Abstract
Persons at-risk for autosomal dominant neurodegenerative diseases provide the opportunity to efficiently test preventive interventions. Only a minority of such persons, however, choose to undergo revealing genetic testing, presenting a challenge to enrollment. Thirty-four preclinical Latinos (n = 26) and non-Latinos at-risk for familial Alzheimer’s disease (FAD) unaware of their genetic status were administered a questionnaire exploring their interest in undergoing revealing genetic testing at baseline and in the context of eligibility for four prevention trials of increasing invasiveness. Forty-four percent of subjects expressed a baseline interest in undergoing revealing testing which increased to 85% in order to be eligible for a study of an oral drug "felt to be very safe.” If there were a 50% chance of receiving placebo, this number dropped to 62% (p = 0.02). For those not interested in a study involving a 50% chance of receiving placebo, a range of 5% to 40% chance of receiving placebo was given as acceptable. For more invasive studies, living in the U.S. (as opposed to Mexico) positively influenced the likelihood of participating. Our data suggests that clinical trial designs in which persons must confront their genetic status prior to enrollment are feasible. Study designs to minimize the likelihood of being placed on placebo or provide the eventual administration of the drug through open-label extensions should be considered.
Keywords: FAD, pre-symptomatic, genetic, testing, trials, prevention
INTRODUCTION
Dementia affects approximately twenty-four million people worldwide[1], with Alzheimer disease (AD) comprising 60–70% of all cases[2]. The clinical manifestations of AD are preceded by a 15 – 20 year period of silent pathology that includes accumulation of fibrillar beta amyloid and development of neurofibrillary tangles, and ultimately results in synaptic and neuronal loss that produce cognitive impairment[3]. Because reversing the neuronal loss caused by AD is difficult and may ultimately prove impossible, there are increased efforts at identifying interventions to prevent the clinical manifestation of AD. Delaying onset of AD dementia by 2 years would lead to 2 million fewer cases in the U.S. after 50 years[4]. AD prevention studies, however, present several challenges. For prevention trials to be informative, sufficient numbers of participants must develop dementia to power comparisons of intervention to placebo. Prevention trials may therefore “enrich’ the study population for persons more likely to develop AD (e.g. with a family history of the disorder[5, 6]) but even so, they must recruit several thousand participants and follow them for many years[7]. Studying a population in whom the disease can be more reliably predicted would greatly augment the performance of prevention studies.
Early onset familial AD (FAD) is a rare, fully penetrant, autosomal dominant form of AD [8] due to mutations in the PSEN1, APP, or PSEN2 genes. The typical age of onset is in the mid-30s to late-50s [5] and can be highly consistent within mutation-carrying kindreds[9]. Though affected individuals or pre-symptomatic individuals at-risk for a known familial mutation can undergo genetic testing, such testing is not currently widely offered, at least in part due to the unavailability of effective interventions[10].
One way to perform efficient prevention trials in AD is to enroll presymptomatic FAD mutation carriers. The number of such individuals who decide to undergo predictive, pre-symptomatic testing, however, is relatively low[11]. In one study, less than 10% of eligible persons from families with known pathogenic mutations for frontotemporal dementia (FTD) or FAD decided to undergo predictive testing[12]. As persons at-risk for FAD do not typically desire to undergo genetic testing, one cannot identify appropriate subjects in whom exposure to a potentially toxic treatment is justified[11]. Additionally, the risk of being placed into the placebo arm of a controlled study may be too high for an individual to risk learning that they will develop the disease[11]. The decision to undergo genetic testing prior to such trial participation is therefore a difficult one and performing prevention studies ethically such that subjects are truly informed regarding the scope of risks and benefits presents challenges[11].
The design of prevention trials in FAD will be improved by enhanced understanding of protocol features that affect at-risk persons’ desire to undergo genetic testing. We examined what aspects of study design are important to individuals at-risk for FAD in determining whether they would be willing to undergo genetic testing, learn the results, and participate in the study. We also explored the effect of potential assignment to placebo and participants’ reasoning behind their decisions.
