This review discusses MMP-9 structure, regulation, activation, and function in cardiovascular disease.
Abstract
Matrix metalloproteinase (MMP)-9, one of the most widely investigated MMPs, regulates pathological remodeling processes that involve inflammation and fibrosis in cardiovascular disease. MMP-9 directly degrades extracellular matrix (ECM) proteins and activates cytokines and chemokines to regulate tissue remodeling. MMP-9 deletion or inhibition has proven overall beneficial in multiple animal models of cardiovascular disease. As such, MMP-9 expression and activity is a common end point measured. MMP-9 cell-specific overexpression, however, has also proven beneficial and highlights the fact that little information is available on the underlying mechanisms of MMP-9 function. In this review, we summarize our current understanding of MMP-9 physiology, including structure, regulation, activation, and downstream effects of increased MMP-9. We discuss MMP-9 roles during inflammation and fibrosis in cardiovascular disease. By concentrating on the substrates of MMP-9 and their roles in cardiovascular disease, we explore the overall function and discuss future directions on the translational potential of MMP-9 based therapies.
Matrix metalloproteinases (MMPs) are a family of zinc-dependent endopeptidases responsible for both physiological and pathophysiological tissue remodeling. MMPs cleave all structural elements of the extracellular matrix (ECM), as well as process a variety of non-ECM substrates. Currently, there are 25 family members described in vertebrates, with 22 found in humans. MMPs were initially provided with descriptive names, based on substrate specificity, and were classified into five groups: collagenases, gelatinases, stromelysins, matrilysins, and membrane type (114). A numbering system corresponding to the order of discovery was adapted after it was realized that more MMPs existed than originally expected.
Normal myocardium possesses a number of ECM proteins, including collagens, laminins, fibronectin, and low levels of matricellular proteins, all of which play a role in the physiological performance of the heart. Collagen, the most abundant ECM protein, forms a complex network to provide three-dimensional structure and tensile strength to the cardiac muscle fibers. In cardiovascular disease, the cardiac muscle is subjected to tissue remodeling to preserve cardiac function and integrity, which involves breakdown of the collagen network. The MMPs that can cleave collagen include MMP-1, -2, -8, -9, and -14 (31, 109).
MMP-9, first termed 92-kDa type IV collagenase or gelatinase B, plays a major role in the degradation of ECM in a large spectrum of physiology and pathophysiology processes that involve tissue remodeling. For example, MMP-9 expression is important for embryo implantation, starting from the trophoblastic invasion during the early gestation period (12). MMP-9 is present in developing cardiac tissue in humans and rodents, and is expressed between 16 and 18 days of embryogenesis (61, 99). MMP-9 is also reported to play a significant role in neovascularization through the proteolytic degradation of the proteins in basal lamina of the blood vessels and a release of the biologically active form of vascular endothelial growth factor (6).
MMP-9 plays important roles in immune cell function. MMP-9 deletion promotes the recruitment of eosinophils and Th2 cells into the lungs during allergen challenge (74). In pathophysiological conditions, MMP-9 is upregulated during development and wound healing, as well as during pathologies that involve inflammatory processes, including arthritis, diabetes, and cancer (38). In these pathophysiological conditions, MMP-9 proteolytic properties contribute to stimulate the immune response to initiate pathogenesis and exacerbate disease progression. MMP-9 robustly increases during several cardiovascular diseases, including hypertension, atherosclerosis, and myocardial infarction (MI). The large number of publications on MMP-9 highlight the importance of this enzyme in the list of prospective and important biomarkers, which could be used in combination with other biomarkers to improve diagnosis or accelerate drug discovery (38). In this review, we discuss the structure, activity, and regulation of MMP-9, as well as its roles in common cardiovascular pathologies and potential for translational applications.
MMP-9 Structure and Activity
MMP family members share similar fundamental structural characteristics and are classified according to their substrate specificity. By this classification, MMP-9 belongs to the gelatinase subgroup and is known as gelatinase B due to its ability to degrade gelatin.
Human MMP-9 consists of an NH2-terminal pro-domain, a catalytic domain, a linker domain, and a COOH-terminal hemopexin-like domain that combine to form a 92-kDa pro- and 88-kDa active enzyme in humans (90). The catalytic domain of MMP-9 contains two zinc ions, five calcium ions, and three repeats homologous to the type II module of fibronectin. One of the two zinc ions of the catalytic domain and cysteine switch motif of the pro-domain are structurally coordinated to keep MMP-9 inactive (108). The catalytic zinc ion is essential for proteolytic activity.
MMP-9 has a unique domain termed the fibronectin-like domain, which consists of three repeats of fibronectin type II of ∼58 amino acids. This domain is heavily O-glycosylated and contains elongated linker between catalytic and hemopexin-like domain (89). The fibronectin-like domain is essential in binding to denatured collagen or gelatin (83). Hemopexin-like domain shares sequence similarity to plasma hemopexin and is present in MMP-9. In pro-MMP-9, the hemopexin-like domain forms a tight complex with TIMP-1 and TIMP-3 though their COOH-terminal domains (79). Pro-MMP-9 is complexed with TIMP-1 in the Golgi apparatus of the cell before secretion (104). TIMP-1 is bound to the pro-MMP-9 via COOH-terminal domain, leaving the NH2 terminus capable of inhibiting other MMPs.
MMP-9-null and MMP-9 overexpression mice have been developed. Mouse MMP-9 shares 72% identity and 99% homology with human MMP-9. Human MMP-9 contains a cysteine residue at the 87 amino acid that permits it to bind to neutrophil gelatinase-associated lipocalin, whereas mouse MMP-9 has a serine in this domain (54, 70, 136). Mouse MMP-9 contains 23 extra amino acids, mainly between amino acids 486–501 and 705–711. This results in mouse MMP-9 having an apparent molecular weight of 105 kDa (pro-) and 95 kDa (active).
Cell Expression of MMP-9
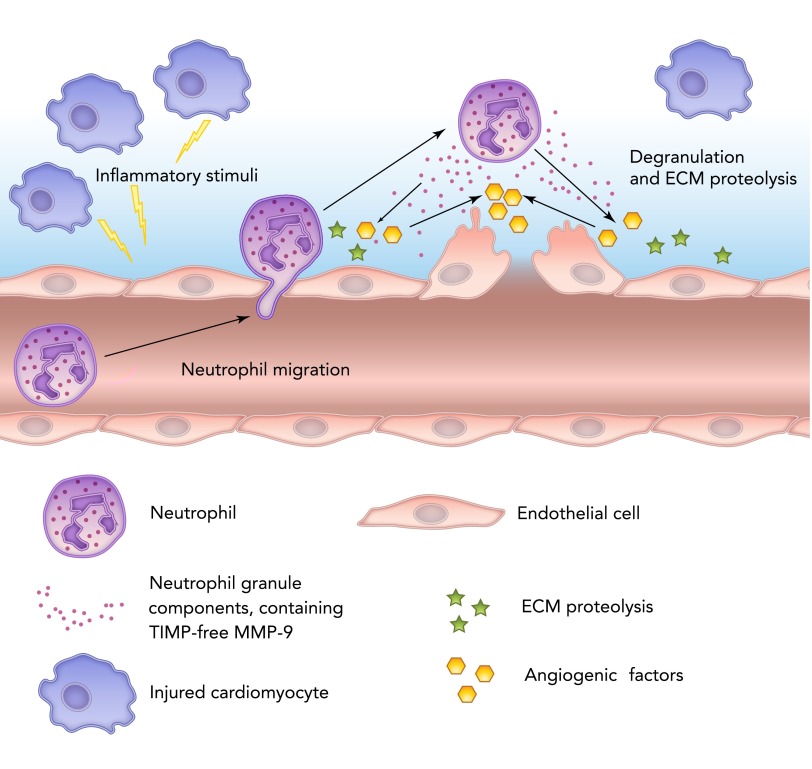
MMP-9 is secreted by a wide number of cell types, including neutrophils, macrophages, and fibroblasts. Neutrophils contain multiple proteases, such as serine proteases (elastase, cathepsin G, and proteinase 3), MMPs (MMP-8 and -9), and urokinase plasminogen activator (uPA). All proteases released from neutrophils promote MMP-9 activation (118). In neutrophils, MMP-9 is synthetized during granulocyte differentiation in the bone marrow. In humans but not rodents, neutrophil MMP-9 is covalently linked with lipocalin, which protects it from proteolytic degradation (21, 52). MMP-9 degrades ECM with subsequent activation of major proangiogenic factors such as vascular endothelial growth factor and fibroblast growth factor-2 (FIGURE 1) (4).
FIGURE 1.
Schematic representation of neutrophil roles in inflammation and MMP-9 release
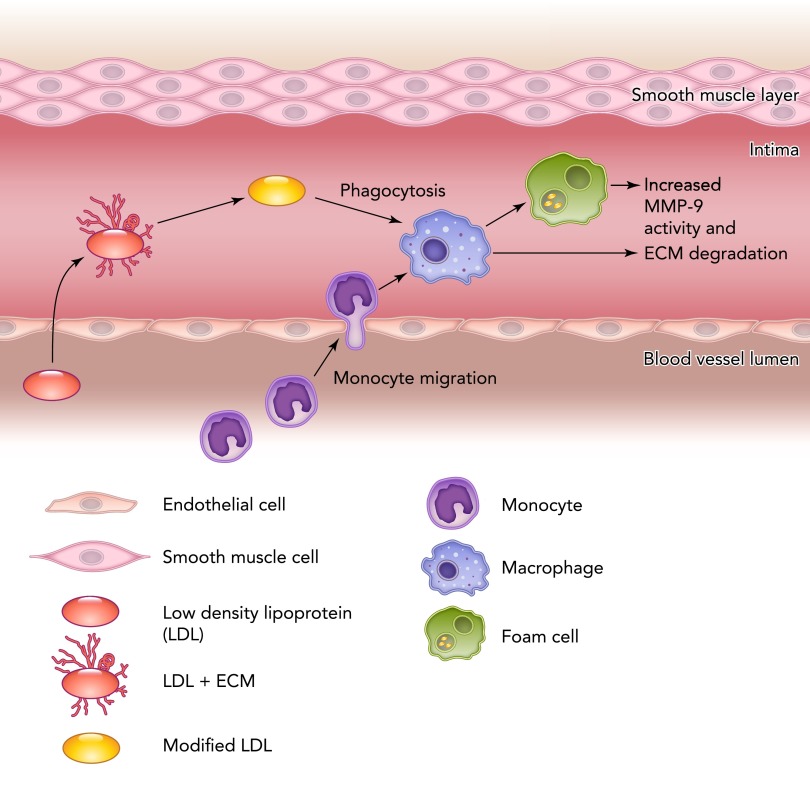
Macrophages are a potent source of MMP-9. Fang and colleagues showed that differentiated macrophages from circulating monocytes isolated from patients with acute MI or stable angina had a twofold increase in mRNA and protein levels of MMP-9 compared with the control groups (25). Monocyte entry into the tissue delineates the transition into the macrophage, at which time MMP-9 expression increases. The release of MMP-9 by macrophages in apoE-deficient mice greatly enhanced elastin degradation and induced plaque disruption (FIGURE 2). Among the macrophage phenotypes, foam cells are a predominant source of active MMP-9 (81). In MI, MMP-9-deficient mice showed reduced rupture rate and attenuated ventricular dilation, which was associated with reduced macrophage infiltration (23).
