Abstract
Background
In a seminal study of gene-environment interaction, childhood maltreatment predicted antisocial behavior more strongly in males carrying an MAOA promoter variant of lesser, compared to higher, transcriptional efficiency. Many further investigations have been reported, including studies of other early environmental exposures and females. Here we report a meta-analysis of studies testing the interaction of MAOA genotype and childhood adversities on antisocial outcomes in predominantly non-clinical samples.
Method
Included were 27 peer-reviewed, English-language studies published through August, 2012, that contained indicators of maltreatment or “other” family (e.g., parenting, sociodemographic) hardships; MAOA genotype; indices of aggressive and antisocial behavior; and statistical test of genotype-environment interaction. Studies of forensic and exclusively clinical samples, clinical cohorts lacking proportionally matched controls, or outcomes non-specific for antisocial behavior were excluded. The Liptak-Stouffer weighted Z-test for meta-analysis was implemented to maximize study inclusion and calculated separately for male and female cohorts.
Results
Across 20 male cohorts, early adversity presaged antisocial outcomes more strongly for low, relative to high, activity MAOA genotype (P=.0044). Stratified analyses showed the interaction specific to maltreatment (P=.0000008) and robust to several sensitivity analyses. Across 11 female cohorts, MAOA did not interact with combined early life adversities, whereas maltreatment alone predicted antisocial behaviors preferentially, but weakly, in females of high activity MAOA genotype (P=.02).
Conclusions
We found common regulatory variation in MAOA to moderate effects of childhood maltreatment on male antisocial behaviors, confirming a sentinel finding in research on gene-environment interaction. An analogous, but less consistent, finding in females warrants further investigation.
Keywords: MAOA, antisocial behavior, childhood maltreatment, genetics, gene-environment interaction, meta-analysis
Two widely cited reports of putative gene-environment (GxE) interaction, published in 2002 and 2003, described genotype-dependent environmental influences on risk for antisocial behavior and depression, respectively, in a well-characterized, longitudinally studied birth cohort(1-2). In the second of these studies, recent stressful life events and childhood maltreatment predicted depression in young adults in proportion to the number of “short” (deletion) alleles carried of a 44-basepair (bp) insertion/deletion (long/short) polymorphism in the regulatory region of the serotonin transporter gene (5-HTTLPR)(2). Two prominent meta-analyses published in 2009 failed to confirm replication of this most frequently cited instance of gene-stress interaction(3-4). Some authors cautioned that these analyses incorporated only a fraction of relevant investigations and relied disproportionately on studies of self-reported life events, rather than contextually sensitive interviews or objective indicators of stress(5-6). In a more comprehensive meta-analysis published subsequently, Karg et al(7) found the 5-HTTLPR to moderate effects of adversity on clinical depression and depressive symptomatology across all published studies (P = .00002), but with some variation among studies of differing methodology. In stratified analyses, 5-HTTLPR genotype interacted with self-reported life events only marginally in predicting depression, whereas the interaction proved robust for studies of childhood maltreatment and of cohorts uniformly exposed to the same stressor or where life events were assessed by structured interview.
The purpose of this paper is to extend meta-analytic review to the first of the two GxE interactions described by Caspi and colleagues(1). There, exposure to maltreatment in childhood predicted later aggressive and antisocial behaviors among males as a function of regulatory variation in the gene encoding monoamine oxidase-A (MAOA). A degradative enzyme, MAOA preferentially deaminates the neurotransmitters, serotonin and norepinephrine, and the MAOA gene contains a 30-bp repeating sequence (Variable Number of Tandem Repeats) in the 5′-flanking region conferring allele-specific variation in MAOA promoter activity(8-10). In this study, early indicators of maltreatment, such as boys’ physical or sexual abuse, maternal rejection, or harsh physical punishment, more strongly predicted later conduct problems, antisocial disposition, and violent offending among persons carrying the MAOA repeat variant (allele) of lesser transcriptional efficiency (“low activity” MAOA genotype) than in those of an alternate (“high activity”) genotype. This finding has since been cited over 2800 times and prompted similar studies by other investigators. In 2007, Taylor and Kim-Cohen(11) confirmed the interaction of early maltreatment and MAOA genotype on antisocial outcomes by meta-analysis of the original study and seven attempted replications(12-18). These studies all included male participants recruited from largely normal populations (viz., excluding forensic or predominantly clinical samples) and contained either a single or composite index of antisocial behavior. Studies also included a measure of participants’ childhood exposure to abuse, neglect, or other harm within the family environment, and all reported such exposure positively associated with study outcomes. Consistent with the initial report of Caspi et al(1), the pooled estimate of correlation between family adversity and indices of later antisocial behavior was greater in individuals of low, compared to high, activity MAOA genotype (P < .0001)(11).
