Abstract
We tested whether microgravity affects mouse development during a period when gravity cues chick and frog embryo development. A rotating vessel developed ~0.1% simulated microgravity (MGS) for embryos. Microgravity simulation resulted in blocked cell accumulation in E2.5 embryos. E1.5 and E3.5 embryos showed lesser effects. For E1.5/2.5 embryos, cell accumulation block was followed by lethality at 48 hours after MGS. For E3.5 embryos, MGS blocked development without lethality but with apoptosis. E1.5–3.5 embryos from the rotational control developed lesser effects than MGS embryos. Embryonic stress-activated protein kinase (SAPK) was phosphorylated during MGS and mediated apoptosis. Increased pSAPK suggested that lethality is due to cellular stress induced by MGS, unlike the dysfunctional development after gravitational disorientation in frog and chick embryos. Thus, MGS causes lethality, a novel phenotype not often observed in microgravity or MGS. Embryonic lethality at E2.5 and apoptosis at E3.5 are associated with SAPK function, suggesting that MGS causes a general stress response that immediately affects many aspects of development. In addition, MGS and many aspects of In vitro fertilization/assisted reproductive technologies (IVF/ART) produce nonphysiological, nonevolutionary stresses that are mediated by SAPK, suggesting the primacy of this protein kinase in a wide range of mechanisms mediating negative reproductive outcomes in IVF/ART and potentially in spaceflight.
Keywords: Microgravity, preimplantation, signal transduction, stress activated protein kinase (SAPK/JNK), trophoblast
INTRODUCTION
Gravity is an essential cue for development of early embryos in frog1–4 and chick.5 There is no evidence that gravity is a cue for mammalian embryos, and mammalian fertilization appears normal under conditions of simulated microgravity.6 However, Kojima and colleagues found that early postfertilization development was slowed by microgravity simulation (MGS).
In spaceflight, frog and chick embryos compensate for the lost gravity cues that are essential for proper early postfertilization development on earth.7–9 In addition, sea urchins have nearly normal fertilization and postfertilization development in spaceflight.10–12 However, mammalian embryonic development at the gravity-sensitive period in chicks and frogs has not been studied in space flight. Postgastrulation rats at E8.5 are the earliest gestational age for mammals tested in space flight and developed normally.13 Typically, aspects of gravitational effects are studied in experimental models on earth using MGS before more costly models in space flight are used.
Microgravity simulation moves the specimen through orbits in a fluid-filled disk-shaped vessel in a clinostat or rotating wall vessel that creates low-shear, solid body movement of a fluid medium.14,15 When rotating about its center axis parallel to the ground, the specimen is placed inside liquid in the vessel and begins to rotate. The specimen is slightly heavier than liquid, hence it falls. It is then subject to forces from its weight and the pressure and shear from the liquid. A microgravity condition is generated when these three forces nearly balance and the specimen feels “weightless.”
In humans, 70% of embryos are lost before birth,16 and most of this lost occurs soon after implantation at 4.5 days after fertilization (E4.5). In the mouse, the first major peak period of lethality for single gene knockouts is just after implantation, but it is likely that the lethality is delayed by gene products stored in the egg and the crucial period of development is before implantation.17,18 Large new programs of messenger RNA (mRNA) synthesis are crucial from onset of zygotic transcription (2-cell-8-cell stage) and at the blastocyst stage.19,20 Cellular stress can have large effects in diverting energy from new transcription and translation.21–23 This makes the preimplantation period, exemplified by new global waves of zygotic macromolecular synthesis20 very sensitive to cellular stress.
The implanting blastocyst is a fluid-filled sphere of cells with an outer placental epithelium and an inner cell mass (ICM) of embryonic cells. Compaction is the event at the 8-cell stage (E2.0-E2.5), where the epithelium is first formed. Compaction is such a crucial event that a molecule responsible for epithelialization, ECadherin, is a rare preimplantation null lethal.24 Other key developmental events are the initiation of embryonic transcription at the 2-cell stage (E1.5) and forming the fluid-filled cavity (cavitation; E2.5–4.5), and hatching from the outer acellular protein shell, the zona pellucida (E4.5). It has become clear that patterning of the embryonic mesoderm and ectoderm that initiates at E6.7 at the start of mouse gastrulation25 is preceded by endoderm patterning initiating in the E3.5-E4.5 embryo.18,26–29 Thus, this time period may also be important in pregastrulation patterning in mammals, similar to the requirement for the endodermal Nieuwkoop center required to pattern Spemann’s organizer in frog. This patterning step occurring in the E3.5-E4.5 blastocyst would be a target of altered gravity.
Cellular stress induction has been observed in MGS and in space flight. Stress-induced protein kinase (SAPK)α/junC N-terminal kinase (JNK2) mRNA is induced 3.4-fold during space flight (SAPK/JNK will be referred to as SAPK here).30,31 Other stress-induced genes such as tumor necrosis factor (TNF) receptor were modulated, although a large number of shear stress and heat shock regulated genes were not induced. SAPK is a mediator of extracellular stressors such as hyperosmolarity and cytokines such as TNFα, as well as by DNA damage.32–37 SAPK phosphorylation at amino acid residues Thr183/Tyr185 is a marker for activation and is causal for stress-induced apoptosis and cell cycle arrest in somatic cells,38 and in embryos and their stem cells.39–42 SAPK1/2 isoforms are expressed throughout preimplantation development, in mouse and human placental cell lines and in early gestation human placenta,43 and increase in phosphorylated SAPK (pSAPK) is negatively proportional to preimplantation development rate.44
MATERIALS AND METHODS
Reagents
Potassium simplex optimized medium supplemented with amino acids (KSOMaa) was from Specialty Media (Phillipsburg, NJ). All media and embryo-tested mineral oil (Sigma) were equilibrated overnight at 37°C in 5% CO2 before embryo culture.
SAPK Antibodies and Inhibitors
The primary antibodies for SAPK Thr183/Tyr185 (CS9251) antibodies were from Cell signaling (Danvers, MA). These antibodies have previously been used in immunofluorescence and immunoblotting studies in mouse embryos.40,41,44
We used SAPK inhibitors as we have described previously.39,40,42 The specific inhibitors for SAPK, LJNKI1, the protease-resistant DJNKI1, and the penetration control TAT-FITC,45–47 were from Alexis (San Diego, CA). LJNKI1 and DJNKI1 are based on the peptide sequence of IB1/JIP1 that binds SAPK and leads to its inhibition in trophoblast stem cells and embryos.39,40,42,45 TAT is the delivery peptide derived from the HIV retro-virus. Thus, DJNKl1 is TAT-JIP1. DJNKl1 or TAT-FITC were preloaded for 2 hours and used at 1 μmol/L as previously described.42
DNTT dUTP Nick End Labeling (TUNEL) Assays
The embryos were washed, fixed, permeabilized, and the TUNEL assay (DeadEnd™ Fluorometric TUNEL System, Promega, Madison, WI, USA) was used as previously described.41 The fraction of TUNEL-positive cells was quantitated in embryos by visually inspecting them using the Z-axis control of an epifluorescent microscope (Leica DM IRE2, Germany). The criteria for assigning a positive status was the co-localization of TUNEL product around a single DAPI-stained nucleus, above the background level of TUNEL staining in normal, cultured embryos.
Collection of Mouse Embryos
Standard techniques were used for obtaining mouse embryos.48 Female MF-1 mice were superovulated and mated with C57BL/6J x SJL/J F1 males as previously described.40,41 Noon of the day following coitus was considered day E0.5. Embryos were obtained at the following stages: 2-cell stage (E1.5), 8-cell stage (E2.5), and morulaearly blastocyst (E3.5). The animal-use protocols were approved by the Wayne State University Animal Investigation Committee (AIC).
