Abstract
Adaptive immunity is characterized by the ability to form long-lived immunological memory. Upon re-exposure to antigen, memory T cells respond more rapidly and robustly than naïve T cells, providing better clearance of pathogens. Recent reviews have reinforced the text-book view that memory T cells arise from effector cells. Although this notion is teleologically appealing, emerging data is more consistent with a model where naïve cells directly develop into memory cells without transitioning through an effector stage. A clear understanding of the lineage relationships between memory and effector cells has profound implications for the design of vaccines and for the development of effective T cell-based therapies.
Introduction
Immunological memory is the key distinguishing hallmark of the adaptive immune system [1]. Through their expression of massively diverse receptors, T cells are capable of exquisite specificity. The rearrangement of the genes encoding these T cell receptors (TCR) occurs in the thymus, which generates ‘naïve’ cells endowed with considerable epigenetic plasticity. Following antigenic stimulation, naïve CD8+ T cells can differentiate into ‘effector’ cells that produce inflammatory cytokines and cytotoxic molecules and into ‘memory’ cells, which are capable of an enhanced response to subsequent encounters with their cognate antigen. The widely held concept that effector T cells give rise to memory cells [2,3] has a certain intuitive and teleological appeal because memory T cells should arise from the effector cells that eliminated pathogens after a primary infection. This reasoning is also consistent with the observed natural history of a CD8+ T cell response in which there is a massive expansion of effector cells that is coincident with the elimination of the pathogen and later, over time, there is a ‘transition’ into the predominance of memory cells. It also seems plausible to some that effector cells do not give rise to memory cells but rather represent a terminally differentiated state, ie memory cells come developmentally before effector cells and not vice versa [4-7]. This model of differentiation, which has analogies to developmental systems, might involve asymmetric division of progenitor cells [8] and it may result from progressive differentiation of naïve cells into memory cells and ultimately effector cells [5,6].
Roadblocks in the determination of T cell lineage relationships
It is surprising that there continues to be a great deal of debate about the lineage relationship between effector and memory T cells. Despite the importance of understanding these relationships – and a growing body of knowledge of the molecular aspects of T cell immunobiology – there remains a robust debate in the field about the relationships of effector and memory T cells [9,10]. As with many debates, the most forcefully held opinions are sometimes held where the information available is most sparse.
How is it that the question of the developmental biology of post-thymic T cells can be so murky whereas other adult systems are more clearly understood? We feel that a major roadblock in the study of T cell maturation and differentiation is simply the lack of clear anatomical relationships among T cell subsets. In most other biological systems, the developmental biology of cellular constituents can be determined in large part by observing the anatomical locations of the cell experiencing maturation. The location and movement of cells within any given anatomical location can provide clues as to the lineage relationships of cells (Figure 1A and B). Differentiation of cell types from stem cells continues in adult organisms, where histologic structures can provide rich evidence for cellular differentiation pathways.
Figure 1. The linage relationship of T cell subsets is complicated by the lack of anatomical clues.
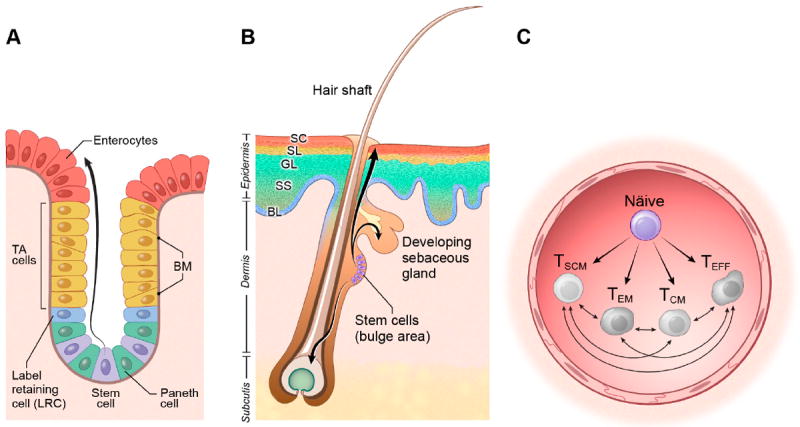
A) The intestinal crypt-villus unit. Intestinal stem cells reside at the base of the crypt between Paneth cells. As cells proliferate and differentiate into transient amplifying (TA) progenitor cells and mature enterocytes, they move upwards to cover the villus. B) The skin. Epidermal stem cells are located in the bulge region of the hair follicle, the base of the sebaceous gland, and the basal layer of the interfollicular epidermis. As cells proliferate and differentiate into keratinocytes, they move upward to form the stratum spinosus (SS), the granular layer (GL), the stratum lucidum (SL) and the stratum corneum (SC). C) T cells. Following antigen-stimulation, naïve T cells differentiate generating the full diversity T cell subsets. The existence of cells at different developmental stages, all of which are moving within the same anatomical space, does not offer an easy static snapshot that provides clues about their lineage relationships which are still the subject of controversy in the field.