Many additional investigations have been reported since publication of Taylor and Kim-Cohen(11). These include further GxE studies of early maltreatment(19-25), studies of other environmental moderators (e.g., neighborhood and family socioeconomic disadvantage, peer deviance, parenting styles, commonly experienced life events, maternal prenatal smoking(25-32)); and studies including females(20-21, 23, 29-31, 33-38) or primarily non-White samples(24, 33-34). Here, we report a further meta-analysis of the accumulated literature addressing interactions of MAOA variation and environmental risk factors in the prediction of aggressive and antisocial outcomes. To allow comparison with the parallel literature on 5-HTTLPR variation, life stress and depression (i.e., the second GxE literature emerging from the two seminal studies of Caspi and colleagues(1-2)), we have followed the same analytic procedures employed by Karg et al(7). This approach maximizes inclusion of studies of differing design and analytic strategy or of limited statistical reporting or data availability through application of the common Liptak-Stouffer weighted Z-test for meta-analysis, which combines published reports by tests of statistical significance(7, 39-42).
METHODS
Studies
We sought all peer-reviewed, English-language studies published through August 2012 from: a) reference lists of prior meta-analysis, narrative reviews and individual studies; and b) major publication databases (e.g., PubMed), using as keywords: monoamine oxidase-A or MAOA and childhood maltreatment, abuse, adversity or family/family environment and antisocial behavior, conduct disorder/problems, delinquency, externalizing behavior, aggression or violence. We only included studies that had genotyped the same MAOA VNTR reported by Caspi et al(1), those in which the interaction of MAOA genotype and early adversity was tested explicitly; and studies for which outcomes included behaviors or disorders on an externalizing or antisocial spectrum (but not solely alcohol or substance abuse). Following Taylor and Kim-Cohen(11), studies of forensic populations(43-45), exclusively clinical samples or clinical samples lacking proportionally matched controls were excluded(46-47). Also excluded were two studies in which indicators of antisocial behavior could not be distinguished from other life outcomes or events (e.g., financial losses, accidents, unspecified relationship problems, or socioeconomic attainments)(48-49).
We identified 27 independent investigations meeting the foregoing criteria and totaling >18,400 study participants. Of these, 12 studies included only male participants(12-16, 19, 22, 24-25, 27-28, 32), 11 included participants of both sexes(1, 17-18, 20-21, 23, 29-31, 33-34), and 4 included only females(26, 36-38). Study samples were mainly all white (23), with 4 studies of mixed ethnicity or primarily non-white samples(17, 19, 24, 33). To determine if outcomes might vary by similarity to the first reported GxE interaction for MAOA, we stratified investigations into 2 groups: studies focusing specifically on early maltreatment and studies of other childhood adversities. Assignment to the “maltreatment” group was made when factors such as physical or sexual abuse, assault or other victimization, severe physical punishment, other exposures to violence, neglect or court-mediated family interventions predominated in indices of early childhood environment. Studies of sociodemographic variables, peer affiliations, maternal prenatal smoking, general life events, or parenting styles, and those with only minor or oblique representation of maltreatment indicators were assigned to the category of “other childhood adversities”. When studies reported on measurements from both categories, these were treated as independent tests in stratified analyses.
Extraction of P-values
The authors independently extracted P-values from each study, without discrepancy. When non-significant findings were reported without exact P-values, we requested more precise values from study authors. If these data were not available or authors declined, we assigned a P-value of 1 (indicating absence of an interaction implicating either low or high activity MAOA genotype). Because interaction terms were occasionally collapsed over sex or ethnicity, we also requested P-values from study authors for males and females analyzed separately, and similarly for white and nonwhite segments of multi-ethnic samples. With respect to outcome measures, for primary analyses we used the P-value associated with the most general (e.g., composite) measure of antisocial behavior or computed a weighted mean P-value when multiple dependent variables and/or multiple environmental moderators were analyzed separately in the published report.