Embryo Culture and Evaluation
For each analysis, groups of 30 embryos were cultured at 37°C in an atmosphere of 5% CO2 in air for 24 to 48 hours 30 μL of media overlayed with mineral oil. Number of embryos developed to the 4-cell to 8-cell (compaction), morula, blastocyst, and hatched blastocyst stages were monitored under microscope (Leica DM IRE2, Germany). The criteria used for evaluating compaction, morula, blastocyst formation, and hatching were done as previously described.49
Indirect Immunocytochemistry and Nuclear Staining
For immunocytochemical analysis, the blastocysts were stained with rabbit polyclonal anti-phosphorylated SAPK/JNK Thr183/Tyr185 (Cell Signaling Technology, Inc, Beverly, MA) as previously described.40,41 The antibodies used in embryos yielded bands of the correct sizes with Western blot analysis.39–41,44 Nuclear counter-staining was done with Hoechst 33258 (10 μg/mL). Photomicrography was done with a Leica DM IRE2 epifluorescence microscope controlled electronically by Spot-Advanced software (Vysis, Downers Grove, IL). Photomicrographs were formatted using Adobe Photo-shop 6.0 (San Jose, CA). FITC intensity measurement and comparison was done with SimplePCI software (Compix Inc, Imaging Systems, Cranberry Township, PA).
Microgravity Simulation
To decrease the net gravitational force on preimplantation mouse embryos, we used a Synthecon (Houston, TX) rotating wall vessel apparatus (Rotary Cell Culture System, RCCS, with 10 mL high-aspect-ratio vessel (HARV) disposable vessels) designed by the National Aeronautics and Space Administration (NASA) for simulating microgravity for cultured cells.15,50 The detailed structure of this bioreactor has been described.51 Briefly, the HARV (Figure 1A) is a 9-cm diameter, 2-cm wide, hollow, plastic, disk-shaped vessel, perfused through a semipermeable membrane on one side of the disk, and with a clear plastic wall on the other side of the disk. Embryos placed in this apparatus without rotation progressed the same as in static controls (data not shown).
Figure 1.

Microgravity simulation (MGS) was performed using a rotating wall vessel in the vertical spin of the “Ferris wheel orientation” and the rotational control (RC) was performed with the same setup operating in horizontal spin of the “centrifuge” orientation. Volumes of oil and of microdrops of KSOMaa embryo media (arrows) were the same in MGS (A) and RC (B), and in static control (SC) as described in materials and methods. A 10-mL capacity HARV (flask) was used. When the axis of rotation of the simulator is parallel to the ground, the embryo continuously falls through the microdrop because it is heavier than the culture media. The embryo is then subject to forces from its weight and the pressure and shear from the liquid. A microgravity condition is generated when these 3 forces nearly balance and the specimen senses “weightlessness.”
An experiment consisted of MGS, rotational control (RC), and static culture (SC) control groups. For MGS, embryos were treated using the Synthecon RCCS1 as previously described41 except 30 μL microdroplets were used instead of the 60 μL microdroplets used before. The 30 μL volume creates less resistance and travels in a near-circular orbit with the oil in the filled 10 mL HARV. However, only 1 speed and 1 level of MGS can be created as there is 1 point of equilibrium between the denser aqueous microdroplet and lighter oil. Thus, instead of performing an MGS dose-response, we tested 3 stages of preimplantation embryo using a single level of MGS.
After turning the bioreactor on, angular force is applied from the rigid vessel wall to the oil and then the microdroplet containing the embryos and a low shear inertial mass equilibrium is set up within 10 minutes. The bioreactor was designed on the principle that the net force vector an object is exposed to at any point in the vessel’s rotation is a combination of force vectors created by the vessel’s rotation, the pressure and viscosity of the fluids, and the gravitational vector.14 If the speed of rotation and the size of microdroplets are adjusted correctly, the force vectors interact so that the microdroplets were in a synchronous, near circular orbit. There was also some rotation of the embryo-containing aqueous microdroplet around its center. This can be visually verified through the clear Teflon disk of the HARV. A circular shape to the orbit is indicative of a net force vector that has the same magnitude at all points in the rotation, but whose direction is constantly changing.52
Because we have verified that the shape of the orbit is approximately circular at 7.5 rotations per minute (RPM), the net force that the embryos “feel” can be approximated by the centripetal acceleration. The centripetal acceleration vector is directed toward the center of the bioreactor and is the primary force responsible for keeping the embryos in a circular orbit. The minimum and maximum centripetal accelerations, based on the minimum and maximum possible radius of the circular orbit, can be calculated. (v = velocity, a = acceleration, m = meters, s = second, T = period of 1 revolution of HARV, r = radius of orbit).
| Minimum Orbital Velocity = vmin | Maximum Orbital Velocity = vmax |
|
| |
| V = 2πr min/T | V = 2πr max/T |
| r min = 0.025 m | r max = 0.045 m |
| T = 8 s | T = 8s |
| v min = 0.0196 m/s | v max = 0.0353m/s |
|
| |
| r min and r max = the minimum and maximum radius of an orbit inside the HARV | |
|
| |
| Minimum centripetal acceleration = a min | Maximum centripetal acceleration = a max |
| a min = v min2/r min = a min = 0.01962/0.025 | a max = v max2/r max = a max = 0.03532/0.045 |
| a min = m/s2 = 0.00157g | a max = m/s2 = 0.00283 g |
So the simulated microgravity we got in this study is approximately 1.6 × 10−3g to 2.8 × 10−3g.
Because of a higher angular velocity of oil at the outer tangent of the microdroplet (near the outer rim of the HARV) compared with the inner tangent (nearer the center of the HARV) of the microdroplet, a counterclockwise rotation of the droplet imparts a small shear on the embryo less than an approximate 10−2 dynes/cm2 estimate. This is ~1%of the shear stress level for embryos generated in our previous experiments.41
Embryo Culture and Evaluation
We used 2 kinds of control groups: RC group and SC group. Rotational control is a motion control for turbulence, shear forces, and vibrations with a gravity vector at 1G. Static culture is a culture control for incubator/media conditions at 1g without motion. After 24 hours of culture or rotation, embryos were examined under microscope (Leica DM IRE2, Germany) for number of embryos developed to the 4-cell to 8-cell (compaction), morula, blastocyst, and hatched blastocyst stages. After the 24-hour stimulus period, embryos isolated on E1.5 and E2.5 were subjected to normal culture for another 24 to 48 hours and the developmental morphology was continuously monitored. Embryos isolated on E3.5 were subjected to indirect immunocytochemistry (ICC) with antiphosphorylated SAPK to determine the stress they had been exposed to during the past 24 hours of culture under different conditions on a molecular level. Quantitative immunofluorescence was performed by analyzing micrographs using the DNN module of Simple PCI software (Cranberry Township, PA) Embryos isolated at E3.5 were also cultured for another 24 hours under normal conditions and assayed for development using morphological criteria and cell number. SAPK Thr183/Tyr185 levels are similar when immunofluorescence or immunoblots are quantitated.41,44 The criteria used for evaluating compaction, morula, blastocyst formation, blastocyst size, and hatching were described previously.41,53
Statistical Analysis
The data in this study are representative of 3 independent biological experiments and indicated as mean ± SD. Statistical significance of differences between different treated samples were calculated by 1-way analysis of variance (ANOVA; SPSS 10.0).
