Abstract
Background
Increased exposure to endogenous estrogen and/or insulin may partly explain the relationship of obesity, physical inactivity, and alcohol consumption and postmenopausal breast cancer. However, these potential mediating effects have not been formally quantified in a survival analysis setting.
Methods
We combined data from two case–cohort studies based in the Women’s Health Initiative- Observational Study with serum estradiol levels, one of which also had insulin levels. A total of 1,601 women (601 cases) aged 50 to 79 years who were not using hormone therapy at enrollment were included. Mediating effects were estimated by applying a new method based on the additive hazard model.
Results
A five-unit increase in body mass index (BMI) was associated with 50.0 [95% confidence interval (CI), 23.2–76.6] extra cases per 100,000 women at-risk per year. Of these, 23.8% (95% CI, 2.9–68.4) could be attributed to estradiol and 65.8% (95% CI, 13.6–273.3) through insulin pathways. The mediating effect of estradiol was greater (48.8%; 95% CI, 18.8–161.1) for BMI when restricted to estrogen receptor positive (ER+) cases. Consuming 7+ drinks/wk compared with abstinence was associated with 164.9 (95% CI, 45.8–284.9) breast cancer cases per 100,000, but no significant contribution from estradiol was found. The effect of alcohol on breast cancer was restricted to ER+ breast cancers.
Conclusions
The relation of BMI with breast cancer was partly mediated through estradiol and, to a greater extent, through insulin.
Impact
The findings provide support for evaluation of interventions to lower insulin and estrogen levels in overweight and obese postmenopausal women to reduce breast cancer risk.
Introduction
Postmenopausal breast cancer risk has been related to lifestyle factors such as obesity, physical inactivity, and alcohol consumption (1–7). In particular, being overweight or obese has been consistently linked to the risk of postmenopausal breast cancer (3, 4). A reduced risk associated with physical activity has previously been established in the Women’s Health Initiative (WHI; ref. 7) and a recent systematic review confirmed an inverse association (5). Likewise, meta-analyses of alcohol and breast cancer have reported increasedrisksof9%to11%in women starting at an intake of around 1 drink/d and the risk increases linearly (1, 2).
Postmenopausal breast cancer is a hormone-related disease, and estrogen levels are predictive of breast cancer risk (8, 9). A recent trial of the estrogen lowering drug exemestane found a 65% reduction in breast cancer incidence (10). Furthermore, alcohol, obesity, and physical activity have all been associated with estrogen levels (11, 12), raising the hypothesis that the relation of these lifestyle factors with breast cancer risk is mediated by estrogen. Data on alcohol consumption and estrogen levels have been mixed in previous observational studies (13, 14), but controlled feeding studies have reported elevated levels of estrogen among participants who were supplied moderate amounts of alcohol compared with placebo (15, 16). Adiposity also directly influences levels of many circulating hormones such as estrogens, testosterone, and insulin (17, 18). The association between body mass index (BMI) and breast cancer is substantially diminished following adjustment for serum estradiol levels, indicating that endogenous hormones are important contributors to the underlying association (4, 19); however, recent data suggest that insulin could also be a mediator (19). Finally, higher physical activity has been linked to lowered estrogen levels in postmenopausal women even after adjustment for BMI, suggesting an independent effect of physical activity (17, 20).
Given the aforementioned evidence, a causal pathway from lifestyle factors to breast cancer that operates through serum estrogen and/or insulin levels is plausible. However, few studies have evaluated the whole pathway from lifestyle factors via estrogens to breast cancer. In this study, we investigate how much of the effect of lifestyle factors on risk of breast cancer is mediated through endogenous levels of estradiol and the extent to which the association of obesity with breast cancer is mediated through insulin levels. This study applies newly developed methods to separate the direct effects of these lifestyle factors (through other pathways) and the indirect effect operating through estrogen. Assuming proper confounding control and no measurement error, the measure has a direct causal interpretation and quantifies additional breast cancer events per unit of time due to a given exposure through direct and indirect (mediated) pathways (21).
Materials and Methods
Study population
The WHI included 4 clinical trials and an observational study (WHI-OS) to examine the risk factors and determinants of cardiovascular disease, cancer, and other health problems of postmenopausal women. General eligibility included age between 50 and 79 years, accessible for follow-up and estimated survival of ≥3 years. The clinical trials had additional eligibility requirements largely related to medical history. The current study population includes data from a subset of the WHI-OS (22, 23). Information on demographic and behavioral factors, medical history, and medication use was collected by a questionnaire and a physical examination at baseline. Blood samples were collected following an overnight fast of at least 12 hours with separated sera stored at −70°C within 2 hours of collection (24). When breast cancer cases and subcohort were identified, the mean follow-up time was 77 months, with 1.6% lost to follow-up and 4.7% deceased.
