Abstract
Rationale
Chronic pain is becoming a more common medical diagnosis and is especially prevalent in older individuals. As such, prescribed use of opioids is on the rise, even though the efficacy for pain management in older individuals is unclear.
Objectives
Thus the present preclinical study assessed the effectiveness of chronic fentanyl administration to produce antinociception in aging rats (16, 20, 24 months).
Methods
Animals were tested in a thermal sensitivity procedure known to involve neural circuits implicated in chronic pain in humans. Sensitivity to heat and cold thermal stimulation was assessed during 28 days of fentanyl administration (1.0 mg/kg/day), and 28 days of withdrawal.
Results
Fentanyl resulted in decreased thermal sensitivity to heat but not cold stimulation indicated by more time spent in the hot compartment relative to time spent in the cold or neutral compartments. Unlike previous findings using a hot-water tail withdrawal procedure, tolerance did not develop to the antinociceptive effects of fentanyl over a 28-day period of drug administration. The oldest animals were least sensitive, and the youngest animals most sensitive to the locomotor-stimulating effects of fentanyl. The effect on the antinociceptive response to fentanyl in the oldest group of rats was difficult to interpret due to profound changes in the behavior of saline-treated animals.
Conclusions
Overall, aging modifies the behavioral effects of opioids, a finding that may inform future studies for devising appropriate treatment strategies.
Keywords: Aging, Antinociception, Chronic administration, Geriatric pharmacotherapy, Pain, Thermal sensitivity, Tolerance, Withdrawal
Introduction
Chronic pain is becoming a more common medical diagnosis, rather than simply considered a symptom, and is especially prevalent in older individuals (Bruckenthal et al. 2009; Colliver et al. 2006; McCarthy et al. 2009; Neville et al. 2008; Rustøen et al. 2005; Walid and Zaytseva 2009). Unfortunately for many pain conditions, including lower back pain (Akkaya and Ozkan 2009; Chenot et al. 2007), chronic post-surgical pain (Apkarian et al. 2009), neuropathic pain (Cavenagh et al. 2006; Colombo et al. 2006; Jensen et al. 2009), and chronic pelvic pain (Dalpiaz et al. 2008), there is a lack of effective treatments, although the use of opioids for treating these conditions is increasing (Atluri et al. 2003; Bell et al. 2009; Benyamin et al. 2008; Birxner et al. 2006; Garcia del Pozo et al. 2008; Pergolizzi et al. 2008; Stein, 2013; Trescot et al. 2008). With the number of people in the United States over the age of 65 expected to more than double by 2050 (Kalapatapu and Sullivan 2010), it is expected that an increased number of aged individuals will be more frequently prescribed opioids for longer periods of time.
The influence of age on various effects of opioids (e.g. pain relief, sedation, development of tolerance and dependence) has not been fully elucidated (see below). In humans, opioids are generally thought to provide a greater analgesic effect to older patients primarily because of changes in the pharmacokinetics of opioids in older individuals (Lugo and Kern 2002; Scott and Stanski 1987; Wilder-Smith 2005). However, despite numerous preclinical studies (for review see Gagliese and Melzack 2000), the effects of aging on opioid function are conflicting such that some studies show decreased (Chan and Lai 1982; Crisp et al. 1994; Hoskins et al. 1986; Kavaliers et al. 1983; Kramer and Bodnar 1986; Raut and Ratka, 2009; Webster et al. 1976), increased (Islam et al. 1993; Smith and Gray 2001), or no change in opioid effects in older animals (Smith and Gray 2001; Van Crugten et al. 1997).
Most preclinical studies examining the effects of opioids on nociceptive processing use high intensity, short duration thermal stimulation which tend to activate nociceptive Aδ-fibers (Millan 1999). We and others have used a thermal sensitivity procedure that is believed to selectively activate C-fibers as opposed to Aδ-fibers, as these nociceptive C-fibers are more likely associated with chronic pain in humans (Raja et al. 1988; Schmelz 2009; Staud et al. 2008). Using these procedures, we have shown that older rats are more sensitive to cold temperatures than younger rats (Morgan et al. 2008, 2012; Yezierski et al. 2010), and that inflammatory pain induced by a formalin injection amplifies these differences (Yezierski et al. 2010). We have also demonstrated that acute administration of morphine is less effective in altering thermal sensitivity (in particular heat stimulation) in older rats (Morgan et al. 2012).
To extend these findings, this study investigated the influence of age on the antinociceptive effectiveness of chronic fentanyl administration. Fentanyl was selected due to its increased use in chronic pain conditions (Bell et al. 2009; Pergolizzi et al. 2008; Bhambhani et al. 2010; Manchikanti and Singh 2008) due in part to the development of delivery systems (e.g. transdermal patch) which makes fentanyl easy to administer in an outpatient setting (Grape et al. 2010). Furthermore, previous studies have shown that the fentanyl administration protocol used in this study (1.0 mg/kg/day for 28 days) produces age-related differences in antinociception assessed using a reflexive, hot water tail withdrawal procedure, and secondary effects (e.g. body weight loss and body composition) in rats across a wide age range (Morgan et al. 2008; Mitzelfelt et al. 2011). In this and previous studies from our lab, Fischer 344 × Brown Norway rats (F344×BN) were used, as there are a number of conceptual similarities between age-related changes observed in this strain and what is observed in humans, including changes in body composition, and behavioral, cognitive and physical functioning (e.g. Carter et al. 2002, 2004, 2009; Mitzelfelt et al., 2011; Morgan et al. 2008, 2012), For this study, an age range (16, 20, 24 months) was selected which spans the 80% to 50% survival rate for this strain of rats (which has a maximal lifespan of ~38 months (Turturro et al. 1999; Carter et al., 2002), a time range functionally analgous to humans aged ~65 to 80 years old (Turturro et al., 1999; Carter et al., 2002). Previous studies assessing age differences in thermal sensitivity, demonstrated the greatest difference in sensitivity between 16 and 24 months of age (Morgan et al. 2012; Yezierski et al. 2010).
Materials and Methods
Animals
Male Fischer 344 × Brown Norway rats (F344×BN) (n=27), obtained from the National Institute of Aging colony at Harlan Industries (Indianapolis, IN), at three ages (16, 20, and 24 months of age at time of pump implant; n=10, 8, and 10 respectively) were used. All animals were individually housed in a climate (temperature and humidity) controlled colony with a 12-hr light/dark cycle with food and water available ad libitum. Animals were cared for in accordance with the regulations of the Institutional Animal Care and Use Committee and in accordance with the “Guide” (ILAR 1996). In addition, animals were assessed on a weekly basis for signs of overt health problems by using a standardized form; measures included, but were not limited to, checking for sudden decline in body weight, redness around the eyes and nostrils, ruffled coat, open sores on the tail, and haunched posture. Two animals were removed from the study because of these overt health problems, and their data were excluded from all analyses.