RESULTS
To simulate microgravity, we used a rotating wall vessel (RWV) designed by NASA, which has been used previously to test for effects of microgravity stimulation (MGS) on development of the vestibular apparatus in Zebrafish (Danio rerio) embryos.51 At 7.5 RPM, we estimated a simulation of 1.6–2.8 × 10−3g on the embryos in a 30 μL microdrop that moved with the oil filling the HARV (Materials and Methods) when the rotation axis was horizontal and the rotation was in the “Ferris wheel” orientation (Figure 1A). In a previous study, we used this orientation to develop 1.2 dynes/cm2 shear stress where the microdrop was 60 μL and was stationary as the oil-filled RWV rotated and imparted a large rotating force to the microdrop and created arteriolar velocities on the embryos.41 However, in this study, because the smaller microdrop rotated with the oil in a solid body minimal shear and maximal MGS developed. As a control for rotational effects independent of MGS, the RWV was operated with a vertical axis of rotation in the “Centrifuge orientation” (rotational control = RC, Figure 1B). The RC control and MGS aqueous microdrops both have a very slow counterclockwise rotation due to higher velocity at the outer tangent of the drop compared with the inner tangent. This would lead to less that 10−2 dynes/cm2 shear force which is about 1% of the shear stress we previously reported41 (Materials and Methods). To control for the effects of movement of embryos in the MGS and RC groups, embryos were cultured under standard optimal static conditions in microdroplets under oil at approximately 1 embryo/μL (static control = SC).
Embryos were evaluated for embryo progression through cleavage division from the 8-cell stage. Then embryos were scored for the sequential developmental events; compaction at the 8-cell stage at 2.5 days after fertilization (E2.5), cavitation to form the blastocyst at E3.5, and expansion and hatching of the late blastocyst stage (E4.5).
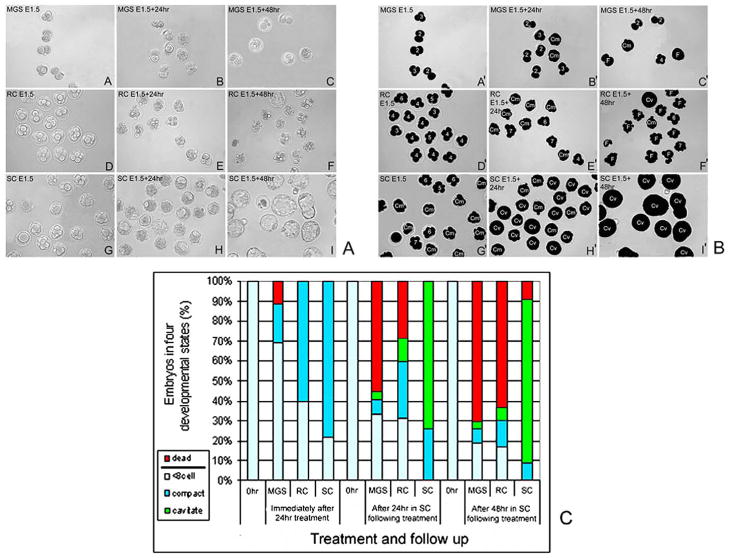
Embryos started 24 hours of treatment at the late 2-cell stage (E1.5). Immediately after 24 hours of treatment, 78.3% ± 6.5% of SC embryos had compacted, but 60.1% ± 5.5% of RC-treated embryos and only 28.7% ± 3.4% of MGS-treated embryos had compacted (Figure 2). Thus, the largest effects at the end of culture were on the MGS group. All treatment groups were cultured for an additional 24 hours and 48 hours to observe further accumulated effects of treatments. By 24 hours following treatment, MGS-treated embryos were less frequently compacted and cavitated (8.1% ± 2.7% and 4.9% ± 2.9%, respectively) compared with RC-treated embryos (28.9% ± 3.1% and 12.3% ± 5.2%, respectively), whereas the SC group had more cavitated embryos (84% ± 7.7%). Microgravity simulation–treated embryos were more frequently fragmented and dead (45.9% ± 3.7%) compared with RC-treated embryos (28.8% ± 1.6%). But, by 48 hours after treatment MGS- and RC-treated embryos had similar frequencies of dead and fragmented embryos (70.4% ± 7.3% vs 63.5% ± 10.7%, respectively).
Figure 2.
E1.5, 2-cell stage embryos are sensitive to microgravity simulation (MGS). Part A. One representative experiment where E1.5, 2-cell stage embryos are shown immediately after (A) MGS, after 24-hour recovery (B), and 48-hour recovery (C). Rotational control (RC)-treated embryos immediately after 24-hour treatment (D), after 24-hour static culture (E), or after 48-hour static culture (F). Embryos were shown immediately after static control (SC) (G), after an additional 24-hour (H), and after an additional 48-hour (I). Part B. Embryos in micrograph panels from part A were blackened to heighten contrast and then assigned numbers indicated cell number, or Cm to show compaction, Cv to show cavitation or F to show fragmentation prior to embryo death. A′–I′ correspond to A–I in part B. Part C. The histograms show 4 criteria for embryo development outlined in Figure 2. The data shown are from 3 experiments; significance measured by ANOVA, is discussed in the Results. For each experiment, in each group there were 30 embryos at Tzero.
Next we used 24 hours of treatment to test embryos at the compacted, 8-cell stage (E2.5). Immediately after 24 hours of treatment, 40.8% ± 4.1% of SC embryos had cavitated and rest were in the compacted-morula stage, but 30.2% ± 5.8% of RC-treated embryos and 90.1% ± 9.4% of MGS embryos had reversed development and decompacted (Figure 3, note that small groups of embryos (<10%) were late precompaction at the start of treatment). Thus the largest effects at the end of culture were on the MGS group as at E1.5, but these effects were more severe and development was reversed. All treatment groups were cultured for an additional 24 hours and 48 hours to observe further accumulated effects of treatments. By 24 hours following treatment, MGS-treated embryos had fewer compacted embryos (6.5% ± 5.2%) and fragmented and dead embryos had appeared (17.4% ± 6.0%), and more RC embryos had decompacted (38.1 ± 3.8) but no fragmented or dead embryos were observed. But, by 48 hours all embryos in both MGS and RC decompacted and this ultimately leads to embryo death. As with the E1.5 embryos, initial MGS treatment caused the highest immediate effects, but by 48 hours it was clear that all RC embryos had sustained sufficient cellular stress to cause the same fatal outcome as for all MGS embryos.
Figure 3.
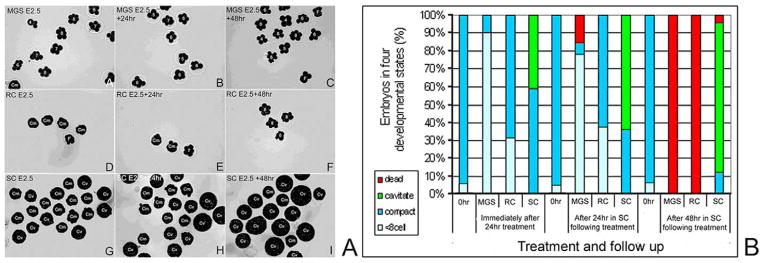
E2.5 embryos were immediately arrested in development and died between 24 and 48 hours after microgravity simulation (MGS). Part A. Embryos were blackened and assigned embryo evaluations as in Figure 2. Microgravity simulation–treated embryos immediately after 24 hours (A), 24 hours after MGS (B), and 48 hours after MGS (C). Rotational control (RC)-treated embryos immediately after treatment (D), 24 hours after RC (E), and 48 hours after RC (F). Static control (SC) treated embryos immediately after treatment (G), after and additional 24 hours of SC (H), and after an additional 48 hours of SC (I). Part B. The histograms show 4 criteria for embryo development from data gathered from 3 experiments, significance measured by ANOVA is discussed in the Results. For each experiment, in each group there were 30 embryos at Tzero.
