Abstract
Background
Fatty acid (FA)-alterations may mediate the mutual association between Major Depressive Disorder (MDD) and cardiovascular disease (CVD). However, etiology of observed FA-alterations in MDD and CVD remains largely unclear. An interesting candidate may be a mutation in the fatty acid–binding protein 2 (FABP2)-gene, because it regulates dietary FA-uptake. Therefore, we aimed to test the hypotheses that in MDD-patients the FABP2 Ala54Thr-polymorphism would be (I) more prevalent than in sex- and age-matched controls, (II) associated with observed alterations in FA-metabolism, and (III) associated with CVD-risk factor waist circumference.
Methods
We measured concentrations of 29 different erythrocyte FAs, FABP2-genotype, and waist circumference in recurrent MDD-patients and matched never-depressed controls.
Results
FABP2-genotype distribution did not significantly differ between the 137 MDD-patients and 73 matched controls. However, patients with the Ala54Thr-polymorphism had (I) higher concentrations of especially eicosadienoic acid (C20:2ω6; P=.009) and other 20-carbon FAs, and associated (II) lower waist circumference (P=.019). In addition, FABP2-genotype effects on waist circumference in patients seemed (I) mediated by its effect on C20:2ω6, and (II) different from controls.
Conclusions
Although Ala54Thr-polymorphism distribution was not associated with recurrent MDD, our results indicate that FABP2 may play a role in the explanation of observed FA-alterations in MDD. For Ala54Thr-polymorphism patients, potentially adaptive conversion of increased bioavailable dietary precursors into eicosadienoic acid instead of arachidonic acid might be related to a low waist circumference. Because this is the first investigation of these associations, replication is warranted, preferably by nutrigenetic studies applying lipidomics and detailed dietary assessment.
Introduction
Major depressive disorder (MDD) - in particular its recurrent form (MDD-R) - is a major cause of disability and excess mortality worldwide [1]. The leading cause of this excess mortality in MDD is cardiovascular disease (CVD) [2]. Accordingly, the primary CVD-risk factor waist circumference - reflecting abdominal obesity and insulin resistance - is strongly associated with MDD [3–7]. Improved understanding of mechanisms underlying the MDD-CVD relationship might lead to novel life-prolonging (preventive) treatment strategies.
An important mechanism underlying the MDD-CVD relationship may be fatty acid (FA)-metabolism, because it regulates e.g. inflammation, thrombosis and neurotransmitter-signaling [8–10]. FA-composition of cell membranes is altered in both MDD and CVD, with decreased ω3 polyunsaturated fatty acids (PUFAs) and increased ω6/ω3 PUFA ratios, e.g. increased arachidonic acid (C20:4ω6; ARA) relative to eicosapentaenoic acid (C20:5ω3; EPA) [8,11–13]. We corroborated these findings in a sample of 137 MDD-R patients, and extended them by showing additional changes in other FA-classes, e.g. lower overall FA-unsaturation, -chain length and -peroxidation [14,15]. However, etiology of FA-alterations in MDD and CVD remains largely unclear.
FA-metabolism is influenced by many different factors, including genotype [16], dietary intake (e.g. essential ω3 FAs from fatty fish), lifestyle (physical activity, smoking), and hormonal regulation [14,17]. Interestingly, we previously reported that FA-alterations in MDD-R follow a bimodal distribution [18]. Instead of being unimodally distributed, with regard to FA-alterations, MDD-R-patients seemed to consist of two separate groups reflected by two unimodal distributions, a phenomenon also observed in schizophrenia [19]. This implies a dichotomous causal factor that divides patients in two groups and thereby underlies these bimodally distributed FA-alterations [19]. A possible example of such a dichotomous causal factor may be a mutation in a gene involved in FA-metabolism.
An interesting location for such a mutation may be the fatty acid–binding protein 2 (FABP2) gene. FABP2 is mainly expressed in small intestine enterocytes, where it is responsible for uptake of dietary FAs. A transition G to A at FABP2-codon 54 results in an alanine (Ala) to threonine (Thr) amino acid substitution (Ala54 to Thr54) [20]. This single nucleotide polymorphism is common, with a Thr54 allelic frequency of 30% in most populations, resulting in altered FABP2 FA-affinity. Homozygous Thr54-carriers show altered dietary FA-uptake, with increased postprandial concentrations of 14–18-carbon fatty acids [21]. Because of the (patho)physiological role of FAs in metabolism, this altered FA-uptake has been suggested to explain the association of FABP2 with increased insulin resistance and FA-oxidation, supporting observations suggesting a role of the FABP2 Ala54Thr-polymorphism in CVD-etiology (e.g. increased waist circumference and atherosclerosis) [21–25].
Considering this involvement of FABP2 in FA-metabolism, the FABP2 Ala54Thr-polymorphism may also be particularly interesting in the explanation of FA-metabolism alterations in MDD-R patients, because - as stated above - we observed (I) increased concentrations of 14–18-carbon FAs of several FA-subclasses [14], and (II) highly significantly lower overall FA-chain length [15], which was (III) bimodally distributed [18]. Surprisingly, previous studies in healthy and CVD-populations found no consistent associations of FA-concentrations with the FABP2 Ala54Thr-polymorphism [24,26,27]. This may be because, to our knowledge, not all members of the different FA-subclasses (e.g. long chain saturated and monounsaturated FA’s) were studied, especially not in a specific psychiatric (e.g. depressed) population with known bimodally distributed FA-alterations.
Therefore, the aim of the present study was to test the hypotheses that in MDD-R-patients the Thr54-polymorphism in the FABP2-gene would be (I) more prevalent than in sex-and age-matched controls, (II) associated with observed (bimodally distributed) alterations in FA-metabolism, and (III) associated with CVD-risk factor waist circumference.
Materials and Methods
2.1. Ethics Statement
All participants provided written informed consent prior to enrolment. The ethics committee of the Academic Medical Center of the University of Amsterdam approved the study.
2.2. Subjects
The current study was part of the DELTA-study, a randomized clinical trial on the effect of an 8-week cognitive therapy on MDD-recurrence, described previously [28,29]. This trial has been registered in the ISRCTN registry as ISRCTN68246470. As part of DELTA-study’s 2-year follow-up measurements, we invited subjects to participate in the current study. At the start of the DELTA-study, participants had to (I) be aged between 18 and 65, (II) have had ≥2 previous major depressive episodes in the last five years, and (III) be in remission of MDD. Exclusion criteria were current or previous mania or hypomania, any psychotic disorder, alcohol or drug abuse, and predominant anxiety disorder. Since any form of therapy (e.g. antidepressant-use) was no inclusion or exclusion criterion for the trial, the DELTA-sample can be considered representative for MDD-R patients with respect to this characteristic.
In addition to the MDD-R patient sample, we recruited age- and gender- matched healthy control subjects, without a personal and/or family history of MDD, as described previously [14].