Genotypes of MAOA
Because MAOA is located on the X chromosome (Xp11.4-Xp11.3), all males are hemizygous for a single allele of the upstream VNTR, for which variants of 2, 3, 3.5, 4 and 5 repeats have been described. In samples of European ancestry, the 3- and 4-repeat alleles account for >95% of variation. The 2, 3 and 5 repeat variants are commonly grouped as “low activity” alleles and contrasted with “high activity” alleles of 3.5 or 4 repeats, based on in vitro studies of MAOA promoter activity(8, 10). Functional characterization of the 5-repeat is somewhat controversial(10, 20), however, though its rarity suggests a negligible effect on study outcomes resulting from differences in allele grouping. Also, some studies disregarded the rare variants altogether, analyzing only the 3- and 4-repeat alleles.
In females, uncertainty regarding extent of X-inactivation at the MAOA locus(50-51) has occasioned differing analytic strategies for comparing MAOA genotypes, and in some instances, served as rationale for excluding females from study analyses(15). When tested in females, some investigators compared only individuals homozygous for low or high activity variants(17, 21, 33-34, 37), whereas others included a heterozygous grouping defined by presence of both a low and high activity allele(18, 20, 23, 26, 29-31, 36, 38). Here, we treat MAOA genotypes in females as they were operationalized in each study, although when a P-value for the contrast of homozygous low and high activity participants was available in studies including heterozygotes, we used this value in the meta-analysis to enhance comparability among studies. Finally, because the sentinel report by Caspi et al(1) addressed risk for antisocial behavior specifically in males, and owing to the more variable classification of MAOA genotypes in studies of females, analyses were conducted separately for each sex.
Statistical analysis
Like the Karg et al(7) meta-analysis of 5-HTTLPR variation, stress and depression, we combined investigations by the Liptak-Stouffer z score procedure to yield an aggregate outcome based on significance tests from each study, adjusted for sample size. Extracted P-values were first expressed as 1-tailed metrics, where P-values less than .50 corresponded to liability for antisocial behavior associated with low activity MAOA genotype and a P-value greater than .50 with high activity MAOA genotype. More precisely, a study outcome was considered consistent with Caspi et al(1) when the dependent variable associated more strongly with an environmental risk factor among participants of low, relative to high, activity MAOA genotype. As in Karg et al(7), P-values were next converted to z-scores, to which positive and negative signs were attached, respectively, for P-values less than and greater than .50. Finally, a composite z score was calculated by the formula:
where zi denotes z scores of the individual studies, wi refers to the study sample size, and k is the number of studies included in the analysis. The outcome, zw, is then tested for two-tailed significance by reference to the standard normal distribution.
This statistic was first calculated for all studies together and then, in stratified analyses, for studies partitioned by category of environmental moderator (i.e., “maltreatment”; “other adversities”). As noted, these analyses were run separately by sex. In 4 samples that included both males and females, sex-specific P-values were unavailable(17, 33-34). We excluded these investigations in primary analyses, but because males comprised a majority of participants in each, we included these with all other male studies in a secondary analysis.
Any initially significant finding was probed for disproportionate influence of single investigations by re-computing zw after removing each study individually, and if found unaltered by deletion of individual studies, the following additional sensitivity analyses were conducted. As noted previously, the P-value entered into analysis was occasionally averaged over tests involving more than one environmental moderator or, more commonly, over multiple outcomes, such as clinical diagnoses, forensic status (e.g., criminal convictions), and dimensional measures of informant and self-reported aggressive, antisocial, or other externalizing behavior. To determine if results were robust to this variation, we repeated the analysis by: a) iteratively substituting individual outcome variables for composite measures or averaged P-values; and b) running 1000 additional iterations of the meta-analysis, in each of which a single dependent measure was selected randomly from all studies with multiple outcomes. We then also ran analyses separately for: a) continuous and dichotomous measures of antisocial behavior; b) outcomes occurring in childhood/adolescence (≤18) and those of adulthood; c) outcomes of overt aggression or violence, non-violent antisocial behaviors (e.g., vandalism, theft), and measures combining violent and non-violent indicators (e.g., Conduct and Antisocial Personality Disorder diagnoses or symptom counts); and d) investigations based on cross-sectional and longitudinally studied cohorts. Regarding maltreatment studies, we also conducted analyses separately for those in which environmental exposures were assessed by family (self or parent) report only and studies including non-familial informant sources (e.g., official record, observation). Finally, to gauge potential publication bias, we: a) computed a fail-safe N by direct computation and calculated the ratio of failsafe N to the number of published studies; and b) followed up with re-analyses stratified by sample size and date of publication.
