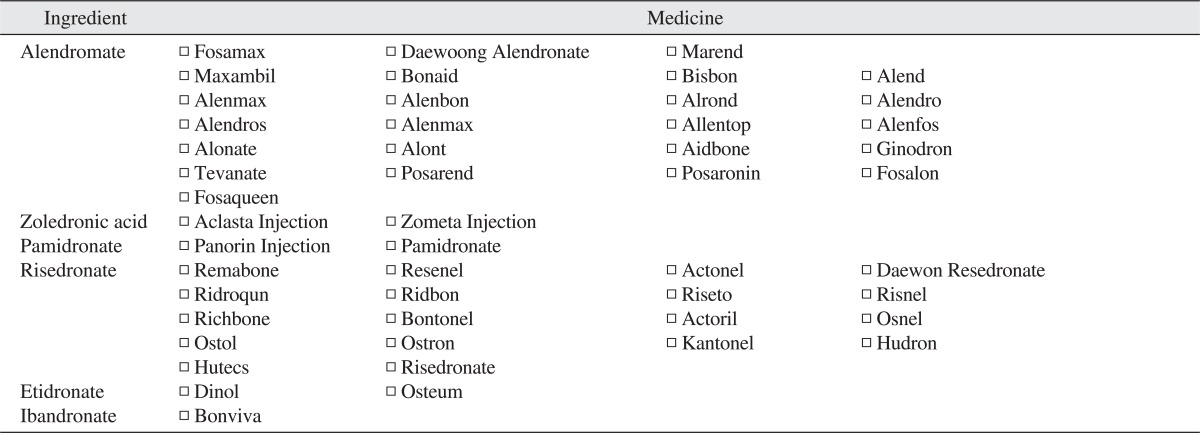
BRONJ is defined as the condition wherein 1) the maxillary bone (jaw bone) is exposed but not treated for more than 8 weeks regardless of proper treatment, 2) the patient had taken - or is taking - BP, and 3) the patient did not receive radiation therapy in the maxillary area4. As shown in the attached Table 1, the name of patients' medicine was surveyed accurately.
Table 1.
Various kinds of bisphosphonates (BPs) according to the official name of BP
III. Results
1. Participating institutions
At least 10 Oral and Maxillofacial Surgery (OMFS) departments of dental schools, 4 OMFS of medical schools, and 1 OMFS of a dental hospital participated in the nationwide survey to collect BRONJ patients' data.
1) Departments of Oral and Maxillofacial Surgery of Dental Schools (in alphabetical order)
Chonbuk National University
Chonnam National University
Chosun University
Dankook University
Gangneung-Wonju National University
Kyung Hee University
Kyungpook National University
Pusan National University
Seoul National University
Wonkwang University
2) Departments of Oral and Maxillofacial Surgery of Medical Schools (in alphabetical order)
Ajou University
Catholic University of Daegu
Gachon University Gil Medical Center
Hallym University Kangdong Sacred Heart Hospital
3) Department of Oral and Maxillofacial Surgery of a Dental Hospital
Sun Dental Hospital
2. Patients
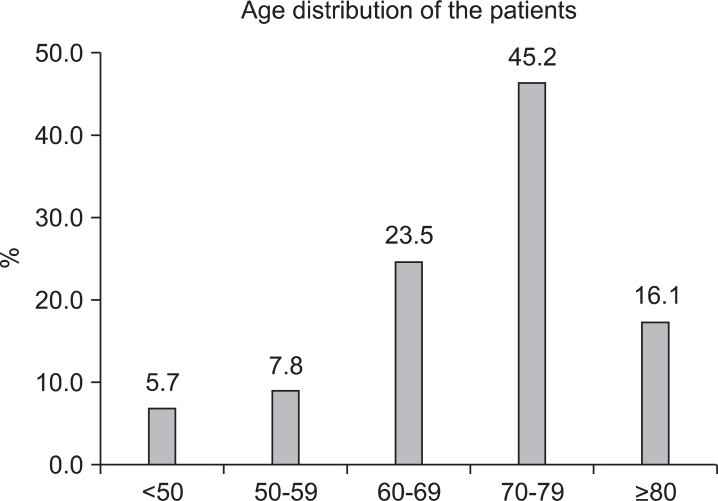
A total of 254 patients were diagnosed with BRONJ in 2010 at the 14 aforesaid institutions. The average age was 70.0±10.1 (38-88 years old) (Fig. 1), with 78.7% receiving BP orally and 21.3% receiving BP through intravenous injection. Alendronate was the most popular drug related to BRONJ, followed by zolendronate.(Fig. 2)
Fig. 1.
