Abstract
Background
Inflammatory factors and low HDL-C relate to CHD risk, but whether inflammation attenuates any protective association of high HDL-C is unknown.
Objective
Investigate inflammatory markers' individual and collective impact on the association of HDL-C with incident coronary heart disease (CHD).
Methods
In 3,888 older adults without known cardiovascular disease (CVD), we examined if the inflammatory markers C-reactive protein (CRP), interleukin-6 (IL-6), and lipoprotein-associated phospholipase A2 (Lp-PLA2) modify the relation of HDL-C with CHD. HDL-C, CRP, IL-6, and Lp-PLA2 values were grouped as using gender-specific tertiles. Also, an inflammation index of z-score sums for CRP, IL-6, and Lp-PLA2 was categorized into tertiles. We calculated CHD incidence for each HDL-C/inflammation group and performed Cox regression, adjusted for standard CVD risk factors and triglycerides to examine the relationship of combined HDL-C-inflammation groups with incident events.
Results
CHD incidence (per 1,000 person years) was higher for higher levels of CRP, IL-6, and the index, and lower for higher levels of HDL-C. Compared to high HDL-C/low-inflammation categories (referent), adjusted HRs for incident CHD were increased for those with high HDL-C and high CRP (HR=1.50, p<0.01) or highest IL-6 tertile (HR=1.40, p<0.05), but not with highest Lp-PLA2 tertile. Higher CHD incidence was similarly seen for those with intermediate or low HDL-C accompanied by high CRP, high IL-6, or a high inflammatory index.
Conclusion
The protective relation of high HDL-C for incident CHD appears to be attenuated by greater inflammation.
Keywords: High Density Lipoprotein, Inflammation, C-Reactive Protein, Coronary Heart Disease
High-density lipoprotein cholesterol (HDL-C) has been shown to be associated with protection from coronary heart disease (CHD) through possible mechanisms of reverse cholesterol transport and reduction of low-density lipoprotein oxidative stress.1, 2 Increased inflammation has a prominent role in the atherosclerotic process. C-reactive protein (CRP), interleukin-6, and other inflammatory markers have been shown to be independently related to progression of atherosclerosis and CHD. 3-7 Lipoprotein-associated phospholipase A2 (Lp-PLA2), a proatherogenic inflammatory marker, has also been shown to be associated with risk for CHD, independent of traditional cardiovascular risk factors.8 We recently demonstrated in US adults from the cross-sectional National Health and Nutrition Examination Survey that CRP levels > 3 mg/L are associated with a greater prevalence of CHD at all levels of HDL-C.9
While low HDL-C is related to future risk of CHD and cardiovascular disease (CVD), it is unknown if the relationship of HDL-C levels with incident CHD and CVD is influenced by inflammation.10 Accordingly, we investigated the influence of inflammatory markers CRP, IL-6 and Lp-PLA2, individually and collectively, on the association of HDL-C with incident CHD and CVD in older individuals without CVD at baseline from the Cardiovascular Health Study (CHS).
Methods
The CHS is a prospective National Institutes of Health-sponsored study focused on identifying CVD risk factors and outcomes in an older community-dwelling sample recruited from Health Care Financing Administration Medicare eligibility lists and from other household members in four US geographic regions (Forsyth County, North Carolina; Sacramento County, California; Washington County, Maryland; and Pittsburgh, Pennsylvania). Initial enrollment during 1989–1990 included 5,201 participants. In 1992-1993 a second cohort of 687 African-American participants was recruited, bringing the total sample size to 5,888. Baseline data were collected from standardized questionnaires, a structured physical examination, and fasting blood analyses.
The methodology and design of CHS have been previously reported.11,12 Our study included subjects with baseline values for age, gender, race, family history of ‘heart attack’, smoking history, diabetes, use of lipid and hypertension medication, blood pressure, waist size, triglycerides, HDL-C, low density lipoprotein cholesterol (LDL-C), and the inflammatory biomarkers CRP, IL-6, and Lp-PLA2, but who were free of known cardiovascular disease at baseline.
Laboratory methods
HDL-C and LDL-C were analyzed from aliquots (0.5 mL) of EDTA plasma which were stored at -80°C at the CHS central laboratory and shipped on dry ice to LipMed, Inc. for NMR lipoprotein subclass analysis. CRP was measured from stored frozen serum samples using a validated high-sensitivity enzyme-linked-immunosorbent-assay (analytical coefficient of variation 8.9%).13 IL-6 was measured from stored frozen serum samples using a commercial assay (Quantikine HS Human IL-6 Immunoassay, R&D Systems, Minneapolis, MN). Lp-PLA2 mass was measured at the Laboratory for Clinical Biochemistry Research (University of Vermont, Burlington, VT) using the PLAC™ Test (Diadexus, San Francisco, CA): analytical coefficient of variation was 6.3%. Triglycerides were measured by enzymatic methods on an Olympus Demand System (Olympus Corp., Lake Success, N.Y.).
