Abstract
Novel classical antifolates (3 and 4) and 17 nonclassical antifolates (11-27) were synthesized as antitumor and/or antiopportunistic infection agents. Intermediates for the synthesis of 3, 4, and 11-27 were 2,4-diamino-5-alkylsubstituted-7H-pyrrolo[2,3-d]pyrimidines, 31 and 38, prepared by a ring transformation/ring annulation sequence of 2-amino-3-cyano-4-alkyl furans to which various aryl thiols were attached at the 6-position via an oxidative addition reaction using I2. The condensation of α-hydroxy ketones with malonodinitrile afforded the furans. For the classical analogues 3 and 4, the ester precursors were deprotected, coupled with diethyl-l-glutamate, and saponified. Compounds 3 (IC50 = 60 nM) and 4 (IC50 = 90 nM) were potent inhibitors of human DHFR. Compound 3 inhibited tumor cells in culture with GI50 ≤ 10−7 M. Nonclassical 17 (IC50 = 58 nM) was a potent inhibitor of Toxoplasma gondii (T. gondii) DHFR with >500-fold selectivity over human DHFR. Analogue 17 was 50-fold more potent than trimethoprim and about twice as selective against T. gondii DHFR.
Introduction
Dihydrofolate reductase (DHFR) along with thymidylate synthase (TS) forms part of the system responsible for the synthesis of 2′-deoxythymidine-5′-monophosphate (dTMP), a key component in DNA biosynthesis and cell replication. TS catalyzes the de novo synthesis of dTMP from 2′-deoxyuridine-5′-monophosphate (dUMP). The cofactor, N5,N10-methylene-tetrahydrofolate (N5,N10-CH2-THF), serves as the donor of the methyl group as well as the reductant for this step and is itself oxidized to 7,8-dihydrofolate (7,8-DHF). The recyclization of 7,8-DHF to 5,6,7,8-tetrahydrofolate (5,6,7,8-THF) is catalyzed by DHFR2 for which NADPH acts as the source of the reductant. Thus inhibition of DHFR and/or TS leads to “thymineless death”.a
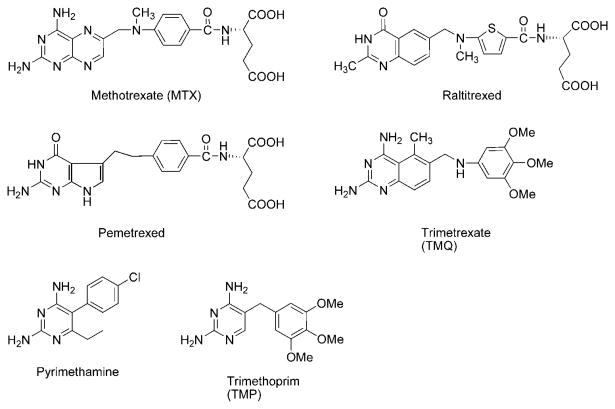
Pneumocystis jiroνecii(P. jiroνecii) previously known as Pneumocystis carinii(P. carinii)3,4 [Note: P. jiroνecii is the strain that infects humans, while P. carinii refers to the strain that infects rats] and Toxoplasma gondii(T. gondii)3,4 are often fatal opportunistic infections in AIDS patients. Mycobacterium aνium(M. aνium) complex (MAC),3,4 a group of organisms that is responsible for disseminated infections in AIDS patients, additionally decreases the quality of life of patients with AIDS. Several DHFR and TS inhibitors have found clinical utility as antitumor and antiopportunistic agents.5 Classical antifolates like, methotrexate6 (MTX) (Figure 1), raltitrexed,7 and pemetrexed8, are clinically used as antitumor agents. Nonclassical antifolates like trimetrexate (TMQ), pyrimethamine, and trimethoprim (TMP) are clinically used as antiopportunistic infection agents.4
Figure 1.
The combination of a weak DHFR inhibitor (TMP, py-rimethamine), along with a potent dihydropteroate synthase (DHPS) inhibitor (sulfamethoxazole), is currently used to treat infections caused by opportunistic pathogens in AIDS patients.9 However, the combination therapy is successful in only 50-75% of the AIDS population; up to 60% are unable to tolerate the combination therapy due to severe, adverse drug reactions.10 Trimetrexate is coadministered with leucovorin, the classical folate cofactor (6R,6S)-5-formyl-5,6,7,8-THF, which selectively rescues the host cell from the toxicity caused by nonselective, TMQ.11
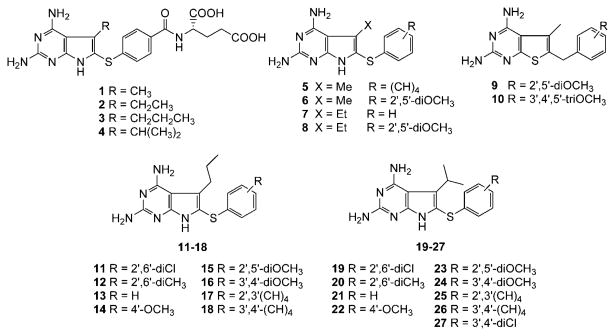
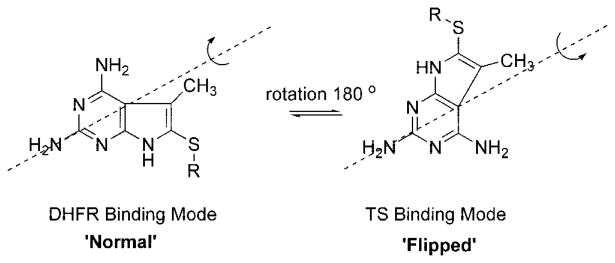
Gangjee et al.12 recently reported 1 (Figure 2) as a dual inhibitor of human DHFR (IC50 = 0.21 μM) and human TS (IC50 = 0.54 μM). The 5-CH3 moiety of 1 was incorporated to provide hydrophobic interaction with Val115 in human DHFR. Compound 1 was designed as a nonpolyglutamylatable DHFR inhibitor. However, unexpectedly, 1 had reasonable folyl poly-γ-glutamate synthetase (FPGS) substrate activity. Molecular modeling using SYBYL 6.813 suggested that 1 binds to human DHFR in the normal 2,4-diamino mode (Figure 3) while it could bind to human TS in the flipped mode. In addition, molecular modeling also indicated that the 5-CH3 group in 1 could provide hydrophobic interaction with Trp109 in human TS. Compound 1 was a reasonably potent inhibitor of the growth of human CCRF-CEM leukemia cells in culture with an EC50 value of 190 nM as compared with MTX (EC50 = 12.5 nM). In 11 of the 60 tumor cell lines evaluated at the National Cancer Institute (NCI) preclinical screening program, compound 1 showed GI50 values of ≤ 10−7 M (Table 3). Homologation of the 5-methyl group in 1 to a 5-ethyl group as in 2 (Figure 2) afforded a 3-fold more potent human DHFR inhibitor (IC50 = 0.066 μM).14 Surprisingly, compound 2 was devoid of any significant TS, inhibitory activity (37% inhibition @ >17 μM). However, compound 2 demonstrated increased tumor cell growth inhibitory activities against certain tumor cell lines compared to 1 in the NCI preclinical screening program (Table 3). Thus the size of the alkyl group attached to the 5-position of the 2,4-diamino, pyrrolo[2,3-d]pyrimidine scaffold dictates the activity against DHFR and/or TS as well as tumor cell growth inhibitory potency.
Figure 2.
Figure 3.
The “Normal” and “Flipped” modes of pyrrolo[2,3-d]pyr-imidine.
Table 3. Cytotoxicity Evaluation (GI50, M) of Compounds 1-4 against Selected Tumor Cell Lines29.
| cell line | R =Me (1) | R = Et (2) | R = Pr (3) | R = iPr (4) |
|---|---|---|---|---|
| Leukemia | ||||
| CCRF-CEM | 2.21 × 10−7 | 5.36 × 10−7 | ||
| HL-60 × (TB) | 4.35 × 10−8 | 3.61 × 10−8 | 1.28 × 10−6 | 5.65 × 10−6 |
| K-562 | 2.95 × 10−8 | 9.52 × 10−7 | 9.32 × 10−7 | |
| MOLT-4 | <1.00 × 10−8 | 1.94 × 10−5 | 1.31 × 10−6 | |
| RPMI-8226 | <1.00 × 10−8 | 1.4 × 10−7 | 7.74 × 10−6 | |
| Nonsmall Cell Lung Cancer | ||||
| NCI-H460 | 6.31 × 10−7 | 2.44 × 10−7 | 2.67 × 10−7 | 6.45 × 10−6 |
| Colon Cancer | ||||
| HT29 | 1.60 × 10−7 | 5.4 × 10−8 | 9.58 × 10−6 | 5.76 × 10−6 |
| SW-620 | 1.24 × 10−7 | 9.0 × 10−8 | 1.21 × 10−6 | 4.71 × 10−6 |
| HCT 15 | 5.95 × 10−5 | 6.7 × 10−7 | >1.0 × 10−4 | 3.48 × 10−5 |
| Central Nervous System Cancer | ||||
| U251 | 8.24 × 10−7 | 1.6 × 10−6 | 7.24 × 10−6 | |
| SF268 | 7.47 × 10−5 | 2.2 × 10−7 | 1.61 × 10−6 | 1.78 × 10−5 |
| Melanoma | ||||
| LOX IMVI | 1.71 × 10−7 | 4.9 × 10−8 | <1.00 × 10−8 | 1.14 × 10−6 |
| Prostrate Cancer | ||||
| PC-3 | 4.36 × 10−8 | >1.0 × 10−4 | >1.0 × 10−4 | |
| Breast Cancer | ||||
| MCF-7 | >1 × 10−4 | 5.9 × 10−7 | 1.64 × 10−7 | |
| Renal Cancer | ||||
| ACHN | 2.42 × 10−5 | 4.5 × 10−7 | 1.52 × 10−5 | 7.39 × 10−6 |
| CAKI-1 | >1 × 10−4 | 1.25 × 10−7 | 3.76 × 10−7 | 4.25 × 10−6 |
| 786-0 | 1.28 × 10−8 | 5.58 × 10−6 | ||
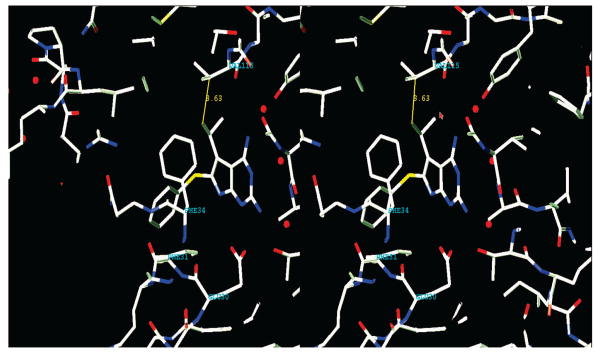
Molecular modeling (SYBYL 6.91)13 further indicated that the 5-alkyl group could be homologated to a 5-propyl or 5-isopropyl. This homologation could further enhance the van der Waals interaction with Val115 in human DHFR (Figure 4) in the normal DHFR binding mode. To determine the optimum size of the 5-alkyl group for DHFR and/or TS inhibitory activity as well as tumor cell growth inhibitory potency, the 2,4-diamino-5-propyl-6-arylthio-7H-pyrrolo[2,3-d]pyrimidine (3) and 2,4-diamino-5-isopropyl-6-arylthio-7H-pyrrolo[2,3-d]pyrimidine (4) classical analogues (Figure 2) were designed and synthesized.
Figure 4.
Stereo view of compound 4 in human DHFR (PDB code 1U72).30 The hydrophobic interaction between 5-isopropyl and Val 115 is shown.
The existing regimen used to treat opportunistic infections in AIDS and other immunocompromised patients is suppressive rather than curative and the therapy must be continued indefinitely.3,4 Thus, it is of considerable interest to design single agents that have both the desired selectivity of TMP and the, potency of TMQ. Such agents could be used as single agents to treat opportunistic infections in immunocompromised patients to decrease cost and increase patient compliance. Because patients with AIDS are often infected with multiple opportunistic infections, it is highly desirable to develop single agents that simultaneously target two or more opportunistic pathogen DHFR.