Surgery and Drug Delivery
Osmotic minipumps (Alzet, Durect Corp., Cupertino, CA) filled with either fentanyl or saline (n=5 in each 16- and 24-month old group; n=4 in each 20-month old group) were implanted subcutaneously in the hindquarter under isoflurane anesthesia (~1.5% at 1.0 L/min O2). Minipumps delivered fluid at a rate of 2.58 μl/hr for 28 days. Fentanyl was delivered at a dose of 1 mg/kg/day. Four weeks after pump implantation, animals were lightly anesthetized and the pumps removed. Fentanyl was generously supplied by the National Institute on Drug Abuse (Research Triangle Park, NC).
Thermal Sensitivity
Apparatus
The thermal sensitivity chambers (see Figure 1) were custom-made and a description of the individual components follows: two aluminum plates (29.2 cm × 17.8 cm; Smalls Design and Manufacturing, Portland, TN) that contain channels throughout were connected to an input and output valve. These valves were connected to recirculating water baths (Model RTE-7; Thermo Scientific) which maintained water at particular temperatures ranging from −25 to 150°C via insulated Tygon® tubing. The plates were surrounded by a Plexiglas chamber with two compartments separated by a removable partition with a cutout (8.9 cm × 8.9 cm) allowing the animal to traverse between the two compartments freely (Magnum Wood LLC, Gainesville, FL). Each chamber was opaque, 43.2 cm tall, and had no cover, allowing an overhead camera and Ethovision 7 (Noldus Information Technology, Wageningen, Netherlands) to record behavior.
Figure 1.
Two testing stations are shown (the one on the right has the chamber removed). Each station consists of 2 aluminum plates connected to hoses that go to/from the water chiller/heaters, surrounded by an opaque 2-compartment chamber. An overhead camera records behavior and Ethovision software monitors position and distance traveled.
Procedure
Upon arrival in the colony, the animals were given 2 weeks to acclimate to the housing facility before thermal sensitivity testing began. Animals were then given ten days of acclimation to the testing apparatus where the thermal plates were set to different temperatures ranging from 20–36°C. Throughout all phases of testing, animals were non-systematically assigned to start on the “hot” or “cold” plate, the start side was counterbalanced within each group, and no animal could start on the same side more than two days in a row to prevent the development of a side bias. After this training, sensitivity to various temperatures was determined by setting one plate to 27.5°C (neutral) and the other plate to a temperature ranging from 10–45°C in 3°C increments. Temperatures for each day were non-systematically determined, with each temperature condition tested once, and a temperature warmer or cooler than 27.5°C could not be tested more than two days in a row, to prevent development of side biases.
After this initial sensitivity testing, animals were tested under three conditions: “cold/neutral” (16 and 27.5°C), “hot/neutral” (45 and 27.5°C), and “hot/cold” (45 and 16°C). These conditions allowed for the independent testing of sensitivity to heat, sensitivity to cold, as well as preference/aversion for heat or cold. Animals were tested six days a week, with each condition tested twice a week, resulting in a total of 8 sessions of each comparison during the four-week drug administration and withdrawal periods. Sessions were 15 minutes in duration, and were conducted at the same time each day (the middle of their light cycle) to eliminate alterations in sensitivity due to photoperiod (e.g. Kavaliers and Hirst, 1983). Data from animals that never changed compartments, i.e. remained in the starting compartment, were excluded from the analysis for that particular session (~3–4% of trials throughout the experiment).
Animals within each age group were then randomized to receive osmotic minipumps containing either saline (n=14) or fentanyl (n=13). Animals were given 24 hours to recover then thermal sensitivity testing resumed. Animals were tested under the same three conditions (hot/cold, hot/neutral, coldneutral) during 4 weeks of drug administration (to assess the potential development of tolerance or hyperalgesia), and during 4 weeks of withdrawal.
Locomotor Activity
Activity levels were measured to assess potential sedative effects which could influence the thermal sensitivity measurements, and represent a potential detrimental side effect of fentanyl administration. The average distance traveled per session was calculated, and the group means for each week are presented as a percent of baseline.
Data Analysis and Statistics
For body weight data collected during and after chronic drug administration, separate two-way ANOVAs were performed for each age group with test session and drug as the main factors. A subsequent two-way, repeated measures ANOVA was performed with age and test session as factors.
The relative side preference was calculated by taking the time (in seconds) spent on the hotter side during the trial, and subtracting 450 (50% of trial time in seconds). This number was then divided by 450, and multiplied by 100. Using this calculation, animals that spent equal time on each plate had a relative preference of zero, whereas preference for the hotter plate is above the zero line, and preference for the colder plate falls below the zero line.
For the initial thermal sensitivity, a 2-way repeated-measures ANOVA, with age and temperature setting as the factors was performed, as well as one-way ANOVAs across ages for each temperature setting. For thermal sensitivity data collected during and after drug administration, data were separated by temperature comparison. Separate two-way ANOVA with repeated measures were performed for each age with drug administered and test session as main factors. A separate two-way ANOVA with repeated measures was performed on the fentanyl-treated animals with age and test session as factors. Locomotor data were analyzed separately for the drug administration and withdrawal phases of testing.
Differences were considered statistically significant with a p-value of less than 0.05. Student-Newman-Keuls post-hoc tests were performed when appropriate. For clarity, if there was a significant interaction, no main effects are reported, and when main effects are reported, there was no significant interaction. All statistical testing was performed using SigmaStat version 3.11 (Systat Software, Inc, San Jose, CA).
Results
Baseline sensitivity to thermal stimulation
Temperatures ranging from 10–25°C (cooler than neutral) and 30–45°C (hotter than neutral) were compared to a neutral temperature (27.5°C) to assess initial sensitivity to thermal stimulation (Figure 2, left panel). There was a main effect of temperature (cooler than neutral: F5, 117 = 7.78, p < 0.001; hotter than neutral: F5, 116 = 7.88, p < 0.001), such that all animals, regardless of age demonstrated an aversion (i.e. decreased time on that plate) towards more extreme temperatures. There were no differences between ages, 16° and 45°C were deemed equally aversive, and were selected as the temperatures for the “cold” and “hot” plates during all subsequent thermal sensitivity testing.