Variable Definitions and Categories
Baseline HDL-C, CRP, IL-6, and Lp-PLA2 values were categorized into gender-specific tertiles. An inflammatory index was calculated based on the conversion of each individual's corresponding three inflammation values into z-score values using gender specific values. The sums of each individual's three inflammatory z-scores were then categorized into tertile sub-groups. Additionally, baseline HDL-C values were categorized into ≥60 mg/dl (high), 40–59 mg/dl (intermediate), and <40 mg/dl (low) sub-groups based on guidelines from the National Cholesterol Education Program Adult Treatment Panel III.14 Similarly, baseline CRP values were categorized into <1 mg/L (low CRP), 1-3 mg/L (intermediate CRP), and >3 mg/L (high CRP) categories based on previous recommendations for healthcare professionals.15.
Diabetes mellitus was defined as having a fasting glucose level ≥6.99 mmol/l (126 mg/dl), taking oral hypoglycemic medication, or self-reported use of insulin. Baseline CVD was defined as a history of angina, stroke, MI, congestive heart failure, coronary artery angioplasty, coronary artery bypass grafting (CABG), or peripheral vascular disease (PVD) as determined from the participant's self-report and/or medical records. Subsequent CVD events (angina, MI, claudication/PVD, and congestive heart failure), as well as the cause of death, were adjudicated by the CHS Cardiac Events Committee, using standardized criteria. The primary outcome of our study's analysis is incident CHD defined as angina, myocardial infarction, coronary artery angioplasty, coronary bypass surgery, or coronary heart disease death; we also conducted secondary analyses involving incident CVD as defined above. For those without an event, we defined patient's time-to-survival as time-to-death (from non-CVD causes), time-to-last follow up, or time-to-the end of the study.
Statistical analysis
For patients with and without incident CHD, we compared baseline laboratory values and past cardiovascular history using the chi-square test for categorical variables and Student's t-test for continuous variables. Incident CHD event rates were calculated according to tertile HDL-C categories paired with respective CRP, IL-6, Lp-PLA2, and inflammatory index tertile categories per 1,000 person years. Cox proportional hazards regression was used to examine the hazard ratios (HRs) and corresponding 95% confidence intervals for CHD events using the highest HDL-C/lowest inflammation group as a reference. Models were adjusted for age, sex, African American race, recruitment site, family history of ‘heart attack’, low-density lipoprotein cholesterol (LDL-C), hypertension medication, systolic and diastolic blood pressure, lipid- lowering medication, current or past smoking status, waist size, diabetes, and triglycerides. We further evaluated unadjusted and adjusted multiplicative interaction terms between HDL-C groups and each inflammatory factor group for CHD incidence. Additionally, we used previously described guideline cut-offs for HDL-C and CRP to calculate adjusted hazard ratio values.
Separately, we normalized values of HDL, CRP, IL-6, and Lp-PLA2 using log scale. Adjusted Cox regression analysis calculated hazard ratios utilizing standardized continuous measures of HDL and each respective inflammation marker or the created inflammatory index for CHD incidence. We further evaluated interaction terms between HDL-C and each inflammatory factor for CHD incidence. Finally, using the same HDL-C/inflammation groups, we evaluated adjusted models using the broader outcome of incident total CVD events. SAS statistical software (version 9.1.3; SAS, Cary, NC) was used for all statistical analyses.16
Results
Study population
We identified 4,426 adults with no baseline CVD among the 5,888 CHS cohort participants. An additional 538 individuals were excluded due to incomplete baseline data, leaving 3,888 individuals with complete data for our analysis. Those developing incident CHD had lower HDL-C and higher LDL-C, diabetes prevalence, systolic blood pressure, waist size, triglycerides, and inflammatory biomarkers as compared to the non-CHD incident group at baseline (p<0.05 for LDL-C, p<0.01 for hs-CRP and Lp-PLA2, and p<0.001 for all others) (Table 1). Of the 3,888 included individuals, 27.1% had a family history of heart attack or stroke, 52.2% were either a past or current smoker, and 13.1% had diabetes. Females had higher HDL-C levels and lower inflammatory factors of IL-6 and Lp-PLA2 compared to males (all p<0.001, data not shown). For those with incident CHD, median (interquartile range) values of CRP, IL-6 and Lp-PLA2 were 1.96 mg/L (1.00-3.42), 1.68 pg/ml (1.24-2.55), and 332.23 ng/ml (265.99-411.66) respectively. For those without incident CHD, median (interquartile range) values of CRP, IL-6 and Lp-PLA2 were 1.70 mg/L (0.87-3.03), 1.55 pg/ml (1.07-2.34), and 318.13 ng/ml (252.11-395.11) respectively.