RESULTS
Male Studies
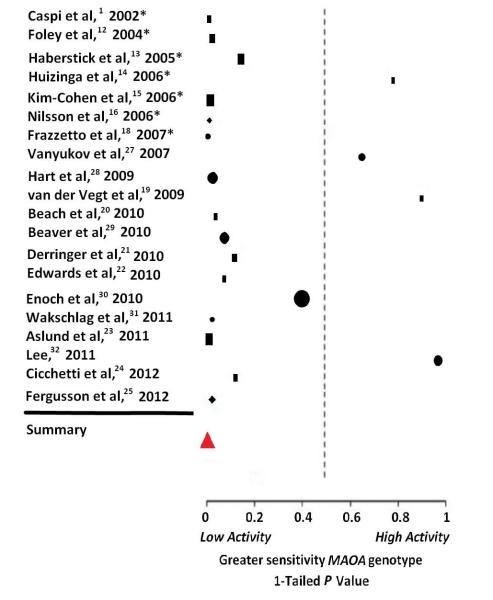
Our search identified 20 studies of exclusively male samples or mixed sex samples for which results were available in males separately (Table 1). These studies included 11,064 subjects (Table 2). The meta-analysis showed MAOA genotype to moderate an association of early life adversities (maltreatment plus “other adversities”) with later aggressive and antisocial outcomes across all male cohorts (P = .0044) (Figure 1). This effect persisted: a) on removal of each study individually (2.5 × 10−6 < P < .018) (Table 2); and b) with iterative substitution of individual outcome variables for composite measures or averaged P-values (6.6 × 10−5 < P < .014). Additional analyses showed the interaction significant in all but one of 1000 random combinations of study-specific dependent variables and environmental moderators (7.1 × 10−6 < P < .06). Results were significant for outcomes indexed to either childhood/adolescence (P = .032) or adulthood (P = .008); outcomes of aggression/violence (P = 3.9 × 10−5), non-violent antisocial behaviors (P = 7.2 × 10−4), or combined indices (P = .022); for dependent measures of continuous (P = .008), but not dichotomous distribution (P = .36); and for studies of both cross-sectional samples (P < .0045) and longitudinally studied cohorts (P = .019). Findings were unaffected by deletion of 2 non-white samples(19, 24) (P = .0044) or inclusion of 4 additional cohorts (N=806) of majority-male, mixed sex samples(17, 33-34) (P = .0034).
Table 1.
Description of MAOA, Early Life Adversity and Antisocial Behavior Studies Included in the Meta-Analysis
| Moderator |
Outcome |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Source, Year |
n | Male % |
Race | Study Design | Adversity Measure |
Informant | Age | Antisocial Behavior |
Informant | Age | Findinga | 1 Tailed P Valueb |
| Caspi et a,l(1) 2002 |
442 | 100% | White | Longitudinal | Maltreatment | INT(C), OBS, PR |
3 to 11 | CD, Violent Conviction, APD sx, Violent Disposition |
INF, INT(C), OR, SR |
11 to 18 | Positive | 0.0050 |
| 229 | 0% | CD | INT(C) | Negative | 0.1285** | |||||||
| Foley et al,(12) 2004 |
514 | 100% | White | Longitudinal | Maltreatment | INT(C, P) | <18 | CD | INT(C, P) | <18 | Positive | 0.0200 |
| Haberstick et al,(13) 2005 |
772 | 100% | White | Longitudinal | Maltreatment | INT(C), SR | <18 | Conduct Problems, Violent Convictions |
INT, OR | 16, 17 & 22 |
Negative | 0.1423* |
| Huizinga et al,(14) 2006 |
277 | 100% | White | Longitudinal | Maltreatment | SR | <17 | CD, Violent Conviction, APD sx, Violent Disposition |
INF, INT(C), OR, SR |
14 to 28 | Negative (Opposite) |
0.7794* |
| Kim-Cohen et al,(15) 2006 |
975 | 100% | White | Longitudinal | Maltreatment | INT (P) | ≤ 7 | ASB; Attention/ Hyperactivity; Emotional Problems |
PR, TR | 7 | Positive | 0.0145 |