MATERIALS AND METHODS
Participants
Thirty-four participants of a comprehensive study of pre- and symptomatic FAD being performed at UCLA completed a questionnaire exploring their interest in undergoing genetic testing in multiple contexts. All participants were at 50% risk of inheriting FAD due to known mutations in PSEN1, APP, or PSEN2 by virtue of being the first-degree relative of someone affected by the illness in a family shown to carry such mutations. This observational study seeks to characterize cognitive, behavioral, imaging (via positron emission tomography and multi-modal magnetic resonance imaging), and biochemical (plasma and cerebrospinal fluid) changes occurring during the pre- and symptomatic stages of FAD. In this study participants undergo genetic testing for the mutation for which they are at-risk but in the context of the study, are not told the results. All participants are offered clinical testing outside the study at no expense to them. Only non-demented participants (Clinical Dementia Rating Scale[13] score less than 1) who were unaware of their mutation status were administered the questionnaire. The population included Mexicans living in Mexico (n = 10), Mexican-Americans (n = 9), other Latinos residing in the U.S. (n = 7), and non-Latino Caucasians residing in the U.S. (n = 8). The questionnaire was created both in English and Spanish and subjects completed it in the language in which they were most proficient. Questionnaires were completed during a research visit or at home and were returned by mail. All subjects sent the questionnaire by mail (n = 10) or asked to complete the questionnaire during the research visit (n = 24) completed the questionnaire. No additional incentives were provided to subjects to complete this sub-study. All study procedures were approved by the UCLA Institutional Review Board.
Questionnaire
A written questionnaire collected background demographic information and explored at-risk persons’ baseline attitudes about genetic testing and clinical trial participation. Willingness to undergo genetic testing in the context of eligibility for four hypothetical prevention trials of “promising interventions” of increasing level of invasiveness was then explored. These hypothetical studies were modeled after currently on-going trials in AD. In each of the four hypothetical scenarios, it was explicitly explained that subjects would have to learn their genetic status and only mutation carriers would be eligible to participate. Subjects read that “In such studies, it may be necessary to assign some subjects to receive placebo (an inert, inactive intervention, or "sugar pill") in order to demonstrate that persons receiving the active drug develop AD at a lower rate.”
The questionnaire was initially written in English and then translated into Spanish by a fluently bilingual person of Puerto Rican origin (author LDM). It was then back-translated to English by a bilingual native of Colombia working as a neuropsychologist in Mexico (author YA-R). Differences in the back-translated version were discussed and edits made to reconcile discrepancies.
Hypothetical Study #1
Study #1 was described as follows: “A drug company is looking for participants for a research study for a medication with substantial promise in preventing AD. The medication has been studied extensively in animals and humans and is felt to be very safe. The treatment is a pill, taken twice a day that would most likely be required for the rest of your life.”
Hypothetical Study #2
Study #2 was described as follows: “A research study is looking at the effects of a vaccination that is given once per year for the rest of your life and hopefully will provide protection from the development of AD. Earlier studies of this vaccination in people have shown a 5% risk of brain inflammation that leads to permanent neurological disability (like a stroke) in 1% of subjects.”
Hypothetical Study #3
Study #3 was described as follows: “A drug company wants to test a medication that would be administered intravenously every three months for the rest of your life. Similar to the vaccination study, prior research in people has shown a 5% risk of brain inflammation that leads to permanent neurological disability (like a stroke) in 1% of subjects.”
Hypothetical Study #4
Study #4 was described as follows: “A research study is looking for participants for a high-risk clinical trial involving brain surgery. In this study, a neurosurgeon would drill small holes, one on each side of your skill while you are asleep under anesthesia. They would then implant the cells deep into your brain. The risks of the surgery and anesthesia can be high, and may include death. Results cannot be guaranteed. However, if the treatment worked, you would not develop AD or it would develop later in life. Therefore, the benefits could be as high as the risks.”
After each hypothetical study description, participants were asked, “knowing that there is a potential (but not a guarantee) to stop the development of AD, but that you would have to be told that you in fact are carrying the gene that causes FAD, would you participate in this study?” If subjects indicated they were interested in a given scenario, they then chose to endorse, or not, the reasons. They were given the following options, “The possible benefits outweigh the risks of being made aware of genetic status”, “To help future generations,” or “Other” and they were given space to provide their reasons. If they chose not to participate in a given scenario, they were also asked to endorse, or not, the following options, “I do not wish to know my genetic status and the possible benefits aren't worth it, “The risks and side effects are too high to justify possible benefits,”, “I do not want to risk being told I am a carrier and then be placed in the placebo group,” and “Other” and were given space to provide their reasons. Subsequent questions explored how the possibility of a 50% risk of being assigned to placebo would affect their decision and if that was unacceptable, what an acceptable chance of receiving placebo would be.