Table 1. Baseline Characteristics of study subjects with vs without incident CHD: Cardiovascular Health Study.
| Incident CHD Mean (SD) or N (%) |
|||
|---|---|---|---|
| Positive | Negative | P-value | |
| Overall | 1184 (100) | 2704 (100) | |
| Age (years) | 72.7 (5.2) | 72.0 (5.3) | <0.001 |
| Gender | <0.001 | ||
| Male | 523 (44.2) | 937 (34.7) | |
| Female | 661 (55.8) | 1767 (65.3) | |
| Race | 0.17 | ||
| White | 1,021 (86.3) | 2,263 (83.7) | |
| African American | 156 (13.2) | 422 (15.6) | |
| Other | 7 (0.3) | 19 (1.6) | |
| Family history of “heart attack” | 366 (33.8) | 687 (28.0) | <0.001 |
| Smoking history | 635 (53.6) | 1392 (51.5) | 0.22 |
| Diabetes | 205 (17.3) | 305 (11.3) | <0.001 |
| Lipid Lowering Medication | 60 (5.1) | 111 (4.1) | 0.18 |
| Hypertension Medication | 518 (43.8) | 989 (36.9) | <0.01 |
| SBP (mm Hg) | 139.2 (22.1) | 134.4 (20.7) | <0.001 |
| Waist size (cm) | 95.7 (12.9) | 92.9 (13.3) | <0.001 |
| Triglycerides (mg/dl) | 140.9 (61.6) | 129.0 (57.6) | <0.001 |
| LDL cholesterol (mg/dl) | 132.2 (36.7) | 129.4 (34.8) | <0.05 |
| HDL-C cholesterol (mg/dL) | 53.3 (14.7) | 57.1 (16.0) | <0.001 |
| Inflammatory Biomarkers | |||
| HS-CRP (mg/L) | 3.4 (5.1) | 2.9 (4.6) | <0.01 |
| IL-6 (pg/ml) | 2.3 (2.3) | 2.0 (1.8) | <0.001 |
| Lp-PLA2 (ng/ml) | 348.7 (113.6) | 335.7 (116.8) | <0.01 |
Abbreviations: CHD, coronary heart disease; HDL-C, high-density lipoprotein; HS-CRP, high sensitivity C-reactive protein; IL-6, interleukin-6; LDL, low-density lipoprotein; Lp-PLA2, lipoprotein-associated phospholipase A2; MI, myocardial infarction; SBP, systolic blood pressure.
Incident CHD by inflammation/HDL-C categories
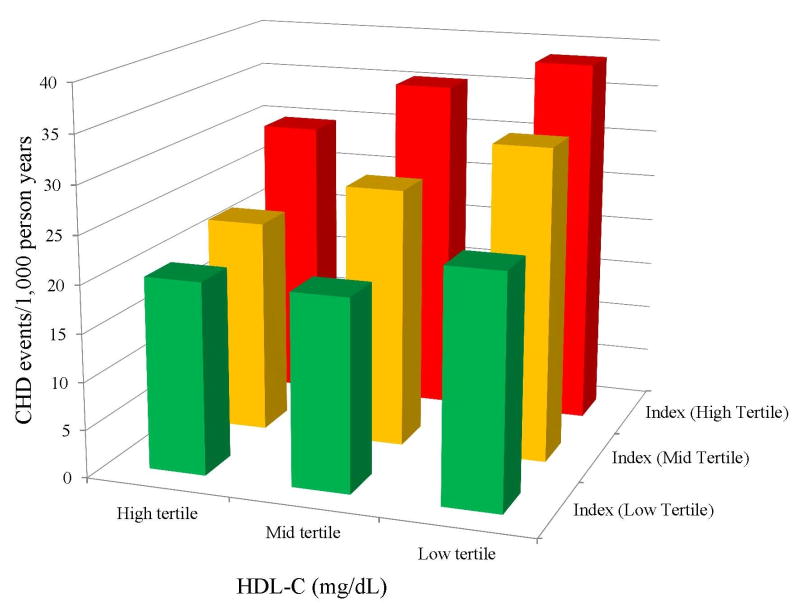
Mean follow-up time for those both with and without incident CHD was 11.1 years, and was 10.3 years for those with and without incident CVD. Across groups defined by HDL-C categories paired with CRP, IL-6, Lp-PLA2, and the inflammatory index categorizations, CHD event rates (per 1,000 person years) were generally higher for participants with higher inflammation levels (Figures 1a-1c), although among those with high HDL-C, the relation of Lp-PLA2 with CHD risk was less consistent (Figure 1c). Similarly, CHD event rates were greater in participants with lower HDL-C levels across increasing tertiles of inflammation index (Figure 2).
Figure 1.
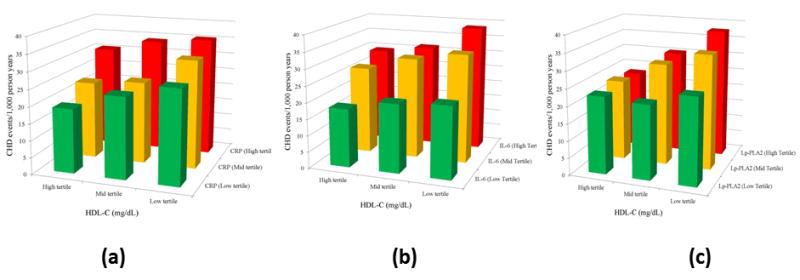
Incidence of CHD events per 1,000 patient years by HDL-C and a) CRP, b) Il-6, and c) LpPla2 tertiles. Coronary Heart Disease, CHD; High Density Lipoprotein Cholesterol, HDL-C; C-Reactive Protein, CRP; Interluekin-6, IL-6; Lipoprotein-associated phospholipase A2, Lp-PLA2.
Figure 2.
Incidence of CHD events per 1,000 patient years by HDL-C and Inflammatory Index tertiles. CHD event rates were higher for participants with higher inflammation index levels according to low, intermediate and high HDL-C categories. Coronary Heart Disease, CHD; High Density Lipoprotein Cholesterol, HDL-C.