2.3. Measurements
We took 20ml blood by venipuncture from subjects in the nonfasting state. As a model of brain FA-concentrations, we used washed erythrocytes, stored at −80 °C until analyses by capillary gas chromatography, as described previously [14]. This resulted in data on 29 different FAs, expressed in pmol/106 erythrocytes [15]. We operationalized FA-metabolism in two steps. First we tested five main FAs: linoleic acid, arachidonic acid, α-linolenic acid, eicosapentaenoic acid and docosahexaenoic acid, together with three indices which describe overall FA-characteristics: the Unsaturation (UI), Peroxidation (PI) and Chain Length Indices (CLI) [15,30]. Subsequently, we exploratively tested 24 other FAs. This approach reduces the multiple testing problem, because it guides interpretation of effects as explorative or a priori selected [15,31].
DNA was isolated from blood using a filter-based method (QIAamp DNA Mini Kit, Qiagen Ltd, United Kingdom). Polymerase chain reaction (PCR) primers were designed using Primer 3. PCR primer sequence TGACAATTTGAAGCTGACAATTA and AATCAAGAATGCATTGCTCAT, PEX primer sequence bioAT AAA TTC ACA GTC (L)AA GAA TCA AGC. Genotyping was done using a Matrix Assisted Laser Desorption Ionization Time Of Flight (MALDI-TOF) mass spectrometer from Bruker Daltonics [32]. All samples were genotyped in duplicate to increase reliability. Genotyping error rate based on these duplicates was 3.7%. We operationalized FABP2-genotype in three categories: GG homozygous, AG heterozygous and AA homozygous. An A-allele results in the Thr54-polymorphism.
We determined waist circumference in centimeters using a standard operating procedure [3,4].
2.4. Statistical analyses
2.4.1. Missing data
We used multiple imputation to prevent bias possibly introduced by missing or non-detectable FA- or genotype-data, which resulted in 5 imputed datasets as described previously [15,33].
2.4.2. Hardy-Weinberg Equilibrium (HWE)
We tested deviations from HWE separately in patients and controls using an online calculator χ2-test, available on http://www.oege.org/software/hwe-mr-calc.html.
2.4.3. Subject characteristics
We compared patients’ and controls’ subject characteristics using χ2-tests or independent Student’s t-tests as appropriate.
2.4.4. Hypotheses testing
For the first hypothesis, we used χ2-tests to test whether the distribution of observed genotype frequencies for patients and controls differed from expected frequencies. In order to test the association of the Thr54-polymorphism with the bimodally distributed FA-alterations in MDD-R-patients (2nd hypothesis), we used linear mixed models with genotype as predictor variable and a FA-concentration as outcome variable [34]. In case the overall F-test for a given FA was significant, we reported parameter estimates for the distinct genotypes with GG as reference category. For the association of Thr54-polymorphism with CVD-risk (3rd hypothesis), we applied a similar model, except that we entered waist circumference as outcome variable. We performed no correction for confounders, because these effects concern genetic effects and genotype is not expected to be subject to confounding factors.
In addition, we planned several post-hoc tests. First, in case FA-alterations would show linear associations with FABP2 genotype, e.g. GG: lowest value, AG: middle value, AA, highest value, we would test the effect of FABP2 on FA-alterations in linear mixed models with FABP2 recoded as scale variable as predictor variable and the FAs as outcome variable. Second, if FA-concentrations and waist circumference would be associated with FABP2-genotype, we would test correlations between these FA-alterations and waist circumference. If these alterations would be significantly correlated, in order to distinguish the direction of these effects, we would perform mediation analyses. To this end, we would use linear mixed models, first with FABP2 and waist circumference as predictor variables and the FA as outcome variable, subsequently with FABP2 and the FA as predictor variables and waist circumference as outcome variable. If the effect of FABP2 on the FA or waist circumference would disappear after inclusion of the other factor (the FA or waist circumference) in the model, this would imply that this other factor mediates the influence of FABP2. Finally, third, if FABP2 would affect FA-concentrations or waist circumference in the MDD-patients, we would test whether this effect would differ from the effect in the matched controls. In order to test this, we would build another set of linear mixed models with FABP2 and patients-status (patient or control) and their interaction (FABP2×patient-status) as predictor variables and the FA or waist circumference as outcome variable. Because the patient-status factor in these models may be subject to confounding, in contrast to the genetic FABP2-factor, we corrected observed effects for possibly confounding differences between patients and controls in age, sex, marital status, educational level, social class, ethnicity, 17-item Hamilton depression rating scale (HDRS)-score, and smoking. To prevent losing power, we used propensity scores, which enable correction for multiple potentially confounding factors while retaining power. Propensity scores represent for each case the saved predicted probability of being a patient or a control, which we calculated using a binary logistic model with patient-status as dependent, and the chosen potential confounders as predictor variables.
Although often not dealt with in FA-research, the multitude of FAs makes multiple comparisons inherent in investigating FA-metabolism, potentially causing type-I errors [15]. To reduce this problem we (I) applied pathophysiologically driven data reduction using the UI, CLI and PI (10), and (II) a priori designated several outcomes as hypothesis based, and others as explorative. Although still subject to debate, Bender and Lange (2001) suggest to perform correction for multiple testing primarily in confirmatory studies, while explorative results should be clearly indicated as such [31]. In line with their advice, and because e.g. Bonferroni correction likely would be too strict considering the relatively strong assumed correlations between the different outcomes in this study and therefore may induce type-II errors, we consequently chose to correct neither the results of the a priori hypothesized outcomes, nor the explorative results, for multiple comparisons [31]. Therefore, particularly the explorative results of this first investigation of associations between FABP2 and several FAs - particularly in a psychiatric population - should be interpreted as such.
2.4.5. Power analyses
We performed sensitivity power analyses. With power=.80 and two-sided alpha=.05, we were able to detect effects with a small effect size (w>0.214;f>0.216;f2>0.080) for all analyses, except for the differences between the different genotype classes in FA-measures and waist circumference in the patient group, for which we were able to detect medium effect sizes (f>0.268).
2.4.6. Software
We performed multiple imputation using the package Amelia II, implemented in R [33]. We performed analyses using SPSS Statistics v.20 (IBM). For multiple imputation results that are not pooled by SPSS, we used Rubin’s rules [35]. For the linear mixed models, these were implemented in a macro available at http://www.amcpsychiatrie-depressie.nl/cms/downloadfile.asp, as described previously. We used G*Power 3.1.3 (Kiel, Germany) to perform power calculations.
Results
3.1. Included sample and missing data
The inclusion procedure resulted in 137 patient and 73 control participants. Of these subjects, 8 patients and 3 controls had no valid FABP2-genotype due to technical reasons. Missingness in FA-data has been described previously [15]. Standard diagnostics by Amelia II suggested successful imputation.
3.2. Subject characteristics
FABP2-genotype was in HWE in controls (P>.05). Equal sex and age distributions among patients and controls indicate successful matching (P>.05). Patients had lower educational level (P<.001) and greater waist circumference (P=.025) (Table S1).
