Abstract
Despite substantial progress in understanding the cancer signaling network, effective therapies remain scarce due to insufficient disruption of oncogenic pathways, drug resistance and drug-induced toxicity. New and more creative approaches are therefore required for the treatment of cancer. MicroRNAs (miRNAs) are a family of small noncoding RNAs that regulate gene expression by sequence-selective targeting of mRNAs, leading to a translational repression or mRNA degradation. Experimental evidence demonstrates that dysregulation of specific miRNAs leads to drug resistance in different cancers and correction of these miRNAs using miRNA mimics or antagomiRs can normalize the gene regulatory network and signaling pathways and sensitize cancerous cells to chemotherapy. Therefore, miRNA-based gene therapy provides an attractive anti-tumor approach for integrated cancer therapy. Here, we will discuss the involvement of microRNAs in chemotherapy resistance and focus on recent advancements in the development and delivery of miRNA-based cancer therapeutics.
Keywords: MicroRNA, Chemotherapy, Cancer, Drug resistance
1. Mechanisms of drug resistance
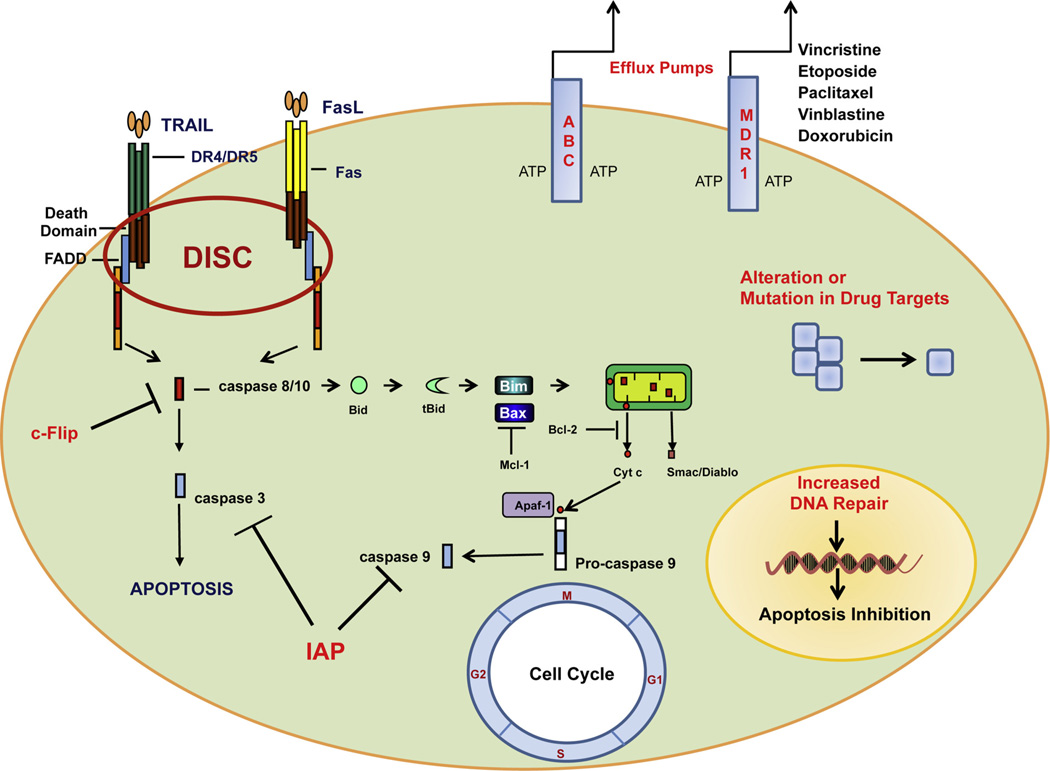
Chemotherapy is the preferred treatment for malignancies. However, a successful long-term use of chemotherapy is often prevented by the development of drug resistance. Drug resistance can occur at many levels, including increased drug efflux, alterations in drug target, DNA repair, cell cycle regulation and evasion of apoptosis (Fig. 1).
Fig. 1.
Mechanisms of drug resistance. Drug efflux, by ATP-dependent multidrug transporters, is one of the most significant forms of resistance against the variety of currently used antineoplastic agents. Aterations in drug targets, increased DNA repair, cell cycle dysregulation and evasion of apoptosis are also responsible for drug resistance.
1.1. Increased drug efflux
One of the most significant forms of resistance against the variety of currently used antineoplastic agents is by the action of a group of membrane proteins which extrude cytotoxic molecules, keeping intracellular drug concentration below a cell-killing threshold. These ATP-dependent multidrug transporters belong to the ubiquitous superfamily of ATP-binding cassette (ABC) proteins which modulate absorption, distribution and excretion of many pharmacological compounds (Fig. 1). The ABC proteins have been grouped into 7 subclasses ranging from ABCA to ABCG (Kast and Gros, 1997; Dean et al., 2001). The prototypical representative of this family, the 170-kDa P-glycoprotein (P-gp), was first characterized in the plasma membrane of Chinese hamster ovary cells and identified as being encoded by the ABCB1 (MDR1) gene (Juliano and Ling, 1976). P-gp can transport a large variety of different molecules, including cytostatic drugs and endogenous substrates (steroid hormones, cytokines) against a drug concentration gradient at the expense of ATP hydrolysis (Borst et al., 2000).
Another subfamily of the ABC transporter family, the human multidrug resistance-associated proteins (MRPs), includes at least seven members that are MRP-related genes and have an established role in multidrug transport, particularly glutathione (GSH)-conjugated derivatives of several toxic compounds (the so-called GS-X pumps) (Ishikawa et al., 2000). MRPs are transport systems that recognize anionic drugs (i.e., methotrexate) and neutral drugs conjugated to acidic ligands, such as GSH, glucuronate, or sulfate, whereas P-gp has a low affinity for negatively charged compounds (Jedlitschky et al., 1996).
The glutathione S-transferases (GSTs) are a multigene family of dimeric enzymes that have a significant role in the detoxification of electrophilic species by catalytic conjugation with reduced glutathione (GSH) (Hayes and Pulford, 1995). Based on their amino acid sequence and substrate specificity, eight classes of GSTs (namely GST alpha, mu, pi, theta, sigma, zeta, kappa and omega) have been identified in mammals (Ketterer, 2001). Currently emerging as the principal focus of research activity within the family, however, is the pi isoform (GSTP), primarily because it is overexpressed in many human tumors (Satoh et al., 2001; Howie et al., 1990), and thus represents a putative cancer biomarker (Tsuchida et al., 1997). Increased glutathione (GSH) may cause resistance by binding/inactivating cisplatin, enhancing DNA repair, or reducing cisplatin-induced oxidative stress. GSTs, especially GSTP1, may augment drug resistance by catalyzing GSH-drug binding (Wu et al., 2010).
1.2. Alterations in drug targets
Alterations in drug targets can be also a cause of drug resistance (Fig. 1). These alterations may be quantitative (e.g., level of expression) or qualitative (e.g., mutation). Key determinants of drug activity are enzymes of DNA functions or proteins of the cellular replication apparatus. In the case of antimetabolites that interfere with various steps in nucleic acid metabolism through inhibition of key enzymes (thymidylate synthase, ribonucleotide reductase, DNA polymerase), an increased content of the target enzyme may result in drug resistance. An example is fluorouracil resistance due to increased level of thymidylate synthase (Longley et al., 2003). By contrast, downregulation of the DNA topoisomerase is expected to decrease sensitivity to important antitumor agents, including anthracyclines and camptothecin (Larsen and Skladanowski, 1998). Alterations in topo-II enzyme activity through decreased levels of topo-II protein or topo-II mutations have been found in cell lines resistant to topo-II-targeted drugs (Deffie et al, 1992). Resistance to taxol can be also associated with multiple alterations of its intracellular target, including modification of tubulin levels and acetylation of α-tubulin (Zunino et al., 1999). Another way through which cells may become resistant, for example to cisplatin, is by developing an enhanced ability to repair cisplatin-induced lesions, through the action of DNA repair proteins (Fig. 1). Nucleotide excision repair (NER) is the major pathway for platinum drug-induced DNA damage. NER involves several proteins and among them is the excision repair cross-complementing 1 protein (ERCC1), which several pre-clinical studies have demonstrated plays an important role in determining cisplatin sensitivity. Indeed, increased expression of ERCC1 is associated with cisplatin resistance (Youn et al, 2004).
1.3. Alterations in DNA repair pathways
Cells respond to DNA damage by activating checkpoint pathways that ultimately block the activity of cyclin-dependent kinases (CDKs) and consequently cause an arrest in cell cycle progression. The G1, S, G2 checkpoints ensure that the cell does not begin DNA replication unless DNA is undamaged (Fig. 1). An understanding of the mechanisms by which anticancer agents influence the cell cycle may offer insights into strategies for sensitizing cancer cells to current therapeutics and can also provide a rationale for the administration of combinations of drugs (Sampath and Plunkett, 2001).
1.4. Evasion of apoptosis
Apoptotic evasion represents one of the true hallmarks of cancer and appears to be a vital component in the chemotherapeutic and radiotherapeutic resistance that characterizes the most aggressive of human cancers (Hanahan and Weinberg, 2000). A central step in the induction of apoptosis involves the activation of caspases. There are two main pathways for the activation of caspases: the extrinsic pathway regulated by ‘death receptors’ of the TNF-receptor family, such as Fas (CD95/APO-1), DR4 (TNF-related apoptosis-inducing ligand receptor 1, TRAIL-R1), and DR5 (TRAIL-R2) and the intrinsic pathway regulated by Bcl-2 proteins. After the binding with their ligands the death receptors recruit caspase 8 via the adapter molecule FADD (Fas-associated death domain) to form the DISC (death-inducing signaling complex) (Nagata, 1999) (Fig. 1). At the DISC, caspase 8 molecules become activated and can subsequently activate the caspase cascade, triggering apoptosis (Fig. 1). Death receptor-mediated apoptosis can be inhibited by decoy receptors: decoy receptor 3 or DCR3 in the case of Fas, and DCR1 and DCR2 in the case of the TRAIL receptors (Ashkenazi, 2002). These decoy receptors lack the intracellular domains (Death Domains) necessary for DISC formation and therefore they cannot trigger apoptosis. Apoptosis mediated by both Fas and DR4/DR5 can also be inhibited by cytoplasmic factors, such as c-FLIP (FLICE-like inhibitory protein), which binds to the DISC and inhibits caspase 8 activation (Krueger et al., 2001).
The intrinsic apoptotic pathway is activated in response to a number of stress stimuli including growth-factor deprivation, cytokine withdrawal, calcium flux or DNA damage, caused by UV or gamma-irradiation other than triggered by members of the tumor necrosis factor family member such as Fas, TNF or TRAIL. Based on their function, the members of the BCL2 family can be divided into pro-apoptotic and pro-survival proteins. Pro-survival proteins contain up to four BCL2 homology (BH) domains, i.e. BCL2, BCL-XL, MCL1, BCL-W. Pro-apoptotic BCL2 family members can be subdivided into multi-domain class proteins, harboring three out of four BH-domains (BH1, 2, 3) i.e. BAK, BAX and BOK and those that only contain the BH3-domain and are referred to as BH3-only proteins, including BIM/BOD, BID, BMF, PUMA/BBC3, NOXA/APR, BAD, BIK/NBK/BLK and HRK/DP5 (Youle and Strasser, 2008). Inhibition of the anti-apoptotic BCL2 family members by BH3-only proteins leads to activation of BAX/BAK, subsequent mitochondrial dysfunction, and cytosolic release of apoptogenic proteins such as cytochrome c (Cyto c) and SMAC/Diablo (Fig. 1). Caspase-8 can also trigger the intrinsic pathway through the cleavage of Bid. Cleaved Bid induces cytosolic release of cytochrome c and SMAC/Diablo. Cytochrome c binds the adaptor proteins APAF1 and pro-caspase-9, consequently forming an apoptosome, which activates caspase-9 and caspase-3, resulting in apoptosis. Members of the IAP (inhibitors of apoptosis) family, which include cIAP1, cIAP2, XIAP, and survivin (Salvesen and Duckett, 2002) can bind directly to caspases, such as caspases 3, 7 and 9, and inhibit their activity, blocking apoptosis (Fig. 1).
2. MicroRNAs
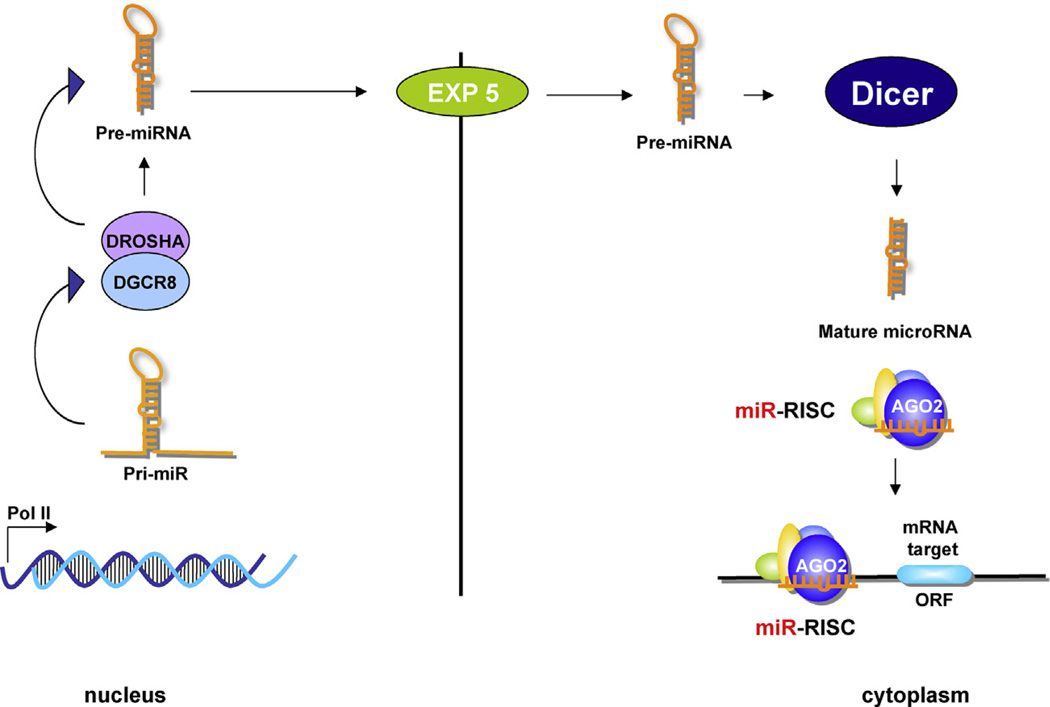
MicroRNAs (miRNAs) are small (19–22 nucleotides) non-coding RNAs, discovered in 1993 to control developmental timing in Caenorhabditis elegans (Lee et al., 1993). They bind to the 3’ untranslated region (3’-UTR) of target messenger RNAs (mRNAs), causing either degradation or inhibition of translation, effectively silencing their target genes. Animal miRNAs are identified as part of an 80-nucleotide RNA with a stem-loop structure, known as a pre-miRNA, included in primary miRNA precursors (pri-miRNAs) that are several hundreds or thousands of nucleotides long (Fig. 2).