Adjusted hazard ratios for incident CHD by HDL-C and inflammation factor categories
Overall, when examined in separate Cox regression models, those with high CRP (as compared to low CRP referent group) had a higher CHD incidence (adjusted HR=1.34, p<0.001). Both those within the highest tertile of IL-6 (adjusted HR=1.42, p<0.0001) and within the highest tertile of Lp-PLA2 (adjusted HR=1.20, p<0.05) overall also had an increased CHD incidence as compared to their respective lowest tertiles.
Adjusted hazard ratios for incident CHD by inflammation/HDL-C paired groups
Table 2 shows adjusted HRs for CHD incidence among HDL-C and inflammation categories. The HR for CHD incidence was higher for the high HDL-C/high CRP category (HR=1.50, p<0.01) as compared to the high HDL-C/low CRP group. Similarly, greater CHD risk (HR=1.40, p<0.05) was observed for the high IL-6 when paired with high HDL-C. The intermediate HDL-C category was associated with higher CHD incidence when paired with high CRP (HRs=1.53, p<0.01), high and intermediate IL-6 (HRs=1.36 and1.46 respectively, both p<0.05), and the high inflammatory index category (HR=1.41, p<0.01), as compared to the high HDL-C/low respective inflammation group. The low HDL-C category was associated with higher CHD incidence with intermediate and high CRP (HRs=1.39 and 1.45 respectively, both p<0.05), with intermediate and high IL-6 (HRs=1.42 and1.61 respectively, p<0.01), and high inflammatory index levels (HR=1.46, p<0.01) as compared to the high HDL-C/low respective inflammation group. There was no consistent association of higher levels of Lp-PLA2 with CHD risk across HDL-C categories. There was also no significant interaction between HDL-C and inflammation groups and the risk of CHD, indicating that the impact of inflammation on CHD was consistent across HDL-C tertiles.
Table 2.
Adjusted Hazard Ratios (95% Confidence Limits) for the Likelihood of an Overall Coronary Heart Disease Event by Inflammatory Markers and HDL-C Groupings.
| Hazard ratio (95% confidence interval) frequency of CHD incidence (total N) |
||||||
|---|---|---|---|---|---|---|
|
| ||||||
| HDL (High Tertile) | HDL (Mid Tertile) | HDL (Low Tertile) | ||||
|
| ||||||
| CRP (Low Tertile) | 1.00 | 1.13 (0.87-1.48) | 1.16 (0.88-1.55) | |||
| 126 (563) | 121 (424) | 98 (305) | ||||
|
| ||||||
| CRP (Mid Tertile) | 1.08 (0.82-1.43) | 1.11 (0.85-1.43) | 1.39 (1.07-1.81)‡; | |||
| 100 (381) | 133 (469) | 162 (454) | ||||
|
| ||||||
| CRP (High Tertile) | 1.50 (1.14-1.98)†; | 1.53 (1.18-1.98)†; | 1.45 (1.11-1.88)†; | |||
| 107 (342) | 154 (438) | 183 (512) | ||||
|
| ||||||
| IL-6 (Low Tertile) | 1.00 | 1.06 (0.81-1.38) | 1.03 (0.76-1.39) | |||
| 123 (555) | 119 (451) | 80 (291) | ||||
|
| ||||||
| IL-6 (Medium Tertile) | 1.31 (1.00-1.71) | 1.46 (1.13-1.88)† | 1.42 (1.09-1.85)† | |||
| 118 (409) | 154 (459) | 157 (426) | ||||
|
| ||||||
| IL-6 (High Tertile) | 1.40 (1.05-1.88)‡; | 1.36 (1.04-1.78)‡ | 1.61 (1.24-2.08)* | |||
| 92 (322) | 135 (421) | 206 (554) | ||||
|
| ||||||
| Lp-PLA2 (Low Tertile) | 1.00 | 0.83 (0.63-1.09) | 0.87 (0.66-1.15) | |||
| 121 (468) | 110 (419) | 116 (409) | ||||
|
| ||||||
| Lp-PLA2 (Medium Tertile) | 0.97 (0.74-1.26) | 1.09 (0.83-1.41) | 1.08 (0.83-1.41) | |||
| 120 (447) | 143 (440) | 150 (409) | ||||
|
| ||||||
| Lp-PLA2 (High Tertile) | 0.82 (0.61-1.11) | 1.08 (0.84-1.41) | 1.25 (0.96-1.63) | |||
| 92 (371) | 155 (472) | 177 (453) | ||||
|
| ||||||
| Index (Low Tertile) | 1.00 | 0.89 (0.67-1.17) | 0.95 (0.71-1.25) | |||
| 131 (545) | 101 (405) | 98 (345) | ||||
|
| ||||||
| Index (Medium Tertile) | 0.97 (0.74-1.28) | 1.12 (0.87-1.44) | 1.23 (0.95-1.61) | |||
| 102 (403) | 148 (479) | 151 (414) | ||||
|
| ||||||
| Index (High Tertile) | 1.24 (0.94-1.64) | 1.41 (1.10-1.82)† | 1.46 (1.13-1.89)† | |||
| 100 (338) | 159 (447) | 194 (512) | ||||
p< 0.001,
p<0.01,
p<0.05 when compared with reference group Coronary heart disease event is defined as angina, myocardial infarction, coronary artery angioplasty, coronary bypass surgery, or coronary heart disease death. Analyses adjusted for age, sex, African American race, recruitment site, family history of ‘heart attack’, low-density lipoprotein cholesterol, hypertension medication, blood pressure, lipid-lowering medication, current smoking, former smoking, waist size, diabetes, and triglycerides. Respective upper cutpoints for 1st and 2nd tertiles for males were the following: HDL (43 and 55 mg/dL), CRP (1.06 and 2.29 mg/L), IL-6 (1.38 and 2.17 pg/ml), and Lp-PLA2 (290.58 and 387.275 ng/ml).