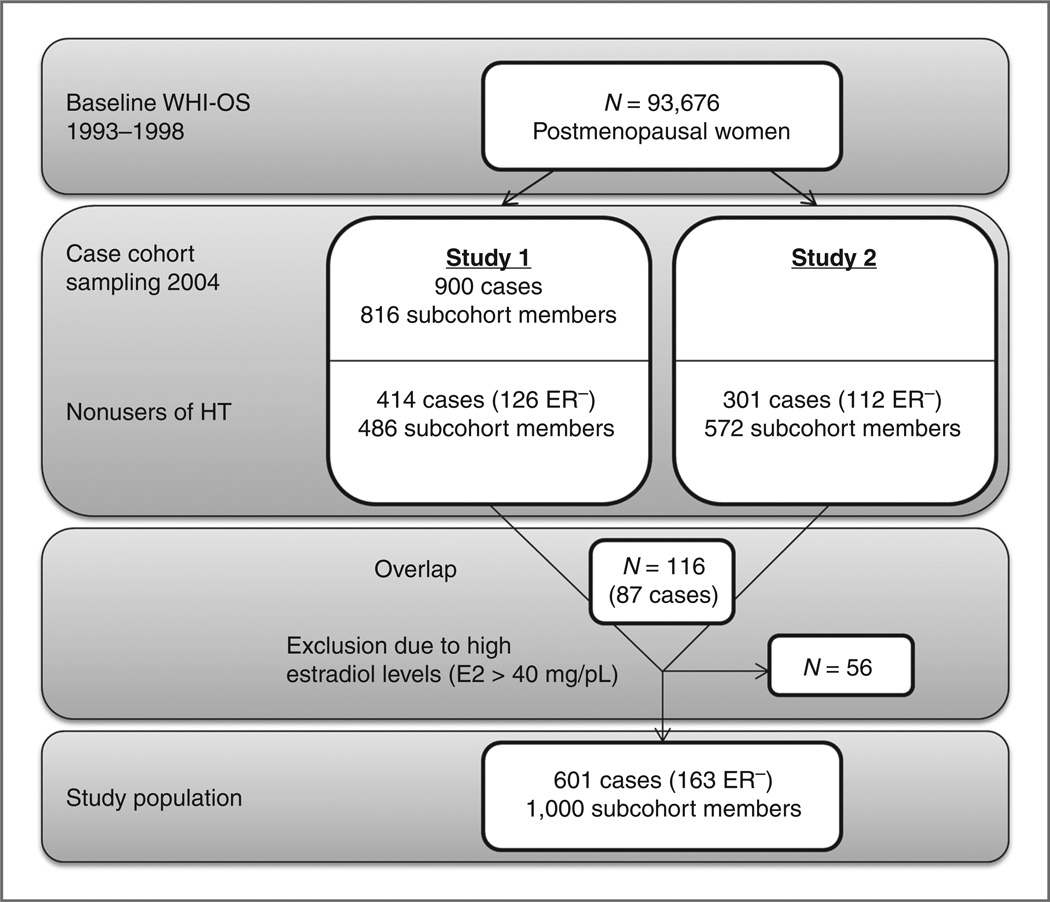
The analyses combined data from 2 case-cohort ancillary studies in the WHI-OS (study 1 and study 2) where baseline endogenous estradiol (E2) levels were available and one (study 1) in which fasting insulin determinations were available. The selection process is described in Fig.1. Study 1 randomly sampled 900 invasive breast cancer cases from the approximately 1,800 cases available on February 29, 2004. A representative subcohort of 816 women from among all participants of the WHI-OS at baseline was randomly selected as the comparison group (controls). We excluded current users of hormone therapy at baseline. Among the selected subjects, 414 cases and 486 subcohort members were current nonusers of hormone therapy. Of these cases, 126 were estrogen receptor (ER)-negative. E2 levels were measured with the use of a Vitros-Eci Immunodiagnostic assay with a sensitivity of 5 pg/mL from Esoterix. Serum insulin levels were measured for participants in study 1 using ELISAs with a sensitivity of 0.26 mIU/mL. For the analysis of insulin as a mediating factor, we excluded diabetics (n = 54; ref. 19). Similarly, study 2 included 301 invasive breast cancers and a subcohort of 572 (end of follow-up August 31, 2004). The 301 breast cancer cases included all ER-negative breast cancers among eligible women in the WHI-OS (n = 112) and a random sample of ER-positive cases. The subcohort was randomly selected from all WHI-OS participants who were non–hormone therapy users at baseline. E2 concentrations in study 2 were quantified using radioimmunoassays following organic solvent extraction and Celite column partition chromatography with a sensitivity of 2 pg/mL at the Reproductive Endocrine Research Laboratory (University of Southern California, Los Angeles, CA; ref. 25). Eighty-seven cases and 29 subcohort members overlapped between the 2 studies. These women were included with estradiol results from study1.Women with estradiol levels above 40 pg/mL (n = 56) were excluded to ensure postmenopausal status. Combining the 2 studies yielded a total of 601 breast cancer cases and 1,000 subcohort members.
Figure 1.
Selection of study participants. HT, hormone therapy; ER−, estrogen receptor negative
Lifestyle factors
At baseline, weight was measured on a balance beam scale and height was measured using a wall-mounted stadiometer with BMI calculated as weight (kg) divided by squared height (m2; ref. 24). Alcohol consumption was assessed in a self-administered food frequency questionnaire as number of servings of each alcohol type (beer, wine, liquor) per week during the preceding 3 months. The total weekly intake was given by the sum of the beverage-specific intakes. Data on physical activity were assessed by questionnaire asking about the frequency, duration, and intensity of exercise. Metabolic equivalent values (MET) were assigned for the activities and multiplied by the hours exercised at that intensity level per week. A total physical activity measure (MET-h/wk) was obtained by summing the values for all types of activities (26).
Breast cancer incidence
Information on breast cancer incidence was initially collected through annual self-administered questionnaires. Subsequently, breast cancer status and clinical and pathologic characteristics of the tumors were confirmed through centralized reviews of hospital discharge summaries, operative reports, history and physical examination, radiology reports, and oncology consultant reports (27).
Other covariates
Covariates were assessed in the self-administered baseline questionnaire and included age, ethnicity, education, marital status, smoking, age at menarche, age at menopause, parity, age at first child’s birth, and first-degree relative with breast cancer.
Statistical analysis
The 2 case–cohort studies were combined by an aggregated pooled analysis technique allowing for the calculation of a single exposure–disease effect estimate while stratifying by study origin (28). Follow-up was calculated from baseline to the date of breast cancer diagnosis, the date of death, or end of follow-up, whichever came first. The case–cohort sampling design was taken into account in the analyses by weighting according to Kalbfleisch and Lawless in order for the cases and subcohort members to represent the total study population (29, 30). Thus, ER-negative cases were included with weight 1 given the sampling strategy, whereas ER-positive cases and subcohort members were weighted with inverse sampling probabilities.
To avoid reduction in sample size and bias related to listwise deletion, missing values were imputed by means of multiple imputation using the MI procedure of SAS version 9.2 (31). Continuous data were imputed by the Markov Chain Monte Carlo method (32). Subsequently, missing values of categorical data were imputed by the logistic regression method. The variables BMI, alcohol intake, and physical activity were logarithmically transformed to accommodate the multivariate normal assumption of the model and reverse-transformed for analysis. The imputation model included all variables involved in the subsequent analyses as predictors as well as the outcome. Results were combined by the PROC MIANA-LYZE procedure, thereby obtaining inferences reflecting the uncertainty about the predictions of the missing data. Because the proportion of missing values was low (BMI, 2%; alcohol, 0.2%; and physical activity, 1.4%),10 imputed data sets were considered appropriate (33).
Differences in distribution of baseline characteristics and hormone levels between cases and subcohort members were compared by Wilcoxon signed rank-sum test (continuous data) and Pearson χ2 test (categorical data). Overall associations between lifestyle factors and breast cancer were evaluated by the Cox proportional hazards model. We evaluated whether the lifestyle factors were linearly associated with breast cancer by visual inspection of the variables grouped according to the quintiles of their distribution. The mediating effect of estradiol or insulin was estimated by applying a newly developed method for mediation analysis in a survival setting based on the Aalen additive hazards model (21). Like the standard Cox model, the Aalen model with time-constant effects has an unspecified baseline hazard and effects of each covariate are modeled by a single parameter (the HRvs. the additive effect). Thus, the 2 models are equally flexible, but the interpretation of effect measures is of course different. For a given exposure, for example alcohol consumption, the absolute incidence rate change for a given alcohol intake provides an estimate of additional cases compared with abstainers which can be decomposed into a direct pathway (i.e., effect of alcohol consumption on breast cancer) and an indirect pathway (e.g., mediated through endogenous estradiol levels; ref. 34). The analyses only focus on indirect effects of E2 and insulin; thus, the direct effect associated with one mediator can (partly) constitute the indirect effect of another, possibly unmeasured, mediator.