Fig. 2.
MicroRNA biogenesis. MicroRNAs (miRNAs) biogenesis is a coordinated process operated by different groups of enzymes and associated proteins in the nucleus or cytoplasm. The pri-miRNA, located in the nucleus, is converted in pre-miRNA by the RNase III enzyme Drosha. The produced pre-miRNA is exported by the Exportin 5 to the cytoplasm where is processed in ~18–22-nucleotide miRNA duplexes by the cytoplasmic RNase III Dicer. One strand of the duplex is degradated and the other one, the mature microRNA, interacts with the RISC (RNA induced silencing complex). Perfect or nearly perfect complementarities between miRNA and its target 3′ UTR induce RISC to cleave the target mRNA, whereas imperfect base matching induces mainly translational silencing of the target. DGCR8, DiGeorge syndrome critical region gene 8 protein.
The first step of miRNA biogenesis involves the transcription of the pri-miRNA mediated by RNA polymerase II (Pol II) (Lee et al., 2004) (Fig. 2), although a minor group of miRNAs can be transcribed by RNA polymerase III (Pol-III) (Borchert et al., 2006). The pri-miRNA is then processed in the nucleus by the RNase III enzyme Drosha and the protein Pasha/DGCR8 into ~70 nucleotides pre-miRNAs (Lee et al., 2003). The pre-miRNA undergoes an additional processing step within the cytoplasm, and a small double-stranded RNA structure approximately 22 nucleotides in length is excised from the pre-miRNA hairpin by another RNase III enzyme, Dicer (Hutvágner et al., 2001; Ketting et al., 2001) (Fig. 2). The mature single-stranded miRNA is then loaded into the RISC (RNA-induced silencing complex), which mediates the degradation or translation inhibition of mRNA’s target gene (Bartel, 2004).
MicroRNAs are novel classes of cellular regulators that can repress the expression of multiple proteins and have been involved in various biological and pathological processes such as development, differentiation, cell proliferation, apoptosis and carcinogenesis (Becam et al., 2011; Meng et al., 2012; Yan et al., 2012; Garofalo et al., 2011; Alder et al., 2012). Recently, some studies have highlighted miRNAs linked to chemoresistant phenotype of different tumors, mainly through abnormal regulation of apoptosis (Xie et al., 2012), cell cycle distribution, (Yamanaka et al., 2012) and activity of drug efflux transporters (Zhu et al., 2008). Dysfunctional miRNAs are commonly found in a variety of solid cancers and are attractive candidates for next-generation therapeutics. Aberrant expression of miRNAs is correlated with the development and progression of tumors, and the reversal of their expression has been shown to modulate the cancer phenotype, suggesting the potential of miRNAs as targets for anti-cancer drugs. Here we describe the putative role(s) of microRNAs in the development of chemoresistance (Table 1). Moreover, we discuss recent discoveries that make them a promising class of drug targets for chemoprevention and therapeutic intervention in cancer.
Table 1.
MicroRNAs involved in drug resistance.
| Type of resistance | microRNAs | Cancer | Drug | Targets | References |
|---|---|---|---|---|---|
| Drug efflux | miR-519c | Colon cancer | MXR | ABCG2 | To et al. (2008) |
| miR-212 | CML | Imatinib | ABCG2 | Turrini et al. (2012) | |
| miR-27a | Gastric cancer | ADR | P-glycoprotein | ||
| miR-101/135 | HCC | N. R. | ABCA1 | Borel et al. (2012) | |
| miR-199a/b/-296 | HCC | N. R. | ABCC1 | Borel et al. (2012) | |
| miR-125a/b | HCC | N. R. | ABCC4 | Borel et al. (2012) | |
| miR-101/125a/let-7a | HCC | N. R. | ABCC5 | Borel et al. (2012) | |
| Let-7a/e | HCC | N. R. | ABCC10 | Borel et al. (2012) | |
| miR-26a/-135b/-145 | HCC | N. R. | ABCE1 | Borel et al. (2012) | |
| miR-379 | HCC | Rifampicin | ABCC2 | Haenisch et al. (2011) | |
| miR-328 | Breast cancer | MXR | ABCG2 | Pan et al. (2009) | |
| miR-200c | Breast cancer | ADX | MDR1/P-glycoprotein | Chen et al. (2011a,b) | |
| miR-326 | Breast cancer | VP-16/ADX | MRP-1 | Liang et al. (2009) | |
| miR-451 | Breast cancer | Irinotecan | ABCB1 | Bitarte et al. (2011) | |
| miR-199a | Ovarian cancer | CDD/PTX/ADR | ABCG2 | Cheng et al. (2012) | |
| MiR-27a | Ovarian cancer | PTX | MDR1/P-gp | Li et al. (2010) | |
| Alterations in drug targets | miR-485-3p | ALL | TopII inhibitors | NF-YB | Chen et al. (2011a,b) |
| miR-143/145 | Liposarcoma | N. R. | Top2a | Ugras et al. (2011) | |
| miR-100 | Breast cancer | PTX | β-Tubulin I, IIA, IIB, V | Lobert et al. (2011) | |
| miR-148a | Prostate cancer | N. R. | MSK1 | Fujita et al. (2010) | |
| miR-7 | Lung cancer | TKIs | EGFR | Rai et al. (2011) | |
| miR-30c/221/222 | Lung cancer | TKIs | BIM/APAF-1 | Garofalo et al. (2011) | |
| miR-103/203 | Lung cancer | TKIs | PKC-ε/SRC | Garofalo et al. (2011) | |
| DNA repair | miR-138 | Lung cancer | CDD | ERCC1 | Wang et al. (2011) |
| miR-182 | Breast cancer | PARP1 inhibitor | BRCA1 | Moskwa et al. (2011) | |
| miR-21 | Colon cancer | 5-FU | hMSH2-hMSH6 | Valeri et al. (2010) | |
| miR-21 | Breast cancer | CDD, ADX | HMSH2 | Yu et al. (2010) | |
| Cell cycle | miR-200 | Lung | DOC | E2F3 | Feng et al. (2012) |
| miR-19b/21 | Colon cancer | 5-FU | SFPQ, MYBL2 | Kurokawa et al. (2012) | |
| miR-215 | Colon cancer | MTX, TDX | DTL | Song et al. (2010) | |
| Osteosarcoma | |||||
| miR-34a | Prostate cancer | Camptothecin | CDK6, cyclin D1, E2F3, E2F1 | Fujita et al. (2008) | |
| miR-221/222 | Breast cancer | Fulvestrant | p27 Kip1 | Rao et al. (2011) | |
| miR-221/222 | Breast cancer | TAM | p27 Kip1 | Miller et al. (2008) | |
| Evasion of apoptosis | miR-181a/b | CLL | Fludarabine | MCL-1, Bcl2 | Zhu et al. (2010a,b) |
| miR-497 | Breast cancer | N. R. | Bcl-w | Shen et al. (2012) | |
| miR-136 | Glioma | CDD | AEG-1, Bcl-2 | Yang et al. (2012) | |
| miR-200b/c/429 | Gastric cancer | CDD, VCR | Bcl-2, Xiap | Zhu et al. (2012) | |
| miR-497/181 | Gastric cancer | CDD, VCR | Bcl2 | Zhu et al. (2010a,b, 2011) | |
| miR-34a | Gastric cancer | ADX, CDD, GEM, DOC | Bcl2 | Ji et al. (2008) | |
| miR-15/16 | Gastric cancer | ADR, VP-16, CDD | Bcl-2 | Xia et al. (2008) | |
| miR-512-3p | HCC | TXL | c-Flip | Chen et al. (2010) | |
| miR-21 | GBM | N. R. | Bcl-2/Bax ratio | Shi et al. (2010) | |
| miR-34c-5p | NSCLC | TXL | Bmf | Catuogno et al. (2012) | |
| miR-24 | NSCLC | TRAIL | Xiap | Xie et al. (2012) | |
| miR-25 | Cholangiocarcinoma | TRAIL | DR4 | Razumilava et al. (2012) | |
| miR-221/222 | NSCLC | TRAIL | PTEN, TIMP3 | Garofalo et al. (2009) |
DOC: docetaxel; CDD: cisplatin; TRAIL: TNF-related apoptosis inducing ligand; TKIs: tyrosine kinase inhibitors; 5-FU: fluorouracil; ADX: doxorubicin; TXL: taxolo; VP-16: etoposide; MXR: mitoxantrone; TAM: tamoxifen; ADR: adriamycin; VCR: vincristine; GEM: gemcitabine; PTX: paclitaxel; TMZ: temozolomide; TDX: tomudex; CML: chronic myeloid leukemia; HCC: hepatocarcinoma; GBM: glioblastoma multiforme; ALL: acute lymphoblastic leukemia; CLL: chronic lymphocytic leukemia. N. R.: not reported.
3. Drug efflux transporters and microRNAs
3.1. ABCB1
MicroRNAs have been shown to be involved in chemotherapy resistance through the regulation of ATP-binding cassette (ABC) membrane transporters. Li et al. found that the expression levels of miR-27a and ABCB1 were up-regulated in paclitaxel-resistant ovarian cancer cell line A2780/Taxol as compared with its parental line A2780. Transfection of A2780/Taxol cells with inhibitors of miR-27a decreased the expression of MDR1 mRNA and P-gp protein, increased HIPK2 protein expression, and enhanced the sensitivity of A2780/taxol cells to paclitaxel (Li et al., 2010). Homeodomain-interacting protein kinase-2 (HIPK2) is a serine-threonine kinase that belongs to a family of transcriptional co-repressors. Recently, it has been shown that HIPK2 can down-modulate the expression of hypoxia-inducible factor 1a (HIF-1α), which is overexpressed in many types of tumors and contributes to chemoresistance by activating ABCB1, in normoxic condition and repress HIF-1 transcriptional activity (Nardinocchi et al., 2011). The deregulation of miR-27a may be involved in the development of drug resistance, regulating the expression of ABCB1 – at least in part – by targeting HIPK2 in ovarian cancer cells (Li et al., 2010). Such emerging evidence indicates that miRNAs could be potential biomarkers for predicting a response to systemic therapy and prognosis in clinical settings.
Another study showed that up-regulation of miR-200c enhanced chemosensitivity of MCF7 breast cancer cells to epiru-bicin by reducing the expression of ABCB1. Restoration of miR-200c in MCF7 cells increased intracellular doxorubicin accumulation. Therefore, miR-200c may act as a promising therapeutic target for improvement of responsiveness to chemotherapy in breast cancer (Chen et al., 2011a,b).
Recent evidence suggests that tumors consist of heterogeneous cell populations that have different biological properties. Furthermore, the capacity for tumor formation and growth resides exclusively in a small proportion of tumor cells, termed cancer-initiating cells (CICs) (Reya et al., 2001; Ponti et al., 2005). Tumor proliferation may be driven by cancer stem cells (CSCs), which divide slowly and are relatively resistant to cytotoxic drugs. Thus, many tumors may progress because CSCs are not sensitive to the treatment. Bitarte et al. obtained colon spheres with properties of CSCs from different colon carcinoma cells and miRNA profiling was performed. The results showed that miR-451 was downregulated in colon spheres versus parental cells. Expression of miR-451 caused a decrease in self-renewal, tumorigenicity, and chemoresistance to irinotecan (first-line therapy for metastatic colorectal cancer (mCRC)) of colon spheres. Irinotecan prevents DNA from unwinding by inhibition of topoisomerase 1. MiR-451 suppressed expression of the ABCB1 (also known as MDR1 or P-glycoprotein) drug efflux transporter, resulting in irinotecan sensitization. Moreover, lower expression of miR-451 was observed in patients who did not respond to irinotecan-based first-line therapy compared with patients who did (Bitarte et al, 2011). Therefore, miR-451 could not only be useful as a novel molecular marker for selection of patients that respond to treatment with irinotecan but also serve as a target for the development of novel therapeutic strategies to overcome drug resistance and tumor growth in colorectal cancer.
3.2. ABCG2
ABCG2 overexpression is frequently observed in human cancer cell lines selected with various anticancer drugs (Doyle et al., 1998; Miyake et al., 1999; Robey et al., 2001).