For example cells of the skin are located in specific anatomic relationships relative to other structures. Skin stem cells reside in a structure called the ‘bulge’ and migrate up and down the hair shaft to refresh new dermal structure [11] (Figure 1B). Cells of the small intestine also have precise anatomical locations in the adult animal. Stem like cells reside deep within the crypts and then they move progressively towards the tips of the villi before they slough off and die [12] (Figure 1A). Post-thymic T cells are motile within the blood and lymphoid tissues, so anatomic clues are not readily apparent. Although intravital microscopy may yield new clues [13], T cells existing at a variety of developmental stages can exist within the same anatomical space (Figure 1C).
In addition to the lack of clear anatomical relationships, other problems have complicated the study of the lineage relationships of T cell subsets. One of the conventional surface proteins used to distinguish memory from effector cells – CD62L – is rapidly cleaved upon T cell activation by a disintegrin metalloprotease called ADAM17 [14-16]. It seems untenable to draw conclusions about effector and memory development based on sorting of CD62L+ and CD62L− cells [17] because the lack of CD62L does not identify bona fide effector cells. Both effector cells as well as recently primed but minimally differentiated T cells lack CD62L on their surfaces: the former cells do not express CD62L transcripts, whereas the later population continue to express this gene product but appear to be negative because CD62L protein is cleaved from the cell surfaces.
Lineage relationships of memory and effector cells have also been complicated by the use of reporter systems that have been seriously flawed. Genetic tagging systems controlled by the granzyme B [18,19] or IFN-γ [20] promoters have been employed to indelibly mark effector T cells and track their fate. These reporter systems however are not designed to measure gradients of expression of the molecule being studied. Reporter systems employed to date are too sensitive and provide binary answers. Thus, genes whose expression is turned on in a progressive manner will be reported as ‘yes’ even if the system is measuring small amounts of the gene in question. Thus, it is likely that these genetic tagging systems identify cells that have been activated rather than marking fully differentiated effector cells.
Two competing models for the relationship of effector and memory T cells
It is manifestly obvious that pathogen-specific T lymphocytes will become effector cells after an infection. It is also clear that pathogen-specific T cells will have a phenotype of memory cells after longer periods of time. However, these irrefutable observations do not necessarily indicate that individual virus-specific effector cells morph into memory cells. The question at hand regards the developmental fates and differentiation of individual T cells and not populations of cells.
The dominant model for CD8+ T cell differentiation is that T cells react to pathogens by experiencing a burst of proliferative activity. During this time, T cells become highly activated and produce copious amounts of effector cytokines (such as IFN-γ, TNF-α and GM-CSF). They also become capable of direct cytolysis and are capable of deploying perforin and granzymes. In this model, most of these cells die, but some of them survive to become memory cells. These memory cells purportedly emerge from the effector population stochastically, or are selected because of their superior ‘fitness’ or avidity for the pathogen-associated antigens. This model (shown in Figure 2, left) has tremendous intuitive and teleological appeal because it predicts that the effector cells that actually are capable of destroying the pathogen give rise to memory cells. Proponents of this theory contend that all memory cells indeed were at one point effector cells that experienced a reversion from an active, lytic state into a state that more closely resembles naïve cells. This model of reversion invokes a pattern of ‘off-on-off’ and ‘on-off-on’ changes in the phenotype, metabolism and gene expression patterns within individual T cells [21].
Figure 2. There are two dominant competing models of T cell differentiation.
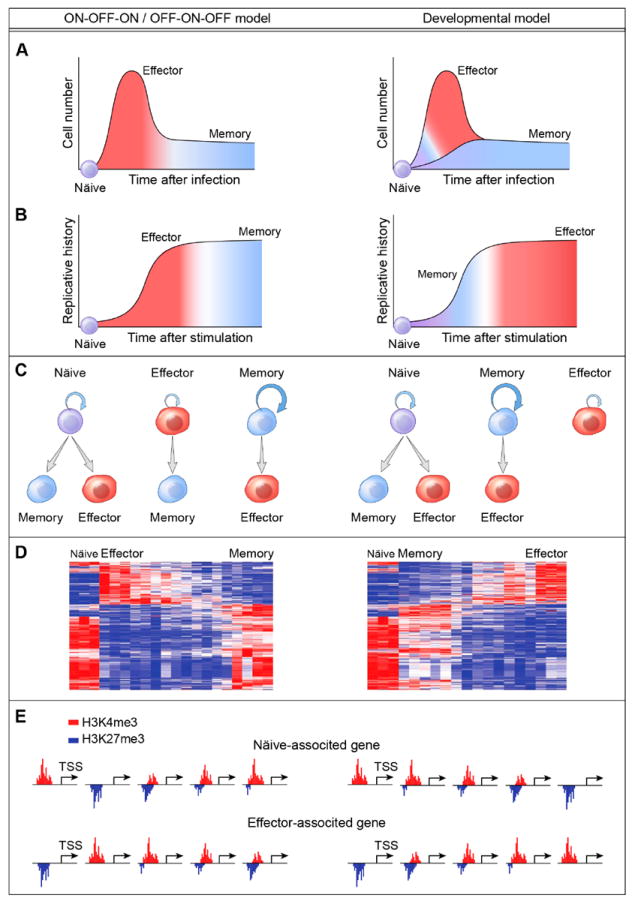
Set of panels shown on the left side depicts the ‘off-on-off’ model, in which all memory T cells are derived from cells that were once effector cells. Shown in the right set of panels is the ‘developmental’ model whereby memory cells arise directly out of naïve precursors without going through an effector stage. These distinct models lead to different predictions, which are explained in detail in the text.