The method involved 2 steps: first, estimation of the effects of exposures on the mediator by a linear regression model, and second, estimation of the effect of both exposure and estradiol on breast cancer incidence by fitting an additive hazard model adjusted for confounders (35). The model was tested for time-dependent effects of covariates as suggested by Martinussen and Scheike (36), and no indication of time-dependent effects for either the exposures or mediator was observed. The total effects (TE) of lifestyle factors on breast cancer were given by the sum of the direct effects (DE) and the indirect effects (IE). The indirect effect (through estradiol) was given by the product of the parameter estimates for the regression of the mediator on the lifestyle factors and the parameter estimate of the effect of the mediator on breast cancer from the additive hazards model. The mediated proportion was computed as IE/TE. For the direct effect, 95% confidence limits were readily available from the model output, whereas limits for the indirect and total effects as well as mediated proportion were computed from the SEs and covariances of the estimated parameters by parametric bootstrap. The bootstrap works by simulating 100,000 replications of the estimated parameters and subsequently computing total and indirect effects as well as mediated proportions for each replication. A 95% confidence interval (CI) can then be obtained as the 2.5th and 97.5th percentiles of the simulated values.
Sensitivity analyses included (i) subset analyses according to ER status on breast cancer cases and according to histologic type, (ii) exclusion of cases occurring within the first 3 years after baseline and women with estradiol >30 pg/mL, (iii) separate analyses for the 2 case–cohort samples, (iv) comparison of the results of the multiply imputed data with complete case analyses, (v) addressing interaction between the 3 lifestyle factors in terms of breast cancer and between alcohol consumption and familial history of breast cancer, and (vi) correlations between logarithmically transformed E2 values of participants overlapping between the 2 ancillary studies by the Pearson correlation coefficient.
As an additional subanalysis, we examined the mediating effect of insulin (log-transformed) on the relation between BMI and breast cancer. This analysis was based on data from study 1 which included 791 nondiabetic women (382 cases and 409 subcohort members) with baseline insulin measurements.
Descriptive statistics and Cox regression analyses were conducted using SAS version 9.2 (SAS Institute). Mediation analyses were conducted in R version 2.10.1 (37). All tests of statistical significance were 2-sided.
Results
The pooled study population comprised 601 cases and 1,000 subcohort members (Table 1). Compared with subcohort members, case subjects had a higher BMI, higher alcohol consumption, and a later onset of menopause. The baseline median levels of estradiol and insulin (among study 1 participants) were higher among cases.
Table 1.
Baseline characteristics and sex hormone levels of breast cancer case subjects and subcohort (Cont'd)
| Variable | Cases (n = 601) | Subcohort (n = 1,000) | Pa |
|---|---|---|---|
| Median BMI, kg/m2 (5th–95th percentile) | 27.6 (20.9–41.0) | 26.8 (20.4–39.6) | 0.03 |
| Missing (%) | 5 (1) | 20 (2) | |
| Alcohol consumption, n (%) | 0.03 | ||
| Abstainers | 242 (40) | 459 (46) | |
| <7 drinks/wk | 265 (44) | 423 (42) | |
| 7+ drinks/wk | 92 (15) | 116 (12) | |
| Missing | 2 (0) | 2 (0) | |
| Median physical activity, METs-h/wk (5th–95th percentile) | 8.8 (0–37.6) | 8.3 (0–41.2) | 0.68 |
| Missing (%) | 10 (2) | 13 (1) | |
| Median age, y (5th–95th percentile) | 67 (53–77) | 66 (53–77) | 0.20 |
| Race/ethnicity, n (%) | 0.15 | ||
| White | 517 (86) | 815 (82) | |
| Black | 50 (8) | 111 (11) | |
| Hispanic | 16 (3) | 35 (4) | |
| Asian/other | 17 (3) | 36 (4) | |
| Missing | 1 (0) | 3 (0) | |
| Highest education level, n (%) | 0.39 | ||
| High school or less | 129 (21) | 244 (24) | |
| College | 299 (50) | 472 (47) | |
| Postgraduate education | 162 (27) | 273 (27) | |
| Missing | 11 (2) | 11 (1) | |
| Marital status, n (%) | 0.11 | ||
| Never married | 37 (6) | 67 (6) | |
| Divorced/separated | 76 (13) | 168 (17) | |
| Widowed | 126 (21) | 214 (21) | |
| Married/marriage-like relationship | 356 (59) | 544 (55) | |
| Missing | 6 (1) | 7 (1) | |
| Smoking status, n (%) | 0.05 | ||
| Never | 311 (52) | 507 (51) | |
| Former | 259 (43) | 412 (41) | |
| Current | 24 (4) | 70 (7) | |
| Missing | 7 (1) | 11 (1) | |
| Age at menarche, n (%) | 0.23 | ||
| ≤10 y | 46 (8) | 80 (8) | |
| 11–12 y | 252 (42) | 379 (38) | |
| ≥ 13 y | 297 (49) | 538 (54) | |
| Missing | 6 (1) | 3 (0) | |
| Age at menopause, n (%) | 0.03 | ||
| ≤42 y | 66 (11) | 150 (15) | |
| 43–48 y | 124 (21) | 239 (24) | |
| 49–51 y | 144 (24) | 215 (22) | |
| ≥52 y | 220 (37) | 327 (32) | |
| Missing | 47 (8) | 69 (7) | |
| Parity, n (%) | 0.89 | ||
| 0 children | 90 (15) | 153 (15) | |
| 1–2 children | 192 (32) | 330 (33) | |
| ≥3 children | 312 (52) | 508 (51) | |
| Missing | 7 (1) | 9 (1) | |
| Age at first child's birth, n (%) | 0.18 | ||
| <20 y | 57 (9) | 100 (10) | |
| 20–24 y | 182 (30) | 348 (35) | |
| 25–29 y | 153 (25) | 211 (21) | |
| ≥30 y | 53 (9) | 76 (8) | |
| Nulliparous | 90 (15) | 153 (15) | |
| Missing | 66 (11) | 112 (11) | |
| First-degree relative with breast cancer, n (%) | 159 (26) | 220 (22) | 0.09 |
| Missing | 24 (4) | 69 (7) | |
| Median serum level of total estradiol, pg/mL | 10.9 (5.0–25.2) | 10.0 (4.3–24.2) | 0.01 |
| Median serum level of total insulin, µIU/mLb | 6.8 (2.5–18.1) | 5.6 (2.3–18.2) | 0.002 |
P value for comparison of cases with subcohort obtained from Wilcoxon signed rank-sum test (continuous data) and Pearson χ2 test (categorical data).
