Abstract
2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) given as a cotreatment with estrogen exhibits antiestrogenic properties on the rodent adult uterus, but less is understood regarding hormonal responsiveness of the adult uterus from animals having been exposed to TCDD during critical periods of development. We characterized the inhibitory effects of TCDD (T) exposure at gestational day 15 (GD15), 4 weeks, and 9 weeks of age (TTT) on the adult uterus following hormone treatment. TTT-exposed mice in response to hormone treatment exhibited a blunted weight increase, had fewer uterine glands, displayed morphological anomalies, and had marked decreases in the hormonal regulation of genes involved in fluid transport (Aqp3 and Aqp5), cytoarchitectural (Dsc2 and Sprr2A), and immune (Lcn2 and Ltf) regulation. To determine if the 9-week exposure was responsible for the blunted uterine response, due to the 7- to 11-day half-life of TCDD in mice, a second set of experiments was performed to examine exposure to TCDD given at GD15, GD15 only (cross-fostered at birth), only during lactation (cross-fostered at birth), or at GD15 and 4 weeks of age. Our studies demonstrate that a single developmental TCDD exposure at GD15 is sufficient to elicit a blunted adult uterine response to estradiol and is due in part to fewer gland numbers and the reduced expression of forkhead box A2 (FoxA2), a gene involved in gland development. Together, these results provide insight regarding the critical nature of in utero exposure and the potential impact on ensuing uterine biology and reproductive health later in life.
Key Words: TCDD, uterus, developmental exposure.
Endocrine disruptors alter the function(s) of the endocrine system and consequently can cause adverse health effects in populations (Diamanti-Kandarakis et al., 2009; Henley and Korach, 2010). These chemicals are widespread in the food chain and the environment and include persistent organic pollutants, biomass combustion, industrial by-products, and other industrial compounds (Jones and de Voogt, 1999). Endocrine disruptors mimic natural hormones, inhibit the actions of hormones, or alter the normal regulatory function of the endocrine system and have potential hazardous effects on humans (Diamanti-Kandarakis et al., 2009). One major activity of estrogen is to regulate growth, development, and reproductive function in males and females; however, the female reproductive system is a significant target for exposure to endocrine-disrupting chemicals. One of the most potent substances known, 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD or dioxin), a ubiquitous environmental contaminant, exhibits antiestrogenic properties in adult mice and rats, including the inhibition of estrogen (E2)-induced uterine growth and proliferation (IARC, 1997; Schecter et al., 1994, 1996; Schecter and Li, 1997; Silkworth and Brown, 1996).
TCDD is released into the environment as a by-product of the manufacturing of polychlorinated phenols, combustion, and metal processing but also from the burning of household waste and forest fires (U.S. EPA, 2009). TCDD does not readily degrade and is highly persistent, widespread, and bioaccumulates throughout the food chain and in lipophilic tissues of animals (IARC, 1986; Lorber et al., 2009; USDHHS, 2005). TCDD is a high-affinity ligand for the aryl hydrocarbon receptor (AhR), which forms a heterodimeric transcription factor complex with the aryl hydrocarbon receptor nuclear translocator (ARNT) (Hoffman et al., 1991; Swanson and Bradfield, 1993). The sex steroids and their cognate receptors dictate the menstrual cycle in mammals and are essential to the establishment and maintenance of pregnancy. Activated AhR/ARNT have been reported to associate with estrogen receptors (ERs), which give rise to adverse E2-related actions of TCDD in cell culture (Ohtake et al., 2003). Conversely, ERα is able to mediate transrepression of AhR-dependent gene regulation (Beischlag and Perdew, 2005).
TCDD given acutely with E2 exhibits antiestrogenic properties on the adult rodent uterus. The uterus undergoes a series of tightly regulated biochemical and biological responses after E2 and progesterone (P4) treatment (Hewitt et al., 2005). Boverhof et al. (2008) acutely cotreated mice with ethynyl estradiol and TCDD for 72h and observed reduced ethynyl estradiol–mediated stromal edema, hypertrophy, and hyperplasia and induced marked epithelial cell apoptosis in the murine uterus when compared with controls (Boverhof et al., 2008). The inhibition of selective E2-mediated gene expression through ER/AhR cross talk was confirmed by microarray and subsequent gene expression analysis. Uterine functions, such as epithelial cell regulation, water/ion transport, cytoarchitectural regulation, and immune regulation, were reported to be inhibited by acute TCDD exposure (Boverhof et al., 2008). Evidence also suggests that TCDD exposure can promote the establishment of endometriosis, an E2-dependent disease resulting from the ectopic invasion of endometrial tissue in the peritoneal cavity (Birnbaum and Cummings, 2002; Rier and Foster, 2003). This disease is associated with chronic pelvic pain and increased rates of infertility in humans (Alvarez et al., 2012). Alterations in the proper regulation of gene expression from hormonal regulation result in dysregulation of uterine function and maintenance of pregnancy (Hewitt et al., 2005). Nayyar et al. (2007) reported that a uterine phenotype in wild-type mice exposed to TCDD (gestational day [GD] 15, 4 weeks, and 9 weeks of age) is similar to that of the uterine phenotype of women with endometriosis.
Due to the above-mentioned studies and the similarities in developmental toxicity exposure to TCDD reported for other estrogenic endocrine disruptors, we hypothesized that the blunted uterine responses observed in mice cotreated with ethynyl estradiol and TCDD would also be present in mice exposed to TCDD at critical periods of life. To further examine the inhibitory effects of TCDD on specific E2-mediated uterine gene responses, mice were first treated with TCDD at GD15, 4 weeks, and 9 weeks and, then as adults, after ovariectomy at 12 weeks, with E2 or E2 + P4 administration (Fig. 1). Data indicated that this exposure scheme to TCDD results in an inhibitory effect on adult E2-induced uterotrophic activity and E2- and E2+P4-mediated gene expression. To determine if the 9-week exposure to TCDD was responsible for inhibitory effects on the uterus, TCDD was administered at GD15, during lactation, and/or at 4 weeks of age to address whether exposure during critical periods of development would alter adult uterine responsiveness to E2 (Fig. 6). Data indicated that a single GD15 exposure to TCDD was sufficient to observe a blunted uterine response to hormonal stimulation as an adult.
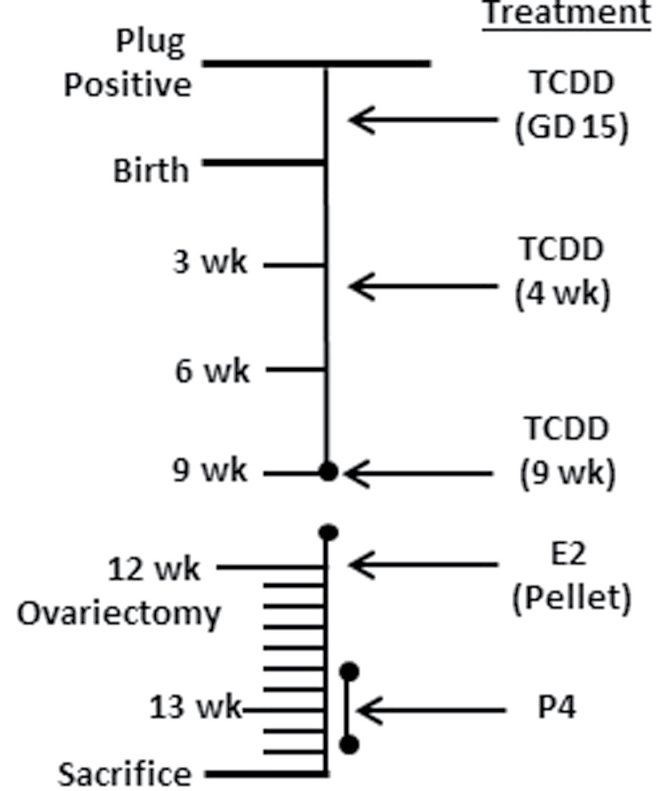
FIG. 1.
Experimental design for study 1 exposure to TCDD at GD15, 4 weeks, and 9 weeks of age with postovariectomy hormone treatment. C57BL6/J mice were time mated/copulation plug positive (GD 0.5). At GD15, pregnant dams were orally administered vehicle (corn oil) or 10 µg/kg of TCDD in corn oil. Animals were weaned at 3 weeks of age. Offspring were orally administered vehicle or TCDD (10 µg/kg) at 4 weeks and 9 weeks of age. Mice were ovariectomized at 12 weeks of age, and a placebo or E2 pellet (0.5mg, 21-day release/mouse) was implanted. Days 6–10 after the pellet, animals received corn oil or P4 (1mg/mouse) subcutaneously for 4 days prior to sacrifice 11 days later.
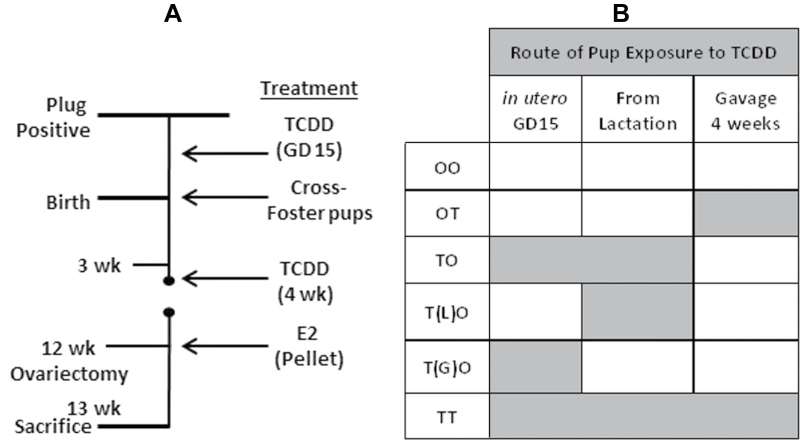
FIG. 6.