| Nilsson et al,(16) 2006 |
79 | 100% | White | Cross-sectional | Maltreatment; Type of Residence |
INT(C), OR |
≤ 16 or 19 | Stealing, Vandalism, Violence |
INT(C) | 16 & 19 | Positive | 0.0078 |
| Widom & Brzustowicz,(17) 2006 |
261 | 67% | White | Longitudinal (Case vs. Control) |
Maltreatment | OR | <12 | CD sx, APD sx, Violent Behaviors |
INT(C), OR, SR |
<18 to 18+ | Positive | 0.0143* |
| 148 | 62% | NW | Negative | 0.5000* | ||||||||
| Frazzetto et al,(18) 2007 |
82 | 100% | White | Cross-Sectional (Case vs. Control) |
Early Adverse Experiences |
SR | <16 | Physical Aggression | SR | m=30.88 | Positive | 0.0020 |
| 153 | 0% | Negative | 0.4230 | |||||||||
| Sjoberg et al,(26) 2007 |
117 | 0% | White | Cross-Sectional | Maltreatment; Type of Residence |
SR | ≤ 16 or 19 | Stealing, Vandalism, Violence |
INT(C) | 16 or 19 | Negative (Opposite) |
0.9082 |
| Vanyukov et al,(27) 2007 |
144 | 100% | White | Longitudinal (High Risk) |
Parental Involvement |
SR | 12 to 18 | CD, ADHD | INT (C, P) | 12 to 18 | Partial Opposite |
0.6517* |
| Ducci et al,(37) 2008 |
187 | 0% | White | Cross-Sectional (Case vs. Control) |
Maltreatment | INT(C), OR |
<16 | APSD sx | INT(C) | m=37.80 | Positive | 0.0002 |
| Hart et al,(28) 2009 |
672 | 100% | NW UNS |
Longitudinal | Neighborhood Characteristics |
OR | r=11-21 | Aggression | SR | r=11-23 | Positive | 0.0250 |
| Prom- Wormley et al,(36) 2009 |
721 | 0% | White | Longitudinal | Maltreatment | PR, SR | <18 | CD | PR, SR | <18 | Opposite | 0.9750 |
| van der Vegt et al,(19) 2009 |
239 | 100% | NW | Cross-Sectional | Maltreatment | INF | ≤15 | Externalizing Behaviors (aggression, delinquency) |
PR | ≤15 | Negative (Opposite) |
0.9000 |
| Weder et al,(33) 2009 |
114 | 66% | NW | Cross-Sectional (Case vs. Control) |
Maltreatment | INT(P, C), SR, OR |
≤15 | Aggression, Rule Breaking, Inattention |
TR | ≤15 | Partial Positive |
0.0359* |
| Beach et al,(20) 2010 |
244 | 100% | White | Longitudinal | Maltreatment | INT (C) | <18 | APD sx | INT(C) | m=46.48 | Negative | 0.0300 |
| 294 | 0% | m=44.95 | Positive | 0.0240 | ||||||||
| Beaver et al,(29) 2010 |
420 | 100% | White | Longitudinal | Protective-Risk Index |
SR | <18 | Incarceration, Anger/ Hostility |
OR, SR | r=24-32 | Partial Positive |
0.0694* |
| 493 | 0% | Negative | 0.1539* | |||||||||
| Derringer et al.,(21) 2010 |
595 | 100% | White | Longitudinal | Maltreatment | INT (C), SR |
<18 | CD sx, APD sx |
INT(C) | 17, 21 & 25 |
Negative | 0.3520* |
| 246 | 0% | Partial Positive |
0.1057* | |||||||||
| Edwards et al,(22) 2010 |
186 | 100% | White | Longitudinal (High Risk) |
Maltreatment | SR | <6 | Externalizing Behaviors (aggression, delinquency) |
PR, SR, TR | 6 to 22 | Negative | 0.0675 |
| Enoch et al,(30) 2010 |
3182 | 100% | White | Longitudinal | Family Adversity, Stressful Life Events |
PR | PN to 7 | Conduct Problems, ADHD |
