Abstract
Lgr5 was identified as a promising gastrointestinal tract stem cell marker in mice. Lineage tracing indicates that Lgr5 + cells may not only be the cells responsible for the origin of tumors; they may also be the so-called cancer stem cells. In the present study, we investigated the presence of Lgr5 + cells and their biological significance in normal human gastric mucosa and gastric tumors. RNAscope, a newly developed RNA in situ hybridization technique, specifically labeled Lgr5 + cells at the basal glands of the gastric antrum. Notably, the number of Lgr5 + cells was remarkably increased in intestinal metaplasia. In total, 76% of gastric adenomas and 43% of early gastric carcinomas were positive for LGR5. Lgr5 + cells were found more frequently in low-grade tumors with active Wnt signaling and an intestinal gland type, suggesting that LGR5 is likely involved in the very early stages of Wnt-driven tumorigenesis in the stomach. Interestingly, similar to stem cells in normal tissues, Lgr5 + cells were often restricted to the base of the tumor glands, and such Lgr5 + restriction was associated with high levels of intestinal stem cell markers such as EPHB2, OLFM4, and ASCL2. Thus, our findings show that Lgr5 + cells are present at the base of the antral glands in the human stomach and that this cell population significantly expands in intestinal metaplasias. Furthermore, Lgr5 + cells are seen in a large number of gastric tumors ; their frequent basal arrangements and coexpression of ISC markers support the idea that Lgr5 + cells act as stem cells during the early stage of intestinal-type gastric tumorigenesis.
Introduction
Cancer stem cell theory has drawn considerable attention since it was first demonstrated in hematologic malignancies. A growing body of evidence from studies of various solid tumors also supports the concept [1]. Because cancer stem cells (CSCs) are believed to be the only tumor cell subpopulation with the potential to establish an entire tumor, especially after chemoradiation therapy, CSC targeting may be a novel approach through which to improve patient outcome [2]. Although many proteins have been proposed as markers of cancer stem cells, the identification and confirmation of such markers in normal human tissues remains challenging. In the gastrointestinal tract, a number of putative stem cell markers such as Lgr5 [3], Bmi-1 [4], and Prominin-1 [5] have been identified. Among these, Lgr5 (Leucine-rich repeat containing G-protein-coupled receptor 5) is the most promising and established marker. Lineage tracing experiments have shown that Lgr5 is an adult stem cell marker expressed in the small intestine, colon, stomach, and hair follicles in mice [3]. Apc-mutant Lgr5 + cells were reported to be the origin of progressively growing adenomas [6]. Additionally, Lgr5 + cells were found to be the multipotent stem cells that produced all other adenoma cell types in intestinal adenomas [7]. Lgr5 seems to be the first reported biomarker for stem cells in both normal intestinal mucosa and corresponding tumor tissues.
For several decades, the isthmus region of the stomach has been widely accepted as a stem cell reservoir, based on indirect evidence such as a high proliferative activity and the presence of immature granule-free cells that resemble embryonic stem cells [8]. However, in vivo lineage tracing revealed that a group of Lgr5+ cells at the base of the pyloric glands were multipotent stem cells that contributed to daily epithelial renewal [9]. The Wnt-driven tumor initiation induced by targeted ablation of Apc tumor suppressor activity was also suspected to occur in the stomach LGR5 + cells. [9]. Despite the discoveries pertaining to Lgr5 as an adult stem cell marker in mice, the relevance of Lgr5 expression in human tissues has not been fully evaluated. This is largely because the in vivo lineage tracing technique, which was used in mice to demonstrate the stem cell activity of candidate cells, cannot be applied to human stem cell population studies [8]. Although several studies have attempted to determine the presence of Lgr5 + cells either with antibodies [10]-[13] or with RNA in situ hybridization (ISH) [14], [15], none of the studies provided convincing evidence supporting the presence of Lgr5 + cells in human tissues. Indeed, the lack of a reliable antibody to LGR5 is the main obstacle in identifying human counterparts of mouse Lgr5+ cells for use in clinical applications.
In the present study, we show that Lgr5 + cells are exclusively located at the base of the antral glands in the human stomach and that this cell population is remarkably expanded in intestinal metaplasias (IMs). This expansion may be a critical factor in the development of tumors from IMs. Most gastric adenomas (GAs) contain a number of LGR5-expressing tumor cells that typically reside at the basal areas of tumor glands in a similar manner to Lgr5 + cells in the intestinal mucosa, as well as in IMs. Furthermore, the lower halves of GA glands, which harbor most of the Lgr5 + cells, specifically coexpress intestinal stem cell markers such as EPHB2, OLFM4, and ASCL2, as well as CD133, thus supporting the hypothesis that LGR5 is a tumor stem cell marker during the early stage of intestinal-type gastric tumorigenesis.
Materials and Methods
Subjects
We analyzed formalin-fixed and paraffin-embedded (FFPE) gastric tumors collected from 159 patients who underwent endoscopic submucosal dissection (ESD) at Seoul National University Hospital, Seoul, Korea, from 2008 to 2010. Clinicopathological data such as patient age and gender, histological tumor type, Lauren’s classification, and evidence of lymphatic invasion were obtained by reviewing the medical charts and pathological records. A normal human skin specimen, including hair follicles, was obtained from a patient with basal cell carcinoma who underwent surgery, and normal small and large intestine samples, which were confirmed to be normal, non-cancerous tissues by histopathological analyses, were obtained from a patient with colon cancer who underwent a colectomy. Unfixed, fresh-frozen, normal gastric tissues were available from 11 patients with gastric cancer who underwent gastrectomy from 2001 to 2005 at Seoul National University Hospital.
Ethical statement
All human specimens were obtained during surgery. The participants did not provide written consent to participate in this study. The retrospective study was performed using the stored samples after the pathologic diagnosis, and all of the samples were anonymized before the study. This retrospective study design was approved by the Institutional Review Board at Seoul National University Hospital under the condition of anonymization (reference: H-1209-037-424).
Tissue microarray (TMA) construction
Core tissue biopsies (2 mm in diameter) were obtained from individual FFPE gastric tumors (donor blocks) and arranged in a new recipient paraffin block (tissue array block) using a trephine apparatus (SuperBioChips Laboratories, Seoul, Korea). Three TMAs were produced, each of which contained 53 gastric tumors that had been removed by ESD, and 7 normal non-tumorous gastric mucosa samples, including the antral glands, fundic glands, and IM. An additional TMA, comprising 30 active gastritis cases, was also constructed from the specimens of the patients with gastric tumors.
RNA in situ hybridization (ISH)
ISH for LGR5, EPHB2, ASCL2, OLFM4, and CDX2 was performed with the RNAscope FFPE assay kit (Advanced Cell Diagnostics, Inc., Hayward, CA, USA) according to the manufacturer’s instructions. Briefly, 4 µm formalin-fixed, paraffin-embedded tissue sections or TMA sections were pretreated with heat and protease digestion and then hybridized with a target probe for LGR5. Thereafter, an HRP-based signal amplification system was hybridized to the target probe before color development with 3,3′-diaminobenzeidine tetrahydrochloride (DAB). Positive staining was defined as the presence of brown punctate dots in the nucleus and/or cytoplasm. The housekeeping gene ubiquitin C (UBC) served as a positive control. The DapB gene, which is derived from a bacterial gene sequence, was used as a negative control. For gastric tumors, LGR5 staining was graded based on the percentage of tumor cells that expressed LGR5 as follows: grade 0, absence of Lgr5+ tumor cells; grade 1, 1%–5% of Lgr5+ tumor cells; grade 2, 6%–25% of Lgr5+ tumor cells; and grade 3, 26%–100% of Lgr5+ tumor cells. The results were grouped as positive (grade 2 or 3) or negative (grade 0 or 1), given that normal gastric mucosa was identified as grade 1 for LGR5 expression.
Immunohistochemistry
Immunohistochemistry was performed on 4 µm TMA sections using a BOND-MAX automated immunostainer and a Bond Polymer Refine Detection kit (Leica Microsystems, Wetzlar, Germany) according to the manufacturer’s instructions. The Ventana BenchMark XT automated staining system (Ventana Medical Systems, Tucson, AZ, USA) was only used for claudin-18 staining. The primary antibodies used were anti-β-catenin (Novocastra Laboratories Ltd., Newcastle, UK; 17C2; 1∶800), anti-CD10 (Novocastra; 56C6; 1∶100), anti-CDX2 (BioGenex, San Ramon, CA, USA; CDX2-88; 1∶500), anti-MUC2 (Novocastra; Ccp58; 1∶300), anti-MUC5AC (Novocastra; CLH2; 1∶300), anti-MUC6 (Novocastra; CLH5; 1∶100), and anti-claudin-18 (Invitrogen, Carlsbad, CA, USA; 34H14L15; 1∶1000) antibodies. Nuclear β-catenin staining was considered positive when more than 10% of the tumor cell nuclei were strongly stained for β-catenin. MUC5AC is expressed in foveolar cells in the stomach, and MUC6 is expressed in mucous cells in the neck of the oxyntic mucosa or in the pyloric glands. MUC2 is expressed in goblet cells with IM in the stomach. CD10 glycoprotein is expressed on the brush borders of intestinal epithelial cells. MUC5AC and MUC6 are gastric phenotypic markers, and MUC2 and CD10 are intestinal phenotypic markers [16]. Based on the phenotypic combinations of mucin expression, gastric tumors were classified into the following 4 groups: combined, gastric, intestinal, and unclassified [17].
