Abstract
Colicin FY is a plasmid encoded toxin that recognizes a yersinia-specific outer membrane protein (YiuR) as a receptor molecule. We have previously shown that the activity spectrum of colicin FY comprises strains of the genus Yersinia. In this study, we analyzed the activity of colicin FY against 110 Yersinia enterocolitica isolates differing in geographical origin and source. All isolates were characterized through analysis of 16S rRNA genes, serotyping, biotyping, restriction profiling of genomic DNA, detection of virulence markers and susceptibility to antibiotics. This confirmed the broad variability of the collection, in which all 110 Y. enterocolitica isolates, representing 77 various strains, were inhibited by colicin FY. Although isolates showed variable levels of susceptibility to colicin FY, it was not associated with any strain characteristic. The universal susceptibility of Y. enterocolitica strains to colicin FY together with the absence of activity towards strains outside the Yersinia genus suggests potential therapeutic applications for colicin FY.
Introduction
Based on both genetic and phenotypic features, Yersinia enterocolitica is considered to be a heterogeneous species [1]–[3]. Y. enterocolitica is often found in aquatic environments and in various animal reservoirs, with swine being a major reservoir of human pathogenic strains. The most frequently isolated human strains belong to bioserotypes 1B/O:8, 2/O:5,27, 2/O:9, and 4/O:3, with 4/O:3 being the most common and typical for Europe [4]–[8].
Infections caused by Y. enterocolitica are the third most common bacterial alimentary infections of humans in the European Union [9]. Yersiniosis ranges from self-limited enteritis to life-threatening systemic infections. The most frequent manifestation is diarrhea, mainly affecting children [4], [10]–[14]. Although antibiotic treatment is recommended for serious cases, the benefits of antibiotic therapy in uncomplicated cases is not well established [5], [15]–[17]. Instead, rehydration and use of probiotics are often suggested for simple diarrheal cases.
Production of bacteriocins has been described in many genera of enteric bacteria including Escherichia, Shigella, Citrobacter, Salmonella, and Yersinia [18]. Although antibacterial activity of various species of genus Yersinia has been previously documented [19]–[24], only three yersinia-produced bacteriocins have been intensively studied and characterized on the molecular level. These include the bacteriocin of Y. pestis (pesticin I; [25]–[30]), a phage tail-like bacteriocin produced by Y. enterocolitica (enterocoliticin; [31], [32]), and a bacteriocin from Y. frederiksenii Y27601 (colicin FY; [33]). Colicin FY is produced by an environmental isolate of Y. frederiksenii, which contains the colicinogenic plasmid pYF27601 (5,574 bp) harboring the colicin FY activity (cfyA) and immunity (cfyI) gene. Colicin FY (54 kDa) recognizes a yersinia-specific outer membrane protein (YiuR) as a receptor molecule in susceptible bacterial strains. YiuR protein is encoded by many yersiniae (e.g. Y. pestis, Y. pseudotuberculosis, Y. enterocolitica, and Y. frederiksenii). YiuR belongs to the family of TonB-dependent proteins with putative iron uptake function. Colicin FY uses the TonB system for translocation, similar to colicins B, D, Ia, and Ib. Lethal activity of colicin FY is exerted by formation of voltage-gated pore in the cytoplasmic membrane. The lethal effect of colicin FY is directed against several nonpathogenic and opportunistic yersiniae (i.e. Y. frederiksenii, Y. aldovae, Y. kristensenii, and Y. intermedia) and also against pathogenic strains of Y. enterocolitica. Previously published data [33] suggested that Y. enterocolitica is widely susceptible to colicin FY; however, the strain collection was limited to only 31 Y. enterocolitica isolates originated in the Czech Republic that were not characterized in detail.
In this study, 110 Y. enterocolitica isolates with different geographical origins and sources were characterized in detail to exclude any potential clonal character of the isolates. Colicin FY inhibited growth of all tested isolates indicating that the vast majority of Y. enterocolitica strains are susceptible to colicin FY.
Materials and Methods
Bacterial strains and growth conditions
Colicin FY producer, Yersinia frederiksenii strain Y27601, was obtained from the National Reference Laboratory for Salmonella, The National Institute of Public Health (NIPH), Prague. The recombinant strain producing colicin FY (Escherichia coli TOP10F'pDS1068) was constructed in our laboratory [33]. Y. enterocolitica isolates were obtained from several institutions including the National Reference Laboratory for Salmonella, NIPH, Prague; the Department of Clinical Microbiology, University Hospital Brno (UHB), Brno; and the Max von Pettenkofer-Institute (MvPI), Ludwig Maximilian University of Munich, Munich. Detailed information for the isolates of Y. enterocolitica used in this work is presented in Table S1. We used a set of 118 Escherichia isolates containing E. coli (39 isolates), E. fergusonii (10 isolates), E. hermanii (42 isolates), and E. vulneris (27 isolates). An additional 18 isolates of enterobacterial species included Budvicia aquatica (24510; from E. Aldová), Citrobacter youngae (42/57; NIPH), C. braakii (B718; UHB), C. freundii (B607; UHB), Enterobacter aerogenes (1832; NIPH), E. cloacae (B604; UHB), Klebsiella pneumoniae (B615; UHB), K. oxytoca (B632; UHB), Kluywera ascorbata (B792; UHB), Leclercia adecarboxylata (2666; from I. Sedláček), Morganella morganii (B619; UHB), Pragia fontium (24613; from E. Aldová), Proteus vulgaris (B635; UHB), Serratia ficaria (B779; UHB), Salmonella enterica subsp. enterica (B753; UHB), Shigella flexneri (strain 4; [66]), S. sonnei (strain 17; from laboratory stock), and S. boydii (U1; from V. Horák).