Next, we treated E3.5 embryos for 24 hours and then cultured an additional 24 to 48 hours after treatment in SC. After 24 hours of treatment, SC embryos had twice the cross-sectional area (Figure 4B) and were maximally expanded. However RC-treated embryos only increased by approximately 33% in the 24 hours after treatment (although this was significant, ANOVA, P < .01), and many cavitated embryos had collapsed and appeared to be compacted embryos (Figure 4A). Microgravity simulation–treated embryos did not significantly increase in size by 24 hours after treatment (ANOVA, P = .7) and many had collapsed and appeared as compacted, not cavitated embryos (Figure 4A). The RC- and MGS-treated embryos expanded at a significantly decreased rate compared with SC embryos (ANOVA, P < .05). The fraction of embryos that progressed to expanded blastocyst stage 24 hours after treatment was significantly lower in RC-treated embryos (60 ± 7.3%, ANOVA, P < .01) (Figure 4C) and significantly lower still in MGS-treated (37.4 ± 6.4%, ANOVA, P < .05) embryos compared with the RC embryos (92.8 ± 4.2%). Significantly fewer MGS-treated and RC-treated embryos progressed to later blastocyst stage (ANOVA, P < .01). Death was not observed at significantly different frequencies in any treatment group, but cavitated embryos that collapse and do not recavitate are morbid (data not shown). We have previously found that embryo collapse occurs with experimental40 or culture stress,42 and if the embryo is healthy is reversed, but if the embryo is not healthy it does not reverse.
Figure 4.
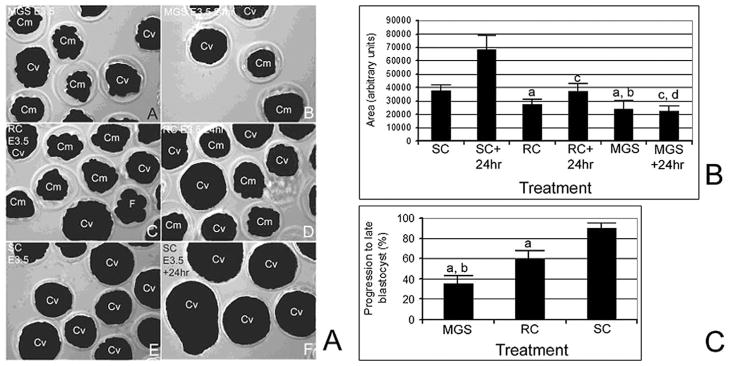
After 24 hours of microgravity simulation (MGS), E3.5 embryos were less developed than in rotational control (RC) or static control (SC) treatments, and this led to morbidity after MGS. Part A. E3.5 embryos immediately after MGS treatment (A), or after an additional 24 hours (B). Embryos immediately after 24 hours of RC treatment (C), or 24 hours later (D). Embryos immediately after 24 hours of SC (E) and 24 hours later (F). Part B. Histograms show quantitated cross-sectional area of micrographed embryos from 3 experiments. (a) Shows a significant difference of RC and MGS compared with SC (ANOVA, P < .01), (c) shows a significant difference for RC and MGS 24 hours after treatment compared with SC embryos (ANOVA, P < .001). (b) Shows no significant difference between RC and MGS immediately after treatment (ANOVA, P = .3), but (d) shows a significant decrease in embryo development for MGS compared with RC 24 hours after treatment (ANOVA, P < .01). Part C. Histograms show embryo development, reversal of development, and arrest in embryos from 3 experiments. Error bars are standard deviations and (a) shows that RC is significantly less than SC and (b) and (c) show that MGS is significantly less than SC and RC, respectively (ANOVA, P < .01). For each experiment, in each group there were 30 embryos at Tzero.
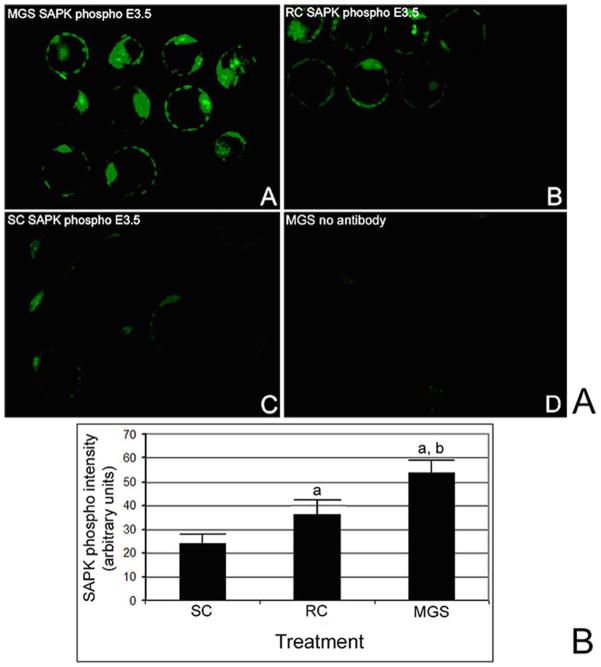
In previous studies of cellular stress, we found that SAPK phosphorylation at thr183/tyr185 was proportional to the amount of embryonic stress.40,41,44,54 SAPK phosphorylation at Thr183/Tyr185 was highest in MGS-treated embryos immediately after 24 hours of treatment (Figure 5B). SAPK phosphorylation was induced 2.2-fold in MGS treated embryos was significantly higher than those in RC treated embryos that were induced 1.5-fold over SC treated embryos. Both had significantly more SAPK phosphorylation than SC-treated embryos (ANOVA for both comparisons was P < .01; Figure 5C).
Figure 5.
Microgravity simulation (MGS)-perturbed E3.5 embryos express higher levels of phosphorylated SAPK than rotational control (RC) embryos which expresses a higher level than static control (SC). Part A. E3.5 embryos were subject to 24 hours of MGS (A), RC (B), or SC (C), fixed, stained and developed using immunocytochemical means for phosphorylated SAPK thr183/tyr185 (SAPK phospho). (D) Shows embryos after 24 hours of MGS, but with no antibody. Part B. Histogram is the immunofluorescence measurements of A–C for 3 experiments that used 95 embryos (error bars are standard deviations). (a) Shows that phosphorylated SAPK is significantly higher in embryos treated with RC or MGS than SC embryos, respectively (ANOVA, P < .01, and P < .005, respectively). (b) Shows that phosphorylated SAPK in embryos treated with MGS is significantly higher than in RC treated embryos (ANOVA, P < .01).