FIGURE 2.
Schematic representation of macrophage roles in inflammation and MMP-9 release
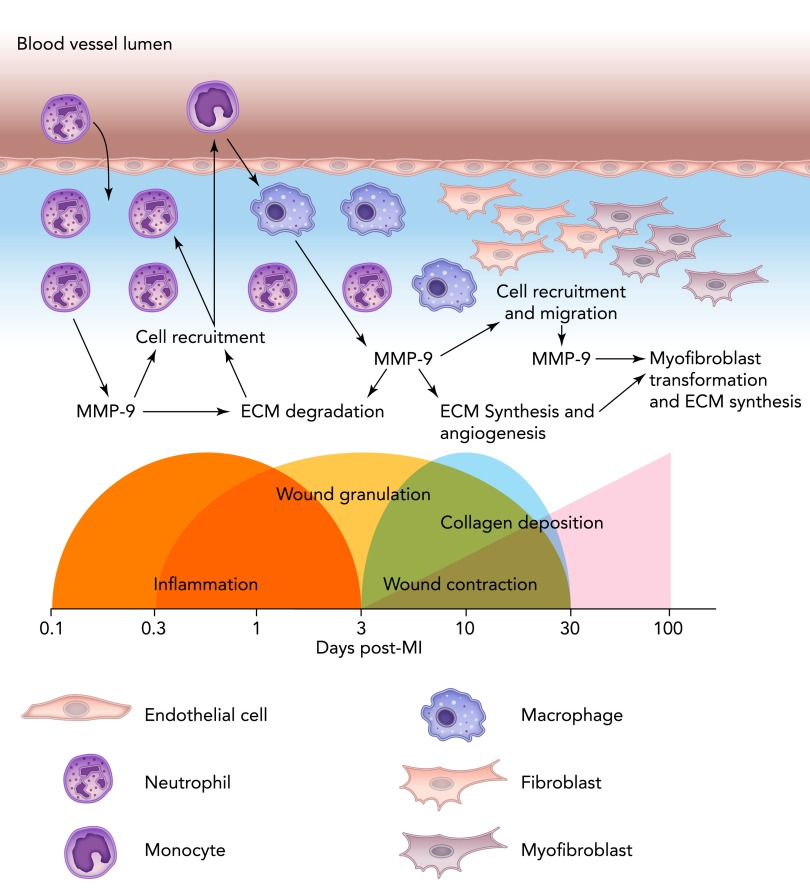
Siwik and colleagues showed an abundance of MMP-9 in cardiac fibroblasts during oxidative stress (112). Cardiac fibroblasts express MMP-9 after stimulation with IL-1β and TNF-α through ERK1/2 and nuclear factor-κB (NF-κB) signaling pathways (11). The activation of MMP-9 expression in cardiac fibroblasts is concomitant with a decrease in collagen synthesis rates to stimulate a net collagenolytic environment. MMP-9 is also actively involved in the cardiac fibroblast migration. Wang et al. showed that cardiac fibroblasts treated with a recombinant protein encoding only the catalytic domain of MMP-9 stimulated cardiac fibroblast migration, increased collagen synthesis, upregulated angiogenic factors, and induced the transition of cardiac fibroblasts to myofibroblasts (128). The role of the myofibroblasts is essential in post-MI healing. Myofibroblasts produce ECM and are able to contract, thus contributing to tissue replacement and scar formation post-MI (FIGURE 3) (121).
FIGURE 3.
Schematic representation of the cells involved in the post-MI inflammatory and wound-healing process
Regulation of MMP-9
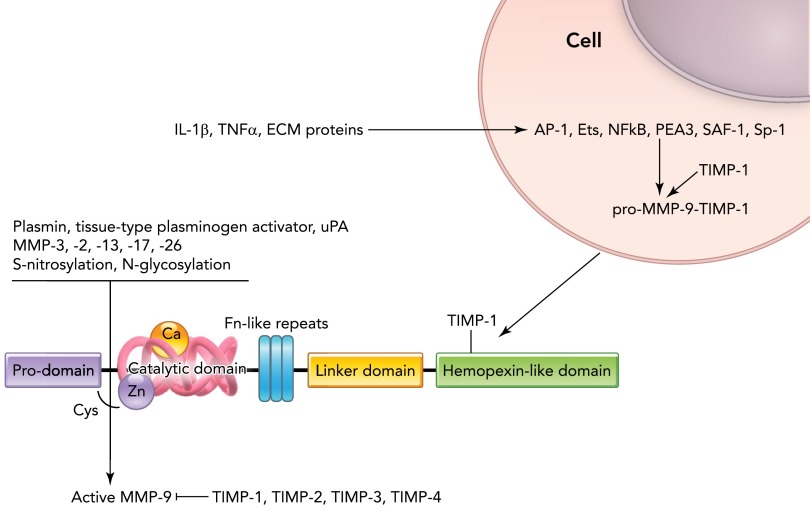
At the transcriptional level, MMP-9 is positively regulated by multiple factors, including E-26 (Ets) transcription factors, NF-κB, polyomavirus enhancer A-binding protein-3 (PEA3), activator protein-1 (AP-1), specificity protein 1 (Sp-1), and serum amyloid A-activating factor (SAF)-1 (16) (FIGURE 4).
FIGURE 4.
MMP-9 structure and factors regulating MMP-9 transcription and translation
Ets, E-26 transcription factor; NF-κB, nuclear factor κB; PEA-3, polyomavirus enhancer A-binding protein-3; AP-1, activator protein 1; SAF-1, serum amyloid A-activating factor 1; HSP60, heat shock protein 60; TIMP, tissue inhibitor of metalloproteinase; uPA, urokinase plasminogen activator.
Ets are a family of transcription factors associated with a variety of biological functions including cellular differentiation, cell migration, proliferation, apoptosis, and angiogenesis. Ets are capable of inducing MMP-9, along with uPA and integrins β2 and β3 (85).
NF-κB is capable of binding to κB DNA on the promoters or enhancers of genes to regulate expression (13). Bond et al. reported an increase in MMP-9 levels in vascular smooth muscle cells via NF-κB mechanisms (8). Inhibition of transcription factor NF-κB reduces MMP-9 production in vascular smooth muscle cells and macrophages (8, 37). Reactive oxygen species can activate MMP-9, both directly and indirectly, by activating transcription factors such as NF-κB (87). Ang II has direct and indirect effects on the expression of MMP-9. In ventricular myocytes, Ang II directly stimulates NF-κB to induce MMP-9 expression (107). Ang II activates epidermal growth factor receptor and the mitogen-activated protein kinase pathway to induce MMP-9 expression (111). Aldosterone, which is produced locally in the myocardium, triggers MMP-9 production through a NF-κB mechanism (67), as does the matricellular protein osteopontin (97).
PEA3 and AP-1 binding sites are present in the MMP-9 promoter (129, 132). AP-1 has two binding sites on MMP-9, and activation of MMP-9 is preceded by a rapid transient increase in AP-1 protein levels (131). Thrombospondins stimulate production of MMP-9 by activating AP-1 (36). Donnini and colleagues showed that fragments of thrombospondin promote MMP-9 production in bovine capillary endothelial cells (22).
Sp-1 binds to the MMP-9 promoter to induce transcription. Sp-1 undergoes several posttranscriptional modifications such as phosphorylation and glycosylation to increase transcription (78, 115). Inhibition of Sp-1 leads to decreased MMP-9 expression (78).
In addition, the transcription factors described above cross-interact to regulate MMP-9 expression, which may be especially important in vivo. In vascular smooth muscle cells, upregulation of MMP-9 is mainly attributed to expression and activation of NF-κB and AP-1 transcription factors (120). Occupation by AP-1 alone, however, is not sufficient for maximal MMP-9 transcription, and the cooperation of either NF-κB or Sp-1 binding proteins upstream of the AP-1 site is required for full MMP-9 transcription induction (5). SAF-1 is an inflammatory responsive transcription factor that induces MMP-9 transcription via cooperation with AP-1 (101). In the MMP-9 promoter region, SAF-1 is located in close proximity to AP-1 elements. Mutation of either SAF-1 or AP-1 greatly affects induction of the MMP-9 promoter and reduces the ability of SAF-1 and AP-1 to activate transcription (101).
Among the cytokines capable of regulating MMP-9 expression, an important role is assigned to TNF-α. Alexander and Acott showed that TNF-α triggers the production of MMP-9 through the protein kinase C signal-transduction pathway (2). Lau et al. showed TNF-α upregulated MMP-9 expression in coronary arteries (62, 63). Heat shock protein 60 has been shown to stimulate TNF-α followed by MMP-9 production in macrophages (58). In rat embryonic cardiomyoblast cell line H9c2, the NF-κB II binding site within the promoter region of MMP-9 (−626/−617) plays a key role in upregulation of MMP-9 expression by TNF-α induction (133). Among other cytokines capable of inducing MMP-9, IL-1β was shown to increase NF-κB and AP-1 in rat myocytes (69). MMP-9 expression has been reported in cardiomyocytes (41).
Classical MMP-9 activation includes disruption of the interaction between the zinc molecule in the catalytic domain and the cysteine switch in the pro-domain. This structural modification leads to cleavage of the pro-form and production of active enzyme. MMP-9 is activated by other MMPs, including MMP-2, -3, -13, -17, and -26 (32, 55, 86, 119). For example, activation of pro-MMP-3 by plasmin, which is generated from plasminogen by uPA bound to the uPA receptor on the plasma membrane, leads to activation of pro-MMP-9 (96). Proteolytic enzymes, such as plasmin, urokinase-type plasminogen activator, and tissue-type plasminogen activator, are capable of cleaving the pro-domain to activate MMP-9 (92).
Another example of indirect pro-MMP-9 activation is through the initial activation of MMP-2 and -13 on the cell surface by membrane type-1-MMP (32). Several serine proteases are capable of pro-MMP-9 activation. Tissue-associated chymotrypsin-like proteinase activates pro-MMP-9 in skin tissues from chronic unhealed wounds (40). Pancreatic trypsin-2 from human carcinoma was an effective pro-MMP-9 activator (113). Posttranslational modifications of MMP-9 are another potent mechanism of increasing extracellular MMP-9 activity, and these include S-nitrosylation and N-glycosylation. MMP-9 has one S-nitrosylation site at cysteine and two N-glycosylation sites at asparagines in positions 38 and 120 (59, 72).
