Abstract
Gefitinib is an epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) of potential use in patients with breast cancer. Unfortunately, in clinical studies, gefitinib is often ineffective indicating that resistance to EGFR inhibitors may be a common occurrence in cancer of the breast. EGFR has been shown to be overexpressed in breast cancer, and in particular remains hyperphosphorylated in cell lines such as MDA-MB-468 that are resistant to EGFR inhibitors. Here, we investigate the cause of this sustained phosphorylation and the molecular basis for the ineffectiveness of gefitinib. We show that reactive oxygen species (ROS), known to damage cellular macromolecules and to modulate signaling cascades in a variety of human diseases including cancers, appear to play a critical role in mediating EGFR TKI-resistance. Furthermore, elimination of these ROS through use of a cell-penetrating catalase derivative sensitizes the cells to gefitinib. These results suggest a new approach for the treatment of TKI-resistant breast cancer patients specifically, the targeting of ROS and attendant downstream oxidative stress and their effects on signaling cascades.
Keywords: Breast cancer, Reactive oxygen species, Tyrosine kinase inhibitors, Epidermal growth factor receptor, Tyrosine kinase
Introduction
Epidermal growth factor receptors (EGFR) are part of the ErbB family (or epidermal growth factor family) composed of receptor tyrosine kinases [1,2]. EGFR is activated by binding of its ligand, epidermal growth factor (EGF), leading to dimerization of the receptor and stimulation of intracellular kinase activity. Upon dimerization, autophosphorylation occurs, eliciting downstream signaling cascades involved in cell survival and increased cellular proliferation [1,2]. In some breast cancers, EGFR is overexpressed, although tyrosine kinase inhibitors targeting EGFR (EGFR TKIs) have yet to demonstrate uniform efficacy in the clinic [3,4]. Interestingly, in breast cancer cell lines resistant to EGFR TKIs, EGFR-phosphorylation is often maintained at high levels in the presence of the TKI [5], indicating that EGFR may continue to signal to promote cell growth and survival. This is supported by evidence that targeted knockdown of EGFR expression can decrease growth and survival of EGFR TKI-resistant breast cancer cells [6].
Mechanisms of EGFR inhibitor resistance in all cancers are the focus of many researchers and specific insights have been realized [7]. The most common mutation known to occur in EGFR is a truncation resulting in a deletion of exons 2–7; the resultant molecule is known as EGFRvIII. Importantly, this mutation occurs in 86% of gliomas and is thought to mediate resistance to EGFR inhibitors [8]. Resistance mechanisms involve upregulation of parallel signaling tyrosine kinase receptors including insulin like growth factor receptor (IGF-1R), vascular endothelial growth factor (VEGFR), and Met as well as the constitutive activation of downstream molecules including Akt and MAPK (reviewed in [7]). These EGFR TKI resistance pathways circumvent EGFR TKIs by the activation of additional signaling pathways.
An additional mechanism to explain the somewhat disappointing overall response of breast tumors to EGFR TKIs is the possibility that the EGFR still has the ability to signal in the absence of innate EGFR kinase activity. Indeed, it has been clearly demonstrated that additional tyrosine kinases can phosphorylate the EGFR independent of ligand. For example, c-Src phosphorylates a kinase-impaired EGFR on its autophosphorylation sites as well as several additional tyrosines [9,10]. In addition, another EGFR family member, HER2, also has the ability to phosphorylate the EGFR on its autophosphorylation sites [11]. Furthermore, it has been shown that a point mutation in the HER2 kinase domain results in constitutive phosphorylation of EGFR in the presence of EGFR TKIs [12]. These data indicate that, even without the EGFR kinase activity, phosphorylation of the EGFR may occur to promote cell growth and survival pathways.
The EGFR TKI-resistance seen in certain cancer cells may result from an ROS-induced inactivation of an EGFR-associated tyrosine phosphatase [13]. In this context, a role for ROS and accompanying oxidative stress in the initiation and progression of breast cancer has been suggested and variously supported in a number of studies [14–16]. Oncogenes with tyrosine kinase activity, including HER2 in breast cancer cells, can induce the upregulation of cellular/oncogenic ROS and influence the metabolic transformation of cancer cells [17]. Furthermore, the cancer cell redox state can affect the function of oncogenic kinases [14].
In order to examine the potential link between cellular ROS and EGFR TKI-resistance, we employed a targeted antioxidant enzyme, CAT-SKL, to reduce oxidant levels in breast cancer cell line models expressing high levels of ROS and EGFR. Our results indicate that CAT-SKL sensitizes previously resistant MDA-MB-468 cells to the anti-cancer effects of the EGFR-targeted TKI, gefitinib. EGFR inhibitors are of great potential value as therapies in breast cancer models; resistance presents a major challenge to this treatment modality. CAT-SKL, a hydrogen peroxide (H2O2) metabolizing protein therapeutic shown to be effective in a number of in vitro and pre-clinical indications [18–20], thus represents a potentially powerful new means of combating this devastating disease.
Materials and methods
Cell culture
SUM149 cells were maintained in Ham’s F-12 supplemented with 5% FBS, 1 µg/mL hydrocortisone, and 5 µg/mL insulin. MDA-MB-468 cells were grown in DMEM supplemented with 10% FBS. Both cell lines were cultured with 2.5 µg/mL amphotericin B and 25 µg/mL gentamicin. For vehicle control treated cells, dimethyl sulfoxide was added at 0.1% final concentration.
SDS-PAGE/immunoblotting
Lysates were prepared from the indicated cells in CHAPS lysis buffer (10 mM CHAPS, 50 mM Tris, pH 8.0, 150 mM NaCl, and 2 mM EDTA with 10 µM NaOVa and 1X protease inhibitor cocktail (EMD Biosciences, Philadelphia, PA)). For immunoblotting, 10 µg of protein lysates were separated by SDS-PAGE and transferred to Immobilon P. Membranes were blocked in either 5% nonfat dry milk or 5% BSA for 1 h at 25 °C. Primary antibodies utilized in this study include: anti-EGFR and anti-pY1068 EGFR from Cell Signaling (Danvers, MA); anti-pMAPK and anti-actin from Sigma (St. Louis, MO); and anti-pTyrosine from Invitrogen (Carlsbad, CA). Anti-mouse and anti-rabbit IgG-HRP was used from Cell Signaling and enhanced chemiluminescence (ECL) reagents were from GE Healthcare Life Sciences (Piscataway, NJ). Experiments were repeated at least three times.