Experimental design for study 2 exposure to TCDD at GD15, lactation, and 4 weeks of age. C57BL/6J mice were time mated/copulation plug positive (GD 0.5). A, At GD15, pregnant dams were orally administered vehicle (corn oil) or 10 µg/kg of TCDD in corn oil. Animals were weaned at 3 weeks of age. At weaning, pups in group T(G)O were cross-fostered to dams that were not exposed to TCDD. Pups in group T(L)O were cross-fostered to dams that were exposed to TCDD during pregnancy at GD15. Groups TO and TT were not cross-fostered. Groups OT and TT additionally received 10 µg/kg of TCDD in corn oil at 4 weeks of age. Mice were ovariectomized at 12 weeks of age and a placebo or E2 pellet (0.5mg, 21-day release/mouse) was implanted and mice were sacrificed 11 days later. B, Table showing schematic of pup exposure to TCDD. Gray boxes denote when pups were exposed to TCDD. Group exposure is as follows: OO—corn oil at GD15 and PND 28; OT—corn oil at GD15, TCDD at PND 28; TO—TCDD at GD15/throughout lactation, corn oil at PND 28; T(G)O—TCDD at GD15, no lactational exposure, and corn oil at PND 28; T(L)O—corn oil GD15, lactational exposure from dams given TCDD at GD15, and corn oil at PND 28; TT—TCDD at GD15, throughout lactation, and at PND 28.
MATERIALS AND METHODS
Chemicals.
TCDD in n-Nonane (chromatographic purity of 99%) was purchased from Cerilliant Corporation (ED-901; Round Rock, Texas). 17β-Estradiol pellets (Estra-1,3,5(10)-triene-3,17β-diol or E2) were from Innovative Research of America (E-121; Sarasota, Florida). P4 (4-pregene-3, 20-dione; P0130) and corn oil (C8267) were purchased from Sigma Chemical Company (St Louis, Missouri).
Animal care and treatment.
All animal studies were conducted in accordance with the National Institutes of Health Guidelines for Humane Use and Care of Animals and with approved National Institute of Environmental Health Sciences animal protocols. Adult male and virgin female C57BL6/J mice (6 weeks of age) were purchased from Jackson Laboratories (Bar Harbor, Maine). Mice were in a controlled temperature range (72°F–74°F) and humidity (40%–50%) on a 12-h light, 12-h dark cycle. Mice received a phytoestrogen-reduced diet (Phytoestrogen Reduced II, Zeigler 5412-01) and water ad libitum.
Virgin female mice were mated, delivered, and cared for a first litter before the experimental protocol began. For experimental breeding, 1 male was randomly housed with 2 female mice for timed mating. Presence of a vaginal plug was considered GD 0.5 of pregnancy and females were then singly housed. Dams were monitored daily for delivery of pups and the date of birth was recorded. On the date of birth (postnatal day [PND] 0.5), the number of pups was counted and the sex of each pup determined, and litter size was standardized to 6 pups per litter. For litter standardization, as many female pups as possible were utilized, thus some females from litters with more than 6 female pups were fostered to dams that did not have 6 females in their litter (male pups were humanely euthanized); all litter standardization was done within the same dose group to allow for uniform lactational exposure.
For the first set of experiments (Fig. 1), at GD15, pregnant dams were weighed and administered corn oil vehicle (O) or 10 μg/kg of TCDD (T) in corn oil at 0.15 μg/100 μl via oral gavage. The dose level of 10 μg/kg of TCDD was chosen based upon previous publications that administered TCDD at this level to pregnant dams (Nayyar et al., 2007). In this paper, they observed no abnormal clinical observations or death of dams. Additionally, both Boverhof et al. (2008) and Nayyar et al. (2007) observed effects on the adult uterus at 10 μg/kg of TCDD. Dams were allowed to deliver, and litters were weaned at 3 weeks of age (PND 21) and pups from different litters were randomized. Four weeks (PND 28) and 9 weeks of age (PND 63) after birth, mice were gavaged with corn oil or 10 μg/kg of TCDD in corn oil. The groups just receiving corn oil are denoted as OOO and the groups receiving TCDD at the 3 different time points are denoted as TTT (n = 5–10 per group). At 12 weeks of age, mice were ovariectomized through two 0.5-cm dorsolateral skin incisions. Mice were anesthetized using isoflurane and oxygen and given buprenorphine (0.1mg/kg) for pain management. Mice at this time were randomly divided into additional treatment groups (oil, E2, or E2+P4). At ovariectomy, mice received placebo or E2 pellet (0.5mg, 21-day release, E-121). Days 6–10 after ovariectomy, mice were injected subcutaneously with 100 μl 0.85% saline/0.25% ethanol vehicle or with 1mg (10mg/ml) of P4 in 100 μl 0.85% saline/0.25% ethanol.
Mice were weighed and then euthanized with CO2. Uteri were removed, weighed, a portion was fixed in 10% formalin, and the remaining uterus was snap-frozen on dry ice and stored at −80°C until use. The liver was removed, weighed, and a portion was snap-frozen on dry ice and stored at −80°C until use.
For the second set of experiments (Fig. 6), the focus of the study was on early-life (GD15 and 4 weeks) exposures to TCDD (10 μg/kg of TCDD in corn oil diluted 0.15 μg/100 μl via oral gavage). The groups are as follows (n = 8–15): OO—corn oil at GD15 and 4 weeks; OT—corn oil at GD15, TCDD at 4 weeks; TO—TCDD at GD15/throughout lactation in milk, corn oil at 4 weeks; T(G)O—TCDD at GD15, no lactational exposure (cross-fostered), and corn oil at 4 weeks; T(L)O—corn oil GD15, lactational exposure from dams given TCDD at GD15, and corn oil at 4 weeks; TT—TCDD at GD15, throughout lactation in milk, and at 4 weeks. Litters were weaned and normalized to 6 pups per litter. At 12 weeks of age, mice were ovariectomized, and postovariectomy treatment and necropsy were as stated above with the exception of the E2+P4 group.
Histology and glandular count.
The same portion of the uterus from each mouse was routinely processed for paraffin embedding. Five micron (5 µm) uterine cross sections were cut and stained with hematoxylin and eosin (H&E) staining. Slides were cut serially and glands were counted every fourth slide from 3 cross sections. Gland numbers from the 3 sections were averaged and used as the gland number for each mouse.
TUNEL analysis.
Uterine cross sections (5 µm) were used for TUNEL staining, which was performed according to the manufacturer’s specification using ApopTag Plus Peroxidase In Situ Apoptosis Staining Kit (Cat. no. S7101, Millipore, Billerica, Massachusetts).
RNA isolation-real-time PCR.
Frozen uterus or liver were pulverized under liquid nitrogen and RNA was isolated using TRIzol as per manufacturer’s instructions (Invitrogen, Carlsbad, California). Using a previously described method, cDNA was synthesized and analyzed by real-time PCR using Fast SYBR (Applied Biosystems, Foster City, California) (Burns et al., 2012). Relative transcript levels were quantified in comparison with the vehicle group and normalized to Rpl7 using the model previously described (Pfaffl, 2001). Primer sequences (Supplementary Table 1) were selected using Primer Express (Applied Biosystems) or Harvard Primer Bank (Harvard University, Boston, Massachusetts) and were purchased from Sigma.
Statistical analysis.
The body weight and tissue weight data were analyzed using studentized residual plots to detect potential outliers in each data set, homogeneity of variance was evaluated by Levene’s test, and if heterogeneity was observed, data were transformed until homogeneity was achieved. One-way ANOVA and Tukey’s post hoc test (all groups compared with each other) were performed for the first study using General Linear Model procedures (SAS, version 9.2; SAS Institute, Inc., Cary, North Carolina). ANOVA followed by Dunnett’s post hoc test (each treatment group compared with their respective control) was performed for the second study using General Linear Model procedures (SAS). Statistical significance is denoted when p < .05.