Statistical Analysis
Descriptive statistics, chi-square analyses, Fisher’s exact test, and the McNemar test for paired proportions were used where appropriate. Variables assessed regarding their impact on desire to be tested and participate in each trial included age, years of education, gender, country of residence, whether they were employed, currently have children, or plan to have more children.
RESULTS
Demographics
Thirty-four participants completed the questionnaire, 10 in Spanish and the remainder in English. The mean age of responders was 35.3 ± 10.3 (Range 19–62). Twenty-six participants (76%) were female and 16 (47.1%) reported having children. Six respondents who did not have children at the time of the study reported plans to have children in the future. Years of education completed ranged from 6 years to 19 years with a mean of 13.9 ± 3.0. Twenty-six (76.5%) participants were employed at the time of completing the questionnaire and 10 (29.4%) reported still being in school.
Questionnaire responses
At baseline, 15 of 34 (44%) respondents reported a desire to learn their genetic status, 12 (35%) did not want to learn their genetic status, and 7 (21%) reported they may be interested in learning their genetic status (Table 1). There was a trend toward females more frequently responding “yes” or “maybe” to undergoing genetic testing at baseline (73% vs. 38% of men, p = 0.07). Eighteen of 22 (82%) respondents with children reported they would consider testing if their children asked them to do so, 1 (5%) reported they would not consider testing, 3 (14%) reported they might consider testing. Also at baseline, 21 (62%) respondents reported that they would be interested in participating in a clinical trial, 9 (26%) reported they may be interested, 3 (9%) reported they would not be interested, and 1 (3%) did not respond to the question. All 16 respondents with children expressed a potential interest in participating in a clinical trial (replied “yes” or “maybe”) relative to 14/17 (82.4%) respondents without children (p = 0.13). Subjects’ age and years of education were not significantly related to the likelihood they wanted to undergo genetic testing at baseline (Table 1).
Table 1.
Demographic information for those wanting or possibly wanting to know their FAD mutation status at baseline and those not wanting to know their FAD mutation status.
| Currently want or maybe want to know their FAD mutation status (n = 22) |
Do not want to know their FAD mutation status (n = 12) |
||
|---|---|---|---|
| # Female (%) | 19 (86%) | 7 (58%) | p = 0.07 |
| Age (s.d.) | 33.7 (10.5) | 38.1 (9.5) | p = 0.24 |
| Education in years (s.d.) | 13.7 (3.2) | 14.3 (2.8) | p = 0.61 |
| # Latino (%, the remainder are non-Latino caucasians) |
11 (50%) | 10 (83%) | p = 0.82 |
Hypothetical Study #1: Oral medication trial
In study #1 of an oral medication “thought to be very safe,” 29 of 34 respondents (85%) indicated they would participate in the trial (Figure). Twenty-four endorsed “the possible benefits outweigh the risks of being made aware of genetic status,” and 27 endorsed “to help future generations” as reasons to participate. One subject provided “I would want to know for my own future planning” and another cited the intervention’s perceived safety as an additional reason to participate. Of the 5 subjects who were not interested in participating, three endorsed concerns about the benefits not outweighing the risks of knowing their status. There were no statistically significant differences in whether a respondent might participate based on spoken language, age, years of education, gender, country of residence, employment status, or whether the respondent had children or was planning to have children.
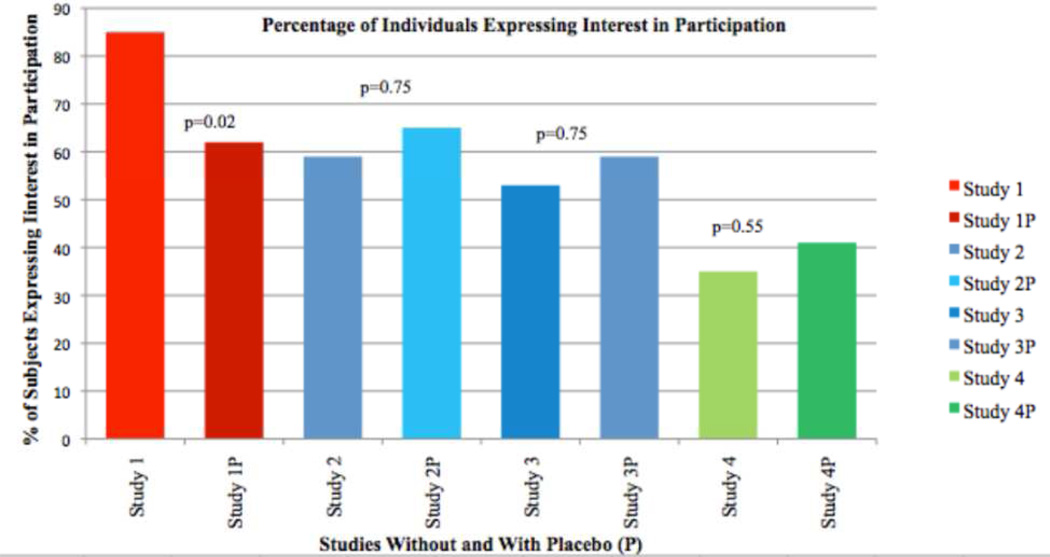
Figure 1.