Dot blot analyses were performed with streptavidin alkaline-phosphatase (1:1000) or anti-catalase antibodies (1:4000) and goat anti-rabbit-alkaline-phosphatase (1:5000) and developed with the NBT/BCIP color development substrate (Thermo Scientific). Protein transduction was performed as follows; 100 nM of biotinylated CAT-SKL was added for 0, 1, 2, and 4 h, cells were washed with PBS, and harvested directly into standard 2X sample buffer. Proteins were separated by 10% SDS-PAGE, and then transferred to a nitrocellulose membrane and blocked for 1 h in 5% nonfat dry milk in Tween-containing, tris buffered saline. Membranes were probed with anti-streptavidin alkaline phosphatase (1:1000) and developed with NBT/BCIP.
Biotinylation of CAT-SKL
CAT-SKL containing an 11 arginine peptide transduction domain and a modified peroxisomal targeting signal was expressed, purified, and biotinylated as described in [18,20–22].
Cell viability and cell growth assays
For testing cell viability, two approaches were employed: enzymatic assays that measure metabolic activity and a dye-exclusion assay that distinguishes live from dead cells. For the generation of IC50 curves, 1000 MDA-MB-468 or SUM149 cells/well were incubated for 72 h with various concentrations of gefitinib (dissolved in dimethyl sulfoxide). At this point, cell viability was determined with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT), as per manufacturer’s protocol. For the sensitivity to H2O2 experiments, cells were plated at 400,000 cells/well, pretreated with CAT-SKL (1 µM) for 4 h, and challenged with H2O2 (1 mM) for 2 h. MDA-MB-468 cell viability was determined using the water soluble tetrazolium cell proliferation assay (WST-1) from Millipore following the manufacturer’s protocol. For the latter, 200,000 cells, pretreated with CAT-SKL and challenged with H2O2 as described above, were incubated with 0.2% trypan blue (final concentration) for 3–5 min and the percentage of non-viable (blue) cells were determined microscopically with a hemocytometer.
Growth assays were performed by plating MDA-MB-468 or SUM149 cells in triplicate in 6-well plates at 35,000 cells/well (day 0). The next day, and every other day thereafter for seven days, the cells were treated with 0.1% dimethyl sulfoxide (vehicle control), or gefitinib, and/or CAT-SKL at the indicated dosages. The number of cells was determined using a Coulter counter on days 1, 4, and 7. Each experiment was repeated at least three times.
Measurement of ROS
H2O2 measurements were performed using the Amplex® Red Hydrogen Peroxide/Peroxidase Assay Kit from Molecular Probes® following the manufacturer’s protocol. After treatment with 1 µM CAT-SKL for 4 h at 37 °C, cells were washed twice with Hank’s balanced salt solution, harvested in the Amplex® Red reagent/horse radish peroxidase working solution, incubated at room temperature for 30 min, and fluorescence was measured on a microplate reader (excitation 530 nm and emission 590 nm). 2′,7′-dichlorofluorescin diacetate (DCF-DA) was used as previously described [23]; live cells were imaged for ROS production. Briefly, cells were treated with or without 1 µM CAT-SKL for 4 h, washed twice with Hank’s balanced salt solution and incubated for 10 min with 25 µM DCF-DA. After washing again, microscopic analysis was performed using the Zeiss LSM 510 META NLO confocal microscope at the Wayne State University School of Medicine Microscopy, Imaging, & Cytometry Resources Core. Cellular ROS was quantified either by counting the cells that stain positive for ROS or by using MetaMorph® (Molecular Devices) image analysis software to quantitate cellular fluorescence intensities.
Results
EGFR remains tyrosine phosphorylated in breast cancer cells resistant to TKIs
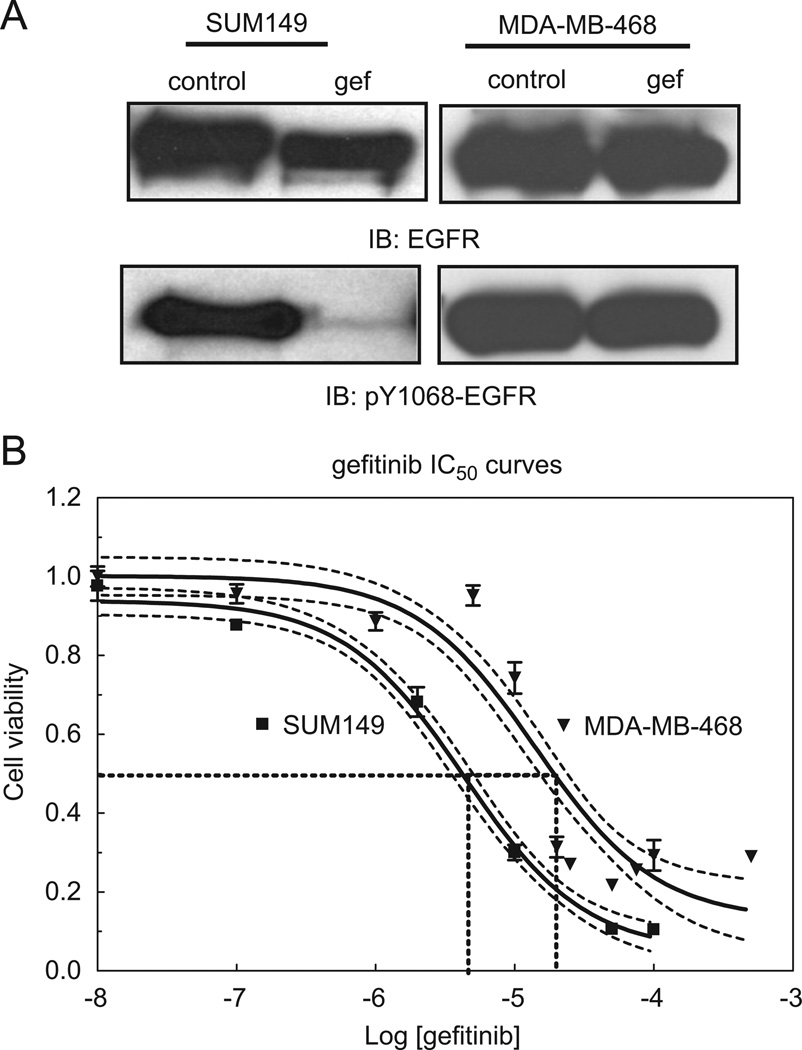
Despite EGFR overexpression in more than 30% of breast cancers, clinical trials using EGFR targeted therapies have failed to show a significant benefit in disease-free and overall survival [3,4]. Breast cancers overexpressing EGFR have a poor clinical outcome, therefore emphasizing a need to more effectively target EGFR in these cancers. To examine the effects of gefitinib on EGFR phosphorylation, we cultured two human breast cancer cell lines in the presence of the TKI. In SUM149 cells, phosphorylation of EGFR at tyrosine 1068 was reduced in response to gefitinib, whereas EGFR phosphorylation at tyrosine 1068 in MDA-MB-468 cells was unaffected (Fig. 1A). Inhibitory growth curves further document the sensitivity of SUM149 cells to gefitinib (IC50 values of 6.8 µM for SUM149 cells and 14.1 µM for the MDA-MB-468 cells (Fig. 1B; dotted lines=95% confidence intervals)). We previously documented this trend of EGFR tyrosine phosphorylation in the presence of gefitinib correlating with resistance to effects on viability by EGFR TKIs and established the inhibition of EGFR kinase activity with gefitinib ([5]; Fig. S1). Here we further demonstrate that in MDA-MB-468 cells, autophosphorylation specifically at tyrosine 1068 is regulated independently of EGFR kinase activity.