RESULTS
Exposure to TCDD at GD15, 4 Weeks, and 9 Weeks of Age (TTT) Alters Uterine Weight Increase Following Hormonal Stimulation
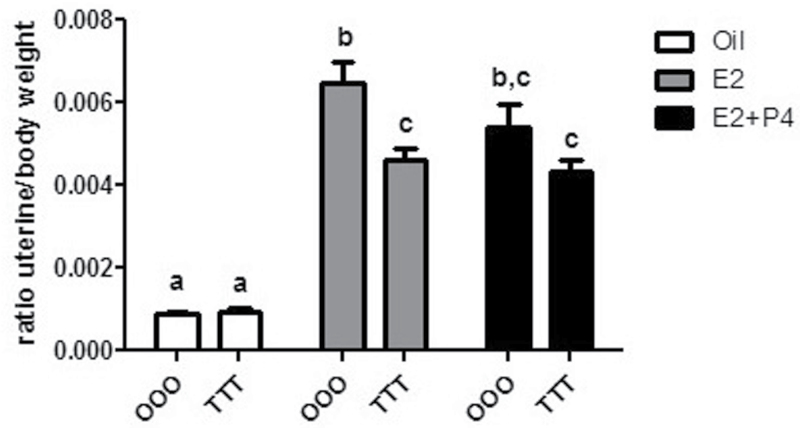
At necropsy, body weight and uterine weight were collected. Uterine weights were observed relative to body weight and, as expected, significant increases in uterine/body weight were observed with E2 treatment in oil-treated control animals (OOO-Oil vs OOO-E2) (Fig. 2). A minor decrease in uterine weight was calculated in OOO-E2+P4 when compared with OOO-E2 (16%). In TTT mice, E2 significantly increased uterine weight (TTT-Oil vs TCDD-E2) (499%), but this weight increase was blunted and decreased significantly (28%) relative to control animals (OOO-E2) not exposed to TCDD. The same blunted weight increase was observed in the TTT-E2+P4 group (20%). These data reveal that GD15, 4-week, and 9-week exposure to TCDD alters the adult uterine response to hormone treatment.
FIG. 2.
GD15, 4 weeks, and 9 weeks of age exposure to TCDD results in a blunted E2-mediated uterine weight increase. Uterine weights were obtained from OOO (corn oil), or TTT (TCDD)-exposed (10 µg/kg) mice after oil (corn oil), E2 (0.5mg, 21-day release/mouse), or E2+P4 (0.5mg, 21-day release/mouse + 1mg/mouse). Data are expressed as a ratio of uterine weight (mg) to body weight (g). Mean ± SD, letters different from each other represent statistical differences (p < .05).
Uteri From Mice Exposed to TCDD at GD15, 4 Weeks, and 9 Weeks of Age (TTT) Have Reduced Hormonal Regulation of Gene Expression
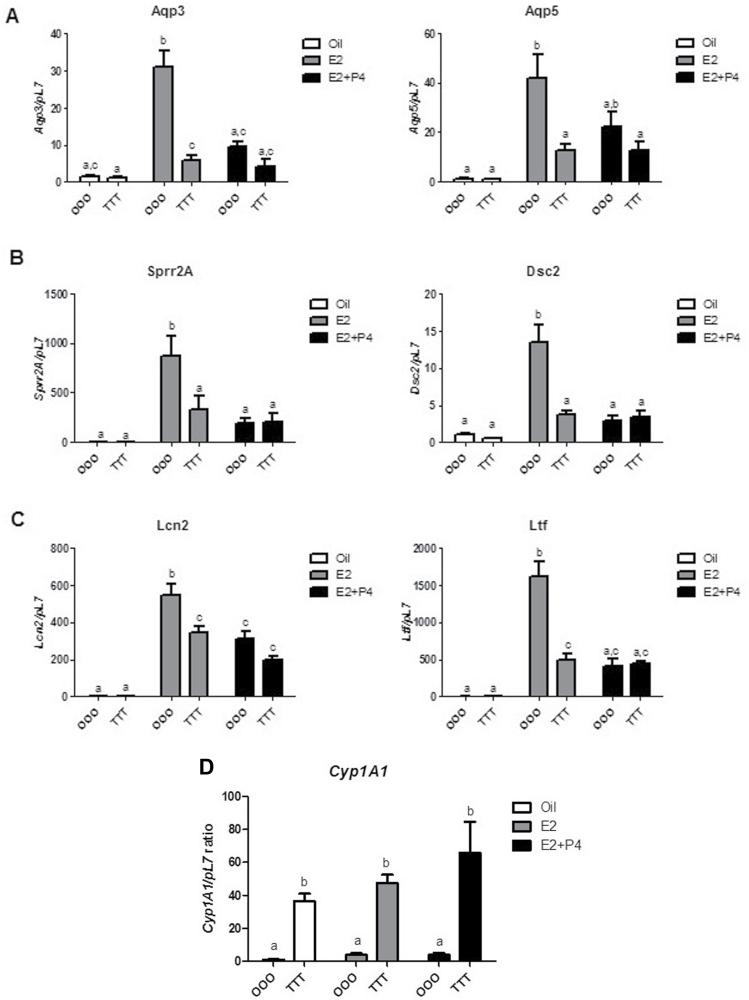
Hormonal regulation of uterine weight increase involves a coordinated proliferative response that is mediated by an orchestrated series of changes in gene expression (Fertuck et al., 2003; Hewitt and Korach, 2011; Kwekel et al., 2005; Moggs et al., 2004). Hormonally responsive genes were chosen from 3 categories, due to the complex physiological changes that occur in the uterus: water uptake, cytoarchitectural, and immune regulation. The aquaporin (Aqp) gene family is important for water transport, which manifests the water imbibition and increased uterine weight response (Richard et al., 2003). Aqp3 and Aqp5 are both increased by E2 and decreased with E2+P4 cotreatment in OOO control animals. In TTT-exposed animals (Fig. 3A), Aqp3 and Aqp5 were significantly less responsive to E2 or E2+P4 treatment. Aqp1, expressed in the myometrium, served as a control and did not change with E2 or E2+P4 or exposure to TCDD (data not shown). Desmocollin-2 (Dsc2) and small proline rich protein 2A (Sprr2a) are involved in uterine cytoarchitectural function (Boverhof et al., 2008). Dsc2 and Sprr2a in OOO control animals were induced with E2 and decreased with E2+P4 treatment (Fig. 3B). These hormonal changes were not observed when the animals were TTT exposed. Two genes involved in immune regulation (Huang et al., 1999; Teng, 1999), lipocalin-2 (Lcn2) and lactoferrin (Ltf), are both markedly induced by E2 and decreased with the addition of P4 in OOO control animals (Fig. 3C). In TTT-exposed animals, the Lcn2 response to E2 and E2+P4 was blunted compared with controls. For Ltf, the E2 and E2+P4 responses were abrogated in the TTT-exposed groups. TTT-exposed animals had a significant increase in hepatic Cyp1A1, a key cytochrome p450 activated by TCDD (Dong et al., 1996; Ko et al., 1996), which suggests continued presence and/or activity of TCDD at 13 weeks of age (Fig. 3D). Corroborating previous reports (DeVito et al., 1992, 1994), ERα expression levels were not altered from TTT exposure (Supplementary Figure 1A). ERβ was slightly elevated in the TTT-E2 group, but this did not reach statistical significance (Supplementary Figure 1B). These data suggest that TTT exposure alters the hormonal responsiveness of some select genes involved in uterine function but not due to alterations in ER expression.
FIG. 3.
Exposure to TCDD at GD15, 4 weeks, and 9 weeks of age results in blunted E2-mediated uterine gene responses. Genes involved in (A) tissue fluid uptake (Aqp3 and Aqp5), (B) cytoarchitectural regulation (Dsc2 and Sprr2a), (C) immune regulation (Lcn2 and Ltf), and (D) Cyp1A1 were examined in OOO and TTT-exposed mice treated postovariectomy with oil (corn oil), E2 (0.5mg, 21-day release/mouse), or E2+P4 (0.5mg, 21-day release/mouse + 1mg/mouse). Data are expressed relative to the pL7. Mean ± SD, letters different from each other represent statistical differences (p < .05).