3.3. FABP2-distribution in patients and controls
The distribution of FABP2-genotype did not differ among patients and controls (P=.627). In patients, genotype frequencies were GG: 57.7%, AG: 34.3%, and AA: 8.0%, and in controls 50.7%, 41.6%, and 7.7%, respectively.
3.4. Association FABP2-FA-concentrations
Regarding the association of the Thr54-polymorphism with FA-metabolism, MDD-R patients’ FA-concentrations of the a priori selected five main FAs and three indices did not differ according to FABP2-genotype (Table 1). In the explorative analyses of the other FAs, two FA-concentrations were significantly associated with FABP2-genotype: eicosadienoic acid (C20:2ω6; F2,222.6=4.801,P=.009) and docosadienoic acid (C22:2ω6; F2,289.1=3.271,P=.039; Table 1). Looking at the parameter estimates for the three subgroups, the AA-genotype had higher eicosadienoic acid (b=0.437,SE b=0.156,t=7.886,P=.006) and docosadienoic acid-concentrations (b=0.236,SE b=0.120,t=2.324,P=.020) compared to the reference GG-category (Figure 1).
Table 1. Differences in fatty acid concentrations, fatty acid indices and waist circumference between GG, AG and AA-carriers of the Ala54Thr fatty acid-binding protein 2 (FABP2) polymorphism, vertically graphically divided in a priori (upper panel) and explorative (lower panel) tests.
| GG (N=79)a | AG (N=47)a | AA (N=11)a | F-value | Df1 | Df2 | P-value | |
|---|---|---|---|---|---|---|---|
| C18:2n6 | 65.42±1.528 | 67.05±1.922 | 65.64±4.198 | 0.215 | 2 | 87.502 | .807 |
| C20:4n6 | 71.07±0.976 | 73.51±1.374 | 71.78±2.819 | 1.003 | 2 | 44.068 | .375 |
| C18:3n3 | 0.826±0.036 | 0.845±0.044 | 0.848±0.091 | 0.073 | 2 | 163.23 | .930 |
| C20:5n3 | 3.381±0.188 | 3.278±0.270 | 3.381±0.534 | 0.049 | 2 | 56.873 | .952 |
| C22:6n3 | 15.19±0.558 | 14.63±0.844 | 14.16±1.535 | 0.287 | 2 | 38.681 | .752 |
| PI | 1.101±0.012 | 1.096±0.016 | 1.107±0.031 | 0.057 | 2 | 106.47 | .945 |
| CLI | 18.31±0.023 | 18.30±0.030 | 18.38±0.065 | 0.601 | 2 | 164.04 | .549 |
| UI | 1.290±0.009 | 1.289±0.012 | 1.305±0.023 | 0.187 | 2 | 125.27 | .830 |
| WC | 91.40±1.556 | 88.04±2.010 | 79.16±4.173 | 4.000 | 2 | 896.61 | .019 |
| C14 | 3.333±0.116 | 3.287±0.154 | 2.817±0.351 | 1.047 | 2 | 83.660 | .355 |
| C16 | 162.8±2.597 | 165.5±3.300 | 157.9±7.915 | 0.466 | 2 | 90.552 | .629 |
| C18 | 103.0±1.206 | 104.6±1.554 | 100.4±3.338 | 0.761 | 2 | 295.71 | .468 |
| C20 | 2.490±0.042 | 2.597±0.054 | 2.705±0.132 | 1.965 | 2 | 46.139 | .152 |
| C22 | 7.568±0.190 | 7.481±0.234 | 8.287±0.501 | 1.085 | 2 | 120.20 | .341 |
| C24 | 14.78±0.496 | 14.64±0.653 | 16.40±1.378 | 0.727 | 2 | 84.733 | .486 |
| C18:4n3 | 0.221±0.028 | 0.280±0.037 | 0.246±0.078 | 0.777 | 2 | 133.36 | .462 |
| C22:5n3 | 8.009±0.196 | 7.913±0.251 | 7.918±0.543 | 0.048 | 2 | 87.828 | .953 |
| C18:3n6 | 0.583±0.026 | 0.589±0.030 | 0.479±0.083 | 0.906 | 2 | 24.758 | .417 |
| C20:3n6 | 8.879±0.284 | 8.796±0.376 | 9.738±0.887 | 0.536 | 2 | 40.268 | .589 |
| C22:4n6 | 10.46±0.310 | 10.67±0.409 | 11.64±0.848 | 0.883 | 2 | 153.36 | .416 |
| C22:5n6 | 1.695±0.070 | 1.738±0.095 | 1.777±0.179 | 0.126 | 2 | 62.253 | .882 |
| C20:2n6 | 1.243±0.049 | 1.377±0.065 | 1.680±0.148 | 4.801 | 2 | 222.65 | .009 |
| C22:2n6 | 0.509±0.036 | 0.470±0.050 | 0.745±0.098 | 3.271 | 2 | 289.13 | .039 |
| C14:1n5 | 0.288±0.036 | 0.308±0.044 | 0.286±0.110 | 0.055 | 2 | 40.243 | .947 |
| C16:1n7 | 3.181±0.172 | 3.068±0.262 | 2.986±0.521 | 0.099 | 2 | 40.381 | .906 |
| C18:1n7 | 7.469±0.169 | 7.651±0.246 | 7.162±0.533 | 0.403 | 2 | 38.358 | .671 |
| C20:1n7 | 0.254±0.029 | 0.304±0.038 | 0.261±0.100 | 0.443 | 2 | 41.976 | .645 |
| C16:1n9 | 0.798±0.149 | 1.124±0.204 | 1.078±0.543 | 0.730 | 2 | 13.743 | .500 |
| C18:1n9 | 74.61±1.183 | 74.92±1.563 | 73.49±3.390 | 0.075 | 2 | 98.188 | .927 |
| C20:1n9 | 1.151±0.042 | 1.207±0.056 | 1.409±0.123 | 2.099 | 2 | 62.402 | .131 |
| C22:1n9 | 1.792±0.241 | 2.042±0.317 | 2.010±0.652 | 0.219 | 2 | 1928.1 | .803 |
| C24:1n9 | 13.16±0.415 | 13.25±0.541 | 14.52±1.208 | 0.624 | 2 | 87.715 | .538 |
| C20:3n9 | 0.397±0.028 | 0.371±0.036 | 0.243±0.076 | 1.849 | 2 | 101.56 | .163 |
| Total FAs | 585.5±6.571 | 593.3±8.685 | 573.3±19.72 | 0.527 | 2 | 984.82 | .590 |
Abbreviations: PI, Peroxidation Index; CLI, Chain Length Index; UI Unsaturation Index; WC, Waist circumference
a N per genotype based on estimation after multiple imputation.
Figure 1. Fatty acid concentrations in recurrent depression according to FABP2 genotype.