Tryptone-yeast (TY) broth consisting of 8 g/l tryptone (Hi-Media, Mumbai, India), 5 g/l yeast extract (Hi-Media), and 5 g/l sodium chloride in water was used throughout the study. For cultivation on solid media, TY broth was supplemented with agar powder (1.5%, w/v; Hi-Media). Mueller-Hinton agar (38 g/l; Hi-Media) was used for analysis of antibiotic susceptibility. TY agar plates supplemented with L-(+)-arabinose (0.2 g/l; Sigma-Aldrich, St. Louis, USA) were used to induce expression of colicin FY in recombinant E. coli TOP10F'pDS1068.
16S rRNA analysis
To analyze the 16S rDNA in Y. enterocolitica isolates, a part of the 16S rRNA gene, consisting of 524 bp, was amplified from a single bacterial colony resuspended in 100 µl of deionized water. The yersinia DNA was amplified using Taq polymerase (New England Biolabs, Beverly, USA) and a pair of 16S rDNA-specific primers (16SRNA-F: 5′-AGTTTGATCATGGCTCAG-3′ and 16SRNA-R: 5′-TTACCGCGGCTGCTGGCA-3′) [34]. Colony PCR started with denaturation at 94°C for 5 min, followed by 40 cycles at 95°C for 30 s, 50°C for 30 s, 72°C for 1 min, and extension at 72°C for 10 min. PCR products were sequenced using Taq DyeDeoxy Terminator Cycle Sequencing Kit (Applied Biosystems, Foster City, USA). The Lasergene program package (DNASTAR, Madison, USA) was used for assembly of the sequencing reads and further data analyses. Isolates were classified to subspecies on the basis of polymorphisms in the 30 bp region of the 16S rDNA [35].
Bioserotype classification
Y. enterocolitica isolates were serotyped and biotyped using previously described methods [1], [2]. Isolates from the Czech Republic were serotyped using diagnostic agglutination antisera O:3, O:5, O:8 and O:9 (ITEST PLUS, Hradec Králové, Czech Republic) and biotyped on the basis of esculin hydrolysis, indole production, xylose and/or trehalose utilization. Bioserotypes of 50 isolates from outside the Czech Republic were provided with isolates. For verification of the provided bioserotype characterization, a random subgroup (n = 15) of these isolates was also bioserotyped.
Pulsed field gel electrophoresis (PFGE)
Overnight TY cultures were centrifuged, diluted in suspension buffer (100 mM Tris (Sigma-Aldrich), 100 mM EDTA (Sigma-Aldrich), pH = 8) to OD600 = 1.4 and mixed with equal volume of 1.6% Pulsed Field Certified Agarose (Bio-Rad Laboratories, Hercules, USA) containing 1% SDS (Sigma-Aldrich). Proteinase K (Sigma-Aldrich) was added to the suspension (to a final concentration of 0.5 mg/ml) and samples were aliquoted into plug molds. Each plug was transferred into 5 ml of lysis buffer (50 mM Tris, 50 mM EDTA, 1% SDS, 500 µg proteinase K, pH = 8) and incubated at 54°C for 2 hours. The plugs were then washed in deionized water at 54°C (2×15 min) followed by washing (4×15 min) with TE buffer (10 mM Tris, 1 mM EDTA, pH = 8). After bacterial lysis, the plugs were digested with 50 U of NotI enzyme (New England Biolabs) at 37°C for 3 hours and were loaded into a 1% Pulsed Field Certified Agarose gel. Electrophoresis was performed using a CHEF-DR II system (Bio-Rad Laboratories) in 0.5% TBE (50 mM Tris, 50 mM boric acid, 1.5 mM EDTA) at 14°C, 6 V/cm and a ramping time of 2.5 s to 25 s over 24 hours. Gels were stained with ethidium bromide (1 µg/ml) and visualized under UV light. Genomic DNA from Salmonella enterica, serotype Braenderup H9812, digested using XbaI enzyme (New England Biolabs), was used as a molecular weight standard.
BioNumerics v6.1 (Applied Maths, Sint Martens Latem, Belgium) was used to analyze restriction profiles (bands from ∼50 kbp to ∼500 kbp). Based on the PFGE data, dendrograms were constructed using Dice's coefficient of similarity and Unweighted Pair Group Method with Arithmetic Mean (UPGMA) clustering at 0.5% tolerance.
Detection of virulence factors
Genomic DNA was isolated from overnight cultures using DNAzol® Reagent (Invitrogen, Carlsbad, USA), according to the manufacturer's instructions. Isolated DNA (1 µl) was used as a template for multiplex PCR [36]. Three different virulence markers – ystA (gene encoding yersinia stable toxin, 134 bp), virF (gene encoding transcriptional activator of yop genes, 231 bp) and ail (attachment and invasion locus, 356 bp), were amplified using 0.5 U Taq polymerase (New England Biolabs) and specific primer pairs (Yst-a: 5′-GTCTTCATTTGGAGGATTCGGC-3′, Yst-b: 5′-AATCACTACTGACTTCGGCTGG-3′, ViF-a: 5′-GCTTTTGCTTGCCTTTAGCTCG-3′, VirF-b: 5′-AGAATACGTCGCTCGCTTATCC-3′, Ail-a: 5′-TGGTTATGCGCAAAGCCATGT-3′, and Ail-b: 5′-TGGAAGTGGGTTGAATTGCA-3′).
PCR started with denaturation at 94°C for 5 min, followed by 35 cycles at 94°C for 30 s, 55°C for 30 s, 72°C for 1 min, and extension at 72°C for 10 min. When multiplex PCR results were negative, virulence markers were analyzed separately under the same PCR conditions.