Exposure to TCDD at GD15, 4 Weeks, and 9 Weeks of Age (TTT) Alters Uterine Appearance and Reduces Uterine Gland Number
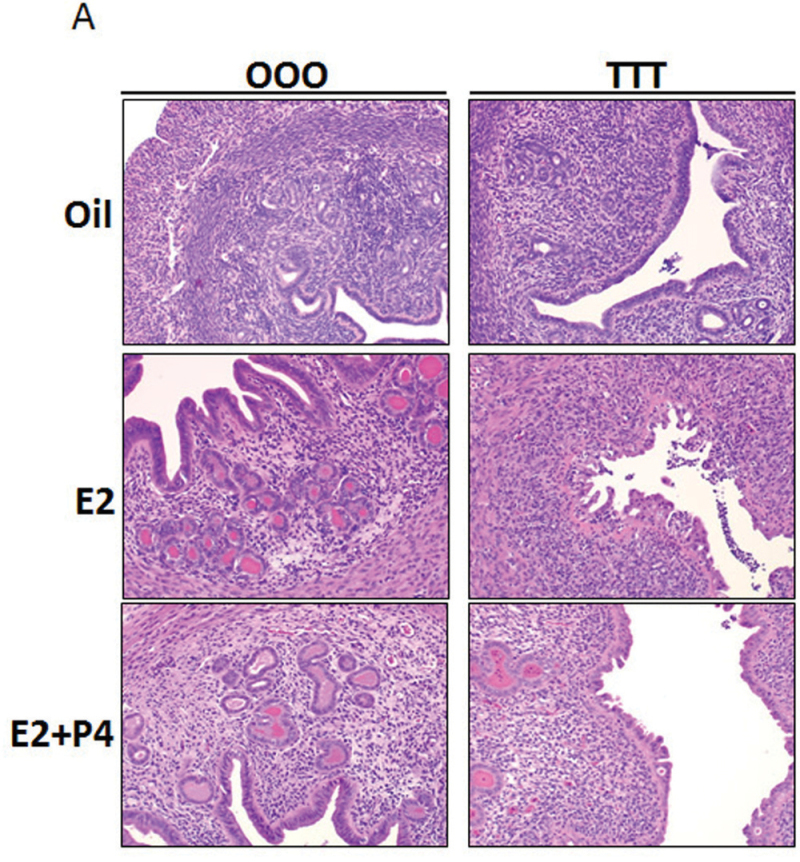
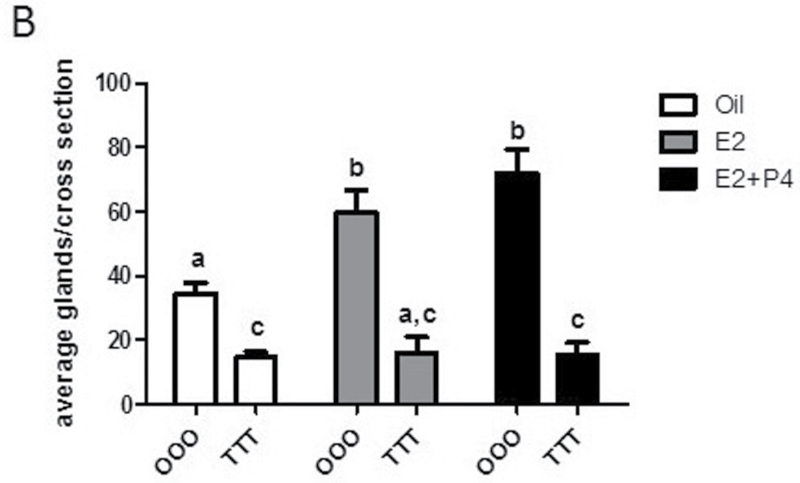
To examine the extent of the uterine changes, uterine cross sections were stained with H&E. Vehicle-treated uteri display classical uterine structures with stroma, epithelial lumen, and glandular structures. With E2 and E2+P4 treatment, stromal edema, hypertrophy, and increase in epithelial compartment height were as expected (Fig. 4A; OOO group). TCDD treatment resulted in severe changes to the uterine phenotype, with the most notable alterations in the TTT-E2 group (Fig. 4A; TTT group). Luminal epithelial cells were no longer columnar in appearance and had altered cell polarity. The TTT exposure displayed a gradation in phenotype severity with some uteri having a normal appearance to the luminal epithelium (Supplementary Figure 2); a range from decreased to complete absence of glandular structures was observed in uteri from TTT-exposed mice. To quantify the marked decrease in uterine glands observed, glands were counted from uterine cross sections as described in Materials and Methods section. TTT-Oil uteri when compared with OOO-Oil showed a decreased number of glands per cross section (Fig. 4B). With hormone treatment postovariectomy in OOO animals (E2 or E2+P4), the uteri develop more glandular structures. In contrast, in the TTT group, hormone treatment postovariectomy (E2 or E2+P4) does not alter the number of glandular structures and the decreases are significantly reduced compared with control uteri. These data reveal that TTT exposure affects uterine morphology and the formation of uterine glands.
FIG. 4.
Exposure to TCDD at GD15, 4 weeks, and 9 weeks of age alters uterine morphology and causes a significant decrease in uterine glands. A, Comparison of uterine morphology by histology. Uterine cross sections were H&E stained. Images are ×200. Representative images are shown. B, Uterine glands were counted as described in Materials and Methods section. Mean ± SD, letters different from each other represent statistical differences (p < .05).
Uteri From Mice Exposed to TCDD at GD15, 4 Weeks, and 9 Weeks of Age (TTT) Have Minimal Levels of Apoptosis
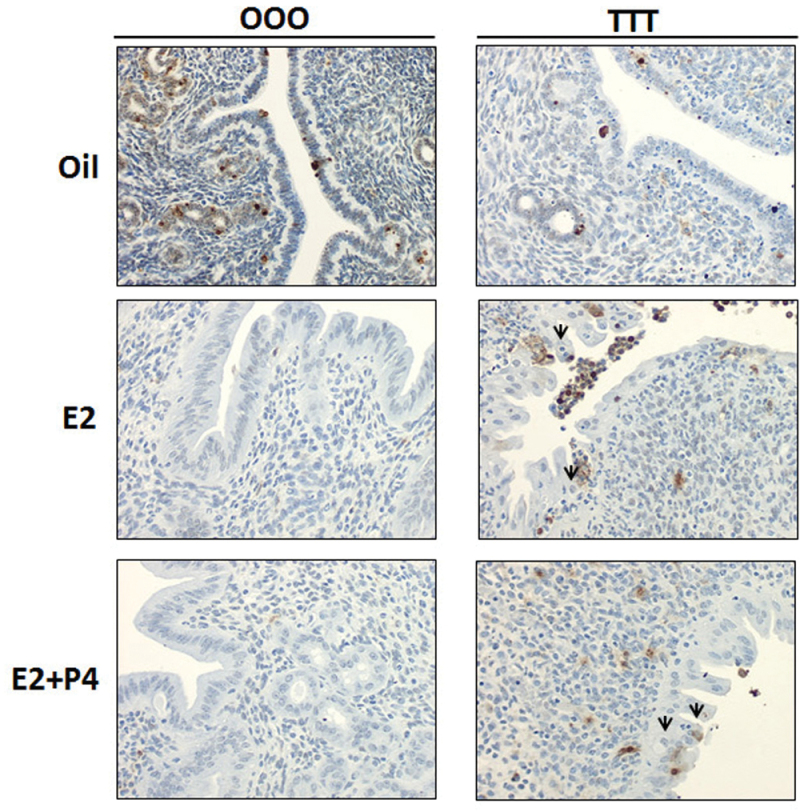
Boverhof et al. (2008) reported a visual phenotype of luminal epithelial cell apoptosis after 72h of ethynyl estradiol (10 µg/kg)/TCDD (30 µg/kg) treatment. Therefore, to determine if an increase in apoptosis was mediating the decrease in uterine weight response in the present study, uterine cross sections were stained by immunohistochemistry for TUNEL to detect apoptotic bodies (Fig. 5). In OOO samples with E2 or E2+P4 treatment, very few cells stained positively for apoptosis (brown color denotes TUNEL positive cells). In TTT samples with E2 or E2+P4, more apoptotic cells/bodies were seen; however, in contrast to previous reports (Boverhof et al., 2008), the areas (Fig. 5; denoted below the black arrows) suspected to be apoptotic bodies did not stain positively for TUNEL. The minimal increase in apoptotic cells in TTT-exposed mice did not fully correlate with the significant decrease in uterine weight after E2 or E2+P4 treatment.
FIG. 5.
Exposure to TCDD at GD15, 4 weeks, and 9 weeks of age slightly increases apoptosis. Uterine cross sections were TUNEL stained as described in the Materials and Methods section. Darker color denotes TUNEL positive cells. Arrows denote cell populations expected to be TUNEL positive based on Boverhof et al. (2008).
Exposure to TCDD at GD15 Blunts the Uterine Response to E2 as Adults
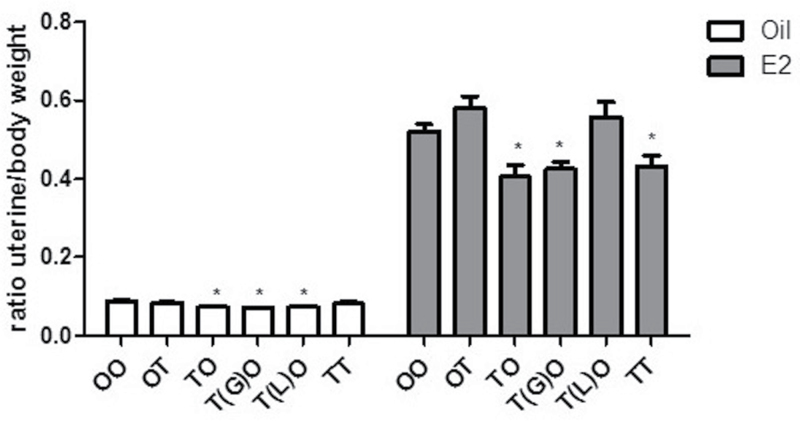
Due to the elevated levels of CYP1A1 message and protein observed at the termination of the first study, we wanted to determine if the 3 doses of TCDD together or the 9 week only exposure to TCDD were responsible for the cumulative effects on the adult uterus. The treatment scheme and dosing are shown in Figure 6. Dams were gavaged with TCDD (10 µg/kg) to expose pups at GD15 and through milk during lactation (TO), at 4 weeks only by gavage (OT), at GD15 with cross-fostering at birth (T(G)O), at lactation only (T(L)O), and at GD15 and 4 weeks (TT). Mice were ovariectomized and treated with corn oil vehicle or E2 (0.5mg, 21-day release pellet) as described above. Due to minimal changes from E2+P4 treatment in the TTT experiment, only E2 treatment was used postovariectomy in the following experiments. At necropsy, body weight and uterine weight were collected. No differences in final body weight were observed. Relative uterine weight (uterus/body weight) is shown in Figure 7. In the postovariectomy treatment with oil, a significant decrease in uterine weight was seen for TCDD exposure groups TO (16%), T(G)O (19%), T(L)O (16%), and TT (20%). This demonstrates a decreased basal uterine weight in these uteri from TCDD exposure. In the postovariectomy E2 treatment group, as expected, a significant increase in uterine weight is seen; however, within this treatment group, TCDD exposure groups, TO (22%), T(G)O (19%), and TT (17%), the increases in uterine weight are significantly decreased and blunted relative to the OO-E2 control group. These data suggest that GD15 TCDD exposure alone results in a blunted uterine response to hormone as adults.