Respective upper cutpoints for 1st and 2nd tertiles for females were the following: HDL (52 and 65 mg/dL), CRP (1.24 and 2.75 mg/L), IL-6 (1.20 and 1.99 pg/ml), and Lp-PLA2 (266.15 and 361.83 ng/ml).
Abbreviations: CRP, C-reactive protein; HDL, high-density lipoprotein; IL-6, interleukin-6; LDL, low-density lipoprotein; Lp-PLA2, lipoprotein-associated phospholipase A2; reference group, ref.
Finally, using established guideline cut-offs for HDL-C and CRP, we found that the HR for CHD incidence was higher for the high HDL-C (≥60 mg/dl)/high CRP (>3 mg/L) category (HR=1.52, p<0.01) as compared to the high HDL-C/low CRP (<1 mg/L) group. Similarly, the intermediate HDL-C category (40-59 mg/dl) was associated with higher CHD incidence when paired with high CRP (HR=1.44, p<0.01), in addition to the low HDL-C category (<40 mg/dl) being associated with higher CHD incidence when paired with intermediate and high higher CRP (HRs=1.46 and 1.64 respectively, p<0.05),
Adjusted hazard ratios for incident CHD by continuous HDL-C and inflammation factor values
From Cox regression utilizing continuous measures, HDL was inversely associated (HR=0.93 per SD, p=0.059) and CRP was positively associated (HR=1.16 per SD, p<0.0001) with the risk of future CHD events. Similar trends were seen in separate models of HDL (HR=0.94 per SD, p<0.10) with IL-6 (HR=1.14 per SD, p<0.0001), HDL (HR=0.92 per SD, p<0.05) with Lp-PLA2 (HR=1.08 per SD, p<0.05), and HDL (HR=0.93 per SD, p<0.10) with the inflammatory index (HR=1.14 per SD, p<0.0001). Adjusted multiplicative interaction terms utilizing continuous measures were only significant for that of Lp-PLA2 with HDL (p<0.05) for CHD incidence. This indicates that the direct relation of Lp-PLA2 with CHD events differs according to HDL-C level (being strongest for those with lower levels of HDL-C).
Adjusted hazard ratios for incident CVD by inflammation/HDL-C categories
Using a broader definition to designate CVD incidence in secondary analyses, we continued to see significantly different HRs among a broader range of HDL-C/inflammatory groups categorized according to gender-specific tertiles as in our CHD analyses. The HR for CVD event incidence was higher for the high HDL-C/high CRP group (HR=1.38, p<0.01) as compared to the high HDL-C/low CRP group. In addition, higher adjusted HRs for CVD incidence were seen for the high HDL-C category with intermediate and high IL-6 (HRs=1.27 and 1.39 respectively, p<0.05) as compared to the high HDL-C/low IL-6 group. For the intermediate HDL-C category, higher CVD incidence was seen with high CRP (HR=1.30, p<0.01) and with intermediate and high IL-6 (HR=1.28 and 1.29 respectively, p<0.001). For the low HDL-C category, higher CVD incidence was seen with high CRP (HRs=1.47, p<0.001), intermediate and high IL-6 (HRs=1.32 and 1.57 respectively, p<0.001), and high Lp-PLA2 (HRs=1.24, p<0.05).
Discussion
We demonstrate in a large sample of older adults that the association of HDL-C with CHD risk diminishes at higher levels of inflammation as those with the highest HDL-C values accompanied by increased hs-CRP and IL-6 had increased CHD risks. Adjusted models showed 50% and 40% greater risks of CHD for those within the highest tertile of CRP and IL-6, respectively, despite being in the highest tertile of HDL, as compared to those in the first tertile for these markers. We also found similarly increased for CHD incidence within high HDL/high inflammation groups when using established clinical cut-offs for HDL and CRP. Additionally, we also have shown the possibility of similar risks among an individual who have high HDL and high inflammation as compared to an individual with low HDL and normal inflammation.. The lack of significant interactions between HDL and inflammatory factors as predictors of CHD risk indicates that the impact of inflammation is similar regardless of HDL-C level.