Gangjee et al.15 also reported nonclassical analogues of 1, including 5 and 6 (Figure 2) as inhibitors of DHFR from opportunistic pathogens. The 5-CH3 moiety was designed to, afford hydrophobic interaction with Ile123 in P. carinii DHFR, Val151 in T. gondii DHFR, and Ile102 in M. avium DHFR on the basis of X-ray crystal structure,16,17 multiple sequence alignment,18,19 and molecular modeling (SYBYL 6.813) studies, respectively. The 5-CH3 group was also suggested to influence the conformations of the 6-arylthio side chain in these inhibitors, thus limiting its flexibility and contributing to the potency of these compounds. Several compounds, including 5 and 6 (Table 2), displayed 10-fold or higher selectivity ratios for T. gondii DHFR and/or M. avium DHFR compared to rat liver (rl), DHFR.15 Compound 6 with a 2′,5′-(OCH3)2 substitution was 16-fold more potent and equally selective compared to TMP against T. gondii DHFR.
Table 2. Inhibitory Concentration (IC50, μM) against Isolated DHFRa and Selectivity Ratiosb.
| cmpd | P. carinii | rat liver | rl/pcb | M. avium | rl/mab |
|---|---|---|---|---|---|
| 3 | 0.00311 | 0.06 | 19.3 | 0.000737 | 81.4 |
| 4 | 0.0048 | 0.186 | 38.8 | 0.000391 | 475.7 |
| 5 | 37.3 | 4.57 | 0.12 | 0.7 | 6.5 |
| 6 | 14.6 | 7.8 | 0.5 | 0.1 | 78 |
| 7 | 21.8 | 5.6 | 0.3 | 0.88 | 6.36 |
| 8 | 6.04 | 4.05 | 0.7 | 0.1043 | 38.8 |
| 11 | 5.35 | 5.95 | 1.1 | 12.3 | 0.5 |
| 12 | 5.32 | 2.43 | 0.5 | 10.4 | 0.23 |
| 13 | 4.21 | 1.25 | 0.3 | 1.31 | 1.0 |
| 14 | 4.21 | 1.25 | 0.3 | 0.63 | 2.0 |
| 15 | 10.4 | 8.9 | 0.9 | 0.6 | 14.8 |
| 16 | 8.45 | 3.45 | 0.4 | 0.21 | 16.4 |
| 17 | 8.6 | 6.5 | 0.8 | 4.1 | 1.59 |
| 18 | 3.5(16%)c | 3.8 | ND | 7.8 | 0.5 |
| 19 | 22(14%) | 2.4 | NDd | 2.7 | 0.9 |
| 20 | 27.2 | 14.8 | 0.5 | 28.6 | 0.52 |
| 21 | 11.2 | 2 | 0.2 | 0.3687 | 5.42 |
| 22 | ND | 1.9 | ND | ND | ND |
| 23 | 35.6 | 50.3 | 1.4 | 2.1 | 24 |
| 24 | 28.2 | 4.6 | 0.2 | 3.4 | 1.3 |
| 25 | 0.68 | 0.91 | 1.3 | 0.24 | 3.8 |
| 26 | 7.1 | 2.1 | 0.3 | 2.54 | 0.83 |
| 27 | 10.9 | 13.5 | 1.2 | 1.14 | 11.84 |
| TMQ | 0.042 | 0.003 | 0.07 | 0.0015 | 2.0 |
| TMP | 12 | 180 | 15 | 0.3 | 600 |
These assays were carried out at 37 °C under conditions of substrate (90 μM dihydrofolic acid) and cofactor (119 μM NADPH) in the presence of 150 mM KCl.
Selectivity ratios, rl/pc = IC50 rat liver dihydrofolate reductase/IC50P. carinii dihydrofolate reductase; rl/ma = IC50 rat liver dihydrofolate reductase/IC50M. aνium dihydrofolate reductase.
Number in parenthesis indicate the percentage inhibition at the given concentration.
ND = not determined.
Rosowsky et al.,20 using a different approach, reported compounds 9 and 10 (Figure 2) with a single carbon atom bridge that displayed fair T. gondii DHFR potency and good selectivity. Analogue 9 (IC50 = 0.07 μM) was the most potent in this series against T. gondii DHFR, while analogue 10 was the most selective for T. gondii DHFR compared to rlDHFR with a selectivity ratio of 81.
A sulfur atom was incorporated in compounds 5 and 6 rather than a carbon atom, as in compounds 9 and 10, to increase the proximity of the 6-arylthio ring to the hydrophobic residues on the pathogen DHFR due to the increased atomic size of the sulfur atom as well as a decrease in the C-S-C angle (98°) compared to a C-C-C angle (109°).15 Compound 6 was 19fold more potent and nearly one-half as selective as the most selective compound (10) of the 6-carbon-bridged analogues. The biological activity of compounds 5 and 6 supported the hypothesis that the 6-arylthio side chain of these compounds indeed interacts more favorably with Phe91 in T. gondii DHFR and Val158 in M. aνium DHFR and that the sulfur bridge increased activity and selectivity. Gangjee et al.14 have also synthesized the ethyl homologues of 5 and 6 with the goal of further increasing the potency and selectivity. Compound 8 (Figure 2), the ethyl homologue of 6, was found to have increased potency and/or selectivity against P. carinii and T. gondii DHFR compared to rlDHFR (Table 2). Similar to their methyl counterparts, the ethyl homologues including 7 and 8 were found to have increased potency and/or selectivity against T. gondii and/or M. aνium DHFR. In most instances, the ethyl homologues tested were found to be more active and/or selective against two or more pathogen DHFR. In an attempt to optimize the size of the 5-alkyl substitution on the potency and selectivity for P. carinii DHFR, T. gondii DHFR, and M. aνium DHFR compounds 11-27 (Figure 2) were also designed and synthesized. Compounds 11-18 contain a 5-propyl group, while compounds 19-27 contain a 5-isopropyl group.
Chemistry
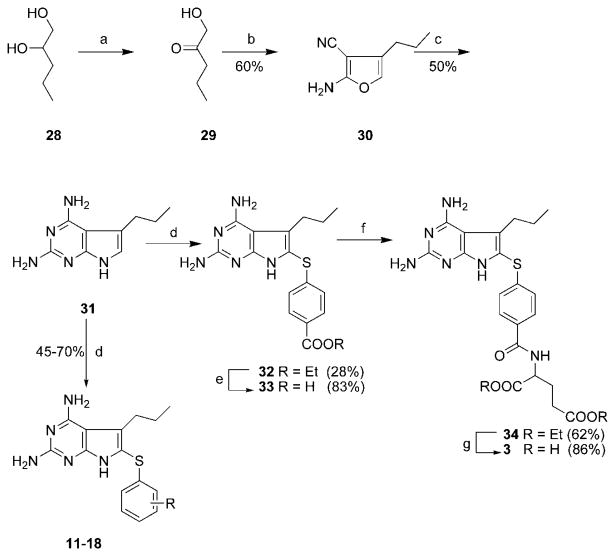
The syntheses of compounds 3 and 11-18 required the synthesis of 2,4-diamino-5-propyl-7H-pyrrolo[2,3-d]pyrimidine, 31 (Scheme 1), while the synthesis of 4 and 19-27 required the synthesis of 2,4-diamino-5-isopropyl-7H-pyrrolo[2,3-d]py-rimidine, 38 (Scheme 2). Taylor et al.21 have reported the synthesis of various 2,4-diamino-5-alkyl-7H-pyrrolo[2,3-d]py-rimidines by a ring transformation/ring annulation sequence of 2-amino-3-cyano-4-alkyl furans. These furans were in turn obtained by the condensation of suitable α-hydroxy ketones with malonodinitrile in the presence of a suitable base such as triethylamine. Gangjee et al.12,14,15,22 and Rosowsky et al.23,24 have also successfully adopted this methodology in their synthesis of pyrrolo[2,3-d]pyrimidine containing antifolates. Extending this general methodology to the synthesis of 31 required the synthesis of 1-hydroxy-2-pentanone, 29 (Scheme 1). Compound 29 was in turn obtained from the commercially available 1,2-pentanediol, 28, by regiospecific oxidation of the secondary alcohol using hexabutyldistannoxane (HBD) and Br2 (Scheme 1).25 Two methods were attempted for the separation of the 1-hydroxy-2-pentanone, 29, from the reaction mixture. The first involved silica gel chromatography on the crude reaction mixture and the second was a direct distillation of the crude reaction mixture. In general, distillation was found to be superior to column chromatography. Condensation of 29 with malonodinitrile using triethylamine as base afforded the 2-amino-4-propyl-furan-3-carbonitrile, 30, in 60% yield. Further, condensation of 30 with guanidine (liberated from guanidine, hydrochloride and NaOMe) afforded 31 in 50% yield. Reaction of 2,4-diamino-5-propyl-7H-pyrrolo[2,3-d]pyrimidine, 31, and ethyl 4,4′-bismercaptobenzoate in EtOH/H2O followed by the addition of I2 at reflux afforded compound 32 in 28% yield.26,27 The disappearance of the 6-vinyl proton at δ 6.38 and the appearance of the characteristic AA′XX′ pattern for the 6-aryl, protons in the 1H NMR spectrum of 32 in DMSO (d6) indicated the success of the oxidative addition reaction.
Scheme 1a.
a Conditions: (a) O(SnBu3)2, Br2, CH2Cl2; (b) malonodinitrile, NEt3, MeOH, 24 h; (c) guanidine hydrochloride, NaOMe, overnight; (d) ArSH, I2, EtOH/H2O (2:1), 100-110 °C; (e) 1N NaOH, 80 °C, 24 h; (f) 2-chloro-4,6-dimethoxy-1,3,5-triazine, N-methylmorpholine, diethyl-L-glutamate hydrochloride, 0 °C to r.t.; (g) 1N NaOH, 0 °C to r.t.
Scheme 2a.
a Conditions: (a) HCHO, 3-ethylbenzothiazolium bromide, Et3N, EtOH, 60 °C, 72 h; (b) malonodinitrile, NEt3, MeOH, 24 h; (c) guanidine hydrochloride, NaOMe, 96 h; (d) ArSH, I2, EtOH/H2O (2:1), 100-110 °C; (e) 1N NaOH, 80 °C, 24 h. (f) 2-chloro-4,6-dimethoxy-1,3,5-triazine, N-methylmorpholine, diethyl-l-glutamate hydrochloride, 0 °C to r.t.; (g) 1N NaOH, 0 °C to r.t.
Hydrolysis of the ester 32 with aqueous 1N NaOH at 80 °C (24 h) followed by acidification gave the required acid, 33, in 83% yield. Peptide coupling21 of the acid 33 with diethyl-l-glutamate using 2,6-dimethoxy-4-chlorotriazine and N-methyl, morpholine, followed by chromatographic purification afforded the coupled product 34 in 62% yield. The 1H NMR spectrum of 34 in DMSO (d6) revealed the newly formed amide NH proton at δ 8.64-8.67 ppm as a doublet. Hydrolysis of the diester 34 with aqueous NaOH at 0 °C (4 h) and then at room temperature (24 h), followed by acidification, gave the desired compound 3 in 86% yield.
Similarly, reaction of 31 with appropriately substituted aryl thiols in a mixture of EtOH/H2O (2:1) followed by addition of I2 at reflux as reported previously27 afforded 11-18 in 45%-70% yields. The yields reveal no apparent correlation between the extent of pyrrolo[2,3-d]pyrimidine substitution and the electron-donating or -withdrawing effects of substituents in the thiophenol.