Fig. 1.
EGFR phosphorylation and TKI sensitivity of breast cancer cell lines. (A) SUM149 and MDA-MB-468 cells were treated with 0.5 µM gefitinib for 30 min. Lysates were then prepared, separated by SDS-PAGE, and immunoblotted using anti-EGFR or anti-pY1068-EGFR antibodies. Control=vehicle control treated; gef=gefitinib treated; IB=immunoblot. (B) SUM149 or MDA-MB-468 cells were cultured with increasing concentrations of gefitinib for 72 h. MTT colorimetric assays were performed to assess cell viability. Fraction of surviving cells was determined and IC50 values were calculated from sigmoidal dose response curves. 95% confidence intervals for each curve appear as dashed lines. Log[geftinib]=log of each dose of gefitinib used in M.
Elevated ROS levels in TKI-resistant breast cancer cells
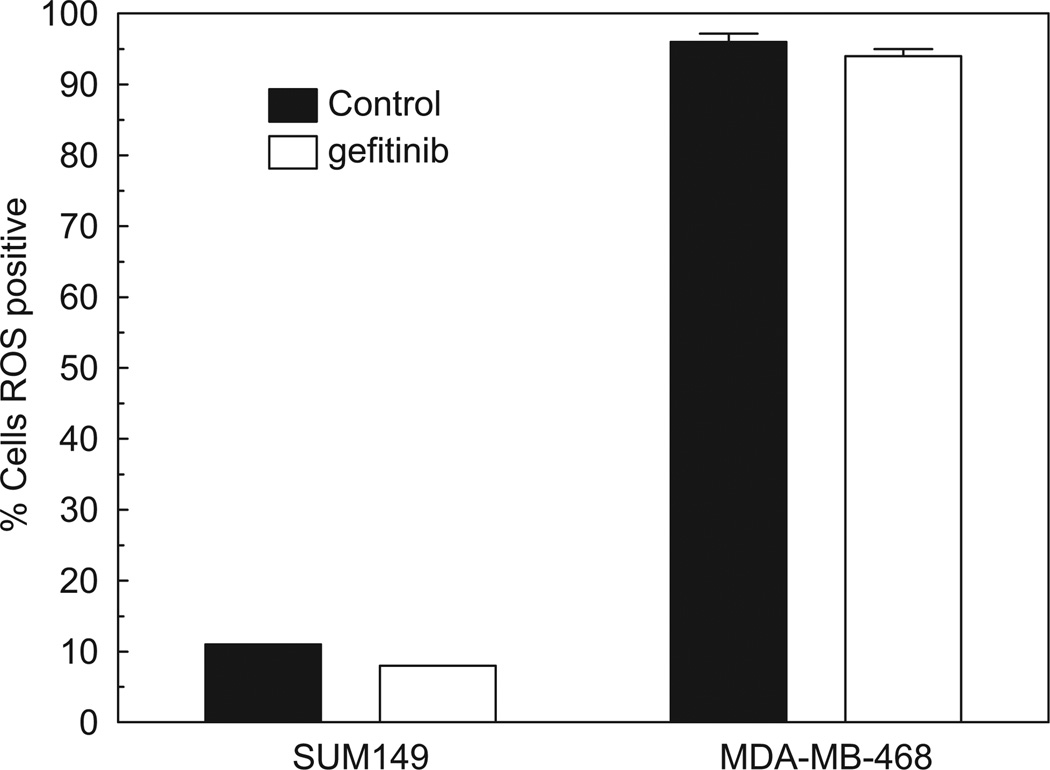
Deregulation of EGFR tyrosine phosphorylation could be elevated by ROS through any number of distinct mechanisms. Therefore, we sought to determine the baseline level of ROS in MDA-MB-468 cells compared to a gefitinib-sensitive breast cancer cell line, in this case, SUM149. Using a DCF-fluorescence assay that measures total intracellular ROS, we found that oxidant levels in MDA-MB-468 cells were nearly 10 fold higher when directly compared to the SUM149 cells (Fig. 2; black bars). To determine if ROS production was altered by gefitinib treatment, we incubated both cell lines with 0.5 µM gefitinib for 30 min. Interestingly, ROS production was not altered by gefitinib in either cell line (Fig. 2; white bars). These results led us to consider that ROS production in MDA-MB-468 cells may regulate EGFR tyrosine phosphorylation and subsequent biological resistance to gefitinib.
Fig. 2.
ROS levels are elevated in TKI-resistant breast cancer cells. SUM149 and MDA-MB-468 cells were treated with 0.5 µM gefitinib for 30min. ROS production was measured using the fluorescent dye, 2′,7′-dichlorofluorescin diacetate (DCF-DA), which measures reactive oxygen on a per cell basis. The percentage of cells positive for ROS was determined from an average of three independent experiments. Control=vehicle control treated; and gefitinib=gefitinib treated.