Among participants in study 1 (cases, n = 382; subcohort, n = 409).
In the Cox analysis, a 5-unit increase in BMI was associated with a 1.14 (95% CI, 1.04–1.25) higher risk of breast cancer. Adjustment for estradiol reduced the HR to 1.11 (95% CI, 1.00–1.22). Compared with women who reported no alcohol consumption, those reporting a weekly consumption of 7+ drinks had a higher breast cancer risk of 1.59 (95% CI, 1.10–2.29). Adjustment for estradiol had little impact on this relation. We observed no association between physical activity and breast cancer in this subpopulation, which is why this factor was omitted from the mediation analyses.
Higher levels of estradiol were observed with increasing levels of BMI and alcohol consumption (Table 2). For every 5-unit change in BMI, a 17.5% higher estradiol level was observed. In women with an alcohol consumption of 7+ drinks/wk, a6% higher level of estradiol was observed compared with abstainers.
Table 2.
Parameter estimates and SEs for the regression of log(estradiol) on BMI and alcohol consumption adjusting for confounders
| Estimate (SE)×102 |
Relative Change in E2 |
95% CI | |
|---|---|---|---|
| BMI (5-unit increase) | 16.15 (1.04) | 17.5% | 15.2–19.9 |
| Alcohol consumption | |||
| Abstainers | 0.00 (ref.) | ||
| <7 drinks/wk | −0.41 (2.72) | −0.5% | −6.6 to 5.0 |
| 7+ drinks/wk | 5.82 (4.28) | 6.0% | −2.5 to 15.3 |
NOTE: Adjusted forage, ethnicity, educational level, marital status, smoking, age at menarche, age at menopause, parity, age at first child's birth, and first-degree relative with breast cancer.
The unadjusted absolute breast cancer rate was 370 cases per 100,000 women per year. The direct effects of BMI and alcohol consumption on breast cancer risk derived from the additive hazards model, and the indirect and total effects are presented in Table 3. The incidence of breast cancer increased with higher BMI. A 5-unit higher BMI was associated with 50.0 (95% CI, 23.2–76.6) extra cases per 100,000 women at-risk per year. Of these, 23.8% (95% CI, 2.9–68.4) could be attributed to the pathway through estradiol. Correspondingly, consuming 7+ drinks/wk compared with alcohol abstinence was associated with 178.3 (95% CI, 59.5–296.7) additional cases per 100,000 of which only 2.4% (95% CI, −1.2 to 10.7) could be attributed to the pathway through estradiol.
Table 3.
Total and direct effects for BMI and alcohol consumption and indirect effects of estradiol on breast cancer derived from linear regression parameter estimates and the additive hazard model
| DE(95%CI)×10−5 | IE (95% CI) ×10−5 | TE (95% CI) ×10−5 | IE/TE (95% CI) | |
|---|---|---|---|---|
| BMI (5-unit increase) | 38.0 (8.1–68.1) | 11.9 (1.7–22.6) | 50.0 (23.2–76.6) | 0.238 (0.029–0.684) |
| Alcohol consumption | ||||
| Abstainers → <7 drinks/wk | 48.8 (−18.2 to 115.7) | −0.3(−5.0 to 4.2) | 48.6 (−18.7 to 115.6) | −0.006 (−0.311 to 0.278) |
| Abstainers → 7+ drinks/wk | 174.0 (55.3 to 292.2) | 4.3 (−1.8 to 13.5) | 178.3 (59.5 to 296.7) | 0.024(−0.012 to 0.107) |
NOTE: Adjusted for age, ethnicity, educational level, marital status, physical activity, smoking, age at menarche, age at menopause, parity, age at first child's birth, and first-degree relative with breast cancer.
Analyses restricted to ER-positive breast cancer cases generally yielded results similar to the overall population (Table 4). However, the proportion of the association between BMI and breast cancer mediated by estradiol was remarkably higher for ER-positive tumors (48.8%; 95% CI, 18.8%–161.1%). The effect of alcohol consumption on ER-positive breast cancers was similar to the main analyses. Results of the analyses restricted to ER-negative breast cancers were very different indicating a negative indirect effect of estradiol. However, this subanalysis was based on a very low number of cases (n = 163) and all estimates were nonsignificant. Separate alcohol analyses according to histologic type suggested a larger effect on ER-positive lobular breast cancers than ER-positive ductal breast cancers. The total effect of 7+ drinks/wk compared with abstainers was 121.3 (95% CI, 55.2–187.2) extra cases per 100,000 for ER-positive lobular breast cancers compared with 86.4 (95% CI, 4.9–167.6) extra cases per 100,000 for ER-positive ductal breast cancers.
Table 4.
Total and direct effects for BMI and alcohol consumption and indirect effects of estradiol on ER-positive and -negative breast cancers]
| DE (95% CI) × 10 −5 | IE (95% CI) × 10 −5 | TE (95% CI) × 10 −5 | IE/TE (95% CI) | |
|---|---|---|---|---|
| ER-positive breast cancers (n = 401) | ||||
| BMI (5-unit increase) | 16.9 (−7.4 to 41.1) | 16.1 (7.5–25.2) | 33.0 (10.3–55.5) | 0.488 (0.188–1.611) |
| Alcohol consumption | ||||
| Abstainers → <7 drinks/wk | 58.0 (−0.3 to 116.2) | −0.3 (−6.3 to 5.7) | 57.8 (−0.9 to 116.3) | −0.005 (−0.255 to 0.191) |
| Abstainers → 7+ drinks/wk | 183.0 (76.0–289.6) | 5.8 (−2.9 to 16.7) | 188.9 (81.4–296.0) | 0.031 (−0.017 to 0.110) |
| ER-negative breast cancers (n = 163) | ||||
| BMI (5-unit increase) | 15.2 (0.9–29.6) | −3.0 (−7.9 to 1.7) | 12.1 (−0.1 to 24.4) | −0.248 (−1.430 to 0.468) |
| Alcohol consumption | ||||
| Abstainers → <7 drinks/wk | −11.9 (−41.7 to 17.8) | 0.1 (−1.6 to 1.8) | −11.9 (−41.6 to 17.9) | −0.008 (−0.394 to 0.398) |
| Abstainers → 7+ drinks/wk | −31.0 (−72.0 to 9.8) | −1.1 (−4.9 to 1.1) | −32.1 (−73.0 to 8.7) | 0.034 (−0.199 to 0.367) |
NOTE: Adjusted for age, ethnicity, educational level, marital status, physical activity, smoking, age at menarche, age at menopause, parity, age at first child's birth, and first-degree relative with breast cancer.