Colorectal cancer (CRC) is one of the most frequently occurring cancers in United States with more than 140,000 new cases and about 50,000 deaths expected to occur in 2010 (Jemal et al., 2010). To and colleagues demonstrated that ABCG2 mRNA adopts a longer 3’UTR in the parental S1 colon cancer cell line than in its mitoxantrone-resistant counterpart and that a miRNA (hsa-miR-519c) decreases endogenous ABCG2 mRNA and protein levels by acting through a putative hsa-miR-519c binding site located within the longer 3’UTR region found only in parental cells. These findings suggest that escape from miRNA-mediated translational repression and mRNA degradation could lead to overexpression of ABCG2 in drug-resistant cancer cells (To et al., 2008).
Despite the enormous success of imatinib in chronic myeloid leukemia (CML), therapy resistance has emerged in a significant proportion of patients, partly because of the overexpression of ABC efflux transporters. Using an array comprising 667 miRNAs, Turrini et al. investigated whether the expression of microRNAs is altered in CML K-562 cells becoming resistant to increasing concentrations of imatinib. ABCG2 protein expression was 7.2-fold elevated after long-term treatment with 0.3 µmol/l imatinib and decreased gradually at higher concentrations whereas miR-212 was down-regulated. Reporter gene assays confirmed miR-212 to target the 3’-UTR region of ABCG2. In contrast, transfection of anti-miR-212 revealed an upregulation of ABCG2 protein expression (Turrini et al, 2012)
Breast cancer is the second leading cause of cancer death in women. Despite improvement in treatment over the past few decades, there is an urgent need for development of targeted therapies. Drug resistance remains a major clinical obstacle to successful treatment of breast cancer. The molecular mechanisms that may contribute to chemotherapeutic resistance in breast cancers include overexpression of ATP-binding cassette transporters, anti-apoptotic factors and cell cycle deregulation. Pan et al. identified a microRNAs (hsa-miR-328) able to downregulate ABCG2 in breast cancer cells, increasing mitoxantrone sensitivity, suggesting that suppressed miR-328 expression may be another underlying mechanism for ABCG2 overexpression in mitoxantrone resistant breast cancer cells (MCF-7/MX100) (Pan et al., 2009).
Epithelial ovarian cancer (EOC) is the sixth most common cancer in women worldwide and, despite advances in detection and therapies, it still represents the most lethal gynecologic malignancy in the industrialized countries (Iorio et al., 2007). In advanced ovarian cancer the first line of chemotherapy is the combination of car-boplatin/cisplatin with paclitaxel or other chemotherapy agents (Cannistra, 2004; Ozols, 2005). Many of the initially responding patients relapse after a few years from the first cycle of therapy. In ovarian cancer, CD44(+)/CD117(+) stem cells, also known as cancer-initiating cells (CICs), are highly proliferative, have a low degree of differentiation, and are resistant to chemotherapeutics. Therefore, the CD44(+)/CD117(+) subpopulation is thought to be an important target for novel therapeutic strategies. Cheng et al. found that miR-199a specifically regulates CD44, and that over-expression of miR-199a inhibited the proliferation of ovarian CICs in vitro and in vivo. Indeed, overexpression of miR-199a significantly increased the chemosensitivity of ovarian CICs to cisplatin, paclitaxel and adriamycin and reduced mRNA expression of the multidrug resistance gene ABCG2 as compared with miR-199a mutant-transfected and untransfected ovarian cells. Moreover, the effect of miR-199a expression on tumor growth was investigated in vivo by subcutaneously inoculating miR-199a–transfected and mutant miR-199a–transfected CD44+/CD117+ ovarian CICs into nude mice. Expression of miR-199a significantly decreased the tumor volume in mice xenografts. More apoptotic cells were detected in tumors formed by miR-199a–transfected cells. Taken together, these results indicated that expression of miR-199a in CD44+/CD117+ ovarian CICs suppressed tumor growth in vivo by reducing cell proliferation and promoting apoptosis (Cheng et al., 2012).
3.3. ABCA1, ABCC and ABCE1
Hepatocellular carcinoma (HCC) is the fifth most common type of cancer worldwide. With a 5-year survival of less than 5%, HCC remains one of the most fatal cancers, and few treatments have proven to be effective (El-Serag and Mason, 1999). Major pitfalls are late diagnosis, tumor recurrence, and resistance to chemotherapeutic treatment, mediated by high expression of ABC transporter family members. Borel et al., identified the up-regulation of 11 and down-regulation of 79 microRNAs, comparing 19 paired hepatocellular carcinomas with healthy tissues. By luciferase assay they found that miR-101 and miR-135b down-regulated ABCA1, miR-199a/b and miR-296 downregulated ABCC1, ABCC4 was a direct target of miR-125a/b, ABCC5 was downregulated by miR-101, miR-125a and let-7a, ABCC10 was a target of let-7a/e, and ABCE1 was directly downmodulated by miR-26a, miR-135b and miR-145 (Borel et al., 2012). Haenisch and colleagues screened the expression of 377 human miRNAs in HepG2 cells after 48 h of treatment with 5 µM rifampicin (a pregnane X receptor (PXR) ligand). MiR-379 increased whereas ABCC2 protein decreased after 72 h of rifampicin treatment (Haenisch et al., 2011).
Liang and colleagues analyzed miRNA expression levels in the ABCC1 (MRP1) overexpressing breast cancer cell line, MCF-7/VP, in comparison with its parent cell line, MCF-7, using a miRNA microarray. MiR-326 was downregulated in MCF-7/VP compared to MCF-7. The elevated levels ofmiR-326 in the mimics-transfected VP-16-resistant cell line, MCF-7/VP, downregulated MRP-1 expression and sensitized these cells to VP-16 and doxorubicin (Liang et al., 2009).
4. Alterations in drug targets and microRNAs
4.1. Topoisomerase II
DNA topoisomerase II (Top2) is an essential nuclear enzyme involved in many cellular processes (Wang et al., 2002; Nitiss, 2009). Mammalian cells have two isoforms of the type II enzymes: Top2α (170 kDa) (Tsai-Pflugfelder et al., 1988), and Top2β (180 kDa) (Chung et al., 1989). Top2-DNA covalent complexes serve as the cytotoxic target for many anticancer drugs such as doxorubicin, etoposide, teniposide, and amsacrine (Beck et al., 1996; Pommier et al., 2010). However, tumors frequently become refractory to treatments with Top2 inhibitors because of the emergence of drug resistance.
Teniposide-resistant human lymphoblastic leukemia CEM cells (CEM/VM-1–5) express reduced Top2α protein compared with parental CEM cells. NF-YB Nuclear factor (NF)-YB, a subunit of the transcription factor nuclear factor Y (NF-Y) complex, binds and activates CCAAT-containing promoters, including Topo2α. Chen et al. found that NF-YB protein expression is increased in CEM/VM-1–5 cells compared to the parental CEM cells, suggesting that increased NF-YB may be a negative regulator of Top2α in CEM/VM-1–5 cells. MicroRNA target prediction programs revealed that the 3’-untranslated region (3’-UTR) of NF-YB harbors a putative hsa-miR-485-3p binding site. Thus, hsa-miR-485-3p mediates drug responsiveness by decreasing NF-YB expression, which in turn negatively regulates Top2α expression. Overexpression of miR-485-3p in CEM/VM-1–5 cells led to reduced expression of NF-YB and corresponding up-regulation of Top2α, with increased sensitivity to the Top2 inhibitors (Chen et al., 2011a,b).
Liposarcoma is the most common mesenchymal cancer, with a mortality rate of 60% among patients with this disease and lack of therapeutic options. Ugras et al. profiled microRNAs expression in samples of normal adipose tissue, well-differentiated liposarcoma, and dedifferentiated liposarcoma. They found in dedifferentiated liposarcomas compared to the adipose tissue two downregulated miRNAs, miR-143 and miR-145. Restoring miR-143 expression in dedifferentiated liposarcoma cells decreased expression of BCL2, TOP2A, PRC1, and PLK1, inhibiting apoptosis, DNA replication and cytokinesis (Ugras et al., 2011).
4.2. β-Tubulin
Antimitotic drugs are key components of combination chemotherapy protocols for hematological and solid tumors. The taxanes (e.g., paclitaxel) bind to the β subunit of the tubulin heterodimer and reduce microtubule dynamics, leading to cell cycle arrest in G2/M. The effectiveness of taxane therapy is severely limited by intrinsic and acquired drug resistance. Lobert et al., showed a significant decrease in the tumor suppressor miR-100 in MCF7 cells in response to paclitaxel treatment. Overexpression of miR-100 in MCF-7 cells significantly reduced β-tubulin I, IIA, IIB and V mRNA, suggesting a possible role for this miR in the response to paclitaxel in breast cancer (Lobert et al., 2011).
4.3. MSK1
Prostate cancer is the second leading cause of cancer related death in men in the United States. Anti-androgen therapy is currently the first line of treatment for patients diagnosed with prostate cancers. Unfortunately, most patients will eventually develop the androgen-independent form of prostate cancers, which is highly metastatic and has poor prognosis. Microtubule stabilizers, such as paclitaxel (PTX), are used in treating patients diagnosed with androgen-independent prostate cancer (Shang et al., 2009) but there are currently few effective approaches for treating chemoresistant prostate cancers. Fujita et al. demonstrated that miR-148a is down-regulated in hormone-refractory prostate cancer cells compared with normal and hormone-sensitive cancer cells. They also identified MSK1 as a direct target of miR-148a. Mitogen- and stress-activated kinase 1 (MSK1), also known as ribo-somal protein S6 kinase, is a serine/threonine kinase that serves as a downstream target of extracellular signal-regulated kinase (ERK) or p38 mitogen-activated protein kinase in response to various stimuli including epidermal growth factor (EGF), phorbol ester (TPA), UV-irradiation, and anisomycin (Wiggin et al., 2002; Soloaga et al., 2003). Activated MSK1 phosphorylates chromatin-related proteins such as histone H3 and HMG-14 and transcription factors such as CREB and NF-κB, (Wiggin et al., 2002; Soloaga et al., 2003; Duncan et al., 2006). In paclitaxel-resistant PC3 cells, miR-148a inhibited malignant phenotypes including paclitaxel-resistance and reduced MSK1 expression via acting on its 3’-UTR, suggesting that miR-148a has potential as a novel therapeutic target for treatment of hormone-refractory prostate cancer especially for drug-resistant prostate cancer (Fujita et al., 2010).
4.4. EGFR
Lung cancer is currently the leading cause of cancer-related death worldwide, accounting for approximately a third of all cancer diagnoses and deaths (Gompelmann et al., 2011). Approximately 70–80% of lung cancers are non-small cell lung cancer (NSCLC), including squamous cell carcinoma, adenocarcinoma, and large cell carcinoma (Pathak et al., 2004) Despite advances made in surgery, radiation therapy, and chemotherapy, the 5-year survival for lung cancer remains at only about 16%. The high mortality rate associated with lung cancer has prompted numerous exhaustive efforts to identify novel therapeutic targets and treatment modalities for this deadly disease. Our understanding of the central role of epidermal growth factor receptor in the development and progression of lung adenocarcinoma has led to the development of molecular agents against this key oncogene that have demonstrated significant clinical efficacy against the disease. Despite these successes, de novo or acquired resistance to these anti-epidermal growth factor receptor agents invariably develops, either through additional mutations in the epidermal growth factor receptor (EGFR) or abnormal regulation of downstream signaling pathways (Kobayashi et al., 2005; Wei, 2011). A strong correlation between activating mutations in the EGFR tyrosine kinase domain and the response to tyrosine kinase inhibitors (TKIs) erlotinib and gefitinib has been reported in several trials. Although most EGFR mutant NSCLCs initially respond to EGFR inhibitors, the majority of these tumors ultimately become resistant to the drugs. Two mechanisms of acquired resistance have been validated in patients: secondary mutations in EGFR itself, including the EGFR T790M “gatekeeper” mutation, observed in 50% of resistant cases; and amplification of the MET oncogene (Takezawa et al., 2010), associated with tumor growth and metastasis, observed in 20% of resistant cases (Engelman et al., 2007).
To overcome resistance, Rai et al. focused on EGFR suppression using miR-7, which target three different sites in the 3’-untranslated region of EGFR mRNA. They analyzed two EGFR-TKI-sensitive cell lines (PC-9 and H3255) and two EGFR-TKI-resistant cell lines harboring T790M (RPC-9 and H1975). They used cationic liposomes to deliver a plasmid expressing miR-7, inhibiting the EGFR signaling and overcoming acquired resistance to EGFR-TKIs, regardless of T790M mutation status (Rai et al, 2011).
Recently, to understand the role of microRNAs in TKI-resistant NSCLCs, our group examined changes in miRNAs that are mediated by tyrosine kinase receptors. A microRNA microarray identified miR-30b, miR-30c, miR-221 and miR-222 modulated by both epidermal growth factor (EGF) and MET receptors, and miR-103 and miR-203 controlled only by MET. We showed that these miRNAs influenced the response to gefitinib-induced apoptosis of NSCLC cells in vitro and in vivo by inhibiting the expression of the genes encoding BCL2-like 11 (BIM), apoptotic peptidase activating factor 1 (APAF-1), protein kinase C ε (PKC-ε) and sarcoma viral oncogene homolog (SRC) (Garofalo et al., 2011). Therefore, it is possible to speculate that the modulation of these microRNAs in conjunction with chemotherapy, could improve the response to TKIs, such as gefitinib and erlotinib, in NSCLC.
5. DNA repair and microRNAs
5.1. DNA excision repair
Several reports indicate that microRNAs may regulate drug resistance by controlling DNA repair. Wang and colleagues showed the dysregulation of 14 miRNAs in A549/DDP (cisplatin (DDP) resistant) cell line compared to the parental A549 cell line. Up-regulation of one of these miRs, miR-138, increased the sensitivity of A549/DDP cells to cisplatin in vitro. Intriguingly, the authors showed that excision repair cross-complementation group 1 (ERCC1) was negatively regulated by miR-138. These findings suggest that miR-138 could play an important role in the development of cisplatin resistance in non-small cell lung cancer (Wang et al., 2011).