EGFR tyrosine phosphorylation is regulated by reactive oxygen
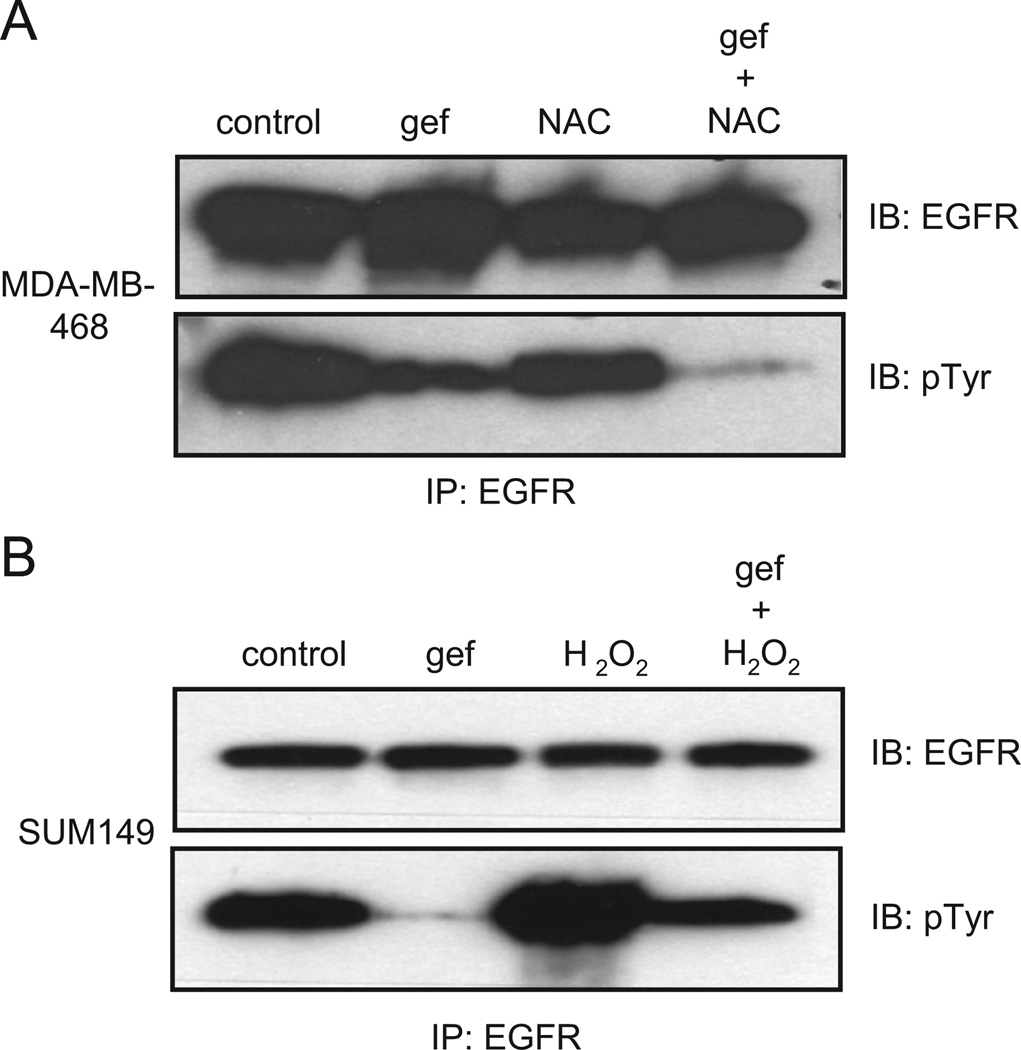
To examine the role of ROS in modulating EGFR tyrosine phosphorylation, we examined the process in cells treated with the antioxidant, N-acetyl cysteine (NAC), or the oxidizer, H2O2, as seen in Fig. 3A and B (immunoblots quantified in Supplementary Fig. S2A and B). Quenching ROS decreased EGFR tyrosine phosphorylation in gefitinib-treated MDA-MB-468 cells. In contrast, enhancing oxidative stress in SUM149 cells with H2O2 rendered them unable to decrease EGFR tyrosine phosphorylation. These results support the model that in breast cancer cells, ROS modulates EGFR phosphorylation in the presence of an EGFR TKI.
Fig. 3.
EGFR tyrosine phosphorylation is regulated by reactive oxygen. (A) MDA-MB-468 cells were treated with 0.5 µM gefitinib for 30min and/or 50mM N-acetyl cysteine for the last 10 min as indicated. Cell lysates were immunoprecipitated using anti-EGFR antibodies and immunoblotted with anti-EGFR or anti-phosphotyrosine antibodies. (B) SUM149 cells were treated with 0.5 µM gefitinib and/or 5mM H2O2. Cell lysates were immunoprecipitated using anti-EGFR antibodies and immunoblotted with anti-EGFR or anti-phosphotyrosine antibodies. Control=vehicle control treated; gef=gefitinib treated; NAC=N-acetyl cysteine treated; IB=immunoblot; and IP=immunoprecipitation.
CAT-SKL transduction of MDA-MB-468 cells
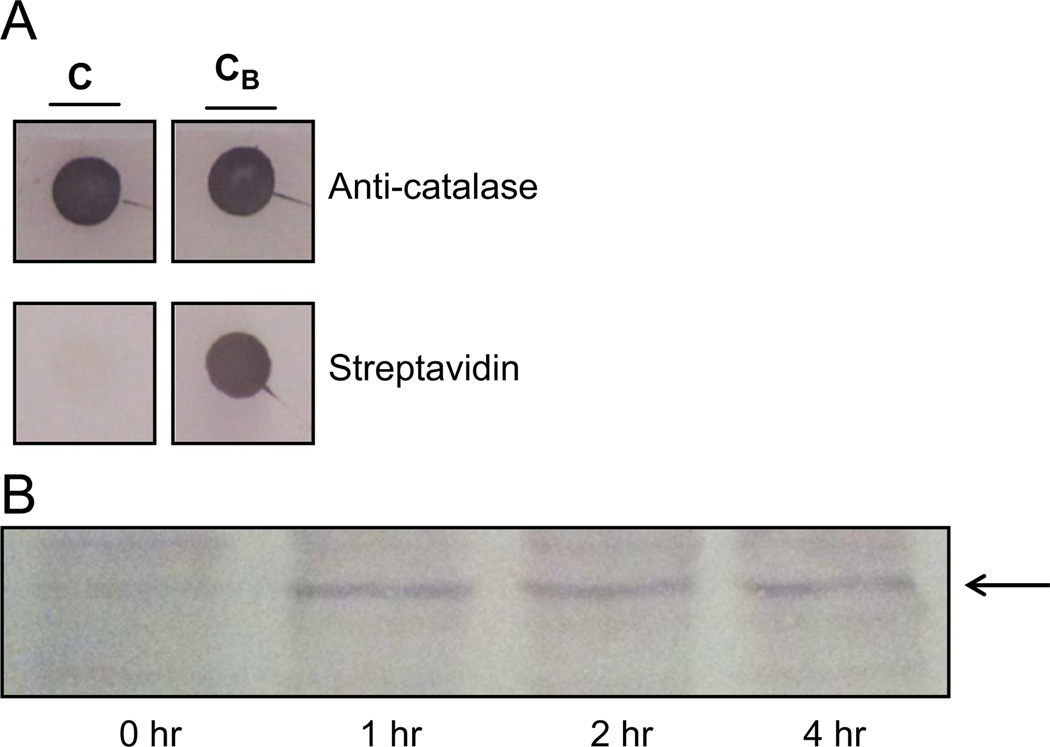
NAC was abandoned as a potential therapeutic due to its toxicity in our long-term growth assays (data not shown). Instead, we employed a novel cell-penetrating catalase derivative, called CAT-SKL [14,24–26]. This molecule is designed to enter cells and, by virtue of a modified targeting signal, efficiently traffic to peroxisomes. Once there, it metabolizes H2O2 produced both within the organelle, as well as elsewhere in the cell, and restores, to varying extents, oxidative balance in cells [19,24–26]. This targeted enzyme approach has shown efficacy in a number of cellular and preclinical indications and works, at least in part, by controlling inflammatory processes [19,20]. In order to demonstrate transduction of MDA-MB-468 breast cancer cells, we biotinylated CAT-SKL and tracked its entry into the cells (Fig. 4A). MDA-MB-468 cells were incubated with 100 nM CAT-SKL for 1–4 h. Protein lysates were isolated and blotted with streptavidin-alkaline phosphatase labeled antibodies. Importantly, biotinylated-CAT-SKL was detected inside the MDA-MB-468 cells after 1 h of incubation (Fig. 4B).