Figure 2.
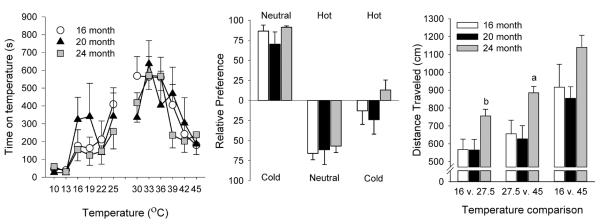
Baseline measures. Initial thermal sensitivity (left panel) displayed as the time spent on each temperature when compared to neutral (27.5°C). Thermal sensitivity (center panel) shown as relative preference (% of session spent on a particular side) during cold/neutral (16 and 27.5°C), hot/neutral (45 and 27.5°C), and hot/cold (45 and 16°C) comparisons. Locomotor activity (right panel) displayed for the three temperature comparisons in the three age groups. All data presented as mean ± s.e.m. a p < 0.05 compared to all other ages, b p < 0.05 compared to 16-month old animals.
Baseline sensitivity to testing conditions
We then tested animals across these temperature comparisons: “cold/neutral” (16 and 27.5°C), “hot/neutral” (45 and 27.5°C), and “hot/cold” (45 and 16°C). The relative preference for each temperature comparison across ages is shown in Figure 2 (center panel). In the cold/neutral comparison and hot/neutral comparison, all animals showed a strong preference for the neutral plate. However, during the hot/cold comparison, there was no preference for a particular side for all ages. There was a main effect of temperature comparison (F2, 46 = 127.62, p < 0.001), but there were no significant differences across ages during this assessment. Thus, the baseline against which the effects of chronic fentanyl administration were assessed did not differ across ages.
Baseline locomotor activity
Horizontal locomotion was assessed during the various temperature comparisons to provide a baseline level of activity (Figure 2, right panel). Activity levels were significantly different for each comparison, with the greatest levels of locomotor activity occurring during the hot/cold sessions, and the least amount of activity during the cold/neutral sessions (main effect of temperature: F2, 48 = 40.76, p < 0.001). The 24-month old animals also showed significantly greater locomotor activity than the other two age groups (main effect of age: F2, 24 = 4.37, p = 0.024). One-way ANOVAs showed there were significant differences between ages for the cold/neutral (F2, 24 = 4.28, p = 0.026) and hot/neutral comparisons (F2, 24 = 4.60, p = 0.020). Post-hoc analysis showed that 24-month old animals had significantly greater levels of activity than 16-month old animals during the cold neutral comparison, and greater activity than both 16- and 20-month old animals during the hot/neutral comparison (Figure 2). Overall, these data demonstrate that older animals have increased locomotor activity when presented with choice situations including extreme thermal stimulation.
Effects of Chronic Fentanyl Administration and Withdrawal
Cold/Neutral thermal sensitivity
The average time spent on the cold plate for each age across 4 weeks of chronic administration and 4 weeks of withdrawal is shown in Figure 3. Overall, chronic fentanyl administration showed little effect on sensitivity to cold. All three ages showed greater time spent on the cold plate during the first session after the start of drug administration; however this effect was not maintained through the rest of drug administration. Statistical analysis showed a main effect of session for the 20-month old animals (F16, 85 = 2.48, p = 0.004). Post-hoc analysis showed that animals spent greater time on the cold plate during first drug session than the fourth drug session, and all withdrawal sessions with the exception of the first withdrawal session (Figure 3, center panel). There was a significant interaction between drug and session for the 24-month old animals (F16, 103 = 2.34, p = 0.005), with fentanyl-treated animals spending more time on the cold plate than saline-treated animals during the first, sixth, and eighth drug session, as well as the first withdrawal session (Figure 3, right panel). Generally, chronic fentanyl administration had little effect, at any age, on sensitivity to cold thermal stimulation.
Figure 3.

Sensitivity to cold during drug (fentanyl or saline) administration and withdrawal across ages (16, 20, and 24 months of age). Time spent on cold plate (16°C) during cold/neutral sessions (mean ± s.e.m.) is shown. These cold/neutral sessions were conducted 2 times per week over 4 weeks (i.e. 8 sessions) during (Drug) and following (Withdrawal) drug administration.. * p < 0.05 compared to saline-treated animals.
Hot/Neutral thermal sensitivity
Fentanyl administration increased the amount of time spent on the hot plate during hot/neutral sessions (Figure 4). Throughout drug administration, fentanyl-treated animals spent more time on the hot plate than on the neutral plate, a reversal from behavior during baseline assessment. However, this change in preference did not extend through the withdrawal period. Interestingly, 24-month old saline-treated animals also showed increased time spent on the hot plate, although this increase was not as great or as consistent as the fentanyl-treated animals.
Figure 4.
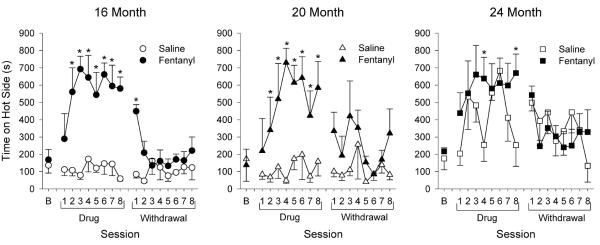
Sensitivity to heat during drug (fentanyl or saline) administration and withdrawal. Time spent on hot plate (45°C) is shown. These hot/neutral sessions were conducted 2 times per week over 4 weeks (i.e. 8 sessions) during (Drug) and following (Withdrawal) drug administration. * p < 0.05 compared to saline-treated animals.
Within each age, there was significant drug × session interaction (16-month: F16, 127 = 6.11, p < 0.001; 20-month: F16, 89 = 2.36, p = 0.006; 24-month: F16, 110 = 2.02, p = 0.018). Fentanyl-treated animals spent significantly more time on the hot plate than saline-treated animals during drug sessions 2 through 8 for both 16- and 20-month old animals, and this increase persisted through the first withdrawal session for the 16-month old animals. Fentanyl-treated animals spent more time on the hot plate than saline animals, but only during drug sessions 4 and 8 for the 24-month old animals. Overall, fentanyl administration decreased sensitivity to hot thermal stimulation in all age groups.