5.2. Homologous recombination
BRCA1 encodes for a protein called breast cancer type 1 susceptibility protein, which is required for DNA double-strand break repair via homologous recombination. If BRCA1 is inactivated by mutation or promoter methylation, damaged DNA is not repaired properly and this increases risks for cancers (Friedenson, 2007). Decreased expression of the BRCA1 has been shown to be common in sporadic basal-like breast cancer (Mueller and Roskelley, 2003) and correlates with poor prognosis of breast cancer patients. The molecular mechanism of BRCA1 suppression in sporadic tumors is unclear. Moskwa et al. showed that overexpression of miR-182, may play a role in BRCA1 downregulation in sporadic breast tumors. Manipulation of miR-182 expression in multiple breast tumor lines impacts BRCA1 levels and sensitivity to PARP1 inhibition, in both cultured cells and in xenograft models (Moskwa et al, 2011).
5.3. Mismatch repair
MicroRNA-21 (miR-21) is linked to a number of human tumors including colorectal cancer, where it appears to regulate the expression of tumor suppressor genes including p21, phosphatase and tensin homolog (PTEN), TGFβ receptor II. Valeri et al. demonstrated that miR-21 targets and down-regulates the core mismatch repair (MMR) recognition protein complex, human mutS homolog 2 (hMSH2) and 6 (hMSH6). The mismatch repair (MMR) system is involved in DNA damage recognition and repair. Human mutS homolog 2 (hMSH2) and human mutL homolog 1 (hMLH1) function as core MMR proteins and form heterodimers with protein homologs hMSH3 or hMSH6 and hMLH3 or hPMS2, respectively (Fishel, 2001). Defects in MMR proteins have been associated with reduced or absent benefit from 5-FU adjuvant chemotherapy in clinical trials (Ribic et al., 2003). MMR impairment appears to cause reduced incorporation of 5-FU metabolites into DNA, leading to reduced G2/M arrest and apoptosis after 5-FU treatment (Meyers et al, 2005). Cells with miR-21 overexpression exhibited significantly reduced 5-fluorouracil (5-FU)-induced G2/M damage arrest and apoptosis that is characteristic of defects in the core MMR component. These results suggest that miR-21-dependent down-regulation of hMSH2-hMSH6 might be responsible for both primary and acquired resistance to 5-FU (Valeri et al., 2010).
TGF-β is a cytokine that plays a tumor suppressive role in normal epithelia by potently inhibiting cell proliferation and inducing apoptosis; conversely, it accelerates progression of established cancer by multiple autocrine and paracrine mechanisms (Derynck et al., 2001). Yu et al. reported that TGF-β downregulated MSH2 in HER2-transformed MCF10A mammary epithelial cells and in breast cancer cells. This was mediated by a TGF-β-induced miRNA, miR-21, which targets the 3’ untranslated region (UTR) of MSH2 mRNA and downregulates its expression. They further found that by downregulating MSH2, TGF-β contributes to resistance of cancer cells to DNA-damaging chemotherapy agents such as cisplatin and doxorubicin, but not docetaxel, a chemotherapy drug targeting the microtubule (Yu et al., 2010).
6. Cell cycle regulation and microRNAs
6.1. E2F3
Docetaxel is widely used in the treatment of advanced NSCLC and other solid tumors. It inhibits microtubule dynamics by enhancing microtubule polymerization, causing the metaphase to anaphase transition arrest, activating the spindle assembly checkpoint, and subsequently leading to apoptosis (Yvon et al., 1999; Wang et al., 2000). However, chemoresistance remains the most important obstacle restricting the clinical application of docetaxel. Feng et al. identified miR-200 as the most down-regulated miRNA in docetaxel-resistant human lung adenocarcinoma cells. They proved that miR-200b could function as a chemosensitivity restorer to docetaxel in human lung adenocarcinoma mediated, at least partially, by targeting the transcription factor E2F3, which is critical for the maintenance of normal cell cycle progression. Therefore, miR-200b may act as a tumor suppressor to reverse docetaxel resistance of human lung adenocarcinoma cells (Feng et al., 2012).
6.2. p27
Fulvestrant is an estrogen receptor antagonist with no agonist effects, which works both by down-regulating and by degrading the estrogen receptor (Kansra et al., 2005) and highly effective antagonist to hormone-sensitive breast cancers following failure of previous tamoxifen or aromatase inhibitor therapies. However, after prolonged fulvestrant therapy, acquired resistance eventually occurs in the majority of breast cancer patients, due to poorly understood mechanisms. Rao et al. identified an upregulation of miR-221/222 in MCF-7 breast cancer cells with acquired fulvestrant resistance compared to the sensitive cells. Transfection with 2’-O-Me-221 and 2’-O-Me-222 or control 2’-O-Me-eGFP antagomiRs increased protein levels of the cell cycle inhibitor p27 (Kip1), suggesting that targeting p27 may be one of the mechanisms by which miR-221/222 confers the fulvestrant-resistant phenotype. Moreover, miR-221/222 overexpression activated the β-catenin pathway and repressed TGF-β-mediated growth inhibition. Therefore, these two ‘oncomirs’ may represent promising therapeutic targets for treating fulvestrant-resistant breast cancer (Rao et al., 2011). Tamoxifen is an antagonist of the estrogen receptor in breast tissue, successfully used to treat women with estrogen receptor-positive breast cancer. Miller et al. performed a miRNA microarray analysis of MCF-7 Tamoxifen sensitive (parental) versus MCF7 tamoxifen-resistant cells. MiR-221 and miR-222 were the most upregulated miRNAs in the resistant compared to the sensitive cells. They showed that ectopic expression of miR-221/222 rendered the parental MCF-7 cells resistant to tamoxifen through downregulation of p27 (Miller et al., 2008).
6.3. c-Myc
In 2007, reports from several laboratories showed that members of the miR-34 family are direct p53 targets, and their upregulation induced apoptosis and cell-cycle arrest (He et al., 2007; Bommer et al., 2007). In mammalians, the miR-34 family comprises three processed miRNAs that are encoded by two different genes: miR-34a is encoded by its own transcript, whereas miR-34b and miR-34c share a common primary transcript. Moreover, the promoter region of miR-34a, −34b and −34c contains CpG islands and aberrant CpG methylation that reduces miR-34 family expression in multiple types of cancer (Lodygin et al., 2008; Chim et al., 2011). Yamamura et al. reported that miR-34a was downregulated in prostate cancer tissues and silenced the expression of the c-Myc oncogene by targeting its 3’ UTR, inhibiting cell proliferation, cell invasion and promoting apoptosis. MiR-34a was found to repress RhoA, a regulator of cell migration and invasion, by suppressing c-Myc-Skp2-Miz1 transcriptional complex that activates RhoA. MiR-34a also suppressed the c-Myc-P-TEFb complex that plays a key role in controlling the elongation phase of transcription by RNA polymerase II (Pol II), indicating one of the mechanisms by which miR-34a has a dramatic effect on cellular function (Yamamura et al, 2012). Fujita et al. showed that miR-34a expression was markedly reduced in p53-null PC3 cells and p53-mutated DU145 cells compared with LNCaP cells expressing wild-type p53. In PC3 cells, ectopic expression of miR-34a decreased the SIRT1 mRNA and protein levels as well as protein levels of known direct target genes, such as CDK6, cyclin D1, E2F3, E2F1, BCL2. Ectopic miR-34a expression resulted in cell cycle arrest and growth inhibition and attenuated chemoresistance to anticancer drug camptothecin by inducing apoptosis, suggesting a potential role of miR-34a for the treatment of p53-defective prostate cancer (Fujita et al., 2008).
6.4. MYBL2 and SFPQ
Kurokawa and colleagues examined the effect of the widely used anticancer drug 5-FU on microRNA expression profiles in colon cancer cells. They identified the up-regulation of specific microRNA expression in response to 5-FU treatment. Particularly, miR-19b and miR-21 were over-expressed in 5-FU-resistant cells. After transfection of miR-19b, specific mRNAs were recruited to microRNA:mRNA complexes isolated with Ago2 antibody and subjected to whole-genome transcriptional analysis. The category “Cell Cycle” was indicated as a probable area of the molecular and cellular function related with 5-FU resistance. They validated MYBL2 and SFPQ as putative targets. MYBL2 (also known as b-Myb) is a member of a family of transcription factors involved in the control of cell cycle progression (Oh and Reddy, 1999). SFPQ has functions at different cell cycle stages to maintain sister chromatid interaction (Kurokawa et al., 2012), and depletion of this gene has been found to cause abnormal accumulation of cells in the S phase of the cell cycle (Salton et al., 2010).
6.5. DTL
Dihydrofolate reductase (DHFR) and thymidylate synthase (TYMS, TS) are two of the most important targets for antifolate-and fluoropyrimidine-based chemotherapies widely used to reduce the recurrence rates and improve the survival of a number of tumors, including osteosarcoma and colorectal cancer. Interestingly, despite the down-regulation of DHFR and TS proteins, ectopic expression of miR-215 resulted in a decreased sensitivity to methotrexate (MTX) and the TS inhibitor Tomudex (TDX). Further studies revealed that over-expression of miR-215 inhibited cell proliferation and triggered cell cycle arrest at G2 phase, and that this effect was accompanied by a p53-dependent up-regulation of p21. Moreover, denticleless protein homolog (DTL), a cell cycle-regulated nuclear and centrosome protein, was confirmed to be one of the critical targets of miR-215, and knock-down of DTL by siRNA resulted in enhanced G2-arrest, p53 and p21 induction, and reduced cell proliferation (Song et al., 2010).
7. Evasion of apoptosis and microRNAs
7.1. BCL2 family members
The first evidence that miRNAs were involved in cancer came from the finding that miR-15 and miR-16 were downregulated or deleted in most patients with chronic lymphocytic leukemia (Calin et al., 2002). Cimmino et al. demonstrated that miR-15a and miR-16-1 negatively regulated the anti-apoptotic protein B-cell lymphoma 2 (BCL2) at a posttranscriptional level, inducing apoptopsis in a leukemic cell line model (Cimmino et al., 2005). Therefore, miR-15 and miR-16 are natural antisense BCL2 interac-tors that could be used for therapy of BCL2-overexpressing tumors. Furthermore, microRNA expression profiles can distinguish normal B cells from malignant B cells in CLL (Calin and Croce, 2006).
MiR-15 and miR-16 are also involved in the development of multidrug resistance in gastric cancer cells. Expression analysis of 342 human miRNAs showed that 10 miRNAs (let-7a, miR-15b, 16a, 17-5p, −20a, −23b, −106a, −106b, −196a, −320) were downregulated more than 2-fold in the multidrug-resistant gastric cancer cell line SGC7901/VCR compared to its parental cell line SGC7901 (Xia et al., 2008). Subsequent validation experiments showed that miR-15b and miR-16, by regulating BCL2 expression levels, modulated the susceptibility of cancer cells to chemotherapeutic drug-induced apoptosis such as adriamycin, etoposide and cisplatin. Cytotoxicity was not affected after 5-FU and mitomycin treatment, suggesting that these drugs trigger apoptosis of gastric cancer cells through a BCL2 independent pathway.
In addition to miR-15a and miR-16-1, several other microRNAs have been shown to play critical roles in cancer therapy response via modulation of anti-apoptotic genes. Zhu et al. performed miRNA expression profile in six Chinese patients with chronic lymphocytic leukemia (CLL), compared to 30 healthy donors. MiR-181a and miR-181b expression was significantly lower and associated with shorter overall survival in CLL patients. Transfection of miR-181a and miR-181b into CLL cells from p53 wildtype patients led to significant increase in fludarabine-induced apoptosis compared to the cells transfected with a miRNA control through the downregulation of BCL2 and MCL1 (Zhu et al., 2010a,b). Shen et al. found that miR-497 expression was decreased in breast cancer compared to normal breast tissues. qRT-PCR and Western blot analysis data indicated that the overexpression of miR-497 resulted in the down-regulation of BCL-W at the mRNA and protein levels. The up-regulation of miR-497 caused cellular growth inhibition and apoptotic enhancement, suggesting its use as a potential therapeutic target for the treatment of breast cancer (Shen et al., 2012).
Yang et al. reported that miR-136 is downregulated in human glioma and that miR-136 overexpression promotes apoptosis of glioma cells induced by cisplatin. Two anti-apoptotic genes, BCL2 and AEG1, previously found to be upregulated in human gliomas and correlated with the development and progression of the disease (Liu et al., 2010), were identified as targets of miR-136. Restoration of BCL2 or AEG1 expression suppressed miR-136-enhanced apoptosis (Yang et al., 2012).
Zhu and colleagues, in three separated studies, found that miR-200b/c/-429 cluster, miR-497 and miR-181b regulated the response to vincristine and cisplatin of gastric and lung cancer cells. MiR-200b/c and miR-429 sensitized vincristine-resistant SGC7901/VCR and cisplatin-resistant A549/CDDP cells to vincristine- and cisplatin-induced apoptosis, at least in part via targeting the anti-apoptotic genes BCL2 and XIAP, respectively (Zhu et al, 2012). Enforced miR-497 and miR-181 expression reduced BCL2 protein level and sensitized SGC7901/VCR and A549/CDDP cells to VCR-induced and CDDP-induced apoptosis, respectively (Zhu et al., 2010a,b, 2011).
Ji et al. reported that restoration of miR-34 in Kato III cells rendered the cells 2–3-fold more sensitive to the four chemotherapeutic agents used in gastrointestinal cancer chemotherapy (doxorubicin, cisplatin, gemcitabine, and docetaxel) by downregulating BCL2. The same results were observed with BCL2 siRNA transfection (Ji et al., 2008).