Although MMP-9 is synthesized and secreted in a pro-form, there is evidence that MMP-9 may also be activated intracellularly. Pereira and colleagues reported that activated MMP-9 accumulates in cells undergoing apoptosis, although this does not rule out the possibility that MMP-9 had been activated extracellularly and taken back up (93). Tissue inhibitor of metalloproteinase (TIMP)-1-free MMP-9 has been shown to accumulate in microvascular endothelial cells in endothelial vesicles after phorbol myristate acetate stimulation (82). Future studies are warranted to determine whether MMP-9 can be activated intracellularly.
Inhibition of MMP-9 is performed by TIMPs binding to the zymogen forms of the enzyme (34). All TIMPs are known to interact with MMP-9 and inhibit its activity (9). TIMP-1 binds to pro-MMP-9, in addition to inhibiting its active form (104). In circulation, α2 macroglobulin inhibits MMP-9 to prevent systemic MMP-9 activation.
MMP-9 Roles in Cardiovascular Disease
Cardiovascular diseases involve inflammation and altered tissue remodeling associated with the reorganization of ECM and the activation of MMP-9.
MMP-9 Gene Polymorphism in Cardiovascular Disease
T allele carriers of C-1562T gene polymorphism are associated with an increased level of blood pressure and aortic stiffness in a hypertensive population (146) (Table 1). Carriers for the R279Q polymorphism show susceptibility to the increase in aortic stiffness and an increased risk for the development of hypertension (138). Another MMP-9 gene polymorphism, 836GA, alone and in haplotype with C-1562T, was shown to contribute to the development of hypertension and aortic stiffness (71).
Table 1.
Gene polymorphisms associated with increased MMP-9 expression and cardiovascular disease
| Disease | Gene Polymorphisms |
|---|---|
| Hypertension | C-1562T, R279Q, 836GA |
| Atherosclerosis | C-1562T, a number of microsatellite (CA)n repeats of >22, R279Q |
| Myocardial infarction | C-1562T, R279Q, R668Q |
Gene polymorphisms associated with increased MMP-9 expression and cardiovascular disease (15, 21, 38, 43, 56, 76, 80, 96–97, 107, 112, 114).
Genetic variation in promoter polymorphisms imposes allele-specific effects on the gene expression of MMP-9 (50, 144). In particular, the C-1562T polymorphism has been reported to increase gene expression of MMP-9 and was associated with severity of coronary atherosclerosis. The individuals carrying the T allele were predisposed to increased plaque instability through increased ECM degradation. Another functional polymorphism in the promoter region of MMP-9 is microsatellite (CA)n. (CA)n is located near the −90 position, which corresponds to a sequence of cytosine-adenine repeats (20). The number of CA repeats of 22 or more in the microsatellite of MMP-9 promoter was associated with carotid atherosclerosis and the formation of plaques with a thin fibrous cap (28). A combination of C-1562T in the promoter region and a G/A transition in exon 6 (R279Q) was reported to be a major haplotype among the Caucasian population with different stages of atherosclerosis (100).
The C-1562T polymorphism in the MMP9 gene promoter is associated with an elevated MMP-9 expression and increased susceptibility to MI (56, 127). The R279Q polymorphism of MMP-9 gene is not independently associated with MI; however, in combination with smoking, it has a synergistic effect and is significantly associated with the risk for MI (126). Another polymorphism in MMP-9, R668Q has been suggested to relate to the development of MI; however, Rodius and colleagues evaluated 1,049 patients and showed the frequency of MMP-9 R668Q was ∼25% and was not increased in patients with MI (105). The C-1562T R668Q polymorphisms in the MMP-9 gene also have been associated with heart failure (HF) and mortality (70).
Hypertension
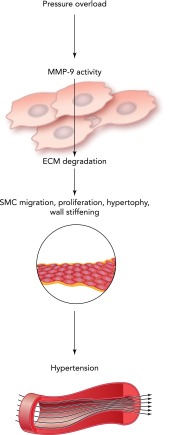
Hypertension is defined clinically as increased arterial blood pressure over 139 systolic and/or 89 diastolic mmHg. Hypertension is associated with alterations in cardiac, renal, neural, and vascular control systems which increase cardiac output, arterial stiffness, and peripheral resistance. Hypertensive patients express higher levels of MMP-9 in serum, which positively correlate with aortic stiffness (FIGURE 5) (116). MMP-9 activity is induced very early with the development of hypertension, contributing to collagen breakdown and arterial distensibility. An increase in fibrillar collagen in the compensated stage of hypertension is associated with increased MMP-9 activity (130). An increased arterial pressure and altered remodeling in the blood vessels lead to a pressure overload of the heart. Under these conditions, both vascular and cardiac tissues undergo additional compensatory remodeling. MMP-9 activity is increased in arteries with high pressure compared with vessels under normal pressure (64). Compensatory cardiac hypertrophy that develops in response to an increased pressure overload in hypertension is an established risk factor for atrial fibrillation, diastolic and systolic HF, and sudden death (53). Compensatory hypertrophy of the heart is associated with increased MMP-9 activity. Li et al. showed increased MMP-9 activity during compensatory hypertrophy in spontaneously hypertensive rats (66). ECM degradation increases during the transition stage from compensation to clinically apparent HF and associates with increased MMP-9 activity (1).
FIGURE 5.
Schematic representation of MMP-9 involvement in hypertension
Atherosclerosis
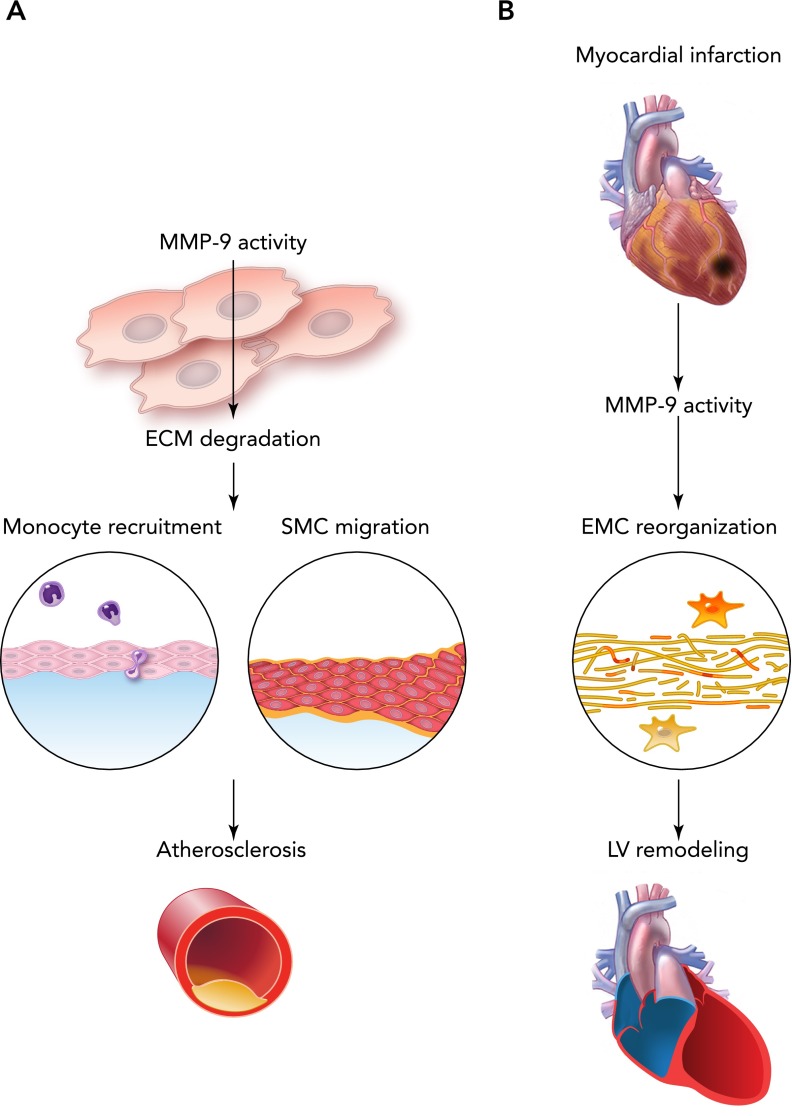
Atherosclerosis is defined clinically as buildup of fats and cholesterol in the arterial wall, which progresses to a plaque formation and restriction of blood flow. Human atherosclerotic plaques contain mostly collagen type I, III, IV, V, XI, and XVI (102). MMP-9 plays divergent roles in the formation and destabilization of atherosclerotic plaques (FIGURE 6A) (80). Plaque ruptures are associated with increased MMP-9 proteolytic activity (10, 60). Arterial remodeling is also associated with increased collagen I and IV accumulation and degradation. Flamant et al. reported increased deposition of collagen IV in the carotid arteries of MMP-9-null mice treated with Ang II, along with reduced arterial compliance, compared with wild-type mice (29). In the plaque, the major source for MMP-9 is macrophage-derived foam cells, and MMP-9 associates with the formation of a vulnerable thin fibrous cap (125). In apolipoprotein E-null mice, MMP-9 levels were highly correlated with incidence of plaque rupture (35). An increase in systemic MMP-9 levels is highly correlated with cardiovascular mortality in patients with atherosclerosis (7). Serum MMP-9 levels correlate with C-reactive protein, interleukin-6, and fibrinogen levels and serve as an identification marker for patients at risk for future MI (26, 47). These findings suggest deleterious effects of the MMP-9 overexpression on the progression of atherosclerosis. Interestingly, MMP-9-null mice show increased deposition of fibrin, which suggests that fibrin is degraded by MMP-9 (65). In atherosclerosis, increased levels of plasminogen activator inhibitor accelerate the process by allowing fibrin deposition in developing lesions. Since MMP-9 levels are reduced in plasminogen-deficient mice, it is possible that plasminogen regulates fibrin through MMP-9 signaling (17). Thus MMP-9 signaling may contribute to a reduction in thrombus size and play a beneficial role in suppressing the progression of atherosclerosis by inhibiting fibrin deposition.
FIGURE 6.
Schematic representations of MMP-9 involvement
Schematic representation of MMP-9 involvement in the atherosclerosis (A) and MI (B).