Hot/Cold thermal sensitivity
Fentanyl administration increased the time spent on the hot plate during hot/cold tests for all ages (Figure 5), although 24-month old, saline-treated animals also showed increased preference for the hot plate during these sessions. For 16-month old animals, there was a significant drug × session interaction (F16, 127 = 1.84, p = 0.033), with fentanyl animals spending more time on the hot plate during all drug sessions except session 2, and withdrawal sessions 2, 6, 7, and 8 (Figure 5, left panel). 20-month old animals showed main effects for both drug and session (drug: F1, 6 = 14.41, p = 0.009; session: F16, 95 = 2.30, p = 0.007), with fentanyl-treated animals spending a greater amount of time on the hot plate compared to saline-treated animals (Figure 5, center panel). There was a main effect of session for the 24-month old animals (F16, 106 = 3.50, p < 0.001), with animals spending a greater amount of time on the hot plate during drug sessions 3 through 8 and withdrawal sessions 1, 3, 5, 6, and 7, when compared to baseline (Figure 5, right panel). Consistent with the above findings, fentanyl administration decreased sensitivity to hot thermal stimulation in all age groups.
Figure 5.
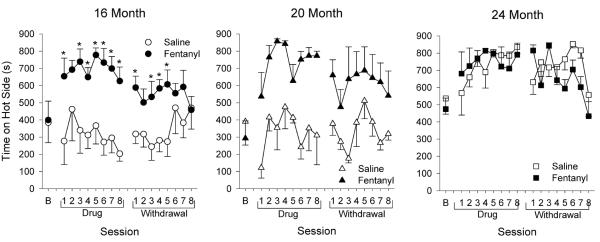
Thermal sensitivity during drug administration and withdrawal for hot/cold sessions. Time spent on hot plate (45°C) is shown. These hot/cold sessions were conducted 2 times per week over 4 weeks (i.e. 8 sessions) during (Drug) and following (Withdrawal) drug administration.. * p < 0.05 compared to saline-treated animals.
Locomotor activity
Locomotor activity for each age, treatment condition, and phase of the experiment (drug treatment or withdrawal) is shown in Figure 6. In general, fentanyl administration increased locomotor activity during drug administration (F1,93 = 72.82, p < 0.001), which decreased during drug withdrawal. There was a significant drug × week interaction (F3,93 = 5.80, p = 0.001) during chronic drug treatment with fentanyl-treated animals showing significantly increased locomotor activity during drug administration weeks 2, 3, and 4 compared to week 1 (and week 4 was increased relative to week 2). Overall, activity in the 16-month old animals was higher than the 24-month old animals (F2,93 = 4.46, p = 0.014). During the 4-week withdrawal period, fentanyl-treated animals demonstrated higher levels of locomotion compared to saline-treated controls (F1,84=40.99; p < .001), although the magnitude of this increase was clearly less than observed during fentanyl administration. In summary, locomotor activity was increased during fentanyl administration, thereby showing that there were no sedative effects of fentanyl, and the oldest age group of animals was somewhat less sensitive to this effect of fentanyl.
Figure 6.
Locomotor activity. Horizontal locomotion is shown as average values during a week (6 testing sessions) for each age and treatment group. * p < 0.05 compared to saline-treated animals.
Body weight
Changes in body weight due to drug administration and withdrawal are shown in Table 1. In general, fentanyl administration resulted in a loss of body weight by the 4th week of drug administration (data are averages from the last day of the experimental phase), which recovered during withdrawal. For all three ages, there was a significant drug × test phase interaction (16-month: F2, 16 = 8.82, p = 0.003; 20-month: F2, 12 = 15.42, p < 0.001; 24-month: F2, 14 = 18.08, p < 0.001), suggesting no change in saline-treated animals, with decreases in the fentanyl-treated animals which recovered during the withdrawal period. Additional post-hoc analyses showed there was a significant difference between saline and fentanyl-treated animals for both the 20- and 24-month old animals at the end of drug administration, but only for the 24-month old animals at the end of the withdrawal period.
Table 1.
Body weight (means and s.e.m.) at baseline, during drug administration, and withdrawal
| Age | Group | Baseline | Treatment | Withdrawal |
|---|---|---|---|---|
| 16 | Saline | 451.2 (± 21.2) | 448.4 (± 21.8) | 460.8 (± 21.2) |
| Fentanyl | 441.6 (± 9.0) | 407.4a(± 10.3) | 448.0 (± 10.5) | |
| 20 | Saline | 464.0 (± 18.8) | 467.8 (± 18.5) | 480.3 (± 19.8) |
| Fentanyl | 469.5 (± 18.4) | 405.8a, b (± 5.3) | 449.8a (± 9.8) | |
| 24 | Saline | 534.4 (± 17.5) | 527.8 (± 16.6) | 525.8 (± 23.8) |
| Fentanyl | 499.0 (± 9.3) | 414.8a, b (± 11.8) | 451.3a,b (± 11.5) |
p < 0.05 compared to baseline conditions for that experimental group
p < 0.05 compared to saline group during that phase
Discussion
A potential medical crisis is approaching with increases in the age of the population (Colliver et al. 2006; Kalapatapu and Sullivan 2010), the rates of chronic pain (Bruckenthal et al. 2009; Colliver et al. 2006; McCarthy et al. 2009; Neville et al. 2008; Rustøen et al. 2005; Walid and Zaytseva 2009), and the use of opioids to treat chronic pain (Caudill-Slosberg et al. 2004; Ghodse 2003; Kalso et al. 2003; Rowbotham et al. 2003) resulting in greater numbers of aged individuals taking opioids for longer periods of time. Unfortunately, how older individuals respond to chronic opioid administration compared with younger individuals has not been extensively studied. The purpose of these experiments was to assess the relationship between age and the sensitivity to the antinociceptive effects of chronic fentanyl administration using an operant-based thermal sensitivity procedure.
Baseline Differences
At baseline, there were no differences in sensitivity to the various temperatures across ages. Although age differences in sensitivity to thermal stimulation have been reported by us (e.g. Yezierski et al., 2010) and others (e.g. Scarpace, 1997), lack of differences in the present study may be due to the relatively small age range examinned.
Body Weight
During fentanyl administration animals of all ages lost weight and this effect increased with age, with older animals losing weight more quickly, and losing a greater percent of their body weight during drug administration. These results were not unexpected, as chronic opioid agonist administration has previously been shown to decrease body weight (Binsack et al. 2006; Levine et al. 1998; Li et al. 2010; Mitzelfelt et al. 2011), and suggest that fentanyl has a greater effect on older individuals than younger individuals, which is a concern in a clinical setting.