Percentage of subjects expressing an interest in undergoing revealing genetic testing in order to participate in clinical trials to prevent FAD of increasing invasiveness, without or with (P) a 50% chance of receiving placebo.
When participants were asked to consider hypothetical study #1 in the context of a 50% chance of receiving placebo, the number of respondents willing to undergo testing and participate dropped to 21 (62%, p = 0.02, Figure) with 11 individuals electing not to participate (33%), and 2 respondents not answering the question (5%). Of the eleven individuals not willing to undergo a 50% chance or receiving placebo, 6 provided acceptable risks of receiving placebo and were “0%” (n = 3), “10%” (n = 2) and” 25%” and “30%” (one subject each).
Hypothetical Study #2: Vaccine trial
Twenty (59%) respondents indicated they would participate in Study #2 (Figure). Seventeen endorsed “the possible benefits outweigh the risks of being made aware of genetic status” and 16 “To help future generations” as reasons. Of the 14 who were not willing to participate in this study, all endorsed concerns regarding the risk outweighing the benefits. Those willing were no different from those unwilling based on gender, years of education, employment, or whether the respondent had children or planned to have children. English-speakers (71%), however, more frequently endorsed a willingness to participate than did Spanish-speakers (30%, p=0.03) and those residing in the US (75%) more frequently endorsed a willingness to participate than those living in Mexico, (22%, p=0.006). Additionally, those willing to participate in Hypothetical study #2 were significantly younger than those choosing not to participate (32.3 years vs. 39.6 years, p = 0.04).
For the vaccine study, the number of respondents willing to participate increased non-significantly to 22 (65%, p = 0.75, Figure) when asked to consider the trial with a 50% possibility of random assignment to a placebo group. Of the 12 who refused participation, 10 endorsed concerns about the risks of the study outweighing the benefits. Regarding an acceptable chance of receiving placebo, 3 indicated “0%”, 1 a “10%”, and 1 a “25%” chance. Of the 20 subjects who had indicated a willingness to participate in a study without a placebo arm, 4 declined when there was a 50% chance of receiving placebo. Of the 14 who indicated they would not participate without placebo, 6 indicated they would if they had a 50% chance of being assigned to placebo. All endorsed “the possible benefits outweigh the risks of being made aware of genetic status” and “to help future generations” as reasons to participate.
Hypothetical Study #3: Intravenous drug trial
Eighteen of 34 respondents (53%) indicated they would participate in Trial #3 (Figure). Seventeen endorsed “The possible benefits outweigh the risks of being made aware of genetic status” and 13 “to help future generations” as reasons to participate. No differences between willing and unwilling respondents were apparent with regard to language, gender, age, years of education, or employment status. However, there were statistically significant differences in whether respondents were willing to participate when analyzing the data in relation to country of residence or whether they had children. Persons living in the U.S., were more likely to endorse a willingness to participate (67% vs. 22%, p = 0.02) and persons with children more frequently endorsed a willingness to participate than did those without children (75% vs. 33%, p = 0.02).
The number of respondents who indicated they were willing to participate in the intravenous drug study increased non-significantly from 18 to 20 (59%, p = 0.75. Figure) in the setting of a 50% possibility of randomization to placebo. Of the 18 who indicated a willingness to participate without placebo, 4 declined when there was a 50% chance of receiving placebo. Of the 16 who refused the study without placebo, 6 said they would if there was a 50% chance of receiving placebo. Of these 6, 5 endorsed “The possible benefits outweigh the risks of being made aware of genetic status” and “to help future generations” as motivations. Eleven of 14 persons who refused the study with placebo endorsed the risks of the study outweighing the benefits as the reason. One subject wrote “Being vaccinated every 3 mo for the rest of my life would be hard for me and I would be scared of side effects.” Regarding an acceptable chance of receiving placebo, four indicated “0%”, four indicated “10%”, and one indicated “25%.”