The alternative to this model has it that memory cells do not arise from effector population, but rather are derived directly from activated naïve cells that never experience a full-blown highly cytotoxic effector state. In this model, rather than differentiating to memory cells, effector cells represent a terminally differentiated state that can only give way to more effectors or to senescent or dead cells. This is model is reminiscent of developmental models that include stem cells (Figure 2, right panel) that maintain themselves while also giving rise to progeny cells that experience progressive differentiation into mature functional tissues.
The models represent polar opposites of T cell development and lead to radically different predictions for the behavior of individual T cells. The debate is not merely one of nomenclature and semantics. The ‘off-on-off’ model predicts that individual T cells become granule-containing, highly cytotoxic effector cells after exposure to antigen, and that after elimination of the antigen most of these cells die but some experience a gradual transition to memory cells, whereas the developmental model would have effector cells dying off and memory cells arising as a separate population of cells that never experiences a full-blown effector state (Figure 2A). In this later model, memory cells are activated, but do not experience full-strength or repeated antigenic stimulation in a highly inflammatory milieu. These conditions exist towards the end of an infectious event, and are consistent with experiments showing that naïve antigen-specific T cells added during the tail-end of an infection are more prone to form memory [22].
The two models also have very different predictions for the replicative capabilities of individual T cells. The off-on-off model predicts that memory cells have proliferated more or the same extent of the effector cells from which they were purportedly derived. This view, however, is at odds with the findings that length of telomeres [23-25] and activity of telomerase [25] are both reduced in effector compared to memory cells. These data are, instead, consistent with the developmental model, which predicts that individual memory cells have proliferated less than the effector cells that will be derived from their progeny (Figure 2B). The predicted multipotency of cells in the off-on-off model is that effector cells are capable of becoming memory cells [2,17] whereas the developmental model predicts that this effector to memory cell transition does not happen and that effector cells exist in a terminal state that results in cell death but not formation of memory [26] (Figure 2C). Here again, the developmental model is more consistent with varieties of experimental data. Repeated antigenic stimulation of T cells leads to increased effector functions but impaired memory [27,28]. Conversely, memory T cell subsets are demonstrably capable of differentiating into both memory and effector subsets whereas effector cells are incapable of differentiating into memory cells in vitro [26].
As its name implies, the off-on-off model would predict that gene expression in individual cells that are turned on upon antigenic stimulation are gradually turned off upon resolution of a pathogenic infection. Of course, this model also implies that genes associated with naïve cells are turned off during infection and turned on again during the gradual reversion of effector cells into memory cells [29] (Figure 2D). The aforesaid also applies to changes in chromatin structure – where T cells experience massive changes to their chromatin which are substantially undone during their hypothesized transition into memory cells [21] (Figure 2E). This ‘flip-flopping’ of chromatin would represent a significant departure from patterns of progressive chromatin changes that are observed in other developmental systems [30]. By sharp contrast, a model where naïve T cells first transition into memory cells and then transition into effector cells would be more in line with other biological systems [26,31,32].
It seems difficult or impossible to reconcile the notion that there effector to memory transition is correct given that many genetic or environmental stimuli that promote T cell effector differentiation are accompanied by impairment in memory formation and vice versa [33-47].
What we have learned from the analysis of individual T cells
Emerging technologies enable the integration of large amounts of new data that influence perceptions of these new models for cellular differentiation. The differentiation of CD8+ T cells has now been studied at the level of individual T cells. Adoptive transfer of T cells individually labeled with heritable congenic markers or DNA ‘barcodes’ has recently enabled investigators to trace the progeny of single T cells and gain new insights on developmental models of memory generation [48-50]. These studies have revealed an extreme heterogeneity in the behavior of individual naïve cells in response to infection. Specifically, T cells that experienced massive proliferation tend to generate short-lived KLRG-1+ effector cells, whereas minimally expanded T cells preferentially form long-lived CD62L+, CD27+ cells [48-50]. These findings are consistent with a developmental model where proliferation and differentiation are tightly linked. In this model, naïve cells proliferate to generate memory cells first and then effector cells, a concept we think is further supported by recent findings posthumously published by Leo Lefrançois. His team tracked the fate of individual naïve and memory T cells upon adoptive transfer in the same hosts. As predicted by a model where T cells experience progressive differentiation, the descendants of single memory cells were largely short-lived KLRG-1+ effector cells, whereas the progeny derived from single naïve CD8+ T cells were preferentially memory cells [50].