To test for the role of SAPK in the effects of MGS, we treated embryos for 24 hours of MGS with or without SAPK inhibitor and then assayed for cell number and apoptosis. SC embryos started at 32.4 ± 6.4 cells at time zero and were 84.7 ± 9.9 cells after 24 hours of culture (Figure 6A). However, MGS embryos had 37.2 ± 11.4 cells, significantly less than SC embryos (ANOVA, P < .001), and not significantly more than the cell number of embryos before treatment (ANOVA, P = .8). Embryos exposed to MGS but preloaded and cultured with 1uM DJNKl1 (as previously done)40–42 had significantly more cells (57.1 ± 7.8) than MGS alone, but significantly less cells than SC. For cells/embryos undergoing apoptosis, there were 40.5 ± 8.5 apoptotic cells for MGS-treated embryos, 7.4 ± 6.3 for SC treated embryos, and 17.7 ± 6.9 cells in RC treated embryos (Figure 6B). As with the cell number outcome, blocking SAPK function reduced cell death significantly during MGS treatment, but significantly more cells were TUNEL positive than in SC controls. Thus, SAPK inhibitors reversed the 56.2% cell accumulation decrease by 41.9% and reversed the 5.5fold increase in TUNEL by 71.9%.
Figure 6.
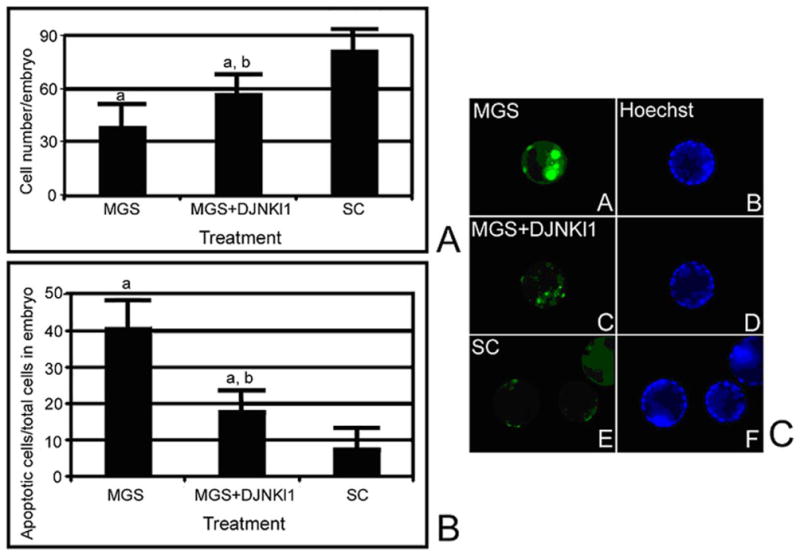
Inhibition of SAPK function significantly reverses decreased cell accumulation and increased apoptosis in E3.5 embryos after 24 hours of microgravity simulation (MGS) treatment. E3.5 embryos were treated with 24 hours of static control (SC) or MGS with or without 1 μmol/L SAPK inhibitor DJNKl1 and then analyzed for cell number (A) or TUNEL (B). In (A, B) error flags show standard deviations from 3 experiments that used 87 embryos. In (A), (a, b) show that embryos with MGS + DJNKl1 had significantly fewer cells than SC and more cells than embryos treated with MGS alone (ANOVA, P < .001), respectively. In (B), (a) show that embryos with MGS + DJNKl1 had significantly more TUNEL-positive cells than SC (ANOVA, P < .01), and (b) shows that MGS + DJNKl1 had significantly fewer TUNEL-positive cells than MGS alone (ANOVA, P < .001).
DISCUSSION
In the studies reported here, 24hr of MGS at 10−3g causes severe pathogenic outcomes for embryos at the late 2-cell, 8-cell stage, and blastocyst stage, and the most severe effect occurs at the 8-cell stage. As suggested from previous work, shear stress in the RC also causes pathogenic effects, although these were less severe and/or slower than MGS effects at all 3 embryonic stages tested. As for previous cellular stressors such as IVF media, shear stress, hyperosmolar sorbitol, and benzo(a)pyrene cigarette tar,40,41,44,54 pSAPK was induced and mediated increased apoptosis and decreased cell accumulation. Because SAPK(/JNK2 mRNA is the predominant mRNA in human and mouse periimplantation trophoblast stem cells, placenta, and or embryos and these mRNA levels increase during spaceflight,30,31 anticipated SAPK activation in embryos during spaceflight would be deleterious.
This research was aimed at testing for MGS effects on mammalian development during a period when perturbation of a gravity axis perturbs frog and chick development by causing sublethal repositioning of gravity-affected cues or mechanisms.1–4 Altered cues ramified into later developmental lethality by dysmorphogenesis, but not the immediate cessation of cell number increase and later death reported here. Thus, MGS causes a more rapid, severe, pathogenic response in mouse embryos, not the slower developmental effects observed in frog and chick embryogenesis.
A previous study reported that MGS during fertilization had no effect on later mouse development.6 But, this study reported that MGS deranged early cleavage divisions and a substantial number of embryos were retarded from fertilization to the morula stage. The phenotype reported here was much more severe than in the previous report. In the 2 periods traversed by embryos in the previous report and this one, 2-cell and 8-cell/morula stages, there was a severe growth arrest, failure to compact, decompaction, and ultimate death in most embryos observed here, but not in the previous report. This could be due to several reasons, difference in mouse strain, media, and magnitude of MGS are possible differences.
In a separate study focused on the effects of shear stress on the preimplantation embryo,41 the droplet size was increased until it fell through the moving oil at a velocity equal to the rotation of the oil. Thus the aqueous drop was stationary and this maximized shear stress at 1.2 dynes/cm2 and minimized MGS to near zero.41 This level of shear stress was over 100-fold greater than the shear stress in RC here, so we anticipate that RC should have minimal shear and effects due to shear. The MGS here causes rapid cessation of cell accumulation at E1.5 and E2.5 immediately after stimulation but death did not occur until 24 to 48 hours after stimulation. Similarly for E3.5 embryos, MGS caused attenuation of embryo development, but decreases in embryo development and attenuation in cell accumulation became apparent in the 24 hours after stimulus. In contrast with the previous study on shear stress, there was little embryo death here whereas there was a rapid death that became irreversible for E3.5 embryos by 12 hours of rotation and all embryos were dead by the end of a 24 hours of shear stress.41 This suggests that high shear stress can be potently lethal, with a more rapid lethality than 1 mol/L high-dose hyperosmolar stress, and 1/100th shear stress here contributes to the RC-triggered lethality and partially to the MGS-triggered lethality. These data together suggest that earlier embryos, known to be more susceptible to stressful perturbations, die due to a failure to maintain cellular homeostasis, due to MGS with a lesser component of shear stress, rather than suffer a developmental misprogramming.