Hypothetical Study #4: Neurosurgery trial
When asked about participation in the trial involving brain surgery, 12 of 34 respondents (35%) indicated they would participate (Figure). Nine endorsed “the possible benefits outweigh the risks of being made aware of genetic status” and 11 “To help future generations” as reasons. One indicated “I would want to wait until 5 years from the time I am likely to die of AD,” and one “I think that I would do most anything that was reasonably safe and has been studied well.” All but one of the 22 participants refusing participation endorsed concerns regarding the risks outweighing the benefits. There were no differences in whether or not a respondent would participate in the trial based on language, gender, age, years of education, country of residence, employment, or baseline interest in participating in clinical trials. A greater proportion of persons with children (50%) than persons without children (22%) expressed an interest in participating, though this difference did not reach statistical significance (p = 0.09).
When respondents considered trial #4 in the setting of a 50% chance of random assignment to placebo, the number willing to participate increased non-significantly to 14 (41%, p = 0.55, Figure). One respondent elected not to answer the question (3%). Of the 11 subjects willing to participate in Study #4 without the possibility of assignment to placebo, four refused participation if there was a 50% chance of being assigned to placebo. Of 22 who refused participation without the possibility of assignment to placebo, 7 indicated a willingness to participate if there was a 50% chance of being assigned to placebo. Of these seven, 6 endorsed both “the possible benefits outweigh the risks of being made aware of genetic status” and 11 “To help future generations” as reasons. All but one of the 19 subjects who refused participation when there was a chance of receiving placebo endorsed concerns about the risks outweighing the benefits. Specific reasons given were “This whole procedure seems dangerous and painful,” “The risk of death for a placebo is too high,” and “If anesthesia is involved, I want the treatment and not the placebo.” Acceptable chances of receiving placebo were given as “0%” by four, “5%” by two, “10%” by two, and “40%” by one subject.
Overall, 22 of the 34 subjects completing the questionnaire answered the same with regards to whether or not they would participate in studies with a 50% chance of receiving placebo across all trials. Of these, 13 said they would participate in all trials and 9 said they would not participate in any.
DISCUSSION
The purpose of the study was to explore the willingness to undergo revealing genetic testing in the setting of clinical trials for experimental interventions of FAD. These results may be generalizable to prevention studies in other fully-penetrant autosomal dominant neurodegenerative disease of adult onset such as Huntington’s disease.
The availability of prevention studies might serve as incentive for persons at-risk for FAD to undergo genetic testing. Nearly half of respondents expressed interest in undergoing revealing genetic testing. The proportion willing to undergo testing was increased in three out of four hypothetical clinical trial scenarios, even when trials were placebo-controlled. In two of the hypothetical studies, including trials of a vaccine and of an intravenously infused medication, residing in the U.S. (as opposed to Mexico) was associated with higher rates of interest in participation. For the infused medication trial and a trial of neurosurgical intervention, persons with children were more motivated, possibly related to their expressed interest in helping future generations.
As the potential for complications increases, the number of subjects interested in undergoing testing to participate decreases. Only trial #1 was explicitly described as safe and only trial #4 was explicitly described as being high risk. Surprisingly, the number of respondents interested in participating in the more invasive studies 2, 3, and 4 increased slightly when the possibility of receiving placebo was introduced. Though this may represent a random effect, some people may participate out of altruism rather than for any possible benefit to themselves and respondents may have viewed the possibility of receiving placebo as a reduction in the overall risk of participation. The fact that the vast majority of these subjects explicitly endorsed “to help future generations” as motivation to participate supports this possibility. A few subjects unwilling to participate in a study in which there was a 50% chance of receiving placebo indicated a willingness to participate if the risk was reduced to between 5 and 40%. This suggests that trial designs in which the chance of receiving placebo is less than 50% may increase enrollment.
Limitations of the study include the small sample size and the highly selected nature of the population. All subjects had previously participated in, or were currently participating in, a comprehensive observational study of the presymptomatic state in FAD and therefore represent a highly motivated group. This is reflected in the high level of interest in potentially undergoing genetic testing at baseline (44%) which is substantially higher than the 8% previously observed in a clinic-based study[12]. Therefore, the high level of interest in participating in clinical trials in which genetic status is revealed is unlikely to represent the opinion of the general population at-risk for FAD, which includes those who have opted not to participate in observational studies.