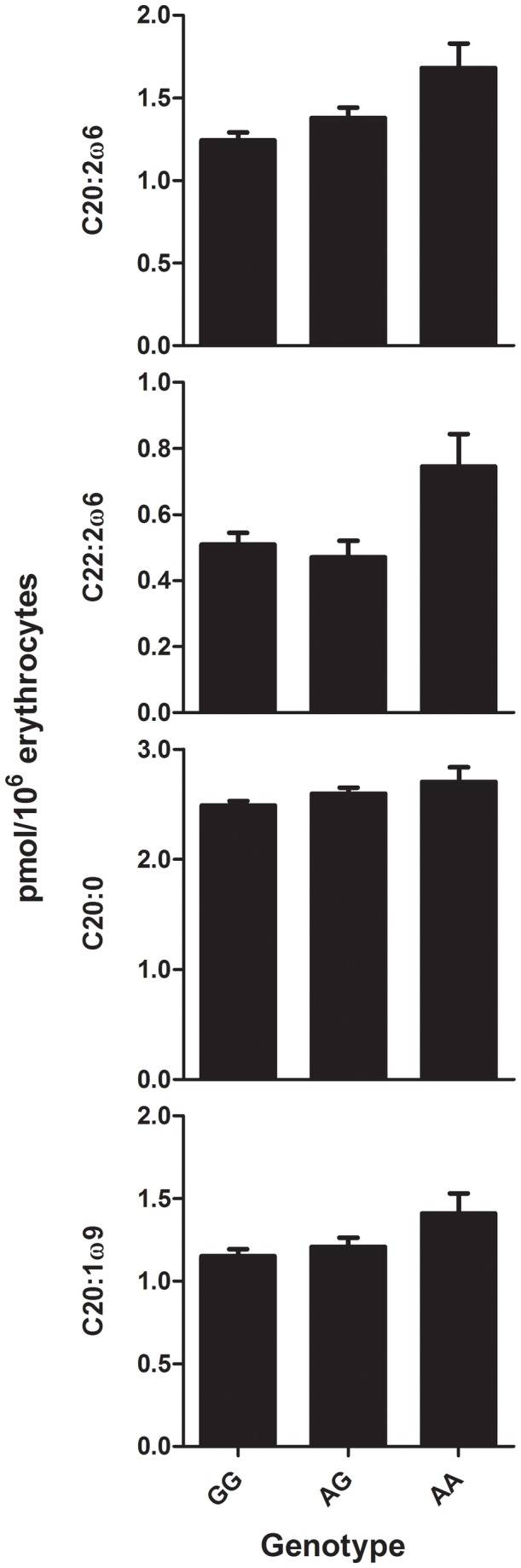
Concentrations of four fatty acids [eicosadienoic acid (C20:2ω6), docosadienoic acid (C22:2ω6), arachidic acid (C20:0) and gondoic acid (C20:1ω9)] in 137 recurrently depressed patients according to Ala54Thr fatty acid-binding protein 2 (FABP2) polymorphism genotype (GG, AG, AA).
3.5. Association FABP2-waist circumference
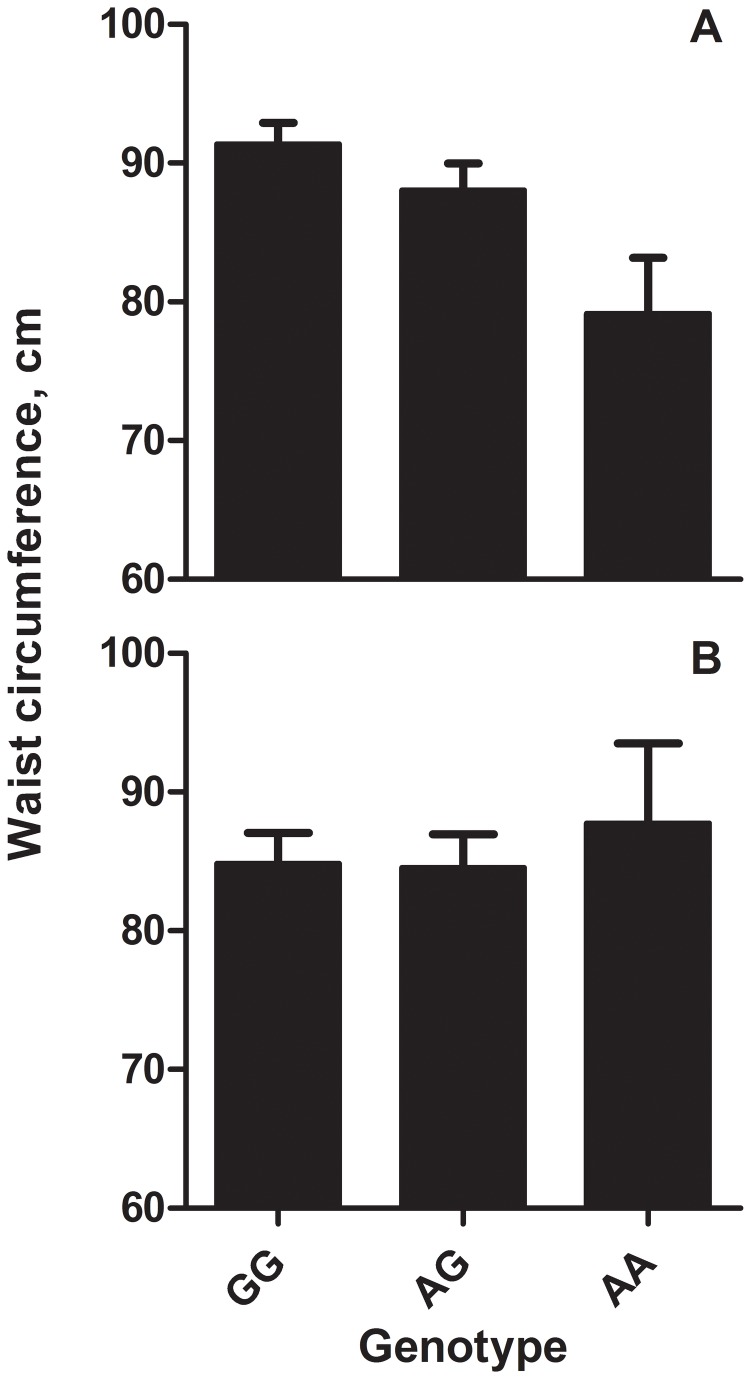
Considering the third hypothesis, MDD-R patients’ waist circumference significantly differed according to FABP2-genotype (F 2,896,6=4.000,P=.019). AA-genotype was associated with lower waist circumference estimate compared to the reference GG category (b=-12.243,SE b=4.474,t=-2.737,P=.006; Figure 2, Panel A).
Figure 2. Waist circumference according to FABP2 genotype compared between recurrently depressed patients and healthy controls.
Waist circumference according to Ala54Thr fatty acid-binding protein 2 (FABP2) polymorphism genotype (GG, AG, AA) in 137 recurrently depressed patients (Panel A) and 73 non-depressed controls (Panel B).