Laser-capture microdissection and RNA extraction
For each patient, the upper and lower portions of the tumor glands and the normal gastric and intestinal mucosa were isolated from 5 to 6 sections. Briefly, 4 µm paraffin-embedded tissue sections were obtained, and the areas of interest were selectively microdissected using a laser microdissection device (ION LMD, Jung Woo International Co., Seoul, Korea) without deparaffinization or staining procedures in order to minimize further cellular RNA damage. Total RNA was extracted from the laser-captured areas with an RNeasy FFPE Kit (Qiagen, Valencia, CA, USA) according to manufacturer’s instructions, with a slight modification involving extended proteinase K digestion for at least 17 hours after the deparaffinization step [18].
Quantitative real-time PCR
cDNA was prepared from 0.5–1 µg of total RNA with random hexamer primers and the GoScript reverse transcription system (Promega, Madison, WI, USA). PCR reactions were performed with Premix EX Taq (Takara Bio, Shiga, Japan) according to the manufacturer’s recommendations and with the following cycling conditions: initial denaturation for 30 s at 95°C, followed by 40–50 cycles of 95°C for 5 s and 60°C for 34 s, in an Applied Biosystems 7500 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). The data were analyzed using the 7500 system SDS software program (v1.4; Applied Biosystems). The following TaqMan gene expression assays were used: Hs00173908_m1 (LGR4), Hs00173664_m1 (LGR5), Hs00663887_m1 (LGR6), Hs00362096_m1 (EPHB2), Hs00270888_s1 (ASCL2), Hs00197437_m1 (OLFM4), Hs01009250_m1 (PROM1), Hs010780810_m1 (CDX2), Hs00212584_m1 (CLDN18), and Hs0275899_g1 (GAPDH). GAPDH served as the endogenous control. All experiments were performed in duplicate.
Statistical analysis
Statistical analyses were performed using the PASW 18.0 statistical software program (IBM SPSS Statistics, Chicago, IL, USA) and Prism version 5.0 (GraphPad Software, Inc., San Diego, CA, USA). The correlations between LGR5 positivity and clinicopathological parameters were tested using the χ2-test or Fisher’s exact test. Between-group comparisons of the real-time PCR data were performed using Student’s t-test. The significance of the relationship between LGR5 and CD133 expression was assessed with the Pearson correlation test. The correlation between the grades according to RNA ISH and the LGR5 transcripts measured by RT-PCR was evaluated by the Spearman correlation test. The results were considered significant when p<0.05.
Results
1. RNAscope specifically identifies LGR5 + stem cells in human tissues
Lgr5 + cells have been well documented in the stem cell niches of knock-in mouse models, including the crypt bases of the small and large intestines and hair follicle bulges [3], [19]. However, the identification of Lgr5 + cells in human clinical specimens has not been very successful, possibly because the sensitivities and specificities of LGR5 antibodies and RNA ISH techniques are insufficient. In the present study, we applied RNAscope, a novel RNA ISH technology, to examine human FFPE tissues [20]. To validate this method before investigating the distribution of Lgr5 + cells in the stomach, we assessed whether RNAscope could specifically mark Lgr5 + cells in well-established niches. As expected, groups of Lgr5 + cells were detected at the exact intestinal and hair follicular locations that had been observed in mice. Almost every small intestinal crypt harbored several Lgr5 + cells in the basal area (Fig. 1A), and Lgr5 + cells were interspersed with Paneth cells (Fig. 1B). Most colon crypts contained Lgr5 + cells, even though the LGR5 staining intensities were weaker than in the small intestine (Fig. 1C, D). Many Lgr5 + cells were spread along the hair follicle bulges (Fig. 1E, F). We also confirmed LGR5 expression in tumors that arose from these tissues, such as basal cell carcinoma (Fig. S1A) and colonic adenoma (Fig. S1B), as reported in previous studies [15], [21]. These findings demonstrated the ability of RNAscope to specifically identify Lgr5 + stem cells in human FFPE specimens. To further establish the robustness of RNAscope, we showed that CDX2-expressing tumor cells were positively stained by RNAscope while CDX2-negative adjacent normal gastric epithelial cells were not stained (Fig. S2).
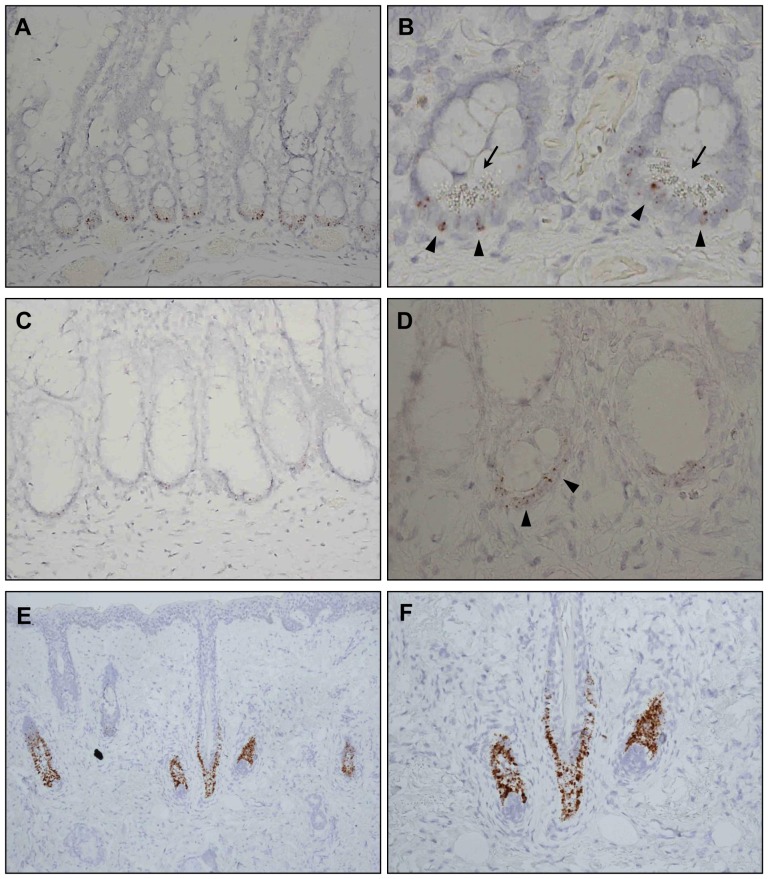
Figure 1. Validation of RNA in situ hybridization (ISH) for the identification of LGR5 + cells in established niches in normal human tissues.
RNA ISH was performed to detect LGR5-expressing cells in formalin-fixed and paraffin-embedded small intestine, colon, and hair follicle samples. LGR5 + cells are indicated by the brown colored dots. (A) LGR5 + cells were observed at the base of all small bowel crypts. (B) LGR5 + cells, indicated by arrowheads, were located next to or between Paneth cells, which are distinguished by their characteristic cytoplasmic granules and marked by arrows. (C, D) LGR5 + cells in the colonic crypts had lower LGR5 expression than LGR5 + cells in the small intestine. (E, F) Many LGR5 + cells were noted in the hair follicle bulges. Magnifications: A, C, F, ×200; B, D, ×400; E, ×100.
2. IM in the stomach is associated with a marked expansion of the LGR5 + cell population
We then investigated the spatial distribution of Lgr5 + cells in the non-tumorous gastric mucosa. Consistent with the findings in mice, a sparse Lgr5 + cell population was observed only at the base of the antral glands (Fig. 2A, B), but not at the isthmus or neck region. No Lgr5 + cells were noted at the fundic glands (Fig. 2C, D), suggesting that Lgr5 + cells only comprise a small group of stem cells restricted to the antrum. Remarkably, IM was associated with a dramatic increase in the number of Lgr5 + cells (Fig. 2E, F). Interestingly, Lgr5 + cells were also detected in IM in the corpus, where no Lgr5 + cells were present (Fig. 2G, H). Lgr5 + cells were mostly restricted to the basal areas of the metaplastic glands, similar to the pattern observed in the small intestine. Figure S3 shows an additional set of pictures of Lgr5 + cells in the gastric mucosa. Active gastritis with or without Helicobacter pylori infection had no effect on the Lgr5 + cell population (Fig. 2I). When counting the number of glands with Lgr5 + cells among 20 consecutive glands in both the gastric and intestinal mucosa, we surprisingly discovered that the frequency of glands with Lgr5 + cells in IM was almost as high as that in the small intestine (Fig. 2I), indicating that IM of the stomach seems to recapitulate intestinal mucosal features with regard to the stem cell population.