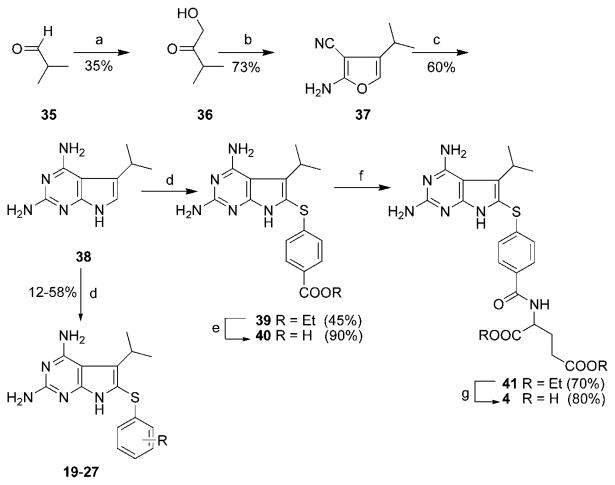
Analogous to 31, the synthesis of 37 (Scheme 2) required the synthesis of 1-hydroxy-3-methyl-2-butanone, 36. Thiazolium salt-catalyzed benzoin condensation of isopropyl aldehyde 35 with paraformaldehyde catalyzed by N-ethylbenzothiazolium bromide and triethylamine afforded the α-hydroxy ketone, 36, after distillation, in 35% yield.28 Compounds 4 and 19-27 were synthesized as shown in Scheme 2 starting with 36 in essentially the same way as described for 3 and 11-18 in Scheme 1.
The yields in Scheme 2, as before for Scheme 1, reveal no apparent correlation between the extent of pyrrolo[2,3-d]pyri-midine substitution, and the electronic nature of the substituents in the thiophenol. The lower yields of 18 (Scheme 1) and 19 (Scheme 2) may be a result of unfavorable steric interactions between the bulky 5-isopropyl group in 37 and the 2′,6′-disubstitution present on the thiophenols, which makes 6-sub-stitution more difficult.
Biological Evaluation and Discussion
Compounds 3, 4, and 11-27 were evaluated as inhibitors of human (h), Escherichia coli (E. coli), and T. gondii DHFR and TS. The inhibitory potency (IC50) values are compared with MTX, pemetrexed, TMP, pyrimethamine, and the previously synthesized 1 and 2 (Table 1). Compounds 3 and 4 are good inhibitors of hDHFR with nanomolar IC50 values and were about 3-fold and 4-fold less potent as hDHFR inhibitors, respectively, compared with MTX and about 25-fold and 17-fold more potent respectively than pemetrexed. Compound 3 was equipotent with the previously synthesized 2 and about 3.5-fold more potent than 1. The biological data of 1-4 indicate that an ethyl, propyl, or isopropyl group at the 5-position are all conducive for potent hDHFR inhibition. The potent hDHFR activity of 2-4 compared to 1 could be attributed to increased hydrophobic interaction of the bulkier alkyl groups in 2-4 with Val115 in hDHFR. The increased activity of 2-4 may also result from favorable orientation of the 6-position thioaryl side chain when bound to hDHFR. Against hTS, 3 and 4 had similar inhibitory potency as MTX but were 4-fold less inhibitory than pemetrexed. Compounds 3 and 4 were about 63-74-fold less potent than 1 as inhibitors of hTS. These results indicate that homologation of the 5-methyl group in 1 to larger alkyl groups as in 2-4 is detrimental to hTS inhibition. This decrease in potency may be due to steric hindrance between the larger alkyl groups in 2-4 and Trp109 in hTS and/or due to unfavorable orientation of the 6-position side chains for interaction with the hTS in the presence of the bulkier 5-alkyl moiety.
Table 1. Inhibitory Concentration (IC50,μM) and Selectivity Ratiosj against Isolated TS and DHFRa.
| TS | DHFR | Selectivity ratiosj | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||
| cmpd | humanb | E. colib | T. gondiic | humand | E. colie | T. gondiic | h/ecj | h/tgj |
| 1f | 0.54 | >180 | 1.8 | 0.21 | 0.016 | 0.17 | 13 | 1.2 |
| 2g | >17 (37)h | 0.066 | 0.002 | 33 | ||||
| 11 | >22 (0) | >22 (0) | ND | 2.6 | 1.3 | ND | 2 | |
| 12 | >24 (0) | >24 (0) | ND | 3.0 | 1.5 | ND | 2 | |
| 13 | >14 (0) | >14 (0) | >1.4 (0) | 33 | 1.3 | 0.12 | 25 | 275 |
| 14 | >13 (0) | >13 (0) | >13 (12) | >30 (15) | 0.61 | 0.23 | >49 | >130 |
| 15 | >12 (0) | >12 (0) | >12 (0) | >28 (13) | 1.4 | 1.4 | >20 | >20 |
| 16 | >11 (0) | >11 (0) | >11 (16) | >26 (35) | 0.5 | 1.0 | >52 | >26 |
| 17 | >12 (0) | >12 (0) | >12 (16) | >29 (17) | 1.5 | 0.058 | >19 | >500 |
| 18 | >12 (0) | >12 (0) | >15 (12) | >15 (30) | 0.60 | 0.15 | >25 | >100 |
| 19 | >22 (18) | >2.2 (0) | >2.2 (0) | >26 (37) | 2.1 | 0.52 | >12 | >50 |
| 20 | >13 (0) | >13 (0) | >13 (0) | >30 (10) | 3.0 | 0.30 | >10 | >100 |
| 21 | >27 (0) | >2.7 (0) | >2.7 (0) | 16 | 0.13 | 0.064 | 123 | 250 |
| 22 | >25 (0) | >2.5 (0) | >2.5 (0) | 30 | 0.15 | 0.60 | 200 | 50 |
| 23 | >23 (0) | >2.3 (0) | >2.3 (0) | >28 (0) | 0.11 | 1.4 | >254 | >20 |
| 24 | >23 (0) | >2.3 (0) | >2.3 (0) | 27 | 0.11 | 2.7 | 245 | 10 |
| 25 | >12 (0) | >12 (0) | >12 (0) | >29 (27) | 0.15 | 0.12 | > 193 | >241 |
| 26 | >24 (39) | >2.4 (0) | >2.4 (0) | >29 (36) | 0.29 | 1.5 | >100 | >19 |
| 27 | >23 (14) | >2.3 (0) | >2.3 (0) | >27 (0) | 0.14 | 1.4 | > 193 | >19 |
| MTX | 29 | 90 | 18 | 0.022 | 0.0066 | 0.011 | 3.3 | 2 |
| pemetrexedi | 9.5 | 76 | 2.8 | 1.5 | 230 | 0.46 | 0.006 | 3.26 |
| TMP | 680 | 0.020 | 2.9 | 34000 | 234 | |||
| pyrimethamine | 6.0 | 2.0 | 0.080 | 3 | 75 | |||
The percent inhibition was determined at a minimum of four inhibitor concentrations within 20% of the 50% point. The standard deviations for determination of 50% points were within ± 10% of the value given.
Kindly provided by Dr. Frank Maley, New York State Department of Health, Albany, NY.
Kindly provided by Dr. K. Anderson, Yale University.
Kindly provided by Dr. J. H. Freisheim, Medical College of Ohio, Toledo, OH.
Kindly provided by Dr. R. L. Blakley, St. Jude Children's Hospital, Memphis, TN.
Data taken from ref 12.
Data taken from ref 14.
Number in parenthesis indicated % inhibition at that concentration.
Kindly provided by Dr. Chuan Shih, Eli Lilly & Co., Indianapolis IN.
Selectivity ratios, h/ec = IC50 human dihydrofolate reductase/IC50E. coli dihydrofolate reductase; h/tg = IC50 human dihydrofolate reductase/IC50T. gondii dihydrofolate reductase.
Compound 3 was a poor inhibitor of hTS and E. coli TS (Table 1) but showed moderate inhibition against T. gondii TS (equipotent to pemetrexed). Compound 3 was a good inhibitor of all three DHFR tested. In addition, 3 is also a dual inhibitor of T. gondii DHFR and T. gondii TS. The nonclassical analogues, 11-27 were all poor inhibitors of all three TS tested. They were however reasonably potent inhibitors of E. coli DHFR and T. gondii DHFR. Most of the analogues were weak or poor inhibitors of hDHFR.
E. coli DHFR (Table 1)
In general the 5-isopropyl compounds (19-27) with the exceptions of 19 and 20 were more potent and selective against E. coli DHFR than the corresponding 5-propyl compounds (11-18). In the 5-propyl series, analogue 16 with a 3,4-dimethoxyphenyl side chain was the most potent and selective compound against E. coli DHFR. In the 5-isopropyl series, analogues 21-27 were potent against E. coli DHFR. A number of the nonclassical compounds in the 5-isopropyl series showed good selectivity for E. coli DHFR as compared to hDHFR. Compounds 21-27 were 100-fold to 254-fold more selective for E. coli DHFR than hDHFR. Thus the 5-isopropyl-6-substituted phenyl analogues were reasonably selective for bacterial DHFR.
T. gondii DHFR (Table 1)
In general the 5-propyl compounds (13-18) with the exception of 13 were more potent and selective against T. gondii DHFR than the corresponding 5-isopropyl compounds (21-26). In the 5-propyl series, analogue 17 with a 1-naphthyl side chain was the most potent compound against T. gondii DHFR and was 50-fold more potent than TMP, 5-fold less potent than MTX and equipotent with pyrimethamine. Compound 17 was also the most selective compound against T. gondii DHFR with >500-fold selectivity over human DHFR. Thus 17 is 50-fold more potent than TMP and about twice as selective against T. gondii DHFR. Compound 13 with a phenyl side chain was 20-fold more potent than TMP and equally selective against T. gondii DHFR compared with human DHFR. In the 5-isopropyl series, analogue 21 with an unsubstituted phenyl side chain was the most potent analogue against T. gondii DHFR and was 45-fold more potent than TMP and equally selective. Analogue 25 with a 1-naphthyl side chain was 24fold more potent than TMP and equally selective.
Compounds 3, 4, 11-27, MTX, PYR, and TMP were also assayed against T. gondii DHFR using protocols described for Table 2 (data for T. gondii DHFR not shown), as well as under the conditions described for Table 1. Of the 20 compounds jointly assayed, MTX, 3, and 4 were identified as the three most potent compounds by both protocols; both laboratories also placed TMP, 23, 24, and 27 as among the six least potent compounds. Selectivity could only be directly compared for the compounds in Table 1 that had defined selectivity values. Assays under both sets of conditions identified TMP, 13, and 21 as the most selective compounds in this set of nine compounds and MTX as the least selective. Considering all compounds inde pendently assayed as described in Table 2 for T. gondii, DHFR, the most selective compounds are TMP, 21, 15, 20, 13, 17, and 23; this list is consistent with the results in Table 1, except that the selectivity of compounds 15 and 20 is artificially depressed in Table 1 by the inability to generate full inhibition curves for the human reference enzyme.
Compounds 3, 4, and 11-27 were also evaluated as inhibitors of P. carinii DHFR, M. avium DHFR, and rlDHFR, which served as the mammalian surrogate under slightly different assay conditions. The inhibitory potency (IC50) values are compared with TMQ, TMP, and the previously synthesized 5-8 (Table 2). Several compounds displayed 10-fold or higher selectivity ratios for M. avium DHFR. Against P. carinii DHFR, in, general, the 5-propyl nonclassical analogues (11-18) were more potent and selective than the corresponding 5-isopropyl analogues (19-27). Against M. aνium DHFR, the nature of the phenyl, substitution along with the 5-alkyl group determined the potency and selectivity of the compound.
P. carinii DHFR (Table 2)
Against P. carinii DHFR the most potent analogues bore an unsubstituted phenyl (in 13) or a 4′-methoxyphenyl substitution (in 14) in the 5-propyl (11-18) series. Other substitutions such as a 2,6-dichloro 11, 2,6-dimethyl 12, 3,4-dimethoxy 16, or 2,5-dimethoxy 15 caused a slight drop in activity. The 1-naphthyl substitution in 17 was found to display moderate DHFR inhibitory activity, while a 2-naphthyl substitution in 18 was found to be slightly detrimental for activity compared to 13. In the 5-isopropyl (19-27) series, the most potent analogue contained a 1-naphthyl side chain 25 and displayed submicromolar inhibitory potency. All other substitutions in the side chains as in 19-27, with the exception of 25, displayed micromolar or higher inhibitory potency. Compounds 11-27 were not selective against P. carinii DHFR and increasing the size of the 5-alkyl group did not improve the selectivity, however, it increased the potency of the compounds against P. carinii DHFR compared to the corre sponding methyl (compare 5 with 26 or 6 with 15) and ethyl (compare 7 with 13 or 21) analogues. The biological data of analogues 11-21 indicate that the homologation to a 5-propyl or 5-isopropyl group is conducive for potent inhibition of P. carinii DHFR, however, it does not improve the selectivity.