Age distribution of bisphosphonate-related osteonecrosis of the jaw patients.
Fig. 2.
Percentage of bisphosphonate-related osteonecrosis of the jaw patients according to medication.
IV. Discussion
BP is very effective in curbing the spread of osteoporosis and malignant bone tumor. In Korea, BP accounts for 85% of osteoporosis medicines; the most popular products are alendronate and risedronate5. Korea is estimated to suffer socioeconomic loss of 1.50 trillion won yearly due to the treatment of osteoporosis fracture6. Therefore, considering the fact that the sales of all BP products in 2008 were pegged at 114.2 billion won, the socioeconomic profit of medication apparently outweighs the loss. Nonetheless, the long-term administration of BP reportedly caused BRONJ; since the first case reported by Marx7 in 2003, many clinical and basic research studies have been conducted. In Korea, since the first case report in the oral and maxillofacial area in 20098, each school has accumulated case series9-11. Recently, the Japan Association of Oral and Maxillofacial Surgeons reported to the Journal of Oral and Maxillofacial Surgery in 2011 that it collected statistics of 248 training hospitals in Japan covering 568 cases1.
With respect to foreign cases, 11 Departments of Oral and Maxillofacial Surgery in Europe collected statistics of 470 cases during 4 years and 4 months (2004-2008)2; 8 Departments of Oral and Maxillofacial Surgery in Italy reported statistics of 672 cases during 4 years and 5 months (2004-2008)3. Considering the fact that some institutions and training hospitals in the country did not participate in this study, the number of actual cases may be much more than the aforesaid 253 cases. The number of collected cases in Korea is far greater than the number of cases in foreign countries.
According to the research of Hoff et al.12 as one of the most comprehensive BRONJ research studies on cancer patients so far, 2.4% of multiple myeloma patients and 1.2% of breast cancer patients who received intravenous BP had BRONJ, for an average incidence of 0.8-1.2%. On the other hand, Woo et al.13 reported that a BRONJ incidence of 21% among patients who received IV BP for more than 3 years, with Bamias et al.14 suggesting an incidence ratio of 6.7%. Similarly, the 11incidence of BRONJ among cancer patients was collected relatively well, and the average value was 2-11%. Since BRONJ caused by BP injected into osteoporosis patients is relatively rare, however, incidence or prevalence is hard to trace. According to the data of Merck & Co. (Whitehouse Station, NJ, USA, 2006), a pharmaceutical company, the annual incidence of BRONJ caused by oral BPs (alendronate) was 1.6-1.94/100,000, whereas the statistics of AAOMS report a 0.7/100,000 incidence15.
The American Society of Bone and Mineral Research (ASBMR) task force estimated the incidence of BRONJ among osteoporosis patients to be 1/10,000-1/100,00016, with the European Society on Clinical and Economic Aspects of Osteoporosis and other Bone Diseases reporting an incidence of 1/20,000-110,000/year17. According to the statistics in Australia, during the period 2004-2005, there were a total of 158 BRONJ patients in Australia. Incidence among malignant tumor patients was 1/87-114 (0.88-1.15%), whereas that among osteoporosis patients was 1/2,260-8,470 (0.01-0.04%)18. Lo et al.19 reported the prevalence of BRONJ associated with injection of oral BP to be 0.1%, based on the result of the survey among 8,572 patients.