FIG. 7.
Exposure to TCDD at GD15 blunts the adult uterine weight increase after E2 treatment. Uterine weights were obtained from OO, OT, TO, T(G)O, T(L)O, and TT groups after oil (corn oil) or E2 (0.5mg, 21-day release/mouse) treatment. Data are expressed as a ratio of uterine weight (mg) to body weight (g). Mean ± SD, *, represents statistical differences compared with control group mean (p < .05).
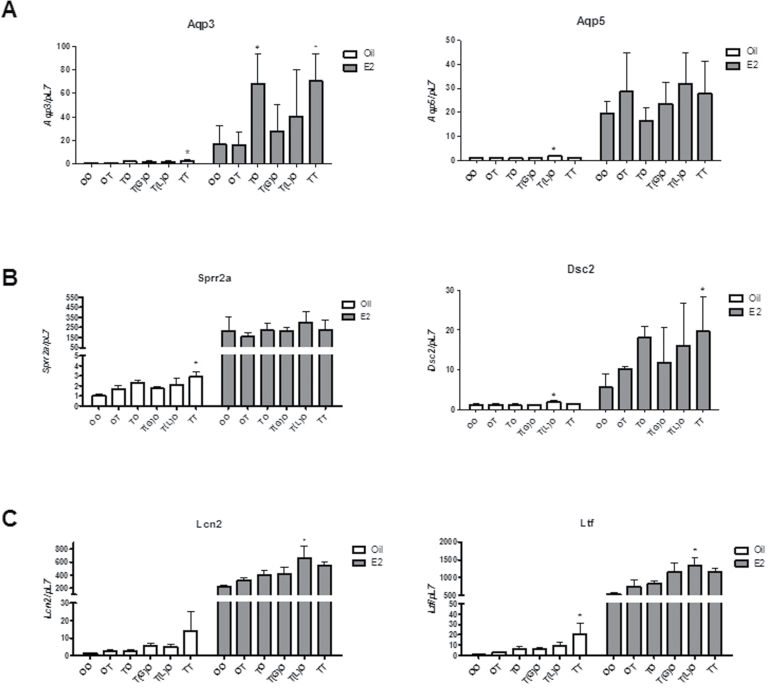
Exposure to TCDD at GD15 or 4 Weeks of Age Does Not Alter Uterine Gene Expression Changes in Response to E2
We next examined the same select set of genes reported in Figure 3. Overall, we did not observe consistent significant changes in gene expression from developmental exposure to TCDD relative to the non-TCDD-treated controls (Fig. 8). All genes responded similarly to E2 treatment as did animals not exposed to TCDD, albeit, a range in responses was observed in animals exposed to TCDD. These results suggest that the changes in hormonal response of the adult uterus of these select genes can be attributed to TCDD exposure at 9 weeks. The GD15 and 4-week exposure to TCDD did not induce Cyp1A1 message (data not shown) or CYP1A1 protein levels at the time of collection (Supplementary Figure 3) at 11 weeks of age and suggest that these periods in development are not altering the hormonal responsiveness of these select genes in the adult uterus.
FIG. 8.
Exposure to TCDD at GD15 does not consistently alter the adult uterine gene expression response to E2. Genes involved in (A) tissue fluid uptake (Aqp3 and Aqp5), (B) cytoarchitectural regulation (Dsc2 and Sprr2a), and (C) immune regulation (Lcn2 and Ltf) were examined in mice treated postovariectomy with oil (corn oil) or E2 (0.5mg, 21-day release/mouse). Data are expressed relative to the pL7. Mean ± SD, *, represents statistical differences compared with control group mean (p < .05).
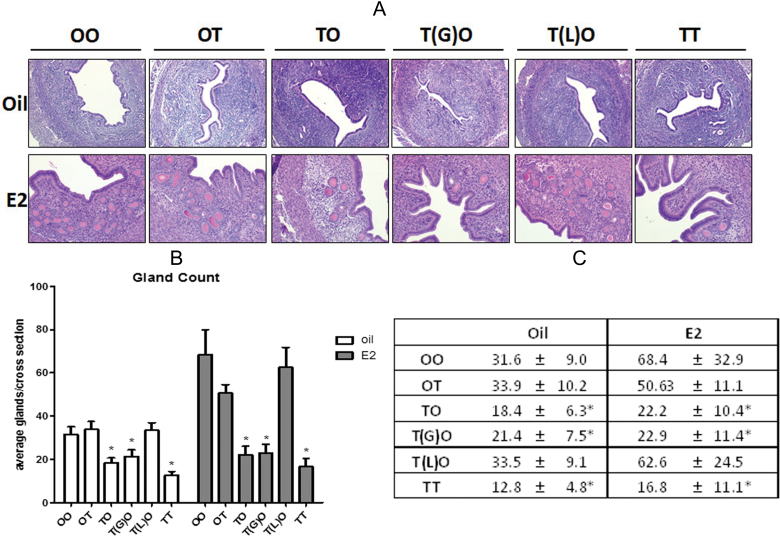
Exposure to TCDD at GD15 Results in Decreased Uterine Gland Numbers in Adult Uteri
Uterine sections were blinded and the epithelial cell layers were evaluated and uterine glands counted. All TCDD-exposed uteri were comparable with control uteri after ovariectomy as adults (Fig. 9A) with oil or E2 treatment. Uteri were larger and demonstrate an increase in epithelial height with E2 treatment. Significantly fewer gland numbers were noted. Figure 9B shows that animals in the oil group exposed to TCDD at TO, T(G)O, and TT have reduced number of uterine glands. With E2 treatment, an increase in gland number is observed in the OO-E2 (Fig. 9C). Increases in gland numbers, like OO, are observed also in OT and T(L)O groups treated with E2. TCDD groups TO, T(G)O, and TT do not have an increase in gland number with E2 and remain fewer as was seen in the oil group. These data suggest a basal decrease in gland number as well as the inhibition of gland formation in TCDD groups TO, T(G)O, and TT after hormonal stimulation.
FIG. 9.
Exposure to TCDD at GD15 alters uterine gland number. A, Comparison of uterine morphology by histology. Uterine cross sections were H&E stained. Images are ×200. Representative images are shown. B, Uterine gland counts. Mean ± SD, *, represents statistical differences compared with control group mean (p < .05). C, Table of full statistical analysis of uterine gland counts. Mean ± SD, *, represents statistical differences from OO-Oil control (p < .05).
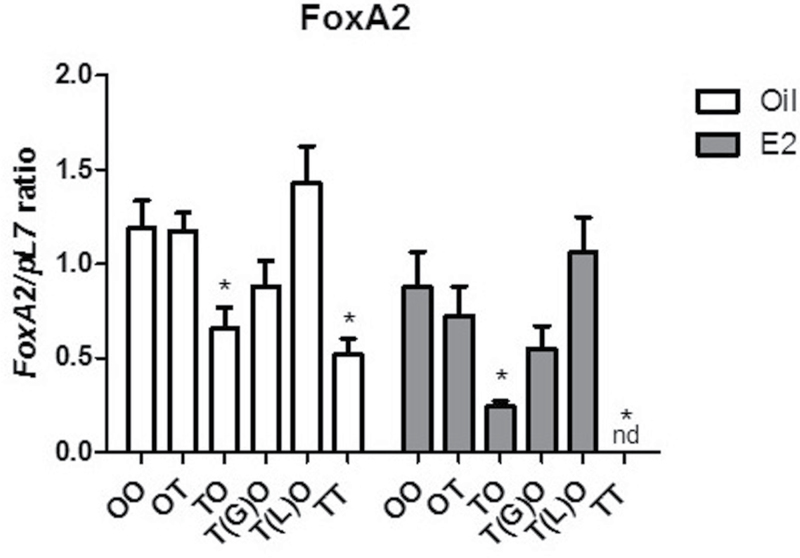
To evaluate the potential effects of TCDD on uterine gland development, forkhead box A2 (FoxA2), a known marker of uterine gland development (Filant et al., 2012; Jeong et al., 2010), was examined by real-time PCR (Fig. 10). The levels of FoxA2 were significantly reduced in TCDD groups TO and TT treated with oil or E2, corresponding to the decrease in uterine gland counts. Interestingly, FoxA2 was not detectable in the TT-E2 samples. The FoxA2 levels were reduced in the T(G)O groups but did not reach statistical significance. Groups OT and T(L)O were not different than the OO-Oil control group correlating to the gland numbers. Additionally, we confirmed a decrease in FoxA2 in the TTT animals from the first experiment (Supplementary Figure 1C). These data suggest that the expression of Foxa2 is blunted (TO-Oil, TT-Oil, TO-E2) or inhibited (TT-E2) by early-life exposure to TCDD.
FIG. 10.
Exposure to TCDD at GD15 decreases uterine message levels of FoxA2. Data are expressed relative to pL7. Mean ± SD, *, represents statistical differences compared with control group mean (p < .05).