PR | 4 & 7 | Negative | 0.3936* |
| 3976 | 0% | Partial Positive |
0.1635* | |||||||||
| Wakschlag et al,(31) 2010 |
78 | 100% | White | Longitudinal (High Risk) |
Prenatal Smoking | DT, PR | PN | CD sx | PR | m=15 | Positive | 0.0160* |
| 99 | 0% | Opposite | 0.9990* | |||||||||
| Aslund et al,(23) 2011 |
780 | 100% | White | Cross-Sectional | Maltreatment | INT (C) | <18 | Stealing, Vandalism, Violence |
SR | r=17-18 | Positive | 0.0048* |
| 882 | 0% | Opposite | 0.9995* | |||||||||
| Lee,(32) 2011 |
672 | 100% | White | Longitudinal | Deviant Peer Behavior |
SR | m=15.65 | Overt/Covert ASB | SR | mr=15-22 | Partial Opposite |
0.9641* |
| Reti et al,(34) 2011 |
283 | 52% | White | Cross-Sectional | Maltreatment | INT (C) | <18 | APD sx | INT(C) | 18+ | Opposite | 0.9236 |
| Cicchetti et al,(24) 2012 |
312 | 100% | NW | Cross-Sectional (High Risk) |
Maltreatment | OR | ≤ 12 | CD sx, ASB, Externalizing Behaviors (aggression, delinquency) |
INF, INT(C), TR |
≤ 12 | Partial Positive |
0.1190* |
| Fergusson et al,(25) 2012 |
351 | 100% | White | Longitudinal | Maltreatment; PN Smoking; Material Deprivation |
INT(C), SR | PN to 15 | Violent/Property Offenses, Conduct Problems, Hostility |
INT(C), SR, OR |
15 to 21; 25 & 30 |
Positive | 0.0192* |
| McGrath et al,(38) 2012 |
192 | 0% | White | Cross-Sectional (High Risk) |
Maltreatment | SR | <18 | Conduct Problems, Impulsive-Sensation Seeking, Interpersonal Aggression, PN Smoking |
SR | m=42.9 | Negative (Opposite) |
0.9066 |
Abbreviations: ADHD, Attention Deficit Hyperactivity Disorder; APD, Antisocial Personality Disorder; ASB, Antisocial Behaviors; C, Child; CD, Conduct Disorder; DT, Drug Test; INF, Other Informant; INT, Interview; m, mean age; mr, mean age range; n, number of participants; NW, Non-white; OBS, Observation; OR, Official Record; P, Parent; PN, Prenatal; PR, Parent-Report; r, age range; SR, Self-Report; sx, Symptom Count; TR, Teacher-Report.
“Positive” indicates a significant (P<.05) interaction effect with the low-activity MAOA variant, as presented in the original study report, “Negative” indicates no interaction effect (P>.05), and “Opposite” indicates a significant (P<.05) interaction with the high-activity MAOA variant. “Partial” indicates a significant interaction effect for one or more, but not all, measures tested in a study with multiple dependent variables.
One-tailed P-value, with smaller values (P<.50) indicating greater sensitivity among low-activity MAOA subjects and larger values (P>.50) indicating greater sensitivity among high-activity MAOA subjects.
Denotes a weighted mean P-value created when multiple dependent variables and/or multiple environmental moderators were analyzed separately in the published report.
From footnoted observations that were not a component of primary analyses in Caspi et al,(1) 2002.
Table 2.