Previously, Corsetti et al. showed that males from the Prevention of Renal and Vascular End-Stage Disease study with high HDL-C and high CRP levels are a subgroup at relatively high-risk for incident CVD.17 Further, our prior study from the NHANES cross-sectional survey shows the prevalence and likelihood of CVD to be greater when CRP is elevated both in those with normal and high HDL-C levels.10 The current study corroborates and extends these findings to incident CHD in a large, US population-based prospective study of older men and women with a wider array of inflammatory factors. Although higher CRP and IL-6 were strongly associated with CHD incidence, the risk associated with greater Lp-PLA2 was less consistent. This may be due to the fact that Lp-PLA2 activity rather than purely mass has been shown to be a better predictor of vascular events.18 Another potential explanation for this finding could be its dual role in inflammation. Lp-PLA2, although primarily proinflammatory or proatherogenic when associated with LDL, has also been shown to have anti-inflammatory properties when associated with HDL-C.19,20 However, in our continuous models, which may have more statistical power, the relation of LpPla2 levels with CHD risk did appear to differ according to HDL-C level, consistent with a stronger role for LpPla2 in promoting atherosclerosis when dyslipidemia (eg low HDL-C) is also present.
Despite the beneficial reverse cholesterol transport and inflammatory-modulating properties of HDL-C, it has been well established that clinical events often manifest in patients with normal ranges of HDL-C. In the Framingham Heart Study, 44% of clinical events occurred in men with HDL-C>40 mg/dL and 43% of events in women with HDL-C >50 mg/dL.21,22 A recent meta-analysis showed that increasing levels of circulating of HDL-C are not associated with reduced CHD events, CH0D death or total deaths.23 Also, while there are abundant epidemiologic evidence for the inverse association between HDL-C and CHD risk, drugs which increase HDL-C have shown little benefit for coronary risk.24,25 This in turn has led many to believe that increasing HDL-C in the hope of achieving atheroprotection may be futile.26,27 The results of our study suggest that those with increased levels of inflammation may not show the benefits typically expected with higher levels of HDL-C. Our observations of attenuated protective effects of HDL-C on CHD in the presence of increased levels of inflammation could be due to inflammation promoting dysfunctional HDL, or possibly HDL could simply be less effective in protecting against CHD when inflammation is present in the coronary circulation.
The lack of a protective association of HDL-C with CVD may be due to oxidation and enzymatic alteration of HDL-C proteins and lipids during times of substantial inflammation.28 Past studies have suggested that oxidative stress-related processes can lead to modified or displaced HDL particles, specifically the particles apoA1 and lecithin cholesterol acyl-transferase.29 In fact, it has been suggested that at sites of inflammation, myeloperoxidase binding to HDL-C converts it to a proinflammatory particle.30 Alternatively, increased levels of inflammation as identified by elevations in hs-CRP and IL-6 in our study in particular, may reflect a low grade inflammation of the vasculature, which would presumably render the vasculature less protected by increases in HDL-C. Past studies using mouse models have suggested that in the presence of systemic inflammatory states such as atherosclerosis, HDL-C becomes proinflammatory due to the alteration of apoliporotein A1.31 This in turn relates to the fact that plasma HDL-C cholesterol levels do not always accurately predict the true function of reverse cholesterol transport and modulation of inflammation by HDL particles during times of chronic inflammation states.30
Our study has important strengths and limitations. CHS is a well-characterized cohort of mostly Caucasian individuals who were aged 65 and greater at baseline; thus our results may not be entirely generalizable to younger persons or those of other ethnicities. As only those persons who survived to an age of 65 were included in our sample it is not surprising that a larger proportion of females (62.4%) with higher HDL-C values were observed. Also, our study was limited to investigating hs-CRP, Il-6, and LpPla2 as other inflammatory measures were not available on the entire cohort. Additionally, as contemporary populations have greater prevalence of lipid-lowering and other cardioprotective medications that may modify levels of inflammation, it is possible that findings would be stronger in the absence of any such therapies. This may be especially relevant with the increasing usage of these medications during the CHS time period (from the baseline examination in 1990-1992 to the present day), indicating a possible change in patient's inflammatory biomarker values from baseline to follow-up. Lastly, we were unable to identify or exclude those participants who may have had increased inflammation due to recent intense exercise, injury, or to other chronic conditions.
Conclusions
We demonstrate that the protective relation of HDL-C with incident CHD and CVD is attenuated when increased levels of inflammation are present and increased risks are observed when levels of inflammation are high, even with HDL-C is elevated. Currently, guidelines suggest the use of the inflammatory factors such as CRP and Lp-PLA2 as measures for cardiovascular risk assessment in those at intermediate risk for developing a CHD event.32 Further study is needed to determine whether the influence of inflammation in increasing risk at all levels of HDL-C is direct, or mediated at least partially by an adverse effect on HDL function, and if other inflammatory factors may be more important in this process. Finally, the extent to which persistent inflammation may prevent or attenuate any protection afforded by therapeutic efforts to raise HDL-C is an intriguing issue worthy of further investigation in the wake of results from recent clinical trials that have attempted to raise HDL-C to prevent CVD events.
Highlights.