DISCUSSION
This study, divided into 2 main experiments, was conducted to examine the potential inhibitory effects of TCDD exposure during critical periods of development and evaluate hormone-mediated uterine changes. The first experiments were designed to examine the cumulative effects of TCDD exposure at GD15, 4 weeks, and 9 weeks of age. The second set of experiments was designed to closely examine the effects of TCDD exposure at particular life stages and evaluate the potential effects on the adult uterus.
Inhibition of Hormone-Mediated Uterine Growth, Gland Number, and Gene Expression in TTT-Exposed Mice
Boverhof et al. (2008) reported that a 3-day cotreatment with TCDD (30 µg/kg) and ethynyl estradiol (10 µg/kg) reduced stromal edema, hypertrophy, and hyperplasia in uteri of mice ovariectomized at PND 20 and cotreated at PND 24; however, in our study, the treatment regimen of TCDD was not simultaneous with E2 or E2+P4, and strikingly similar results were obtained. The half-life of TCDD in mice is 7–11 days (Birnbaum, 1986) and the uteri of mice in our study exposed to TCDD at GD15, 4 weeks, and 9 weeks of age exhibited a blunted uterine weight increase after E2 or E2+P4 treatment at 13 weeks of age similar to the cotreatment dose of TCDD at 30 µg/kg. The approximate levels of TCDD at necropsy (1.25–2.5 µg/kg), based on half-life estimations, was sufficient to increase Cyp1A1. These data importantly demonstrate that TCDD does not have to be administered concurrently or at higher dosing regimens with E2 or E2+P4 to observe reduced adult uterine responsiveness.
TCDD affects only a subset of ethynyl estradiol–mediated response and does not a global antiestrogenic effect (Boverhof et al., 2008). Due to the specific inhibitory response of TCDD on E2-mediated differentially expressed genes, we chose a subset of altered targets from their microarray analysis. With the significant decrease in uterine weight most likely due to decreased secretion or water imbibition, Aqp3 and Aqp5 (Moller et al., 2010; Richard et al., 2003) were examined. Both of these aquaporins, increased by E2 and inhibited by P4, play a strong role in epithelial cell responses and are integral in water imbibition of the uterus (Jablonski et al., 2003; Richard et al., 2003). The decreases in Aqp3 and Aqp5 in uteri of mice after TTT exposure suggest that the function of these genes is disrupted, contributing to the decreased uterine weight after hormone stimulation. On the other hand, Aqp1 is myometrial specific (Jablonski et al., 2003) and shows no regulation by E2 or E2+P4 and no altered regulation by TTT exposure (data not shown). These results demonstrate further specificity to either target genes and/or cellular compartments after TCDD exposure (Buchanan et al., 2000).
Histological examination of the uterus revealed visual differences in the severity of phenotype penetrance. Some uteri of TTT-exposed mice appeared very similar to control uteri (Supplementary Figure 2); however, others, completely lacked uterine glands, had less edema, and the epithelial cell layer lacked organization and cell polarity. Dsc2 and Sprr2a are two E2 responsive genes involved in cell junctions and cytoarchitectural changes, respectively (Hong et al., 2004; Marsden et al., 1997). Dsc2 is a member of the cadherin superfamily and the desmocollin subfamily, which functions in epithelial cells to provide cell adhesion (Kurihara et al., 2007). The decrease in Dsc2 with TTT exposure likely contributes to the alteration of the cytoarchitecture, which normally changes to accommodate proliferation and growth in response to E2. Additionally, TCDD cotreatment inhibits the E2 regulation of Sprr2a, a small proline-rich protein important for cytoarchitectural changes (Boverhof et al., 2008; Hong et al., 2004). Two secreted factors, Lcn2 and Ltf, were examined. Lcn2 is a secreted immune modulator important in the innate immune response to bacterial infection and is E2 responsive (Lin et al., 2011). Ltf is known to kill bacteria, play an immunomodulatory role, and the levels in the uterus increase with circulating E2 (Teng et al., 2002). The blunted response of the uteri of TTT-exposed mice suggests that genes involved in cellular architectural and secreted factors are affected comparably to the toxicity of cotreatment with TCDD (Boverhof et al., 2008; Buchanan et al., 2000).
Cotreatment of TCDD and ethynyl estradiol was reported to visually induce epithelial cell apoptosis (Boverhof et al., 2008); therefore, we performed TUNEL assay to determine if increased apoptosis was responsible for the blunted uterine response. In contrast to the previous study, our uteri did not show a marked increase in TUNEL positive cells in mice exposed to TTT. Furthermore, we concluded that apoptosis is not the main driving factor in the blunted uterine weight response after hormone treatment. Conversely, uterine glands are critically important for fetal development, growth, water imbibition, and proliferation (Gray et al., 2001). A dramatic decrease in the number of glands seen per uterine cross section was observed from TTT exposure compared with the numbers observed from OOO control animals. This is in contrast to mice gestationally exposed to estrogenic drug diethylstilbestrol, which exhibit premature formation of uterine glands and alteration in Hox genes (Block et al., 2000; Taylor, 2008; Wordinger et al., 1991) suggesting agonistic and antagonist mechanisms of action of these toxicants in the uterus.
Gestational (GD15) Exposure to TCDD Results in a Blunted Uterine Response to E2
In the second set of experiments, we examined the potential effects of TCDD on early-life exposures to determine what period of exposure results in altered uterine function as adults. When comparing the first set of experiments GD15 with the second set of experiments, we find the investigated decreases in gene expression found in the TTT groups are most likely due to the 9-week exposure to TCDD. However, the second set of experiments suggests that gestational exposure to TCDD is sufficient to reprogram responsiveness and alter the response of the adult uterus to hormones. Beyond the scope of these experiments, future studies will focus on microarray analysis to determine what uterine genes are altered from GD15 and/or 4-week exposure to TCDD. Our current data are in agreement with a study where mice exposed to high doses of TCDD (60 µg/kg), in utero and during lactation only, exhibited decreased uterine weight during estrus and diestrus (Theobald and Peterson, 1997). Additionally, in utero TCDD (1 µg/kg) exposure is known to alter the reproductive morphology and development of rats. Female rats exhibit reduced time to pregnancy, age-associated reproductive loss, and a vaginal “thread,” but the response to hormone was not determined (Gray et al., 1997; Gray and Ostby, 1995).
TCDD, due to its resistance to metabolism and lipophilicity, bioaccumulates in mammary tissue and is ingested by infants through breastfeeding with a daily intake 50 times higher than adults (Patandin et al., 1999). In this study, groups of animals were cross-fostered to dams that had or had not received TCDD at GD15 so that these 2 groups of animals would receive TCDD only during gestation (T(G)O) or only through lactation (T(L)O). Invagination of the uterine epithelium is required for uterine gland development, which, in the mouse, begins around PND 7 with bud formation; by PND 15, the histoarchitecture of the uterus resembles that of the adult (Filant et al., 2012; Gray et al., 2001). Exposure though lactational exposure alone did not alter uterine responses examined; however, gestational exposure alone was able to alter adult uterine function. This is similar to a study in rats exposed at GD15 (1 μg/kg) that revealed reduced mammary gland primary branches, decreased epithelial elongation, and fewer alveolar buds and branches demonstrating a critical time period for consistent inhibition of epithelial development (Fenton et al., 2002). In our study, GD15 TCDD exposure reduced gland numbers (TO, T(G)O, and TT), which did not increase after E2 treatment. Examination of FoxA2, a gene important for uterine gland development because the FoxA2 knockout mice have no uterine glands (Filant et al., 2012; Jeong et al., 2010), suggests that the decrease in this gene from exposure to TCDD at GD15 alone may play an important role in the development of uterine glands in adult uteri. These windows of susceptibility signify the critical nature of lasting reproductive defects from in utero and developmental exposures to TCDD.
Chronic exposure of TCDD to rats leads to delayed puberty, loss of reproductive cyclicity, fewer shed ova, and decreased serum estradiol concentrations leading to a loss of reproductive function with age (Hutz et al., 2006; Shi et al., 2007). Our study did not assess fertility and mice were ovariectomized, but our results suggest that the decreased fertility associated with TCDD exposure can also be attributed to alterations to the uterus. TCDD has a long half-life (~7–11 years), bioaccumulates in humans, and it is considered a risk factor contributing to endometriosis (Rier et al., 1993), a disease that negatively impacts fertility in humans (Anger and Foster, 2008; Foster, 2008). In mice, perinatal plus adult exposure to TCDD (3 µg/kg) increases the size of surgically induced endometriosis (Cummings et al., 1999). An epidemiological study examining the levels of serum TCDD in women with endometriosis found no association (Niskar et al., 2009), but this study did not account for potential in utero exposure levels. The fetal basis of adult disease in which prenatal exposures to toxicants can cause or be associated with increased susceptibility to clinical disease later in life is in agreement with our results, which suggest permanent alterations can occur early in life in the uterus after developmental TCDD exposure. These early-life exposures may contribute to a persistent altered adult uterine phenotype. As with the previous implications for TCDD to affect reproduction, additional well-controlled studies need to be performed to determine if TCDD plays a role in endometriosis, the potential for infertility, or other clinical diseases. Our studies suggest that additional early-life TCDD exposure studies are warranted due to a single gestational exposure eliciting an altered adult uterine phenotype.