These studies using single cell analysis together with T cell subpopulation studies previously published by our group and others all point to the same conclusion. Like other developmental systems in adult organisms that employ stem cells, CD8+ T cell differentiation is largely a linear and unidirectional process [5,6,51] (Fig 3). This process is associated with characteristic changes in cell surface molecules and a shift in metabolism from one based on lipid oxidation to one based on glycolysis [52-54] (Fig 3). In this model, T cell receptor engagement in the context of an inflammatory milieu stimulates T cells to proceed on a pathway of differentiation. In this model supported by a wealth of evidence, it is the strength of the signal that determines how much differentiation T cells experience. The full panoply of inputs that T cells integrate to embark on differentiation is complex and incompletely understood. For purposes here, some parameters that comprise inputs to ‘signal strength’ are the density and affinity of antigen that is engaged by the TCR, the frequency of this engagement, and the costimulation and the amount and types of inflammatory cytokines present during antigenic encounter [5,6,51].
Figure 3. The progressive differentiation CD8+ T cell differentiation is largely a linear and unidirectional process.
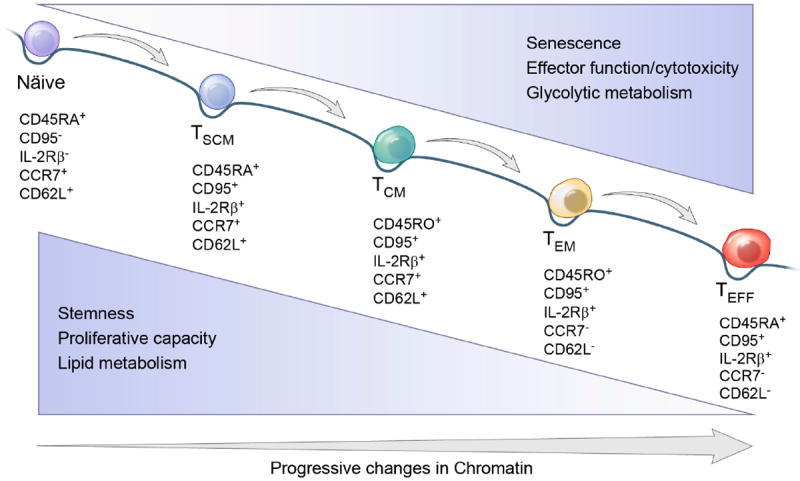
The differentiation of cells proceeding efficiently in mainly one direction has been analogized to a ball rolling down a hill, with the gradual loss of potential. For T cells, this process leads to characteristic changes in cell surface molecules shown. In addition, the model includes observations that cellular differentiation, ie the acquisition of effector functions, is eventually accompanied by senescence. At the same time, there is a loss of ‘stemness’ – the capacity of cells to be multipotent and self-renewing – as well as a diminution in proliferative capacity. Cells move from a metabolism that is based on lipid oxidation and oxidative phosphorylation to one based on glycolysis.
Caveats and clinical implications
The developmental model should not be over interpreted or treated as some orthodox dogma. We explicitly do not want to minimize the potential importance of dedifferentiation of T cells in the physiologic and non-physiologic settings. This is important when considering possible T cell de-differentiation when T cells experience situations in vivo where certain niches are unoccupied [55], especially as a result of certain disease conditions, chemotherapy or total body irradiation. Moreover, T cell differentiation should not be confounded with the ‘off-on-off’ changes that are associated with T cell activation, clonal expansion and quiescence of memory T cell subsets. Clearly, cells must experience transient changes in the metabolism of a T cell that enables clonal expansion such as the generation of ‘building blocks’ including the synthesis of nucleic acid precursors, organelles, membranes and other cellular components involved in T cell mitosis [56,57].
A deeper understanding of T cell differentiation is paramount for the development of effective vaccines and therapies that rely on memory CD8+ T cell formation. In the setting of metastatic malignancy, clinical trials have already shown that factors associated with objective response include longer telomeres of the infused cells [58,59], the number of CD27+ CD8 T cells infused [59,60], and the persistence of the cells in the circulation one month after transfer [59,61]. These observations are all consistent with the notion that transferred cells with a less differentiated phenotype are associated with a greater likelihood of objective response in adoptive cell transfer, findings that are corroborated in pre-clinical work [26,27,42,62-65]. Experiments using new techniques and even carefully-designed clinical trials may begin to resolve the long-standing debate about the lineage relationship between effector and memory T cells.