The alkylating agent temozolomide (TMZ) has been shown to provide significant survival benefits for patients with glioblastoma multiforme (GBM) (Stupp et al, 2005). The molecular mechanisms underlying TMZ resistance are incompletely understood, and therapies aimed at overcoming it have attracted considerable research effort (Bocangel et al., 2002; Tentori and Graziani, 2009). Shi et al. showed that GBM cells with acquired TMZ-resistance (R-D54MG) exhibited significantly higher levels of miR-21 expression than their parental control cells, probably by reducing the BCL2-associated X (BAX)/BCL2 ratio and caspase-3 activity in treatment-naive GBM cells (Shi et al., 2010).
7.2. TRAIL
TNF-related apoptosis-inducing ligand (TRAIL) induces programmed cell death in cancer cells. However, a significant proportion of cancers are resistant to TRAIL-induced apoptosis and often this resistance could be associated with the overexpression of antiapoptotic proteins or the low expression of TRAIL receptors. An attractive and preclinically successful strategy, therefore, is to identify combination treatments that sensitize otherwise resistant cancers to TRAIL. TRAIL and agonistic antibodies raised against TRAIL death receptors are highly promising new anticancer agents. Soluble rhTRAIL (also named dulanermin), the TRAIL-R1-targeting monoclonal agonistic antibody mapatumumab, and the TRAIL-R2-targeting monoclonal agonistic antibodies lexatu-mumab, conatumumab, tigatuzumab, and DAB4 have entered clinical studies. A large number of phase I and phase II clinical trials have been undertaken with these agents by now or are still ongoing, either as monotherapy or in combination with other chemotherapeutic drugs in both solid and non solid malignant neoplasms. In this regard, microRNAs, by regulating gene expression, have been shown to sensitize cancer cells to TRAIL-induced apoptosis, representing a promising tool to increase TRAIL effects in many tumors.
Xie and colleagues demonstrated that miR-24 directly down-regulated XIAP protein expression by targeting its 3’ UTR, inducing sensitivity to TRAIL-induced apoptosis in lung TRAIL-resistant cells (Xie et al., 2012). Razumilava et al. identified elevated miR-25 expression in malignant cholangiocarcinoma cell lines as well as patient samples. Functionally, miR-25 was shown to protect cells against TRAIL-induced apoptosis. They confirmed, by immunoblot and luciferase assay, that the Death Receptor 4 (DR4) was a direct target of miR-25 (Razumilava et al., 2012).
Activation of MET signaling is a frequent genetic event observed in lung cancer development (Benedettini et al., 2010). Our group reported that the MET oncogene, through activation of c-Jun and JNK, regulates miR-221&222 levels, which, in turn, by downregulating PTEN and TIMP3 promote tumorigenesis and TRAIL resistance in lung cancer (Garofalo et al., 2009). Taken together the results suggest that therapeutic intervention, involving the use of microRNAs, should not only sensitize tumor cells to drug-inducing apoptosis but also inhibit survival, proliferation, and invasive capabilities of different cancers.
7.3. FLIP
Chen et al. disclosed that the FLICE-like inhibitory protein (FLIP) was overexpressed in HepG2 hepatocellular carcinoma cells and downregulation of FLIP enhanced taxol-induced apoptosis. Luciferase reporter assay demonstrated that miR-512-3p negatively regulated FLIP expression via a conserved miRNA-binding site in 3’ untranslated region (3’UTR) (Chen et al., 2010).
7.4. BMF
In order to dissect miRNAs implicated in the pro-apoptotic pathway, Catuogno et al. engineered an NSCLC-derived cell line to induce caspase-8-dependent cell death. By using a functional selection, they identified miRNAs whose expression may protect cells from undergoing apoptosis. Among them miR-34c-5p markedly increased resistance to paclitaxel-induced apoptosis. Furthermore, they demonstrated that BCL2-modifying factor (BMF) is a target of miR-34c-5p, and that its silencing, together with that of c-Myc, contributes to resistance to apoptosis induced by paclitaxel through p53 downregulation (Catuogno et al., 2012).
8. MicroRNAs as therapeutic strategy
Results generated in recent years from genomic and proteomic approaches have changed our view on cancer. As a result, approaches to cancer therapies are shifting to accommodate these new findings and to devise more effective and safer remedies. Conventional therapies, still the prevailing form of therapy, are frequently deemed too toxic or inadequate due to chemoresistance. However, many targeted therapies alone are insufficiently effective and will have to be used in combination. This confirms that cancer is the product of various genetic and epigenetic changes and requires interference with multiple oncogenic pathways for successful intervention.
MicroRNA therapeutics appears as a novel field in which miRNA activity is the major target of the intervention (Hemida et al., 2010; Sibley et al., 2010; Kota and Balasubramanian, 2010). The rationale for developing miRNA therapeutics is based on the premise that aberrantly expressed miRNAs play key roles in the development of human disease and resistance to the actual chemotherapy and that correcting these miRNA deficiencies by either antagonizing or restoring miRNA function may provide a therapeutic benefit. Approximately 3% of human genes encode for miRNAs, and up to 60% of human protein coding genes may be regulated by miRNAs (Fabian et al., 2010). One therapeutically interesting concept is that a single miRNA can negatively regulate multiple target proteins through interaction with different target mRNAs. Thus far, researchers have successfully used both miRNA mimics and anti-miRNAs in order to restore normal gene networks in cancer cell lines and animal xenograft models. These findings suggest that miRNA manipulations may be valid anti-cancer therapies.
9. AntagomiRs and miRNA mimics
The therapeutic application of miRNAs involves two systems. One system aims to inhibit oncogenic miRNAs by using miRNA antagonists, such as anti-miRs, locked-nucleic acids (LNA) or antagomiRs. MiRNA antagonists are single-stranded RNA molecules of about 21–23 nucleotides in length and complementary to the mature target miRNA. They specifically silence miRNA expression resulting in the upregulation of hundreds of genes predicted to be repressed by the down-modulated microRNA. The second strategy, miRNA replacement, involves the re-introduction of a tumor suppressor miRNA mimic to restore a loss-of-function (Bader et al., 2010).
The therapeutic use of miRNAs has gained much attention because they control tens to hundreds of genes and by controlling several cellular pathways at once. Although simultaneous targeting of multiple disease genes may be viewed as a powerful trait of therapeutic miRNA mimics, it also raises concerns about potential toxicity, especially because delivery of miRNA mimics will lead to an accumulation of exogenous miRNA in normal cells. Even though these assumptions are understandable, in vivo evidence for toxicity induced by miRNA mimics is still lacking. Mouse studies that evaluated the therapeutic delivery of tumor suppressor miRNAs failed to find adverse events associated with the miRNA and suggest that delivery of miRNA to normal tissues was well tolerated (Kota et al., 2009; Wiggins et al., 2010). Indeed, administration of therapeutic miRNA mimics is only an insignificant incremental increase of what is already present in normal cells. Moreover, normal cells are not addicted to oncogenic pathways and manage to recover from the therapeutic dose used. The main challenge for successful translation into the clinic remains in vivo delivery, which will be the focus of future therapeutic development efforts to harness the full potential of miRNAs.
10. MicroRNA delivery
The transition from bench to bedside of a miRNA-based cancer therapy depends on the availability of a clinically relevant delivery system. Systemic delivery of siRNA or miRNA as anti-cancer approaches in preclinical models has made use of liposomes (Rai et al, 2011), viral vectors (Kota et al, 2009) and nanoparticles (Su et al., 2011).
10.1. Liposomes
Wiggins et al. enabled systemic delivery of miR-34a mimics using a neutral lipid emulsion (NLE) that has the potential to be translated into the clinic (Wiggins et al., 2010). Unlike most lipid-based delivery systems, NLE does not contain cationic lipids, and therefore, may bypass some of the shortcomings that can be attributed to charge. For instance, particles based on neutral lipids are less likely to form aggregates in biofluids, be filtered by the liver or adhere to the endothelium (Landen et al., 2005). In accordance, NLE did not lead to a preferential accumulation of miRNA in liver, but instead, displayed excellent delivery to normal lung and lung tumors. Systemic delivery of miR-34a mimics led to an accumulation of miR-34a in tumor tissues, repression of direct miR-34a targets and robust inhibition of NSCLC xenografts in mice. In another study, Trang and colleagues showed LNE therapeutic benefit in mouse models of lung cancer. Therapeutic delivery was demonstrated using mimics of the tumor suppressors, microRNA-34a (miR-34a) and let-7, both of which are often down regulated or lost in lung cancer. Of note, systemic treatment of a K-ras-activated autochthonous mouse model of non-small cell lung cancer (NSCLC) led to a significant decrease in tumor burden, supporting the development of these let-7 and miR-34 formulations as novel targeted therapies for lung cancer patients (Trang et al., 2011).
miRNA therapeutics screened multiple existing delivery systems and developed the best liposomal miRNA delivery technology, the NOV340 technology, employing an ionizable liposome that forms a particle with a diameter of ~120 nm. The lipids and miRNA mimics are mixed under acidic conditions to facilitate efficient miRNA encapsulation and liposome formation. The pharmacology of the NOV340/miR-34a formulation has already been tested in an orthotopic model of hepatocellular carcinoma. Treatment of mice carrying existing tumors led to a significant tumor regression and prolonged survival (Bader, 2012). Histology of the livers revealed that several of the mice appeared to be tumor-free compared to mice dosed with NOV340 particles with a scrambled control. The NOV340/miR-34a–treated animals did not have any drug-related side effects or toxicity. The company anticipates initiating clinical trials in 2013 for miR-34 that may be one of the first miRNA mimics to reach the clinic.
10.2. Viral vectors
Viruses have been widely used for gene therapy. Modified adenovirus, adeno-associated virus, and lentiviruses have been employed to successfully deliver siRNA/shRNA into cells and stably integrate siRNA/shRNA into targeted genomes (Rubinson et al., 2003). Similar to human chronic lymphocytic leukemia (CLL), the de novo New Zealand Black (NZB) mouse model has a genetically determined age-associated increase in malignant B-1 clones and decreased expression of microRNAs miR-15a and miR-16 in B-1 cells. Kasar et al. employed lentiviral vectors in vivo to restore miR-15a/16 in NZB. Of note, mice treated with the miR-expressing lentivirus had decreased disease and the lentivirus had little systemic toxicity while preferentially targeting B-1 cells (Kasar et al., 2012).
10.3. Nanoparticles
Nanotechnology has enabled significant advances in the areas of cancer therapy. The field of drug delivery is a sterling example, with nanoparticles being increasingly used for generating therapeutic formulations of poorly water-soluble, yet potent anticancer drugs. Emerging evidence shows that nanoparticle delivery is not only as effective as but also less toxic than the previously mentioned vehicles (Bisht et al., 2008). Su et al. showed that systemic delivery of a chemically stabilized anti-miR-122 complexed with interfering nanoparticles (iNOPs) effectively silenced the liver-expressed miR-122 in mice. Intravenous administration of 2mg/kg chemically modified anti-miR-122 complexed with iNOP-7 resulted in specific silencing of miR-122, accompanied by a decrease of plasma cholesterol. The specific silencing of miR-122 was long lasting and did not induce an immune response, demonstrating that iNOPs can successfully deliver anti-miR to specifically target and silence miRNA in clinically acceptable and therapeutically affordable doses (Su et al., 2011).
10.4. LNA-modified oligonucleotides
Lanford and colleagues found that treatment of chronically infected chimpanzees with a locked nucleic acid (LNA)-modified oligonucleotide complementary to miR-122 led to long-lasting suppression of HCV viremia, with no side effects in the treated animals (Lanford et al., 2010). This inhibitor of miR-122 (Miravirsen) is currently in Phase II clinical trials, becoming the first microRNA therapeutic in humans. The anti-viral Proof-of-Concept (PoC) study demonstrated that four out of nine patients treated at the highest dose (7mg/kg) with miravirsen became Hepatitis C Virus (HCV) RNA undetectable within four weeks of dosing (http://mobile.ilcapp.eu/EASL161/poster_24294/program.aspx). These data provide clinical evidence that miravirsen’s unique mechanism-of-action offers a high barrier to viral resistance and the potential for cure with monotherapy.
11. Conclusions
Recent data demonstrate that selective modulation of microRNA activity can improve the response to chemotherapy. Although RNA interference-based therapeutic strategies employing siRNAs have huge potential, the toxicity of siRNAs in preclinical mouse models is of great concern for the ongoing phase I/II clinical trials. In contrast, miRNAs are believed to be relatively safe and have been more effective in cancer treatment in early preclinical studies. Although there are still several obstacles to overcome before clinical testing of miRNA therapeutics, such as delivery and chemical modification of miRNA modulators, it can be expected that in the near future miRNAs and miRNA-targeting oligonucleotides may become promising tools in the fight against cancer. Moreover, the discovery of more miRNA targets will be useful to better define cancer cell signaling and to identify new and more effective drug targets.
Acknowledgements
We thank Justin Middleton and Gianpiero Di Leva for editing of the manuscript and helpful discussions. M.G. is recipient of the Kimmel Scholar Award 2011.
Contributor Information
Michela Garofalo, Email: Michela.garofalo@osumc.edu.
Carlo M. Croce, Email: Carlo.Croce@osumc.edu.