Fig. 4.
CAT-SKL transduction of MDA-MB-468 cells. (A) CATSKL was biotinylated as described [18,21,22]. CAT-SKL (“C”) and CAT-SKL after biotinylation (“CB”) were dot-blotted onto nitrocellulose and probed with anti-catalase antibodies (and alkaline phosphatase secondary antibodies) or streptavidin alkaline phosphatase as indicated. (B) MDA-MB-468 cells were incubated with biotinylated CAT-SKL (100 nM) for 0, 1, 2, and 4 h as indicated. Cells were then washed, harvested, and blotted with streptavidin-alkaline phosphatase. Arrow indicates migration of biotinylated-CAT-SKL.
CAT-SKL reduces ROS in MDA-MB-468 cells
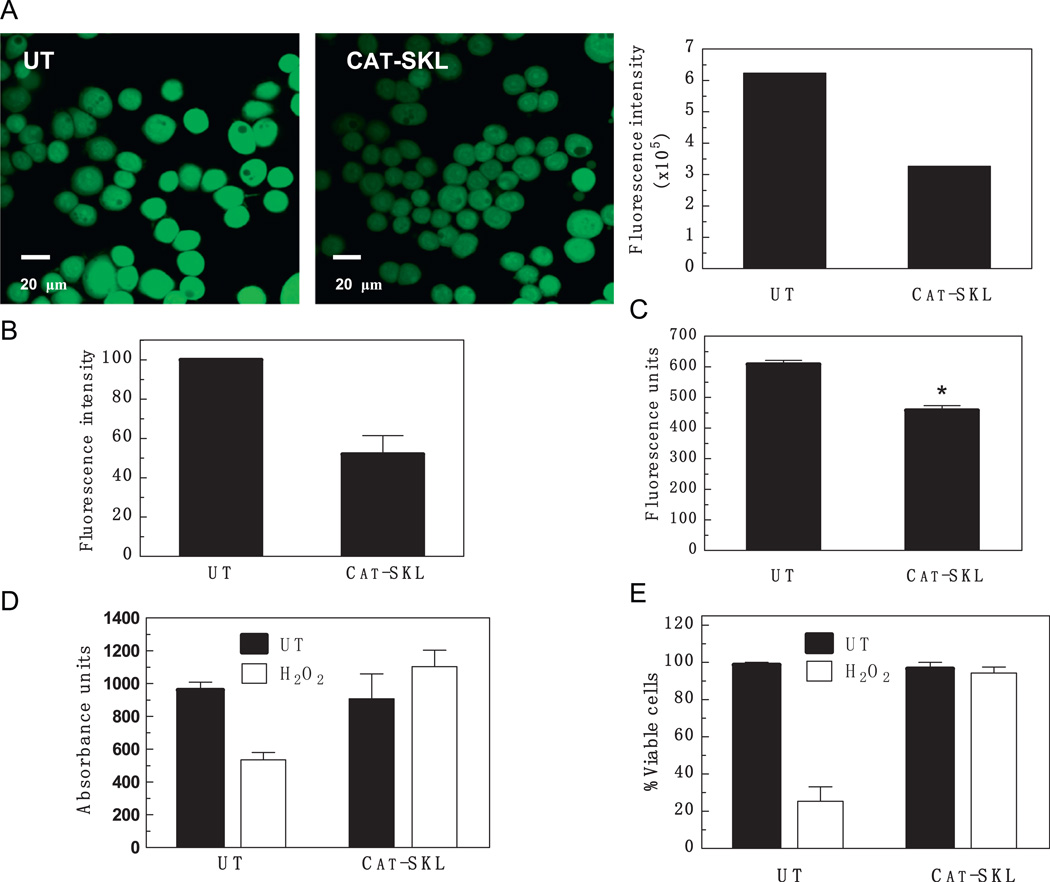
Although we have shown that CAT-SKL was able to enter MDA-MB-468 cells, we needed to confirm that the enzyme was able to quench ROS in this breast cancer model. To accomplish this, MDA-MB-468 cells were incubated with 1 µM CAT-SKL for 4 h and ROS production was determined by measuring DCF-fluorescence (Fig. 5A and B). Importantly, CAT-SKL was able to decrease ROS production by about 48% in MDA-MB-468 cells (Fig. 5B). In addition, when H2O2 specifically was measured using an Amplex® Red based assay, a 21% decline was seen (Fig. 5C). Taken together, these experiments demonstrate that CAT-SKL is able to enter MDA-MB-468 cells and significantly reduce ROS production.
Fig. 5.