Antibiotic susceptibility assay
Yersiniae were tested for susceptibility to 14 antibiotics using the disc diffusion method and the National Committee for Clinical Laboratory Standards guidelines [37]. Susceptibility assays with antibiotic disks (Oxoid, Basingstoke, UK) were performed on Mueller–Hinton agar at 37°C. The following antimicrobial drugs and quantities were used: ampicillin (AMP; 10 mg), cephalothin (KF; 30 mg), doxycycline (DO; 30 mg), cefuroxime (CXM; 30 mg), ciprofloxacin (CIP; 5 mg), sulfamethoxazole-trimethoprim (SXT; 25 mg), oxolinic acid (OA; 30 mg), gentamicin (CN; 10 mg), cefotaxime (CTX; 30 mg), ceftazidime (CAZ; 30 mg), amoxicillin with clavulanic acid (AMC; 30 mg), aztreonam (ATM; 30 mg), chloramphenicol (C; 30 mg), and colistin sulphate (CT; 10 mg).
Colicin activity assay
Detection of colicin activity was performed as described previously [38]. Briefly, the agar plates were inoculated with a stab from the bacterial culture (Y. frederiksenii Y27601, E. coli TOP10F' carrying pDS1068, and E. coli TOP10F' carrying pBAD-A), incubated at 37°C for 48 hours and the resulting macrocolonies were killed with chloroform vapors. Each plate was overlaid with a 0.75% TY agar (3 ml) containing 1×108 cells of a tested isolate. Simultaneously, the level of bacterial susceptibility to colicin FY was detected by spotting of serial dilutions (diluting factor 0.25) of purified colicin FY [33] on the same agar plates. The plates were then incubated at 25°C overnight and zones of growth inhibition were read. The reciprocal value of the highest dilution of the purified colicin causing complete and partial growth inhibition (clear and turbid zone, respectively) of susceptible bacteria was considered to be the colicin titer (in arbitrary units, A.U.).
Detection of colicin FY immunity gene
To analyze for the presence of the colicin FY immunity gene in Y. enterocolitica isolates, a part of the cfyI gene, consisting of 162 bp, was amplified from a single bacterial colony resuspended in 100 µl of deionized water. Bacterial suspensions (1 µl) were boiled and used as a DNA templates, which were amplified using Taq polymerase (New England Biolabs) and specific primers (immFy-F: 5′-GGACGTTACCGCCTACGG-3′ and immFy-R: 5′-ACCCTGAAAGCGAACGAC-3′). Colony PCR started with denaturation at 94°C for 5 min, which was followed by 40 cycles at 95°C for 30 s, 58°C for 30 s, 72°C for 1 min, and finished by extension at 72°C for 10 min.
Sequencing of yiuR and tonB genes
The yiuR gene from nine Y. enterocolitica isolates (YE48, YE61, YE66, YE73 YE81,YE84, YE85, YE93, and YE97) was amplified from a single colony using Taq polymerase (New England Biolabs) and specific primers (YE1461SD-F: 5′-ACCGAAATAAATGGCTAAGGCCTTTAGG-3′, and YE1461-R: 5′-TTAGAAATCGTAGCTGGCGC-3′). Colony PCR started with denaturation at 94°C for 5 min, followed by 40 cycles at 95°C for 30 s, 58°C for 2 min, 72°C for 1 min, and extension at 72°C for 10 min. Additionally, the tonB gene was amplified using specific primers (tonB_YE2222-F: 5′-ATGCAGCTAAATAAATTTTTCTTGGG-3′ and tonB_YE2222-R: 5′-TTAGTCCATTTCCGTCGTG-3′). All PCR products were sequenced using a Taq DyeDeoxy Terminator Cycle Sequencing Kit (Applied Biosystems). The Lasergene program package (DNASTAR) was used for assembly of the sequencing reads and further data analyses.
Results
Sequencing of 16S rRNA genes, serological and biochemical characterization of isolates
Sequencing analysis of the 16S rRNA coding DNA confirmed that all 110 tested isolates belonged to the Y. enterocolitica species (data not shown). Moreover, sequencing results were used to classify isolates into subspecies; 108 isolates were identified as Y. enterocolitica subsp. palearctica and 2 isolates as Y. enterocolitica subsp. enterocolitica (Fig. S1). Seven different serotypes and all six different biotypes (1A, 1B, 2, 3, 4, and 5) were present within the isolates (Fig. S1). Common human pathogenic serotypes, (i.e. O:3, O:5,27, O:8, and O:9) and also several atypical serotypes (i.e. O:5, O:6,30 and O:36) were present in our collection. Y. enterocolitica bioserotype 4/O:3 was the most frequent (77%).
PFGE analysis
To determine the genetic variability of Y. enterocolitica isolates, restriction profiles of all isolates were determined (Fig. S1). Altogether, 41 various pulsotypes (at the 85% similarity level) were identified (Fig. 1). Moreover, 24 different pulsotypes were found within the most abundant bioserotype 4/O:3 subgroup.
Figure 1. Identified pulsotypes among Y. enterocolitica isolates.
Similarities (%) between restriction patterns were calculated using the Dice's index and are shown as the numbers close to nodes. The data were sorted using the UPGMA method. For construction of this dendrogram, 41 Y. enterocolitica pulsotypes with similarity lower than 85% were selected from a dendrogram containing all 110 isolates (Fig. S1). Susceptibility to colicin FY is shown in the right panel, followed by additional strain characteristics. Colicin FY titers are shown as the reciprocal exponent of the highest four-fold dilution causing clear (light grey) and turbid (dark grey) zones of inhibition. Subspecies: P = palearctica, E = enterocolitica. *Serotypes O:1 and O:2 have been combined to O:3 serotype according to [1].