MI
MI is an acute event characterized by irreversible myocardial tissue injury that develops after prolonged disruption of blood supply to the myocardium. MI is characterized by increased inflammation that replaces necrotic tissue with a fibrotic scar (134). Type III collagen increase is observed as early as 2 days post-MI in rats and is aimed to preserve the integrity of the injured myocardium (15). The ratio of collagen deposition post-MI shifts toward the predominance of collagen I (FIGURE 6B) (147). All of these adverse changes in collagen deposition are observed in HF in vivo models and in humans. Higher MMP-9 levels play an important role during the early stages of acute MI and progression to HF, when observed in decompensated patients (51). MMP-9 levels increase as early as several minutes post-MI and remain increased for the first week in many animal models of MI (24, 106). The early increases in MMP-9 levels post-MI correlated with increased numbers of neutrophils, and later increases at days 2–4 with the infiltration of macrophages. These changes show an important role of MMP-9 in different stages of the inflammatory response. MMP-9-null deletion reduces the number of macrophages post-MI leading to attenuated enlargement of the left ventricle (LV) and reduced collagen accumulation (23). Targeted MMP-9 deletion in mice stimulates neovascularization and improves LV remodeling in the permanent occlusion model of MI (68). Interestingly, transgenic overexpression of MMP-9 in the macrophages unexpectedly shows improved cardiac function and attenuated inflammatory response at day 5 post-MI in mice, suggesting that MMP-9 regulates both macrophage pro- and anti-inflammatory phenotypes and contributes to LV remodeling (142).
MMP-9 Substrates
Cardiac and vascular remodeling include the reorganization of ECM, which is composed of collagen fibers, fibronectin, elastin, and matricellular proteins (Table 2). The deposition of ECM proteins in the LV or vasculature can lead to structural and functional changes due to both direct effects of the ECM proteins as well as indirect effects of ECM proteolytic processing. For example, fragments generated due to MMP-9 cleavage of collagen have suppressive effects on pathological angiogenesis, as well as stimulatory effects on both mRNA and protein levels of MMP-9 (27, 39).
Table 2.
A partial list of ECM, chemokines, cytokines, and substrates degraded, activated or inactivated by MMP-9
| MMP-9 | Substrate |
|---|---|
| Degrades | Collagen type I, II, III, IV, V, XI, XVI, fibronectin, laminin, osteopontin, thrombospondin-1, tenascin-C, galectin-3, decorin |
| Activates | CXCL5, CXCL8, TNF-α, IL-1β, TGF-β |
| Inactivates | CXCL1, CXCL4, CXCL5, CXCL7, CXCL12, IL-1β |
A partial list of ECM, chemokines, cytokines, and substrates degraded, activated or inactivated by MMP-9 (20, 23, 25, 31, 33–34, 36, 54, 58–59, 61, 64, 79, 84, 89, 92-92, 106, 109, 111). IL, interleukin; TNF-α, tumor necrosis factor α; TGF-β, transforming growth factor β; CXCL, chemokine (C-X-C motif) ligand.
Several ECM proteins are proteolytically processed by MMP-9, including collagen, fibronectin, and laminin. ECM fragments are known to express bioactive properties and regulate cardiac and vascular remodeling (117). Laminin is a well known MMP-9 substrate, and its levels negatively correlate with increased levels of MMP-9 (46). Laminin fragments support adhesion and differentiation of stem cells in vitro, playing an important role in wound healing (77). Laminins increase early post-MI and are associated with decreased cardiac rupture. They may impair macrophage infiltration and delay wound healing (73). In patients with hypertension, fibronectin levels are increased in the tunica media of the arteries, whereas laminin levels remain unchanged (103). In post-MI conditions, the levels of fibronectin and laminin are increased (143). Fibronectin fragments generated by MMP-9 cleavage act as chemoattractants for a variety of cell types involved in infarct healing and trigger a feedback mechanism to induce fibronectin expression (143).
Thrombospondin and tenascin-C regulate cell-matrix interactions. Thrombospondin-1 increases 7–28 days after ischemia-reperfusion and is known to inhibit MMP-9 activity (30). The higher level of thrombospondin-1 in the LV of MMP-9-null mice post-MI may explain the beneficial effects of MMP-9 deletion on angiogenesis (68). Tenascin-C plays an important role in the proliferative phase of the infarct healing. Tenascin-C often co-localizes with the MMP-9 site of active remodeling, and the deletion of MMP-9 was reported to increase the production of this matricellular protein (143). Tenascin-C weakens the adhesive interactions involving cardiomyocytes and contributes to reverse cardiac remodeling (30).
Galectin-3 is a carbohydrate-binding protein that contains a collagen-like domain susceptible for direct and rapid cleavage by MMP-9 (84). Galectin-3 serves as a diagnostic biomarker for MMP-9 activity. Galectin-3 is capable of binding to laminin, fibronectin, and collagen IV and is expressed in foam cells and macrophages (137). Higher concentrations of galectin-3 and increased MMP-9 activity are associated with cardiac fibrosis and increased risk for HF and mortality (45).
MMP-9 is known to process a number of inflammatory chemokines through the proteolysis. MMP-9 processes CXCL5 at the NH2 terminus and increases its chemotactic activity twofold (123). MMP-9 increases the chemotactic properties of CXCl8 (122). MMP-9 inactivates CXCL1, CXCL4, CXCL5, CXCL7, and CXCL12 (75, 122, 123). CXCL6 has been shown to be cleaved by MMP-9, but a change in its biological properties has not been reported (123). Evaluated properties of the described chemokines have been performed ex vivo in soluble proteins; however, in vivo these chemokines are immobilized on the ECM or cell surface by binding to glycosaminoglycans via positively charged domains (124).
MMP-9 processes a number of cytokines, including TNF-α, IL-1β, and TGF-β. MMP-9 was shown to release active TNF-α from the cell surface via proteolysis, which results in the production of a biologically active mature form (33). IL-1β is produced in an inactive form and is cleaved mainly by the caspase-1 to its active form by removing the NH2 terminus of the cytokine. MMP-9 both cleaves the inactive form of IL-1β to its active state and degrades its active form to decrease its biological activity (110). TGF-β is an anti-inflammatory cytokine and is released in the extracellular space in the latent form. A latent form of TGF-β is cross-linked to the ECM, and its maturation is associated with several mechanisms, including proteolysis. MMP-9 is capable of cleaving the latent pro-form of TGF-β to its active state (140). Another possible mechanism of TGF-β activation is through the degradation of the decorin, a small collagen-associated proteoglycan. Decorin serves as a depot for TGF-β in ECM, and its degradation by MMP-9 leads to a proteolysis of the cytokine and its activation (48).
Recent advances into the identification of ECM proteins in cardiovascular diseases suggest that MMP-9 has a wide array of potential substrates (3). However, the specificity and the functions of peptides generated from these substrates have yet to be evaluated.
Translational Applications
The initial approach to translating MMP-9 research concentrated on inhibiting the enzyme. Among all the MMP inhibitors designed and generated, only nonspecific doxycycline (periostat) has been approved by the Food and Drug Administration (94). Selective inhibitors to MMP-9 have been designed based on motifs of the active site (HWGF, CRRHWGFEFC, and CTTHWGFTLC). These peptides are susceptible to active proteolysis in vivo, which limits their suitability for experimental clinical settings (44, 57). A few selective MMP-9 inhibitors have been generated and successfully tested in the models of cardiovascular disease. In the cardiac injury setting, salvianolic acid, a selective MMP-9 inhibitor, prevented cardiac remodeling in spontaneously hypertensive rats (49). However, MMP-9 possesses not only deleterious effects but also beneficial effects depending on the time of the progression of the cardiovascular disease. Inhibition of MMP-9 in the acute stages of the cardiovascular disease may provide favorable outcomes, but MMP-9 inhibition in the later stages of the disease may alter the compensatory remodeling and contribute to a progression into HF.
A number of medications used in the treatment of patients with atherosclerosis, hypertension, and MI overlap in their efficacy to inhibit the production and activity of MMP-9. Medications targeting the renin-angiotensin-aldosterone system include ACE inhibitors, angiotensin receptor blockers, and aldosterone antagonists, and inhibit MMP-9 activities in animals and patients with MI and/or HF (66, 88). The aldosterone receptor-blocker eplerenone decreased MMP-9 activity in dogs with HF (98). ACE inhibitors reduced MMP-9 activity by directly binding to the MMP-9 active site (135). Beta blockers decrease MMP-9 expression and activity. Carvedilol was reported to reduce plasma levels of MMP-9 in patients with idiopathic cardiomyopathy and improved LV remodeling in a mouse model of acute myocarditis (91). Statins, pravastatin in particular, reduce serum levels of MMP-9 in post-MI patients (139).
Conclusions and Future Directions
Although selective MMP-9 inhibition is still a concept that is under development, targeting other mechanisms that stimulate MMP-9 activation may be beneficial. Administration of an endothelin-1 receptor blocker 3 days after MI reduced MMP-9 levels and activity, decreased TIMP-1 levels, and prevented LV dilation (95). Targeting serine proteases and the plasminogen system is another approach to regulate MMP-9 activity. Mice deficient in uPa or treated with plasminogen activator inhibitors showed protection against cardiac rupture that was mediated through reduced MMP-9 activation (42, 43). Other serine proteases, including serine elastase, trypsin, and cathepsin G, also induce MMP-9 activity and are capable of destroying the inhibitory activity of TIMPs. Inhibition of serine elastase was reported to reduce neutrophil accumulation into the ischemic myocardium and suppress MMP-9 activity (19, 141). There are, therefore, multiple indirect mechanisms of inhibiting MMP-9 function.
A better understanding of ECM fragments and MMP-9 activity may provide new opportunities to regulate inflammation by binding their chemotactic properties and attenuating cardiac remodeling. (76). Several studies have already reported the beneficial use of intracardiac injection of ECM-derived collagen and fibronectin in the post-MI setting (Table 3) (18, 76). Finding new MMP-9 substrates is another promising approach. Barallobre-Barreiro and colleagues identified more than 100 ECM proteins expressed in the focal region of the injured heart in ischemic cardiomyopathy patients, suggesting that there may be more substrates for MMP-9 activity than expected (3).
Table 3.
Effects of intracardiac-injected substances on myocardial structure and function after injury
| Substance Injected | Effects |
|---|---|
| ECM-derived Hep I | Promotes cell attachment, migration, and proliferation; induces Erk1/2 activation; promotes angiogenesis and arteriogenesis |
| ECM-derived Hep III | Promotes cell attachment, migration, and proliferation; induces Erk1/2 activation; promotes angiogenesis and arteriogenesis; prevents worsening of LV function; interacts with other Hep III peptides and ECM proteins; forms polymer-like matrix |
| ECM-derived RDG | Promotes cell attachment, migration, and proliferation; induces Erk1/2 activation; promotes angiogenesis and arteriogenesis |
| ECM-derived FC/HV | Adherent cells noticeable within 24 h; induces Erk1/2 activation |
| Injection of decellularized matrix | Thickens the LV infarcted wall; prevents LV systolic dysfunction; improves EF |
| Fibrin | Preserves infarcted wall thickness and cardiac function |
| Tannic acid | Prevents collagen degradation via cross-linking; inhibits MMP-9 activity |
Another possible approach is targeting ECM components directly at the time of injury. For example, intracardiac injections of tannic acid and fibrin have been shown to stabilize the ECM and preserve cardiac structure and function (14, 145). The results obtained to date provide new perspectives to the translational applications of both diagnostic and therapeutic strategies related to MMP-9 activity.