Effect of CAT-SKL on ROS levels in MDA-MB-468 cells. (A) Cells were pretreated or not (“UT”) with CAT-SKL (1 µM) for 4 h and then examined for the presence of ROS using DCF-DA (25 µM). The accompanying histogram represents MetaMorph®-based quantification of the cellular fluorescence intensities seen in the ROS images. (B) Cellular fluorescence intensities from three experiments (including the one shown in (A)) were pooled and normalized to the untreated control value (arbitrarily set at 100) to permit comparison. Results presented are the mean±1 SD. (C) H2O2 was specifically measured employing the Amplex® Red Reagent (10-acetyl-3,7-dihydroxy-phenoxazine). The decline is statistically significant; *p-value<0.01. (D) and (E) MDA-MB-468 cells were pretreated with CAT-SKL (1 µM) for 4 h and then challenged with H2O2 for 2 h (1mM). Cell viability was determined either using the water soluble tetrazolium cell proliferation assay (WST-1) (D), or by quantitative trypan blue exclusion assays (E).
To determine if H2O2 decreased viability of the MDA-MB-468 cells, 1 mM H2O2 was added to the cells for 2 h and the effects on cell viability were assessed by the MTT assay, a measure of mitochondrial reductase activity. As expected, H2O2 decreased viability in the MDA-MB-468 cells by almost 50% (Fig. 5D; left white bar). In contrast, in cells pretreated with CAT-SKL, exogenously added H2O2 was largely without effect (Fig. 5D). CAT-SKL treatment alone had no effect on cell viability, demonstrating that the enzyme is not toxic to human breast cancer cells. Essentially similar results were obtained when trypan blue (dye) exclusion assays were employed to directly determine cell viability (Fig. 5E).
CAT-SKL sensitizes MDA-MB-468 cells to gefitinib
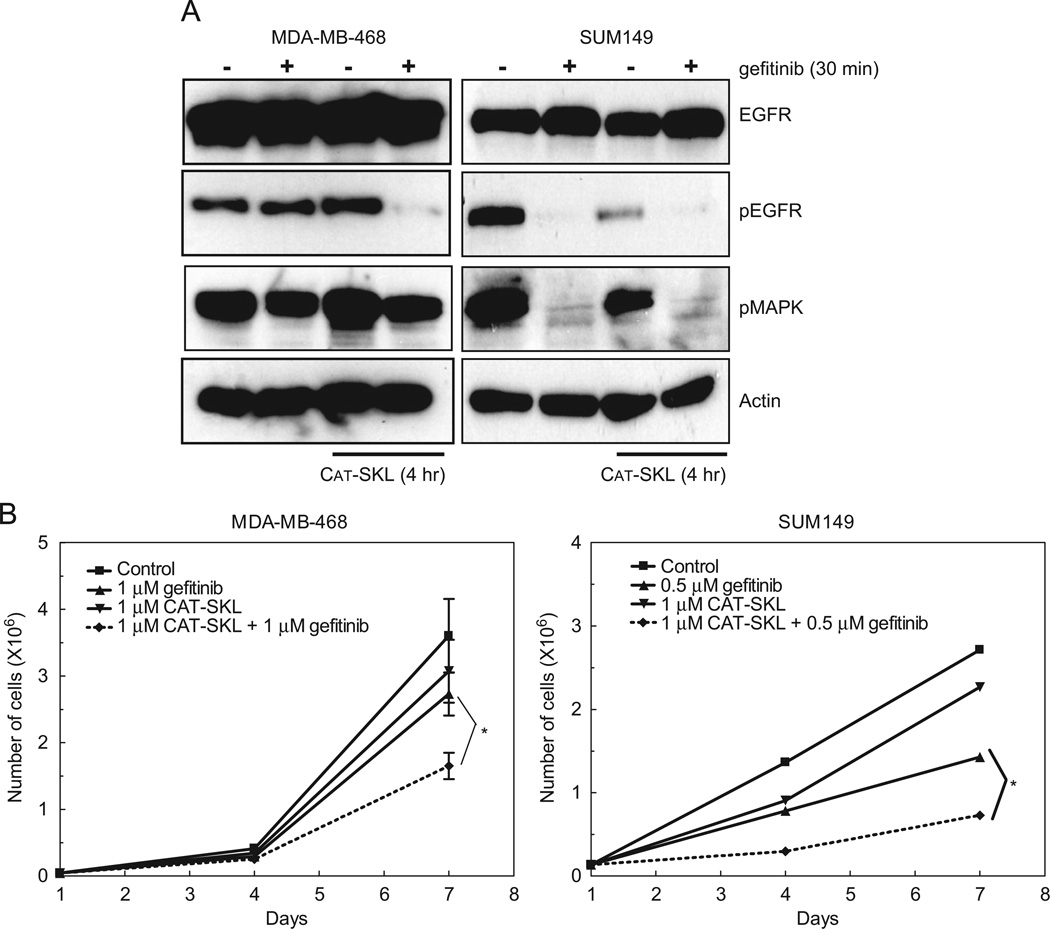
To determine if CAT-SKL reduction of ROS production is sufficient to reduce EGFR tyrosine phosphorylation in the presence of 1 µM gefitinib, we measured EGFR phosphorylation at tyrosine 1068 in MDA-MB-468 cells after a 4 h treatment with 1 µM CAT-SKL. Once again, EGFR tyrosine phosphorylation was not reduced with gefitinib treatment; however, co-treatment with gefitinib and CAT-SKL resulted in an almost complete loss of EGFR tyrosine phosphorylation at 1068 (Fig. 6A; left panel). This is in contrast to the treatment of SUM149 cells with gefitinib and CAT-SKL. Specifically, EGFR tyrosine phosphorylation is abrogated with gefitinib treatment and actually modestly inhibited by CAT-SKL itself (Fig. 6A; right panel). These data suggest that the general phenomenon of inhibiting ROS production in cancer cells may decrease tyrosine phosphorylation of key pro-growth and survival proteins.
Fig. 6.
CAT-SKL decreases EGFR tyrosine phosphorylation in the presence of gefitinib and sensitizes MDA-MB-468 cells to the TKI. (A) MDA-MB-468 and SUM149 cells were incubated with 1 µM CAT-SKL for 4 h followed by 30min with 1 µM or 0.5µM gefitinib, respectively. Cell lysates were prepared, separated by SDS-PAGE, and immunoblotted using anti-EGFR, anti-pY1068-EGFR, anti-pMAPK, or anti-β-actin antibodies. (B) MDA-MB-468 and SUM149 cells were treated with 1 µM or 0.5µM gefitinib, respectively, 1 µM CAT-SKL, or the combination for 7 days. Cells were counted on days 1, 4 and 7 as described in Section 2. Growth curves were plotted over time. *p-value=0.05.