References
- Alder H, Taccioli C, Chen H, Jiang Y, Smalley KJ, Fadda P, Ozer HG, Huebner K, Farber JL, Croce CM, Fong LY. Dysregulation of miR-31 and miR-21 induced by zinc deficiency promotes esophageal cancer. Carcinogenesis. 2012;9:1736–1744. doi: 10.1093/carcin/bgs204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashkenazi A. Targeting death and decoy receptors of the tumour-necrosis factor superfamily. Nature Reviews Cancer. 2002;2:420–430. doi: 10.1038/nrc821. [DOI] [PubMed] [Google Scholar]
- Bader AG, Brown D, Winkler M. The promise of microRNA replacement therapy. Cancer Research. 2010;70:7027–7030. doi: 10.1158/0008-5472.CAN-10-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader AG. miR-34– a microRNA replacement therapy is headed to the clinic. Frontiers in Genetics. 2012;3:120. doi: 10.3389/fgene.2012.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- Becam I, Rafel N, Hong X, Cohen SM, Milán M. Notch-mediated repression of bantam miRNA contributes to boundary formation in the Drosophila wing. Development. 2011;138:3781–3789. doi: 10.1242/dev.064774. [DOI] [PubMed] [Google Scholar]
- Beck J, Niethammer D, Gekeler V. MDR1, MRP, topoisomerase IIalpha/beta, and cyclin A gene expression in acute and chronic leukemias. Leukemia. 1996;(Suppl. 3):S39–S45. [PubMed] [Google Scholar]
- Benedettini E, Sholl LM, Peyton M, Reilly J, Ware C, Davis L, Vena N, Bailey D, Yeap BY, Fiorentino M, Ligon AH, Pan BS, Richon V, Minna JD, Gazdar AF, Draetta G, Bosari S, Chirieac LR, Lutterbach B, Loda M. Met activation in non-small cell lung cancer is associated with de novo resistance to EGFR inhibitors and the development of brain metastasis. American Journal of Pathology. 2010;177:415–423. doi: 10.2353/ajpath.2010.090863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisht S, Feldmann G, Koorstra JB, Mullendore M, Alvarez H, Karikari C, Rudek MA, Lee CK, Maitra A, Maitra A. In vivo characterization of a polymeric nanoparticle platform with potential oral drug delivery capabilities. Molecular Cancer Therapeutics. 2008;7:3878–3888. doi: 10.1158/1535-7163.MCT-08-0476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitarte N, Bandres E, Boni V, Zarate R, Rodriguez J, Gonzalez-Huarriz M, Lopez I, Javier Sola J, Alonso MM, Fortes P, Garcia-Foncillas J. MicroRNA-451 is involved in the self-renewal, tumorigenicity, and chemoresistance of colorectal cancer stem cells. Stem Cells. 2011;29:1661–1671. doi: 10.1002/stem.741. [DOI] [PubMed] [Google Scholar]
- Bocangel DB, Finkelstein S, Schold SC, Bhakat KK, Mitra S, Kokkinakis DM. Multifaceted resistance of gliomas to temozolomide. Clinical Cancer Research. 2002;8:2725–2734. [PubMed] [Google Scholar]
- Bommer GT, Gerin I, Feng Y, Kaczorowski AJ, Kuick R, Love RE, Zhai Y, Giordano TJ, Qin ZS, Moore BB, MacDougald OA, Cho KR, Fearon ER. p53-mediated activation ofmiRNA34 candidate tumor-suppressor genes. Current Biology. 2007;15:1298–1307. doi: 10.1016/j.cub.2007.06.068. [DOI] [PubMed] [Google Scholar]
- Borel F, Han R, Visser A, Petry H, van Deventer SJ, Jansen PL, Konstanti-nova P. Adenosine triphosphate-binding cassette transporter genes up-regulation in untreated hepatocellular carcinoma is mediated by cellular microRNAs. Hepatology. 2012;3:821–832. doi: 10.1002/hep.24682. [DOI] [PubMed] [Google Scholar]
- Borchert GM, Lanier W, Davidson BL. RNA polymerase III transcribes human microRNAs. Nature Structural & Molecular Biology. 2006;12:1097–1101. doi: 10.1038/nsmb1167. [DOI] [PubMed] [Google Scholar]
- Borst P, Evers R, Kool M, Wijnholds J. A family of drug transporters: the multidrug resistance-associated proteins. Journal of the National Cancer Institute. 2000;16:1295–1302. doi: 10.1093/jnci/92.16.1295. [DOI] [PubMed] [Google Scholar]
- Calin GA, Dumitru CD, Shimizu M, Bichi R, Zupo S, Noch E, Aldler H, Rattan S, Keating M, Rai K, Rassenti L, Kipps T, Negrini M, Bullrich F, Croce CM. Frequent deletions and down-regulation of micro-RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proceedings of the National Academy of Sciences of the United States of America. 2002;24:15524–15529. doi: 10.1073/pnas.242606799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calin GA, Croce CM. Genomics of chronic lymphocytic leukemia microRNAs as new players with clinical significance. Seminars in Oncology. 2006;2:167–173. doi: 10.1053/j.seminoncol.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Cannistra SA. Cancer of the ovary. New England Journal of Medicine. 2004;351(24):2519–2529. doi: 10.1056/NEJMra041842. [DOI] [PubMed] [Google Scholar]
- Catuogno S, Cerchia L, Romano G, Pognonec P, Condorelli G, de Franciscis V. miR-34c may protect lung cancer cells from paclitaxel-induced apoptosis. Oncogene. 2012 doi: 10.1038/onc.2012.51. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Chen CF, He X, Arslan AD, Mo YY, Reinhold WC, Pommier Y, Beck WT. Novel regulation of nuclear factor-YB by miR-485-3p affects the expression of DNA topoisomerase IIα and drug responsiveness. Molecular Pharmacology. 2011a;4:735–744. doi: 10.1124/mol.110.069633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Zhu HH, Zhou LF, Wu SS, Wang J, Chen Z. Inhibition of c-FLIP expression by miR-512-3p contributes to taxol-induced apoptosis in hepatocellular carcinoma cells. Oncology Reports. 2010;5:1457–1462. doi: 10.3892/or_00000784. [DOI] [PubMed] [Google Scholar]
- Chen J, Tian W, Cai H, He H, Deng Y. Down-regulation of microRNA-200c is associated with drug resistance in human breast cancer. Medical Oncology. 2011b doi: 10.1007/s12032-011-0117-4. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Cheng W, Liu T, Wan X, Gao Y, Wang H. MicroRNA-199a targets CD44 to suppress the tumorigenicity and multidrug resistance of ovarian cancer-initiating cells. FEBS Journal. 2012;279:2047–2059. doi: 10.1111/j.1742-4658.2012.08589.x. [DOI] [PubMed] [Google Scholar]
- Chim CS, Wan TS, Wong KY, Fung TK, Drexler HG, Wong KF. Methylation of miR-34a, miR-34b/c, miR-124-1 and miR-203 in Ph-negative myeloproliferative neoplasms. Journal of Translational Medicine. 2011;9:197. doi: 10.1186/1479-5876-9-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung TD, Drake FH, Tan KB, Per SR, Crooke ST, Mirabelli CK. Characterization and immunological identification of cDNA clones encoding two human DNA topoisomerase II isozymes. Proceedings of the National Academy of Sciences of the United States of America. 1989;23:9431–9435. doi: 10.1073/pnas.86.23.9431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimmino A, Calin GA, Fabbri M, Iorio MV, Ferracin M, Shimizu M, Wojcik SE, Aqeilan RI, Zupo S, Dono M, Rassenti L, Alder H, Volinia S, Liu CG, Kipps TJ, Negrini M, Croce CM. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:13944–13949. doi: 10.1073/pnas.0506654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean M, Rzhetsky A, Allikmets R. The human ATP-binding cassette (ABC) transporter superfamily. Genome Research. 2001;11:1156–1166. doi: 10.1101/gr.184901. [DOI] [PubMed] [Google Scholar]
- Deffie AM, McPherson JP, Gupta RS, Hedley DW, Goldenberg GJ. Multifactorial resistance to antineoplastic agents in drug-resistant P388 murine leukemia, Chinese hamster ovary, and human HeLa cells, with emphasis on the role of DNA topoisomerase II. Biochemistry and Cell Biology. 1992;5:354–364. doi: 10.1139/o92-055. [DOI] [PubMed] [Google Scholar]
- Derynck R, Akhurst RJ, Balmain A. TGF-beta signaling in tumor suppression and cancer progression. Nature Genetics. 2001;2:117–129. doi: 10.1038/ng1001-117. [DOI] [PubMed] [Google Scholar]
- Doyle LA, Yang W, Abruzzo LV, Krogmann T, Gao Y, Rishi AK, Ross DD. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proceedings of the National Academy of Sciences of the United States of America. 1998;26:15665–15670. doi: 10.1073/pnas.95.26.15665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan EA, Anest V, Cogswell P, Baldwin AS. The kinases MSK1 and MSK2 are required for epidermal growth factor-induced, but not tumor necrosis factor-induced, histone H3 Ser10 phosphorylation. Journal of Biological Chemistry. 2006;18:12521–12525. doi: 10.1074/jbc.M513333200. [DOI] [PubMed] [Google Scholar]
- El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. New England Journal of Medicine. 1999;10:745–750. doi: 10.1056/NEJM199903113401001. [DOI] [PubMed] [Google Scholar]
- Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, Lindeman N, Gale CM, Zhao X, Christensen J, Kosaka T, Holmes AJ, Rogers AM, Cappuzzo F, Mok T, Lee C, Johnson BE, Cantley LC, Jänne PA. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- Fabian MR, Sonenberg N, Filipowicz W. Regulation of mRNA translation and stability by microRNAs. Annual Review of Biochemistry. 2010;79:351–379. doi: 10.1146/annurev-biochem-060308-103103. [DOI] [PubMed] [Google Scholar]
- Feng B, Wang R, Song HZ, Chen LB. MicroRNA-200b reverses chemoresistance of docetaxel-resistant human lung adenocarcinoma cells by targeting E2F3. Cancer. 2012;118:3365–3376. doi: 10.1002/cncr.26560. [DOI] [PubMed] [Google Scholar]
- Fishel R. The selection for mismatch repair defects in hereditary nonpolyposis colorectal cancer: revising the mutator hypothesis. Cancer Research. 2001;20:7369–7374. [PubMed] [Google Scholar]
- Friedenson B. The BRCA1/2 pathway prevents hematologic cancers in addition to breast and ovarian cancers. BMC Cancer. 2007;6(7):152. doi: 10.1186/1471-2407-7-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita Y, Kojima K, Hamada N, Ohhashi R, Akao Y, Nozawa Y, Deguchi T, Ito M. Effects of miR-34a on cell growth and chemoresistance in prostate cancer PC3 cells. Biochemical and Biophysical Research Communications. 2008;1:1. doi: 10.1016/j.bbrc.2008.09.086. [DOI] [PubMed] [Google Scholar]
- Fujita Y, Kojima K, Ohhashi R, Hamada N, Nozawa Y, Kitamoto A, Sato A, Kondo S, Kojima T, Deguchi T, Ito M. MiR-148a attenuates pacli-taxel resistance of hormone-refractory, drug-resistant prostate cancer PC3 cells by regulating MSK1 expression. Journal of Biological Chemistry. 2010;25:19076–19084. doi: 10.1074/jbc.M109.079525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garofalo M, Di Leva G, Romano G, Nuovo G, Suh SS, Ngankeu A, Taccioli C, Pichiorri F, Alder H, Secchiero P, Gasparini P, Gonelli A, Costinean S, Acunzo M, Condorelli G, Croce CM. miR-221&222 regulate TRAIL resistance and enhance tumorigenicity through PTEN and TIMP3 downregulation. Cancer Cell. 2009;6:498–509. doi: 10.1016/j.ccr.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Garofalo M, Romano G, Di Leva G, Nuovo G, Jeon YJ, Ngankeu A, Sun J, Lovat F, Alder H, Condorelli G, Engelman JA, Ono M, Rho JK, Cascione L, Volinia S, Nephew KP, Croce CM. EGFR and MET receptor tyrosine kinase-altered microRNA expression induces tumorigenesis and gefitinib resistance in lung cancers. Nature Medicine. 2011;18:74–82. doi: 10.1038/nm.2577. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Gompelmann D, Eberhardt R, Herth FJ. Advanced malignant lung disease: what the specialist can offer. Respiration. 2011;2:111–123. doi: 10.1159/000329703. [DOI] [PubMed] [Google Scholar]
- Haenisch S, Laechelt S, Bruckmueller H, Werk A, Noack A, Bruhn O, Remm-ler C, Cascorbi I. Down-regulation of ATP-binding cassette C2 protein expression in HepG2 cells after rifampicin treatment is mediated by microRNA-379. Molecular Pharmacology. 2011;2:314–320. doi: 10.1124/mol.110.070714. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;1:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Hayes JD, Pulford DJ. The glutathione S-transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Critical Reviews in Biochemistry and Molecular Biology. 1995;6:445–600. doi: 10.3109/10409239509083491. [DOI] [PubMed] [Google Scholar]
- He L, He X, Lim LP, de Stanchina E, Xuan Z, Liang Y, Xue W, Zender L, Magnus J, Ridzon D, Jackson AL, Linsley PS, Chen C, Lowe SW, Cleary MA, Hannon GJ. A microRNA component of the p53 tumour suppressor network. Nature. 2007;7148:1130–1134. doi: 10.1038/nature05939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemida MG, Ye X, Thair S, Yang D. Exploiting the therapeutic potential of microRNAs in viral diseases: expectations and limitations. Molecular Diagnosis & Therapy. 2010;14:271–282. doi: 10.1007/BF03256383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howie AF, Forrester LM, Glancey MJ, Schlager JJ, Powis G, Beckett GJ, Hayes JD, Wolf CR. Glutathione S-transferase and glutathione peroxidase expression in normal and tumour human tissues. Carcinogenesis. 1990;3:451–458. doi: 10.1093/carcin/11.3.451. [DOI] [PubMed] [Google Scholar]