An important aspect of our study was that the population was largely Latino of Mexican origin. It is important to understand the attitudes of this population that is typically underrepresented in research but the applicability of our results to other ethnic groups may be limited. In future studies it will be necessary to evaluate the opinions of other ethnicities. Additionally, the majority of respondents were female, due to higher numbers of female participants in the observational study. Rates of participation in genetic studies are generally higher in women[14] though the degree to which this applies to clinical trials of prevention is unknown.
Though we exerted every effort to make the English and Spanish versions of the questionnaire equivalent, the degree to which we achieved this is unknown. It is therefore impossible to eliminate language as a potential confounder or to determine to what extent language or culture account for the observed differences among persons from different nations. Unfortunately, it is difficult to predict how such a bias might influence the outcomes of this survey. Finally, the degree to which subjects’ responses on this hypothetical questionnaire predict their real-life behavior is unknown. It is very possible that persons at-risk for FAD will respond differently when faced with actual trial opportunities.
Clinical trials for the prevention of FAD are underway[11, 15, 16]. In order to avoid the necessity of revealing persons’ genetic status for enrollment, one possible study design is to perform testing but not reveal the results to subjects, non-randomly assigning all non-mutation carriers to placebo. Though this design has important strengths, a potential pitfall is that, should mutation carriers who do not want to know their genetic status develop adverse effects thought to be related to drug, they would (potentially incorrectly) infer information they may not have wanted to receive.
Our data suggests that clinical trial designs in which persons must confront their genetic status prior to enrollment are feasible. However, in order to uphold the principle of autonomy, these vulnerable subjects need to be thoroughly educated prior to making decisions regarding participation. Additionally, to uphold the principle of beneficence, study designs to minimize the likelihood of being placed on placebo and eventual provision of the drug (e.g. via open-label extensions) must be considered.
CONCLUSIONS
Our results indicate that the availability of trials to prevent FAD, and possibly other fully-penetrant autosomal dominant neurodegenerative diseases of late-onset, will provide motivation for subjects to undergo revealing genetic testing in all but the most invasive protocols. This suggests that such studies in which genetic status is revealed are feasible. Latinos living in the U.S. were more likely to participate in the studies of intermediate invasiveness then their counterparts in Mexico, possibly reflecting an effect of the adoption of the vales of Western medicine in the course of acculturation. Though the possibility of receiving placebo can decrease subjects’ willingness to participate, this may not hold for interventions perceived to be more dangerous and designs in which the risk of receiving placebo is less than 50% can encourage participation. Altruism appears to be an important factor influencing at-risk persons desire to undergo genetic testing and participate. These observations should provide guidance in the design of and recruitment strategies for such prevention studies.
Supplementary Material
Acknowledgements
We would like to thank the participants most of all. This study was supported by PHS K08 AG-22228, The Dominantly Inherited Alzheimer Network U01 AG-032338, the UCLA Clinical Translational Research Institute 1UL1-RR033176, Alzheimer's Disease Research Center Grant P50 AG-16570, General Clinical Research Centers Program M01-RR00865, and the Easton Consortium for Alzheimer's Disease Drug Discovery and Biomarker Development.
Lists of Abbreviations
- AD
Alzheimer disease
- FAD
early-onset familial Alzheimer’s disease
- HD
Huntington disease
- FTD
frontotemporal dementia
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interests: The authors report no competing interests.