In this review, we summarized and discussed the roles of MMP-9 in normal development and in multiple cardiovascular diseases, including hypertension, atherosclerosis, MI, and HF. The ability of currently used medications in cardiac disease to directly or indirectly affect the production and activity of MMP-9 suggests that this metalloproteinase plays a key role in many of the molecular mechanisms underlying these pathophysiological conditions. However, selective inhibition of MMP-9 remains an open issue. Future successful in vivo studies using selective MMP-9 inhibitors may provide new insights and perspective to intervene on ECM remodeling in humans.
Footnotes
We acknowledge support from the NIH/NHLBI HHSN 268201000036C (N01-HV-00244) for the San Antonio Cardiovascular Proteomics Center, HL-051971, and R01 HL-075360, and from the Biomedical Laboratory Research and Development Service of the Veterans Affairs Office of Research and Development Award 5I01BX000505.
No conflicts of interest, financial or otherwise, are declared by the author(s).
Author contributions: A.Y. prepared figures; A.Y. and M.L.L. drafted manuscript; A.Y., Y.M., R.P.I., M.E.H., and M.L.L. edited and revised manuscript; M.L.L. approved final version of manuscript.
References
- 1.Ahmed SH, Clark LL, Pennington WR, Webb CS, Bonnema DD, Leonardi AH, McClure CD, Spinale FG, Zile MR. Matrix metalloproteinases/tissue inhibitors of metalloproteinases: relationship between changes in proteolytic determinants of matrix composition and structural, functional, and clinical manifestations of hypertensive heart disease. Circulation 113: 2089–2096, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Alexander JP, Acott TS. Involvement of protein kinase C in TNFalpha regulation of trabecular matrix metalloproteinases and TIMPs. Invest Ophthalmol Vis Sci 42: 2831–2838, 2001 [PubMed] [Google Scholar]
- 3.Barallobre-Barreiro J, Didangelos A, Schoendube FA, Drozdov I, Yin X, Fernandez-Caggiano M, Willeit P, Puntmann VO, Aldama-Lopez G, Shah AM, Domenech N, Mayr M. Proteomics analysis of cardiac extracellular matrix remodeling in a porcine model of ischemia/reperfusion injury. Circulation 125: 789–802, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Bekes EM, Schweighofer B, Kupriyanova TA, Zajac E, Ardi VC, Quigley JP, Deryugina EI. Tumor-recruited neutrophils and neutrophil TIMP-free MMP-9 regulate coordinately the levels of tumor angiogenesis and efficiency of malignant cell intravasation. Am J Pathol 179: 1455–1470, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benbow U, Brinckerhoff CE. The AP-1 site and MMP gene regulation: what is all the fuss about? Matrix Biol 15: 519–526, 1997 [DOI] [PubMed] [Google Scholar]
- 6.Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z, Hanahan D. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nature Cell Biol 2: 737–744, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blankenberg S, Rupprecht HJ, Poirier O, Bickel C, Smieja M, Hafner G, Meyer J, Cambien F, Tiret L. Plasma concentrations and genetic variation of matrix metalloproteinase 9 and prognosis of patients with cardiovascular disease. Circulation 107: 1579–1585, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Bond M, Chase AJ, Baker AH, Newby AC. Inhibition of transcription factor NF-kappaB reduces matrix metalloproteinase-1, -3 and -9 production by vascular smooth muscle cells. Cardiovasc Res 50: 556–565, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Brew K, Nagase H. The tissue inhibitors of metalloproteinases (TIMPs): an ancient family with structural and functional diversity. Biochim Biophys Acta 1803: 55–71, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown DL, Hibbs MS, Kearney M, Loushin C, Isner JM. Identification of 92-kD gelatinase in human coronary atherosclerotic lesions. Association of active enzyme synthesis with unstable angina. Circulation 91: 2125–2131, 1995 [DOI] [PubMed] [Google Scholar]
- 11.Brown RD, Jones GM, Laird RE, Hudson P, Long CS. Cytokines regulate matrix metalloproteinases and migration in cardiac fibroblasts. Biochem Biophys Res Commun 362: 200–205, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Canete-Soler R, Gui YH, Linask KK, Muschel RJ. Developmental expression of MMP-9 (gelatinase B) mRNA in mouse embryos. Dev Dyn 204: 30–40, 1995 [DOI] [PubMed] [Google Scholar]
- 13.Chen LF, Greene WC. Shaping the nuclear action of NF-kappaB. Nature Rev Mol Cell Biol 5: 392–401, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Christman KL, Fok HH, Sievers RE, Fang Q, Lee RJ. Fibrin glue alone and skeletal myoblasts in a fibrin scaffold preserve cardiac function after myocardial infarction. Tissue Eng 10: 403–409, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Cleutjens JP, Verluyten MJ, Smiths JF, Daemen MJ. Collagen remodeling after myocardial infarction in the rat heart. Am J Pathol 147: 325–338, 1995 [PMC free article] [PubMed] [Google Scholar]
- 16.Crawford HC, Matrisian LM. Mechanisms controlling the transcription of matrix metalloproteinase genes in normal and neoplastic cells. Enzyme Protein 49: 20–37, 1996 [DOI] [PubMed] [Google Scholar]
- 17.Creemers EE, Cleutjens JP, Smits JF, Daemen MJ. Matrix metalloproteinase inhibition after myocardial infarction: a new approach to prevent heart failure? Circ Res 89: 201–210, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Dai W, Gerczuk P, Zhang Y, Smith L, Kopyov O, Kay GL, Jyrala AJ, Kloner RA. Intramyocardial injection of heart tissue-derived extracellular matrix improves postinfarction cardiac function in rats. J Cardiovasc Pharmacol Therap 18: 270–279, 2013 [DOI] [PubMed] [Google Scholar]
- 19.Delyani JA, Murohara T, Lefer AM. Novel recombinant serpin, LEX-032, attenuates myocardial reperfusion injury in cats. Am J Physiol Heart Circ Physiol 270: H881–H887, 1996 [DOI] [PubMed] [Google Scholar]
- 20.Demacq C, Vasconcellos VB, Marcaccini AM, Gerlach RF, Silva WA, Jr, Tanus-Santos JE. Functional polymorphisms in the promoter of the matrix metalloproteinase-9 (MMP-9) gene are not linked with significant plasma MMP-9 variations in healthy subjects. Clin Chem Lab Med 46: 57–63, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Di Carlo A. Evaluation of neutrophil gelatinase-associated lipocalin (NGAL), matrix metalloproteinase-9 (MMP-9) and their complex MMP-9/NGAL in sera and urine of patients with kidney tumors. Oncol Lett 5: 1677–1681, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Donnini S, Morbidelli L, Taraboletti G, Ziche M. ERK1–2 and p38 MAPK regulate MMP/TIMP balance and function in response to thrombospondin-1 fragments in the microvascular endothelium. Life Sci 74: 2975–2985, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Ducharme A, Frantz S, Aikawa M, Rabkin E, Lindsey M, Rohde LE, Schoen FJ, Kelly RA, Werb Z, Libby P, Lee RT. Targeted deletion of matrix metalloproteinase-9 attenuates left ventricular enlargement and collagen accumulation after experimental myocardial infarction. J Clin Invest 106: 55–62, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Etoh T, Joffs C, Deschamps AM, Davis J, Dowdy K, Hendrick J, Baicu S, Mukherjee R, Manhaini M, Spinale FG. Myocardial and interstitial matrix metalloproteinase activity after acute myocardial infarction in pigs. Am J Physiol Heart Circ Physiol 281: H987–H994, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Fang L, Du XJ, Gao XM, Dart AM. Activation of peripheral blood mononuclear cells and extracellular matrix and inflammatory gene profile in acute myocardial infarction. Clin Sci (Lond) 119: 175–183, 2010 [DOI] [PubMed] [Google Scholar]
- 26.Ferroni P, Basili S, Martini F, Cardarello CM, Ceci F, Di Franco M, Bertazzoni G, Gazzaniga PP, Alessandri C. Serum metalloproteinase 9 levels in patients with coronary artery disease: a novel marker of inflammation. J Investig Med 51: 295–300, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Fichter M, Korner U, Schomburg J, Jennings L, Cole AA, Mollenhauer J. Collagen degradation products modulate matrix metalloproteinase expression in cultured articular chondrocytes. J Orthop Res 24: 63–70, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Fiotti N, Altamura N, Fisicaro M, Carraro N, Uxa L, Grassi G, Torelli L, Gobbato R, Guarnieri G, Baxter BT, Giansante C. MMP-9 microsatellite polymorphism and susceptibility to carotid arteries atherosclerosis. Arterioscler Thromb Vasc Biol 26: 1330–1336, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Flamant M, Placier S, Dubroca C, Esposito B, Lopes I, Chatziantoniou C, Tedgui A, Dussaule JC, Lehoux S. Role of matrix metalloproteinases in early hypertensive vascular remodeling. Hypertension 50: 212–218, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Frangogiannis NG. Matricellular proteins in cardiac adaptation and disease. Physiol Rev 92: 635–688, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovasc Res 53: 31–47, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Fridman R, Toth M, Pena D, Mobashery S. Activation of progelatinase B (MMP-9) by gelatinase A (MMP-2). Cancer Res 55: 2548–2555, 1995 [PubMed] [Google Scholar]
- 33.Gearing AJ, Beckett P, Christodoulou M, Churchill M, Clements J, Davidson AH, Drummond AH, Galloway WA, Gilbert R, Gordon JL. Processing of tumour necrosis factor-alpha precursor by metalloproteinases. Nature 370: 555–557, 1994 [DOI] [PubMed] [Google Scholar]