Locomotor Activity
At baseline, activity levels were highest in hot/cold temperature comparison, and across comparisons the oldest animals had higher levels of activity potentially indicating a greater sensitivity to thermal stimulation. One of the most common side effects of opioid administration is sedation (Manchikanti and Singh 2008), therefore, the locomotor activity of animals during drug administration was recorded. After one week of fentanyl administration, locomotor activity increased for all fentanyl-treated animals. These results suggest that sedation may not be a major concern with chronic fentanyl administration, and similar results have been observed in clinical setting with human patients (Agarwal et al. 2007). Interestingly, activity levels continued to increase throughout the drug treatment period, and these increases in activity were lowest in the oldest animals (although by week 4 activity levels were similar across ages), suggesting that opioids have a diminished effect with increasing age. Importantly, we have shown that under conditions of calorie restriction, animals increase their physical activity (Carter et al. 2009). Therefore, one explanation for increased locomotor activity in the fentanyl treated groups may be a result of decreased body weight which is directly tied to decreased food intake (i.e. caloric restriction). However, the oldest animals actually experienced greater loss of body weight but experienced less of an increase in physical activity, suggesting that the change in locomotor activity may be a direct effect of fentanyl administration rather than loss of body weight/food intake per se (Carter et al. 2009; Mitzelfelt et al. 2011).
Thermal Sensitivity
Overall, chronic fentanyl administration had a greater effect on sensitivity to heat as opposed to cold. Animals showed an increase in time spent on the cold plate initially, but quickly became tolerant to these effects (i.e. within one week). Interestingly, animals still displayed increased locomotor activity during cold/neutral trials, even though their thermal sensitivity did not change. This suggests that the opioid-dependent changes in locomotor activity and thermal sensitivity are dissociable and likely mediated through different neurobiological systems.
Chronic fentanyl administration did alter tolerance for heat, as all animals increased their time on the hot plate during fentanyl administration. The most striking finding was that chronic saline administration produced exactly the same effect (decreased sensitivity to heat stimulation) in the oldest animals. For this reason, assessment of the effects of fentanyl were difficult to obtain (i.e. there were near maximal effects of saline administration), and makes the change in thermal sensitivity in the oldest animals difficult to interpret. In the fentanyl-treated groups, all ages showed similar levels of antinociception assessed by a decreased sensitivity to heat.
The overall results with fentanyl are similar to previous studies in our lab (Morgan et al. 2012) assessing the effects of acute morphine on thermal sensitivity across ages. Both studies demonstrated that opioid administration, either acutely or chronically, decreased aversion to hot temperatures, yet had little effect on cold sensitivity. Of particular interest is that the antinociceptive effects of fentanyl against heat were maintained throughout 4 weeks of drug administration, suggesting that changes in heat sensitivity are resistant to the development of opioid tolerance. These findings are in stark contrast to previous studies that assessed the effects of chronic fentanyl administration on reflexive tail withdrawal from hot water (Morgan et al. 2008). In fact, that particular study demonstrated a rapid development of tolerance to the antinociceptive effects of fentanyl during chronic administration using similar experimental parameters (e.g. various ages, same sex and rat strain, similar drug administration protocols). Together, these data highlight the suggestion that there are distinct physiological systems mediating nociception assessed using these two behavioral procedures (i.e. thermal sensitivity vs. hot-water tail withdrawal), and add to a growing literature demonstrating qualitative differences in findings assessed using operant and reflexive procedures (Kwilasz and Negus 2012; Martin et al. 2004; Neubert et al. 2007; Stevenson et al. 2009; Yeomans et al. 1996). Importantly, it has been argued that findings using operant procedures are more clinically relevant and predictive of the human condition (Morgan et al. 2008; Negus et al. 2006; Vierck et al. 2008). Furthermore, as in the human clinical situation, the development of tolerance is not a necessary outcome of chronic opioid treatment suggesting that there may be situations in which long-term opioid use for chronic pain conditions is appropriate.
Implications and Future Directions
Older individuals are being prescribed opioids in greater numbers to alleviate pain, and taking them for longer periods of time. However, how increased age affects the antinociceptive properties of opioids is not fully understood. The results of this study suggest that opioid efficacy decreases with advancing age, at least in respects to antinociception. It should be noted however that the age range for this study was limited and even the youngest age tested could be considered an aged rat. This was done to assess animals across the range of middle-aged to pre-senescent, similar to the current age range of the “baby boomer” generation. However, to understand the relationship between aging and opioids, greater age ranges will need to be tested.
This study measured changes in thermal sensitivity in males. It is important to extend these studies to females as well for two reasons. One, females generally live longer than males, and therefore will make up a greater portion of the aged population. Second, some chronic pain conditions are more common in females (McCarthy et al. 2009), and therefore animal studies should be conducted in females in order to better model the conditions that are observed in clinical settings. Previous studies using the thermal sensitivity procedure have shown sex differences (Vierck et al. 2008), and these differences between males and females need to be assessed following acute and chronic opioid administration as well.
This study used an operant-based procedure for pain assessment in animals. While this procedure is increasing in use (e.g. Marcinkiewcz et al. 2009; Morgan et al. 2012; Vierck et al. 2008; Yezierski et al. 2010), it is important to note that opioid effects with this procedure have not been fully characterized, regardless of sex or age. Therefore, it is important to continue to test a range of doses of fentanyl with this procedure, but also the effects of other opioid and non-opioid analgesic drugs need to be characterized. Two important drugs to consider are duloxetine and pregabalin, non-opioid drugs that are commonly prescribed for the treatment of chronic pain. With the thermal sensitivity procedure designed to selectively recruit primary afferents (i.e. C-fibers as opposed to Aδ fibers) involved in chronic pain, it is important to assess the effects of drugs commonly used in the treatment of chronic pain and to potentially assist in the development of novel medications.
Acknowledgements
This research was primarily supported by a grant from the NIH (R21DA023022 to DM). JDM was supported by a T32 training grant (AG00196) and the University of Florida Alumni Fellowship. This article was used in partial fulfillment for the requirements of the doctoral degree at the University of Florida for the first author.