REFERENCES
- 1.Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, Hall K, Hasegawa K, Hendrie H, Huang Y, et al. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366(9503):2112–2117. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fratiglioni L, Launer LJ, Andersen K, Breteler MM, Copeland JR, Dartigues JF, Lobo A, Martinez-Lage J, Soininen H, Hofman A. Incidence of dementia and major subtypes in Europe: A collaborative study of population-based cohorts. Neurologic Diseases in the Elderly Research Group. Neurology. 2000;54(11 Suppl 5):S10–S15. [PubMed] [Google Scholar]
- 3.Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, Marcus DS, Cairns NJ, Xie X, Blazey TM, et al. Clinical and biomarker changes in dominantly inherited Alzheimer's disease. N Engl J Med. 2012;367(9):795–804. doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brookmeyer R, Gray S, Kawas C. Projections of Alzheimer's disease in the United States and the public health impact of delaying disease onset. Am J Public Health. 1998;88(9):1337–1342. doi: 10.2105/ajph.88.9.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meinert CL, McCaffrey LD, Breitner JC. Alzheimer's Disease Anti-inflammatory Prevention Trial: design, methods, and baseline results. Alzheimers Dement. 2009;5(2):93–104. doi: 10.1016/j.jalz.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murrell J, Ghetti B, Cochran E, Macias-Islas MA, Medina L, Varpetian A, Cummings JL, Mendez MF, Kawas C, Chui H, et al. The A431E mutation in PSEN1 causing Familial Alzheimer's Disease originating in Jalisco State, Mexico: an additional fifteen families. Neurogenetics. 2006;7(4):277–279. doi: 10.1007/s10048-006-0053-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thal LJ. Prevention of Alzheimer disease. Alzheimer Dis Assoc Disord. 2006;20(3 Suppl 2):S97–S99. doi: 10.1097/00002093-200607001-00015. [DOI] [PubMed] [Google Scholar]
- 8.Campion D, Dumanchin C, Hannequin D, Dubois B, Belliard S, Puel M, Thomas-Anterion C, Michon A, Martin C, Charbonnier F, et al. Early-onset autosomal dominant Alzheimer disease: prevalence, genetic heterogeneity, and mutation spectrum. Am J Hum Genet. 1999;65(3):664–670. doi: 10.1086/302553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fox NC, Kennedy AM, Harvey RJ, Lantos PL, Roques PK, Collinge J, Hardy J, Hutton M, Stevens JM, Warrington EK, et al. Clinicopathological features of familial Alzheimer's disease associated with the M139V mutation in the presenilin 1 gene. Pedigree but not mutation specific age at onset provides evidence for a further genetic factor. Brain. 1997;120(Pt 3):491–501. doi: 10.1093/brain/120.3.491. [DOI] [PubMed] [Google Scholar]
- 10.Williamson J, LaRusse S. Genetics and genetic counseling: recommendations for Alzheimer's disease, frontotemporal dementia, and Creutzfeldt-Jakob disease. Curr Neurol Neurosci Rep. 2004;4(5):351–357. doi: 10.1007/s11910-004-0081-x. [DOI] [PubMed] [Google Scholar]
- 11.Ringman JM, Grill J, Rodriguez-Agudelo Y, Chavez M, Xiong C. Commentary on "a roadmap for the prevention of dementia II: Leon Thal Symposium 2008" Prevention trials in persons at risk for dominantly inherited Alzheimer's disease: opportunities and challenges. Alzheimers Dement. 2009;5(2):166–171. doi: 10.1016/j.jalz.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steinbart EJ, Smith CO, Poorkaj P, Bird TD. Impact of DNA testing for early-onset familial Alzheimer disease and frontotemporal dementia. Arch Neurol. 2001;58(11):1828–1831. doi: 10.1001/archneur.58.11.1828. [DOI] [PubMed] [Google Scholar]
- 13.Morris JC. Clinical dementia rating: a reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. Int Psychogeriatr. 1997;(9 Suppl 1):173–176. doi: 10.1017/s1041610297004870. discussion 177–178. [DOI] [PubMed] [Google Scholar]
- 14.Koehly LM, Peterson SK, Watts BG, Kempf KK, Vernon SW, Gritz ER. A social network analysis of communication about hereditary nonpolyposis colorectal cancer genetic testing and family functioning. Cancer Epidemiol Biomarkers Prev. 2003;12(4):304–313. [PubMed] [Google Scholar]
- 15.Reiman EM, Langbaum JB, Fleisher AS, Caselli RJ, Chen K, Ayutyanont N, Quiroz YT, Kosik KS, Lopera F, Tariot PN. Alzheimer's Prevention Initiative: a plan to accelerate the evaluation of presymptomatic treatments. J Alzheimers Dis. 2011;(26 Suppl 3):321–329. doi: 10.3233/JAD-2011-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bateman RJ, Aisen PS, De Strooper B, Fox NC, Lemere CA, Ringman JM, Salloway S, Sperling RA, Windisch M, Xiong C. Autosomal-dominant Alzheimer's disease: a review and proposal for the prevention of Alzheimer's disease. Alzheimers Res Ther. 2011;2(6):35. doi: 10.1186/alzrt59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.