Mycobacterium aνium DHFR (Table 2)
Analogue 16 con taining a 3′,4′-dimethoxy substitution in the phenyl ring was the most potent and selective analogue in the 5-propyl series. The second best analogue 15 had a 2′,5′-dimethoxy substitution in the side chain. Substitution of the phenyl ring with other substitutions as in 11-14, 17, and 18 resulted in analogues that were considerably less potent and selective. In the 5-isopropyl series, the most potent analogue 25 contains a 1-naphthyl side chain. The 2-naphthyl substituted analogue 26 was 10-fold less potent than 25. The most selective analogue was 23, with a 2′,5′-dimethoxy substitution in the side chain. The electron donating 3,4-dimethoxy substituted analogue 24 was found to be devoid of any selectivity. In sharp contrast, the analogue with electron withdrawing 3,4-dichloro substitution 27 had the second best selectivity. Again, the biological data of 11-27 indicate that both the alkyl group present at the 5-position as well as the substituents present on the 6-position thioaryl side chain play a role in determining the potency and selectivity of the analogues against M. aνium DHFR.
Rat liver DHFR (Table 2)
In the 5-propyl series, analogues 13 and 14 with an unsubstituted phenyl and a 4′-methoxy substitution, respectively, were the most potent. Dimethoxy substituted analogues 15 and 16 were 2-fold less potent than the monomethoxy 14. Substitution of the phenyl ring with bulky groups such as 1-naphthyl 17 and 2-naphthyl 18 also resulted in 2-fold less potent compounds compared to 13. However, substitution of the phenyl ring with either a 2′,6′-dichloro 11 or 2′,6′-dimethyl 12 substitution maintained activity compared to 13. In the 5-isopropyl series, the 1-naphthyl substituted analogue 25 was the most potent. The 2-naphthyl substituted analogue 26 was 10-fold less potent than 25. Replacing the 1-naphthyl substituent with an unsubstituted phenyl 21 or substitution of the phenyl ring with various electron donating methoxy (23, 24), methyl (20) or electron withdrawing chloro (19, 27) substitution also afforded analogues that were consider ably less potent.
Compounds 3 and 4 were selected by the National Cancer Institute (NCI) for evaluation in its in vitro preclinical antitumor screening program.29 The ability of compounds 3 and 4 to inhibit the growth of tumor cells was measured as GI50 values, the, concentration required to inhibit the growth of tumor cells in culture by 50% compared to a control. In 6 of the 60 tumor cell lines evaluated, compound 3 showed GI50 values of <1 × 10−6 M (Table 3). While in only 2 of the 60 tumor cell lines evaluated, compound 4 showed GI50 values of <1 × 10−6 M. It is noteworthy that compound 3 was not a general cell poison but showed selectivity both within a type of tumor cell line and across different tumor cell lines, with inhibitory values which in some instances differed by 10000-fold. In the melanoma LOX IMVI cell line and the renal cancer cell line 786-0, compound 3 displayed GI50 values of ≤1 × 10−8 M. It can be seen from the tumor cell growth inhibitory activity (Table 3) of compounds 1-4 that the tumor cell growth inhibitory potency, in certain instances, were more potent than either their human DHFR and/or human TS inhibitory activity alone (Table 1) and could be the result of a synergistic effect of dual inhibitory activities against TS and DHFR and/or that poly-glutamylation increases inhibitory activity against TS and/or DHFR in tumor cell systems. Against the outgrowth of tumor cells in culture compound, 2 was in general the most potent compound followed by 3 then 1, and the least potent is com pound 4. Though a strict structure-activity relationship cannot be considered for tumor cells in culture the 5-ethyl is clearly superior to a methyl, propyl or isopropyl.
In summary, homologation of a 5-methyl (compound 1) to a 5-propyl (compound 3) or 5-isopropyl (compound 4) in 2,4-diamino-6-thiobenzoyl-5-alkylpyrrolo[2,3-d]pyrimidines in creases the human DHFR inhibitory activity but is detrimental to the human TS inhibitory activity. We have found that in classical N-[4-[(2,4-diamino-5-alkyl-7H-pyrrolo[2,3-d]pyrimi-din-6-yl)-thio]-benzoyl]-l-glutamic acid containing analogues the size of the alkyl group at the 5-position dictates inhibition of TS and/or DHFR activity as well as tumor cell growth inhibitory activity. The fact that homologated 5-alkyl substit-uents such as ethyl, propyl, and isopropyl are not tolerated by human TS indicates that homologation of the 5-alkyl group beyond a methyl is not conducive for dual human TS-DHFR inhibition in classical 5-alkyl-6-arylthiosubstituted pyrrolo[2,3-d]pyrimidines. Homologation however maintains dual DHFR-TS inhibitory activity against the bifunctional enzyme derived from T. gondii. In the nonclassical series, homologation of the 5-alkyl group is highly conducive for potent inhibition of P. carinii DHFR; however, it does not improve the selectivity of the analogues. Homologation of the 5-alkyl group to a propyl or isopropyl is highly conducive for potent and selective inhibition of T. gondii DHFR compared to human DHFR. The size of the alkyl group present at the 5-position of the pyrrolo[2,3-d]pyrimidine ring system along with the nature of the lipophilic substituents present on the 6-arylthio side chain determines the potency and selectivity against T. gondii DHFR and M. aνium DHFR.
Experimental Section
All evaporations were carried out in vacuum with a rotary evaporator. Analytical samples were dried in vacuum (0.2 mmHg) in an Abderhalden drying apparatus over P2O5 at 70 °C. Thin-layer chromatography (TLC) was performed on silica gel plates (What man 250 μM PE SiLG/UV) with fluorescent indicator. Spots were visualized by UV light (254 and 365 nm) or by staining with a solution of KMnO4 in EtOH. All analytical samples were homo geneous on TLC in at least two different solvent systems. Purification by column and flash chromatography was carried out using Merck silica gel 60 (200-400 mesh). The amount (weight) of silica gel for column chromatography was in the range of 50-100 times the amount (weight) of the crude compounds being separated.
Columns were dry-packed unless specified otherwise. Solvent systems are reported as volume percent of mixture. Melting points were determined on a Mel-Temp II melting point apparatus and are uncorrected. Proton nuclear magnetic resonance (1H NMR) spectra were recorded on a Bruker WH-300 (300 MHz) spectrom eter. The chemical shift (δ) values are reported as parts per million (ppm) relative to tetramethylsilane as internal standard; s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet, br = broad singlet. Elemental analyses were performed by Atlantic Microlab, Inc., Norcross, GA. Elemental compositions were within ± 0.4% of the calculated values. Fractional moles of water or organic solvents frequently found in some analytical samples of antifolates could not be removed despite 24 h of drying in vacuum and were confirmed, where possible, by their presence in the 1H NMR spectrum. High-resolution mass spectra (HRMS), using electron impact (EI), were recorded on a VG Autospec (Fisons Instruments) micromass (EBE Geometry) double-focusing mass spectrometer. All solvents and chemicals were used as received.
1-Hydroxy-2-pentanone (29)
To a solution of 1,2 pentanediol, 28 (2.1 g, 20 mmol) and hexabutyldistannoxane (HBD) (15.5 g, 26 mmol) in anhydrous CH2Cl2 (100 mL), Br2 (4.16 g, 26 mmol) solution in CH2Cl2 (10 mL) was added dropwise at room temper ature with stirring under N2 atmosphere. The mixture was stirred for 3 h at room temperature. The solvent was evaporated under reduced pressure, and the resulting oil was distilled under reduced pressure to give 29 as a colorless oil, bp = 68-70° (16 mmHg) [lit.28 bp = 70° (20 mmHg)].
2-Amino-4-propyl-furan-3-carbonitrile (30)
A mixture of malonodinitrile (2.65 g, 40 mmol) and N(C2H5)3 (5.58 mL, 40 mmol) in anhydrous MeOH (120 mL) was added dropwise to a solution of the α-hydroxy ketone 29 (4 g, 40 mmol) in MeOH, and the resulting dark-red solution was stirred at room temperature for 24 h. To this solution was added silica gel (10 g), and the solvent was evaporated to dryness under reduced pressure to afford a dry silica gel plug, which was loaded on top of a wet (hexane) silica gel column and eluted first with hexane and then with 2:1 hexane/EtOAc to afford 1.88 g (60%) of the furan 30 as a red-cream solid: mp 58.8-62.5 °C; TLC Rf = 0.51 (hexane/EtOAc, 2:1). 1H NMR (DMSO-d6): δ 0.85-0.94 (t, 3 H, 4-CH2CH2CH3), 1.47-1.56 (m, 2 H, 4-CH2CH2CH3), 2.21 - 2.26 (t, 2 H, 4-CH2CH2CH3), 6.74 (s, 1 H, 5-CH), 7.20 (s, 2 H, 2-NH2). Anal. calcd for (C8H10N2O) C, H, N.
2,4-Diamino-5-propyl-7H-pyrrolo[2,3-d]pyrimidine (31)
Amino nitrile furan 30 (1.8 g, 12 mmol) was added to a solution of guanidine hydrochloride (2.5 g, 26 mmol) and NaOMe (1.4 g, 26 mmol) in anhydrous EtOH (100 mL). The resulting dark-red solution was stirred under reflux overnight, during which time it became dark brown. To this solution was added silica gel (5 g), and the solvent was evaporated to dryness under reduced pressure to afford a dry silica gel plug, which was loaded on top of a wet (CHCl3) column and eluted first with CHCl3 and then with a gradient of 1-5% MeOH in CHCl3 to afford 1.15 g (50%) of 31 as a dark-brown solid: mp 215-220 °C; TLC Rf = 0.41 (CHCl3/MeOH, 5:1, with 2 drops of conc NH4OH). 1H NMR (DMSO-d6): δ 0.83-0.89 (t, 3 H, 5-CH2CH2CH3), 1.50-1.60 (m, 2 H, CH2CH2CH3), 2.57-2.62 (t, 2 H, CH2CH2CH3), 5.36 (s, 2 H, 2/4-NH2), 5.91 (s, 2 H, 2/4-NH2), 6.38 (s, 1 H, 6-CH), 10.36 (s, 1 H, 7-NH). Anal. calcd for (C9H13N5·0.1H2O) C, H, N.
Ethyl 4-[2,4-Diamino-5-propyl-7H-pyrrolo[2,3-d]pyrimidin-6-yl)sulfanyl]benzoate (32)
To a suspension of 31 (1.1 g, 5.7 mmol) in a mixture of EtOH/H2O (2:1, 75 mL) was added diethyl 4,4′-dithiobis(benzoate) (2.2 g, 6 mmol) and the suspension was heated to 100-110 °C, then I2 (3 g, 12 mmol) was added and the reaction was monitored (TLC) for completion (3 h). To this solution was added excess sodium thiosulfate and the solution was evapo rated to dryness under reduced pressure and the resulting residue was washed with water and air-dried. This residue was then dissolved in MeOH (100 mL) and to this was added silica gel (15 g) and the resulting suspension was evaporated to dryness under reduced pressure to afford a dry silica gel plug that was loaded on top of a wet silica gel (CHCl3) column and eluted first with CHCl3, and then with a gradient of 1-5% MeOH in CHCl3. Fractions containing the desired spot (TLC) were pooled and evaporated to dryness to afford 610 mg (28%) of 32 as a white solid: mp = 260.6-261 °C; TLC Rf = 0.54 (CHCl3/MeOH, 5:1). 1H NMR (DMSO-d6): δ 0.82 (t, 3 H, CH2CH2CH3), 1.29 (t, 3 H, CH2CH3), 1.41 - 1.43 (m, 2 H, CH2CH2CH3), 2.76 (t, 2 H, CH2CH2CH3), 4.27-4.29 (q, 2 H, CH2CH3), 7.09 (s, 2 H, 2/4-NH2), 7.74 (s, 2 H, 2/4-NH2), 7.13-7.16 (d, 2 H, C6H4), 7.84-7.87 (d, 2 H, C6H4), 12.08 (s, 1 H, 7NH). Anal. calcd for (C18H21N5O2S) C, H, N, S.