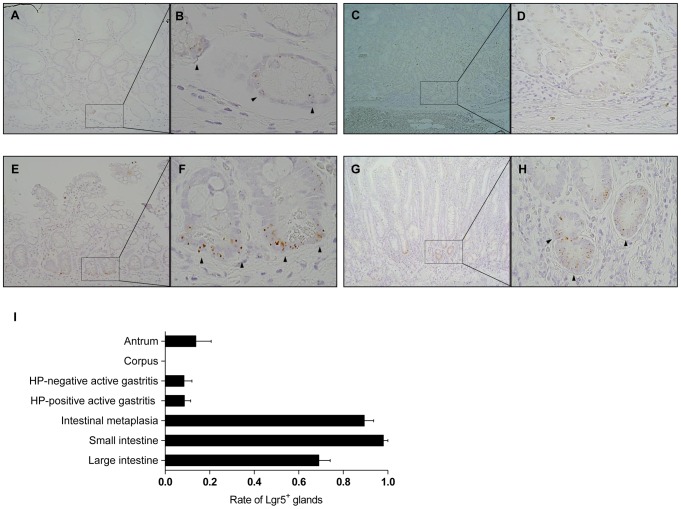
Figure 2. Localization of LGR5 + cells in the gastric antrum and intestinal metaplasia.
We examined many different types of non-tumorous gastric mucosa (GM), including GM without inflammation or intestinal metaplasia (IM) (antrum, n = 4; corpus, n = 4), GM with active gastritis (n = 12), and GM with IM in the antrum (n = 5), as well as small bowel (n = 5) and colon (n = 5). (A, B) Rarely, a few LGR5 + cells were observed in the basal region of the gastric antrum. (C, D) No LGR5 + cells were noted in the corpus. (E, F) The LGR5 + cells population, which is located primarily in the lower regions of glands, dramatically increased in IM of the antral glands. (G, H) Interestingly, IM in the corpus had as many LGR5 + cells as IM in the antrum. Arrows indicate the fundic glands. (E) When the ratio of LGR5 + cell-containing glands in 20 consecutive glands was assessed, IM showed a similar ratio to the small intestine. LGR5 + cells are indicated by arrowheads. HP, Helicobacter pylori. Magnifications: A, C, E, G, ×100; B, F, ×600; D, H, ×400.
3. The Lgr5+ cell population specifically increases with IM and positively correlates with CD133 expression
To provide further evidence of the close relationship between the appearance of Lgr5 + cells and IM, we collected 11 fresh-frozen, non-tumorous gastric tissues and divided them into 2 groups according to the CDX2 expression level, either CDX2-low and CDX2-high, because CDX2 is highly expressed in IM of the stomach [22] (Fig. 3A). The expression of claudin-18, known as gastric-type claudin, was also examined. However, there was no significant correlation between claudin-18 and CDX2 expression (Fig. S4). When LGR4, 5, and 6 expression levels were compared between the 2 groups, only the LGR5 level was significantly higher in the CDX2-high group, thus confirming a specific association between LGR5 expression and gastric mucosal intestinalization (Fig. 3B, C, and D). The CD133 expression level was also higher in the CDX2-high group, although the difference was not statistically significant (p = 0.055; Fig. 3E), which led us to hypothesize that the increased number of Lgr5 + cells was associated with CD133 expression, because CD133 is a stem cell marker in many different types of tumors [23]. Indeed, we found a positive correlation between LGR5 and CD133 expression when analyzing the RT-PCR data from the normal gastric mucosa (Pearson correlation coefficient = 0.47, p = 0.02) (Fig. 3F). In a mouse study, Lgr5 + cells were the cells of origin of a Wnt-driven GA [9]. Most human GAs occur from an IM background [24], and most have APC gene mutations [25]. Based on these findings, we postulated that IM in the stomach enhances the risk of tumor development by increasing the Lgr5 + cell population, which is highly susceptible to transformation by APC mutation.
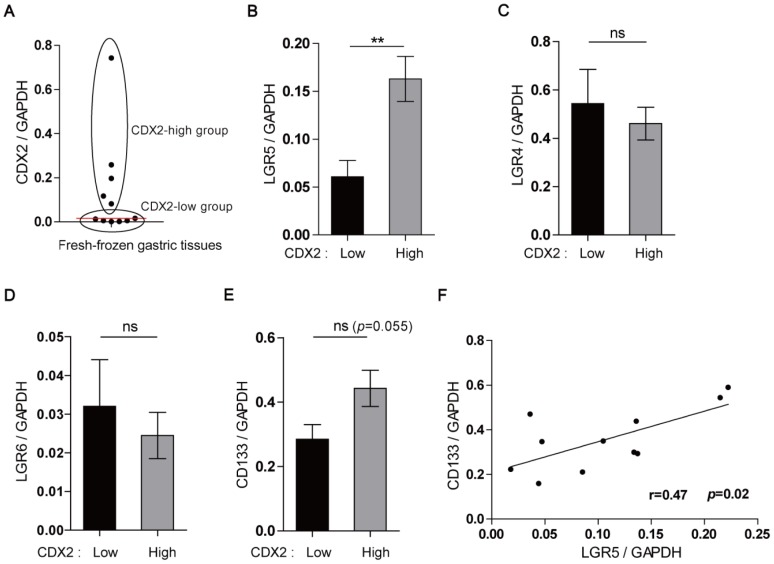
Figure 3. Increased LGR5 expression with gastric intestinalization.
Semi-quantitative real-time PCR was performed with fresh-frozen normal gastric tissues (n = 11) to analyze the expression of LGR4, LGR5, LGR6, CDX2, and CD133. (A) The samples were divided into the CDX2-high and -low groups according to the median CDX2 value (red line). LGR5 expression was significantly higher in the CDX2-high group (B), whereas there were no differences in the levels of LGR4 (C) and LGR6 (D) between the groups. (E) CD133 expression was higher in the CDX2-high group, although this was not statistically significant (p = 0.055). (F) A positive correlation was observed between LGR5 and CD133 expression (Pearson correlation coefficient = 0.47, p = 0.02).
4. LGR5 + tumor cells are often confined to the basal area of tumor glands, reminiscent of normal stem cell niches
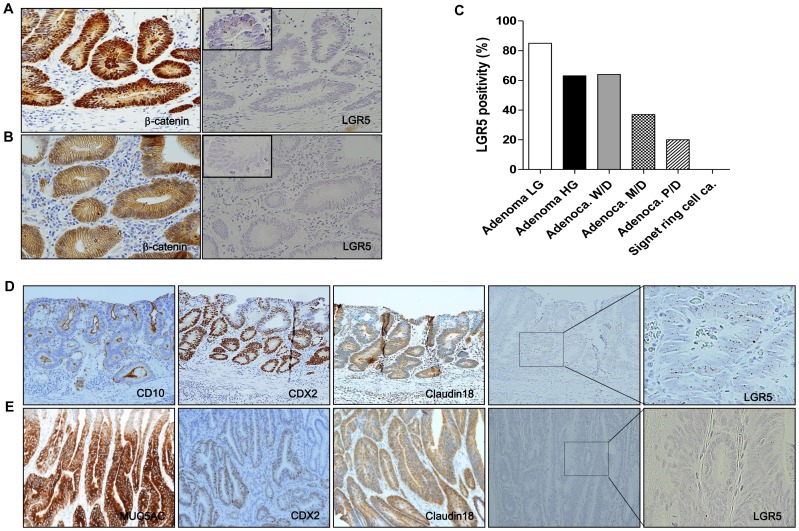
To explore the effects of Lgr5 + cells on tumor development in the human stomach, we constructed tissue microarrays (Fig. S5A) and examined the expression of LGR5 in gastric tumors obtained by ESD, including GAs and early gastric carcinomas (EGCs). The LGR5 expression levels were graded according to the percentage of LGR5 + tumor cells (Fig. 4A, B and Fig. S5B, C). Tumors above grade 2 were considered positive. The degree and pattern of LGR5 expression varied between the gastric tumors. The LGR5-positive tumors could be roughly divided into 2 types based on the distribution of Lgr5 + cells: tumors with basal (Fig. 4C) or diffuse (Fig. 4D) patterns. In the basal pattern tumors, the Lgr5 + cells were mostly restricted to the base of the adenoma segment, which was reminiscent of both IM and the normal crypt architecture. More than half of the tumors exhibited a basal accumulation of Lgr5 + cells, regardless of the histological progression of the gastric tumors, although the overall frequency of LGR5 positivity declined (Fig. S8). This observation led us to speculate that these Lgr5 + cells function as tumor stem cells. In a mouse study, the same tendency of Lgr5 + cells to localize toward the base of the adenoma segment was reported in the intestinal adenomas, and their stem cell-like properties were directly demonstrated by the coexpression of other stem cell markers such as OLFM4 and ASCL2, as well as by stem cell activity [7]. Additionally, to confirm the results from RNA ISH, total RNAs were obtained from FFPE samples of each gastric tumor grade, non-tumorous gastric mucosa, and intestinal mucosa, and the LGR5 transcripts were subsequently measured by semi-quantitative RT-PCR (Fig. 4E). The LGR5 transcript levels in IM of the stomach were similar to those in the small intestine. The RNA ISH assay grades correlated well with the LGR5 mRNA levels determined by RT-PCR (Spearman correlation coefficient = 0.96; p<0.0001) (Fig. 4F).