3.6. Post-hoc analyses
3.6.1. Tests for linear FABP2–FA-associations
In the analyses of the association of the Thr54-polymorphism with FA-metabolism, we observed that while overall effect of genotype was not significant, parameter estimates for specific genotypes on some FAs were significant (P<.05) and suggested a linear associations of FA-concentrations across FABP2 genotype, e.g. GG: lowest value, AG: middle value, AA, highest value. This affected especially FAs with a 20-carbon chain length. We therefore tested this linear relationship using linear mixed models with FABP2-genotype recoded as a scale variable (GG=1, AG=2, AA=3). These models showed significant linear effects of FABP2-genotype on C20 (b=0.106,SE b=0.052,t 135=2.032,P=.045), C20:2ω6 (b=0.182,SE b=0.062,t 135=2.944,P=.004), and C20:1ω9 (b=0.099,SE b=0.049,t 135=2.001,P=.046; Figure 1).
3.6.2. Relationship FABP2, FA-concentrations, and waist circumference
In order to disentangle FABP2’s effects on FA-concentrations and waist circumference, we performed correlation-analyses between waist circumference and FA-concentrations, and mediation analyses concerning the effect of FABP2 on FA-concentrations and waist circumference. In line with the effects of FABP2-genotype on both eicosadienoic acid and waist circumference, a post-hoc Pearson’s correlation test showed that eicosadienoic acid (r=-.243, P=.005) was significantly negatively correlated with waist circumference in patients. Subsequently, in a linear mixed model with both FABP2-genotype and waist circumference as predictors of eicosadienoic acid, we observed that both FABP2 (F2,317.47=3.285,P=.039,b AA vs. GG=0.362,SE b=0.155,t 173.915=2.342,P=.020) and waist circumference (F1,3184.09=4.790,P=.029,b=-0.006,SE b=0.003,t 3184.09=-2.189,P=.029) independently predicted eicosadienoic acid concentrations (overall F3,799.79=4.890,P=.002). However, the other way around, the predictive effect of FABP2 on waist circumference lost significance (P=.102) after including eicosadienoic acid as a [significant (P=.031)] predictor in the model. This indicates that FABP2’s effect on waist circumference is mediated by its effect on eicosadianoic acid, i.e. FABP2’s effect on waist circumference seems to consist of higher eicosadienoic acid concentrations in A-allele carriers which on their turn result in lower waist circumference.
3.6.3. Interactions between patient-status and FABP2 on C20:2ω6 and waist circumference
To test whether the relation between FABP2 and C20:2ω6 and waist circumference differed between patients and controls, we performed linear mixed models with patient-status (yes/no) and FABP2-genotype (GG/AG/AA) and their interaction as predictor variables, and C20:2ω6 or waist circumference as outcome variables. There was no patient-status×FABP2-genotype interaction for C20:2ω6 (F 2,882,788=1.069, P=.344). For waist circumference, the interaction was also non-significant (F 2,640,047=2.055, P=.129), but the patient×AA-genotype parameter estimate was significant at trend level (b=-15.140,SE b=7.719,t 3184.09=-1.961,P=.051), suggesting that in patients AA-genotype was associated with a relatively lower waist circumference compared to controls (Figure 2, Panel A and B). After correction - using propensity scores - for possibly confounding differences between patients and controls in e.g. educational level, social class, HDRS-score, and smoking, the overall genotype effect remained non-significant (F 2,477,338=2.129, P=.120). However, the patient×AA-genotype parameter estimate now just gained significance (b=-15.681,SE b=7.946,t 242.59=-1.974,P=.050).
Discussion
4.1. Summary of results
In the present paper, we examined FABP2’s role in MDD-R, and particularly its relation with bimodally distributed FA-alterations and CVD-risk factor waist circumference. FABP2 Ala54Thr-polymorphism distribution did not differ between 137 patients with recurrent MDD and 73 matched healthy controls without an MDD-history. However, A-allele carrying MDD-R patients had (I) higher concentrations of several 20-carbon FAs, especially eicosadienoic acid (C20:2ω6), and associated (II) lower waist circumference. In addition, post-hoc analyses suggested that effects of FABP2 on waist circumference in patients (I) were mediated by its effect on C20:2ω6, and (II) were different from controls.
4.2. FABP2 genotype distribution
Although it is increasingly recognized that FA-metabolism may play an important role in psychiatric disease, this is – to the best of our knowledge – the first investigation of the role of FABP2 in a psychiatric population. Contrary to our first hypothesis, genotype distribution did not differ from matched controls. This may imply that the Ala54Thr-polymorphism plays no role in vulnerability for MDD-R, as opposed to what have been suggested for other disorders including obesity and atherosclerosis [21–25].
4.3. Association between FABP2 and FA-metabolism
However, with regard to our second hypothesis, although the earlier observed bimodally distributed FA-metabolism parameters [18] were not associated with the Ala54Thr-polymorphism, we observed specific associations of the A-allele with the biologically mutually related FAs eicosadienoic acid (C20:2ω6) and its elongation product docosadienoic acid (C22:2ω6) [14]. In addition, also other 20-carbon FAs (C20, C20:2ω6, and C20:1ω9) showed a significant linear relation of increased concentrations with one or two A-alleles. Interestingly, previous research in healthy and CVD-subjects did not find consistent associations of the Ala54Thr-polymorphism with FA-concentrations [24,26,27]. This could be explained by lack of power [24,27], use of other sample mediums [24,26,27], lack of measurement of eicosadienoic acid (C20:2ω6) and docosadienoic acid (C22:2ω6), or inclusion of different patient populations [24,26,27].
Regarding other FAs, two of these previous studies found no associations [24,26], while one other study [27] found lower palmitoleic acid (C16:1ω7) and higher α-linolenic (C18:3ω3) and lignoceric acid (C24:0) in phospholipids to be associated with threonine coding FABP2-alleles. Interestingly, a study investigating FABP2’s effect on FA-uptake suggested that threonine coding FABP2-alleles are associated with an increased uptake of particularly 14-18-cabon FAs, including α-linoleic acid (C18:2ω6) [21], which can be enzymatically elongated to eicosadienoic acid (C20:2ω6) and docosadienoic acid (C22:2ω6) [14]. It may therefore be hypothesized that the increased red blood cell membrane concentration of eicosadienoic acid in our patients with the AA-genotype may reflect an adaptive conversion of increasedly bioavailable α-linoleic acid (C18:2ω6), to prevent accumulation of its other, and supposedly more bioactive (as main precursor of pro-inflammatory eicosanoids), conversion product arachidonic acid (ARA; C20:4ω6) [14]. Of note in this respect, increased ARA has been associated with (visceral) obesity and CVD-risk [36,37].