To determine if CAT-SKL could sensitize MDA-MB-468 cells to the anti-proliferation effects of gefitinib, we treated cells with vehicle or 1 µM gefitinib in the presence or absence of 1 µM CAT-SKL in a 7 day proliferation assay. MDA-MB-468 cells continued to proliferate with 1 µM gefitinib or 1 µM CAT-SKL treatment alone (Fig. 6B; left panel; triangles). However, co-treatment of MDA-MB-468 cells with gefitinib and CAT-SKL decreased cell proliferation by nearly 50% (Fig. 6B; left panel; diamonds). This decrease in proliferation was statistically significant and provides evidence, for the first time, of a targeted antioxidant enzyme sensitizing cancer cells to a TKI. Taken together, these data suggest that CAT-SKL may represent a new therapeutic approach for the treatment of EGFR TKI-resistant breast cancers.
Previously we have shown that SUM149 cells are sensitive to EGFR inhibitors including gefitinib (Fig. 1B and 3B). Here we demonstrate that gefitinib treatment decreases cell proliferation by 50% in the SUM149 cells (Fig. 6B; right panel). As with the MDA-MB-468 cells, CAT-SKL alone failed to inhibit cell proliferation in the SUM149 cells. However, combining gefitinib and CAT-SKL produced an additional 25% decrease in cell proliferation compared to gefitinib treatment alone (Fig. 6B; right panel; diamonds). Taken together, these data suggest that for breast cancers that express high levels of EGFR and ROS, a combination of antioxidant and TKI treatment strategies may elicit a greater effect than, or overcome resistance to, EGFR-targeted TKI therapy.
Discussion
In this report we have provided evidence of a role for ROS production in mediating resistance to EGFR inhibitors through maintenance of EGFR tyrosine phosphorylation. Specifically, we found that inhibiting ROS production using a novel inhibitor of H2O2 production, CAT-SKL, sensitized resistant MDA-MB-468 breast cancer cells to EGFR kinase inhibition by gefitinib. In doing so, EGFR tyrosine phosphorylation, and cell proliferation were reduced. As EGFR inhibitors have yet to show consistent value in the clinical treatment of breast cancer, these data provide striking evidence for the efficacy of a combination of CAT-SKL and gefitinib in the treatment of EGFR expressing tumors.
How CAT-SKL elicits its effect remains to be further investigated, but in light of our observations that CAT-SKL treatment has a role in attenuating EGFR phosphorylation, a plausible mechanistic explanation may involve CAT-SKL-dependent reactivation of a phosphatase (PTP) that targets the EGFR. It is known that PTPs contain an active cysteine that is required for phosphorylation and that ROS modify this site (reviewed in [27]). Inhibition of ROS production by antioxidants has been shown to “reactivate” tyrosine PTPs; including PTP1B, TCPTP, SHP2, SHP1, CD45, PTPκ, and PTP-PEST [27]. Importantly, PTP1B, SHP1, and PTPκ have been shown to dephosphorylate EGFR. In addition, SHP1 and PTPκ have been implicated as tumor suppressors, implying that loss of the function of these PTPs promotes cancer progression. Future work will address the hypothesis that down-regulation of ROS by CAT-SKL treatment can reverse PTP inactivation and thus allow for dephosphorylation of EGFR, and reestablishment of sensitivity to gefitinib.
The work of others has established that dysregulated ROS production/metabolism plays a crucial role in many human diseases, including cancer [14,16]. Furthermore, ROS have been linked to aberrant EGFR phosphorylation resulting in modifications of downstream signaling cascades. Our work here has shown that treatments with antioxidants such as NAC were able to reduce ROS and potentiate the effects of gefitinib on EGFR phosphorylation. However, when growth curves were performed with NAC, cell viability was compromised, suggesting it is a poor clinical option for breast cancer patients. Our results indicate that transduced CAT-SKL was able to significantly reduce cellular ROS and gefitinib-sensitize otherwise resistant breast cancer cells that express high levels of ROS without compromising cell viability. Breast cancer treatment is limited for certain patients due to insensitivity to EGFR TKIs. Furthermore, constitutively active EGFR phosphorylation is a common characteristic of many breast cancers. Reducing ROS production with a safe, targeted enzyme approach employing CAT-SKL may represent a new treatment modality for a deadly disease.
Supplementary Material
Acknowledgments
We are grateful to the Microscopy & Imaging Resources Laboratory Shared Resource facility for providing services. The Microscopy and Imaging Resources Laboratory is supported, in part, by the NIH Center grants no. P30ES06639 to the Institute of Environmental Health Sciences, no. P30CA22453 to the Karmanos Cancer Institute, Wayne State University, and no. U54RR020843 to the Center for Proteolytic Pathways, the Burnham Institute, and the Perinatology Research Branch of the National Institutes of Child Health and Development, Wayne State University. This work was supported in part by Susan G. Komen for the Cure Career Catalyst Grant (no. KG081416; JLB).
Footnotes
Disclosure of potential conflicts of interest
SRT is a cofounder of EXT Life Sciences, Inc., a Michigan-based biotechnology company owned, in part, by Wayne State University. SRT retains an equity interest in the company that is developing CAT-SKL, one of the antioxidants used in this report. Other authors state no conflict of interest.
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.yexcr.2012.06.001.
Contributor Information
Stanley R. Terlecky, Email: srterlecky@med.wayne.edu.
Julie L. Boerner, Email: boernerj@karmanos.org.