This contention is supported by the increase in pSAPK, a reaction at this stage of development to stress triggered by MGS, and to a lesser extent rotation without MGS. The nature of the mechanism of SAPK induction is unclear. But, we have found that pSAPK is induced by poor media such as M16 and Ham’s F-10 + bovine serum albumin (BSA) with preimplantation development from 2-cell to blastocyst-stage negatively affected proportional to pSAPK levels.44 It is unlikely that SAPK is part of any essential normal preimplantation development as recently reported for p38 mitogen-activated protein kinase (p38MAPK).55 This is suggested by data showing that SAPK inhibitors have no effect on development of embryos cultured from the 2-cell to blastocyst-stage in optimal media ([KSOMaa]42), but embryos cultured in KSOMAaa with p38MAPK inhibitors are blocked at the 8-cell stage.55 Because the MGS experiments were performed with optimal KSOMaa that induces little SAPK phosphorylation, SAPK was induced by some aspect of MGS. Because pSAPK increase is not a required developmental event, it is most likely a cellular stress response. Cellular stress responses have been reported for cells in MGS and in space flight at the level of SAPK phosphorylation and the induction of SAPKα/JNK2.30,31,56
There are a number of candidate processes, aside from pSAPK, and mechanisms that have been identified that may lead to cellular stress and death in MGS and space-flight. Changes in cell cycle commitment, TUNEL/apoptosis, growth factor sensitivity, signal transduction, and cytoskeleton integrity are observed in MGS and spaceflight.57–64 Phosphorylated SAPK elevation can be involved with all of these mechanisms, and we have also observed some actin cytoskeleton derangement (unpublished data). A kinase related to SAPK, p38MAPK regulates actin polymerization at the 8-cell stage and inhibiting p38MAPK blocks embryo progression.55 P38MAPK is induced during stressful embryo culture and by hyperosmolar stress.65,66 It has not been established how mammalian cells sense gravity, or MGS perturbation, but structural (cytoskeletal) components such as actin normally sensing dense cellular bodies are candidates,67 as these have already been established in the columella cells in the plant root tip that are geotropic (and sense gravity).68
CONCLUSIONS
(1) MGS is most lethal at 8-cell, less at 2-cell (but still lethal), and without lethality at E3.5 although apoptosis is induced. (2) Cell cycle arrest occurs without lethality during 24 hours of rotation during MGS. Cell cycle arrest is more profound in MGS compared with RC when perturbation starts at the 2-cell stage. Decompaction, a reversal of development, is more severe for 8-cell embryos for MGS than for RC, after 24 hours of MGS. But, RC embryos finally also decompact and die between 24 hours and 48 hours after treatment. (3) The SAPK is proportional to lethality and is probably involved. Also, elevated pSAPK suggests that this lethality is not a developmental response at the embryonic or cell lineage level, but part of a general homeostatic effect in response to stress at the cellular level. (4) Lesser RC lethality is probably due to a low amount of shear stress that was at levels about 1% of those previously reported.41
Future studies will include the study of TUNEL/apoptosis as a mediator of death and the causal role of SAPK in the induction of TUNEL/apoptosis. An alternate apparatus or experiments during spaceflight are important to establish dose-dependent effects and validate MGS findings. We have established that SAPK is causal in shear stress-mediated lethality,41 and mediates hyperosmolarity-induced lethality and cell cycle arrest.39 However, alternate mechanisms, such as cytoskeletal disruption, are likely to contribute to pathogenesis. Cytoskeleton disruptors that lead to chronic SAPK activation for 12 hours in endothelial cells are sufficient to induce apoptosis,69 suggesting an interesting exacerbation by 2 stress inducers, MGS and shear stress, operating via SAPK and cytoskeletal disruption.
In addition, it is important to understand how MGS and other stressors affect other segments of preimplantation development. Essential processes in the zygote such as genome activation are likely to be affected by MGS and its associated stress. Implantation itself may be influenced by cues mediated by gravity and affected by stress responses generated by the alteration of gravity.
Finally, it is clear that SAPK is activated by many physiological stimuli of embryos and their constituent trophoblast stem cells.54 However, a growing list of non-physiological stimuli also activate SAPK and its dependent negative effects. These include embryo culture media used in IVF, pipetting during handling, shear stress, dioxin, benzo(a)pyrene,41,44,54,70 and now MGS. SAPK may have been selected during evolution to mediate stress responses to physiological stressors, but its common response during nonevolutionary, nonphysiological stimuli suggest that it will be important to analyze and perhaps manage during any protocol in IVF/ART.
Acknowledgments
We thank Mike Kruger for advice on statistical analysis. We also thank Dr Josh Zimmerberg of NICHHD for the use of two additional RCCS1 microgravity simulators and additional HARVS. We are also indebted to Dr Richard Tasca, Dr Horacio Croxatto, and Dr Anne McLaren for helpful discussion and criticisms of the manuscript. This research was supported by grants from NASA (NRA-NAG2-1503) and the National Institute of Child Health and Human Development, NIH, (R01-HD40972).
Footnotes
For reprints and permissions queries, please visit SAGE’s Web site at http://www.sagepub.com/journalsPermissions.nav
References
- 1.Gerhart JC, Vincent JP, Scharf SR, Black SD, Gimlich RL, Danilchik M. Localization and induction in early development of Xenopus. Philos Trans R Soc Lond B Biol Sci. 1984;307(1132):319–330. doi: 10.1098/rstb.1984.0134. [DOI] [PubMed] [Google Scholar]
- 2.Vincent JP, Gerhart JC. Subcortical rotation in Xenopus eggs: an early step in embryonic axis specification. Dev Biol. 1987;123(2):526–539. doi: 10.1016/0012-1606(87)90411-8. [DOI] [PubMed] [Google Scholar]
- 3.Pitts GC. Effects of gravity on ontogeny in animals. Life Sci Space Res. 1973;11:171–176. [PubMed] [Google Scholar]
- 4.Cooke J. Permanent distortion of positional system of Xenopus embryo by brief early perturbation in gravity. Nature. 1986;319(6048):60–63. doi: 10.1038/319060a0. [DOI] [PubMed] [Google Scholar]
- 5.Kochav S, Eyal-Giladi H. Bilateral symmetry in chick embryo determination by gravity. Science. 1971;171(975):1027–1029. doi: 10.1126/science.171.3975.1027. [DOI] [PubMed] [Google Scholar]
- 6.Kojima Y, Sasaki S, Kubota Y, Ikeuchi T, Hayashi Y, Kohri K. Effects of simulated microgravity on mammalian fertilization and preimplantation embryonic development in vitro. Fertil Steril. 2000;74(6):1142–1147. doi: 10.1016/s0015-0282(00)01583-1. [DOI] [PubMed] [Google Scholar]