- 34.Goldberg GI, Strongin A, Collier IE, Genrich LT, Marmer BL. Interaction of 92-kDa type IV collagenase with the tissue inhibitor of metalloproteinases prevents dimerization, complex formation with interstitial collagenase, and activation of the proenzyme with stromelysin. J Biol Chem 267: 4583–4591, 1992 [PubMed] [Google Scholar]
- 35.Gough PJ, Gomez IG, Wille PT, Raines EW. Macrophage expression of active MMP-9 induces acute plaque disruption in apoE-deficient mice. J Clin Invest 116: 59–69, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Greenwood JA, Pallero MA, Theibert AB, Murphy-Ullrich JE. Thrombospondin signaling of focal adhesion disassembly requires activation of phosphoinositide 3-kinase. J Biol Chem 273: 1755–1763, 1998 [DOI] [PubMed] [Google Scholar]
- 37.Grimm T, Chovanova Z, Muchova J, Sumegova K, Liptakova A, Durackova Z, Hogger P. Inhibition of NF-kappaB activation and MMP-9 secretion by plasma of human volunteers after ingestion of maritime pine bark extract (Pycnogenol). J Inflamm 3: 1, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Halade GV, Jin YF, Lindsey ML. Matrix metalloproteinase (MMP)-9: a proximal biomarker for cardiac remodeling and a distal biomarker for inflammation. Pharmacol Therap 139: 32–40, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hamano Y, Zeisberg M, Sugimoto H, Lively JC, Maeshima Y, Yang C, Hynes RO, Werb Z, Sudhakar A, Kalluri R. Physiological levels of tumstatin, a fragment of collagen IV alpha3 chain, are generated by MMP-9 proteolysis and suppress angiogenesis via alphaV beta3 integrin. Cancer Cell 3: 589–601, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Han YP, Nien YD, Garner WL. Tumor necrosis factor-alpha-induced proteolytic activation of pro-matrix metalloproteinase-9 by human skin is controlled by down-regulating tissue inhibitor of metalloproteinase-1 and mediated by tissue-associated chymotrypsin-like proteinase. J Biol Chem 277: 27319–27327, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He BJ, Joiner ML, Singh MV, Luczak ED, Swaminathan PD, Koval OM, Kutschke W, Allamargot C, Yang J, Guan X, Zimmerman K, Grumbach IM, Weiss RM, Spitz DR, Sigmund CD, Blankesteijn WM, Heymans S, Mohler PJ, Anderson ME. Oxidation of CaMKII determines the cardiotoxic effects of aldosterone. Nature Med 17: 1610–1618, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heymans S, Lupu F, Terclavers S, Vanwetswinkel B, Herbert JM, Baker A, Collen D, Carmeliet P, Moons L. Loss or inhibition of uPA or MMP-9 attenuates LV remodeling and dysfunction after acute pressure overload in mice. Am J Pathol 166: 15–25, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heymans S, Luttun A, Nuyens D, Theilmeier G, Creemers E, Moons L, Dyspersin GD, Cleutjens JP, Shipley M, Angellilo A, Levi M, Nube O, Baker A, Keshet E, Lupu F, Herbert JM, Smits JF, Shapiro SD, Baes M, Borgers M, Collen D, Daemen MJ, Carmeliet P. Inhibition of plasminogen activators or matrix metalloproteinases prevents cardiac rupture but impairs therapeutic angiogenesis and causes cardiac failure. Nature Med 5: 1135–1142, 1999 [DOI] [PubMed] [Google Scholar]
- 44.Higashi S, Miyazaki K. Identification of a region of beta-amyloid precursor protein essential for its gelatinase A inhibitory activity. J Biol Chem 278: 14020–14028, 2003 [DOI] [PubMed] [Google Scholar]
- 45.Ho JE, Liu C, Lyass A, Courchesne P, Pencina MJ, Vasan RS, Larson MG, Levy D. Galectin-3, a marker of cardiac fibrosis, predicts incident heart failure in the community. J Am Coll Cardiol 60: 1249–1256, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Horstmann S, Kalb P, Koziol J, Gardner H, Wagner S. Profiles of matrix metalloproteinases, their inhibitors, and laminin in stroke patients: influence of different therapies. Stroke 34: 2165–2170, 2003 [DOI] [PubMed] [Google Scholar]
- 47.Hou ZH, Lu B, Gao Y, Cao HL, Yu FF, Jing N, Chen X, Cong XF, Roy SK, Budoff MJ. Matrix metalloproteinase-9 (MMP-9) and myeloperoxidase (MPO) levels in patients with nonobstructive coronary artery disease detected by coronary computed tomographic angiography. Acad Radiol 20: 25–31, 2013 [DOI] [PubMed] [Google Scholar]
- 48.Imai K, Hiramatsu A, Fukushima D, Pierschbacher MD, Okada Y. Degradation of decorin by matrix metalloproteinases: identification of the cleavage sites, kinetic analyses and transforming growth factor-beta1 release. Biochem J 322: 809–814, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jiang B, Li D, Deng Y, Teng F, Chen J, Xue S, Kong X, Luo C, Shen X, Jiang H, Xu F, Yang W, Yin J, Wang Y, Chen H, Wu W, Liu X, Guo DA. Salvianolic acid A, a novel matrix metalloproteinase-9 inhibitor, prevents cardiac remodeling in spontaneously hypertensive rats. PLos One 8: e59621, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jones CB, Sane DC, Herrington DM. Matrix metalloproteinases: a review of their structure and role in acute coronary syndrome. Cardiovasc Res 59: 812–823, 2003 [DOI] [PubMed] [Google Scholar]
- 51.Jong GP, Ma T, Chou P, Chang MH, Wu CH, Lis PC, Lee SD, Liu JY, Kuo WW, Huang CY. Serum MMP-9 activity as a diagnosing marker for the developing heart failure of post MI patients. Chinese J Physiol 49: 104–109, 2006 [PubMed] [Google Scholar]
- 52.Jonsson S, Lundberg A, Kalvegren H, Bergstrom I, Szymanowski A, Jonasson L. Increased levels of leukocyte-derived MMP-9 in patients with stable angina pectoris. PLos One 6: e19340, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Katholi RE, Couri DM. Left ventricular hypertrophy: major risk factor in patients with hypertension: update and practical clinical applications. Intl J Hypertens 2011: 495349, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kjeldsen L, Bainton DF, Sengelov H, Borregaard N. Structural and functional heterogeneity among peroxidase-negative granules in human neutrophils: identification of a distinct gelatinase-containing granule subset by combined immunocytochemistry and subcellular fractionation. Blood 82: 3183–3191, 1993 [PubMed] [Google Scholar]
- 55.Knauper V, Smith B, Lopez-Otin C, Murphy G. Activation of progelatinase B (proMMP-9) by active collagenase-3 (MMP-13). Eur J Biochem 248: 369–373, 1997 [DOI] [PubMed] [Google Scholar]
- 56.Koh YS, Chang K, Kim PJ, Seung KB, Baek SH, Shin WS, Lim SH, Kim JH, Choi KB. A close relationship between functional polymorphism in the promoter region of matrix metalloproteinase-9 and acute myocardial infarction. Int J Cardiol 127: 430–432, 2008 [DOI] [PubMed] [Google Scholar]
- 57.Koivunen E, Arap W, Valtanen H, Rainisalo A, Medina OP, Heikkila P, Kantor C, Gahmberg CG, Salo T, Konttinen YT, Sorsa T, Ruoslahti E, Pasqualini R. Tumor targeting with a selective gelatinase inhibitor. Nature Biotechnol 17: 768–774, 1999 [DOI] [PubMed] [Google Scholar]
- 58.Kol A, Sukhova GK, Lichtman AH, Libby P. Chlamydial heat shock protein 60 localizes in human atheroma and regulates macrophage tumor necrosis factor-alpha and matrix metalloproteinase expression. Circulation 98: 300–307, 1998 [DOI] [PubMed] [Google Scholar]
- 59.Kotra LP, Zhang L, Fridman R, Orlando R, Mobashery S. N-Glycosylation pattern of the zymogenic form of human matrix metalloproteinase-9. Bioorg Chem 30: 356–370, 2002 [DOI] [PubMed] [Google Scholar]
- 60.Kramsch DM, Franzblau C, Hollander W. The protein and lipid composition of arterial elastin and its relationship to lipid accumulation in the atherosclerotic plaque. J Clin Invest 50: 1666–1677, 1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.LAVBNakao LS, Ramos SG, Filho AP, Murta LO, Jr, Ingberman M, Tefe-Silva C, Precoma DB. Assessment of MMP-9, TIMP-1, and COX-2 in normal tissue and in advanced symptomatic and asymptomatic carotid plaques. Thromb J 9: 6, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lau AC, Duong TT, Ito S, Wilson GJ, Yeung RS. Inhibition of matrix metalloproteinase-9 activity improves coronary outcome in an animal model of Kawasaki disease. Clin Exp Immunol 157: 300–309, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lau AC, Duong TT, Ito S, Yeung RS. Matrix metalloproteinase 9 activity leads to elastin breakdown in an animal model of Kawasaki disease. Arthritis Rheum 58: 854–863, 2008 [DOI] [PubMed] [Google Scholar]
- 64.Lehoux S, Lemarie CA, Esposito B, Lijnen HR, Tedgui A. Pressure-induced matrix metalloproteinase-9 contributes to early hypertensive remodeling. Circulation 109: 1041–1047, 2004 [DOI] [PubMed] [Google Scholar]
- 65.Lelongt B, Bengatta S, Delauche M, Lund LR, Werb Z, Ronco PM. Matrix metalloproteinase 9 protects mice from anti-glomerular basement membrane nephritis through its fibrinolytic activity. J Exp Med 193: 793–802, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li H, Simon H, Bocan TM, Peterson JT. MMP/TIMP expression in spontaneously hypertensive heart failure rats: the effect of ACE- and MMP-inhibition. Cardiovasc Res 46: 298–306, 2000 [DOI] [PubMed] [Google Scholar]
- 67.Li YY, McTiernan CF, Feldman AM. Interplay of matrix metalloproteinases, tissue inhibitors of metalloproteinases and their regulators in cardiac matrix remodeling. Cardiovasc Res 46: 214–224, 2000 [DOI] [PubMed] [Google Scholar]
- 68.Lindsey ML, Escobar GP, Dobrucki LW, Goshorn DK, Bouges S, Mingoia JT, McClister DM, Jr, Su H, Gannon J, MacGillivray C, Lee RT, Sinusas AJ, Spinale FG. Matrix metalloproteinase-9 gene deletion facilitates angiogenesis after myocardial infarction. Am J Physiol Heart Circ Physiol 290: H232–H239, 2006 [DOI] [PubMed] [Google Scholar]