Studies Included in the All Male Group Meta-Analysis
| Source, Year | Total No. of Participants |
1-Tailed P Value |
Fisher P Value After Study Exclusion |
|---|---|---|---|
| Caspi et al,(1) 2002 | 442 | 0.0050 | 1.02×10−2 |
| Foley et al,(12) 2004 | 514 | 0.0200 | 9.32×10−3 |
| Haberstick et al,(13) 2005 | 772 | 0.1423 | 7.15×10−3 |
| Huizinga et al,(14) 2006 | 277 | 0.7794 | 3.50×10−3 |
| Kim-Cohen et al,(15) 2006 |
975 | 0.0145 | 1.73×10−2 |
| Nilsson et al,(16) 2006 | 79 | 0.0078 | 5.11×10−3 |
| Frazzetto et al,(18) 2007 | 82 | 0.0020 | 5.27×10−3 |
| Vanyukov et al,(27) 2007 | 144 | 0.6517 | 4.10×10−3 |
| Hart et al,(28) 2009 | 672 | 0.0250 | 1.08×10−2 |
| van der Vegt et al,(19) 2009 |
239 | 0.9000 | 3.28×10−3 |
| Beach et al,(20) 2010 | 244 | 0.0300 | 6.14×10−3 |
| Beaver et al,(29) 2010 | 420 | 0.0694 | 6.73×10−3 |
| Derringer et al,(21) 2010 | 595 | 0.3520 | 4.65×10−3 |
| Edwards et al,(22) 2010 | 186 | 0.0675 | 5.44×10−3 |
| Enoch et al,(30) 2010 | 3182 | 0.3936 | 2.48×10−6 |
| Wakschlag et al,(31) 2010 | 78 | 0.0160 | 4.95×10−3 |
| Aslund et al,(23) 2011 | 780 | 0.0048 | 1.78×10−2 |
| Lee,(32) 2011 | 672 | 0.9641 | 1.28×10−3 |
| Cicchetti et al,(24) 2012 | 312 | 0.1190 | 5.61×10−3 |
| Fergusson et al,(25) 2012 | 399 | 0.0192 | 8.05×10−3 |
| Total | 11064 | ||
| Average Sample Size | 553 | .0044 |
Figure 1.
Forest plot of 20 male samples for the interaction of MAOA genotype and early life adversities on aggressive and antisocial behavior. Icons indicate the 1-tailed P value for each sample, where lower values denote a greater sensitivity to adversity with low-activity MAOA genotype and high values denote a greater sensitivity with high-activity MAOA genotype. The size of the icon reflects relative sample size. Squares mark studies that indexed adversity specifically to childhood maltreatment; circles indicate studies of other childhood adversities; and diamonds indicate studies that included both maltreatment and other childhood adversities. The red triangle depicts the overall result of the meta-analysis for all-male samples (2-tailed). Studies marked with an asterisk were included in the prior meta-analysis by Taylor and Kim-Cohen11.
Stratified Analyses
Low activity MAOA genotype heightened risk for antisocial behavior among individuals exposed to maltreatment specifically (P = .0000008), but not in tests of other childhood adversities (P = .40). The interaction with maltreatment remained highly significant: a) when deleting each study individually (P=2.2 × 10−5 < P < 2.7 × 10−7) (Table 3); b) with iterative substitution of individual outcome measures for composite indices or mean P-values (P=3.5 × 10−5 < P < 1.4 × 10−7); and c) across all of 1000 random combinations of individual outcome measures (1.8 × 10−9 < P < .005). Among maltreatment studies, findings were again significant when analyzed separately for child/adolescent (P = 3.6 × 10−5) and adult outcomes (P = .05); for outcomes of aggression/violence (P = .01), non-violent antisocial behaviors (P = 4.0 × 10−4), and combined indices (P = 3.6 × 10−6). They were significant also for dependent variables of both continuous (P = 1.4 × 10−6) and dichotomous distribution (P = .02); in cross-sectional studies (P = .01) and studies of longitudinal cohorts (P = 3.5 × 10−5); and where assessment of maltreatment exposure rested on family-based report only (P = 7.8 × 10−7) or included nonfamilial informant sources (P = .022). Here, too, results were unaltered by removal of 2 non-white cohorts(19, 24) (P = 5.4 × 10−7) or addition of the 4 majority-male mixed sex samples(17, 33-34) (P = 5.7 × 10−7).
Table 3.