Interpretation of increased eicosadienoic acid concentrations in AA-genotype patients as an adaptive process to prevent ARA-accumulation corresponds with the observed negative association of eicosadienoic acid and waist circumference. In addition, eicosadienoic acid mediated the lower waist circumference in AA-patients: the relation between FABP2 and waist circumference lost significance when eicosadienoic acid was incorporated in the model. This might imply that for AA-patients, conversion of increasedly bioavailable α-linoleic acid (C18:2ω6) into eicosadienoic acid (C20:2ω6) instead of ARA (C20:4ω6) helps in maintaining a low waist circumference thereby possibly reducing CVD-risk.
4.4. Association between FABP2 and waist circumference
Our finding of an interaction at trend level between patient-status and FABP2-genotype on waist circumference is intriguing. Contrary to our third hypothesis and results in controls, AA-genotype was significantly associated with a lower waist circumference in the MDD-patients. This may have several explanations. First, patients’ metabolic constitution may be influenced by other genes, e.g. mutations in the 1-carbon-cycle, interacting with FABP2’s effect on waist circumference [38]. In addition, stress may affect FA-metabolism leading to different associations in patients, through the association between the HPA-axis and FA-metabolism [39]. Finally, FABP2’s effects may interact with dietary availability of nutrients, which could differ between patient and controls [16]. Future research should investigate these possibilities.
Interestingly, several studies also indicate that the Ala54Thr-polymorphism may be associated with a beneficial CVD-risk profile in response to certain dietary regimens, including eicosapentaenoic acid supplementation [40], or moderate-fat or high-polyunsaturated fat diets [41,42]. In these studies, Ala54Thr-polymorphism carriers had better metabolic responses and e.g. a larger reduction in waist circumference. This may indicate that different dietary preferences in patients played a role in the interaction between patient-status and FABP2 on waist circumference. Unfortunately, a main limitation of the present study is that no dietary data has been collected. Nevertheless, given the genetic nature of our observed effects, dietary intake likely is not a confounding factor, but rather interacts with FABP2-genotype to explain observed relations. In addition, erythrocyte FA-concentrations are thought to be more stable than plasma FAs, thereby reflecting long-term FA-metabolism, instead of dietary fluctuations [17]. Future nutrigenetic studies combining FABP2 and dietary assessment will further elucidate their interaction in explaining altered FA-metabolism and waist circumference in MDD(-R).
4.5. Additional limitations
Some additional limitation should be mentioned. Analyses did not differentiate between FA-subclasses, e.g. sphingomyelin or phosphatidylcholine. More advanced lipidomic analyses may provide better insight into the impact of FAB2 on FA-metabolism. However, this is the first report including a wide range of different FAs and indices into the analyses. This brings up another important point: the issue of multiple testing. Because of the multitude of FAs, studying FA-metabolism usually entails multiple comparisons. Although often overlooked in FA-literature, multiple comparisons may lead to type-I errors [15]. We dealt with this intrinsic problem by (I) applying pathophysiologically driven data reduction using the UI, CLI and PI [15], and (II) a priori designating several outcomes as hypothesis based, and others as explorative. However, despite these precautions, it remains important to keep the multiple testing problem in mind when interpreting results. None of the reported significant results would have survived Bonferroni correction. However, this correction likely would have been too strict considering the relatively strong assumed correlations between the different outcomes in this study. For that reason, strict correction in this sample could have induced type-II errors, and therefore we consequently chose to correct neither the results of the a priori hypothesized outcomes, nor the explorative results, for multiple comparisons [31]. Therefore, particularly explorative first results should be interpreted as such, and replication in further investigations is warranted. In addition, while power calculations showed that we had adequate power to detect small effect sizes for almost all analyses, we had insufficient power to detect small effect sizes in the analyses on the differences between the different genotype classes in FA-measures and waist circumference in the patient group, for which we were able to detect medium effect sizes (f>0.268). Therefore, we may have missed additional small effects of FABP2 on FA-measures or waist circumference. However, for these analyses, we additionally operationalized the effects of FABP2 as a linear function, which resulted in adequate power to detect small effect sizes (f2>0.080). So, in conclusion, the only effects for which our set-up may have precluded detection of small effect sizes would be non-linear effects of FABP2 on FA-measures and/or waist circumference. However, both (I) existing literature on the physiological role of FABP2, and (II) data from our sample presented in table 1, do not suggest such non-linear relationships.
4.6. Study strengths
Our study also has particular strengths. To our knowledge FABP2 was investigated in a psychiatric population for the first time, in a specific sample of MDD-R-patients with known bimodally distributed FA-alterations. In addition, an advanced multiple imputation procedure reduced the possibility that missing data have influenced results [15,33]. Finally, this is the first report of an association between FABP2 and two specific mutually biologically related FAs, namely eicosadienoic acid (C20:2ω6) and docosadienoic acid (C22:2ω6), which increases knowledge on FABP2’s role in health and disease.
Conclusion
Although Ala54Thr-polymorphism distribution was not associated with MDD, A-allele carrying patients had (I) higher concentrations of several 20-carbon FAs, particularly eicosadienoic acid (C20:2ω6), and associated (II) lower waist circumference. Therefore, for AA-patients, potentially adaptive conversion of increasedly bioavailable dietary precursors into eicosadienoic acid might mediate maintenance of a low waist circumference, which may guide future investigations of CVD-prevention in these patients. Considering the explorative nature of this first investigation of these associations, replication is warranted, preferably by nutrigenetic studies applying lipidomics and detailed dietary assessment.
Supporting Information
Subject Characteristics. a Educational level is defined as: low, primary education or preparatory middle-level applied education; middle, higher general continued education or middle-level applied education; and high, preparatory scientific education, higher applied education, or scientific education. b based on occupation: Class 1, e.g. cleaner; Class 2, e.g. nurse; Class 3, e.g. general manager. Abbreviations: HDRS, Hamilton depression rating scale; TCA, tricyclic antidepressant; SSRI, selective serotonin reuptake inhibitor.