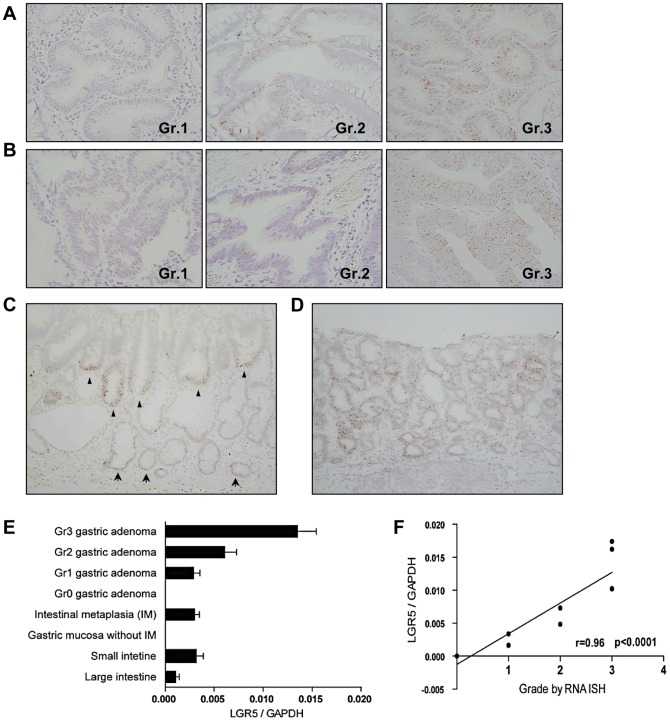
Figure 4. LGR5-expressing cells in gastric tumors.
Tissue microarrays were constructed from gastric tumors, including gastric adenomas (GAs) (n = 75) and early gastric carcinomas (EAGs) (n = 68), that had been removed by endoscopic submucosal dissection. LGR5 expression in GAs (A) and EGCs (B) was classified according to the percentage of LGR5 + tumor cells as grades 0, 1, 2, and 3. (C) Some GAs had a distinct LGR5 + cell distribution that was restricted to the bases of tumor glands (marked by arrowheads); this distribution was quite similar to that of LGR5 + cells in IM (indicated by arrows) observed around the tumor. (D) However, some GAs contained LGR5-expressing tumor cells in a relatively diffuse or patchy distribution pattern. (E) Semi-quantitative real-time PCR from formalin-fixed and paraffin-embedded GAs (n = 11), IM in the antrum (n = 3), GM without IM in the corpus (n = 4), and small (n = 4) and large (n = 4) intestinal tissues was performed to confirm the RNA in situ hybridization (ISH) results. (F) There was a positive correlation between the LGR5 grades in GAs as determined by RNA ISH and LGR5 transcript levels in RT-PCR (Spearman correlation coefficient = 0.96; p<0.0001). Magnifications: A, B, ×400.
5. LGR5 positivity is associated with nuclear β-catenin, histological differentiation, and mucin type in gastric tumors
Correlations of LGR5 positivity with various clinicopathological factors were evaluated and summarized for GAs (Table 1) and EGCs (Table S1). A total of 57 GA cases (76%) and 31 EGC cases (43%) were positive for LGR5. LGR5 expression was strongly and positively associated with nuclear β-catenin in both GAs (p = 0.015) (Fig. 5A, B and Fig. S6) and EGCs (p = 0.000), which was consistent with previous reports that documented a relationship between LGR5 and Wnt pathway activation [26], [27]. LGR5 expression was higher in low-grade adenomas than in high-grade adenomas (p = 0.025) (Table 1), and the expression tended to decline with tumor dedifferentiation (p = 0.036) (Fig. 5C). Nuclear β-catenin expression also significantly decreased with tumor progression (Table S2). Moreover, LGR5 expression was higher in adenomas with intestinal-type glands than in those with gastric-type glands (Fig. 5D and E and Fig. S7) (p = 0.024). Thus, our findings indicate that Lgr5 + cells occur more frequently in low-grade tumors with active Wnt pathway signaling and an intestinal gland type, suggesting that LGR5 is more likely involved in the very early stages of Wnt-driven tumorigenesis in the stomach.
Table 1. Assessment of LGR5 expression in gastric adenomas.
| Total (%) | LGR5 | p-value | ||
| Negative (%) | Positive (%) | |||
| Patients | 75 (100) | 18 (24) | 57 (76) | |
| Age | ||||
| ≥65 | 41 (55) | 10 (24) | 31 (76) | 1.000† |
| <65 | 34 (45) | 8 (24) | 26 (76) | |
| Gender | ||||
| Female | 22 (29) | 4 (18) | 18 (82) | 0.560† |
| Male | 53 (71) | 14 (26) | 39 (74) | |
| Grade | ||||
| Low | 47 (63) | 7 (15) | 17 (85) | 0.025† |
| High | 30 (37) | 11 (37) | 40 (63) | |
| β-catenin | ||||
| Nuclear stain | 44 (59) | 6 (14) | 38 (84) | 0.015† |
| No nuclear stain | 31 (41) | 12 (39) | 19 (61) | |
| Mucinous type | ||||
| Gastric | 13 (17) | 7 (54) | 6 (46) | 0.024# |
| Intestinal | 30 (40) | 4 (13) | 26 (87) | |
| Mixed | 23 (31) | 5 (22) | 18 (78) | |
| Unclassified | 9 (12) | 1 (11) | 8 (89) | |
#Pearson Chi-Square. †Fisher's exact test.
Figure 5. Relationships between LGR5 positivity and nuclear β-catenin, histological differentiation, and mucinous type in gastric adenomas.
Gastric adenomas with strong cytoplasmic and nuclear β-catenin expression (n = 44) were highly likely to be positive for LGR5 (A), whereas adenomas with normal β-catenin expression levels (n = 31) had relatively low LGR5 positivity (B). (C) As the tumors progressed and dedifferentiated, LGR5 positivity declined (n = 143). Intestinal-type adenomas (n = 30) (A) generally had higher levels of LGR5 expression than gastric-type adenomas (n = 13) (E). CD10 and MUC2 expression refers to the intestinal tumor gland phenotype, and MUC5AC and MUC6 mucin expression represents the gastric gland phenotype. LG, low grade; HG, high grade; Adenoca, adenocarcinoma; W/D, well differentiated; M/D, moderately differentiated; P/D, poorly differentiated. Magnifications: A, B, D, E, ×200.
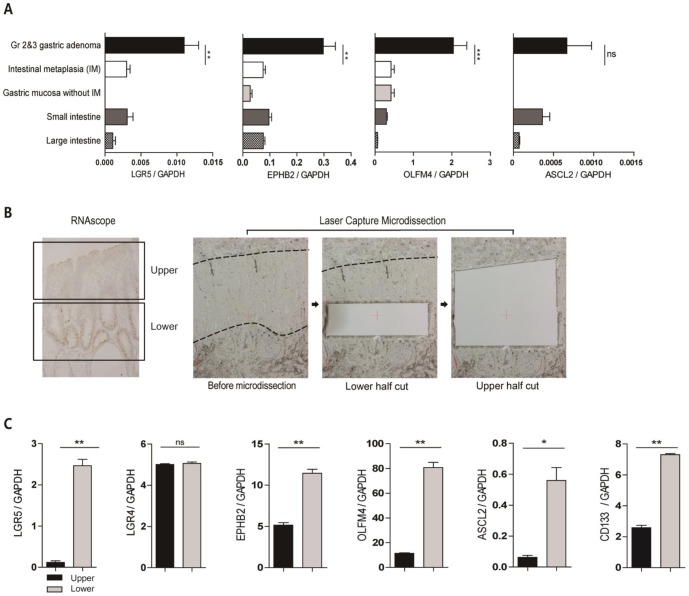
6. LGR5 expression is associated with the levels of other intestinal stem cell (ISC) markers
The distinct basal restriction of LGR5+ cells in some GAs is interesting because it strikingly resembles the restriction of Lgr5 + stem cells to the niche in normal tissues. To further investigate whether the Lgr5 + cells in human GAs had any stem cell properties, we analyzed the levels of ISC markers such as ASCL2, EPHB2, and OLFM4, which are highly expressed in stem-like cells from human colorectal cancers [28], as well as in murine intestinal adenomas [7]. In general, GAs expressed significantly higher levels of EPHB2 and OLFM4 than the non-tumorous gastric mucosa (Fig. 6A). To clarify whether the upregulated expression of ISC markers was related to the LGR5 + tumor cells, we microdissected the tumor glands into upper and lower regions (Fig. 6B) and compared the marker transcript levels in both areas. The results confirmed that LGR5 expression was higher in the lower region, whereas the expression of LGR4, which is a close relative of the LGR5 gene, did not differ between the 2 regions (Fig. 6C). The expression levels of all examined ISC markers were higher in the lower region, where the majority of Lgr5 + cells exist (Fig. 6C). Furthermore, CD133 expression was higher in the basal regions of the tumor glands (Fig. 6C). Thus, these findings support the hypothesis that Lgr5 + cells restricted to the bases of tumor glands retain stem cell properties in human GAs.
Figure 6. LGR5 + cells, restricted to the lower regions of tumor glands, are associated with high levels of intestinal stem cell (ISC) markers.