- 7.Young RS, Tremor JW. Weightlessness and the developing frog egg. Life Sci Space Res. 1968;6:87–93. [PubMed] [Google Scholar]
- 8.Ubbels GA, Berendsen W, Narraway J. Fertilization of frog eggs on a Sounding Rocket in space. Adv Space Res. 1989;9(11):187–197. doi: 10.1016/0273-1177(89)90073-2. [DOI] [PubMed] [Google Scholar]
- 9.Suda T. Lessons from the space experiment SL-J/FMPT/L7: the effect of microgravity on chicken embryogenesis and bone formation. Bone. 1998;22(5 suppl):73S–78S. doi: 10.1016/s8756-3282(98)00021-0. [DOI] [PubMed] [Google Scholar]
- 10.Schatten H, Chakrabarti A, Taylor M, et al. Effects of space-flight conditions on fertilization and embryogenesis in the sea urchin Lytechinus pictus. Cell Biol Int. 1999;23(6):407–415. doi: 10.1006/cbir.1999.0371. [DOI] [PubMed] [Google Scholar]
- 11.Marthy HJ, Schatt P, Santella L. Fertilization of sea urchin eggs in space and subsequent development under normal conditions. Adv Space Res. 1994;14(8):197–208. doi: 10.1016/0273-1177(94)90404-9. [DOI] [PubMed] [Google Scholar]
- 12.Steffen S, Fiser R, Simerly C, Schatten H, Schatten G. Microgravity effects on sea urchin fertilization and development. Adv Space Res. 1992;12(1):167–173. doi: 10.1016/0273-1177(92)90280-b. [DOI] [PubMed] [Google Scholar]
- 13.Ronca AE, Alberts JR. Effects of prenatal spaceflight on vestibular responses in neonatal rats. J Appl Physiol. 2000;89(6):2318–2324. doi: 10.1152/jappl.2000.89.6.2318. [DOI] [PubMed] [Google Scholar]
- 14.Kessler JO. Theory and experimental results on gravitational effects on monocellular algae. Adv Space Res. 1992;12(1):33–42. doi: 10.1016/0273-1177(92)90261-u. [DOI] [PubMed] [Google Scholar]
- 15.Klaus DM. Clinostats and bioreactors. Gravit Space Biol Bull. 2001;14(2):55–64. [PubMed] [Google Scholar]
- 16.Cross JC, Werb Z, Fisher SJ. Implantation and the placenta: key pieces of the development puzzle. Science. 1994;266(5190):1508–1518. doi: 10.1126/science.7985020. [DOI] [PubMed] [Google Scholar]
- 17.Copp AJ. Death before birth: clues from gene knockouts and mutations. Trends Genet. 1995;11(3):87–93. doi: 10.1016/S0168-9525(00)89008-3. [DOI] [PubMed] [Google Scholar]
- 18.Rappolee DA. It’s not just baby’s babble/Babel: recent progress in understanding the language of early mammalian development: a minireview. Mol Reprod Dev. 1999;52(2):234–240. doi: 10.1002/(SICI)1098-2795(199902)52:2<234::AID-MRD15>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 19.Hamatani T, Carter MG, Sharov AA, Ko MS. Dynamics of global gene expression changes during mouse preimplantation development. Dev Cell. 2004;6(1):117–131. doi: 10.1016/s1534-5807(03)00373-3. [DOI] [PubMed] [Google Scholar]
- 20.Wang QT, Piotrowska K, Ciemerych MA, et al. A genome-wide study of gene activity reveals developmental signaling pathways in the preimplantation mouse embryo. Dev Cell. 2004;6(1):133–144. doi: 10.1016/s1534-5807(03)00404-0. [DOI] [PubMed] [Google Scholar]
- 21.Hardie DG. Minireview: the AMP-activated protein kinase cascade: the key sensor of cellular energy status. Endocrinology. 2003;144(12):5179–5183. doi: 10.1210/en.2003-0982. [DOI] [PubMed] [Google Scholar]
- 22.Liu L, Cash TP, Jones RG, Keith B, Thompson CB, Simon MC. Hypoxia-induced energy stress regulates mRNA translation and cell growth. Mol Cell. 2006;21(4):521–531. doi: 10.1016/j.molcel.2006.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weston CR, Davis RJ. The JNK signal transduction pathway. Curr Opin Genet Dev. 2002;12(1):14–21. doi: 10.1016/s0959-437x(01)00258-1. [DOI] [PubMed] [Google Scholar]
- 24.Larue L, Ohsugi M, Hirchenhain J, Kemler R. E-cadherin null mutant embryos fail to form a trophectoderm epithelium. Proc Natl Acad Sci U S A. 1994;91(17):8263–8267. doi: 10.1073/pnas.91.17.8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lawson KA, Meneses JJ, Pedersen RA. Clonal analysis of epiblast fate during germ layer formation in the mouse embryo. Development. 1991;113(3):891–911. doi: 10.1242/dev.113.3.891. [DOI] [PubMed] [Google Scholar]
- 26.Rossant J. Lineage development and polar asymmetries in the peri-implantation mouse blastocyst. Semin Cell Dev Biol. 2004;15(5):573–581. doi: 10.1016/j.semcdb.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 27.Lu CC, Brennan J, Robertson EJ. From fertilization to gastrulation: axis formation in the mouse embryo. Curr Opin Genet Dev. 2001;11(4):384–392. doi: 10.1016/s0959-437x(00)00208-2. [DOI] [PubMed] [Google Scholar]
- 28.Thomas PQ, Brown A, Beddington RS. Hex: a homeobox gene revealing peri-implantation asymmetry in the mouse embryo and an early transient marker of endothelial cell precursors. Development. 1998;125(1):85–94. doi: 10.1242/dev.125.1.85. [DOI] [PubMed] [Google Scholar]
- 29.Labelle-Dumais C, Jacob-Wagner M, Pare JF, Belanger L, Dufort D. Nuclear receptor NR5A2 is required for proper primitive streak morphogenesis. Dev Dyn. 2006;235(12):3359–3369. doi: 10.1002/dvdy.20996. [DOI] [PubMed] [Google Scholar]
- 30.Hammond TG, Benes E, O’Reilly KC, et al. Mechanical culture conditions effect gene expression: gravity-induced changes on the space shuttle. Physiol Genomics. 2000;3(3):163–173. doi: 10.1152/physiolgenomics.2000.3.3.163. [DOI] [PubMed] [Google Scholar]
- 31.Hammond TG, Lewis FC, Goodwin TJ, et al. Gene expression in space. Nat Med. 1999;5(4):359. doi: 10.1038/7331. [DOI] [PubMed] [Google Scholar]
- 32.Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103(2):239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 33.Whitmarsh AJ, Davis RJ. Signal transduction by MAP kinases: regulation by phosphorylation-dependent switches. Sci STKE. 1999;1999(1):PE1. doi: 10.1126/stke.1999.1.pe1. [DOI] [PubMed] [Google Scholar]
- 34.Whitmarsh AJ, Davis RJ. Regulation of transcription factor function by phosphorylation. Cell Mol Life Sci. 2000;57(8–9):1172–1183. doi: 10.1007/PL00000757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kyriakis JM, Avruch J. Sounding the alarm: protein kinase cascades activated by stress and inflammation. J Biol Chem. 1996;271(40):24313–24316. doi: 10.1074/jbc.271.40.24313. [DOI] [PubMed] [Google Scholar]
- 36.Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001;81(2):807–869. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- 37.Rappolee DA. Signal transduction. In: Krawetz S, Womble D, editors. Introduction to Bioinformatics. A Theoretical and Practical Approach. 2. Totowa, NJ: Humana Press; 2003. pp. 55–71. [Google Scholar]
- 38.Woodgett JR, Avruch J, Kyriakis J. The stress activated protein kinase pathway. Cancer Surv. 1996;27:127–138. [PubMed] [Google Scholar]
- 39.Zhong W, Xie Y, Wang Y, et al. Use of hyperosmolar stress to measure stress-activated protein kinase activation and function in human HTR cells and mouse trophoblast stem cells. Reprod Sci. 2007;14(6):534–547. doi: 10.1177/1933719107307182. [DOI] [PubMed] [Google Scholar]
- 40.Xie Y, Zhong W, Wang Y, et al. Using hyperosmolar stress to measure biologic and stress-activated protein kinase responses in preimplantation embryos. Mol Hum Reprod. 2007;13(7):473–481. doi: 10.1093/molehr/gam027. [DOI] [PubMed] [Google Scholar]