Acknowledgments
The authors would like to thank the late Leo Lefrançois and Rafi Ahmed for robust discussion and debate regarding the lineage relationships of memory and effector T cells. We also thank Christopher A. Klebanoff, Joseph G. Crompton and Rahul Roychoudhuri for insightful input of this review. We would also like to thank Alan Hoofring and Lydia V. Kibiuk for creating the illustrations used in this piece.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 2.Lefrancois L, Obar JJ. Once a killer, always a killer: from cytotoxic T cell to memory cell. Immunol Rev. 2010;235:206–218. doi: 10.1111/j.0105-2896.2010.00895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antia R, Ganusov VV, Ahmed R. The role of models in understanding CD8+ T-cell memory. Nat Rev Immunol. 2005;5:101–111. doi: 10.1038/nri1550. [DOI] [PubMed] [Google Scholar]
- 4.Fearon DT, Manders P, Wagner SD. Arrested differentiation, the self-renewing memory lymphocyte, and vaccination. Science. 2001;293:248–250. doi: 10.1126/science.1062589. [DOI] [PubMed] [Google Scholar]
- 5.Gattinoni L, Klebanoff CA, Restifo NP. Paths to stemness: building the ultimate antitumour T cell. Nat Rev Cancer. 2012;12:671–684. doi: 10.1038/nrc3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lanzavecchia A, Sallusto F. Progressive differentiation and selection of the fittest in the immune response. Nat Rev Immunol. 2002;2:982–987. doi: 10.1038/nri959. [DOI] [PubMed] [Google Scholar]
- 7.Klebanoff CA, Gattinoni L, Restifo NP. CD8+ T-cell memory in tumor immunology and immunotherapy. Immunol Rev. 2006;211:214–224. doi: 10.1111/j.0105-2896.2006.00391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang JT, Palanivel VR, Kinjyo I, Schambach F, Intlekofer AM, Banerjee A, Longworth SA, Vinup KE, Mrass P, Oliaro J, et al. Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science. 2007;315:1687–1691. doi: 10.1126/science.1139393. [DOI] [PubMed] [Google Scholar]
- 9.Ahmed R, Bevan MJ, Reiner SL, Fearon DT. The precursors of memory: models and controversies. Nat Rev Immunol. 2009;9:662–668. doi: 10.1038/nri2619. [DOI] [PubMed] [Google Scholar]
- 10.Reiner SL, Sallusto F, Lanzavecchia A. Division of labor with a workforce of one: challenges in specifying effector and memory T cell fate. Science. 2007;317:622–625. doi: 10.1126/science.1143775. [DOI] [PubMed] [Google Scholar]
- 11.Blanpain C, Fuchs E. Epidermal homeostasis: a balancing act of stem cells in the skin. Nat Rev Mol Cell Biol. 2009;10:207–217. doi: 10.1038/nrm2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12**.Clevers H. The intestinal crypt, a prototype stem cell compartment. Cell. 2013;154:274–284. doi: 10.1016/j.cell.2013.07.004. Clevers and colleagues have tracked differentiation and fate of cells in the gut using R26R-Confetti ‘brainbow’ mice that utilize combinatorial expression of fluorescent proteins. They found that fluorescently-labeled cells that appear first from stem cells at the base of the crypt, then form ‘clonal conveyor belt’ with cells migrating up the crypts and differentiating, eventually populating the entire surface of the intestine. The oldest cells are jettisoned via apoptosis at the villus tip. [DOI] [PubMed] [Google Scholar]
- 13.Kastenmuller W, Brandes M, Wang Z, Herz J, Egen JG, Germain RN. Peripheral prepositioning and local CXCL9 chemokine-mediated guidance orchestrate rapid memory CD8+ T cell responses in the lymph node. Immunity. 2013;38:502–513. doi: 10.1016/j.immuni.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jung TM, Gallatin WM, Weissman IL, Dailey MO. Down-regulation of homing receptors after T cell activation. J Immunol. 1988;141:4110–4117. [PubMed] [Google Scholar]
- 15.Peschon JJ, Slack JL, Reddy P, Stocking KL, Sunnarborg SW, Lee DC, Russell WE, Castner BJ, Johnson RS, Fitzner JN, et al. An essential role for ectodomain shedding in mammalian development. Science. 1998;282:1281–1284. doi: 10.1126/science.282.5392.1281. [DOI] [PubMed] [Google Scholar]
- 16.Yang S, Liu F, Wang QJ, Rosenberg SA, Morgan RA. The shedding of CD62L (L-selectin) regulates the acquisition of lytic activity in human tumor reactive T lymphocytes. PLoS One. 2011;6:e22560. doi: 10.1371/journal.pone.0022560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wherry EJ, Teichgraber V, Becker TC, Masopust D, Kaech SM, Antia R, von Andrian UH, Ahmed R. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;4:225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 18.Jacob J, Baltimore D. Modelling T-cell memory by genetic marking of memory T cells in vivo. Nature. 1999;399:593–597. doi: 10.1038/21208. [DOI] [PubMed] [Google Scholar]