In a subanalysis of the mediating effect of insulin for the relation between BMI and breast cancer (study 1 subjects only), we found higher levels of insulin with increasing BMI. For every 5-unit change in BMI, a 26.0% higher insulin level was observed (95% CI, 22.6–29.3). A 5-unit increase in BMI was associated with 52.0 (95% CI, 12.1–91.3) additional cases per 100,000 women at-risk per year (Table 5). The proportion mediated by estradiol was 21.0% (95% CI, −3.8 to 119.4) whereas insulin mediated 65.8% (95% CI, 13.6–273.3) of this relation.
Table 5.
Direct, indirect, and total effects for BMI on breast cancer based on data from study 1 (N = 791)
| DE (95% CI) × 10 −5 | IE (95% CI) × 10 −5 | TE (95% CI) × 10 −5 | IE/TE (95% CI) | |
|---|---|---|---|---|
| BMI (5-unit increase) | 6.9 (−46.6 to 61.1) | 52.0 (12.1–91.3) | ||
| Estradiol | 10.9 (−1.8 to 24.6) | 0.210 (−0.038 to 1.194) | ||
| Insulin | 34.2 (9.4–59.0) | 0.658 (0.136–2.733) |
NOTE: Adjusted for age, ethnicity, educational level, marital status, physical activity, smoking, age at menarche, age at menopause, parity, age at first child's birth, and first-degree relative with breast cancer.
Subset analyses of the 2 case𣀓cohort samples were conducted to exclude heterogeneity between study-specific effects. The effects of the lifestyle factors on breast cancer risk were generally similar across studies, and including interaction terms between the exposures and study origin in the Cox model did not indicate between-study differences in the relative risk of breast cancer by BMI (Pinteraction = 0.47), physical activity (Pinteraction = 0.72), or alcohol consumption (Pinteraction = 0.23). Mediation analyses for the 2 studies separately revealed stronger effects for the lifestyle factors in study 1 than in study 2. However, the mediated proportions of estradiol were very similar.
Comparing the multiple imputation analysis with the complete case analysis indicated little difference in results between the 2 approaches. Excluding cases occurring within the first 3 years following baseline (n = 247) and all women with estradiol levels above 30 pg/mL (n = 44) did not affect the strength of the observed associations. Tests for 3-factor interaction between BMI, alcohol consumption, and physical activity indicated that none of these factors modified the effect of the other on breast cancer risk. When excluding women with a family history of breast cancer, the increased risk of breast cancer with higher alcohol intake became more pronounced; the total effect of a weekly alcohol consumption of 7+ drinks was 205.4 (95% CI, 70.5–339.9) additional cases per 100,000 women at-risk per year compared with abstainers, but the indirect effect of estradiol remained negligible. Because the 2 ancillary studies used different assay methods (with different sensitivities) for measuring estradiol, we compared values for the overlapping participants between the 2 studies. The mean values of the log-transformed E2 values from the 2 assessments overlapping were 2.6 (SD = 0.4) and 2.7 (SD = 0.5), and a strong-to-moderate correlation in the estradiol measurements (r = 0.60, P < 0.001) was observed. Also, similar associations of E2 levels and breast cancer were observed between the 2 studies.
Discussion
This analysis of postmenopausal women indicated that an intervention that could modify the estradiol and insulin levels for women with high BMI to the estradiol and insulin levels of women with lower BMI has the potential to reduce a substantial number of breast cancer cases in obese women.
Our finding on the relation between BMI and postmenopausal breast cancer is consistent with previous studies (3). Data on the mediating effect of estrogen and/or insulin are sparse and, as mentioned, appropriate quantifications of the mediated proportion are, to our knowledge, lacking. However, 2 large scale pooled analyses examining the mediating effect of estrogen based on the traditional approach to mediation analysis concluded that estrogen largely explained the association between BMI and breast cancer (4, 38). In our analysis, when restricted to ER-positive breast cancer cases, the proportion of the association mediated by estradiol was more pronounced, which supports the causal interpretation. However, as only approximately 55% of the risk appeared to be mediated by estradiol, our findings also suggest that other important mediators play a role in this relation. A previous analysis based on data from study 1 found hyperinsulinemia to be an independent risk factor for breast cancer after adjustment for estradiol and other risk factors. Furthermore, control for insulin attenuated the BMI–breast cancer relation to a greater degree than adjustment for estradiol (19). Our analysis, which quantifies these relations, indicates that insulin plays a greater role than estradiol in the obesity–breast cancer relationship. These findings, which require confirmation, add to the growing awareness that hyperinsulinemia represents a significant biologic contributor in breast cancer. Other potential mediating pathways to be explored include androgens and inflammatory factors—both of which are known to be upregulated in obesity and have been linked to breast cancer risk (12, 39).
Previous studies on alcohol consumption and breast cancer also reported an elevated risk with higher consumption (1, 2, 40). Our results are compatible with indirect effects via estradiol ranging from none to as much as 15%, so at least a substantial proportion of the alcohol–breast cancer relation seemed to be mediated through other mechanisms than estradiol. A previous large WHI study on alcohol and breast cancer subtypes found alcohol to be more strongly related to lobular than ductal carcinomas and to hormone receptor–positive tumors than to hormone receptor–negative tumors (40), which was supported by our further analyses. Also, a large meta-analysis investigated effects of alcohol on breast cancer according to estrogen and progesterone receptor (PR) status (41) and found a stronger effect of alcohol on ER+ than ER− tumors. The risk was mostly pronounced for ER+/PR− tumors. A similar pattern was observed in a recent case–control study (42). It has been proposed that classical ER-mediated estrogenic action is related to PR expression (43); However, a positive association between alcohol consumption and ER+/PR− tumors indicates that the relation cannot be explained by the estrogen pathway only (41). Another suggested mechanism is the induction of carcinogenesis in breast tissue caused by acetaldehyde generated during alcohol metabolism (44). However, it is also likely that the measurements in our study did not accurately capture the elevated estradiol levels caused by alcohol consumption. We observed a weak association between alcohol consumption and endogenous estradiol levels which is not in accordance with findings of previous studies (11, 14, 45). The effect of alcohol ingestion on endogenous estradiol levels is rather acute and if the alcohol is primarily consumed in the evening or during weekends and blood is collected during the day, this relation would be attenuated (12, 46). In support of this explanation, a pronounced effect of alcohol on ER+ tumors was observed whereas no effect was found for ER− tumors. Also, the reported alcohol consumption among women in this study was extremely low which may explain the weak association with estradiol.