- 69.Long CS. The role of interleukin-1 in the failing heart. Heart Fail Rev 6: 81–94, 2001 [DOI] [PubMed] [Google Scholar]
- 70.Ma Y, Yabluchanskiy A, Lindsey ML. Neutrophil roles in left ventricular remodeling following myocardial infarction. Fibrogenesis Tissue Repair 6: 11, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mahmud A, Zhou S, Ryan AW, Jerrard-Dunne P, Feely J. A haplotype at the MMP-9 locus is associated with high-blood pressure and arterial stiffness in patients with essential hypertension. Artery Res 3: 17–23, 2009 [Google Scholar]
- 72.Martinez-Ruiz A, Lamas S. S-nitrosylation: a potential new paradigm in signal transduction. Cardiovasc Res 62: 43–52, 2004 [DOI] [PubMed] [Google Scholar]
- 73.Matsumura S, Iwanaga S, Mochizuki S, Okamoto H, Ogawa S, Okada Y. Targeted deletion or pharmacological inhibition of MMP-2 prevents cardiac rupture after myocardial infarction in mice. J Clin Invest 115: 599–609, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McMillan SJ, Kearley J, Campbell JD, Zhu XW, Larbi KY, Shipley JM, Senior RM, Nourshargh S, Lloyd CM. Matrix metalloproteinase-9 deficiency results in enhanced allergen-induced airway inflammation. J Immunol 172: 2586–2594, 2004 [DOI] [PubMed] [Google Scholar]
- 75.McQuibban GA, Butler GS, Gong JH, Bendall L, Power C, Clark-Lewis I, Overall CM. Matrix metalloproteinase activity inactivates the CXC chemokine stromal cell-derived factor-1. J Biol Chem 276: 43503–43508, 2001 [DOI] [PubMed] [Google Scholar]
- 76.Mihardja SS, Lee RJ. Using extracellular matrix-derived peptides to alter the microenvironment for myocardial repair. Curr Vasc Pharmacol 10: 342–346, 2012 [DOI] [PubMed] [Google Scholar]
- 77.Miyazaki T, Futaki S, Suemori H, Taniguchi Y, Yamada M, Kawasaki M, Hayashi M, Kumagai H, Nakatsuji N, Sekiguchi K, Kawase E. Laminin E8 fragments support efficient adhesion and expansion of dissociated human pluripotent stem cells. Nature Comm 3: 1236, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Murthy S, Ryan AJ, Carter AB. SP-1 regulation of MMP-9 expression requires Ser586 in the PEST domain. Biochem J 445: 229–236, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res 69: 562–573, 2006 [DOI] [PubMed] [Google Scholar]
- 80.Newby AC. Dual role of matrix metalloproteinases (matrixins) in intimal thickening and atherosclerotic plaque rupture. Physiol Rev 85: 1–31, 2005 [DOI] [PubMed] [Google Scholar]
- 81.Newby AC, George SJ, Ismail Y, Johnson JL, Sala-Newby GB, Thomas AC. Vulnerable atherosclerotic plaque metalloproteinases and foam cell phenotypes. Thromb Haemost 101: 1006–1011, 2009 [PMC free article] [PubMed] [Google Scholar]
- 82.Nguyen M, Arkell J, Jackson CJ. Active and tissue inhibitor of matrix metalloproteinase-free gelatinase B accumulates within human microvascular endothelial vesicles. J Biol Chem 273: 5400–5404, 1998 [DOI] [PubMed] [Google Scholar]
- 83.O'Farrell TJ, Pourmotabbed T. The fibronectin-like domain is required for the type V and XI collagenolytic activity of gelatinase B. Arch Biochem Biophys 354: 24–30, 1998 [DOI] [PubMed] [Google Scholar]
- 84.Ochieng J, Fridman R, Nangia-Makker P, Kleiner DE, Liotta LA, Stetler-Stevenson WG, Raz A. Galectin-3 is a novel substrate for human matrix metalloproteinases-2 and -9. Biochemistry 33: 14109–14114, 1994 [DOI] [PubMed] [Google Scholar]
- 85.Oda N, Abe M, Sato Y. ETS-1 converts endothelial cells to the angiogenic phenotype by inducing the expression of matrix metalloproteinases and integrin beta3. J Cell Physiol 178: 121–132, 1999 [DOI] [PubMed] [Google Scholar]
- 86.Ogata Y, Enghild JJ, Nagase H. Matrix metalloproteinase 3 (stromelysin) activates the precursor for the human matrix metalloproteinase 9. J Biol Chem 267: 3581–3584, 1992 [PubMed] [Google Scholar]
- 87.Okamoto T, Akaike T, Sawa T, Miyamoto Y, van der Vliet A, Maeda H. Activation of matrix metalloproteinases by peroxynitrite-induced protein S-glutathiolation via disulfide S-oxide formation. J Biol Chem 276: 29596–29602, 2001 [DOI] [PubMed] [Google Scholar]
- 88.Onal IK, Altun B, Onal ED, Kirkpantur A, Gul Oz S, Turgan C. Serum levels of MMP-9 and TIMP-1 in primary hypertension and effect of antihypertensive treatment. Eur J Intern Med 20: 369–372, 2009 [DOI] [PubMed] [Google Scholar]
- 89.Opdenakker G, Van den Steen PE, Dubois B, Nelissen I, Van Coillie E, Masure S, Proost P, Van Damme J. Gelatinase B functions as regulator and effector in leukocyte biology. J Leukoc Biol 69: 851–859, 2001 [PubMed] [Google Scholar]
- 90.Papazafiropoulou A, Tentolouris N. Matrix metalloproteinases and cardiovascular diseases. Hippokratia 13: 76–82, 2009 [PMC free article] [PubMed] [Google Scholar]
- 91.Pauschinger M, Rutschow S, Chandrasekharan K, Westermann D, Weitz A, Peter Schwimmbeck L, Zeichhardt H, Poller W, Noutsias M, Li J, Schultheiss HP, Tschope C. Carvedilol improves left ventricular function in murine coxsackievirus-induced acute myocarditis association with reduced myocardial interleukin-1beta and MMP-8 expression and a modulated immune response. Eur J Heart Fail 7: 444–452, 2005 [DOI] [PubMed] [Google Scholar]
- 92.Pepper MS. Role of the matrix metalloproteinase and plasminogen activator-plasmin systems in angiogenesis. Arterioscler Thromb Vasc Biol 21: 1104–1117, 2001 [DOI] [PubMed] [Google Scholar]
- 93.Pereira AM, Strasberg-Rieber M, Rieber M. Invasion-associated MMP-2 and MMP-9 are up-regulated intracellularly in concert with apoptosis linked to melanoma cell detachment. Clin Exp Metastasis 22: 285–295, 2005 [DOI] [PubMed] [Google Scholar]
- 94.Peterson JT. Matrix metalloproteinase inhibitor development and the remodeling of drug discovery. Heart Fail Rev 9: 63–79, 2004 [DOI] [PubMed] [Google Scholar]
- 95.Podesser BK, Siwik DA, Eberli FR, Sam F, Ngoy S, Lambert J, Ngo K, Apstein CS, Colucci WS. ET(A)-receptor blockade prevents matrix metalloproteinase activation late postmyocardial infarction in the rat. Am J Physiol Heart Circ Physiol 280: H984–H991, 2001 [DOI] [PubMed] [Google Scholar]
- 96.Ramos-DeSimone N, Hahn-Dantona E, Sipley J, Nagase H, French DL, Quigley JP. Activation of matrix metalloproteinase-9 (MMP-9) via a converging plasmin/stromelysin-1 cascade enhances tumor cell invasion. J Biol Chem 274: 13066–13076, 1999 [DOI] [PubMed] [Google Scholar]
- 97.Rangaswami H, Bulbule A, Kundu GC. Nuclear factor-inducing kinase plays a crucial role in osteopontin-induced MAPK/IkappaBalpha kinase-dependent nuclear factor kappaB-mediated promatrix metalloproteinase-9 activation. J Biol Chem 279: 38921–38935, 2004 [DOI] [PubMed] [Google Scholar]
- 98.Rastogi S, Mishra S, Zaca V, Alesh I, Gupta RC, Goldstein S, Sabbah HN. Effect of long-term monotherapy with the aldosterone receptor blocker eplerenone on cytoskeletal proteins and matrix metalloproteinases in dogs with heart failure. Cardiovasc Drugs Ther 21: 415–422, 2007 [DOI] [PubMed] [Google Scholar]
- 99.Ratajska A, Cleutjens JP. Embryogenesis of the rat heart: the expression of collagenases. Basic Res Cardiol 97: 189–197, 2002 [DOI] [PubMed] [Google Scholar]
- 100.Rauch I, Iglseder B, Paulweber B, Ladurner G, Strasser P. MMP-9 haplotypes and carotid artery atherosclerosis: an association study introducing a novel multicolour multiplex RealTime PCR protocol. Eur J Clin Invest 38: 24–33, 2008 [DOI] [PubMed] [Google Scholar]
- 101.Ray A, Bal BS, Ray BK. Transcriptional induction of matrix metalloproteinase-9 in the chondrocyte and synoviocyte cells is regulated via a novel mechanism: evidence for functional cooperation between serum amyloid A-activating factor-1 and AP-1. J Immunol 175: 4039–4048, 2005 [DOI] [PubMed] [Google Scholar]
- 102.Rekhter MD. Collagen synthesis in atherosclerosis: too much and not enough. Cardiovasc Res 41: 376–384, 1999 [DOI] [PubMed] [Google Scholar]
- 103.Rodella L, De Ciuceis C, Rizzoni D, Porteri E, Rezzani R, Boari G, Borsani E, Favero G, Platto C, Tiberio G, Giulini S, Agabiti Rosei E. Fibronectin and laminin content in the tunica media of subcutaneous small resistance arteries of patients with essential hypertension: Pp11428. J Hypertens 28: e179, 2010 [Google Scholar]
- 104.Roderfeld M, Graf J, Giese B, Salguero-Palacios R, Tschuschner A, Muller-Newen G, Roeb E. Latent MMP-9 is bound to TIMP-1 before secretion. Biol Chem 388: 1227–1234, 2007 [DOI] [PubMed] [Google Scholar]
- 105.Rodius S, Mulliert M, Azuaje F, Devaux Y, Wagner DR. Matrix metalloproteinase 9 polymorphism and outcome after myocardial infarction [Online]. Cardiogenetics 1: July, 2011; http://www.pagepressjournals.org/index.php/cardiogen/article/view/12 [Google Scholar]
- 106.Romanic AM, Burns-Kurtis CL, Gout B, Berrebi-Bertrand I, Ohlstein EH. Matrix metalloproteinase expression in cardiac myocytes following myocardial infarction in the rabbit. Life Sci 68: 799–814, 2001 [DOI] [PubMed] [Google Scholar]
- 107.Rouet-Benzineb P, Gontero B, Dreyfus P, Lafuma C. Angiotensin II induces nuclear factor- kappa B activation in cultured neonatal rat cardiomyocytes through protein kinase C signaling pathway. J Mol Cell Cardiol 32: 1767–1778, 2000 [DOI] [PubMed] [Google Scholar]
- 108.Rowsell S, Hawtin P, Minshull CA, Jepson H, Brockbank SM, Barratt DG, Slater AM, McPheat WL, Waterson D, Henney AM, Pauptit RA. Crystal structure of human MMP9 in complex with a reverse hydroxamate inhibitor. J Mol Biol 319: 173–181, 2002 [DOI] [PubMed] [Google Scholar]