- 19.Bannard O, Kraman M, Fearon DT. Secondary replicative function of CD8+ T cells that had developed an effector phenotype. Science. 2009;323:505–509. doi: 10.1126/science.1166831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harrington LE, Janowski KM, Oliver JR, Zajac AJ, Weaver CT. Memory CD4 T cells emerge from effector T-cell progenitors. Nature. 2008;452:356–360. doi: 10.1038/nature06672. [DOI] [PubMed] [Google Scholar]
- 21**.Youngblood B, Hale JS, Ahmed R. T-cell memory differentiation: insights from transcriptional signatures and epigenetics. Immunology. 2013;139:277–284. doi: 10.1111/imm.12074. A recent and clear discussion of the alternative model to the one espoused in the present piece. Youngblood and colleagues forward the view that T cell memory is derived from effector T cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.D’Souza WN, Hedrick SM. Cutting edge: latecomer CD8 T cells are imprinted with a unique differentiation program. J Immunol. 2006;177:777–781. doi: 10.4049/jimmunol.177.2.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 24.Papagno L, Spina CA, Marchant A, Salio M, Rufer N, Little S, Dong T, Chesney G, Waters A, Easterbrook P, et al. Immune activation and CD8+ T-cell differentiation towards senescence in HIV-1 infection. PLoS Biol. 2004;2:E20. doi: 10.1371/journal.pbio.0020020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romero P, Zippelius A, Kurth I, Pittet MJ, Touvrey C, Iancu EM, Corthesy P, Devevre E, Speiser DE, Rufer N. Four functionally distinct populations of human effector-memory CD8+ T lymphocytes. J Immunol. 2007;178:4112–4119. doi: 10.4049/jimmunol.178.7.4112. [DOI] [PubMed] [Google Scholar]
- 26.Gattinoni L, Lugli E, Ji Y, Pos Z, Paulos CM, Quigley MF, Almeida JR, Gostick E, Yu Z, Carpenito C, et al. A human memory T cell subset with stem cell-like properties. Nat Med. 2011;17:1290–1297. doi: 10.1038/nm.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gattinoni L, Klebanoff CA, Palmer DC, Wrzesinski C, Kerstann K, Yu Z, Finkelstein SE, Theoret MR, Rosenberg SA, Restifo NP. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J Clin Invest. 2005;115:1616–1626. doi: 10.1172/JCI24480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wirth TC, Xue HH, Rai D, Sabel JT, Bair T, Harty JT, Badovinac VP. Repetitive antigen stimulation induces stepwise transcriptome diversification but preserves a core signature of memory CD8(+) T cell differentiation. Immunity. 2010;33:128–140. doi: 10.1016/j.immuni.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Best JA, Blair DA, Knell J, Yang E, Mayya V, Doedens A, Dustin ML, Goldrath AW. Transcriptional insights into the CD8(+) T cell response to infection and memory T cell formation. Nat Immunol. 2013;14:404–412. doi: 10.1038/ni.2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30**.Zhu J, Adli M, Zou JY, Verstappen G, Coyne M, Zhang X, Durham T, Miri M, Deshpande V, De Jager PL, et al. Genome-wide chromatin state transitions associated with developmental and environmental cues. Cell. 2013;152:642–654. doi: 10.1016/j.cell.2012.12.033. This paper provides evidence that cells experience progressive changes in chromatin with differentiation. The authors perform detailed epigenetic studies on a large and diverse collection of human tissues and stem cells to find that developmental specification is accompanied by progressive chromatin restriction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Willinger T, Freeman T, Hasegawa H, McMichael AJ, Callan MF. Molecular signatures distinguish human central memory from effector memory CD8 T cell subsets. J Immunol. 2005;175:5895–5903. doi: 10.4049/jimmunol.175.9.5895. [DOI] [PubMed] [Google Scholar]