References
- Agarwal S, Polydefkis M, Block B, Haythornwaite J, Raja SN. Transdermal fentanyl reduces pain and improves functional activity in neuropathic pain states. Pain Med. 2007;8:554–562. doi: 10.1111/j.1526-4637.2006.00246.x. [DOI] [PubMed] [Google Scholar]
- Akkaya T, Ozkan D. Chronic post-surgical pain. Agri. 2009;21:1–9. [PubMed] [Google Scholar]
- Apkarian AV, Baliki MN, Geha PY. Towards a theory of chronic pain. Prog Neurobiol. 2009;87:81–97. doi: 10.1016/j.pneurobio.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atluri S, Boswell MV, Hansen HC, Trescot AM, Singh V, Jordan AE. Guidelines for the use of controlled substances in the management of chronic pain. Pain Physician. 2003;6:233–257. [PubMed] [Google Scholar]
- Bell JS, Klaukka T, Ahonen J, Hartikainen S. National utilization of transdermal fentanyl among community-dwelling older people in Finland. Am J Geriatr Pharmacother. 2009;7:355–61. doi: 10.1016/j.amjopharm.2009.11.006. [DOI] [PubMed] [Google Scholar]
- Benyamin R, Trescot AM, Datta S, Buenaventura R, Adlaka R, Sehgal N, et al. Opioid complications and side effects. Pain Physician. 2008;11:S105-S120–S105-S120. [PubMed] [Google Scholar]
- Bhambhani Y, Gross DP, Haykowsky M, Rashiq S. Effect of opioid administration on cardiorespiratory and muscle oxygenation during lifting in chronic back pain patients. Eur J Appl Physiol. 2010;109:241–250. doi: 10.1007/s00421-009-1332-y. [DOI] [PubMed] [Google Scholar]
- Binsack R, Zheng ML, Zhang ZS, Yang L, Zhu YP. Chronic morphine drinking establishes morphine tolerance, but not addiction in Wistar rats. Journal of Zhejiang University. Science. 2006;B 7:892–898. doi: 10.1631/jzus.2006.B0892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brixner DI, Oderda GM, Roland CL, Rublee DA. Opioid expenditures and utilization in the Medicaid system. J Pain Palliat Care Pharmacother. 2006;20:5–13. [PubMed] [Google Scholar]
- Bruckenthal P, Reid MC, Reisner L. Special issues in the management of chronic pain in older adults. Pain Med. 2009;10(Suppl 2):S67–78. doi: 10.1111/j.1526-4637.2009.00667.x. [DOI] [PubMed] [Google Scholar]
- Carter CS, Sonntag WE, Onder G, Pahor M. Physical performance and longevity in aged rats. J Gerontol A Biol Sci Med Sci. 2002;57:B193–197. doi: 10.1093/gerona/57.5.b193. [DOI] [PubMed] [Google Scholar]
- Carter CS, Cesari M, Ambrosius WT, Hu N, Diz D, Oden S, Sonntag WE, Pahor M. Angiotensin-converting enzyme inhibition, body composition, and physical performance in aged rats. J Gerontol A Biol Sci Med Sci. 2004;59:416–423. doi: 10.1093/gerona/59.5.b416. [DOI] [PubMed] [Google Scholar]
- Carter CS, Leeuwenburgh C, Daniels M, Foster TC. Influence of calorie restriction on measures of age-related cognitive decline: role of increased physical activity. J Gerontol A Biol Sci Med Sci. 2009;64:850–859. doi: 10.1093/gerona/glp060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caudill-Slosberg MA, Schwartz LM, Woloshin S. Office visits and analgesic prescriptions for musculoskeletal pain in US: 1980 vs. 2000. Pain. 2004;109:514–519. doi: 10.1016/j.pain.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Cavenagh J, Good P, Ravenscroft P. Neuropathic pain: are we out of the woods yet? Intern Med J. 2006;36:251–5. doi: 10.1111/j.1445-5994.2006.01046.x. [DOI] [PubMed] [Google Scholar]
- Chan SH, Lai YY. Effects of aging on pain responses and analgesic efficacy of morphine and clonidine in rats. Exp Neurol. 1982;75:112–119. doi: 10.1016/0014-4886(82)90011-5. [DOI] [PubMed] [Google Scholar]
- Chenot JF, Becker A, Leonhardt C, Keller S, Donner-Banzhoff N, Baum E, et al. Use of complementary alternative medicine for low back pain consulting in general practice: a cohort study. BMC Complement Altern Med. 2007;7:42. doi: 10.1186/1472-6882-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colliver JD, Compton WM, Gfroerer JC, Condon T. Projecting drug use among aging baby boomers in 2020. Ann Epidemiol. 2006;16:257–265. doi: 10.1016/j.annepidem.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Colombo B, Annovazzi PO, Comi G. Medications for neuropathic pain: current trends. Neurol Sci. 2006;27(Suppl 2):S183–9. doi: 10.1007/s10072-006-0598-7. [DOI] [PubMed] [Google Scholar]
- Crisp T, Stafinsky JL, Hoskins DL, Dayal B, Chinrock KM, Uram M. Effects of aging on spinal opioid-induced antinociception. Neurobiol Aging. 1994;15:169–74. doi: 10.1016/0197-4580(94)90108-2. [DOI] [PubMed] [Google Scholar]
- Dalpiaz O, Kerschbaumer A, Mitterberger M, Pinggera G, Bartsch G, Strasser H. Chronic pelvic pain in women: still a challenge. BJU Int. 2008;102:1061–5. doi: 10.1111/j.1464-410X.2008.07771.x. [DOI] [PubMed] [Google Scholar]
- Gagliese L, Melzack R. Age differences in nociception and pain behaviours in the rat. Neurosci Biobehav Rev. 2000;24:843–854. doi: 10.1016/s0149-7634(00)00041-5. [DOI] [PubMed] [Google Scholar]
- Garcia del Pozo J, Carvajal A, Viloria JM, Velasco A, Garcia del Pozo V. Trends in the consumption of opioid analgesics in Spain. Higher increases as fentanyl replaces morphine. Eur J Clin Pharmacol. 2008;64:411–5. doi: 10.1007/s00228-007-0419-9. [DOI] [PubMed] [Google Scholar]
- Ghodse H. Pain, anxiety and insomnia--a global perspective on the relief of suffering: comparative review. Br J Psychiatry. 2003;183:15–21. doi: 10.1192/bjp.183.1.15. [DOI] [PubMed] [Google Scholar]
- Grape S, Schug SA, Lauer S, Schug BS. Formulations of fentanyl for the management of pain. Drugs. 2010;70:57–72. doi: 10.2165/11531740-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Hoskins B, Burton CK, Ho IK. Differences in morphine-induced antinociception and locomotor activity in mature adult and aged mice. Pharmacol Biochem Behav. 1986;25:599–605. doi: 10.1016/0091-3057(86)90148-6. [DOI] [PubMed] [Google Scholar]