(A) LGR5-positive gastric adenomas (n = 8) expressed substantially higher levels of ISC markers such as EPHB2, OLFM4, and ASCL2 than non-tumorous gastric mucosa (IM, n = 4; GM without IM in the corpus, n = 4) and the small (n = 3) and large (n = 3) intestines, although the difference in ASCL2 expression was not statistically significant. (B) Lower and upper regions were laser-capture microdissected from GAs with basal LGR5 + cells. (C) Semi-quantitative real-time PCR of the dissected tissues revealed that the basal regions that harbored the most LGR5 + cells had higher levels of ISC markers and CD133, whereas no difference in LGR4 expression was observed between the lower and upper regions.
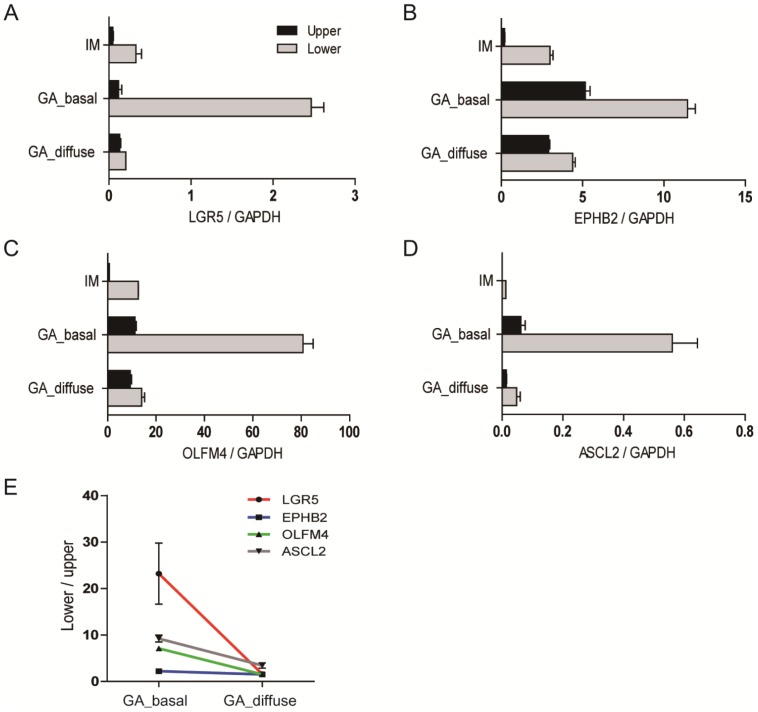
7. Basal restriction of LGR5 + cells in GAs correlates with the differential expression of ISC markers along the gland axes
To strengthen the hypothesis that the Lgr5 + cells restricted to the bases of tumor glands are essential for the distinct ISC marker expression patterns in the upper and lower regions, we examined a GA with relatively diffuse LGR5 distribution and a slight basal accentuation of LGR5 expression. LGR5 expression in the upper and lower halves of the gland differed little in the adenoma, unlike that in IM and GAs with basal LGR5 expression (Fig. 7A). Indeed, the wide regional variation in the expression of all ISC markers that was observed in the GAs and IM with basal LGR5 expression was remarkably reduced in the GA with diffuse LGR5 expression (Fig. 7B, C, and D). The expression ratios of the lower regions to the upper regions for all ISC markers, including EphB2 (p = 0.018), OLFM4 (p = 0.002), and ASCL2 (p = 0.025), were significantly decreased in the GA with diffuse LGR5 expression, compared to a GA with basal LGR5 expression (Fig. 7E). These findings indicate that the basal restriction of Lgr5 + cells in GAs is closely correlated with the gradient expression of ISC markers along the gland axes.
Figure 7. Comparison of ISC marker expression in gastric adenomas with different LGR5 distribution patterns.
The upper and lower gland regions were selectively microdissected from IM (n = 3) and the GA with diffusely distributed LGR5 + cells (GA_diffuse) to compare the differential expression of ISC markers to those of the GA with basally restricted LGR5 + cells (GA_basal). RT-PCR analysis of LGR5 (A), EPHB2 (B), OLFM4 (C), and ASCL2 (D) expression demonstrated that the ISC marker expression gradient between the lower and upper gland regions was remarkably reduced in the GA with a diffuse pattern (E).
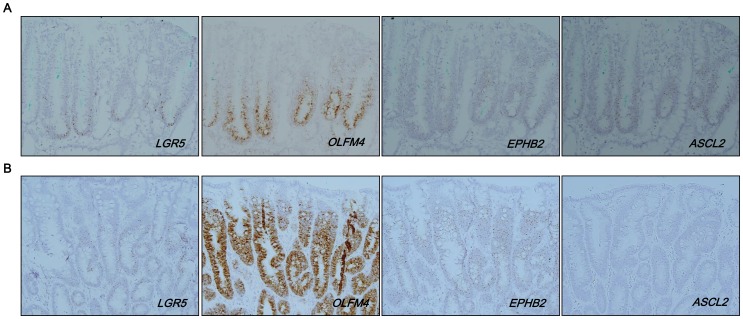
8. Spatial correlation of LGR5 expression with ISC markers in GAs
To confirm the RT-PCR results that indicated an association between LGR5 expression and the expression of other ISC markers, we performed RNA in situ hybridization on GAs with basal or diffuse patterns. For a GA with a diffuse pattern, we selected one with strong and diffuse LGR5 expression for improved visualization at a low power view. The expression of OLFM4, EPHB2, and ASCL2 was relatively restricted to the basal region of tumor glands in the GA with basal LGR5 expression (Fig.8A), but the expression of these markers was diffuse in the GA with a diffuse LGR5 expression pattern (Fig. 8B). Notably, in the GA with a basal pattern, LGR5 expression was confined to the very lower area of the tumor glands when other stem cell markers were expressed by a larger population of cells beyond the base (Fig. 8A and Fig. S9), suggesting that LGR5 is a marker for a specific group of cells with stem cell features. On the other hand, for the GA with diffuse LGR5 expression in which the majority of tumor cells expressed ISC markers as well, it remains unclear whether all Lgr5 + cells are tumor stem cells.
Figure 8. Correlation of LGR5 + cells with other intestinal stem cell markers.
In RNA in situ hybridization, intestinal stem cell markers (OLFM4, EPHB2, and ASCL2) were mainly expressed at the basal area of tumor glands in the GA with a basal pattern (A). In contrast, the GA with a diffuse pattern (n = 2) showed diffuse expression of the markers throughout the tumor (B). Magnification: A, B, ×200.
Discussion
Considerable evidence supports that Lgr5 is a normal gastrointestinal tract stem cell marker in mice, with a corresponding role in murine tumors. Investigations of LGR5 expression in human tissues, however, have been limited, and an appropriate histological method to identify Lgr5 + cells in human organs has not been established. Previous studies that attempted to use antibodies or RNA ISH on human FFPE tissues did not seem to provide accurate results with regard to the specific labeling of LGR5+ cells [10]–[15]. For example, Uehara et al. [29] and Wu et al. [30] recently reported the distribution of LGR5 cells in human gastric glands using commercially available LGR5 antibodies. However, an appropriate positive control stain to validate the specificity of the antibodies was not provided, and the results showed nonspecific staining of parietal cells or hematopoietic cells in the lamina propria of the stomach. Simon et al. generated monoclonal antibodies to LGR5 to investigate the histo-anatomical distribution of Lgr5+ cells with gastric cancer progression and validated its specificity by western blot analysis of LGR5-transfected cell lines [11]. However, the immunostaining of colonic mucosa as a positive control was not entirely convincing because of the strong positive staining of stromal and endothelial cells. More importantly, none of these studies demonstrated the specific localization of LGR5 cells at the basal glands of normal gastric antrum, as shown in mice. Nakata et al. [31] and Becker et al. [12] did provide a positive control stain for LGR5 antibodies with the intestinal mucosa before using the antibodies in brain and colonic tumors, but the quality of the staining was not sufficient to ensure the sensitivity and specificity of the antibodies. Although RNA ISH has specifically shown the Lgr5 + cells restricted at the crypt base in studies of human and murine intestines, the visualization quality of Lgr5+ cells in FFPE tissues was not satisfying, and the technique has not been used in human gastric tissues [14], [15], [32].
In the present study, we used RNAscope to determine the presence of Lgr5 + cells in various gastric lesions after validating the technique. We specifically identified Lgr5 + cells at the same locations in human gastric mucosa, intestines, and hair follicles as previously shown in mice. Moreover, the quality of the staining was much better than with conventional RNA ISH, which made it easier to evaluate Lgr5 + cells in their cellular context. Consequently, we expect that this method will facilitate studies of Lgr5 + cells in other archived human samples, which could in turn accelerate investigations into the practical significance of LGR5 in a variety of human diseases. However, RNA ISH only detects cells that contain LGR5 transcripts; it is unknown whether LGR5 transcripts are sufficiently translated into proteins that play functional roles in determining stem cell properties. It remains a possibility that LGR5 serves mainly as a surrogate stem cell marker without any functional implications. Although LGR5 acts as a receptor for ligands such as R-spondins and helps augment Wnt signaling [33], [34], the functional relevance of LGR5 in humans with regard to stem cell activity has yet to be determined.