REFERENCES
- 1.DeYulia GJ, Carcamo JM. EGF receptor-ligand interaction generates extracellular hydrogen peroxide that inhibits EGFR-associated protein tyrosine phosphatases. Biochem. Biophys. Res. Commun. 2005;334(1):38–42. doi: 10.1016/j.bbrc.2005.06.056. [DOI] [PubMed] [Google Scholar]
- 2.Jorissen RN, et al. Epidermal growth factor receptor: mechanisms of activation and signalling. Exp. Cell Res. 2003;284(1):31–53. doi: 10.1016/s0014-4827(02)00098-8. [DOI] [PubMed] [Google Scholar]
- 3.Baselga J, et al. Phase II and tumor pharmacodynamic study of gefitinib in patients with advanced breast cancer. J. Clin. Oncol. 2005;23(23):5323–5333. doi: 10.1200/JCO.2005.08.326. [DOI] [PubMed] [Google Scholar]
- 4.Tan AR, et al. Evaluation of biologic end points and pharmacokinetics in patients with metastatic breast cancer after treatment with erlotinib, an epidermal growth factor receptor tyrosine kinase inhibitor. J. Clin. Oncol. 2004;22(15):3080–3090. doi: 10.1200/JCO.2004.08.189. [DOI] [PubMed] [Google Scholar]
- 5.Mueller KL, Hunter LA, Ethier SP, Boerner JL. Met and c-Src cooperate to compensate for loss of epidermal growth factor receptor kinase activity in breast cancer cells. Cancer Res. 2008;68(9):3314–3322. doi: 10.1158/0008-5472.CAN-08-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Irwin ME, Mueller KL, Bohin N, Ge Y, Boerner JL. Lipid raft localization of EGFR alters the response of cancer cells to the EGFR tyrosine kinase inhibitor gefitinib. J. Cell Physiol. 2011;226(9):2316–2328. doi: 10.1002/jcp.22570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wheeler DL, Dunn EF, Harari PM. Understanding resistance to EGFR inhibitor-impact on future treatment strategies. Nat. Rev. Clin. Oncol. 2010;7(9):493–507. doi: 10.1038/nrclinonc.2010.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lo HW. EGFR-targeted therapy in malignant glioma: novel aspects and mechanisms of drug resistance. Curr. Mol. Pharmacol. 2010;3(1):37–52. doi: 10.2174/1874467211003010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amos S, Martin PM, Polar GA, Parsons SJ, Hussaini IM. Phorbol 12-myristate 13-acetate induces epidermal growth factor receptor transactivation via protein kinase Cdelta/c-Src pathways in glioblastoma cells. J. Biol. Chem. 2005;280(9):7729–7738. doi: 10.1074/jbc.M409056200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stover DR, Becker M, Liebetanz J, Lydon NB. Src phosphorylation of the epidermal growth factor receptor at novel sites mediates receptor interaction with Src and P85 alpha. J. Biol. Chem. 1995;270(26):15591–15597. doi: 10.1074/jbc.270.26.15591. [DOI] [PubMed] [Google Scholar]
- 11.Spivak-Kroizman T, et al. Heterodimerization of c-erbB2 with different epidermal growth factor receptor mutants elicits stimulatory or inhibitory responses. J. Biol. Chem. 1992;267(12):8056–8063. [PubMed] [Google Scholar]
- 12.Wang SE, et al. HER2 kinase domain mutation results in constitutive phosphorylation and activation of HER2 and EGFR and resistance to EGFR tyrosine kinase inhibitors. Cancer Cell. 2006;10(1):25–38. doi: 10.1016/j.ccr.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 13.Chen CH, et al. Reactive oxygen species generation is involved in epidermal growth factor receptor transactivation through the transient oxidization of Src homology 2-containing tyrosine phosphatase in endothelin-1 signaling pathway in rat cardiac fibroblasts. Mol. Pharmacol. 2006;69(4):1347–1355. doi: 10.1124/mol.105.017558. [DOI] [PubMed] [Google Scholar]
- 14.Benhar M, Engelberg D, Levitzki A. ROS, stress-activated kinases and stress signaling in cancer. EMBO Rep. 2002;3(5):420–425. doi: 10.1093/embo-reports/kvf094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bollig-Fischer A, et al. HER-2 signaling, acquisition of growth factor independence, and regulation of biological networks associated with cell transformation. Cancer Res. 2010;70(20):7862–7873. doi: 10.1158/0008-5472.CAN-10-1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liou GY, Storz P. Reactive oxygen species in cancer. Free Radical Res. 2010;44(5):479–496. doi: 10.3109/10715761003667554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee SR, Kwon KS, Kim SR, Rhee SG. Reversible inactivation of protein-tyrosine phosphatase 1B in A431 cells stimulated with epidermal growth factor. J. Biol. Chem. 1998;273(25):15366–15372. doi: 10.1074/jbc.273.25.15366. [DOI] [PubMed] [Google Scholar]
- 18.Koepke JI, et al. Restoration of peroxisomal catalase import in a model of human cellular aging. Traffic. 2007;8(11):1590–1600. doi: 10.1111/j.1600-0854.2007.00633.x. [DOI] [PubMed] [Google Scholar]
- 19.Undyala V, Terlecky SR, Vander Heide RS. Targeted intracellular catalase delivery protects neonatal rat myocytes from hypoxia-reoxygenation and ischemia-reperfusion injury. Cardiovasc. Pathol. 2011;20(5):272–280. doi: 10.1016/j.carpath.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Young CN, et al. Reactive oxygen species in tumor necrosis factor-alpha-activated primary human keratinocytes: implications for psoriasis and inflammatory skin disease. J. Invest. Dermatol. 2008;128(11):2606–2614. doi: 10.1038/jid.2008.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Terlecky SR. In vitro analysis of peroxisomal protein import. Curr. Protocol Cell Biol. 2002;15(Chapter 11:Unit 11) doi: 10.1002/0471143030.cb1115s14. [DOI] [PubMed] [Google Scholar]
- 22.Terlecky SR, Legakis JE, Hueni SE, Subramani S. Quantitative analysis of peroxisomal protein import in vitro. Exp. Cell Res. 2001;263(1):98–106. doi: 10.1006/excr.2000.5111. [DOI] [PubMed] [Google Scholar]
- 23.Legakis JE, et al. Peroxisome senescence in human fibroblasts. Mol. Biol. Cell. 2002;13(12):4243–4255. doi: 10.1091/mbc.E02-06-0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Price M, Terlecky SR, Kessel D. A role for hydrogen peroxide in the pro-apoptotic effects of photodynamic therapy. Photochem. Photobiol. 2009;85(6):1491–1496. doi: 10.1111/j.1751-1097.2009.00589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Terlecky SR, Koepke JI. Drug delivery to peroxisomes: employing unique trafficking mechanisms to target protein therapeutics. Adv. Drug Deliv. Rev. 2007;59(8):739–747. doi: 10.1016/j.addr.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 26.Wood CS, et al. Hypocatalasemic fibroblasts accumulate hydrogen peroxide and display age-associated pathologies. Traffic. 2006;7(1):97–107. doi: 10.1111/j.1600-0854.2005.00358.x. [DOI] [PubMed] [Google Scholar]
- 27.Tonks NK. Protein tyrosine phosphatases: from genes, to function, to disease. Nat. Rev. Mol. Cell Biol. 2006;7(11):833–846. doi: 10.1038/nrm2039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.