4-[2,4-Diamino-5-propyl-7H-pyrrolo[2,3-d]pyrimidin-6-yl)sul-fanyl]benzoic acid (33)
To a suspension of 32 (330 mg, 0.9 mmol) in EtOH (30 mL) was added aqueous 1N NaOH (12 mL) and the reaction mixture was stirred at 80 °C for 24 h. At this time, TLC indicated the disappearance of the starting ester at Rf = 0.54 (CHCl3/MeOH, 5:l) and formation of one major spot at the origin. The solvent was evaporated to dryness under reduced pressure, and the resulting sodium salt (yellow oil) was dissolved in water (15 mL) and carefully acidified to pH 4 by dropwise addition of 3N HCl. The resulting suspension was filtered and washed carefully with cold water and dried over P2O5 to afford 295 mg (83%) of 33 as a white solid. This was directly used in the next step without further characterization.
Diethyl N-[4-[(2,4-Diamino-5-propyl-7H-pyrrolo[2,3-d]pyri-midin-6-yl)sulfanyl]-benzoyl]-l-glutamate (34)
To a suspension of the acid 33 (344 mg, 1 mmol) in anhydrous DMF (15 mL) under N2 was added N-methyl morpholine (145 μL, 1.33 mmol) and the resulting suspension was cooled to 0 °C. At this point, 2-chloro-4,6-dimethoxy-1,3,5-benzotriazine (235 mg, 1.33 mmol) was added and the suspension was stirred for 2 h at 0 °C; during this time, it formed a solution. The reaction mixture was again cooled to 0 °C and diethyl-l-glutamic acid (317 mg, 1.33 mmol) was added followed by N-methyl morpholine (145 μL, 1.33 mmol). The solution was slowly allowed to warm to room temperature with stirring and left at room temperature for a total of 24 h. At this time, TLC indicated the formation of one major spot at Rf = 0.58 (CHCl3/MeOH, 5:l). To the resulting solution was added silica gel (5 g), and the DMF was evaporated to dryness at room temperature using an oil pump. The silica gel plug was loaded on a wet (CHCl3) silica gel column and eluted with a gradient of 1-3% MeOH in CHCl3. Fractions containing the desired spot (TLC) were pooled and evaporated to dryness under vacuum to afford 330 mg (62%) of 34 as a white solid: mp 260-260.5 °C; TLC R f = 0.58 (CHCl3/MeOH, 5:1). 1H NMR (DMSO-d6): δ 0.80-0.84 (t, 3 H, CH2CH2CH3), 1.13-1.19 (m, 6 H, CH2CH3), 1.43 - 1.45 (m, 2 H, CH2CH2CH3), 1.98-2.09 (m, 2 H, Glu β-CH2), 2.42-2.50 (t, 2 H, Glu γ-CH2), 2.72 (t, 2 H, CH2CH2CH3), 4.00-4.10 (m, 4 H, CH2CH3), 4.40 (m, 1 H, Glu α-CH), 5.64 (s, 2 H, 2/4-NH2), 6.22 (s, 2 H, 2/4-NH2), 7.04-7.07 (d, 2 H, C6H4), 7.75-7.77 (d, 2 H, C6H4), 8.64-8.67 (d, 1 H, CONH), 11.06 (s, 1 H, 7-NH). Anal. calcd for (C25H32N6SO5) C, H, N, S.
N-[4-[(2,4-Diamino-5-propyl-7H-pyrrolo[2,3-d]pyrimidin-6-yl)sulfanyl]benzoyl]-l-glutamic Acid (3)
To a suspension of 34 (200 mg, 0.4 mmol) in EtOH (15 mL) was added 1N NaOH (6 mL) at 0 °C and the resulting suspension was stirred at 0 °C (4 h) and then at room temperature for 24 h. At this point, TLC showed the disappearance of the starting ester at Rf =0.58 (CHCl3/MeOH, 5:l) and formation of one major spot at the origin. The solvent was evaporated to dryness under reduced pressure, and the sodium salt (yellow oil) was dissolved in water (5 mL) and the solution was cooled in an ice bath and acidified carefully to pH 4.0 with dropwise addition of 3N HCl. The resulting suspension was frozen using dry ice/acetone and the reaction flask was kept at 5 °C for 24 h and filtered. The residue was washed carefully with cold water and dried over P2O5 to afford 160 mg (86%) of 3 as a white solid: mp 259.5-260 °C. 1H NMR (DMSO-d6): δ 0.74-0.82 (t, 3 H, CH2CH2CH3), 1.43 - 1.45 (m, 2 H, CH2CH2CH3), 1.93-2.08 (m, 2 H, Glu β-CH2), 2.51 (t, 2 H, Glu γ-CH2), 2.99 (t, 2 H, CH2CH2CH3), 4.37 (m, 1 H, Glu α-CH), 5.67 (s, 2 H, 2/4-NH2), 6.26 (s, 2 H, 2/4-NH2), 7.04-7.07 (d, 2 H, C6H4), 7.76-7.78 (d, 2, H, C6H4), 8.51-8.53 (d, 1 H, CONH), 11.09 (s, 1 H, 7-NH), 12.37 (bs, 2 H, COOH). Anal. calcd for (C21H24N6SO5 · 1.0 H2O) C, H, N, S.
1-Hydroxy-3-methyl-2-butanone (36)
In a 1000 mL flask were placed paraformaldehyde (9.45 g, 0.3 mol), 3-ethylbenzothiazolium bromide (7.32 g, 0.03 mol), isobutraldehyde, 35, (27.5 mL, 0.3 mol), anhydrous EtOH (300 mL), and Et3N (4.2 mL, 0.03 mol), and then dry N2 gas was bubbled into the reaction mixture. The mixture was then heated in an oil bath at 60 °C for 72 h, during which time the color of the reaction mixture changed to dark reddish-brown. The solvent was evaporated to dryness under reduced pressure, and to the resulting residue was added EtOAc (20 mL). The resulting suspension was filtered, and the solid was washed repeatedly with EtOAc. The filtrate was evaporated under reduced pressure to a dark-brown oil, which was distilled under low pressure to afford 10.7 g (35%) of 36 as a colorless oil: bp = 65-68 °C (16 mmHg) [lit.28 bp = 65 °C (20 mmHg)]. 1H NMR (CDCl3-d): δ 1.15 - 1.17 (d, 6 H, CH(CH3)2), 2.60-2.69 (m, 1 H, CH(CH3)2), 3.13 (bs, 1 H, OH), 4.32 (s, 2 H, CH2).
2-Amino-4-isopropyl-furan-3-carbonitrile (37)
A mixture of malonodinitrile (12.55 g, 190 mmol) and Et3N (19.19 g, 190 mmol) in MeOH (220 mL) was added dropwise to a solution of the a-hydroxy ketone 36 (19.4 g, 190 mmol) in MeOH (10 mL) and the resulting solution was stirred at room temperature for 24 h. To this solution was added silica gel (50 g), and the solvent was evaporated under reduced pressure to afford a dry silica gel plug, which was loaded on top of a wet (hexane) silica gel column and eluted first with hexane and then with 2:1 hexane/EtOAc to afford the furan 37 (11.1 g, 73%) as a reddish-brown solid: mp 62-62.5 °C; TLC Rf = 0.64 (hexane/EtOAc, 2:1). 1H NMR (DMSO-d6): δ 1.13 - 1.15 (d, 6 H, CH(CH3)2), 2.59-2.65 (m, 1 H, CH(CH3)2), 6.71 (s, 1 H, 5-CH), 7.23 (s, 2 H, 2-NH2) Anal. calcd for (C8H10N2O) C, H, N.
2,4-Diamino-5-isopropyl-7H-pyrrolo[2,3-d]pyrimidine (38)
Amino nitrile furan 37 (1.5 g, 10 mmol) was added to a solution prepared from guanidine hydrochloride (1.43 g, 15 mmol) and NaOMe (0.81 g, 15 mmol) in anhydrous EtOH (100 mL). The resulting dark-red reaction mixture was stirred under reflux for 96 h, during which time it turned dark-brown. To this solution was added silica gel (15 g), and the solvent was evaporated to dryness under reduced pressure to afford a silica gel plug, which was loaded on top of a wet (CHCl3) silica gel column and eluted first with CHCl3 and then with a gradient of 1-5% MeOH in CHCl3 to give 1.15 g (60%) of 38 as a white solid: mp 221 - 222 °C; TLC Rf = 0.38 (CHCl3/MeOH, 5:1). 1H NMR (DMSO-d6): δ 1.17-1.19 (d, 6 H, CH(CH3)2), 3.17 (m, 1 H, CH(CH3)2),5.33 (s, 2 H, 2/4-NH2), 5.90 (s, 2 H, 2/4-NH2), 6.39 (s, 1 H, 6-CH), 10.35 (s, 1 H, 7-NH). Anal. calcd for (C9H13N5) C, H, N.
Ethy l4-[2,4-Diamino-5-isopropyl-7H-pyrrolo[2,3-d]pyrimi-din-6-yl)sulfanyl]-benzoate (39)
To a suspension of 38 (1.0 g, 5.2 mmol) in a mixture of EtOH/H2O (2:1, 75 mL) was added diethyl 4,4′-dithiobis(benzoate) (2.2 g, 6 mmol) and the suspension was heated to 100-110 °C, then I2 (3 g, 12 mmol) was added and the reaction was monitored for completion (3 h). To this solution was added excess sodium thiosulfate and the solution was evaporated to dryness under reduced pressure and the resulting residue was washed with water and air-dried. This residue was then dissolved in MeOH (100 mL) and to this was added silica gel (15 g), and the resulting suspension was evaporated to dryness under reduced pressure to afford a dry silica gel plug, which was loaded on top of a wet (CHCl3) silica gel column and eluted first with CHCl3 and then with a gradient of 1-5% MeOH in CHCl3. Fractions containing the desired spot (TLC) were pooled and evaporated to dryness to afford 890 mg (45%) of 39 as a white solid: mp = 263-264.7 °C; TLC Rf = 0.61 (CHCl3/MeOH, 5:1). 1H NMR (DMSO-d6): δ 1.23-1.29 (m, 9 H, CH2CH3 and CH(CH3)2), 3.33-3.41 (m, 1 H, CH(CH3)2), 4.23-4.29 (q, 2 H, CH2CH3), 5.65 (s, 2 H, 2/4-NH2), 6.15 (s, 2 H, 2/4-NH2), 7.05-7.08 (d, 2 H, C6H4), 7.82-7.84 (d, 2 H, C6H4), 11.03 (s, 1 H, 7-NH). Anal. calcd for (C18H21N5O2S·0.4H2O) C, H, N, S.
4-[2,4-Diamino-5-isopropyl-7H-pyrrolo[2,3-d]pyrimidin-6-yl)-sulfanyl]-benzoic acid (40)
To a suspension of 39 (530 mg, 1.43 mmol) in EtOH (50 mL) was added aqueous 1N NaOH (20 mL) and the reaction mixture was stirred at 80 °C for 24 h. At this point, TLC indicated the disappearance of the starting ester at Rf = 0.54 (CHCl3/MeOH, 5:l) and formation of one major spot at the origin. The solvent was evaporated to dryness, and the resulting sodium salt (yellow oil) was dissolved in water (15 mL) and carefully acidified to pH 4 by dropwise addition of 3N HCl. The resulting suspension was filtered and washed carefully with cold water and dried over P2O5 to afford 436 mg (90%) of 40 as a white solid. 1H NMR (DMSO-d6): δ 1.24-1.25 (d, 6 H, CH(CH3)2), 3.35 (m, 1 H, CH(CH3)2), 5.60 (s, 2 H, 2/4-NH2), 6.07 (s, 2 H, 2/4-NH2), 6.93-6.95 (d, 2 H, C6H4), 7.75-7.76 (d, 2 H, C6H4), 10.98 (s, 1 H, 7-NH). Anal. calcd for (C16H17N5O2S) MS (EI) calcd m/z = 343.110297; found m/z = 343.109307 (M+).