We observed Lgr5 + cells at almost all crypts of the small intestine and colon and at the hair follicle bulges, which confirmed that Lgr5 + cells are a major stem cell population in these tissues. However, Lgr5 + cells were barely detectable in the antral glands of the normal gastric mucosa, suggesting that Lgr5 + cells in the stomach comprise only a small fraction of the total stem cell population. The stomach can be divided into 4 major parts, depending on its histo-anatomical features, and all proposed stem cell markers to date are present in a limited area of the stomach [35]. Therefore, the stomach likely contains many heterogeneous stem cell populations with distinct characteristics and different markers. The intestinal epithelium is also believed to contain 2 distinct pools of stem cells, the Bmi1-expressing +4 cells and the Lgr5 + crypt-based columnar cells [36].
Lgr5 + cells in the normal antral glands were sparse and unaffected by inflammation or Helicobacter pylori infection. In contrast, the number of Lgr5 + cells and the staining intensity were remarkably increased in IM to nearly the same levels observed in the small intestine. Interestingly, Lgr5 + cells also appeared in IM of the fundic glands, in which Lgr5 + cells are normally absent, implying that intestinalized gastric epithelial cells, including the Lgr5 + cells in IM, might not arise from the existing Lgr5 + stem cells. Spasmolytic polypeptide-expressing metaplasia (SPEM), another type of stomach metaplasia, does not arise from Lgr5+ cells either [37]. Instead, SPEM transdifferentiates from mature chief cells in the murine [38] and human [39], [40] stomach. Moreover, Lgr5+ stem cells arise from Bmi-1-expressing stem cells in the intestinal epithelium [36]. Collectively, these findings suggest that the Lgr5 + cell expansion in IM arises from another group of stem cells, although no stem cell population is yet known to generate Lgr5 + cells in the stomach.
Intestinalized gastric glands are similar in many aspects to the small intestinal epithelium. However, IM is not identical to the intestinal mucosa. One significant difference is that Paneth cells are less frequently found in IM. Paneth cells may be essential for the maintenance of Lgr5 + stem cells in intestinal crypts [41]. However, in IM, Lgr5 + cells usually exist in the absence of Paneth cells, indicating that Lgr5 + cells in IM do not rely on Paneth cells as a source of signaling factors, such as Wnt ligands, Notch ligands, and epidermal growth factor, for survival. Even when Paneth cells are present in IM and GAs, Lgr5 + cells are not necessarily located next to Paneth cells (Fig. S10). Instead of Paneth cells, Lgr5 + cells have redundant sources of survival signals. For instance, Wnt ligands from mesenchymal cells can compensate for the loss of signals from Paneth cells [42], and c-kit+ secretory cells have been identified as a colonic counterpart of Paneth cells that could support Lgr5 + stem cells [43]. Additionally, it was recently shown that Paneth cells are not required to sustain Lgr5 + cells in vivo [44]. Thus, Lgr5 + stem cells in IM might be maintained by an undefined epithelial or mesenchymal cell population that substitutes for Paneth cells.
We speculate that the marked increase in the number of Lgr5 + cells during the process of IM has profound biological significance for tumor initiation in the stomach. A study of Cdx2-transgenic mice demonstrated that IM plays a significant role in the genesis of gastric carcinoma and that the cancer cells originated from intestinal metaplastic epithelial cells that had been entirely transformed from gastric cells by Cdx2 [45]. In addition, an increased number of Lgr5 + cells were observed in the normal intestinal tissues of Apc mutant mice and proposed as the cause of more severe polyposis [14]. Accordingly, given that Lgr5 + cells are the cells of origin of Wnt-driven tumors in the murine stomach [9], the expanded pool of Lgr5 + stem cells might be one of the major factors of IM that contributes to tumor development in the human stomach. However, because a lineage tracing study is not applicable for human tissues at this time, direct evidence that human GAs derive from Lgr5 + cells cannot be obtained. Therefore, future studies on the relationship of Lgr5 + cells and GA development are required.
LGR5 + cells were present in human GAs, where they accounted for 0 to >90% of the tumor cells with 2 main distribution patterns. In addition to tumors with a basally restricted pattern of LGR5 + cells, we also observed adenomas with Lgr5+ cells that were dispersed throughout the tumor mass; this was not noted in a mouse study, in which approximately 5–10% of the tumor cells in most of the intestinal adenomas were Lgr5 + cells, which were mainly located at the basal areas of the tumor glands [7]. This was probably because the intestinal adenomas in the mice all arose from the same Apc mutation, whereas human adenomas develop in response to many different types of Wnt-pathway abnormalities, likely in combination with other genetic abnormalities, which results in tumors with different levels of Wnt signaling and varying numbers of Lgr5 + cells. In fact, the level of Lgr5 expression in adenomas of 2 intestinal tumorigenesis mouse models, Apc 1322T (1322T) and Apc R850X (Min), differed: 1322T tumors had higher Lgr5 expression levels than Min tumors, and more than half of the epithelial portion of the adenomas showed Lgr5 expression [14]. Therefore, in GAs, both the distribution pattern and the number of Lgr5 + cells are likely to differ according to the degree of Wnt pathway activation.
In most of the low-grade adenomas, at least 5% of the tumor cells were LGR5 + cells. We might consider Lgr5 + cells in the niches of normal tissues as stem cells; however, Lgr5 + cells within a tumor mass do not necessarily serve as tumor stem cells. EPHB2- and LGR5-enriched cells reportedly comprise a stem-like cell population in human colorectal cancers [28]. However, it has not been evaluated whether Lgr5 + cells in human gastric tumors retain stem cell properties. To investigate whether Lgr5 + cells in gastric tumors had any stem cell features, we selected GAs with basally restricted Lgr5 + cells that were reminiscent of the normal crypt and IM, and then divided the tumor glands into upper and lower regions by laser capture microdissection. Our study demonstrated that the basal regions of tumor glands, which have a majority of the Lgr5 + cells, express significantly higher levels of ISC markers and CD133 than the upper region. In contrast, the differential expression of ISC markers was substantially attenuated in GAs with diffusely distributed Lgr5 + cells, which was confirmed by the co-localization of LGR5 and ISC markers in RNA in situ hybridization. These findings indicate that LGR5 expression is closely linked to increased expression of ISC markers and provide compelling evidence that basally restricted Lgr5 + cells in GAs act as stem cells. However, the question remains whether the Lgr5 + cells that are distributed diffusely across the adenoma and co-express ISC markers also have stem cell features; they seem too numerous to be stem cells and lack the spatial restriction to the base of the glands. If the diffuse expression only derives from strong Wnt pathway activation, as we mentioned earlier, LGR5 may not be appropriate for use as a stem cell marker. Clearly, further studies are required to unravel the stem cell properties of Lgr5 + cells in gastric tumors.
Although we have successfully demonstrated the stem cell phenotype of Lgr5 + cells in GAs, there are certain limitations regarding the interpretation and generalization of our findings. First, because technical limitations precluded the specific microdissection of Lgr5 + cells, we compared the levels of ISC markers in the upper and lower regions of tumor glands and not in LGR5-positive and -negative tumor cells. Thus, we cannot exclude the possibility that other stem cell populations are present and intermingled with Lgr5 + cells in the lower regions of the gland; these cells might affect the results of stem cell-related gene expression profiling. Second, we only analyzed low-grade GAs with basally restricted Lgr5 + cells, which represent a very early stage during intestinal-type gastric carcinogenesis. Thus, it remains unknown whether Lgr5 + cells retain their stem cell characteristics during tumor progression. The finding that LGR5 positivity declines with tumor progression and dedifferentiation suggests that Lgr5 + cells no longer act as tumor stem cells during the later stages of tumor progression. In fact, tumor stem cells might evolve during tumor progression in response to changing environmental cues [46]. Finally, functional experiments were not performed in this study; instead, indirect phenotypic features related to stem cell-related gene expression patterns were investigated. Nevertheless, we believe our findings provide valuable data that support the use of LGR5 as a stem cell marker.
Supporting Information
LGR5 + cells in the basal cell carcinoma of skin and adenoma of colon. (A) LGR5 stem cells (marked by arrow) are seen at the bulge of hair follicle, and the majority of tumor cells of basal cell carcinoma (indicated by arrow heads) which developed nearby express LGR5. (B) Colonic adenoma cells exhibit LGR5 expression. Inlet pictures show a representative area at higher magnification. Magnification: A, ×100; B, ×200.
(TIF)
Validation of RNAscope with CDX2 expressing gastric adenomas. CDX2 expressing gastric adenoma cells (indicated by arrows), shown as strong nuclear stain by immunohistochemistry (A and C), are specifically identified by RNAscope (B and D), whereas normal gastric epithelial cells adjacent to tumor cells (indicated by arrow heads), negative for CDX2, do not express CDX2 transcripts. Original magnification: A, B, C, D ×200.
(TIF)
Expanded population of LGR5 + cells in the intestinal metaplasia of the stomach (A) A few LGR5 + cells are located at the basal area of antral glands. (B) No LGR5 + cells are found in the body. (C) The LGR5 + cell population remarkably increases when IM occurs in gastric antrum. (D) Notably, LGR5 + cells also appear at the basal part of metaplastic glands that have developed in the body. Arrows indicate the fundic glands. LGR5 + cells are indicated by arrowheads. Magnifications: A, B, C, D, ×400.