Recently, according to a Japan BRONJ position paper, around 1 million osteoporosis patients in Japan received oral BP; the estimated incidence was 0.01-0.02%, and that caused by the injection of IV BP was approximately 1-2%19. According to the statistics in Japan, 57.8% of BRONJ patients contracted the disease after receiving IV BP; 39.5% had BRONJ after receiving oral BP1,20. It is very different with the result of Europe, America, and Australia, i.e., only 5% of cases were oral BP-related13,18,21. In Korea, 78.7% were oral BP-related BRONJ, the highest percentage in the world. It may be concluded that there are more Asians with BRONJ caused by oral BP than westerners. In this study, alendronate showed the highest relation, alendronate being the most popular medicine. According to the sales of each BP ingredient in 2008, alendronate constituted 62%, risedronate, 29%, ibandronate, 7%, pamidronate, 2% and zoledronate, 1%5. Considering the fact that more than 50% of oral BP is discharged from the body within 5 days, 5% remains in the body for more than 1 year22, and alendronate's skeletal half-life is 10.9 years23, the incidence of BRONJ by medicine should be investigated further.
In the statistical research in 2008, 10.3% of the Korean population were older than 65. This means that Korea is already in the aging era (more than 7% of the total population are older than 65), and most of them are potential users of osteoporosis medicine. According to the analysis of osteoporosis prescription by the Health Insurance Review and Assessment Service (HIRA) and Korean Society of Bone Metabolism based on the data of the examination conducted during the period 2004-2008, 300,918 out of a total of 603,870 cases were associated with BP medicines24,25. After applying the osteoporosis patient discrimination algorithm recognized by doctors based on the data of health insurance review, the number of osteoporosis patients older than 50 in 2007 as recognized by doctors was 1,230,580; 61.6% were prescribed an osteoporosis indication medicine26. According to the HIRA, 83% of 1,560,000 osteoporosis patients were on medication as of 2008; 50% of the total prescriptions were BP. In the same year, the number of total osteoporosis patients was 657,07325. Therefore, the total number of patients who received BP is assumed to be 600,000, and the expected BRONJ incidence as calculated by substituting the collected number of patients from only the participating domestic Departments of Oral and Maxillofacial Surgery will be at least 0.04% (1/2,300 people).
The following were the limitations of this study: 1) approval of the Institutional Review Board for the delivery of patients' information by each institution; 2) need to consult with the academy's board of directors regarding the scope, level, and method of information sharing, 3) need for each institution to designate a professor to manage BRONJ research to conduct prospective research continuously, and; 4) need to integrate the data collection system and statistics process.
The Japan Association of Oral and Maxillofacial Surgeons conducted the first nationwide survey in 2006 and the second survey in 2008, and then reviewed all cases collected from 248 training hospitals. As a result, it obtained a relatively significant result as to the level of disease and prognosis of treatment1. Moreover, for atypical femoral fracture (subtrochanteric fracture), which has been regarded as another complication aside from BRONJ, orthopedists and endocrinologists reported nationwide statistics based on cooperation with the HIRA27. In 2009, the Korean Endocrine Society, Korean Society of Bone metabolism, Korean Society of Osteoporosis, and Korean Association of Oral and Maxillofacial Surgeons made a position statement on BRONJ28 and suggested such as a guideline for BRONJ treatment.
V. Conclusion
The results suggest that the estimated incidence of BRONJ in Korea is higher than that of other countries. Considering such trends of international academies, more advanced prospective research studies should be carried out by KAOMS.