- 32.Holmes S, He M, Xu T, Lee PP. Memory T cells have gene expression patterns intermediate between naive and effector. Proc Natl Acad Sci U S A. 2005;102:5519–5523. doi: 10.1073/pnas.0501437102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kalia V, Sarkar S, Subramaniam S, Haining WN, Smith KA, Ahmed R. Prolonged interleukin-2Ralpha expression on virus-specific CD8+ T cells favors terminal-effector differentiation in vivo. Immunity. 2010;32:91–103. doi: 10.1016/j.immuni.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 35.Hand TW, Cui W, Jung YW, Sefik E, Joshi NS, Chandele A, Liu Y, Kaech SM. Differential effects of STAT5 and PI3K/AKT signaling on effector and memory CD8 T-cell survival. Proc Natl Acad Sci U S A. 2010;107:16601–16606. doi: 10.1073/pnas.1003457107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rutishauser RL, Martins GA, Kalachikov S, Chandele A, Parish IA, Meffre E, Jacob J, Calame K, Kaech SM. Transcriptional repressor Blimp-1 promotes CD8(+) T cell terminal differentiation and represses the acquisition of central memory T cell properties. Immunity. 2009;31:296–308. doi: 10.1016/j.immuni.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Masson F, Minnich M, Olshansky M, Bilic I, Mount AM, Kallies A, Speed TP, Busslinger M, Nutt SL, Belz GT. Id2-mediated inhibition of E2A represses memory CD8+ T cell differentiation. J Immunol. 2013;190:4585–4594. doi: 10.4049/jimmunol.1300099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Knell J, Best JA, Lind NA, Yang E, D’Cruz LM, Goldrath AW. Id2 influences differentiation of killer cell lectin-like receptor G1(hi) short-lived CD8+ effector T cells. J Immunol. 2013;190:1501–1509. doi: 10.4049/jimmunol.1200750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim EH, Sullivan JA, Plisch EH, Tejera MM, Jatzek A, Choi KY, Suresh M. Signal integration by Akt regulates CD8 T cell effector and memory differentiation. J Immunol. 2012;188:4305–4314. doi: 10.4049/jimmunol.1103568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jeannet G, Boudousquie C, Gardiol N, Kang J, Huelsken J, Held W. Essential role of the Wnt pathway effector Tcf-1 for the establishment of functional CD8 T cell memory. Proc Natl Acad Sci U S A. 2010;107:9777–9782. doi: 10.1073/pnas.0914127107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou X, Yu S, Zhao DM, Harty JT, Badovinac VP, Xue HH. Differentiation and persistence of memory CD8(+) T cells depend on T cell factor 1. Immunity. 2010;33:229–240. doi: 10.1016/j.immuni.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gattinoni L, Zhong XS, Palmer DC, Ji Y, Hinrichs CS, Yu Z, Wrzesinski C, Boni A, Cassard L, Garvin LM, et al. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat Med. 2009;15:808–813. doi: 10.1038/nm.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim MV, Ouyang W, Liao W, Zhang MQ, Li MO. The transcription factor foxo1 controls central-memory CD8(+) T cell responses to infection. Immunity. 2013;39:286–297. doi: 10.1016/j.immuni.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Michelini RH, Doedens AL, Goldrath AW, Hedrick SM. Differentiation of CD8 memory T cells depends on Foxo1. J Exp Med. 2013;210:1189–1200. doi: 10.1084/jem.20130392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cui W, Liu Y, Weinstein JS, Craft J, Kaech SM. An interleukin-21-interleukin-10-STAT3 pathway is critical for functional maturation of memory CD8+ T cells. Immunity. 2011;35:792–805. doi: 10.1016/j.immuni.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ji Y, Pos Z, Rao M, Klebanoff CA, Yu Z, Sukumar M, Reger RN, Palmer DC, Borman ZA, Muranski P, et al. Repression of the DNA-binding inhibitor Id3 by Blimp-1 limits the formation of memory CD8+ T cells. Nat Immunol. 2011;12:1230–1237. doi: 10.1038/ni.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47**.Yang CY, Best JA, Knell J, Yang E, Sheridan AD, Jesionek AK, Li HS, Rivera RR, Lind KC, D’Cruz LM, et al. The transcriptional regulators Id2 and Id3 control the formation of distinct memory CD8+ T cell subsets. Nat Immunol. 2011;12:1221–1229. doi: 10.1038/ni.2158. Together with references 48. Gerlach C, et al and 49. Plumlee CR, et al, this paper comprises an analysis of the fate and differentiation of individual T cells during infection. These papers contain data that is consistent with a model of progressive T cell differentiation, where memory cells are progenitors of effector cells, and not vice versa. Buchholz et al propose a model of T cell differentiation that is consistent with one published by Klebanoff, et al in 2006 (Reference 7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Buchholz VR, Flossdorf M, Hensel I, Kretschmer L, Weissbrich B, Graf P, Verschoor A, Schiemann M, Hofer T, Busch DH. Disparate individual fates compose robust CD8+ T cell immunity. Science. 2013;340:630–635. doi: 10.1126/science.1235454. [DOI] [PubMed] [Google Scholar]
- 49.Gerlach C, Rohr JC, Perie L, van Rooij N, van Heijst JW, Velds A, Urbanus J, Naik SH, Jacobs H, Beltman JB, et al. Heterogeneous differentiation patterns of individual CD8+ T cells. Science. 2013;340:635–639. doi: 10.1126/science.1235487. [DOI] [PubMed] [Google Scholar]