The study was limited by the fact that the women may have had subclinical disease at the time of measurement of hormone levels (baseline). However, excluding breast cancers occurring in the first 3 years after baseline did not affect the estimates. Also, individual measurements of hormone concentrations are subject to error due to assay variations and short-term fluctuations in serum levels of estradiol and insulin. Even random error in measuring the mediator would bias the results as the indirect effect would be underestimated whereas the direct effect of lifestyle factors would be overestimated. Repeated measurements would have increased the precision of participants’ estradiol and insulin levels. However, previous studies have found high correlations between measurements of plasma estrogen and insulin over at least 3 years in postmenopausal women (47,48), indicating that misclassification of estradiol and insulin levels, at least in the medium term, is limited. As discussed above, breast cancer subtypes may have different etiology and risk factors. While our data enabled us to conduct separate analyses according to ER subtype, we did not have sufficient statistical power to address effects according to joint ER and PR status. In addition, the analyses were based on pooled data from 2 different case–cohort samples. Such a procedure requires homogeneity between the effects of exposures on the outcome. Since both samples were derived from the same cohort and all exposures and covariates have been measured through the same questionnaire, we found it reasonable to pool the data (28). Comparing results from individual studies did not reveal discernible differences, although the effect estimates for alcohol consumption were attenuated by pooling the data as no effect was observed in study 2. The observed difference in effect of alcohol between the 2 studies may be explained by the fact that study 2 included relatively more ER− cases. Insulin measurements were only available for a subset of data and confidence bounds for these estimates were very wide. Additional studies with fasting insulin are needed to further address this hypothesis. Finally, the WHI-OS consists of women who were ineligible or uninterested in participating in the randomized trials, which may raise concern about the generalizability of our results. However, Hays and colleagues have previously provided a detailed comparison of baseline information for the different study components of the WHI and did not find marked differences (22).
In summary, this study applied a new analytic tool for estimating mediating effects. Traditional approaches to mediation analysis typically involve the comparison of Cox models with and without adjustment for the mediator. Such an approach is limited, most importantly because the estimates do not have a causal interpretation and is not mathematically consistent (49, 50). This study applied a causally interpretable measure of mediation with CIs for the proportion mediated. Our results may prove useful in supporting weight loss interventions and identifying biomarker pathways of breast cancer risk in overweight and obese women.
Acknowledgments
The authors thank the following WHI key investigators:
Program Office: (National Heart, Lung, and Blood Institute, Bethesda, Maryland) Jacques Rossouw, Shari Ludlam, Dale Burwen, Joan McGowan, Leslie Ford, and Nancy Geller
Clinical Coordinating Center: (Fred Hutchinson Cancer Research Center, Seattle, WA) Garnet Anderson, Ross Prentice, Andrea LaCroix, and Charles Kooperberg
Investigators and Academic Centers: (Brigham and Women’s Hospital, Harvard Medical School, Boston, MA) JoAnn E. Manson; (Med-Star Health Research Institute/Howard University, Washington, DC) Barbara V. Howard; (Stanford Prevention Research Center, Stanford, CA) Marcia L. Stefanick; (The Ohio State University, Columbus, OH) Rebecca Jackson; (University of Arizona, Tucson/Phoenix, AZ) Cynthia A. Thomson; (University at Buffalo, Buffalo, NY) Jean Wactawski-Wende; (University of Florida, Gainesville/Jacksonville, FL) Marian Limacher; (University of Iowa, Iowa City/Davenport, IA) Robert Wallace; (University of Pittsburgh, Pittsburgh, PA) Lewis Kuller; (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker
Women’s Health Initiative Memory Study: (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker.
Grant Support
This work was supported by the National Cancer Institute (grant number R01-CA93881-01 to H.D. Strickler); the Da Costa International Funds for Breast Cancer Prevention; the National Center for Research Resources (NCRR; grant number UL1 RR024146); and NIH Roadmap for Medical Research (to J.S. Lee). U.A. Hvidtfeldt is supported by the Commission of Social Inequality in Cancer (grant no. SU08004). M.J. Gunter and H.D. Strickler are supported, in part, by the Albert Einstein Cancer Center. The WHI program is funded by the National Heart, Lung, and Blood Institute; NIH; US Department of Health and Human Services through contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
Disclosure of Potential Conflicts of Interests
M.S. Freiberg has employment (other than primary affiliation; e.g., consulting) in VA health care system as clinical investigator. No potential conflicts of interest were disclosed by the other authors.
Authors' Contributions
Conception and design: U.A. Hvidtfeldt, T. Lange, A. Tjønneland, N.H. Rod
Development of methodology: U.A. Hvidtfeldt, T. Lange, N. Keiding
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): M.J. Gunter, R.T. Chlebowski, D. Lane, J.S. Lee, A. Tjønneland, M.Z. Vitolins, S. Wassertheil-Smoller
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): U.A. Hvidtfeldt, M.J. Gunter, T. Lange, R.T. Chlebowski, G.N. Farhat, M.S. Freiberg, N. Keiding, R. Prentice, H.D. Strickler, N.H. Rod
Writing, review, and/or revision of the manuscript: U.A. Hvidtfeldt. M.J. Gunter, T. Lange, R.T. Chlebowski, D. Lane, G.N. Farhat, M.S. Freiberg, N. Keiding, J.S. Lee, R. Prentice, A. Tjønneland, M.Z. Vitolins, S. Wassertheil-Smoller, H.D. Strickler, N.H. Rod