(TIF)
Claudin-18 expression in non-tumorous gastric mucosa. (A) RT-PCR shows a differential expression of CLD18 in fresh-frozen gastric tissues. (n = 11) (B) CDX2 low group tends to express higher CLD18 than CDX2 high group although it is not statistically significant (p = 0.399).
(TIF)
LGR5 expressing cells in gastric tumors. (A) Tissue microarrays were constructed from gastric tumors. LGR5 expression in GAs (B) and EGCs (C) was classified according to the percentage of LGR5 + tumor cells as grades 0, 1, 2, and 3. Magnification: A, B, C, D, ×200.
(TIF)
Relationship between LGR5 expression and nuclear β-catenin Gastric adenomas with strong cytoplasmic and nuclear β-catenin expression tend to be positive for LGR5 (A), whereas adenomas with normal β-catenin levels show relatively low LGR5 positivity (B). Inlet pictures show a representative area at higher magnification. Magnifications: A, B, ×200.
(TIF)
Correlation between LGR5 positivity and mucinous type of gastric adenomas. Intestinal-type adenomas (A) have higher levels of LGR5 expression than gastric-type adenomas (B). CD10, CDX2 and MUC2 expression refers to the intestinal tumor gland phenotype and MUC5AC mucin expression represents the gastric gland phenotype. Magnifications: A, B, ×200.
(TIF)
Basal arrangement of LGR5 + cells in gastric tumors. LGR5 expressing tumor cells are often restricted at the basal part of tumor glands or at the interface between muscularis mucosa and submucosa in the gastric tumors including low grade adenoma (A), high grade adenoma (B), well differentiated adenocarcinoma (C), and moderately differentiated adenocarcinoma (D). (E) Around half of tumors showed basal distribution pattern of LGR5 + cells regardless of histological type. Magnification: A, B, D ×200; C ×100.
(TIF)
Spatial correlation of LGR5 expression with intestinal stem cell markers in a gastric adenoma with basally distributed LGR5 + cells. OLFM4, EPHB2, and ASCL2 expressions tend to gradually increase along the axis of tumor glands in a gastric adenoma in which LGR5 + cells are restricted at the base of tumor. Magnification: A, B, C, D, ×100.
(TIF)
Spatial relationship of LGR5 + cells with regard to Paneth cells. To analyze the association of LGR5 + cells with Paneth cells, we performed combined RNA ISH and IHC. LGR5 + cells are marked with brown dots and Paneth cells are indicated by α-defensin stain as red in the cytoplasm. (A, B) In the small intestine, LGR5 + cells are always adjacent to Paneth cells at the bas β e of crypts. (C, D) However, some LGR5 + cells that appear in the IM are not located near Paneth cells. (E, F) LGR5 + gastric adenoma cells tend to locate in the tumor regardless of adenoma Paneth cells. Boxed areas in figure A, C, and E are shown at higher magnification in figure B, D, and F respectively. LGR5 + cells are marked by arrow heads. Magnification: A, C, E ×100; B, D, F ×400.
(TIF)
Assessment of LGR5 expression in early gastric carcinomas.
(TIF)
LGR5 and nuclear β-catenin with the progression of gastric cancers.
(TIF)
Acknowledgments
We would like to thank Hyun Ju Park for her technical support.
Funding Statement
The study was supported by the SNUH Research Fund, grant no 2013-0393. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Visvader JE, Lindeman GJ (2008) Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer 8: 755–768. [DOI] [PubMed] [Google Scholar]
- 2.Wicha MS, Liu S, Dontu G (2006) Cancer stem cells: an old idea—a paradigm shift. Cancer Res 66: 1883–1890; discussion 1895–1886. [DOI] [PubMed]
- 3. Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, et al. (2007) Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449: 1003–1007. [DOI] [PubMed] [Google Scholar]
- 4. Yan KS, Chia LA, Li X, Ootani A, Su J, et al. (2012) The intestinal stem cell markers Bmi1 and Lgr5 identify two functionally distinct populations. Proc Natl Acad Sci U S A 109: 466–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Snippert HJ, van Es JH, van den Born M, Begthel H, Stange DE, et al.. (2009) Prominin-1/CD133 marks stem cells and early progenitors in mouse small intestine. Gastroenterology 136: 2187–2194 e2181. [DOI] [PubMed]
- 6. Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, et al. (2009) Crypt stem cells as the cells-of-origin of intestinal cancer. Nature 457: 608–611. [DOI] [PubMed] [Google Scholar]
- 7. Schepers AG, Snippert HJ, Stange DE, van den Born M, van Es JH, et al. (2012) Lineage tracing reveals Lgr5+ stem cell activity in mouse intestinal adenomas. Science 337: 730–735. [DOI] [PubMed] [Google Scholar]
- 8. Leushacke M, Barker N (2012) Lgr5 and Lgr6 as markers to study adult stem cell roles in self-renewal and cancer. Oncogene 31: 3009–3022. [DOI] [PubMed] [Google Scholar]
- 9. Barker N, Huch M, Kujala P, van de Wetering M, Snippert HJ, et al. (2010) Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell 6: 25–36. [DOI] [PubMed] [Google Scholar]
- 10. von Rahden BH, Kircher S, Lazariotou M, Reiber C, Stuermer L, et al. (2011) LgR5 expression and cancer stem cell hypothesis: clue to define the true origin of esophageal adenocarcinomas with and without Barrett's esophagus? J Exp Clin Cancer Res 30: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Simon E, Petke D, Boger C, Behrens HM, Warneke V, et al. (2012) The spatial distribution of LGR5+ cells correlates with gastric cancer progression. PLoS One 7: e35486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Becker L, Huang Q, Mashimo H (2008) Immunostaining of Lgr5, an intestinal stem cell marker, in normal and premalignant human gastrointestinal tissue. Scientific World Journal 8: 1168–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang T, Yeoh KG, Salto-Tellez M (2012) Lgr5 expression is absent in human premalignant lesions of the stomach. Gut 61: 1777–1778. [DOI] [PubMed] [Google Scholar]
- 14. Lewis A, Segditsas S, Deheragoda M, Pollard P, Jeffery R, et al. (2010) Severe polyposis in Apc(1322T) mice is associated with submaximal Wnt signalling and increased expression of the stem cell marker Lgr5. Gut 59: 1680–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Uchida H, Yamazaki K, Fukuma M, Yamada T, Hayashida T, et al. (2010) Overexpression of leucine-rich repeat-containing G protein-coupled receptor 5 in colorectal cancer. Cancer Sci 101: 1731–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wakatsuki K, Yamada Y, Narikiyo M, Ueno M, Takayama T, et al. (2008) Clinicopathological and prognostic significance of mucin phenotype in gastric cancer. J Surg Oncol 98: 124–129. [DOI] [PubMed] [Google Scholar]
- 17. Namikawa T, Hanazaki K (2010) Mucin phenotype of gastric cancer and clinicopathology of gastric-type differentiated adenocarcinoma. World J Gastroenterol 16: 4634–4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bonin S, Hlubek F, Benhattar J, Denkert C, Dietel M, et al. (2010) Multicentre validation study of nucleic acids extraction from FFPE tissues. Virchows Arch 457: 309–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jaks V, Barker N, Kasper M, van Es JH, Snippert HJ, et al. (2008) Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat genet 40: 1291–1299. [DOI] [PubMed] [Google Scholar]
- 20. Wang F, Flanagan J, Su N, Wang LC, Bui S, et al. (2012) RNAscope: a novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J Mol Diagn 14: 22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tanese K, Fukuma M, Yamada T, Mori T, Yoshikawa T, et al. (2008) G-protein-coupled receptor GPR49 is up-regulated in basal cell carcinoma and promotes cell proliferation and tumor formation. Am J Pathol 173: 835–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yuasa Y (2003) Control of gut differentiation and intestinal-type gastric carcinogenesis. Nat Rev Cancer 3: 592–600. [DOI] [PubMed] [Google Scholar]
- 23. Mizrak D, Brittan M, Alison M (2008) CD133: molecule of the moment. J Pathol 214: 3–9. [DOI] [PubMed] [Google Scholar]
- 24. Abraham SC, Montgomery EA, Singh VK, Yardley JH, Wu TT (2002) Gastric adenomas: intestinal-type and gastric-type adenomas differ in the risk of adenocarcinoma and presence of background mucosal pathology. Am J Surg Pathol 26: 1276–1285. [DOI] [PubMed] [Google Scholar]
- 25. Lee JH, Abraham SC, Kim HS, Nam JH, Choi C, et al. (2002) Inverse relationship between APC gene mutation in gastric adenomas and development of adenocarcinoma. Am J Pathol 161: 611–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Takahashi H, Ishii H, Nishida N, Takemasa I, Mizushima T, et al. (2011) Significance of Lgr5(+ve) cancer stem cells in the colon and rectum. Ann Surg Oncol 18: 1166–1174. [DOI] [PubMed] [Google Scholar]
- 27. Fan XS, Wu HY, Yu HP, Zhou Q, Zhang YF, et al. (2010) Expression of Lgr5 in human colorectal carcinogenesis and its potential correlation with beta-catenin. Int J Colorectal Dis 25: 583–590. [DOI] [PubMed] [Google Scholar]