- 41.Xie Y, Wang F, Zhong W, Puscheck E, Shen H, Rappolee DA. Shear stress induces preimplantation embryo death that is delayed by the zona pellucida and associated with stress-activated protein kinase-mediated apoptosis. Biol Reprod. 2006;75(1):45–55. doi: 10.1095/biolreprod.105.049791. [DOI] [PubMed] [Google Scholar]
- 42.Xie Y, Puscheck EE, Rappolee DA. Effects of SAPK/JNK inhibitors on preimplantation mouse embryo development are influenced greatly by the amount of stress induced by the media. Mol Hum Reprod. 2006;12(4):217–224. doi: 10.1093/molehr/gal021. [DOI] [PubMed] [Google Scholar]
- 43.Zhong W, Sun T, Wang Q, et al. SAPK/JNK1, 2, but not SAPK/JNK3 mRNA transcripts, are expressed in early gestation human placenta and mouse eggs, preimplantation embryos, and trophoblast stem cells. Fertility and Sterility. 2004;82:1140–1148. doi: 10.1016/j.fertnstert.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 44.Wang Y, Puscheck EE, Wygle DL, et al. Increases in phosphorylation of SAPK/JNK and p38MAPK correlate negatively with mouse embryo development after culture in different media. Fertility and Sterility. 2005;83:1144–1154. doi: 10.1016/j.fertnstert.2004.08.038. [DOI] [PubMed] [Google Scholar]
- 45.Bonny C, Oberson A, Negri S, Sauser C, Schorderet DF. Cell-permeable peptide inhibitors of JNK: novel blockers of beta-cell death. Diabetes. 2001;50(1):77–82. doi: 10.2337/diabetes.50.1.77. [DOI] [PubMed] [Google Scholar]
- 46.Dickens M, Rogers JS, Cavanagh J, et al. A cytoplasmic inhibitor of the JNK signal transduction pathway. Science. 1997;277(5326):693–696. doi: 10.1126/science.277.5326.693. [DOI] [PubMed] [Google Scholar]
- 47.Bonny C, Nicod P, Waeber G. IB1, a JIP-1-related nuclear protein present in insulin-secreting cells. J Biol Chem. 1998;273(4):1843–1846. doi: 10.1074/jbc.273.4.1843. [DOI] [PubMed] [Google Scholar]
- 48.Hogan B, Beddington R, Constantini F, Lacy B. Manipulating the mouse embryo, A laboratory manual. 3. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory; 2002. [Google Scholar]
- 49.Nagy A, Gertsenstein M, Vintersten K, Behringer RR. Manipulating the mouse embryo. A laboratory manual. 3. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; 2003. [Google Scholar]
- 50.Klaus DM, Todd P, Schatz A. Functional weightlessness during clinorotation of cell suspensions. Adv Space Res. 1998;21(8–9):1315–1318. doi: 10.1016/s0273-1177(97)00404-3. [DOI] [PubMed] [Google Scholar]
- 51.Moorman SJ, Burress C, Cordova R, Slater J. Stimulus dependence of the development of the zebrafish (Danio rerio) vestibular system. J Neurobiol. 1999;38(2):247–258. [PubMed] [Google Scholar]
- 52.Halliday Ra. Fundamentals of Physics. 2. Vol. 1. New York City, NY: Wiley and Sons; 1977. [Google Scholar]
- 53.Chai N, Patel Y, Jacobson K, McMahon J, McMahon A, Rappolee DA. FGF is an essential regulator of the fifth cell division in preimplantation mouse embryos. Dev Biol. 1998;198(1):105–115. doi: 10.1006/dbio.1997.8858. [DOI] [PubMed] [Google Scholar]
- 54.Xie Y, Liu J, Proteasa S, et al. Transient stress and stress enzyme responses have practical impacts on parameters of embryo development, from IVF to directed differentiation of stem cells. Mol Reprod Dev. 2008;75(4):689–697. doi: 10.1002/mrd.20787. [DOI] [PubMed] [Google Scholar]
- 55.Natale DR, Paliga AJ, Beier F, D’Souza SJ, Watson AJ. p38 MAPK signaling during murine preimplantation development. Dev Biol. 2004;268(1):76–88. doi: 10.1016/j.ydbio.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 56.Hughes-Fulford M. The role of signaling pathways in osteoblast gravity perception. J Gravit Physiol. 2002;9(1):257–260. [PubMed] [Google Scholar]
- 57.de Groot RP, Rijken PJ, den Hertog J, et al. Nuclear responses to protein kinase C signal transduction are sensitive to gravity changes. Exp Cell Res. 1991;197(1):87–90. doi: 10.1016/0014-4827(91)90483-b. [DOI] [PubMed] [Google Scholar]
- 58.d’Ascanio P, Balaban E, Pompeiano M, Centini C, Pompeiano O. Fos and FRA protein expression in rat precerebellar structures during the Neurolab Space Mission. Brain Res Bull. 2003;62(3):203–221. doi: 10.1016/j.brainresbull.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 59.Boonstra J. Growth factor-induced signal transduction in adherent mammalian cells is sensitive to gravity. Faseb J. 1999;13(suppl):S35–S42. doi: 10.1096/fasebj.13.9001.s35. [DOI] [PubMed] [Google Scholar]
- 60.Hughes-Fulford M, Tjandrawinata R, Fitzgerald J, Gasuad K, Gilbertson V. Effects of microgravity on osteoblast growth. Gravit Space Biol Bull. 1998;11(2):51–60. [PubMed] [Google Scholar]
- 61.Komazaki S. Gravitational effects on apoptosis of presumptive ectodermal cells of amphibian embryo. J Exp Zoolog Part A Comp Exp Biol. 2004;301(3):204–211. doi: 10.1002/jez.a.20025. [DOI] [PubMed] [Google Scholar]
- 62.Lewis ML, Reynolds JL, Cubano LA, Hatton JP, Lawless BD, Piepmeier EH. Spaceflight alters microtubules and increases apoptosis in human lymphocytes (Jurkat) Faseb J. 1998;12(11):1007–1018. doi: 10.1096/fasebj.12.11.1007. [DOI] [PubMed] [Google Scholar]
- 63.Ontiveros C, McCabe LR. Simulated microgravity suppresses osteoblast phenotype, Runx2 levels and AP-1 transactivation. J Cell Biochem. 2003;88(3):427–437. doi: 10.1002/jcb.10410. [DOI] [PubMed] [Google Scholar]
- 64.Sato A, Hamazaki T, Oomura T, et al. Effects of microgravity on c-fos gene expression in osteoblast-like MC3T3-E1 cells. Adv Space Res. 1999;24(6):807–813. doi: 10.1016/s0273-1177(99)00076-9. [DOI] [PubMed] [Google Scholar]
- 65.Fong B, Watson PH, Watson AJ. Mouse preimplantation embryo responses to culture medium osmolarity include increased expression of CCM2 and p38 MAPK activation. BMC Dev Biol. 2007;7:2. doi: 10.1186/1471-213X-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang Y, Puscheck EE, Lewis JJ, Trostinskaia AB, Wang F, Rappolee DA. Increases in phosphorylation of SAPK/JNK and p38MAPK correlate negatively with mouse embryo development after culture in different media. Fertil Steril. 2005;83(suppl 1):1144–1154. doi: 10.1016/j.fertnstert.2004.08.038. [DOI] [PubMed] [Google Scholar]
- 67.Todd P, Klaus DM, Stodieck LS, et al. Cellular responses to gravity: extracellular, intracellular and in-between. Adv Space Res. 1998;21(8–9):1263–1268. doi: 10.1016/s0273-1177(97)00397-9. [DOI] [PubMed] [Google Scholar]
- 68.Staehelin LA, Zheng HQ, Yoder TL, Smith JD, Todd P. Columella cells revisited: novel structures, novel properties, and a novel gravisensing model. Gravit Space Biol Bull. 2000;13(2):95–100. [PubMed] [Google Scholar]
- 69.Hu YL, Li S, Shyy JY, Chien S. Sustained JNK activation induces endothelial apoptosis: studies with colchicine and shear stress. Am J Physiol. 1999;277(4 pt 2):H1593–H1599. doi: 10.1152/ajpheart.1999.277.4.H1593. [DOI] [PubMed] [Google Scholar]
- 70.Xie Y, Wang F, Puscheck EE, Rappolee DA. Pipetting causes shear stress and elevation of phosphorylated stress-activated protein kinase/jun kinase in preimplantation embryos. Mol Reprod Dev. 2007;74(10):1287–1294. doi: 10.1002/mrd.20563. [DOI] [PubMed] [Google Scholar]