Identification of virulence markers
Two chromosome- and one plasmid-encoded virulence markers (ail, ystA, and virF, respectively) were detected using PCR (Fig. S1). In contrast to the chromosomal genes, which were detected in more than 90% of isolates (91% and 96% for ail and ystA, respectively), the virF gene was found in only 50% of Y. enterocolitica isolates. All three determinants were identified in 52 isolates (47%) and only four isolates were negative for all tested markers. Taken together, most of the collected isolates could be considered to be potentially pathogenic Y. enterocolitica.
Susceptibility to antibiotics
All tested yersiniae were susceptible to ciprofloxacin, cefotaxime, ceftazidime, aztreonam, and colistin sulphate. About 20% of isolates showed an intermediate susceptibility to cefuroxime and amoxicillin with clavulanic acid. In addition, more than 90% of isolates were susceptible to doxycycline, sulfamethoxazole-trimethoprim, oxolinic acid, gentamicin, and chloramphenicol. Less than 10% of yersiniae were susceptible to ampicillin and cephalothin. Altogether, 20 different antibiograms were identified (Table S2), with the most frequent antibiogram, A1, identified in 46% of isolates (Fig. S1). Isolates with antibiogram A1 were susceptible to all tested antibiotics with the exception of ampicillin and cephalothin.
Susceptibility to colicin FY
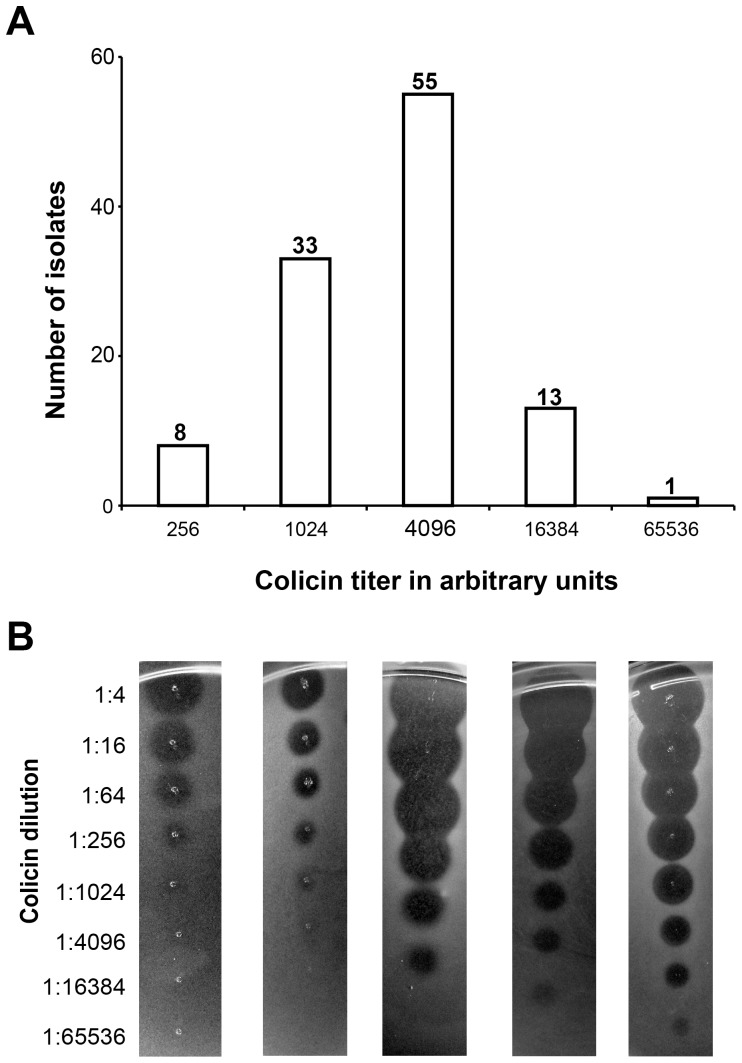
Colicin FY producers, Y. frederiksenii Y27601 and a recombinant strain of E. coli carrying pDS1068 [33], inhibited growth of all tested Y. enterocolitica isolates. All Y. enterocolitica isolates were also susceptible to purified colicin FY. Degree of susceptibility to purified colicin FY (shown as a colicin titer in A.U.) varied from 64 to 1024 and from 256 to 65536 for clear and turbid zones of growth inhibition, respectively. The susceptibility of individual isolates of Y. enterocolitica to colicin FY is shown in Fig. S1 and the distribution of colicin FY susceptibility among Y. enterocolitica is shown in Fig. 2. The susceptibility of Y. enterocolitica isolates to colicin FY showed a nonrandom distribution (chi-squared goodness of fit test; p≤0.001), where the majority of isolates were susceptible to 4096 A.U. for turbid zones of growth inhibition. Sequence analysis of two proteins (YiuR and TonB) in five highly and four less susceptible isolates identified variability within isolates (26 and 18 amino acid positions, respectively). However, not a single variable position of YiuR or TonB was associated with the difference in susceptibility to colicin FY (Fig. 3). All 110 isolates did not encode a colicin FY immunity protein, which should affect the susceptibility to colicin FY.
Figure 2. Susceptibility of Y. enterocolitica isolates to colicin FY.
A) Distribution of colicin FY susceptibility levels. Colicin FY susceptibility is shown as colicin titers representing the highest dilution causing detectable growth inhibition (last turbid zone). The susceptibility to colicin FY ranged from 256 to 65536 A.U. Half of the Y. enterocolitica isolates showed susceptibility corresponding to titer of 4096 A.U. B) Inhibition zones corresponding to different levels of susceptibility. Isolates YE75, YE24, YE107, YE99, and YE84 were used as representative indicators.
Figure 3. Sequence variability between highly and less susceptible Y. enterocolitica isolates.