- Hutvágner G, McLachlan J, Pasquinelli AE, Bálint E, Tuschl T, Zamore PD. A cellular function for the RNA-interference enzyme Dicer in the maturation of the let-7 small temporal RNA. Science. 2001;293:834–838. doi: 10.1126/science.1062961. [DOI] [PubMed] [Google Scholar]
- Iorio MV, Visone R, Di Leva G, Donati V, Petrocca F, Casalini P, Taccioli C, Volinia S, Liu CG, Alder H, Calin GA, Ménard S, Croce CM. MicroRNA signatures in human ovarian cancer. Cancer Research. 2007;18:8699–8707. doi: 10.1158/0008-5472.CAN-07-1936. [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Kuo MT, Furuta K, Suzuki M. The human multidrug resistance-associated protein (MRP) gene family: from biological function to drug molecular design. Clinical Chemistry and Laboratory Medicine. 2000;9:893–897. doi: 10.1515/CCLM.2000.130. [DOI] [PubMed] [Google Scholar]
- Jedlitschky G, Leier I, Buchholz U, Barnouin K, Kurz G, Keppler D. Transport of glutathione, glucuronate, and sulfate conjugates by the MRP gene-encoded conjugate export pump. Cancer Research. 1996;5:988–994. [PubMed] [Google Scholar]
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics. CA: A Cancer Journal for Clinicians. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- Ji Q, Hao X, Meng Y, Zhang M, Desano J, Fan D, Xu L. Restoration of tumor suppressor miR-34 inhibits human p53-mutant gastric cancer tumorspheres. BMC Cancer. 2008;8:266. doi: 10.1186/1471-2407-8-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano RL, Ling V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochimica et Biophysica Acta. 1976;1:152–162. doi: 10.1016/0005-2736(76)90160-7. [DOI] [PubMed] [Google Scholar]
- Kansra S, Yamagata S, Sneade L, Foster L, Ben-Jonathan N. Differential effects of estrogen receptor antagonists on pituitary lactotroph proliferation and prolactin release. Molecular and Cellular Endocrinology. 2005;239:27–36. doi: 10.1016/j.mce.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Kasar S, Salerno E, Yuan Y, Underbayev C, Vollenweider D, Laurindo MF, Fernandes H, Bonci D, Addario A, Mazzella F, Raveche E. Systemic in vivo lentiviral delivery of miR-15a/16 reduces malignancy in the NZB de novo mouse model of chronic lymphocytic leukemia. Genes and Immunity. 2012;13:109–119. doi: 10.1038/gene.2011.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kast C, Gros P. Topology mapping of the amino-terminal half of multidrug resistance-associated protein by epitope insertion and immunofluorescence. Journal of Biological Chemistry. 1997;272:26479–26487. doi: 10.1074/jbc.272.42.26479. [DOI] [PubMed] [Google Scholar]
- Ketterer B. A bird’s eye view of the glutathione transferase field. Chemico-Biological Interactions. 2001;1:27–42. doi: 10.1016/s0009-2797(01)00277-0. [DOI] [PubMed] [Google Scholar]
- Ketting RF, Fischer SE, Bernstein E, Sijen T, Hannon GJ, Plasterk RH. Dicer functions in RNA interference and in synthesis of small RNA involved in developmental timing in C. elegans . Genes and Development. 2001;15:2654–2659. doi: 10.1101/gad.927801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S, Boggon TJ, Dayaram T, Jänne PA, Kocher O, Meyerson M, Johnson BE, Eck MJ, Tenen DG, Halmos B. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. New England Journal of Medicine. 2005;8:786–792. doi: 10.1056/NEJMoa044238. [DOI] [PubMed] [Google Scholar]
- Kota J, Chivukula RR, O’Donnell KA, Wentzel EA, Montgomery CL, Hwang HW, Chang TC, Vivekanandan P, Torbenson M, Clark KR, Mendell JR, Mendell JT. Therapeutic microRNA delivery suppresses tumorigenesis in a murine liver cancer model. Cell. 2009;137:1005–1017. doi: 10.1016/j.cell.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kota SK, Balasubramanian S. Cancer therapy via modulation of microRNA levels: a promising future. Drug Discovery Today. 2010;15:733–740. doi: 10.1016/j.drudis.2010.07.003. [DOI] [PubMed] [Google Scholar]
- Krueger A, Baumann S, Krammer PH, Kirchhoff S. FLICE inhibitory proteins: regulators of death receptor-mediated apoptosis. Molecular and Cellular Biology. 2001;21:8247–8254. doi: 10.1128/MCB.21.24.8247-8254.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurokawa K, Tanahashi T, Iima T, Yamamoto Y, Akaike Y, Nishida K, Masuda K, Kuwano Y, Murakami Y, Fukushima M, Rokutan K. Role of miR-19b and its target mRNAs in 5-fluorouracil resistance in colon cancer cells. Journal of Gastroenterology. 2012;47:883–895. doi: 10.1007/s00535-012-0547-6. [DOI] [PubMed] [Google Scholar]
- Landen CN, Jr, Chavez-Reyes A, Bucana C, Schmandt R, Deavers MT, Lopez-Berestein G, Sood AK. Therapeutic EphA2 gene targeting in vivo using neutral liposomal small interfering RNA delivery. Cancer Research. 2005;65:6910–6918. doi: 10.1158/0008-5472.CAN-05-0530. [DOI] [PubMed] [Google Scholar]
- Lanford RE, Hildebrandt-Eriksen ES, Petri A, Persson R, Lindow M, Munk ME, Kauppinen S, Ørum H. Therapeutic silencing of microRNA-122 in primates with chronic hepatitis C virus infection. Science. 2010;5962:198–201. doi: 10.1126/science.1178178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen AK, Skladanowski A. Cellular resistance to topoisomerase-targeted drugs: from drug uptake to cell death. Biochimica et Biophysica Acta. 1998;1–3:257–274. doi: 10.1016/s0167-4781(98)00140-7. [DOI] [PubMed] [Google Scholar]
- Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–854. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- Lee Y, Ahn C, Han J, Choi H, Kim J, Yim J, Lee J, Provost P, Rådmark O, Kim S, Kim VN. The nuclear RNase III Drosha initiates microRNA processing. Nature. 2003;425:415–419. doi: 10.1038/nature01957. [DOI] [PubMed] [Google Scholar]
- Lee Y, Kim M, Han J, Yeom KH, Lee S, Baek SH, Kim VN. MicroRNA genes are transcribed by RNA polymerase II. EMBO Journal. 2004;20:4051–4060. doi: 10.1038/sj.emboj.7600385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Hu S, Wang J, Cai J, Xiao L, Yu L, Wang Z. MiR-27a modulates MDR1/P-glycoprotein expression by targeting HIPK2 in human ovarian cancer cells. Gynecologic Oncology. 2010;1:125–130. doi: 10.1016/j.ygyno.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Liang Z, Wu H, Xia J, Li Y, Zhang Y, Huang K, Wagar N, Yoon Y, Cho HT, Scala S, Shim H. Involvement of miR-326 in chemotherapy resistance of breast cancer through modulating expression of multidrug resistance-associated protein 1. Biochemical Pharmacology. 2009;79:817–824. doi: 10.1016/j.bcp.2009.10.017. [DOI] [PubMed] [Google Scholar]
- Liu L, Wu J, Ying Z, Chen B, Han A, Liang Y, Song L, Yuan J, Li J, Li M. Astrocyte elevated gene-1 upregulates matrix metalloproteinase-9and induces human glioma invasion. Cancer Research. 2010;9:3750–3759. doi: 10.1158/0008-5472.CAN-09-3838. [DOI] [PubMed] [Google Scholar]
- Lodygin D, Tarasov V, Epanchintsev A, Berking C, Knyazeva T, Körner H, Knyazev P, Diebold J, Hermeking H. Inactivation of miR-34a by aberrant CpG methylation in multiple types of cancer. Cell Cycle. 2008;16:2591–2600. doi: 10.4161/cc.7.16.6533. [DOI] [PubMed] [Google Scholar]
- Longley DB, Harkin DP, Johnston PG. 5-Fluorouracil: mechanisms of action and clinical strategies. Nature Reviews Cancer. 2003;5:330–338. doi: 10.1038/nrc1074. [DOI] [PubMed] [Google Scholar]
- Lobert S, Jefferson B, Morris K. Regulation of β-tubulin isotypes by microRNA 100 in MCF7 breast cancer cells. Cytoskeleton (Hoboken) 2011;6:355–362. doi: 10.1002/cm.20517. [DOI] [PubMed] [Google Scholar]
- Meng S, Cao J, Wang L, Zhou Q, Li Y, Shen C, Zhang X, Wang C. MicroRNA 107 partly inhibits endothelial progenitor cells differentiation via HIF-1β. PLoS ONE. 2012;7:e40323. doi: 10.1371/journal.pone.0040323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers M, Wagner MW, Mazurek A, Schmutte C, Fishel R, Boothman DA. DNA mismatch repair-dependent response to fluoropyrimidine-generated damage. Journal of Biological Chemistry. 2005;7:5516–5526. doi: 10.1074/jbc.M412105200. [DOI] [PubMed] [Google Scholar]
- Miller TE, Ghoshal K, Ramaswamy B, Roy S, Datta J, Shapiro CL, Jacob S, Majumder S. MicroRNA-221/222 confers tamoxifen resistance in breast cancer by targeting p27Kip1. Journal of Biological Chemistry. 2008;44:29897–29903. doi: 10.1074/jbc.M804612200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake K, Mickley L, Litman T, Zhan Z, Robey R, Cristensen B, Brangi M, Greenberger L, Dean M, Fojo T, Bates SE. Molecular cloning of cDNAs which are highly overexpressed in mitoxantrone-resistant cells: demonstration of homology to ABC transport genes. Cancer Research. 1999;1:8–13. [PubMed] [Google Scholar]
- Moskwa P, Buffa FM, Pan Y, Panchakshari R, Gottipati P, Muschel RJ, Beech J, Kulshrestha R, Abdelmohsen K, Weinstock DM, Gorospe M, Harris AL, Helleday T, Chowdhury D. miR-182-mediateddownregulationofBRCA1 impacts DNA repair and sensitivity to PARP inhibitors. Molecular Cell. 2011;2:210–220. doi: 10.1016/j.molcel.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller CR, Roskelley C. Regulation of BRCA1 expression and its relationship to sporadic breast cancer. Breast Cancer Research. 2003;1:45–52. doi: 10.1186/bcr557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata S. Fas ligand-induced apoptosis. Annual Review of Genetics. 1999;33:29–55. doi: 10.1146/annurev.genet.33.1.29. [DOI] [PubMed] [Google Scholar]
- Nardinocchi L, Puca R, D’Orazi G. HIF-1α antagonizes p53-mediated apoptosis by triggering HIPK2 degradation. Aging (Albany, NY) 2011;1:33–43. doi: 10.18632/aging.100254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitiss JL. Targeting DNA topoisomerase II in cancer chemotherapy. Nature Reviews Cancer. 2009;5:338–350. doi: 10.1038/nrc2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh IH, Reddy EP. The myb gene family in cell growth, differentiation and apoptosis. Oncogene. 1999;18:3017–3033. doi: 10.1038/sj.onc.1202839. [DOI] [PubMed] [Google Scholar]
- Ozols RF. Treatment goals in ovarian cancer. International Journal of Gynecological Cancer. 2005;1:3–11. doi: 10.1111/j.1525-1438.2005.15351.x. [DOI] [PubMed] [Google Scholar]
- Pan YZ, Morris ME, Yu AM. MicroRNA-328 negatively regulates the expression of breast cancer resistance protein (BCRP/ABCG2) in human cancer cells. Molecular Pharmacology. 2009;6:1374–1379. doi: 10.1124/mol.108.054163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak AK, Bhutani M, Mohan A, Guleria R, Bal S, Kochupillai V. Non small cell lung cancer (NSCLC): current status and future prospects. Indian Journal of Chest Diseases and Allied Sciences. 2004;3:191–203. [PubMed] [Google Scholar]
- Pommier Y, Leo E, Zhang H, Marchand C. DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chemistry and Biology. 2010;5:421–433. doi: 10.1016/j.chembiol.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponti D, Costa A, Zaffaroni N, Pratesi G, Petrangolini G, Coradini D, Pilotti S, Pierotti MA, Daidone MG. Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Research. 2005;65:5506–5511. doi: 10.1158/0008-5472.CAN-05-0626. [DOI] [PubMed] [Google Scholar]
- Rai K, Takigawa N, Ito S, Kashihara H, Ichihara E, Yasuda T, Shimizu K, Tanimoto M, Kiura K. Liposomal delivery of MicroRNA-7-expressing plasmid overcomes epidermal growth factor receptortyrosine kinase inhibitor-resistance in lung cancer cells. Molecular Cancer Therapeutics. 2011;10:1720–1727. doi: 10.1158/1535-7163.MCT-11-0220. [DOI] [PubMed] [Google Scholar]
- Rao X, Di Leva G, Li M, Fang F, Devlin C, Hartman-Frey C, Burow ME, Ivan M, Croce CM, Nephew KP. MicroRNA-221/222 confers breast cancer fulvestrant resistance by regulating multiple signaling pathways. Oncogene. 2011;30:1082–1097. doi: 10.1038/onc.2010.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razumilava N, Bronk SF, Smoot RL, Fingas CD, Werneburg NW, Roberts LR, Mott JL. miR-25 targets TNF-related apoptosis inducing ligand (TRAIL) death receptor-4 and promotes apoptosis resistance in cholangiocarcinoma. Hepatology. 2012;2:465–475. doi: 10.1002/hep.24698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- Ribic CM, Sargent DJ, Moore MJ, Thibodeau SN, French AJ, Goldberg RM, Hamilton SR, Laurent-Puig P, Gryfe R, Shepherd LE, Tu D, Redston M, Gallinger S. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. New England Journal of Medicine. 2003;3:247–257. doi: 10.1056/NEJMoa022289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robey RW, Medina-Pérez WY, Nishiyama K, Lahusen T, Miyake K, Lit-man T, Senderowicz AM, Ross DD, Bates SE. Overexpression of the ATP-binding cassette half-transporter, ABCG2 (Mxr/BCrp/ABCP1), in flavopiridol-resistant human breast cancer cells. Clinical Cancer Research. 2001;1:145–152. [PubMed] [Google Scholar]