- 109.Rybakowski JK. Matrix metalloproteinase-9 (MMP9)-A mediating enzyme in cardiovascular disease, cancer, and neuropsychiatric disorders. Cardiovasc Psychiatry Neurol 2009: 904836, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schonbeck U, Mach F, Libby P. Generation of biologically active IL-1 beta by matrix metalloproteinases: a novel caspase-1-independent pathway of IL-1 beta processing. J Immunol 161: 3340–3346, 1998 [PubMed] [Google Scholar]
- 111.Shah BH, Catt KJ. A central role of EGF receptor transactivation in angiotensin II -induced cardiac hypertrophy. Trends Pharmacol Sci 24: 239–244, 2003 [DOI] [PubMed] [Google Scholar]
- 112.Siwik DA, Pagano PJ, Colucci WS. Oxidative stress regulates collagen synthesis and matrix metalloproteinase activity in cardiac fibroblasts. Am J Physiol Cell Physiol 280: C53–C60, 2001 [DOI] [PubMed] [Google Scholar]
- 113.Sorsa T, Salo T, Koivunen E, Tyynela J, Konttinen YT, Bergmann U, Tuuttila A, Niemi E, Teronen O, Heikkila P, Tschesche H, Leinonen J, Osman S, Stenman UH. Activation of type IV procollagenases by human tumor-associated trypsin-2. J Biol Chem 272: 21067–21074, 1997 [DOI] [PubMed] [Google Scholar]
- 114.Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Ann Rev Cell Dev Biol 17: 463–516, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Taheri F, Bazan HE. Platelet-activating factor overturns the transcriptional repressor disposition of Sp1 in the expression of MMP-9 in human corneal epithelial cells. Invest Ophthalmol Vis Sci 48: 1931–1941, 2007 [DOI] [PubMed] [Google Scholar]
- 116.Tan J, Hua Q, Xing X, Wen J, Liu R, Yang Z. Impact of the metalloproteinase-9/tissue inhibitor of metalloproteinase-1 system on large arterial stiffness in patients with essential hypertension. Hypertens Res 30: 959–963, 2007 [DOI] [PubMed] [Google Scholar]
- 117.Trial J, Rossen RD, Rubio J, Knowlton AA. Inflammation and ischemia: macrophages activated by fibronectin fragments enhance the survival of injured cardiac myocytes. Exp Biol Med (Maywood) 229: 538–545, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tschesche H, Zolzer V, Triebel S, Bartsch S. The human neutrophil lipocalin supports the allosteric activation of matrix metalloproteinases. Eur J Biochem 268: 1918–1928, 2001 [DOI] [PubMed] [Google Scholar]
- 119.Uria JA, Lopez-Otin C. Matrilysin-2, a new matrix metalloproteinase expressed in human tumors and showing the minimal domain organization required for secretion, latency, and activity. Cancer Res 60: 4745–4751, 2000 [PubMed] [Google Scholar]
- 120.Valen G, Yan ZQ, Hansson GK. Nuclear factor kappa-B and the heart. J Am Coll Cardiol 38: 307–314, 2001 [DOI] [PubMed] [Google Scholar]
- 121.van den Borne SW, Diez J, Blankesteijn WM, Verjans J, Hofstra L, Narula J. Myocardial remodeling after infarction: the role of myofibroblasts. Nature Rev Cardiol 7: 30–37, 2010 [DOI] [PubMed] [Google Scholar]
- 122.Van den Steen PE, Proost P, Wuyts A, Van Damme J, Opdenakker G. Neutrophil gelatinase B potentiates interleukin-8 tenfold by aminoterminal processing, whereas it degrades CTAP-III, PF-4, and GRO-alpha and leaves RANTES and MCP-2 intact. Blood 96: 2673–2681, 2000 [PubMed] [Google Scholar]
- 123.Van Den Steen PE, Wuyts A, Husson SJ, Proost P, Van Damme J, Opdenakker G. Gelatinase B/MMP-9 and neutrophil collagenase/MMP-8 process the chemokines human GCP-2/CXCL6, ENA-78/CXCL5 and mouse GCP-2/LIX and modulate their physiological activities. Eur J Biochem 270: 3739–3749, 2003 [DOI] [PubMed] [Google Scholar]
- 124.Van Lint P, Libert C. Chemokine and cytokine processing by matrix metalloproteinases and its effect on leukocyte migration and inflammation. J Leukoc Biol 82: 1375–1381, 2007 [DOI] [PubMed] [Google Scholar]
- 125.Wagsater D, Zhu C, Bjorkegren J, Skogsberg J, Eriksson P. MMP-2 and MMP-9 are prominent matrix metalloproteinases during atherosclerosis development in the Ldlr(−/−)Apob(100/100) mouse. Intl J Mol Med 28: 247–253, 2011 [DOI] [PubMed] [Google Scholar]
- 126.Wang L, Ma YT, Xie X, Yang YN, Fu ZY, Li XM, Liu F, Huang Y, Ma X, Chen BD, Yuan S, Sun MH, Peng X, Wang BZ. Interaction between MMP-9 gene polymorphisms and smoking in relation to myocardial infarction in a Uighur population. Clin Appl Thromb Hemost 18: 72–78, 2012 [DOI] [PubMed] [Google Scholar]
- 127.Wang L, Ma Yt, Xie X, Yang Yn, Fu Zy, Liu F, Li Xm, Chen Bd. [Association of MMP9 gene −1562 C/T polymorphism with myocardial infarction in Uighur population of Xinjiang]. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 28: 180–184, 2011 [DOI] [PubMed] [Google Scholar]
- 128.Wang Y, Xu F, Chen J, Shen X, Deng Y, Xu L, Yin J, Chen H, Teng F, Liu X, Wu W, Jiang B, Guo DA. Matrix metalloproteinase-9 induces cardiac fibroblast migration, collagen and cytokine secretion: inhibition by salvianolic acid B from Salvia miltiorrhiza. Phytomedicine 19: 13–19, 2011 [DOI] [PubMed] [Google Scholar]
- 129.Wasylyk C, Gutman A, Nicholson R, Wasylyk B. The c-Ets oncoprotein activates the stromelysin promoter through the same elements as several non-nuclear oncoproteins. EMBO J 10: 1127–1134, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Weber KT, Pick R, Jalil JE, Janicki JS, Carroll EP. Patterns of myocardial fibrosis. J Mol Cell Cardiol 21, Suppl 5: 121–131, 1989 [DOI] [PubMed] [Google Scholar]
- 131.Woessner JF., Jr Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J 5: 2145–2154, 1991 [PubMed] [Google Scholar]
- 132.Wu CY, Hsieh HL, Sun CC, Yang CM. IL-1beta induces MMP-9 expression via a Ca2+-dependent CaMKII/JNK/c-JUN cascade in rat brain astrocytes. Glia 57: 1775–1789, 2009 [DOI] [PubMed] [Google Scholar]
- 133.Wu HT, Sie SS, Kuan TC, Lin CS. Identifying the regulative role of NF-kappaB binding sites within promoter region of human matrix metalloproteinase 9 (mmp-9) by TNF-alpha induction. Appl Biochem Biotechnol 169: 438–449, 2013 [DOI] [PubMed] [Google Scholar]
- 134.Yabluchanskiy A, Chilton RJ, Lindsey ML. Left ventricular remodeling: one small step for the extracellular matrix will translate to a giant leap for the myocardium. Congest Heart Fail 19: E5–E8, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yamamoto D, Takai S, Miyazaki M. Inhibitory profiles of captopril on matrix metalloproteinase-9 activity. Eur J Pharmacol 588: 277–279, 2008 [DOI] [PubMed] [Google Scholar]
- 136.Yan L, Borregaard N, Kjeldsen L, Moses MA. The high molecular weight urinary matrix metalloproteinase (MMP) activity is a complex of gelatinase B/MMP-9 and neutrophil gelatinase-associated lipocalin (NGAL). Modulation of MMP-9 activity by NGAL. J Biol Chem 276: 37258–37265, 2001 [DOI] [PubMed] [Google Scholar]
- 137.Yang RY, Rabinovich GA, Liu FT. Galectins: structure, function and therapeutic potential. Expert Rev Mol Med 10: e17, 2008 [DOI] [PubMed] [Google Scholar]
- 138.Yasmin, McEniery CM, O'Shaughnessy KM, Harnett P, Arshad A, Wallace S, Maki-Petaja K, McDonnell B, Ashby MJ, Brown J, Cockcroft JR, Wilkinson IB. Variation in the human matrix metalloproteinase-9 gene is associated with arterial stiffness in healthy individuals. Arterioscler Thromb Vasc Biol 26: 1799–1805, 2006 [DOI] [PubMed] [Google Scholar]
- 139.Yasuda S, Miyazaki S, Kinoshita H, Nagaya N, Kanda M, Goto Y, Nonogi H. Enhanced cardiac production of matrix metalloproteinase-2 and -9 and its attenuation associated with pravastatin treatment in patients with acute myocardial infarction. Clin Sci (Lond) 112: 43–49, 2007 [DOI] [PubMed] [Google Scholar]
- 140.Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev 14: 163–176, 2000 [PMC free article] [PubMed] [Google Scholar]
- 141.Zaidi SH, You XM, Ciura S, Husain M, Rabinovitch M. Overexpression of the serine elastase inhibitor elafin protects transgenic mice from hypoxic pulmonary hypertension. Circulation 105: 516–521, 2002 [DOI] [PubMed] [Google Scholar]
- 142.Zamilpa R, Ibarra J, de Castro Bras LE, Ramirez TA, Nguyen N, Halade GV, Zhang J, Dai Q, Dayah T, Chiao YA, Lowell W, Ahuja SS, D'Armiento J, Jin YF, Lindsey ML. Transgenic overexpression of matrix metalloproteinase-9 in macrophages attenuates the inflammatory response and improves left ventricular function post-myocardial infarction. J Mol Cell Cardiol 53: 599–608, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zamilpa R, Lopez EF, Chiao YA, Dai Q, Escobar GP, Hakala K, Weintraub ST, Lindsey ML. Proteomic analysis identifies in vivo candidate matrix metalloproteinase-9 substrates in the left ventricle post-myocardial infarction. Proteomics 10: 2214–2223, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhang B, Ye S, Herrmann SM, Eriksson P, de Maat M, Evans A, Arveiler D, Luc G, Cambien F, Hamsten A, Watkins H, Henney AM. Functional polymorphism in the regulatory region of gelatinase B gene in relation to severity of coronary atherosclerosis. Circulation 99: 1788–1794, 1999 [DOI] [PubMed] [Google Scholar]
- 145.Zhang H, Zhu SJ, Wang D, Wei YJ, Hu SS. Intramyocardial injection of tannic acid attenuates postinfarction remodeling: a novel approach to stabilize the breaking extracellular matrix. J Thorac Cardiovasc Surg 137: 216–222, 222e211–212, 2009 [DOI] [PubMed] [Google Scholar]
- 146.Zhou S, Feely J, Spiers JP, Mahmud A. Matrix metalloproteinase-9 polymorphism contributes to blood pressure and arterial stiffness in essential hypertension. J Hum Hypertens 21: 861–867, 2007 [DOI] [PubMed] [Google Scholar]
- 147.Zimmerman SD, Thomas DP, Velleman SG, Li X, Hansen TR, McCormick RJ. Time course of collagen and decorin changes in rat cardiac and skeletal muscle post-MI. Am J Physiol Heart Circ Physiol 281: H1816–H1822, 2001 [DOI] [PubMed] [Google Scholar]