A) Sequence analysis of YiuR protein from various isolates identified variability in 26 amino acid posistions. B) Sequence analysis of TonB from various isolates identified variability in 18 amino acid positions. A consensus sequence is shown at the top of the figure. The numbers correspond to amino acid position in the YiuR and TonB proteins, respectively.
In contrast to the universal susceptibility of Y. enterocolitica strains to colicin FY, no colicin FY-susceptible strains outside the Yersinia genus were identified when 136 isolates belonging to the 13 other enterobacterial genera (i.e. Budvicia, Citrobacter, Enterobacter, Escherichia, Klebsiella, Kluyvera, Leclercia, Morganella, Pragia, Proteus, Salmonella, Serratia, and Shigella) were tested (data not shown).
Discussion
The aim of this study was to evaluate susceptibility of Y. enterocolitica to colicin FY using a set of characterized isolates. The 110 isolates were collected from different geographical areas with the majority of isolates originating in Europe. Most of Y. enterocolitica isolates originated from human clinical material, but veterinary and environmental isolates were also present. To exclude the possibility that the collection of Y. enterocolitica contains multiple isolates of identical strains, further characterization of these isolates was performed including analysis of the 16S rRNA genes, serotyping, biotyping, restriction profiling of genomic DNA, detection of virulence markers and susceptibility to antibiotics. Using these typing techniques, 77 different Y. enterocolitica strains were identified in our collection, from which 59 strains were represented by a single isolate, while the other 18 strains contained more than one isolate with identical characteristics. High genetic variability was shown for nonhuman isolates, while more subtle variability was found within the 4/O:3 subgroup. Almost all tested isolates can be considered to be pathogenic or potentially pathogenic. With the exception of two beta-lactam antibiotics, isolates of Y. enterocolitica were susceptible to all tested antibiotics. As results of the characterization assays are in agreement with other studies on Y. enterocolitica [5], [9], [39]–[48], our set of Y. enterocolitica isolates represents a group of isolates with typical features.
None of the110 isolates of Y. enterocolitica encoded the colicin FY immunity protein (data not shown) and all were susceptible to colicin FY; however they differed in the degree of susceptibility to it. No obvious association between colicin FY susceptibility and other described strain parameters was found, indicating that it is independent of other strains characteristics (Fig. S1). Moreover, no amino acid replacements in the YiuR and TonB proteins were associated with differences in colicin FY susceptibility. Others features (e.g. cell wall composition) seem to affect susceptibility to colicin FY.
Bacterial resistance to bacteriocins is considered to be a successful strategy in antimicrobial competition [49]–[51]. It has been shown that around 75% of E. coli strains isolated from different source populations are resistant to one or more bacteriocin types [52], [53]. Resistance to bacteriocin-like substances produced by yersiniae has been found among Y. enterocolitica strains [21]. In addition, enterocoliticin, a characterized phage tail-like bacteriocin, has been shown to inhibit pathogenic Y. enterocolitica serotypes O:3, O:5,27, and O:9, but not serotype O:8 and various nonpathogenic isolates (biotype 1A) [31]. In contrast, colicin FY appears to inhibit all Y. enterocolitica isolates. A similar finding has been published with respect to an uncharacterized bacteriocin from Y. kristensenii and 35 tested strains of Y. enterocolitica [24], [54], [55]; however, it is not known whether this uncharacterized bacteriocin could, in fact, be colicin FY.
Although the reason for the universal susceptibility of Y. enterocolitica to colicin FY is unknown, it can be speculated that this could be a result of the clonal character of isolates and/or the essential character of the YiuR receptor for yersiniae. The clonal character of Y. enterocolitica was obvious only in pathogenic isolates (e.g. serotype O:3), while nonpathogenic isolates of biotype 1A were more heterogeneous [56], [57]. This explanation therefore appears unlikely. Similarly, the essential character of the YiuR receptor also appears unlikely, since yersiniae possess several iron uptake systems (e.g. Y. pestis harbors several systems and Yiu-mediated transport is not first in the iron uptake hierarchy [58]). Another possible explanation for universal activity of colicin FY against Y. enterocolitica involves a close relationship between Y. enterocolitica and colicin FY producers, with little or no contact between them. A phylogenetic analysis showed that Y. enterocolitica was clearly related to other environmental enterocolitica-like species including Y. frederiksenii [59], [60]. In addition, contact between the two bacterial species appears to be quite rare despite the sporadic co-occurrence of both species [61]–[63]. The separation of Y. enterocolitica strains from environmental colicin FY-producers could explain the absence of colicin FY resistant mutants among Y. enterocolitica strains. In fact, colicin FY resistant colonies of Y. enterocolitica were induced in the presence of colicin FY in laboratory conditions (data not shown). Moreover, the environmental yersiniae (e.g. Y. frederiksenii and Y. kristensenii) that live in the same environment as colicin producers contain both resistant and susceptible strains to colicin FY [33].
There is increasing interest in nonpathogenic microorganisms and their antimicrobial substances that naturally antagonize pathogenic agents. To date, several nonpathogenic Escherichia coli strains have been used as probiotics (e.g. E. coli Nissle 1917; [64]). Although the role of bacteriocin synthesis in probiotic bacteria is not known, production of bacteriocins is a common feature of many of them [65]–[67]. It is therefore tempting to speculate that synthesis of colicin FY could represent an important feature of recombinant probiotic E. coli strains used in cases of diarrhea caused by yersiniae. However, the effect of colicin FY synthesis should be tested using in vivo experiments to see whether colicin FY has therapeutic potential relative to intestinal yersiniosis.
Supporting Information
Dendrogram of Y. enterocolitica isolates. Similarities (%) between restriction patterns were calculated using the Dice's index and are shown as the numbers close to nodes. The data were sorted using the UPGMA method. Susceptibility to colicin FY is shown in the right panel, followed by additional strain characteristics. Colicin FY titers are shown as the reciprocal exponent of the highest four-fold dilution causing clear (light grey) and turbid (dark grey) zones of inhibition. *Serotypes O:1 and O:2 have been combined to O:3 serotype according to [1]. The lines on the right side show isolates with the same characteristics (i.e. considered to be identical strains).