- ILAR . Guide for the Care and Use of Laboratory Animals. National Academies Press; Washington, D.C.: 1996. [PubMed] [Google Scholar]
- Islam AK, Cooper ML, Bodnar RJ. Interactions among aging, gender, and gonadectomy effects upon morphine antinociception in rats. Physiol Behav. 1993;54:45–53. doi: 10.1016/0031-9384(93)90042-e. [DOI] [PubMed] [Google Scholar]
- Jensen TS, Madsen CS, Finnerup NB. Pharmacology and treatment of neuropathic pains. Curr Opin Neurol. 2009;22:467–74. doi: 10.1097/WCO.0b013e3283311e13. [DOI] [PubMed] [Google Scholar]
- Jourdan D, Boghossian S, Alloui A, Veyrat-Durebex C, Coudore MA, Eschalier A, et al. Age-related changes in nociception and effect of morphine in the Lou rat. Eur J Pain. 2000;4:291–300. doi: 10.1053/eujp.2000.0188. [DOI] [PubMed] [Google Scholar]
- Jourdan D, Pickering G, Marchand F, Gaulier JM, Alliot J, Eschalier A. Impact of ageing on the antinociceptive effect of reference analgesics in the Lou/c rat. Br J Pharmacol. 2002;137:813–820. doi: 10.1038/sj.bjp.0704944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalapatapu RK, Sullivan MA. Prescription use disorders in older adults. Am J Addict. 2010;19:515–22. doi: 10.1111/j.1521-0391.2010.00080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalso E, Allan L, Dellemijn PL, Faura CC, Ilias WK, Jensen TS, et al. Recommendations for using opioids in chronic non-cancer pain. Eur J Pain. 2003;7:381–386. doi: 10.1016/S1090-3801(02)00143-X. [DOI] [PubMed] [Google Scholar]
- Kavaliers M, Hirst M. Daily rhythms of analgesia in mice: effects of age and photoperiod. Brain Res. 1983;279:387–393. doi: 10.1016/0006-8993(83)90216-0. [DOI] [PubMed] [Google Scholar]
- Kavaliers M, Hirst M, Teskey GC. Ageing, opioid analgesia and the pineal gland. Life Sci. 1983;32:2279–2287. doi: 10.1016/0024-3205(83)90427-7. [DOI] [PubMed] [Google Scholar]
- Kramer E, Bodnar RJ. Age-related decrements in morphine analgesia: a parametric analysis. Neurobiol Aging. 1986;7:185–191. doi: 10.1016/0197-4580(86)90041-2. [DOI] [PubMed] [Google Scholar]
- Kwilasz AJ, Negus SS. Dissociable effects of the cannabinoid receptor agonists 9-tetrahydrocannabinol and CP55940 on pain-stimulated versus pain-depressed behavior in rats. J Pharmacol Exp Ther. 2012;343:389–400. 62. doi: 10.1124/jpet.112.197780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine AS, Grace M, Billington CJ, Gosnell BA, Krahn DD, Brown DM, et al. Effect of morphine and nalmefene on energy balance in diabetic and non-diabetic rats. Pharmacol Biochem Behav. 1998;29:495–500. doi: 10.1016/0091-3057(88)90010-x. [DOI] [PubMed] [Google Scholar]
- Li P, Maguma HT, Thayne K, Davis B, Taylor DA. Correlation of the time course of development and decay of tolerance to morphine with alterations in sodium pump protein isoform abundance. Biochem Pharmacol. 2010;79:1015–1024. doi: 10.1016/j.bcp.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Lugo RA, Kern SE. Clinical pharmacokinetics of morphine. J Pain Palliat Care Pharmacother. 2002;16:5–18. doi: 10.1080/j354v16n04_02. [DOI] [PubMed] [Google Scholar]
- Manchikanti L, Singh A. Therapeutic opioids: A ten-year perspective on the complexities and complications of the escalating use, abuse, and nonmedical use of opioids. Pain Physician. 2008;11:S63–S88. [PubMed] [Google Scholar]
- Marcinkiewcz CA, Green MK, Devine DP, Duarte P, Vierck CJ, Yezierski RP. Social defeat stress potentiates thermal sensitvity in operant models of pain processing. Brain Res. 2009;1251:112–120. doi: 10.1016/j.brainres.2008.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin TJ, Buechler NL, Kahn W, Crews JC, Eisenach JC. Effects of laparotomy on spontaneuous exploratory activity and conditioned operant responding in the rat: a model for postoperative pain. Anesthesiology. 2004;101:191–203. doi: 10.1097/00000542-200407000-00030. [DOI] [PubMed] [Google Scholar]
- McCarthy LH, Bigal ME, Katz M, Derby C, Lipton RB. Chronic pain and obesity in elderly people: results from the Einstein aging study. J Am Geriatr Soc. 2009;57:115–9. doi: 10.1111/j.1532-5415.2008.02089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan MJ. The induction of pain: an integrative review. Prog Neurobiol. 1999;57:1–164. doi: 10.1016/s0301-0082(98)00048-3. [DOI] [PubMed] [Google Scholar]
- Mitzelfelt JD, DuPree JP, Seo DO, Carter CS, Morgan D. Effects of chronic fentanyl administration on physical performance of aged rats. Exp Gerontol. 2011;46:65–72. doi: 10.1016/j.exger.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D, Carter CS, DuPree JP, Yezierski RP, Vierck CJ. Evaluation of prescription opioids using operant-based pain measures in rats. Exp Clin Psychopharmacol. 2008;16:367–375. doi: 10.1037/a0013520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D, Mitzelfelt JD, Koerper LM, Carter CS. Effects of morphine on thermal sensitivity in adult and aged rats. J Gerontol A Biol Sci Med Sci. 2012;67:705–713. doi: 10.1093/gerona/glr210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Vanderah TW, Brandt MR, Bilsky EJ, Becerra L, Borsook D. Preclinical assessment of candidate analgesic drugs: recent advances and future challenges. J Pharmacol Exp Ther. 2006;319:507–514. doi: 10.1124/jpet.106.106377. [DOI] [PubMed] [Google Scholar]