Diethyl N-[4-[(2,4-Diamino-5-isopropyl-7H-pyrrolo[2,3-d]py-rimidin-6-yl)sulfanyl]-benzoyl]-l-glutamate (41)
To a suspension of the acid 40 (300 mg, 0.87 mmol) in anhydrous DMF (25 mL) under N2 was added N-methylmorpholine (145 μL, 1.33 mmol) and the resulting suspension was cooled to 0 °C. At this point, 2-chloro-4,6-dimethoxy-1,3,5-triazine (235 mg, 1.34 mmol) was added and the suspension was stirred for 2 h, during which time it formed a solution. The reaction mixture was again cooled to 0 °C and diethyl-l-glutamate (317 mg, 1.33 mmol) was added followed by N-methylmorpholine (145 μL, 1.33 mmol). The solution was slowly allowed to warm to room temperature with stirring and left at room temperature for a total of 24 h. To the resulting solution was added silica gel (5 g) and the DMF was evaporated using an oil pump. The silica gel plug was loaded on a wet (CHCl3) silica gel column and eluted with a gradient of 1-3% MeOH in CHCl3. Fractions containing the desired spot (TLC) were pooled and evaporated to dryness under vacuum to give 330 mg (70%) of 41 as a white solid: mp 217.6-218 °C; TLC Rf = 0.53 (CHCl3/MeOH, 5:1). 1H NMR (DMSO-d6): δ 1.12-1.19 (m, 6 H, CH2CH3), 1.24-1.27 (d, 6 H, CH(CH3)2), 1.97-2.07 (m, 2 H, Glu β-CH2), 2.39-2.44 (t, 2 H, Glu γ-CH2), 3.99-4.12 (m, 4 H, CH2CH3), 4.40 (m, 1 H, Glu α-CH), 5.63 (s, 2 H, 2/4-NH2), 6.12 (s, 2 H, 2/4-NH2), 7.02-7.05 (d, 2 H, C6H4), 7.74-7.77 (d, 2 H, C6H4), 8.63-8.65 (d, 1 H, CONH), 11.01 (s, 1 H, 7-NH). Anal. calcd for (C25H32N6O5-S ·0.5H2O) C, H, N, S.
N-[4-[(2,4-Diamino-5-isopropyl-7H-pyrrolo[2,3-d]pyrimidin-6-yl)sulfanyl]-benzoyl]-l-glutamate (4)
To a suspension of 41 (200 mg, 0.37 mmol) in EtOH (15 mL) was added 1N NaOH (6 mL) and the suspension stirred at 0 °C (4 h) and then at room temperature for 24 h. The EtOH was evaporated to dryness under reduced pressure, the yellow oil was dissolved in water (5 mL), and the solution was cooled in an ice-bath and acidified carefully to pH 4.0 with dropwise addition of 3N HCl. This suspension was left at 5 °C for 24 h and filtered. The residue was washed well with water and O(C2H5)2 and then dried over P2O5/vacuum to afford 165 mg (80%) of 4 as a white solid: mp 206.5-207 °C. 1H NMR (DMSO-d6): δ 1.25 - 1.27 (d, 6 H, CH(CH3)2), 1.93-2.06 (m, 2 H, Glu β-CH2), 2.31 - 2.33 (t, 2 H, Glu γ-CH2), 4.36 (m, 1 H, Glu α-CH), 5.78 (s, 2 H, 2/4-NH2), 6.29 (s, 2 H, 2/4-NH2), 7.03-7.06 (d, 2 H, C6H4), 7.76-7.79 (d, 2 H, C6H4), 8.51-8.54 (d, 1 H, CONH), 11.13 (s, 1 H, 7-NH), 12.4 (bs, 2 H, COOH). Anal. calcd for (C21H24N6O5S· 0.2H2O· 1.8C4H10O) C, H, N, S.
2,4-Diamino-5-propyl-6-(2′,6′-dichlorophenylsulfanyl)-7H-pyrrolo[2,3-d]pyrimidine (11)
To a solution of 31 (300 mg, 1.57 mmol) in a mixture of EtOH/water (2:1, 30 mL) was added 2,6-dichlorophenylthiol (540 mg, 3.00 mmol) and the reaction mixture was heated to 100-110 °C, then I2 (750 mg, 3.00 mmol) was added and the heating continued with stirring for a total of 3 h. To this mixture was added an excess of sodium thiosulfate and the reaction mixture concentrated under reduced pressure. To the resulting residue was added silica gel (10 g) and MeOH (50 mL) and the solution evaporated to dryness under reduced pressure to afford a dry silica gel plug, which was loaded on top of a wet (CHCl3) silica gel column and eluted with a gradient of 1-3% MeOH in CHCl3. Fractions containing the desired spot (TLC) were pooled, and evaporated to dryness. The resulting residue was washed with MeOH, filtered, and dried to yield 385 mg (67%) of 11: mp 238-240 °C; TLC Rf = 0.51 (CHCl3/MeOH, 5:1, with 2 drops of NH4OH). 1H NMR (DMSO-d6): δ 0.76 (t, 3 H, 5-CH2CH2CH3), 1.15 (m, 2 H, 5-CH2CH2CH3), 2.64 (t, 2 H, 5-CH2CH2CH3), 5.54 (s, 2 H, 2/4-NH2), 6.08 (s, 2 H, 2/4-NH2), 7.32-7.35 (m, 1 H, C6H3), 7.48-7.51 (d, 2 H, C6H3), 10.93 (s, 1 H, 7-NH). Anal. calcd for (C15H15N5Cl2S·0.17CHCl3) C, H, N, Cl, S.
2,4-Diamino-5-propyl-6-(2′,6′-dimethylphenylsulfanyl)-7H-pyrrolo[2,3-d] pyrimidine (12)
Compound 12 was synthesized as described for 11 using 2,6-dimethylphenylthiol (420 mg, 3.00 mmol) and 31 (300 mg, 1.57 mmol): yield 52%; mp 227-230 °C; TLC Rf = 0.55 (CHCl3/MeOH, 5:1, with 2 drops of NH4OH). 1H NMR (DMSO-d6): δ 0.74-0.78 (t, 3 H, 5-CH2CH2CH3), 1.16-1.25 (m, 2 H, 5-CH2CH2CH3), 2.19-2.25 (t, 2 H, 5-CH2CH2CH3), 2.35 (s, 6 H, 2′,6′-diCH3), 5.46 (s, 2 H, 2/4-NH2), 6.00 (s, 2 H, 2/4-NH2), 7.07 (m, 3 H, C6H3), 10.75 (s, 1 H, 7-NH). Anal. calcd for (C17H21N5S·0.6CH3OH) C, H, N, S.
2,4-Diamino-5-propyl-6-(phenylsulfanyl)-7H-pyrrolo[2,3-d]py-rimidine (13)
Compound 13 was synthesized as described for 11 using phenylthiol (280 mg, 2.00 mmol) and 31 (200 mg, 1.04 mmol): yield 65%; mp 252.2-252.7 °C; TLC Rf = 0.53 (CHCl3/MeOH, 5:1, with 2 drops of NH4OH). 1H NMR (DMSO-d6): δ 0.81-0.86 (t, 3 H, 5-CH2CH2CH3), 1.43 - 1.45 (m, 2 H, 5-CH2CH2CH3), 2.70-2.75 (t, 2 H, 5-CH2CH2CH3), 5.59 (s, 2 H, 2/4-NH2), 6.15 (s, 2 H, 2/4-NH2), 7.00-7.02 (d, 2 H, C6H5), 7.12-7.14 (m, 1 H, C6H5), 7.24-7.29 (m, 2 H, C6H5), 10.98 (s, 1 H, 7-NH). Anal. calcd for (C15H17N5S·0.2H2O) C, H, N, S.
2,4-Diamino-5-propyl-6-(4′-methoxyphenylsulfanyl)-7H-pyr-rolo[2,3-d]pyrimidine (14)
Compound 14 was synthesized as described for 11 using 4-methoxyphenylthiol (280 mg, 2.00 mmol) and 31 (200 mg, 1.04 mmol): yield 50%; mp 247.9-248.2 °C; TLC Rf = 0.63 (CHCl3/MeOH, 5:1, with 2 drops of NH4OH). 1H NMR (DMSO-d6): δ 0.82-0.87 (t, 3 H, 5-CH2CH2CH3), 1.41 (m, 2 H, 5-CH2CH2CH3), 2.74 (t, 2 H, 5-CH2CH2CH3), 3.69 (s, 3 H, 4′-OCH3), 5.55 (s, 2 H, 2/4-NH2), 6.11 (s, 2 H, 2/4-NH2), 6.85-6.88 (d, 2 H, C6H4), 7.04-7.07 (d, 2 H, C6H4), 10.95 (s, 1 H, 7-NH). Anal. calcd for (C16H19N5OS·0.5H2O) C, H, N, S.
2,4-Diamino-5-propyl-6-(2′,5′-dimethoxyphenylsulfanyl)-7H-pyrrolo[2,3-d]pyrimidine (15)
Compound 15 was synthesized as described for 11 using 2,5-dimethoxyphenylthiol (525 mg, 3.00 mmol) and 31 (300 mg, 1.57 mmol): yield 45%; mp 217-218 °C; TLC Rf = 0.56 (CHCl3/MeOH, 5:1, with 2 drops of NH4OH). 1H NMR (DMSO-d6): δ 0.81-0.86 (t, 3 H, 5-CH2CH2CH3), 1.40-1.47 (m, 2 H, 5-CH2CH2CH3), 2.66-2.71 (t, 2 H, 5-CH2CH2CH3), 3.53 (s, 3 H, 2′/5′-OCH3), 3.80 (s, 3 H, 2′/5′-OCH3), 5.59 (s, 2 H, 2/4-NH2), 6.17 (s, 2 H, 2/4-NH2), 5.96 (s, 1 H, C6H3), 6.64-6.67 (d, 1 H, C6H3), 6.89-6.92 (d, 1 H, C6H3), 10.91 (s, 1 H, 7-NH). Anal. calcd for (C17H21N5O2S) C, H, N, S.
2,4-Diamino-5-propyl-6-(3′,4′-dimethoxyphenylsulfanyl)-7H-pyrrolo[2,3-d]pyrimidine (16)
Compound 16 was synthesized as described for 11 using 3,4-dimethoxyphenylthiol (350 mg, 2.00 mmol) and 31 (200 mg, 1.04 mmol): yield 65%; mp >230 °C (dec); TLC Rf = 0.53 (CHCl3/MeOH, 5:1, with 2 drops of NH4OH). 1H NMR (DMSO-d6): δ 0.83-0.88 (t, 3 H, 5-CH2CH2CH3), 1.43-1.45 (m, 2 H, 5-CH2CH2CH3), 2.73-2.75 (t, 2 H, 5-CH2CH2CH3), 3.68 (s, 6 H, 3′,4′-diOCH3), 5.59 (s, 2 H, 2/4-NH2), 6.15 (s, 2 H, 2/4-NH2), 6.59-6.62 (d, 1 H, C6H3), 6.82-6.88 (m, 2 H, C6H3), 10.98 (s, 1 H, 7-NH). Anal. calcd for (C17H21N5O2S·0.2CHCl3) C, H, N, S.