(TIF)
Y. enterocolitica isolates used in this study.
(DOCX)
Antibiotic susceptibility of Y. enterocolitica isolates.
(DOCX)
Funding Statement
This work was supported by the Ministry of Health of the Czech Republic (NT13413-4/2012) to D.S. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Aleksić S, Bockemühl J (1984) Proposed revision of the Wauters et al. antigenic scheme for serotyping of Yersinia enterocolitica . J Clin Microbiol 20: 99–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wauters G, Kandolo K, Janssens M (1987) Revised biogrouping scheme of Yersinia enterocolitica . Contrib Microbiol Immunol 9: 14–21. [PubMed] [Google Scholar]
- 3. Wauters G, Aleksić S, Charlier J, Schulze G (1991) Somatic and flagellar antigens of Yersinia enterocolitica and related species. Contrib Microbiol Immunol 12: 239–243. [PubMed] [Google Scholar]
- 4. Bottone EJ (1997) Yersinia enterocolitica: the charisma continues. Clin Microbiol Rev 10: 257–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bottone EJ (1999) Yersinia enterocolitica: overview and epidemiologic correlates. Microbes and Infection 1: 323–333. [DOI] [PubMed] [Google Scholar]
- 6. Fredriksson-Ahomaa M, Hallanvuo S, Korte T, Siitonen A, Korkeala H (2001) Correspondence of genotypes of sporadic Yersinia enterocolitica bioserotype 4/O:3 strains from human and porcine sources. Epidemiol Infect 127: 37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fredriksson-Ahomaa M, Korkeala H (2003) Low occurrence of pathogenic Yersinia enterocolitica in clinical, food, and environmental samples: a methodological problem. Clin Microbiol Rev 16: 220–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang X, Cui Z, Jin D, Tang L, Xia S, et al. (2009) Distribution of pathogenic Yersinia enterocolitica in China. Eur J Clin Microbiol Infect Dis 28: 1237–1244. [DOI] [PubMed] [Google Scholar]
- 9. European Food Safety Authority (2012) The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2010. EFSA J 10: e2597 10.2903/j.efsa.2012.2597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Marriott DJ, Taylor S, Dorman DC (1985) Yersinia enterocolitica infection in children. Med J Aust 143: 489–492. [DOI] [PubMed] [Google Scholar]
- 11. Lee LA, Gerber AR, Lonsway DR, Smith JD, Carter GP, et al. (1990) Yersinia enterocolitica O:3 infections in infants and children, associated with the household preparation of chitterlings. N Engl J Med 322: 984–987. [DOI] [PubMed] [Google Scholar]
- 12. Lee LA, Taylor J, Carter GP, Quinn B, Farmer JJ, et al. (1991) Yersinia enterocolitica O:3: an emerging cause of pediatric gastroenteritis in the United States. J Infect Dis 163: 660–663. [DOI] [PubMed] [Google Scholar]
- 13. Gray JT, WaKabongo M, Campos FE, Diallo AA, Tyndal C, et al. (2001) Recognition of Yersinia enterocolitica multiple strain infection in twin infants using PCR-based DNA fingerprinting. J Appl Microbiol 90: 358–364. [DOI] [PubMed] [Google Scholar]
- 14. Ray SM, Ahuja SD, Blake PA, Farley MM, Samuel M, et al. (2004) Population-based surveillance for Yersinia enterocolitica infections in FoodNet Sites, 1996–1999: Higher risk of disease in infants and minority populations. Clin Infect Dis 38(s3): S181–S189 10.1086/381585 [DOI] [PubMed] [Google Scholar]
- 15. Pai CH, Gillis F, Tuomanen E, Marks MI (1984) Placebo-controlled double-blind evaluation of trimethoprim-sulfamethoxazole treatment of Yersinia enterocolitica gastroenteritis. J Pediatr 104: 308–311. [DOI] [PubMed] [Google Scholar]
- 16. Hoogkamp-Korstanje JA, Stolk-Engelaar VM (1995) Yersinia enterocolitica infection in children. Pediatr Infect Dis J 14: 771–775. [DOI] [PubMed] [Google Scholar]
- 17. Abdel-Haq NM, Asmar BI, Abuhammour WM, Brown WJ (2000) Yersinia enterocolitica infection in children. Pediatr Infect Dis J 19: 954–958. [DOI] [PubMed] [Google Scholar]
- 18. Cascales E, Buchanan SK, Duché D, Kleanthous C, Lloubès R, et al. (2007) Colicin biology. Microbiol Mol Biol Rev 71: 158–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ben-Gurion R, Hertman I (1958) Bacteriocin-like material produced by Pasteurella pestis . J Gen Microbiol 19: 289–297. [DOI] [PubMed] [Google Scholar]
- 20. Bottone EJ, Sandhu KK, Pisano MA (1979) Yersinia intermedia: temperature-dependent bacteriocin production. J Clin Microbiol 10: 433–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Calvo C, Brault J, Ramos-Cormenzana A, Mollaret HH (1986) Production of bacteriocin-like substances by Yersinia frederiksenii, Y. kristensenii, and Y. intermedia strains. Folia Microbiol 31: 177–186. [DOI] [PubMed] [Google Scholar]
- 22. Cafferkey MT, McClean K, Drumm ME (1989) Production of bacteriocin-like antagonism by clinical isolates of Yersinia enterocolitica . J Clin Microbiol 27: 677–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Csiszár K, Tóth I (1992) Bacteriocin-like antagonism in Yersinia enterocolitica . Acta Microbiol Hung 39: 193–201. [PubMed] [Google Scholar]
- 24. Toora S, Budu-Amoako E, Ablett RF, Smith J (1994) Inhibition and inactivation of pathogenic serogroups of Yersinia enterocolitica by a bacteriocin produced by Yersinia kristensenii . Lett App Microbiol 19: 40–43. [Google Scholar]