CONCLUSIONS
Exposure to TCDD at GD15, 4 weeks, and 9 weeks of age alters uterine morphology and the E2- and E2+P4-mediated selective gene responses in the adult ovariectomized uterus in a manner similar to what is described for cotreatment of TCDD. Exposure to TCDD at GD15 is sufficient for a blunted adult uterine response to E2 postovariectomy and for the decreased development of endometrial glands in adult uteri. Disruption of FoxA2 expression likely contributes to fewer uterine glands. These data provide understanding of phenotypic uterine changes after multiple TCDD exposures and also the implications of a single exposure during a critical period of development. The study of a persistent environmental toxicant provides insight that disruption during critical windows of susceptibility is important for understanding effects on uterine biology and reproductive health later in life.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
FUNDING
Division of Intramural Research of the National Institute of Environmental Health Sciences (Z01 ES70065 to K.S.K. and K.A.B.); National Institute of Environmental Health Sciences contract (N01 ES00005 to L.M.Z.); Division of Intramural Research of the National Cancer Institute at National Institute of Environmental Health Sciences (ZIA BC011476 to L.S.B.).
Supplementary Material
ACKNOWLEDGMENTS
We thank Yin Li, Gregory Scott, and Rick Moore for critical review of this paper. We thank the NIEHS Histology Laboratory for histological assistance. We thank the Comparative Medicine Branch for surgical assistance and ILS research assistants for their contributions to the second study. The authors do not have any conflicts to disclose.
REFERENCES
- Alvarez P., Chen X., Hendrich J., Irwin J. C., Green P. G., Giudice L. C., Levine J. D. (2012). Ectopic uterine tissue as a chronic pain generator. Neuroscience 225, 269–282.10.1016/j.neuroscience.2012.08.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anger D. L., Foster W. G. (2008). The link between environmental toxicant exposure and endometriosis. Front. Biosci. 13, 1578–1593 [DOI] [PubMed] [Google Scholar]
- Beischlag T. V., Perdew G. H. (2005). ER alpha-AHR-ARNT protein-protein interactions mediate estradiol-dependent transrepression of dioxin-inducible gene transcription. J. Biol. Chem. 280, 21607–21611 [DOI] [PubMed] [Google Scholar]
- Birnbaum L. S. (1986). Distribution and excretion of 2,3,7,8-tetrachlorodibenzo-p-dioxin in congenic strains of mice which differ at the Ah locus. Drug Metab. Dispos. 14, 34–40 [PubMed] [Google Scholar]
- Birnbaum L. S., Cummings A. M. (2002). Dioxins and endometriosis: A plausible hypothesis. Environ. Health Perspect. 110, 15–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block K., Kardana A., Igarashi P., Taylor H. S. (2000). In utero diethylstilbestrol (DES) exposure alters Hox gene expression in the developing mullerian system. FASEB J. 14, 1101–1108 [DOI] [PubMed] [Google Scholar]
- Boverhof D. R., Burgoon L. D., Williams K. J., Zacharewski T. R. (2008). Inhibition of estrogen-mediated uterine gene expression responses by dioxin. Mol. Pharmacol. 73, 82–93.10.1124/mol.107.040451 [DOI] [PubMed] [Google Scholar]
- Buchanan D. L., Sato T., Peterson R. E., Cooke P. S. (2000). Antiestrogenic effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin in mouse uterus: Critical role of the aryl hydrocarbon receptor in stromal tissue. Toxicol. Sci. 57, 302–311 [DOI] [PubMed] [Google Scholar]
- Burns K. A., Rodriguez K. F., Hewitt S. C., Janardhan K. S., Young S. L., Korach K. S. (2012). Role of estrogen receptor signaling required for endometriosis-like lesion establishment in a mouse model. Endocrinology 153, 3960–3971.10.1210/en.2012-1294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings A. M., Hedge J. M., Birnbaum L. S. (1999). Effect of prenatal exposure to TCDD on the promotion of endometriotic lesion growth by TCDD in adult female rats and mice. Toxicol. Sci. 52, 45–49 [DOI] [PubMed] [Google Scholar]
- DeVito M. J., Ma X., Babish J. G., Menache M., Birnbaum L. S. (1994). Dose-response relationships in mice following subchronic exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin: CYP1A1, CYP1A2, estrogen receptor, and protein tyrosine phosphorylation. Toxicol. Appl. Pharmacol. 124, 82–90 [DOI] [PubMed] [Google Scholar]
- DeVito M. J., Thomas T., Martin E., Umbreit T. H., Gallo M. A. (1992). Antiestrogenic action of 2,3,7,8-tetrachlorodibenzo-p-dioxin: Tissue-specific regulation of estrogen receptor in CD1 mice. Toxicol. Appl. Pharmacol. 113, 284–292 [DOI] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E., Bourguignon J. P., Giudice L. C., Hauser R., Prins G. S., Soto A. M., Zoeller R. T., Gore A. C. (2009a). Endocrine-disrupting chemicals: An Endocrine Society scientific statement. Endocr. Rev. 30, 293–342.10.1210/er.2009-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong L., Ma Q., Whitlock J. P., Jr (1996). DNA binding by the heterodimeric Ah receptor. Relationship to dioxin-induced CYP1A1 transcription in vivo. J. Biol. Chem. 271, 7942–7948 [DOI] [PubMed] [Google Scholar]
- Fenton S. E., Hamm J. T., Birnbaum L. S., Youngblood G. L. (2002). Persistent abnormalities in the rat mammary gland following gestational and lactational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Toxicol. Sci. 67, 63–74 [DOI] [PubMed] [Google Scholar]
- Fertuck K. C., Eckel J. E., Gennings C., Zacharewski T. R. (2003). Identification of temporal patterns of gene expression in the uteri of immature, ovariectomized mice following exposure to ethynylestradiol. Physiol. Genomics 15, 127–141.10.1152/physiolgenomics.00058.2003 [DOI] [PubMed] [Google Scholar]
- Filant J., Zhou H., Spencer T. E. (2012). Progesterone inhibits uterine gland development in the neonatal mouse uterus. Biol. Reprod. 86, 146, 1–9.10.1095/biolreprod.111.097089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster W. G. (2008). Endocrine toxicants including 2,3,7,8-terachlorodibenzo-p-dioxin (TCDD) and dioxin-like chemicals and endometriosis: Is there a link? J. Toxicol. Environ. Health B Crit. Rev. 11, 177–187.10.1080/10937400701873456 [DOI] [PubMed] [Google Scholar]
- Gray C. A., Bartol F. F., Tarleton B. J., Wiley A. A., Johnson G. A., Bazer F. W., Spencer T. E. (2001). Developmental biology of uterine glands. Biol. Reprod. 65, 1311–1323 [DOI] [PubMed] [Google Scholar]
- Gray L. E., Jr, Ostby J. S. (1995). In utero 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) alters reproductive morphology and function in female rat offspring. Toxicol. Appl. Pharmacol. 133, 285–294 doi:10.1006/taap.1995.1153 [DOI] [PubMed] [Google Scholar]
- Gray L. E., Wolf C., Mann P., Ostby J. S. (1997). In utero exposure to low doses of 2,3,7,8-tetrachlorodibenzo-p-dioxin alters reproductive development of female Long Evans hooded rat offspring. Toxicol. Appl. Pharmacol. 146, 237–244.10.1006/taap.1997.8222 [DOI] [PubMed] [Google Scholar]
- Henley D. V., Korach K. S. (2010). Physiological effects and mechanisms of action of endocrine disrupting chemicals that alter estrogen signaling. Hormones (Athens) 9, 191–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt S. C., Harrell J. C., Korach K. S. (2005). Lessons in estrogen biology from knockout and transgenic animals. Annu. Rev. Physiol. 67, 285–308.10.1146/annurev.physiol.67.040403.115914 [DOI] [PubMed] [Google Scholar]
- Hewitt S. C., Korach K. S. (2011). Estrogenic activity of bisphenol A and 2,2-bis(p-hydroxyphenyl)-1,1,1-trichloroethane (HPTE) demonstrated in mouse uterine gene profiles. Environ. Health Perspect. 119, 63–70.10.1289/ehp.1002347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman E. C., Reyes H., Chu F. F., Sander F., Conley L. H., Brooks B. A., Hankinson O. (1991). Cloning of a factor required for activity of the Ah (dioxin) receptor. Science 252, 954–958 [DOI] [PubMed] [Google Scholar]
- Hong S. H., Nah H. Y., Lee J. Y., Lee Y. J., Lee J. W., Gye M. C., Kim C. H., Kang B. M., Kim M. K. (2004). Estrogen regulates the expression of the small proline-rich 2 gene family in the mouse uterus. Mol. Cells 17, 477–484 [PubMed] [Google Scholar]
- Huang H. L., Chu S. T., Chen Y. H. (1999). Ovarian steroids regulate 24p3 expression in mouse uterus during the natural estrous cycle and the preimplantation period. J. Endocrinol. 162, 11–19 [DOI] [PubMed] [Google Scholar]