References
- 1.Urade M, Tanaka N, Furusawa K, Shimada J, Shibata T, Kirita T, et al. Nationwide survey for bisphosphonate-related osteonecrosis of the jaws in Japan. J Oral Maxillofac Surg. 2011;69:e364–e371. doi: 10.1016/j.joms.2011.03.051. [DOI] [PubMed] [Google Scholar]
- 2.Otto S, Abu-Id MH, Fedele S, Warnke PH, Becker ST, Kolk A, et al. Osteoporosis and bisphosphonates-related osteonecrosis of the jaw: not just a sporadic coincidence--a multi-centre study. J Craniomaxillofac Surg. 2011;39:272–277. doi: 10.1016/j.jcms.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 3.Vescovi P, Campisi G, Fusco V, Mergoni G, Manfredi M, Merigo E, et al. Surgery-triggered and non surgery-triggered bisphosphonate-related osteonecrosis of the jaws (BRONJ): a retrospective analysis of 567 cases in an Italian multicenter study. Oral Oncol. 2011;47:191–194. doi: 10.1016/j.oraloncology.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 4.Ruggiero SL, Mehrotra B, Rosenberg TJ, Engroff SL. Osteonecrosis of the jaws associated with the use of bisphosphonates: a review of 63 cases. J Oral Maxillofac Surg. 2004;62:527–534. doi: 10.1016/j.joms.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Park HM, Lee ES, Kim SM. The use of osteoporosis medications in Korea in 2008. Korean J Bone Metab. 2009;16:87–93. [Google Scholar]
- 6.Ko JM, Kim KS. Guidelines for diagnosis and treatment of osteoporosis. Seoul: Sehung Dublishing; 2008. [Google Scholar]
- 7.Marx RE. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg. 2003;61:1115–1117. doi: 10.1016/s0278-2391(03)00720-1. [DOI] [PubMed] [Google Scholar]
- 8.Choi BJ, Kwon YD, Lee B, Walter C, Al-Nawas B. Maxillary sinusitis as a complication of oral bisphosphonate related osteonecrosis of the jaw: a case report. J Korean Assoc Oral Maxillofac Surg. 2009;35:39–40. [Google Scholar]
- 9.Kim KW, Kim BJ, Lee CH. Clinical study of diagnosis and treatment of bisphosphonate-related osteonecrosis of the jaws. J Korean Assoc Oral Maxillofac Surg. 2011;37:54–61. [Google Scholar]
- 10.Son HJ, Jang HY, Keum YS, Lee JY, Kim HC, Lee SC. Oral bisphosphonates induced osteonecrosis of the mandible: a case report. J Korean Assoc Oral Maxillofac Surg. 2009;35:106–111. [Google Scholar]
- 11.Han YS, Lee IW, Lee H, Suh JW, Kim SM, Myoung H, et al. Retrospective study on the bisphosphonate-related osteonecrosis of jaw. J Korean Assoc Oral Maxillofac Surg. 2011;37:470–476. [Google Scholar]
- 12.Hoff AO, Toth BB, Altundag K, Johnson MM, Warneke CL, Hu M, et al. Frequency and risk factors associated with osteonecrosis of the jaw in cancer patients treated with intravenous bisphosphonates. J Bone Miner Res. 2008;23:826–836. doi: 10.1359/JBMR.080205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woo SB, Hellstein JW, Kalmar JR. Narrative [corrected] review: bisphosphonates and osteonecrosis of the jaws. Ann Intern Med. 2006;144:753–761. doi: 10.7326/0003-4819-144-10-200605160-00009. [DOI] [PubMed] [Google Scholar]
- 14.Bamias A, Kastritis E, Bamia C, Moulopoulos LA, Melakopoulos I, Bozas G, et al. Osteonecrosis of the jaw in cancer after treatment with bisphosphonates: incidence and risk factors. J Clin Oncol. 2005;23:8580–8587. doi: 10.1200/JCO.2005.02.8670. [DOI] [PubMed] [Google Scholar]
- 15.Ruggiero SL, Dodson TB, Assael LA, Landesberg R, Marx RE, Mehrotra B American Association of Oral and Maxillofacial Surgeons. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws-2009 update. J Oral Maxillofac Surg. 2009;67(5 Suppl):2–12. doi: 10.1016/j.joms.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 16.Khosla S, Burr D, Cauley J, Dempster DW, Ebeling PR, Felsenberg D, et al. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2007;22:1479–1491. doi: 10.1359/jbmr.0707onj. [DOI] [PubMed] [Google Scholar]