We examined whether inflammation impacts on the relation of HDL-C with CHD events
We investigated inflammatory factors C-reactive protein, interleukin-6, and LpPla2
Increased inflammation attenuated the protective relation of high HDL-C with CHD
At all levels of HDL-C, increased inflammation was associated with higher CHD risk
Acknowledgments
Presented in part at the National Lipid Association Scientific Sessions, Las Vegas, NV, May 2013. This research was supported by NHLBI contracts HHSN268201200036C, N01-HC-85239, N01-HC-85079 through N01-HC-85086; N01-HC-35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, N01-HC-45133 and NHLBI grant HL080295, with additional contribution from NINDS. Additional support was provided through AG-023629, AG-15928, AG-20098, and AG-027058 from the NIA. See also http://www.chs-nhlbi.org/pi.htm.
Footnotes
Conflicts of Interest: Dr. Wong reports he is a consultant to AVIIR, Inc. and Dr. Lloyd-Jones reports he is a consultant to Medtronic.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abbott RD, Yano K, Hakim AA, Burchfiel CM, Sharp DS, Rodriguez BL, Curb JD. Changes in total and high-density lipoprotein cholesterol over 10- and 20-year periods (the Honolulu Heart Program) Am J Cardiol. 1998;82:172–178. doi: 10.1016/s0002-9149(98)00310-5. [DOI] [PubMed] [Google Scholar]
- 2.Castelli WP, Garrison RJ, Wilson PW, Abbott RD, Kalousdian S, Kannel WB. Incidence of coronary heart disease and lipoprotein cholesterol levels. The Framingham Study. JAMA. 1986;256:2835–2838. [PubMed] [Google Scholar]
- 3.Lagrand WK, Visser CA, Hermens WT, Niessen HW, Verheugt FW, Wolbink GJ, Hack CE. C-reactive protein as a cardiovascular risk factor: more than an epiphenomenon? Circulation. 1999;100:96–102. doi: 10.1161/01.cir.100.1.96. [DOI] [PubMed] [Google Scholar]
- 4.Ridker PM. High-sensitivity C-reactive protein, inflammation, and cardiovascular risk: from concept to clinical practice to clinical benefit. Am Heart J. 2004;148:S19–26. doi: 10.1016/j.ahj.2004.04.028. [DOI] [PubMed] [Google Scholar]
- 5.Bassuk SS, Rifai N, Ridker PM. High-sensitivity C-reactive protein: clinical importance. Curr Probl Cardiol. 2004;29:439–493. [PubMed] [Google Scholar]
- 6.Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000;101:1767–1772. doi: 10.1161/01.cir.101.15.1767. [DOI] [PubMed] [Google Scholar]
- 7.Luc G, Bard JM, Juhan-Vague I, Ferrieres J, Evans A, Amouyel P, Arveiler D, Fruchart JC, Ducimetiere P. C-reactive protein, interleukin-6, and fibrinogen as predictors of coronary heart disease: the PRIME Study. Arterioscler Thromb Vasc Biol. 2003;23:1255–1261. doi: 10.1161/01.ATV.0000079512.66448.1D. [DOI] [PubMed] [Google Scholar]
- 8.Packard CJ, O'Reilly DS, Caslake MJ, McMahon AD, Ford I, Cooney J, Macphee CH, Suckling KE, Krishna M, Wilkinson FE, Rumley A, Lowe GD. Lipoprotein-associated phospholipase A2 as an independent predictor of coronary heart disease. West of Scotland Coronary Prevention Study Group. N Engl J Med. 2000;343:1148–1155. doi: 10.1056/NEJM200010193431603. [DOI] [PubMed] [Google Scholar]
- 9.Bilhorn KR, Luo Y, Lee BT, Wong ND. High-Density Lipoprotein Cholesterol, High-Sensitivity C-Reactive Protein, and Cardiovascular Disease in United States Adults. Am J Cardiol. 2012 doi: 10.1016/j.amjcard.2012.06.056. [DOI] [PubMed] [Google Scholar]
- 10.Wong ND, Malik S, Kashyap ML. Preventive Cardiology: A Practical Approach. New York, NY: McGraw-Hill; 2005. [Google Scholar]
- 11.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 12.Cushman M, Cornell ES, Howard PR, Bovill EG, Tracy RP. Laboratory methods and quality assurance in the Cardiovascular Health Study. Clin Chem. 1995;41:264–270. [PubMed] [Google Scholar]
- 13.Macy EM, Hayes TE, Tracy RP. Variability in the measurement of C-reactive protein in healthy subjects: implications for reference intervals and epidemiological applications. Clin Chem. 1997;43:52–58. [PubMed] [Google Scholar]
- 14.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 15.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, 3rd, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC, Jr, Taubert K, Tracy RP, Vinicor F, Centers for Disease Control and Prevention; American Heart Association Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 16.SAS Procedures Guide. 3rd. Cary, NC: SAS Institute; 1995. [Google Scholar]
- 17.Corsetti JP, Gansevoort RT, Sparks CE, Dullaart RP. Inflammation reduces HDL protection against primary cardiac risk. Eur J Clin Invest. 2010;40:483–489. doi: 10.1111/j.1365-2362.2010.02287.x. [DOI] [PubMed] [Google Scholar]