- Rubinson DA, Dillon CP, Kwiatkowski AV, Sievers C, Yang L, Kopinja J, Rooney DL, Zhang M, Ihrig MM, McManus MT, Gertler FB, Scott ML, Van Parijs L. A lentivirus-based system to functionally silence genes in primary mammalian cells, stem cells and transgenic mice by RNA interference. Nature Genetics. 2003;33:401–406. doi: 10.1038/ng1117. [DOI] [PubMed] [Google Scholar]
- Salton M, Lerenthal Y, Wang SY, Chen DJ, Shiloh Y. Involvement of Matrin3andSFPQ/NONOinthe DNA damage response. Cell Cycle. 2010;9:1568–1576. doi: 10.4161/cc.9.8.11298. [DOI] [PubMed] [Google Scholar]
- Salvesen GS, Duckett CS. IAP proteins: blocking the road to death’s door. Nature Reviews Molecular Cell Biology. 2002;3:401–410. doi: 10.1038/nrm830. [DOI] [PubMed] [Google Scholar]
- Sampath D, Plunkett W. Design of new anticancer therapies targeting cell cycle checkpoint pathways. Current Opinion in Oncology. 2001;6:484–490. doi: 10.1097/00001622-200111000-00011. [DOI] [PubMed] [Google Scholar]
- Satoh T, Nishida M, Tsunoda H, Kubo T. Expression of glutathione S-transferase pi (GST-pi) in human malignant ovarian tumors. European Journal of Obstetrics, Gynecology, and Reproductive Biology. 2001;2:202–208. doi: 10.1016/s0301-2115(00)00473-5. [DOI] [PubMed] [Google Scholar]
- Shang D, Liu Y, Liu Q, Zhang F, Feng L, Lv W, Tian Y. Synergy of 5-aza-2’deoxycytidine (DAC) and paclitaxel in both androgen-dependent and -independent prostate cancer cell lines. Cancer Letters. 2009;278:82–87. doi: 10.1016/j.canlet.2008.12.034. [DOI] [PubMed] [Google Scholar]
- Shen L, Li J, Xu L, Ma J, Li H, Xiao X, Zhao J, Fang L. miR-497 induces apo-ptosis of breast cancer cells by targeting Bcl-w. Experimental and Therapeutic Medicine. 2012;3:475–480. doi: 10.3892/etm.2011.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Chen J, Yang J, Pan T, Zhang S, Wang Z. MiR-21 protected human glioblastoma U87MG cells from chemotherapeutic drug temozolomide induced apoptosis by decreasing Bax/Bcl-2 ratio and caspase-3 activity. Brain Research. 2010;1352:255–264. doi: 10.1016/j.brainres.2010.07.009. [DOI] [PubMed] [Google Scholar]
- Sibley CR, Seow Y, Wood MJ. Novel RNA-based strategies for therapeutic gene silencing. Molecular Therapy. 2010;18:466–476. doi: 10.1038/mt.2009.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soloaga A, Thomson S, Wiggin GR, Rampersaud N, Dyson MH, Hazzalin CA, Mahadevan LC, Arthur JS. MSK2 and MSK1 mediate the mitogen- and stress-induced phosphorylation of histone H3 and HMG-14. EMBO Journal. 2003;11:2788–2797. doi: 10.1093/emboj/cdg273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song B, Wang Y, Titmus MA, Botchkina G, Formentini A, Kornmann M, Ju J. Molecular mechanism of chemoresistance by miR-215 in osteosarcoma and colon cancer cells. Molecular Cancer. 2010;9:96. doi: 10.1186/1476-4598-9-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. New England Journal of Medicine. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- Su J, Baigude H, McCarroll J, Rana TM. Silencing microRNA by interfering nanoparticles in mice. Nucleic Acids Research. 2011;39:e38. doi: 10.1093/nar/gkq1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takezawa K, Okamoto I, Tanizaki J, Kuwata K, Yamaguchi H, Fukuoka M, Nishio K, Nakagawa K. Enhanced anticancer effect of the combination of BIBW2992 and thymidylate synthase-targeted agents in non-small cell lung cancer with the T790M mutation of epidermal growth factor receptor. Molecular Cancer Therapeutics. 2010;9:1647–1656. doi: 10.1158/1535-7163.MCT-09-1009. [DOI] [PubMed] [Google Scholar]
- Tentori L, Graziani G. Recent approaches to improve the antitumor efficacy of temozolomide. Current Medicinal Chemistry. 2009;16:245–257. doi: 10.2174/092986709787002718. [DOI] [PubMed] [Google Scholar]
- To KK, Zhan Z, Litman T, Bates SE. Regulation of ABCG2 expression at the 3’ untranslated region of its mRNA through modulation of transcript stability and protein translation by a putative microRNA in the S1 colon cancer cell line. Molecular and Cellular Biology. 2008;17:5147–5156. doi: 10.1128/MCB.00331-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trang P, Wiggins JF, Daige CL, Cho C, Omotola M, Brown D, Weidhaas JB, Bader AG, Slack FJ. Systemic delivery of tumor suppressor microRNA mimics using a neutral lipid emulsion inhibits lung tumors in mice. Molecular Therapy. 2011;19:1116–1122. doi: 10.1038/mt.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai-Pflugfelder M, Liu LF, Liu AA, Tewey KM, Whang-Peng J, Knutsen T, Huebner K, Croce CM, Wang JC. Cloning and sequencing of cDNA encoding human DNA topoisomerase II and localization of the gene to chromosome region 17q21–22. Proceedings of the National Academy of Sciences of the United States of America. 1988;19:7177–7181. doi: 10.1073/pnas.85.19.7177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchida S, Kimura J, Hayakari M, Ishikawa T. Usefulness of glutathione S-transferase as a tumor marker. Rinsho Byori. Japanese Journal of Clinical Pathology. 1997;12:1125–1132. [PubMed] [Google Scholar]
- Turrini E, Haenisch S, Laechelt S, Diewock T, Bruhn O, Cascorbi I. MicroRNA profiling in K-562 cells under imatinib treatment: influence of miR-212 and miR-328 on ABCG2 expression. Pharmacogenetics and Genomics. 2012;3:198–205. doi: 10.1097/FPC.0b013e328350012b. [DOI] [PubMed] [Google Scholar]
- Ugras S, Brill E, Jacobsen A, Hafner M, Socci ND, Decarolis PL, Khanin R, O’Connor R, Mihailovic A, Taylor BS, Sheridan R, Gimble JM, Viale A, Crago A, Antonescu CR, Sander C, Tuschl T, Singer S. Small RNA sequencing and functional characterization reveals MicroRNA-143 tumor suppressor activity in liposarcoma. Cancer Research. 2011;17:5659–5666. doi: 10.1158/0008-5472.CAN-11-0890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valeri N, Gasparini P, Braconi C, Paone A, Lovat F, Fabbri M, Sumani KM, Alder H, Amadori D, Patel T, Nuovo GJ, Fishel R, Croce CM. MicroRNA-21 induces resistance to 5-fluorouracil by down-regulating human DNA MutS homolog 2 (hMSH2) Proceedings of the National Academy of Sciences of the United States of America. 2010;49:21098–21103. doi: 10.1073/pnas.1015541107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Zhong M, Liu W, Li J, Huang J, Zheng L. Alterations of microR-NAs in cisplatin-resistant human non-small cell lung cancer cells (A549/DDP) Experimental Lung Research. 2011;37:427–434. doi: 10.3109/01902148.2011.584263. [DOI] [PubMed] [Google Scholar]
- Wang TH, Wang HS, Soong YK. Paclitaxel-induced cell death: where the cell cycle and apoptosis come together. Cancer. 2000;88:2619–2628. doi: 10.1002/1097-0142(20000601)88:11<2619::aid-cncr26>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Wang Y, Lynch AS, Chen SJ, Wang JC. On the molecular basis of the thermal sensitivity of an Escherichia coli topA mutant. Journal of Biological Chemistry. 2002;2:1203–1209. doi: 10.1074/jbc.M109436200. [DOI] [PubMed] [Google Scholar]
- Wei X. Mechanism of EGER-related cancer drug resistance. Anti-Cancer Drugs. 2011;10:963–970. doi: 10.1097/CAD.0b013e32834a149c. [DOI] [PubMed] [Google Scholar]
- Wiggin GR, Soloaga A, Foster JM, Murray-Tait V, Cohen P, Arthur JS. MSK1 and MSK2 are required for the mitogen- and stress-induced phosphorylation of CREB and ATF1 in fibroblasts. Molecular and Cellular Biology. 2002;8:2871–2881. doi: 10.1128/MCB.22.8.2871-2881.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins JF, Ruffino L, Kelnar K, Omotola M, Patrawala L, Brown D, Bader AG. Development of a lung cancer therapeutic based on the tumor suppressor microRNA-34. Cancer Research. 2010;70:5923–5930. doi: 10.1158/0008-5472.CAN-10-0655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Wang HD, Guo W, Yang K, Zhao YP, Jiang YG, He P. Up-regulation of Fas reverses cisplatin resistance of human small cell lung cancer cells. Journal of Experimental & Clinical Cancer Research. 2010;29:49. doi: 10.1186/1756-9966-29-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia L, Zhang D, Du R, Pan Y, Zhao L, Sun S, Hong L, Liu J, Fan D. miR-15b and miR-16 modulate multidrug resistance by targeting BCL2 in human gastric cancer cells. International Journal of Cancer. 2008;123:372–379. doi: 10.1002/ijc.23501. [DOI] [PubMed] [Google Scholar]
- Xie Y, Tobin LA, Camps J, Wangsa D, Yang J, Rao M, Witasp E, Awad KS, Yoo N, Ried T, Kwong KF. MicroRNA-24 regulates XIAP to reduce the apoptosis threshold in cancer cells. Oncogene. 2012 doi: 10.1038/onc.2012.258. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamura S, Saini S, Majid S, Hirata H, Ueno K, Deng G, Dahiya R. MicroRNA-34a modulates c-Myc transcriptional complexes to suppress malignancy in human prostate cancer cells. PLoS ONE. 2012;1:e29722. doi: 10.1371/journal.pone.0029722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka S, Campbell NR, An F, Kuo SC, Potter JJ, Mezey E, Maitra A, Selaru FM. Coordinated effects of microRNA-494 induce G2/M arrest in human cholangiocarcinoma. Cell Cycle. 2012;11:2729–2738. doi: 10.4161/cc.21105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan G, Zhang L, Fang T, Zhang Q, Wu S, Jiang Y, Sun H, Hu Y. MicroRNA-145 suppresses mouse granulosa cell proliferation by targeting activin receptor IB. FEBS Letters. 2012 doi: 10.1016/j.febslet.2012.06.048. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Yang Y, Wu J, Guan H, Cai J, Fang L, Li J, Li M. MiR-136 promotes apoptosis of glioma cells by targeting AEG-1 and Bcl-2. FEBS Letters. 2012 doi: 10.1016/j.febslet.2012.08.003. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nature Reviews Molecular Cell Biology. 2008;1:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- Youn CK, Kim MH, Cho HJ, Kim HB, Chang IY, Chung MH, You HJ. Oncogenic H-Ras up-regulates expression of ERCC1 to protect cells from platinum-based anticancer agents. Cancer Research. 2004;64:4849–4857. doi: 10.1158/0008-5472.CAN-04-0348. [DOI] [PubMed] [Google Scholar]
- Yu Y, Wang Y, Ren X, Tsuyada A, Li A, Liu LJ, Wang SE. Context-dependent bidirectional regulation of the MutS homolog 2 by transforming growth factor |3 contributes to chemoresistance in breast cancer cells. Molecular Cancer Research. 2010;12:1633–1642. doi: 10.1158/1541-7786.MCR-10-0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yvon AM, Wadsworth P, Jordan MA. Taxol suppresses dynamics of individual microtubules in living human tumor cells. Molecular Biology of the Cell. 1999;10:947–959. doi: 10.1091/mbc.10.4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu DX, Miao KR, Fang C, Fan L, Zhu W, Zhu HY, Zhuang Y, Hong M, Liu P, Xu W, Li JY. Aberrant microRNA expression in Chinese patients with chronic lymphocytic leukemia. Leukemia Research. 2010a;35:730–734. doi: 10.1016/j.leukres.2010.11.005. [DOI] [PubMed] [Google Scholar]
- Zhu H, Wu H, Liu X, Evans BR, Medina DJ, Liu CG, Yang JM. Role of MicroRNA miR-27a and miR-451 in the regulation of MDR1/P-glycoprotein expression in human cancer cells. Biochemical Pharmacology. 2008;5:582–588. doi: 10.1016/j.bcp.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Shan X, Wang T, Shu Y, Liu P. miR-181b modulates multidrug resistance by targeting BCL2 in human cancer cell lines. International Journal of Cancer. 2010b;127(11):2520–2529. doi: 10.1002/ijc.25260. [DOI] [PubMed] [Google Scholar]
- Zhu W, Zhu D, Lu S, Wang T, Wang J, Jiang B, Shu Y, Liu P. miR-497 modulates multidrug resistance of human cancer cell lines by targeting BCL2. Medical Oncology. 2011;29:384–391. doi: 10.1007/s12032-010-9797-4. [DOI] [PubMed] [Google Scholar]
- Zhu W, Xu H, Zhu D, Zhi H, Wang T, Wang J, Jiang B, Shu Y, Liu P. miR-200bc/429 cluster modulates multidrug resistance of human cancer cell lines by targeting BCL2 and XIAP. Cancer Chemotherapy and Pharmacology. 2012;69:723–731. doi: 10.1007/s00280-011-1752-3. [DOI] [PubMed] [Google Scholar]
- Zunino F, Cassinelli G, Polizzi D, Perego P. Molecular mechanisms of resistance to taxanes and therapeutic implications. Drug Resistance Updates. 1999;6:351–357. doi: 10.1054/drup.1999.0108. [DOI] [PubMed] [Google Scholar]