- 50.Plumlee CR, Sheridan BS, Cicek BB, Lefrancois L. Environmental Cues Dictate the Fate of Individual CD8 T Cells Responding to Infection. Immunity. 2013 doi: 10.1016/j.immuni.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol. 2012;12:269–281. doi: 10.1038/nri3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52**.Sukumar M, Liu J, Ji Y, Subramanian M, Crompton JG, Yu Z, Roychoudhuri R, Palmer DC, Muranski P, Karoly ED, Mohney RP, Klebanoff CA, Lal A, Finkel T, Restifo NP, Gattinoni L. Inhibiting glycolytic metabolism enhances CD8+ T cell memory and antitumor function. Journal of Clinical Investigation. 2013 doi: 10.1172/JCI69589. Together with Pearce, EL, et al, (Ref 54.) this paper describes the metabolic changes that influence the differentiation of CD8+ T cells into memory or effector cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chang CH, Curtis JD, Maggi LB, Jr, Faubert B, Villarino AV, O’Sullivan D, Huang SC, van der Windt GJ, Blagih J, Qiu J, et al. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell. 2013;153:1239–1251. doi: 10.1016/j.cell.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang LS, Jones RG, Choi Y. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460:103–107. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chang HH, Hemberg M, Barahona M, Ingber DE, Huang S. Transcriptome-wide noise controls lineage choice in mammalian progenitor cells. Nature. 2008;453:544–547. doi: 10.1038/nature06965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang R, Green DR. Metabolic checkpoints in activated T cells. Nat Immunol. 2012;13:907–915. doi: 10.1038/ni.2386. [DOI] [PubMed] [Google Scholar]
- 57.Pearce EL, Pearce EJ. Metabolic pathways in immune cell activation and quiescence. Immunity. 2013;38:633–643. doi: 10.1016/j.immuni.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhou J, Shen X, Huang J, Hodes RJ, Rosenberg SA, Robbins PF. Telomere length of transferred lymphocytes correlates with in vivo persistence and tumor regression in melanoma patients receiving cell transfer therapy. J Immunol. 2005;175:7046–7052. doi: 10.4049/jimmunol.175.10.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, Citrin DE, Restifo NP, Robbins PF, Wunderlich JR, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011;17:4550–4557. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huang J, Kerstann KW, Ahmadzadeh M, Li YF, El-Gamil M, Rosenberg SA, Robbins PF. Modulation by IL-2 of CD70 and CD27 expression on CD8+ T cells: importance for the therapeutic effectiveness of cell transfer immunotherapy. J Immunol. 2006;176:7726–7735. doi: 10.4049/jimmunol.176.12.7726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Robbins PF, Dudley ME, Wunderlich J, El-Gamil M, Li YF, Zhou J, Huang J, Powell DJ, Jr, Rosenberg SA. Cutting edge: persistence of transferred lymphocyte clonotypes correlates with cancer regression in patients receiving cell transfer therapy. J Immunol. 2004;173:7125–7130. doi: 10.4049/jimmunol.173.12.7125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Klebanoff CA, Gattinoni L, Torabi-Parizi P, Kerstann K, Cardones AR, Finkelstein SE, Palmer DC, Antony PA, Hwang ST, Rosenberg SA, et al. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc Natl Acad Sci U S A. 2005;102:9571–9576. doi: 10.1073/pnas.0503726102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hinrichs CS, Spolski R, Paulos CM, Gattinoni L, Kerstann KW, Palmer DC, Klebanoff CA, Rosenberg SA, Leonard WJ, Restifo NP. IL-2 and IL-21 confer opposing differentiation programs to CD8+ T cells for adoptive immunotherapy. Blood. 2008;111:5326–5333. doi: 10.1182/blood-2007-09-113050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hinrichs CS, Borman ZA, Cassard L, Gattinoni L, Spolski R, Yu Z, Sanchez-Perez L, Muranski P, Kern SJ, Logun C, et al. Adoptively transferred effector cells derived from naive rather than central memory CD8+ T cells mediate superior antitumor immunity. Proc Natl Acad Sci U S A. 2009;106:17469–17474. doi: 10.1073/pnas.0907448106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Klebanoff CA, Gattinoni L, Palmer DC, Muranski P, Ji Y, Hinrichs CS, Borman ZA, Kerkar SP, Scott CD, Finkelstein SE, et al. Determinants of successful CD8+ T-cell adoptive immunotherapy for large established tumors in mice. Clin Cancer Res. 2011;17:5343–5352. doi: 10.1158/1078-0432.CCR-11-0503. [DOI] [PMC free article] [PubMed] [Google Scholar]