- 25. Ferber DM, Brubaker RR (1979) Mode of action of pesticin: N-acetylglucosaminidase activity. J Bacteriol 139: 495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rakin A, Saken E, Harmsen D, Heesemann J (1994) The pesticin receptor of Yersinia enterocolitica: a novel virulence factor with dual function. Mol Microbiol 13: 253–263. [DOI] [PubMed] [Google Scholar]
- 27. Pilsl H, Killmann H, Hantke K, Braun V (1996) Periplasmic location of the pesticin immunity protein suggests inactivation of pesticin in the periplasm. J Bacteriol 178: 2431–2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rakin A, Boolgakowa E, Heesemann J (1996) Structural and functional organization of the Yersinia pestis bacteriocin pesticin gene cluster. Microbiology 142: 3415–3424. [DOI] [PubMed] [Google Scholar]
- 29. Vollmer W, Pilsl H, Hantke K, Höltje JV, Braun V (1997) Pesticin displays muramidase activity. J Bacteriol 179: 1580–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Patzer SI, Albrecht R, Braun V, Zeth K (2012) Structural and mechanistic studies of pesticin, a bacterial homolog of phage lysozymes. J Biol Chem 287: 23381–23396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Strauch E, Kaspar H, Schaudinn C, Dersch P, Madela K, et al. (2001) Characterization of enterocoliticin, a phage tail-like bacteriocin, and its effect on pathogenic Yersinia enterocolitica strains. Appl Environ Microbiol 67: 5634–5642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Damasko C, Konietzny A, Kaspar H, Appel B, Dersch P, et al. (2005) Studies of the efficacy of enterocoliticin, a phage-tail like bacteriocin, as antimicrobial agent against Yersinia enterocolitica serotype O3 in a cell culture system and in mice. J Vet Med B 52: 171–179. [DOI] [PubMed] [Google Scholar]
- 33. Bosák J, Laiblová P, Šmarda J, Dedicová D, Šmajs D (2012) Novel colicin FY of Yersinia frederiksenii inhibits pathogenic Yersinia strains via YiuR-mediated reception, TonB import, and cell membrane pore formation. J Bacteriol 194: 1950–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kotetishvili M, Kreger A, Wauters G, Morris JG, Sulakvelidze A, et al. (2005) Multilocus sequence typing for studying genetic relationships among Yersinia species. J Clin Microbiol 43: 2674–2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Neubauer H, Aleksic S, Hensel A, Finke EJ, Meyer H (2000) Yersinia enterocolitica 16S rRNA gene types belong to the same genospecies but form three homology groups. Int J Med Microbiol 290: 61–64. [DOI] [PubMed] [Google Scholar]
- 36. Harnett N, Lin YP, Krishnan C (1996) Detection of pathogenic Yersinia enterocolitica using the multiplex polymerase chain reaction. Epidemiol Infect 117: 59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clinical and Laboratory Standards Institute (2012) Performance standards for antimicrobial disk susceptibility tests; Approved standard - Eleventh edition. Clinical and Laboratory Standards Institute.Wayne PA. 58.
- 38. Šmarda J, Šmajs D, Horynová S (2006) Incidence of lysogenic, colicinogenic and siderophore-producing strains among human non-pathogenic Escherichia coli . Folia Microbiol 51: 387–391. [DOI] [PubMed] [Google Scholar]
- 39. Bissett ML, Powers C, Abbott SL, Janda JM (1990) Epidemiologic investigations of Yersinia enterocolitica and related species: sources, frequency, and serogroup distribution. J Clin Microbiol 28: 910–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Batzilla J, Antonenka U, Höper D, Heesemann J, Rakin A (2011) Yersinia enterocolitica palearctica serobiotype O:3/4 - a successful group of emerging zoonotic pathogens. BMC Genomics 12: e348 10.1186/1471-2164-12-348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rahman A, Bonny TS, Stonsaovapak S, Ananchaipattana C (2011) Yersinia enterocolitica: Epidemiological studies and outbreaks. J Pathog 2011: e239391 10.4061/2011/239391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Saken E, Roggenkamp A, Aleksic S, Heesemann J (1994) Characterisation of pathogenic Yersinia enterocolitica serogroups by pulsed-field gel electrophoresis of genomic NotI restriction fragments. J Med Microbiol 41: 329–338. [DOI] [PubMed] [Google Scholar]
- 43. Najdenski H, Iteman I, Carniel E (1994) Efficient subtyping of pathogenic Yersinia enterocolitica strains by pulsed-field gel electrophoresis. J Clin Microbiol 32: 2913–2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Iteman I, Guiyoule A, Carniel E (1996) Comparison of three molecular methods for typing and subtyping pathogenic Yersinia enterocolitica strains. J Med Microbiol 45: 48–56. [DOI] [PubMed] [Google Scholar]
- 45. Asplund K, Johansson T, Siitonen A (1998) Evaluation of pulsed-field gel electrophoresis of genomic restriction fragments in the discrimination of Yersinia enterocolitica O:3. Epidemiol Infect 121: 579–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zheng H, Sun Y, Mao Z, Jiang B (2008) Investigation of virulence genes in clinical isolates of Yersinia enterocolitica . FEMS Immunol Med Microbiol 53: 368–374. [DOI] [PubMed] [Google Scholar]
- 47. Fàbrega A, Vila J (2012) Yersinia enterocolitica: pathogenesis, virulence and antimicrobial resistance. Enferm Infecc Microbiol Clin 30: 24–32. [DOI] [PubMed] [Google Scholar]