- Neubert JK, Rossi HL, Pogar J, Jenkins AC, Caudle RM. Effects of mu- and kappa-2 opioid receptor agonists on pain and rearing behaviors. Behav Brain Funct. 2007;3:49. doi: 10.1186/1744-9081-3-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville A, Peleg R, Singer Y, Sherf M, Shvartzman P. Chronic pain: a population-based study. Isr Med Assoc J. 2008;10:676–80. [PubMed] [Google Scholar]
- Pergolizzi J, Böger RH, Budd K, Dahan A, Erdine S, Hans G, et al. Opioids and the management of chronic severe pain in the elderly: consensus statement of an International Expert Panel with focus on the six clinically most often used World Health Organization step III opioids (buprenorphine, fentanyl, hydromorphone, methadone, morphine, oxycodone) Pain Practice. 2008;8:287–313. doi: 10.1111/j.1533-2500.2008.00204.x. [DOI] [PubMed] [Google Scholar]
- Raja SN, Meyer RA, Campbell JN. Peripheral mechanisms of somatic pain. Anesthesiology. 1988;68:571–590. doi: 10.1097/00000542-198804000-00016. [DOI] [PubMed] [Google Scholar]
- Raut A, Ratka A. Oxidative damage and sensitivity to nociceptive stimulus and opioids in aging rats. Neurobio Aging. 2009;30:910–919. doi: 10.1016/j.neurobiolaging.2007.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowbotham MC, Twilling L, Davies PS, Reisner L, Taylor K, Mohr D. Oral opioid therapy for chronic peripheral and central neuropathic pain. N Engl J Med. 2003;348:1223–1232. doi: 10.1056/NEJMoa021420. [DOI] [PubMed] [Google Scholar]
- Rustøen T, Wahl AK, Hanestad BR, Lerdal A, Paul S, Miaskowski C. Age and the experience of chronic pain: differences in health and quality of life among younger, middle-aged, and older adults. Clin J Pain. 2005;21:513–23. doi: 10.1097/01.ajp.0000146217.31780.ef. [DOI] [PubMed] [Google Scholar]
- Saunders DR, Paolino RM, Bousquet WF, Miya TS. Age-related responsiveness of the rat to drugs affecting the central nervous system. Proc Soc Exp Biol Med. 1974;147:593–595. doi: 10.3181/00379727-147-38395. [DOI] [PubMed] [Google Scholar]
- Scarpace PJ. Thermoregulation with age: role of beta-adrenergic signal transduction. Ann N Y Acad Sci. 1997;813:111–116. doi: 10.1111/j.1749-6632.1997.tb51680.x. [DOI] [PubMed] [Google Scholar]
- Schmelz M. Translating nociceptive processing into human pain models. Exp Brain Res. 2009;196:173–178. doi: 10.1007/s00221-009-1809-2. [DOI] [PubMed] [Google Scholar]
- Scott JC, Stanski DR. Decreased fentanyl and alfentanil dose requirements with age. A simultaneous pharmacokinetic and pharmacodynamic evaluation. J Pharmacol Exp Ther. 1987;240:159–166. [PubMed] [Google Scholar]
- Smith MA, Gray JD. Age-related differences in sensitivity to the antinociceptive effects of opioids in male rats. Influence of nociceptive intensity and intrinsic efficacy at the mu receptor. Psychopharmacology. 2001;156:445–453. doi: 10.1007/s002130100750. [DOI] [PubMed] [Google Scholar]
- Staud R, Bovee CE, Robinson ME, Price DD. Cutaneous C-fiber pain abnormalities of fibromyalgia patients are specifically related to temporal summation. Pain. 2008;139:315–323. doi: 10.1016/j.pain.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein C. Opioids, sensory systems and chronic pain. Eur J Pharmacol. 2013 doi: 10.1016/j.ejphar.2013.01.076. http://dx.doi.org/10.1016//j. ejphar.2013.01.076i. [DOI] [PubMed]
- Stevenson GW, Cormier J, Mercer H, Adams C, Dunbar C, Negus SS, Bilsky EJ. Targeting pain-depressed behaviors in preclinical assays of pain and analgesia: drug effects on acetic acid-depressed locomotor activity in ICR mice. Life Sci. 2009;85:309–315. doi: 10.1016/j.lfs.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trescot AM, Glaser SE, Hansen H, Benyamin R, Patel S, Manchikanti L. Effectiveness of opioids in the treatment of chronic non-cancer pain. Pain Physician. 2008;11:S181–S200. [PubMed] [Google Scholar]
- Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, Hart RW. Growth curves and survival characteristics of the animals used in the Biomarkers of Aging Program. J Gerontol A Biol Sci Med Sci. 1999;54:B492–501. doi: 10.1093/gerona/54.11.b492. [DOI] [PubMed] [Google Scholar]
- Van Crugten JT, Somogyi AA, Nation RL, Reynolds G. The effect of old age on the disposition and antinociceptive response of morphine and morphine-6β-glucuronide in the rat. Pain. 1997;71:199–205. doi: 10.1016/s0304-3959(97)03363-0. [DOI] [PubMed] [Google Scholar]
- Vierck CJ, Acosta-Rua AJ, Rossi HL, Neubert JK. Sex differences in thermal pain sensitivity and sympathetic reactivity for two strains of rat. J Pain. 2008;9:739–749. doi: 10.1016/j.jpain.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierck CJ, Hansson PT, Yezierski RP. Clinical and pre-clinical pain assessment: are we measuring the same thing? Pain. 2008;135:7–10. doi: 10.1016/j.pain.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Walid MS, Zaytseva N. Pain in nursing home residents and correlation with neuropsychiatric disorders. Pain Physician. 2009;12:877–80. [PubMed] [Google Scholar]
- Webster GW, Shuster L, Eleftheriou BE. Morphine analgesia in mice of different ages. Exp Aging Res. 1976;2:221–233. doi: 10.1080/03610737608257178. [DOI] [PubMed] [Google Scholar]
- Wilder-Smith OH. Opioid use in the elderly. European Journal of Pain. 2005;9:137–140. doi: 10.1016/j.ejpain.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Yeomans DC, Cooper BY, Vierck CJ., Jr Effects of systemic morphine on responses of primates to first or second pain sensations. Pain. 1996;66:253–263. doi: 10.1016/0304-3959(96)03082-5. [DOI] [PubMed] [Google Scholar]
- Yezierski RP, King CD, Morgan D, Carter CS, Vierck CJ. Effects of age on thermal sensitivity in the rat. J Gerontol A Biol Sci Med Sci. 2010;65:353–362. doi: 10.1093/gerona/glq024. [DOI] [PMC free article] [PubMed] [Google Scholar]