- 17.Rizzoli R, Burlet N, Cahall D, Delmas PD, Eriksen EF, Felsenberg D, et al. Osteonecrosis of the jaw and bisphosphonate treatment for osteoporosis. Bone. 2008;42:841–847. doi: 10.1016/j.bone.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 18.Mavrokokki T, Cheng A, Stein B, Goss A. Nature and frequency of bisphosphonate-associated osteonecrosis of the jaws in Australia. J Oral Maxillofac Surg. 2007;65:415–423. doi: 10.1016/j.joms.2006.10.061. [DOI] [PubMed] [Google Scholar]
- 19.Lo JC, O'Ryan FS, Gordon NP, Yang J, Hui RL, Martin D, et al. Predicting Risk of Osteonecrosis of the Jaw with Oral Bisphosphonate Exposure (PROBE) Investigators. Prevalence of osteonecrosis of the jaw in patients with oral bisphosphonate exposure. J Oral Maxillofac Surg. 2010;68:243–253. doi: 10.1016/j.joms.2009.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoneda T, Hagino H, Sugimoto T, Ohta H, Takahashi S, Soen S, et al. Bisphosphonate-related osteonecrosis of the jaw: position paper from the Allied Task Force Committee of Japanese Society for Bone and Mineral Research, Japan Osteoporosis Society, Japanese Society of Periodontology, Japanese Society for Oral and Maxillofacial Radiology, and Japanese Society of Oral and Maxillofacial Surgeons. J Bone Miner Metab. 2010;28:365–383. doi: 10.1007/s00774-010-0162-7. [DOI] [PubMed] [Google Scholar]
- 21.Abu-Id MH, Warnke PH, Gottschalk J, Springer I, Wiltfang J, Acil Y, et al. "Bis-phossy jaws" - high and low risk factors for bisphosphonate-induced osteonecrosis of the jaw. J Craniomaxillofac Surg. 2008;36:95–103. doi: 10.1016/j.jcms.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 22.Kimmel DB. Mechanism of action, pharmacokinetic and pharmacodynamic profile, and clinical applications of nitrogen-containing bisphosphonates. J Dent Res. 2007;86:1022–1033. doi: 10.1177/154405910708601102. [DOI] [PubMed] [Google Scholar]
- 23.Khan SA, Kanis JA, Vasikaran S, Kline WF, Matuszewski BK, McCloskey EV, et al. Elimination and biochemical responses to intravenous alendronate in postmenopausal osteoporosis. J Bone Miner Res. 1997;12:1700–1707. doi: 10.1359/jbmr.1997.12.10.1700. [DOI] [PubMed] [Google Scholar]
- 24.Ministry for Health, Welfare and Family Affairs, Korean National Health and Nutrition Examination Survey in 2005. Korea Centers for Disease Control and Prevention [Internet] [cited 2012 Jul 1]. Available from: http://knhanes.cdc.go.kr.
- 25.Choi HJ, Shin CS, Ha YC, Jang S, Jang S, Park C, et al. Burden of osteoporosis in adults in Korea: a national health insurance database study. J Bone Miner Metab. 2012;30:54–58. doi: 10.1007/s00774-011-0280-x. [DOI] [PubMed] [Google Scholar]
- 26.Jang S, Park C, Jang S, Yoon HK, Shin CS, Kim DY, et al. Medical service utilization with osteoporosis. Endocrinol Metab. 2010;25:326–339. [Google Scholar]
- 27.Lee YK, Ha YC, Park C, Yoo JJ, Shin CS, Koo KH. Bisphosphonate use and increased incidence of subtrochanteric fracture in South Korea: results from the National Claim Registry. Osteoporos Int. 2013;24:707–711. doi: 10.1007/s00198-012-2016-8. [DOI] [PubMed] [Google Scholar]
- 28.The Korean Endocrine Society; The Korean Society of Bone Metabolism; The Korean Society of Osteoporosis; The Korean Association of Oral and Maxillofacial Surgeons. Bisphosphonate related osteonecrosis of the jaw (BRONJ)-position statement of Korea. J Korean Endocr Soc. 2009;24:227–230. [Google Scholar]