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): U.A. Hvidtfeldt, G.N. Farhat, S. Wassertheil-Smoller
Study supervision: M.J. Gunter, A. Tjønneland, S. Wassertheil-Smoller
References
- 1.Smith-Warner SA, Spiegelman D, Yaun SS, van den Brandt PA, Folsom AR, Goldbohm RA, et al. Alcohol and breast cancer in women - a pooled analysis of cohort studies. JAMA. 1998;279:535–540. doi: 10.1001/jama.279.7.535. [DOI] [PubMed] [Google Scholar]
- 2.Longnecker MP. Alcoholic beverage consumption in relation to risk of breast cancer: meta-analysis and review. Cancer Causes Control. 1994;5:73–82. doi: 10.1007/BF01830729. [DOI] [PubMed] [Google Scholar]
- 3.van den Brandt PA, Spiegelman D, Yaun SS, Adami HO, Beeson L, Folsom AR, et al. Pooled analysis of prospective cohort studies on height, weight, and breast cancer risk. Am J Epidemiol. 2000;152:514–527. doi: 10.1093/aje/152.6.514. [DOI] [PubMed] [Google Scholar]
- 4.Key TJ, Appleby PN, Reeves GK, Roddam A, Dorgan JF, Longcope C, et al. Body mass index, serum sex hormones, and breast cancer risk in postmenopausal women. J Natl Cancer Inst. 2003;95:1218–1226. doi: 10.1093/jnci/djg022. [DOI] [PubMed] [Google Scholar]
- 5.Monninkhof EM, Elias SG, Vlems FA, van der Tweel I, Schuit AJ, Voskuil DW, et al. Physical activity and breast cancer - a systematic review. Epidemiology. 2007;18:137–157. doi: 10.1097/01.ede.0000251167.75581.98. [DOI] [PubMed] [Google Scholar]
- 6.Hamajima N, Hirose K, Tajima K, Rohan T, Calle EE, Heath CW, Jr, et al. Alcohol, tobacco and breast cancer - collaborative reanalysis of individual data from 53 epidemiological studies, including 58,515 women with breast cancer and 95,067 women without the disease. Br J Cancer. 2002;87:1234–1245. doi: 10.1038/sj.bjc.6600596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McTiernan A, Kooperberg C, White E, Wilcox S, Coates R, dams-Campbell LL, et al. Recreational physical activity and the risk of breast cancer in postmenopausal women - The Women's Health Initiative cohort study. JAMA. 2003;290:1331–1336. doi: 10.1001/jama.290.10.1331. [DOI] [PubMed] [Google Scholar]
- 8.Dorgan JF, Longcope C, Franz C, Stanczyk FZ, Chang LC, Stephen-son HE, et al. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst. 2002;94:606–616. doi: 10.1093/jnci/94.8.606. [DOI] [PubMed] [Google Scholar]
- 9.Thomas HV, Reeves GK, Key TJA. Endogenous estrogen and postmenopausal breast cancer a quantitative review. Cancer Causes Control. 1997;8:922–928. doi: 10.1023/a:1018476631561. [DOI] [PubMed] [Google Scholar]
- 10.Goss PE, Ingle JN, Ales-Martinez JE, Cheung AM, Chlebowski RT, Wactawski-Wende J, et al. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med. 2011;364:2381–2391. doi: 10.1056/NEJMoa1103507. [DOI] [PubMed] [Google Scholar]
- 11.Rod NH, Hansen AM, Nielsen J, Schnohr P, Gronbaek M. Low-risk factor profile, estrogen levels, and breast cancer risk among postmenopausal women. Int J Cancer. 2009;124:1935–1940. doi: 10.1002/ijc.24136. [DOI] [PubMed] [Google Scholar]
- 12.Key TJ, Appleby PN, Reeves GK, Roddam AW, Helzlsouer KJ, Alberg AJ, et al. Circulating sex hormones and breast cancer risk factors in postmenopausal women: reanalysis of 13 studies. Br J Cancer. 2011;105:709–722. doi: 10.1038/bjc.2011.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newcomb PA, Klein R, Klein BE, Haffner S, Mares-Perlman J, Cruickshanks KJ, et al. Association of dietary and life-style factors with sex hormones in postmenopausal women. Epidemiology. 1995;6:318–321. doi: 10.1097/00001648-199505000-00022. [DOI] [PubMed] [Google Scholar]
- 14.Hankinson SE, Willett WC, Manson JE, Hunter DJ, Colditz GA, Stampfer MJ, et al. Alcohol, height, and adiposity in relation to estrogen and prolactin levels in postmenopausal women. J Natl Cancer Inst. 1995;87:1297–1302. doi: 10.1093/jnci/87.17.1297. [DOI] [PubMed] [Google Scholar]
- 15.Dorgan JF, Baer DJ, Albert PS, Judd JT, Brown ED, Corle DK, et al. Serum hormones and the alcohol-breast cancer association in postmenopausal women. J Natl Cancer Inst. 2001;93:710–715. doi: 10.1093/jnci/93.9.710. [DOI] [PubMed] [Google Scholar]
- 16.Vatsalya V, Issa JE, Hommer DW, Ramchandani VA. Pharmacodynamic effects of intravenous alcohol on hepatic and gonadal hormones: influence of age and sex. Alcohol Clin Exp Res. 2012;36:207–213. doi: 10.1111/j.1530-0277.2011.01600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neilson HK, Friedenreich CM, Brockton NT, Millikan RC. Physical activity and postmenopausal breast cancer: proposed biologic mechanisms and areas for future research. Cancer Epidemiol Biomarkers Prev. 2009;18:11–27. doi: 10.1158/1055-9965.EPI-08-0756. [DOI] [PubMed] [Google Scholar]
- 18.Kendall A, Folkerd EJ, Dowsett M. Influences on circulating oestrogens in postmenopausal women: relationship with breast cancer. J Steroid Biochem Mol Biol. 2007;103:99–109. doi: 10.1016/j.jsbmb.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 19.Gunter MJ, Hoover DR, Yu H, Wassertheil-Smoller S, Rohan TE, Manson JE, et al. Insulin, insulin-like growth factor-I, and risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 2009;101:48–60. doi: 10.1093/jnci/djn415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coyle YM. Lifestyle, genes, and cancer. Methods Mol Biol. 2009;472:25–56. doi: 10.1007/978-1-60327-492-0_2. [DOI] [PubMed] [Google Scholar]
- 21.Lange T, Hansen JV. Direct and indirect effects in a survival context. Epidemiology. 2011;22:575–581. doi: 10.1097/EDE.0b013e31821c680c. [DOI] [PubMed] [Google Scholar]
- 22.Hays J, Hunt JR, Hubbell FA, Anderson GL, Limacher M, Allen C, et al. The Women's Health Initiative recruitment methods and results. Ann Epidemiol. 2003;13:S18–S77. doi: 10.1016/s1047-2797(03)00042-5. [DOI] [PubMed] [Google Scholar]
- 23.Design of the Women's Health Initiative clinical trial and observational study. The Women's Health Initiative Study Group. Control Clin Trials. 1998;19:61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 24.Anderson GL, Manson J, Wallace R, Lund B, Hall D, Davis S, et al. Implementation of the Women's Health Initiative study design. Ann Epidemiol. 2003;13:S5–S17. doi: 10.1016/s1047-2797(03)00043-7. [DOI] [PubMed] [Google Scholar]