- 28. Merlos-Suarez A, Barriga FM, Jung P, Iglesias M, Cespedes MV, et al. (2011) The intestinal stem cell signature identifies colorectal cancer stem cells and predicts disease relapse. Cell Stem Cell 8: 511–524. [DOI] [PubMed] [Google Scholar]
- 29. Uehara T, Ma D, Yao Y, Lynch JP, Morales K, et al. (2013) H. pylori infection is associated with DNA damage of Lgr5-positive epithelial stem cells in the stomach of patients with gastric cancer. Dig Dis Sci 58: 140–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wu C, Xie Y, Gao F, Wang Y, Guo Y, et al. (2013) Lgr5 expression as stem cell marker in human gastric gland and its relatedness with other putative cancer stem cell markers. Gene 525: 18–25. [DOI] [PubMed] [Google Scholar]
- 31. Nakata S, Campos B, Bageritz J, Bermejo JL, Becker N, et al. (2013) LGR5 is a marker of poor prognosis in glioblastoma and is required for survival of brain cancer stem-like cells. Brain Pathol 23: 60–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Leedham SJ, Rodenas-Cuadrado P, Howarth K, Lewis A, Mallappa S, et al. (2013) A basal gradient of Wnt and stem-cell number influences regional tumour distribution in human and mouse intestinal tracts. Gut 62: 83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Carmon KS, Gong X, Lin Q, Thomas A, Liu Q (2011) R-spondins function as ligands of the orphan receptors LGR4 and LGR5 to regulate Wnt/beta-catenin signaling. Proc Natl Acad Sci U S A 108: 11452–11457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ruffner H, Sprunger J, Charlat O, Leighton-Davies J, Grosshans B, et al. (2012) R-Spondin potentiates Wnt/beta-catenin signaling through orphan receptors LGR4 and LGR5. PLoS One 7: e40976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Qiao XT, Gumucio DL (2011) Current molecular markers for gastric progenitor cells and gastric cancer stem cells. J Gastroenterol 46: 855–865. [DOI] [PubMed] [Google Scholar]
- 36. Tian H, Biehs B, Warming S, Leong KG, Rangell L, et al. (2011) A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature 478: 255–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nam KT, O'Neal RL, Coffey RJ, Finke PE, Barker N, et al. (2011) Spasmolytic polypeptide-expressing metaplasia (SPEM) in the gastric oxyntic mucosa does not arise from Lgr5-expressing cells. Gut 61: 1678–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nam KT, Lee HJ, Sousa JF, Weis VG, O'Neal RL, et al.. (2010) Mature chief cells are cryptic progenitors for metaplasia in the stomach. Gastroenterology 139: 2028–2037 e2029. [DOI] [PMC free article] [PubMed]
- 39. Lennerz JK, Kim SH, Oates EL, Huh WJ, Doherty JM, et al. (2010) The transcription factor MIST1 is a novel human gastric chief cell marker whose expression is lost in metaplasia, dysplasia, and carcinoma. Am J Pathol 177: 1514–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Capoccia BJ, Jin RU, Kong YY, Peek RM Jr, Fassan M, et al. (2013) The ubiquitin ligase Mindbomb 1 coordinates gastrointestinal secretory cell maturation. J Clin Invest 123: 1475–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, et al. (2011) Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 469: 415–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Farin HF, Van Es JH, Clevers H (2012) Redundant sources of Wnt regulate intestinal stem cells and promote formation of Paneth cells. Gastroenterology 143: 1518–1529 e1517. [DOI] [PubMed]
- 43.Rothenberg ME, Nusse Y, Kalisky T, Lee JJ, Dalerba P, et al.. (2012) Identification of a cKit(+) colonic crypt base secretory cell that supports Lgr5(+) stem cells in mice. Gastroenterology 142: 1195–1205 e1196. [DOI] [PMC free article] [PubMed]
- 44. Kim TH, Escudero S, Shivdasani RA (2012) Intact function of Lgr5 receptor-expressing intestinal stem cells in the absence of Paneth cells. Proc Natl Acad Sci U S A 109: 3932–3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mutoh H, Sakurai S, Satoh K, Tamada K, Kita H, et al. (2004) Development of gastric carcinoma from intestinal metaplasia in Cdx2-transgenic mice. Cancer Res 64: 7740–7747. [DOI] [PubMed] [Google Scholar]
- 46. Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CH, et al. (2006) Cancer stem cells--perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res 66: 9339–9344. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
LGR5 + cells in the basal cell carcinoma of skin and adenoma of colon. (A) LGR5 stem cells (marked by arrow) are seen at the bulge of hair follicle, and the majority of tumor cells of basal cell carcinoma (indicated by arrow heads) which developed nearby express LGR5. (B) Colonic adenoma cells exhibit LGR5 expression. Inlet pictures show a representative area at higher magnification. Magnification: A, ×100; B, ×200.
(TIF)
Validation of RNAscope with CDX2 expressing gastric adenomas. CDX2 expressing gastric adenoma cells (indicated by arrows), shown as strong nuclear stain by immunohistochemistry (A and C), are specifically identified by RNAscope (B and D), whereas normal gastric epithelial cells adjacent to tumor cells (indicated by arrow heads), negative for CDX2, do not express CDX2 transcripts. Original magnification: A, B, C, D ×200.
(TIF)
Expanded population of LGR5 + cells in the intestinal metaplasia of the stomach (A) A few LGR5 + cells are located at the basal area of antral glands. (B) No LGR5 + cells are found in the body. (C) The LGR5 + cell population remarkably increases when IM occurs in gastric antrum. (D) Notably, LGR5 + cells also appear at the basal part of metaplastic glands that have developed in the body. Arrows indicate the fundic glands. LGR5 + cells are indicated by arrowheads. Magnifications: A, B, C, D, ×400.
(TIF)
Claudin-18 expression in non-tumorous gastric mucosa. (A) RT-PCR shows a differential expression of CLD18 in fresh-frozen gastric tissues. (n = 11) (B) CDX2 low group tends to express higher CLD18 than CDX2 high group although it is not statistically significant (p = 0.399).
(TIF)
LGR5 expressing cells in gastric tumors. (A) Tissue microarrays were constructed from gastric tumors. LGR5 expression in GAs (B) and EGCs (C) was classified according to the percentage of LGR5 + tumor cells as grades 0, 1, 2, and 3. Magnification: A, B, C, D, ×200.
(TIF)
Relationship between LGR5 expression and nuclear β-catenin Gastric adenomas with strong cytoplasmic and nuclear β-catenin expression tend to be positive for LGR5 (A), whereas adenomas with normal β-catenin levels show relatively low LGR5 positivity (B). Inlet pictures show a representative area at higher magnification. Magnifications: A, B, ×200.
(TIF)
Correlation between LGR5 positivity and mucinous type of gastric adenomas. Intestinal-type adenomas (A) have higher levels of LGR5 expression than gastric-type adenomas (B). CD10, CDX2 and MUC2 expression refers to the intestinal tumor gland phenotype and MUC5AC mucin expression represents the gastric gland phenotype. Magnifications: A, B, ×200.
(TIF)
Basal arrangement of LGR5 + cells in gastric tumors. LGR5 expressing tumor cells are often restricted at the basal part of tumor glands or at the interface between muscularis mucosa and submucosa in the gastric tumors including low grade adenoma (A), high grade adenoma (B), well differentiated adenocarcinoma (C), and moderately differentiated adenocarcinoma (D). (E) Around half of tumors showed basal distribution pattern of LGR5 + cells regardless of histological type. Magnification: A, B, D ×200; C ×100.
(TIF)
Spatial correlation of LGR5 expression with intestinal stem cell markers in a gastric adenoma with basally distributed LGR5 + cells. OLFM4, EPHB2, and ASCL2 expressions tend to gradually increase along the axis of tumor glands in a gastric adenoma in which LGR5 + cells are restricted at the base of tumor. Magnification: A, B, C, D, ×100.
(TIF)
Spatial relationship of LGR5 + cells with regard to Paneth cells. To analyze the association of LGR5 + cells with Paneth cells, we performed combined RNA ISH and IHC. LGR5 + cells are marked with brown dots and Paneth cells are indicated by α-defensin stain as red in the cytoplasm. (A, B) In the small intestine, LGR5 + cells are always adjacent to Paneth cells at the bas β e of crypts. (C, D) However, some LGR5 + cells that appear in the IM are not located near Paneth cells. (E, F) LGR5 + gastric adenoma cells tend to locate in the tumor regardless of adenoma Paneth cells. Boxed areas in figure A, C, and E are shown at higher magnification in figure B, D, and F respectively. LGR5 + cells are marked by arrow heads. Magnification: A, C, E ×100; B, D, F ×400.
(TIF)
Assessment of LGR5 expression in early gastric carcinomas.
(TIF)
LGR5 and nuclear β-catenin with the progression of gastric cancers.
(TIF)