2,4-Diamino-5-propyl-6-(1′-napthylsulfanyl)-7H-pyrrolo[2,3-d]pyrimidine (17)
Compound 17 was synthesized as described for 11 using 1-naphthylthiol (320 mg, 2.00 mmol) and 31 (200 mg, 1.04 mmol): yield 58%; mp > 255 °C dec; TLC Rf = 0.63 (CHCl3/MeOH, 5:1, with 2 drops of NH4OH). 1H NMR (DMSO-d6): δ 0.80-0.84 (t, 3 H, 5-CH2CH2CH3), 1.43 - 1.45 (m, 2 H, 5-CH2CH2CH3), 2.73-2.75 (t, 2 H, 5-CH2CH2CH3), 5.62 (s, 2 H, 2/4-NH2), 6.19 (s, 2 H, 2/4-NH2), 6.86 (d, 1 H, C10H7), 7.37 (t, 1 H, C10H7), 7.58-7.61 (m, 3 H, C10H7), 7.70 (d, 1 H, C10H7), 7.94 (d, 1 H, C10H7), 11.05 (s, 1 H, 7-NH). Anal. calcd for (C19H19N5S) C, H, N, S.
2,4-Diamino-5-propyl-6-(2′-napthylsulfanyl)-7H-pyrrolo[2,3-d]pyrimidine (18)
Compound 18 was synthesized as described for 11 using 2-naphthylthiol (480 mg, 3.00 mmol) and 31 (300 mg, 1.57 mmol): yield 70%; mp > 250 °C (dec); TLC Rf = 0.56 (CHCl3/MeOH, 5:1, with 2 drops of NH4OH). 1H NMR (DMSO-d6): δ 0.80-0.85 (t, 3 H, 5-CH2CH2CH3), 1.42-1.49 (m, 2 H, 5-CH2CH2CH3), 2.74-2.79 (t, 2 H, 5-CH2CH2CH3), 5.60 (s, 2 H, 2/4-NH2), 6.18 (s, 2 H, 2/4-NH2), 7.17-7.20 (d, 1 H, C10H7), 7.40-7.50 (m, 3 H, C10H7), 7.73-7.76 (d, 1 H, C10H7), 7.81 (s, 1 H, C10H7), 7.84-7.85 (d, 1 H, C10H7), 11.06 (s, 1 H, 7-NH). Anal. calcd for (C19H19N5S·0.5H2O) C, H, N, S.
2,4-Diamino-5-isopropyl-6-(2′,6′-dichlorophenylsulfanyl)-7H-pyrrolo[2,3-d]pyrimidine (19)
To a solution of 38 (300 mg, 1.57 mmol) in a mixture of EtOH/water (2:1, 30 mL) was added 2,6-dichlorophenylthiol (540 mg, 3.00 mmol) and the reaction mixture was heated to 100-110 °C, then I2 (750 mg, 3.0 mmol) was added and the heating continued with stirring for a total of 2 h. To this mixture was added an excess of sodium thiosulfate and the reaction mixture concentrated under reduced pressure. To the resulting residue was added silica gel (10 g) and MeOH (50 mL) and the solution evaporated to dryness under reduced pressure to afford a dry silica gel plug, which was loaded on top of a wet (CHCl3) silica gel column and eluted with a gradient of 1-3% MeOH in CHCl3. Fractions containing the desired spot (TLC) were pooled and evaporated to dryness. The resulting residue was washed with MeOH, filtered, and dried to yield 70 mg (12%) of 19: mp 230-231 °C; TLC Rf = 0.60 (CHCl3/MeOH, 5:1, with 2 drops of NH4OH). 1H NMR (DMSO-d6): δ 1.10-1.12 (d, 6 H, CH(CH3)2), 5.54 (s, 2 H, 2/4-NH2), 5.87 (s, 2 H, 2/4-NH2), 7.31-7.33 (m, 1 H, C6H3), 7.46-7.49 (d, 2 H, C6H3), 10.90 (s, 1 H, 7-NH). Anal. calcd for (C15H15N5Cl2S·0.3H2O) C, H, N, Cl, S.
2,4-Diamino-5-isopropyl-6-(2′,6′-dimethylphenylsulfanyl)-7H-pyrrolo[2,3-d] pyrimidine (20)
Compound 20 was synthesized as described for 19 using 2,6-dimethylphenylthiol (420 mg, 3.00 mmol) and 38 (300 mg, 1.57 mmol): yield 22%; mp 248-248.3 °C; TLC Rf = 0.63 (CHCl3/MeOH, 5:1, with 2 drops of NH4OH). 1H NMR (DMSO-d6): δ 1.11 - 1.14 (d, 6 H, 5-CH(CH3)2), 2.30 (s, 6 H, 2′,6′-diCH3), 5.46 (s, 2 H, 2/4-NH2), 5.79 (s, 2 H, 2/4-NH2), 7.08 (m, 3 H, C6H3), 10.66 (s, 1 H, 7-NH). Anal. calcd for (C17H21N5S·0.2H2O) C, H, N, S.
2,4-Diamino-5-isopropyl-6-(phenylsulfanyl)-7H-pyrrolo[2,3-d]pyrimidine (21)
Compound 21 was synthesized as described for 19 using phenylthiol (330 mg, 3.00 mmol) and 38 (300 mg, 1.57 mmol) except that the compound was washed with hexane and dried. Yield: 45%; mp 228.5-229 °C; TLC Rf = 0.53 (CHCl3/MeOH, 5:1, with 2 drops of NH4OH). 1H NMR (DMSO-d6): δ 1.25 - 1.27 (d, 6 H, 5-CH(CH3)2), 5.39 (bs, 2 H, 2/4-NH2), 5.89 (bs, 2 H, 2/4-NH2), 6.97-7.29 (m, 5 H, C6H5), 10.97 (s, 1 H, 7-NH). Anal. calcd for (C15H17N5S·0.1C6H14) C, H, N, S.
2,4-Diamino-5-isopropyl-6-(4′-methoxyphenylsulfanyl)-7H-pyrrolo[2,3-d]pyrimidine (22)
Compound 22 was synthesized as described for 19 using 4-methoxyphenylthiol (280 mg, 2.00 mmol) and 38 (200 mg, 1.04 mmol): yield 40%; mp 288-289 °C; TLC Rf = 0.58 (CHCl3/MeOH, 5:1, with 2 drops of NH4OH). 1H NMR (DMSO-d6): δ 1.26-1.28 (d, 6 H, 5-CH(CH3)2), 3.41-3.48 (m, 1 H, 5-CH(CH3)2), 3.70 (s, 3 H, 4-OCH3), 5.56 (bs, 2 H, 2/4-NH2), 6.02 (bs, 2 H, 2/4-NH2), 6.86-6.89 (d, 2 H, C6H4), 7.02-7.04 (d, 2 H, C6H4), 10.93 (s, 1 H, 7-NH). Anal. calcd for (C16H19N5-SO·0.1H2O) C, H, N, S.
2,4-Diamino-5-isopropyl-6-(2′,5′-dimethoxyphenylsulfanyl)-7H-pyrrolo[2,3-d]pyrimidine (23)
Compound 23 was synthesized as described for 19 using 2,5-dimethoxyphenylthiol (800 mg, 4.00 mmol) and 38 (350 mg, 2.00 mmol): yield 53%; mp 243-244 °C; TLC Rf = 0.60 (CHCl3/MeOH, 5:1, with 2 drops of NH4OH). 1H NMR (DMSO-d6): δ 1.24-1.26 (d, 6 H, 5-CH(CH3)2), 3.53 (s, 3 H, 2′/5′-OCH3), 3.80 (s, 3 H, 2′/5′-OCH3), 5.59 (bs, 2 H, 2/4-NH2), 5.93 (s, 1 H, C6H3), 6.07 (bs, 2 H, 2/4-NH2), 6.63-6.66 (d, 1 H, C6H3), 6.89-6.92 (d, 1 H, C6H3), 10.87 (s, 1 H, 7-NH). Anal. calcd for (C17H21N5SO2) C, H, N, S.
2,4-Diamino-5-isopropyl-6-(3′,4′-dimethoxyphenylsulfanyl)-7H-pyrrolo[2,3-d]pyrimidine (24)
Compound 24 was synthesized as described for 19 using 3,4-dimethoxyphenylthiol (520 mg, 3.00 mmol) and 38 (300 mg, 1.57 mmol): yield 58%; mp 293-293.5 °C; TLC Rf = 0.58 (CHCl3/MeOH, 5:1, with 2 drops of NH4OH). 1H NMR (DMSO-d6): δ 1.27-1.29 (d, 6 H, 5-CH(CH3)2), 3.67 (s, 3 H, 3′/4′-OCH3), 3.69 (s, 3 H, 3′/4′-OCH3), 5.56 (bs, 2 H, 2/4-NH2), 6.01 (bs, 2 H, 2/4-NH2), 6.56-6.59 (d, 1 H, C6H3), 6.79 (s, 1 H, C6H3), 6.87-6.89 (d, 1 H, C6H3), 10.93 (s, 1 H, 7-NH). Anal. calcd for (C17H21N5SO2 ·0.5H2O) C, H, N, S.
2,4-Diamino-5-isopropyl-6-(1′-napthylsulfanyl)-7H-pyrrolo[2,3-d]pyrimidine (25)
Compound 25 was synthesized as described for 19 using 1-naphthylthiol (320 mg, 2.00 mmol) and 38 (200 mg, 1.04 mmol): yield 45%; mp 267-267.5 °C; TLC Rf= 0.60 (CHCl3/MeOH, 5:1, with 2 drops of NH4OH). 1H NMR (DMSO-d6): δ 1.26-1.28 (d, 6 H, 5-CH(CH3)2), 5.63 (s, 2 H, 2/4-NH2), 6.11 (s, 2 H, 2/4-NH2), 6.76-6.78 (d, 1 H, C10H7), 7.37 (t, 1 H, C10H7), 7.59-7.72 (m, 3 H, C10H7), 7.94 (d, 1 H, C10H7), 8.2 (d, 1 H, C10H7), 11.02 (s, 1 H, 7-NH). Anal. calcd for (C19H19N5S) C, H, N, S.
2,4-Diamino-5-isopropyl-6-(2′-napthylsulfanyl)-7H-pyrrolo[2,3-d]pyrimidine (26)
Compound 26 was synthesized as described for 19 using 2-naphthylthiol (640 mg, 4.00 mmol) and 38 (350 mg, 1.83 mmol): yield 40%; mp 247-247.5 °C; TLC Rf = 0.58 (CHCl3/MeOH, 5:1, with 2 drops of NH4OH). 1H NMR (DMSO-d 6): δ 1.27-1.29 (d, 6 H, 5-CH(CH3)2), 5.63 (s, 2 H, 2/4-NH2), 6.12 (s, 2 H, 2/4-NH2), 7.15-7.18 (d, 1 H, C10H7), 7.44-7.47 (m, 3 H, C10H7), 7.74-7.76 (d, 1 H, C10H7), 7.82-7.85 (d, 2 H, C10H7), 11.05 (s, 1 H, 7-NH). Anal. calcd for (C19H19N5S) C, H, N, S.
2,4-Diamino-5-isopropyl-6-(3′,4′-dichlorophenylsulfanyl)-7H-pyrrolo[2,3-d]pyrimidine (27)
Compound 27 was synthesized as described for 19 using 3,4-dichlorophenylthiol (540 mg, 3.00 mmol) and 38 (300 mg, 1.57 mmol): yield 37%; mp 244-244.5 °C; TLC Rf = 0.58 (CHCl3/MeOH, 5:1, with 2 drops of NH4OH). 1H NMR (DMSO-d6): δ 1.24-1.27 (d, 6 H, 5-CH(CH3)2), 5.67 (bs, 2 H, 2/4-NH2), 6.17 (bs, 2 H, 2/4-NH2), 6.96 (s, 2 H, C6H3), 7.37 (s, 1 H, C6H3), 11.04 (s, 1 H, 7-NH). Anal. calcd for (C15H15N5SCl2) C, H, N, S, Cl.
Supplementary Material
Acknowledgments
This work was supported in part by the National Institutes of Health, grants AI41743 (A.G.); AI44661 (A.G.), CA89300 (A.G.), and AI47759 (A.G.) from the National Institute of Allergy and Infectious Diseases.