(DOCX)
Funding Statement
This study has been made possible due to financial aid of the Netherlands Foundation for Mental Health, Utrecht and the Health Research Development Council, Department Prevention Program (ZonMw). HGR is supported by a NWO/ZonMw VENI-Grant #016.126.059. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Greden JF (2001) The burden of recurrent depression: causes, consequences, and future prospects. J Clin Psychiatry 62 Suppl 22: 5-9. PubMed: 11599650. [PubMed] [Google Scholar]
- 2. Osby U, Brandt L, Correia N, Ekbom A, Sparén P (2001) Excess mortality in bipolar and unipolar disorder in Sweden. Arch Gen Psychiatry 58: 844-850. doi: 10.1001/archpsyc.58.9.844. PubMed: 11545667. [DOI] [PubMed] [Google Scholar]
- 3. Huxley R, Mendis S, Zheleznyakov E, Reddy S, Chan J (2010) Body mass index, waist circumference and waist:hip ratio as predictors of cardiovascular risk--a review of the literature. Eur J Clin Nutr 64: 16-22. doi: 10.1038/ejcn.2009.68. PubMed: 19654593. [DOI] [PubMed] [Google Scholar]
- 4. Vazquez G, Duval S, Jacobs DR, Silventoinen K (2007) Comparison of Body Mass Index, Waist Circumference, and Waist/Hip Ratio in Predicting Incident Diabetes: A Meta-Analysis. Epidemiol Rev 29: 115-128. doi: 10.1093/epirev/mxm008. PubMed: 17494056. [DOI] [PubMed] [Google Scholar]
- 5. Zhao G, Ford ES, Li C, Tsai J, Dhingra S, Balluz LS (2011) Waist circumference, abdominal obesity, and depression among overweight and obese U.S. adults: National Health and Nutrition Examination Survey 2005-2006. BMC Psychiatry 11: 130Available online at: 10.1186/1471-244X-11-130 Available online at: PubMed: 21834955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang C, Rexrode KM, van Dam RM, Li TY, Hu FB (2008) Abdominal Obesity and the Risk of All-Cause, Cardiovascular, and Cancer Mortality: Sixteen Years of Follow-Up in US Women. Circulation 117: 1658-1667. doi: 10.1161/CIRCULATIONAHA.107.739714. PubMed: 18362231. [DOI] [PubMed] [Google Scholar]
- 7. Lok A, Visscher TL, Koeter MW, Assies J, Bockting CL et al. (2010) The 'Weight' of recurrent depression: a comparison between individuals with recurrent depression and the general population and the influence of antidepressants. Psychother Psychosom 79: 386-388. doi : 10.1159/000320898. PubMed: 20829650. [DOI] [PubMed] [Google Scholar]
- 8. Mozaffarian D, Wu JHY (2011) Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol 58: 2047-2067. doi: 10.1016/j.jacc.2011.06.063. PubMed: 22051327. [DOI] [PubMed] [Google Scholar]
- 9. Piomelli D, Astarita G, Rapaka R (2007) A neuroscientist's guide to lipidomics. Nat Rev Neurosci 8: 743-754. doi: 10.1038/nrn2233. PubMed: 17882252. [DOI] [PubMed] [Google Scholar]
- 10. Hibbeln JR, Salem N (1995) Dietary polyunsaturated fatty acids and depression: when cholesterol does not satisfy. Am J Clin Nutr 62: 1-9. PubMed: 7598049. [DOI] [PubMed] [Google Scholar]
- 11. Lin PY, Huang SY, Su KP (2010) A Meta-Analytic Review of Polyunsaturated Fatty Acid Compositions in Patients with Depression. Biol Psychiatry 68: 140-147. doi: 10.1016/j.biopsych.2010.03.018. PubMed: 20452573. [DOI] [PubMed] [Google Scholar]
- 12. McNamara RK, Carlson SE (2006) Role of omega-3 fatty acids in brain development and function: potential implications for the pathogenesis and prevention of psychopathology. Prostaglandins Leukot Essent Fatty Acids 75: 329-349. doi: 10.1016/j.plefa.2006.07.010. PubMed: 16949263. [DOI] [PubMed] [Google Scholar]
- 13. McNamara RK, Hahn CG, Jandacek R, Rider T, Tso P et al. (2007) Selective deficits in the omega-3 fatty acid docosahexaenoic acid in the postmortem orbitofrontal cortex of patients with major depressive disorder. Biol Psychiatry 62: 17-24. doi: 10.1016/j.biopsych.2006.08.026. PubMed: 17188654. [DOI] [PubMed] [Google Scholar]
- 14. Assies J, Pouwer F, Lok A, Mocking RJT, Bockting CLH et al. (2010) Plasma and erythrocyte fatty acid patterns in patients with recurrent depression: a matched case-control study. PLOS ONE 5: e10635. doi: 10.1371/journal.pone.0010635. PubMed: 20498721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mocking RJ, Assies J, Lok A, Ruhé HG, Koeter MW et al. (2012) Statistical Methodological Issues in Handling of Fatty Acid Data: Percentage or Concentration, Imputation and Indices. PubMed: 22446846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mutch DM, Wahli W, Williamson G (2005) Nutrigenomics and nutrigenetics: the emerging faces of nutrition. FASEB J 19: 1602-1616. doi: 10.1096/fj.05-3911rev. PubMed: 16195369. [DOI] [PubMed] [Google Scholar]
- 17. Hodson L, Skeaff CM, Fielding BA (2008) Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog Lipid Res 47: 348-380. doi: 10.1016/j.plipres.2008.03.003. PubMed: 18435934. [DOI] [PubMed] [Google Scholar]
- 18. Mocking RJT, Assies J, Koeter MWJ, Ruhé HG, Lok A et al. (2012) Bimodal distribution of fatty acids in recurrent major depressive disorder. Biol Psychiatry 71: e3-e5. doi: 10.1016/S0006-3223(12)00375-7. PubMed: 21993192. [DOI] [PubMed] [Google Scholar]
- 19. Bentsen H, Solberg DK, Refsum H, Gran JM, Bøhmer T et al. (2011) Bimodal distribution of polyunsaturated fatty acids in schizophrenia suggests two endophenotypes of the disorder. Biol Psychiatry 70: 97-105. doi: 10.1016/j.biopsych.2011.02.011. PubMed: 21546001. [DOI] [PubMed] [Google Scholar]
- 20. Baier LJ, Sacchettini JC, Knowler WC, Eads J, Paolisso G et al. (1995) An amino acid substitution in the human intestinal fatty acid binding protein is associated with increased fatty acid binding, increased fat oxidation, and insulin resistance. J Clin Invest 95: 1281-1287. doi: 10.1172/JCI117778. PubMed: 7883976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Agren JJ, Vidgren HM, Valve RS, Laakso M, Uusitupa MI (2001) Postprandial responses of individual fatty acids in subjects homozygous for the threonine- or alanine-encoding allele in codon 54 of the intestinal fatty acid binding protein 2 gene. Am J Clin Nutr 73: 31-35. PubMed: 11124746. [DOI] [PubMed] [Google Scholar]
- 22. Albala C, Santos JL, Cifuentes M, Villarroel AC, Lera L et al. (2004) Intestinal FABP2 A54T polymorphism: association with insulin resistance and obesity in women. Obes Res 12: 340-345. doi: 10.1038/oby.2004.42. PubMed: 14981227. [DOI] [PubMed] [Google Scholar]