- Hutz R. J., Carvan M. J., Baldridge M. G., Conley L. K., Heiden T. K. (2006). Environmental toxicants and effects on female reproductive function. Trends Reproductive Biol. 2, 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- IARC. (1986). Occupational exposures to chlorophenoxy herbicides. IARC Mongr. Eval. Carcing. Risk Hum. 41, 357–377 [PubMed] [Google Scholar]
- IARC. (1997). IARC working group on the evaluation of carcinogenic risks to humans: Polychlorinated dibenzo-para-dioxins and polychlorinated dibenzofurans. Lyon, France, 4–11 February 1997. IARC Monogr. Eval. Carcinog. Risk Hum. 69, 1–631 [PMC free article] [PubMed] [Google Scholar]
- Jablonski E. M., McConnell N. A., Hughes F. M., Jr, Huet-Hudson Y. M. (2003). Estrogen regulation of aquaporins in the mouse uterus: Potential roles in uterine water movement. Biol. Reprod. 69, 1481–1487.10.1095/biolreprod.103.019927 [DOI] [PubMed] [Google Scholar]
- Jeong J. W., Kwak I., Lee K. Y., Kim T. H., Large M. J., Stewart C. L., Kaestner K. H., Lydon J. P., DeMayo F. J. (2010). Foxa2 is essential for mouse endometrial gland development and fertility. Biol. Reprod. 83, 396–403.10.1095/biolreprod.109.083154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K. C., de Voogt P. (1999). Persistent organic pollutants (POPs): State of the science. Environ. Pollut. 100, 209–221 [DOI] [PubMed] [Google Scholar]
- Ko H. P., Okino S. T., Ma Q., Whitlock J. P., Jr. (1996). Dioxin-induced CYP1A1 transcription in vivo: The aromatic hydrocarbon receptor mediates transactivation, enhancer-promoter communication, and changes in chromatin structure. Mol. Cell Biol. 16, 430–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara I., Lee D. K., Petit F. G., Jeong J., Lee K., Lydon J. P., DeMayo F. J., Tsai M. J., Tsai S. Y. (2007). COUP-TFII mediates progesterone regulation of uterine implantation by controlling ER activity. PLoS Genet. 3, e102.10.1371/journal.pgen.0030102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwekel J. C., Burgoon L. D., Burt J. W., Harkema J. R., Zacharewski T. R. (2005). A cross-species analysis of the rodent uterotrophic program: Elucidation of conserved responses and targets of estrogen signaling. Physiol. Genomics 23, 327–342 doi:10.1152/physiolgenomics.00175.2005 [DOI] [PubMed] [Google Scholar]
- Lin H. H., Liao C. J., Lee Y. C., Hu K. H., Meng H. W., Chu S. T. (2011). Lipocalin-2-induced cytokine production enhances endometrial carcinoma cell survival and migration. Int. J. Biol. Sci. 7, 74–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorber M., Patterson D., Huwe J., Kahn H. (2009). Evaluation of background exposures of Americans to dioxin-like compounds in the 1990s and the 2000s. Chemosphere 77, 640–651.10.1016/j.chemosphere. 2009.08.016 [DOI] [PubMed] [Google Scholar]
- Marsden M. D., Collins J. E., Greenwood M. D., Adams M. J., Fleming T. P., Magee A. I., Buxton R. S. (1997). Cloning and transcriptional analysis of the promoter of the human type 2 desmocollin gene (DSC2). Gene 186, 237–247 [DOI] [PubMed] [Google Scholar]
- Moggs J. G., Tinwell H., Spurway T., Chang H. S., Pate I., Lim F. L., Moore D. J., Soames A., Stuckey R., Currie R., et al. (2004). Phenotypic anchoring of gene expression changes during estrogen-induced uterine growth. Environ. Health Perspect. 112, 1589–1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller F. J., Diel P., Zierau O., Hertrampf T., Maass J., Vollmer G. (2010). Long-term dietary isoflavone exposure enhances estrogen sensitivity of rat uterine responsiveness mediated through estrogen receptor alpha. Toxicol. Lett. 196, 142–153.10.1016/j.toxlet.2010.03.1117 [DOI] [PubMed] [Google Scholar]
- Nayyar T., Bruner-Tran K. L., Piestrzeniewicz-Ulanska D., Osteen K. G. (2007). Developmental exposure of mice to TCDD elicits a similar uterine phenotype in adult animals as observed in women with endometriosis. Reprod. Toxicol. 23, 326–336.10.1016/j.reprotox.2006.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niskar A. S., Needham L. L., Rubin C., Turner W. E., Martin C. A., Patterson D. G., Jr, Hasty L., Wong L. Y., Marcus M. (2009). Serum dioxins, polychlorinated biphenyls, and endometriosis: A case-control study in Atlanta. Chemosphere 74, 944–949.10.1016/j.chemosphere.2008.10.005 [DOI] [PubMed] [Google Scholar]
- Ohtake F., Takeyama K., Matsumoto T., Kitagawa H., Yamamoto Y., Nohara K., Tohyama C., Krust A., Mimura J., Chambon P., et al. (2003). Modulation of oestrogen receptor signalling by association with the activated dioxin receptor. Nature 423, 545–550.10.1038/nature01606. [DOI] [PubMed] [Google Scholar]
- Patandin S., Dagnelie P. C., Mulder P. G., Op de Coul E., van der Veen J. E., Weisglas-Kuperus N., Sauer P. J. (1999). Dietary exposure to polychlorinated biphenyls and dioxins from infancy until adulthood: A comparison between breast-feeding, toddler, and long-term exposure. Environ. Health Perspect. 107, 45–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl M. W. (2001). A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29, e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard C., Gao J., Brown N., Reese J. (2003). Aquaporin water channel genes are differentially expressed and regulated by ovarian steroids during the periimplantation period in the mouse. Endocrinology 144, 1533–1541 [DOI] [PubMed] [Google Scholar]
- Rier S., Foster W. G. (2003). Environmental dioxins and endometriosis. Semin. Reprod. Med. 21, 145–154.10.1055/s-2003-41321 [DOI] [PubMed] [Google Scholar]
- Rier S. E., Martin D. C., Bowman R. E., Dmowski W. P., Becker J. L. (1993). Endometriosis in rhesus monkeys (Macaca mulatta) following chronic exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Fundam. Appl. Toxicol. 21, 433–441 [DOI] [PubMed] [Google Scholar]
- Schecter A., Furst P., Furst C., Papke O., Ball M., Ryan J. J., Hoang D. C., Le C. D., Hoang T. Q., Cuong H. Q., et al. (1994). Chlorinated dioxins and dibenzofurans in human tissue from general populations: A selective review. Environ. Health Perspect. 102(Suppl. 1), 159–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schecter A., Li L. (1997). Dioxins, dibenzofurans, dioxin-like PCBs, and DDE in U.S. fast food, 1995. Chemosphere 34, 1449–1457 [DOI] [PubMed] [Google Scholar]
- Schecter A., McGee H., Stanley J. S., Boggess K., Brandt-Rauf P. (1996). Dioxins and dioxin-like chemicals in blood and semen of American Vietnam veterans from the state of Michigan. Am. J. Ind. Med. 30, 647–654 [DOI] [PubMed] [Google Scholar]
- Shi Z., Valdez K. E., Ting A. Y., Franczak A., Gum S. L., Petroff B. K. (2007). Ovarian endocrine disruption underlies premature reproductive senescence following environmentally relevant chronic exposure to the aryl hydrocarbon receptor agonist 2,3,7,8-tetrachlorodibenzo-p-dioxin. Biol. Reprod. 76, 198–202 [DOI] [PubMed] [Google Scholar]
- Silkworth J. B., Brown J. F., Jr. (1996). Evaluating the impact of exposure to environmental contaminants on human health. Clin. Chem. 42(8 Pt 2), 1345–1349 [PubMed] [Google Scholar]
- Swanson H. I., Bradfield C. A. (1993). The AH-receptor: Genetics, structure and function. Pharmacogenetics 3, 213–230 [DOI] [PubMed] [Google Scholar]
- Taylor H. S. (2008). Endocrine disruptors affect developmental programming of HOX gene expression. Fertil. Steril. 89(2 Suppl.), e57–e58.10.1016/ j.fertnstert.2007.12.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng C. T. (1999). Regulation of lactoferrin gene expression by estrogen and epidermal growth factor: Molecular mechanism. Cell Biochem. Biophys. 31, 49–64.10.1007/BF02738154 [DOI] [PubMed] [Google Scholar]
- Teng C. T., Gladwell W., Beard C., Walmer D., Teng C. S., Brenner R. (2002). Lactoferrin gene expression is estrogen responsive in human and rhesus monkey endometrium. Mol. Hum. Reprod. 8, 58–67 [DOI] [PubMed] [Google Scholar]
- Theobald H. M., Peterson R. E. (1997). In utero and lactational exposure to 2,3,7,8-tetrachlorodibenzo-rho-dioxin: Effects on development of the male and female reproductive system of the mouse. Toxicol. Appl. Pharmacol. 145, 124–135.10.1006/taap.1997.8173 [DOI] [PubMed] [Google Scholar]
- USDHHS. (2005). Report on Carcinogens, National Toxicology Program, Research Triangle Park, NC, 11th ed [Google Scholar]
- U.S. EPA. (2009). Summary of U.S. EPA Dioxin Workshop: February 18–20, 2009, Washington DC. [Google Scholar]
- Wordinger R., Nile J., Stevens G. (1991). Effect of in-utero exposure to diethylstilboestrol on ontogeny of uterine glands in neonatal mice. J. Reprod. Fertil. 92, 209–216 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.