- 48. Stock I, Wiedemann B (1999) An in-vitro study of the antimicrobial susceptibilities of Yersinia enterocolitica and the definition of a database. J Antimicrob Chemother 43: 37–45. [DOI] [PubMed] [Google Scholar]
- 49. Riley MA, Gordon DM (1999) The ecological role of bacteriocins in bacterial competition. Trends Microbiol 7: 129–133. [DOI] [PubMed] [Google Scholar]
- 50. Kerr B, Riley MA, Feldman MW, Bohannan BJM (2002) Local dispersal promotes biodiversity in a real-life game of rock-paper-scissors. Nature 418: 171–174. [DOI] [PubMed] [Google Scholar]
- 51. Nahum JR, Harding BN, Kerr B (2011) Evolution of restraint in a structured rock–paper–scissors community. Proc Natl Acad Sci U S A 108 (s2): 10831–10838 10.1073/pnas.1100296108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Feldgarden M, Riley MA (1998) High levels of colicin resistance in Escherichia coli . Evolution 52: 1270–1276. [DOI] [PubMed] [Google Scholar]
- 53. Gordon DM, Riley MA, Pinou T (1998) Temporal changes in the frequency of colicinogeny in Escherichia coli from house mice. Microbiology 144: 2233–2240. [DOI] [PubMed] [Google Scholar]
- 54. Toora S (1995) Partial purification and characterization of bacteriocin from Yersinia kristensenii . J Appl Bacteriol 78: 224–228. [DOI] [PubMed] [Google Scholar]
- 55. Toora S (1995) Application of Yersinia kristensenii bacteriocin as a specific marker for the rapid identification of suspected isolates of Yersinia enterocolitica . Lett Appl Microbiol 20: 171–174. [DOI] [PubMed] [Google Scholar]
- 56. Bhaduri S, Wesley I, Richards H, Draughon A, Wallace M (2009) Clonality and antibiotic susceptibility of Yersinia enterocolitica isolated from U.S. market weight hogs. Foodborne Pathog Dis 6: 351–356. [DOI] [PubMed] [Google Scholar]
- 57. Sihvonen LM, Jalkanen K, Huovinen E, Toivonen S, Corander J, et al. (2012) Clinical isolates of Yersinia enterocolitica Biotype 1A represent two phylogenetic lineages with differing pathogenicity-related properties. BMC Microbiology 12: 208 10.1186/1471-2180-12-208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kirillina O, Bobrov AG, Fetherston JD, Perry RD (2006) Hierarchy of iron uptake systems: Yfu and Yiu are functional in Yersinia pestis . Infect Immun 74: 6171–6178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chen PE, Cook C, Stewart AC, Nagarajan N, Sommer DD, et al. (2010) Genomic characterization of the Yersinia genus. Genome Biol 11: R1 10.1186/gb-2010-11-1-r1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Reuter S, Thomson NR, McNally A (2012) Evolutionary dynamics of the Yersinia enterocolitica complex. Adv Exp Med Biol 954: 15–22 10.1007/978-1-4614-3561-72 [DOI] [PubMed] [Google Scholar]
- 61. Shayegani M, DeForge I, McGlynn DM, Root T (1981) Characteristics of Yersinia enterocolitica and related species isolated from human, animal, and environmental sources. J Clin Microbiol 14: 304–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Greenwood M, Hooper WL (1987) Human carriage of Yersinia spp. J Med Microbiol 23: 345–348. [DOI] [PubMed] [Google Scholar]
- 63. Soltan-Dallal MM, Moezardalan K (2004) Frequency of Yersinia species infection in paediatric acute diarrhoea in Tehran. East Mediterr Health J 10: 152–158. [PubMed] [Google Scholar]
- 64. Lodinová-Zádniková R, Sonnenborn U (1997) Effect of preventive administration of a nonpathogenic Escherichia coli strain on the colonization of the intestine with microbial pathogens in newborn infants. Biol Neonate 71: 224–232. [DOI] [PubMed] [Google Scholar]
- 65. Patzer SI, Baquero MR, Bravo D, Moreno F, Hantke K (2003) The colicin G, H and X determinants encode microcins M and H47, which might utilize the catecholate siderophore receptors FepA, Cir, Fiu and IroN. Microbiology 149: 2557–2570. [DOI] [PubMed] [Google Scholar]
- 66. Cursino L, Šmajs D, Šmarda J, Nardi RM, Nicoli JR, et al. (2006) Exoproducts of the Escherichia coli strain H22 inhibiting some enteric pathogens both in vitro and in vivo . J Appl Microbiol 100: 821–829. [DOI] [PubMed] [Google Scholar]
- 67. Wooley RE, Gibbs PS, Shotts EB Jr (1999) Inhibition of Salmonella typhimurium in the chicken intestinal tract by a transformed avirulent avian Escherichia coli . Avian Dis 43: 245–250. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Dendrogram of Y. enterocolitica isolates. Similarities (%) between restriction patterns were calculated using the Dice's index and are shown as the numbers close to nodes. The data were sorted using the UPGMA method. Susceptibility to colicin FY is shown in the right panel, followed by additional strain characteristics. Colicin FY titers are shown as the reciprocal exponent of the highest four-fold dilution causing clear (light grey) and turbid (dark grey) zones of inhibition. *Serotypes O:1 and O:2 have been combined to O:3 serotype according to [1]. The lines on the right side show isolates with the same characteristics (i.e. considered to be identical strains).
(TIF)
Y. enterocolitica isolates used in this study.
(DOCX)
Antibiotic susceptibility of Y. enterocolitica isolates.
(DOCX)