- 23. Hegele RA (1998) A review of intestinal fatty acid binding protein gene variation and the plasma lipoprotein response to dietary components. Clin Biochem 31: 609-612. doi: 10.1016/S0009-9120(98)00078-2. PubMed: 9876891. [DOI] [PubMed] [Google Scholar]
- 24. Pratley RE, Baier L, Pan DA, Salbe AD, Storlien L et al. (2000) Effects of an Ala54Thr polymorphism in the intestinal fatty acid-binding protein on responses to dietary fat in humans. J Lipid Res 41: 2002-2008. PubMed: 11108733. [PubMed] [Google Scholar]
- 25. Wanby P, Palmquist P, Brudin L, Carlsson M (2005) Genetic variation of the intestinal fatty acid-binding protein 2 gene in carotid atherosclerosis. Vasc Med 10: 103-108. doi: 10.1191/1358863x05vm609oa. PubMed: 16013194. [DOI] [PubMed] [Google Scholar]
- 26. Erkkilä AT, Lindi V, Lehto S, Pyörälä K, Laakso M et al. (2002) Variation in the fatty acid binding protein 2 gene is not associated with markers of metabolic syndrome in patients with coronary heart disease. Nutr Metab Cardiovasc Dis 12: 53-59. PubMed: 12189904. [PubMed] [Google Scholar]
- 27. Vidgren HM, Sipiläinen RH, Heikkinen S, Laakso M, Uusitupa MI (1997) Threonine allele in codon 54 of the fatty acid binding protein 2 gene does not modify the fatty acid composition of serum lipids in obese subjects. Eur J Clin Invest 27: 405-408. doi: 10.1046/j.1365-2362.1997.1320679.x. PubMed: 9179548. [DOI] [PubMed] [Google Scholar]
- 28. Bockting CL, Schene AH, Spinhoven P, Koeter MW, Wouters LF et al. (2005) Preventing relapse/recurrence in recurrent depression with cognitive therapy: a randomized controlled trial. J Consult Clin Psychol 73: 647-657. doi: 10.1037/0022-006X.73.4.647. PubMed: 16173852. [DOI] [PubMed] [Google Scholar]
- 29. Lok A, Mocking RJT, Ruhé HG, Visser I, Koeter MWJ et al. (2012) Longitudinal hypothalamic-pituitary-adrenal axis trait and state effects in recurrent depression. Psychoneuroendocrinology 37: 892-902. doi: 10.1016/j.psyneuen.2011.10.005. PubMed: 22094110. [DOI] [PubMed] [Google Scholar]
- 30. Mocking RJ, Assies J, Bot M, Jansen EH, Pouwer F (2012) Biological effects of add-on eicosapentaenoic acid supplementation in patients with diabetes mellitus and co-morbid major depression: a randomized, double-blind, placebo-controlled study. [DOI] [PMC free article] [PubMed]
- 31. Bender R, Lange S (2001) Adjusting for multiple testing-when and how? J Clin Epidemiol 54: 343-349. doi: 10.1016/S0895-4356(00)00314-0. PubMed: 11297884. [DOI] [PubMed] [Google Scholar]
- 32. Pusch W, Wurmbach JH, Thiele H, Kostrzewa M (2002) MALDI-TOF mass spectrometry-based SNP genotyping. Pharmacogenomics 3: 537-548. doi: 10.1517/14622416.3.4.537. PubMed: 12164776. [DOI] [PubMed] [Google Scholar]
- 33. Donders AR, van der Heijden GJ, Stijnen T, Moons KG (2006) Review: a gentle introduction to imputation of missing values. J Clin Epidemiol 59: 1087-1091. doi: 10.1016/j.jclinepi.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 34. Gueorguieva R (2004) Move over anova: Progress in analyzing repeated-measures data andits reflection in papers published in the archives of general psychiatry. Arch Gen Psychiatry 61: 310-317. doi: 10.1001/archpsyc.61.3.310. PubMed: 14993119. [DOI] [PubMed] [Google Scholar]
- 35. Marshall A, Altman DG, Holder RL, Royston P Marshall A, Altman DG, Holder RL, Royston P (2009) Combining estimates of interest in prognostic modelling studies after multiple imputation: current practice and guidelines. BMC Med Res Methodol 9: 57 1471-2288-9-57. doi: 10.1186/1471-2288-9-57. PubMed: 19638200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Inoue K, Kishida K, Hirata A, Funahashi T, Shimomura I Inoue K, Kishida K, Hirata A, Funahashi T, Shimomura I (2013) Low serum eicosapentaenoic acid / arachidonic acid ratio in male subjects with visceral obesity. Nutr Metab (Lond) 10: 25 1743-7075-10-25. doi: 10.1186/1743-7075-10-25. PubMed: 23497138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Savva SC, Chadjigeorgiou C, Hatzis C, Kyriakakis M, Tsimbinos G et al. (2004) Association of adipose tissue arachidonic acid content with BMI and overweight status in children from Cyprus and Crete. Br J Nutr 91: 643-649. doi: 10.1079/BJN20031084. PubMed: 15035692. [DOI] [PubMed] [Google Scholar]
- 38. Assies J, Lok A, Bockting CL, Weverling GJ, Lieverse R et al. (2004) Fatty acids and homocysteine levels in patients with recurrent depression: an explorative pilot study. Prostaglandins Leukot Essent Fatty Acids 70: 349-356. doi: 10.1016/j.plefa.2003.12.009. PubMed: 15041026. [DOI] [PubMed] [Google Scholar]
- 39. Mocking RJT, Ruhé HG, Assies J, Lok A, Koeter MWJ et al. (2013) Relationship between the hypothalamic-pituitary-adrenal-axis and fatty acid metabolism in recurrent depression. Psychoneuroendocrinology, 38: 1607–17. PubMed: 23465556 . [DOI] [PubMed] [Google Scholar]
- 40. Pishva H, Amini M, Eshraghian MR, Hosseini S, Mahboob SA (2012) Effects of EPA supplementation on plasma fatty acids composition in hypertriglyceridemic subjects with FABP2 and PPARalpha genotypes. J Diabetes Metab Disord 11: 25 2251-6581-11-25. doi: 10.1186/2251-6581-11-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. de Luis D, Aller R, Izaola O, Sagrado MG, de la Fuente B et al. (2012) Effect of Fatty Acid-Binding Protein 2 Ala54Thr Genotype on Weight Loss and Cardiovascular Risk Factors After a High-Polyunsaturated Fat Diet in Obese Patients. J Investig Med 60: 1194–8. PubMed: 23072901. [DOI] [PubMed] [Google Scholar]
- 42. Martinez-Lopez E, Garcia-Garcia MR, Gonzalez-Avalos JM, Maldonado-Gonzalez M, Ruiz-Madrigal B et al. (2013) Effect of Ala54Thr polymorphism of FABP2 on anthropometric and biochemical variables in response to a moderate-fat diet. Nutrition 29: 46-51. doi: 10.1016/j.nut.2012.03.002. PubMed: 22817827. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Subject Characteristics. a Educational level is defined as: low, primary education or preparatory middle-level applied education; middle, higher general continued education or middle-level applied education; and high, preparatory scientific education, higher applied education, or scientific education. b based on occupation: Class 1, e.g. cleaner; Class 2, e.g. nurse; Class 3, e.g. general manager. Abbreviations: HDRS, Hamilton depression rating scale; TCA, tricyclic antidepressant; SSRI, selective serotonin reuptake inhibitor.
(DOCX)