Footnotes
Supporting Information Available: Elemental analysis. This material is available free of charge via the Internet at http://pubs.acs.org.
Abbreviations: T. gondii, Toxoplasma gondii; DHFR, Dihydrofolatereductase; TS, thymidylate synthase; P. jiroVecii, Pneumocystis jiroVecii; M. avium, Mycobacterium avium; MTX, methotrexate; TMQ, trimetrexate; TMP, trimethoprim; DHPS, dihydropteroate synthase; FPGS, folyl poly-?-glutamate synthetase.
References
- 1.(a) Taken in part from the dissertation submitted by H J to the Graduate School of Pharmaceutical Sciences, Duquesne University, in partial fulfillment of the requirements for the degree of Ph. D. Jul, 2006. [Google Scholar]; (b) Presented in part at the 229th American Chemical Society National Meeting; San Diego, CA. Mar, 2005. pp. 13–17. Abstract MEDI-491. [Google Scholar]; (c) Presented in part at the 13th International Symposium on Chemistry and Biology of Pteridines and Folates, Egmond aan Zee; The Netherlands. Jun, 2005. pp. 20–24. Oral Presentation O04-02. [Google Scholar]; (d) Presented in part at the 13th International Symposium on Chemistry and biology of Pteridines and Folates, Egmond aan Zee; The Netherlands. Jun, 2005. pp. 20–24. Poster number P-09. [Google Scholar]; (e) Presented in part at the 231st American Chemical Society National Meeting; Atlanta, GA. Mar, 2006. pp. 26–30. Abstr MEDI-348. [Google Scholar]
- 2.Schnell JR, Dyson HJ, Wright PE. Structure, Dynamics, and Catalytic Function of Dihydrofolate Reductase. Annu Reν Biophys Biomol Struct. 2004;33:119–140. doi: 10.1146/annurev.biophys.33.110502.133613. [DOI] [PubMed] [Google Scholar]
- 3.Willemot P, Klein MB. Prevention of HIV-associated Opportunistic Infections and Diseases in the Age of Highly Active Antiretroviral Therapy. Expert Reν Anti-Infect Ther. 2004;2:521–532. doi: 10.1586/14787210.2.4.521. [DOI] [PubMed] [Google Scholar]
- 4.Klepser ME, Klepser TB. Drug Treatment of HIV-related Opportunistic Infections. Drugs. 1997;53:40–73. doi: 10.2165/00003495-199753010-00004. [DOI] [PubMed] [Google Scholar]
- 5.Takimoto CH. Antifolates in Clinical Development. Semin Oncol. 1997;24:S18-40–S18-51. [PubMed] [Google Scholar]
- 6.Bertino JR, Kamen B, Romanini A. Folate Antagonists. In: Holland JF, Frei E, Bast RC, Kufe DW, Morton DL, Weichselbaum RR, editors. Cancer Medicine. Vol. 1. Williams & Wilkins; Baltimore, MD: 1997. pp. 907–921. [Google Scholar]
- 7.Jackman AL, Taylor GA, Gibson W, Kimbell R, Brown M, Calvert AH, Judson IR, Hughes LR. ICI D1694 A Quinazoline Antifolate Thymidylate Synthase Inhibitor That is a Potent Inhibitor of L1210 Tumour Cell Growth in Vitro and in Vivo: A New Agent for Clinical Study. Cancer Res. 1991;51:5579–5586. [PubMed] [Google Scholar]
- 8.Taylor EC, Kuhnt D, Shih C, Rinzel SM, Grindey GB, Barredo J, Jannatipour M, Moran R. A Dideazatetrahydrofolate Analogue Lacking a Chiral Center at C-6, N-[4-[2-(2-Amino-3,4-dihydro-4-oxo-7H-pyrrolo[2,3-d]pyrimidin-5-y1)ethylbenzoyl]-l-glutam-ic Acid, Is an Inhibitor of Thymidylate Synthase. J Med Chem. 1992;35:4450–4454. doi: 10.1021/jm00101a023. [DOI] [PubMed] [Google Scholar]
- 9.Podzamczer D, Salazar A, Jimenez J, Consiglio E, Santin M, Casanova A, Rufi G, Gudiol F. Intermittent Trimethoprim-Sulfamethoxazole compared with Dapsone-Pyrimethamine for the Simultaneous Primary Prophylaxis of Pneumocystis Pneumonia and Toxoplasmosis in Patients Infected with HIV. Ann Intern Med. 1995;122:755–761. doi: 10.7326/0003-4819-122-10-199505150-00004. [DOI] [PubMed] [Google Scholar]
- 10.Roudier C, Caumes E, Rogeaux O, Bricaire F, Gentilini M. Adverse Cutaneous Reactions to Trimethoprim-Sulfamethoxazole in Patients with the Acquired Immunodeficiency Syndrome and Pneu-mocystis carinii Pneumonia. Arch Dermatol. 1994;130:1383–1386. [PubMed] [Google Scholar]
- 11.News. FDA Approve Trimetrexate as Second line Therapy of Pneumocystis carinii Pneumonia. Am J Hosp Pharm. 1994;51:591–591. [PubMed] [Google Scholar]
- 12.Gangjee A, Lin X, Kisliuk RL, McGuire JJ. Synthesis of N-{4-[(2,4-Diamino-5-methyl-4,7-dihydro-3H-pyrrolo[2,3-d]pyrimidin-6-yl)thio]benzoyl}-l-glutamic Acid and N-{4-[(2-Amino-4-oxo-5-methyl-4,7-dihydro-3H-pyrrolo[2,3-d]pyrimidin-6-yl)thio]benzoyl}-l-glutamic Acid as Dual Inhibitors of Dihydrofolate Reductase and Thymidylate Synthase and as Potential Antitumor Agents. J Med Chem. 2005;48:7215–7222. doi: 10.1021/jm058234m. [DOI] [PubMed] [Google Scholar]
- 13.Tripos Inc, 1699 South Hanley Road, St. Louis, MO, 63144.
- 14.Gangjee A, Zeng Y, Talreja T, McGuire JJ, Kisliuk RL, Queener SF. Design and Synthesis of Classical and Nonclassical 6-Arylthio-2,4-diamino-5-ethylpyrrolo[2,3-d]pyrimidines as Anti-folates. J Med Chem. 2007;50:3046–3053. doi: 10.1021/jm070165j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gangjee A, Lin X, Queener SF. Design, Synthesis, and Biological Evaluation of 2,4-Diamino-5-methyl-6-substituted-pyrrolo[2,3-d]py-rimidines as Dihydrofolate Reductase Inhibitors. J Med Chem. 2004;47:3689–3692. doi: 10.1021/jm0306327. [DOI] [PubMed] [Google Scholar]
- 16.Cody V, Wojtczak A, Kalman TI, Friesheim JH, Blakley RL. Conformational Analysis of Human Dihydrofolate Reductase Inhibitor Complexes: Crystal Structure Determination of Wild Type and F31 Mutant Binary and Ternary Inhibitor Complexes. Adν Exp Med Biol. 1993;338:481–486. doi: 10.1007/978-1-4615-2960-6_97. [DOI] [PubMed] [Google Scholar]
- 17.Champness JN, Achari A, Ballantine SP, Bryant PK, Delves CJ, Stammers DK. The Structure of Pneumocystis carinii Dihydrofolate Reductase to 1.9 Å Resolution. Structure. 1994;2:915–924. doi: 10.1016/s0969-2126(94)00093-x. [DOI] [PubMed] [Google Scholar]
- 18.Roos DS. Primary Structure of the Dihydrofolate Reductase-Thymidylate Synthase, ene from Toxoplasma gondii. J Biol Chem. 1993;268:6269–6280. [PubMed] [Google Scholar]
- 19.Ginkel SZ, Dooley TP, Suling WJ, Barrow WW. Identification and Cloning of the Mycobacterium aνium FolA Gene, Required for Dihydrofolate Reductase Activity. FEMS Microbiol Lett. 1997;156:69–78. doi: 10.1111/j.1574-6968.1997.tb12707.x. [DOI] [PubMed] [Google Scholar]
- 20.Rosowsky A, Mota CE, Wright JE, Freisheim JH, Huesner JJ, McCormack JJ, Queener SF. 2,4-Diaminothieno[2,3-d]pyrimidine Analogs of Trimetrexate and Piritrexim as Potential Inhibitors of Pneumocystis carinii and Toxoplasma gondii Dihydro-folate Reductase. J Med Chem. 1993;36:3103–3112. doi: 10.1021/jm00073a009. [DOI] [PubMed] [Google Scholar]
- 21.Taylor EC, Patel HH, Jun JG. A One-Step Ring Transformation/Ring Annulation Approach to Pyrrolo[2,3-d]pyrimidines. A New Synthesis of the Potent Dihydrofolate Reductase Inhibitor TNP-351. J Org Chem. 1995;60:6684–6687. [Google Scholar]
- 22.Gangjee A, Ye Z, Queener SF. Synthesis of Three Carbon Atom Bridged 2,4-Diaminopyrrolo[2,3-d]pyrimidines as Nonclassical Di-hydrofolate Reductase Inhibitors. J Heterocycl Chem. 2005;42:1127–1133. [Google Scholar]
- 23.Rosowsky A, Chen H, Fu H, Queener SF. Synthesis of New 2,4-Diaminopyrido[2,3-d]pyrimidine and 2,4-Diaminopyrrolo[2,3-d]pyrimidine Inhibitors of Pneumocystis carinii, Toxoplasma gondii, and Mycobacterium aνium Dihydrofolate Reductase. Bioorg Med Chem. 2003;11:59–67. doi: 10.1016/s0968-0896(02)00325-5. [DOI] [PubMed] [Google Scholar]
- 24.Rosowsky A, Fu H, Queener SF. Synthesis of New 2,4-Diamino-7H-pyrrolo[2,3-d]pyrimidines via the Taylor Ring Transformation/Ring Annulation Strategy. J Heterocycl Chem. 2001;38:1197–1202. [Google Scholar]
- 25.Ueno Y, Okawara M. Oxidation Using Distannoxane I Selective Oxidation of Alcohols. Tetrahedron Lett. 1976;50:4597–4600. [Google Scholar]
- 26.Gangjee A, Jain HD, McGuire JJ, Kisliuk RL. Benzoyl Ring Halogenated Classical 2-Amino-6-methyl-3,4-dihydro-4-oxo-5-sub-stituted Thiobenzoyl-7H-pyrrolo[2,3-d]pyrimidine Antifolates as In hibitors of Thymidylate Synthase and as Antitumor Agents. J Med Chem. 2004;47:6730–6739. doi: 10.1021/jm040144e. [DOI] [PubMed] [Google Scholar]
- 27.Gangjee A, Jain HD, Phan J, Lin X, Song X, McGuire JJ, Kisliuk RL. Dual Inhibitors of Thymidylate Synthase and Dihydro-folate Reductase as Antitumor Agents: Design, Synthesis, and Biologi cal Evaluation of Classical and Nonclassical Pyrrolo[2,3-d]pyrimidine Antifolates. J Med Chem. 2006;49:1055–1065. doi: 10.1021/jm058276a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsumoto T, Ohishi M, Inoue S. Selective Cross-Acyloin Condensation Catalyzed by Thiazolium Salt Formation of 1-Hydroxy-, 2-one from Formaldehyde and Other Aldehydes. J Org Chem. 1985;50:603–606. [Google Scholar]
- 29.We thank the Developmental Therapeutics Program of the National Cancer Institute for performing the in vitro anticancer evaluation.
- 30.Cody V, Luft JR, Pangborn W. Understanding the Role of Leu22 Variants in Methotrexate Resistance: Comparison of Wild-Type and Leu22Arg Variant Mouse and Human Dihydrofolate Reductase Ternary Crystal Complexes with Methotrexate and NADPH. Acta Crystallogr, Sect D: Biol Crystallogr. 2005;61:147–155. doi: 10.1107/S0907444904030422. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.