- 25.Farhat GN, Cummings SR, Chlebowski RT, Parimi N, Cauley JA, Rohan TE, et al. Sex hormone levels and risks of estrogen receptor-negative and estrogen receptor-positive breast cancers. J Natl Cancer Inst. 2011;103:562–570. doi: 10.1093/jnci/djr031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ainsworth BE, Haskell WL, Leon AS, Jacobs DR, Jr, Montoye HJ, Sallis JF, et al. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993;25:71–80. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 27.Curb JD, McTiernan A, Heckbert SR, Kooperberg C, Stanford J, Nevitt M, et al. Outcomes ascertainment and adjudication methods in the Women's Health Initiative. Ann Epidemiol. 2003;13:S122–S128. doi: 10.1016/s1047-2797(03)00048-6. [DOI] [PubMed] [Google Scholar]
- 28.Smith-Warner SA, Spiegelman D, Ritz J, Albanes D, Beeson WL, Bernstein L, et al. Methods for pooling results of epidemiologic studies: the Pooling Project of Prospective Studies of Diet and Cancer. Am J Epidemiol. 2006;163:1053–1064. doi: 10.1093/aje/kwj127. [DOI] [PubMed] [Google Scholar]
- 29.Prentice RL. A case-cohort design for epidemiologic cohort studies and disease prevention trials. Biometrika. 1986;73:1–11. [Google Scholar]
- 30.Kalbfleisch JD, Lawless JF. Likelihood analysis of multi-state models for disease incidence and mortality. Stat Med. 1988;7:149–160. doi: 10.1002/sim.4780070116. [DOI] [PubMed] [Google Scholar]
- 31.SAS Institute Inc. SAS/STAT 9.22 user's guide. Cary, NC: SAS Institute Inc; 2010. The MI Procedure. [Google Scholar]
- 32.Schafer JL. Analysis of incomplete multivariate data. 1st ed. New York, NY: Chapmann and Hall; 1997. [Google Scholar]
- 33.Rubin DB. Multiple imputation after 18+ years. J Am Stat Assoc. 1996;91:473–489. [Google Scholar]
- 34.Hafeman DM, Schwartz S. Opening the Black Box: a motivation for the assessment of mediation. Int J Epidemiol. 2009;38:838–845. doi: 10.1093/ije/dyn372. [DOI] [PubMed] [Google Scholar]
- 35.Aalen OO. A model for non-parametric regression analysis of counting processes. In: Klonecki W, Kozek A, Rosinski J, editors. Lecture notes in statistics-2: mathematical statistics and probability theory. New York, NY: Springer-Verlag; 1980. pp. 1–25. [Google Scholar]
- 36.Martinussen T, Scheike TH. Dynamic regression models for survival data. 1st ed. New York, NY: Springer-Verlag; 2006. [Google Scholar]
- 37.R version 2.10.1. [cited Dec 14 2009]. Available from: http://cran.rproject.org.
- 38.Rinaldi S, Key TJ, Peeters PH, Lahmann PH, Lukanova A, Dossus L, et al. Anthropometric measures, endogenous sex steroids and breast cancer risk in postmenopausal women: a study within the EPIC cohort. Int J Cancer. 2006;118:2832–2839. doi: 10.1002/ijc.21730. [DOI] [PubMed] [Google Scholar]
- 39.Cummings SR, Lee JS, Lui LY, Stone K, Ljung BM, Cauleys JA. Sex hormones, risk factors, and risk of estrogen receptor-positive breast cancer in older women: a long-term prospective study. Cancer Epidemiol Biomarkers Prev. 2005;14:1047–1051. doi: 10.1158/1055-9965.EPI-04-0375. [DOI] [PubMed] [Google Scholar]
- 40.Li CI, Chlebowski RT, Freiberg M, Johnson KC, Kuller L, Lane D, et al. Alcohol consumption and risk of postmenopausal breast cancer by subtype: the women's health initiative observational study. J Natl Cancer Inst. 2010;102:1422–1431. doi: 10.1093/jnci/djq316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suzuki R, Orsini N, Mignone L, Saji S, Wolk A. Alcohol intake and risk of breast cancer defined by estrogen and progesterone receptor status-a meta-analysis of epidemiological studies. Int J Cancer. 2008;122:1832–1841. doi: 10.1002/ijc.23184. [DOI] [PubMed] [Google Scholar]
- 42.Bao PP, Shu XO, Gao YT, Zheng Y, Cai H, Deming SL, et al. Association of hormone-related characteristics and breast cancer risk by estrogen receptor/progesterone receptor status in the shanghai breast cancer study. Am J Epidemiol. 2011;174:661–671. doi: 10.1093/aje/kwr145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Horwitz KB, Koseki Y, McGuire WL. Estrogen control of progesterone receptor in human breast cancer: role of estradiol and antiestrogen. Endocrinology. 1978;103:1742–1751. doi: 10.1210/endo-103-5-1742. [DOI] [PubMed] [Google Scholar]
- 44.Seitz HK, Stickel F. Molecular mechanisms of alcohol-mediated car-cinogenesis. Nat Rev Cancer. 2007;7:599–612. doi: 10.1038/nrc2191. [DOI] [PubMed] [Google Scholar]
- 45.Gavaler JS, Van Thiel DH. The association between moderate alcoholic beverage consumption and serum estradiol and testosterone levels in normal postmenopausal women: relationship to the literature. Alcohol Clin Exp Res. 1992;16:87–92. doi: 10.1111/j.1530-0277.1992.tb00642.x. [DOI] [PubMed] [Google Scholar]
- 46.Gavaler JS, Love K. Detection of the relationship between moderate alcoholic beverage consumption and serum levels of estradiol in normal postmenopausal women: effects of alcohol consumption quantitation methods and sample size adequacy. J Stud Alcohol. 1992;53:389–394. doi: 10.15288/jsa.1992.53.389. [DOI] [PubMed] [Google Scholar]
- 47.Hankinson SE, Manson JE, Spiegelman D, Willett WC, Longcope C, Speizer FE. Reproducibility of plasma hormone levels in postmenopausal women over a 2–3-year period. Cancer Epidemiol Biomarkers Prev. 1995;4:649–654. [PubMed] [Google Scholar]
- 48.Kabat GC, Kim M, Caan BJ, Chlebowski RT, Gunter MJ, Ho GY, et al. Repeated measures of serum glucose and insulin in relation to postmenopausal breast cancer. Int J Cancer. 2009;125:2704–2710. doi: 10.1002/ijc.24609. [DOI] [PubMed] [Google Scholar]
- 49.Cole SR, Hernan MA. Fallibility in estimating direct effects. Int J Epidemiol. 2002;31:163–165. doi: 10.1093/ije/31.1.163. [DOI] [PubMed] [Google Scholar]
- 50.Kaufman JS, Maclehose RF, Kaufman S. A further critique of the analytic strategy of adjusting for covariates to identify biologic mediation. Epidemiol Perspect Innov. 2004;1:4. doi: 10.1186/1742-5573-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.WHI investigators. [cited 2012]. Available from: http://www.whiscience.org/publications/WHI_investigators_shortlist.pdf.