- 18.Ridker PM, MacFadyne JG, Wolfert RL, Koenig W. Relationship of lipoprotein-associated phospholipase A(2) mass and acivity with incident vascular events among primary prevention patients allocated to placebo or statin therapy: an analysis from the JUPITER trial. Clin Chem. 2012;58:877–886. doi: 10.1373/clinchem.2011.180281. [DOI] [PubMed] [Google Scholar]
- 19.Tsimihodimos V, Karabina SA, Tambaki AP, Bairaktari E, Goudevenos JA, Chapman MJ, Elisaf M, Tselepis AD. Atorvastatin preferentially reduces LDL-associated platelet-activating factor acetylhydrolase activity in dyslipidemias of type IIA and type IIB. Arterioscler Thromb Vasc Biol. 2002;22:306–311. doi: 10.1161/hq0202.102918. [DOI] [PubMed] [Google Scholar]
- 20.Theilmeier G, De Geest B, Van Veldhoven PP, Stengel D, Michiels C, Lox M, Landeloos M, Chapman MJ, Ninio E, Collen D, Himpens B, Holvoet P. HDL-associated PAF-AH reduces endothelial adhesiveness in apoE-/- mice. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2000;14:2032–2039. doi: 10.1096/fj.99-1029com. [DOI] [PubMed] [Google Scholar]
- 21.Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. The American journal of medicine. 1977;62:707–714. doi: 10.1016/0002-9343(77)90874-9. [DOI] [PubMed] [Google Scholar]
- 22.Ansell BJ, Navab M, Hama S, Kamranpour N, Fonarow G, Hough G, Rahmani S, Mottahedeh R, Dave R, Reddy ST, Fogelman AM. Inflammatory/antiinflammatory properties of high-density lipoprotein distinguish patients from control subjects better than high-density lipoprotein cholesterol levels and are favorably affected by simvastatin treatment. Circulation. 2003;108:2751–2756. doi: 10.1161/01.CIR.0000103624.14436.4B. [DOI] [PubMed] [Google Scholar]
- 23.Briel M, Ferreira-Gonzalez I, You JJ, Karanicolas PJ, Akl EA, Wu P, Blechacz B, Bassler D, Wei X, Sharman A, Whitt I, Alves da Silva S, Khalid Z, Nordmann AJ, Zhou Q, Walter SD, Vale N, Bhatnagar N, O'Regan C, Mills EJ, Bucher HC, Montori VM, Guyatt GH. Association between change in high density lipoprotein cholesterol and cardiovascular disease morbidity and mortality: systematic review and meta-regression analysis. BMJ. 2009;338:b92. doi: 10.1136/bmj.b92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Haan W, de Vries-van der Weij J, van der Hoorn JW, Gautier T, van der Hoogt CC, Westerterp M, Romijn JA, Jukema JW, Havekes LM, Princen HM, Rensen PC. Torcetrapib does not reduce atherosclerosis beyond atorvastatin and induces more proinflammatory lesions than atorvastatin. Circulation. 2008;117:2515–2522. doi: 10.1161/CIRCULATIONAHA.107.761965. [DOI] [PubMed] [Google Scholar]
- 25.Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJ, Komajda M, Lopez-Sendon J, Mosca L, Tardif JC, Waters DD, Shear CL, Revkin JH, Buhr KA, Fisher MR, Tall AR, Brewer B. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357:2109–2122. doi: 10.1056/NEJMoa0706628. [DOI] [PubMed] [Google Scholar]
- 26.Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes-Nickens P, Koprowicz K, McBride R, Teo K, Weintraub W. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–2267. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- 27.Joy T, Hegele RA. Is raising HDL a futile strategy for atheroprotection? Nature reviews Drug discovery. 2008;7:143–155. doi: 10.1038/nrd2489. [DOI] [PubMed] [Google Scholar]
- 28.Navab M, Hama SY, Cooke CJ, Anantharamaiah GM, Chaddha M, Jin L, Subbanagounder G, Faull KF, Reddy ST, Miller NE, Fogelman AM. Normal high density lipoprotein inhibits three steps in the formation of mildly oxidized low density lipoprotein: step 1. Journal of lipid research. 2000;41:1481–1494. [PubMed] [Google Scholar]
- 29.Ansell BJ, Fonarow GC, Fogelman AM. The paradox of dysfunctional high-density lipoprotein. Curr Opin Lipidol. 2007;18:427–434. doi: 10.1097/MOL.0b013e3282364a17. [DOI] [PubMed] [Google Scholar]
- 30.Undurti A, Huang Y, Lupica JA, Smith JD, DiDonato JA, Hazen SL. Modification of high density lipoprotein by myeloperoxidase generates a pro-inflammatory particle. The Journal of biological chemistry. 2009;284:30825–30835. doi: 10.1074/jbc.M109.047605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Navab M, Van Lenten BJ, Reddy ST, Fogelman AM. High-density lipoprotein and the dynamics of atherosclerotic lesions. Circulation. 2001;104:2386–2387. [PubMed] [Google Scholar]
- 32.Greenland P, Alpert JS, Beller GA, Benjamin EJ, Budoff MJ, Fayad ZA, Foster E, Hlatky MA, Hodgson JM, Kushner FG, Lauer MS, Shaw LJ, Smith SC, Jr, Taylor AJ, Weintraub WS, Wenger NK, Jacobs AK. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2010;122:2748–2764. doi: 10.1161/CIR.0b013e3182051bab. [DOI] [PubMed] [Google Scholar]