Studies Included in the All Male, Maltreatment Group Meta-Analysis
| Source, Year | Total No. of Participants |
1-Tailed P Value |
Fisher P Value After Study Exclusion |
|---|---|---|---|
| Caspi et al,(1) 2002 | 442 | 0.0050 | 8.59×10−6 |
| Foley et al,(12) 2004 | 514 | 0.0200 | 5.63×10−6 |
| Haberstick et al,(13) 2005 | 772 | 0.1423 | 8.20×10−7 |
| Huizinga et al,(14) 2006 | 277 | 0.7794 | 3.40×10−7 |
| Kim-Cohen et al,(15) 2006 |
975 | 0.0145 | 8.59×10−6 |
| Nilsson et al,(16) 2006 | 79 | 0.0183 | 1.23×10−6 |
| van der Vegt et al,(19) 2009 |
239 | 0.9000 | 2.70×10−7 |
| Beach et al,(20) 2010 | 244 | 0.0300 | 2.25×10−6 |
| Derringer et al,(21) 2010 | 595 | 0.3520 | 3.80×10−7 |
| Edwards et al,(22) 2010 | 186 | 0.0675 | 1.51×10−6 |
| Aslund et al,(23) 2011 | 780 | 0.0048 | 2.24×10−5 |
| Cicchetti et al,(24) 2012 | 312 | 0.1190 | 1.59×10−6 |
| Fergusson et al,(25) 2012 | 399 | 0.0051 | 7.46×10−6 |
| Total | 5814 | ||
| Average Sample Size | 447 | .0000008 |
Female Studies
We identified 12 studies involving females only or separately analyzed female cohorts, with a total of 7,588 subjects (Table 1). The meta-analysis showed no significant interaction of MAOA genotype with early life adversities (maltreatment and “other adversities”) across all studies (P = .77).
Stratified Analyses
When analyzed separately, MAOA genotype predicted antisocial outcomes in interaction with childhood maltreatment (P = .020), but not on exposure to other early adversities (P = .32). Unlike males, the interaction with maltreatment reflected an increased risk linked to high activity MAOA genotype. On deletion of each study individually, however, this finding lost significance with removal of either of 2 study cohorts (.004 < P < .97) (Table 4).
Table 4.
Studies Included in the All Female, Maltreatment Group Meta-Analysis
| Source, Year | Total No. of Participants |
1-Tailed P Value |
Fisher P Value After Study Exclusion |
|---|---|---|---|
| Caspi et al,(1) 2002 | 229 | 0.1285 | 9.88×10−3 |
| Sjoberg et al,(26) 2007 | 117 | 0.9494 | 2.85×10−2 |
| Ducci et al,(37) 2008 | 187 | 0.0002 | 3.73×10−3 |
| Prom-Wormley et al,(36) 2009 |
721 | 0.9750 | 1.39×10−1 |
| Beach et al,(20) 2010 | 294 | 0.0240 | 3.98×10−3 |
| Derringer et al,(21) 2010 | 246 | 0.1057 | 8.54×10−3 |
| Aslund et al,(23) 2011 | 882 | 0.9995 | 9.68×10−1 |
| McGrath et al,(38) 2012 | 192 | 0.9066 | 3.08×10−2 |
| Total | 2868 | ||
| Average Sample Size | 359 | .02 |
Publication Bias
Our results corroborate the sentinel observation of Caspi et al(1) that childhood maltreatment predicts antisocial outcomes more strongly in males of low, compared to high, activity MAOA genotype, and do not show this interaction extended to other categories of early life adversity or to females. To render the MAOA x maltreatment interaction in males non-significant (P > .05) would require >93 unpublished analyses or undiscovered studies of null effect (P = .50) and equal average sample size (N = 447). This yields a failsafe ratio of 7 studies not included for each maltreatment study of males included in the meta-analysis. The MAOA x Maltreatment interaction also proved significant in analyses restricted to: a) studies with samples either larger (P = 1.7 × 10−4) or smaller (P = .01) than Caspi et al (2002); and b) either recent (dated 2010-2012; P < 3.0 × 10−4) or early replication attempts (2004-2009; P = .005).
DISCUSSION
In their provocative first study of GxE interaction, Caspi et al(1) reported that common polymorphic variation in MAOA moderated the influence of childhood maltreatment on boys’ later aggressive and antisocial behaviors, as seen in a longitudinally studied, normal population. Our purpose was to determine whether this finding replicated in subsequent research addressed to the same hypothesis, when again examined in primarily nonclinical samples and extended to studies of other early life adversities or to females. Across male cohorts, the meta-analysis showed a moderately reliable interaction of MAOA variation and environmental risk factors, with childhood adversities presaging antisocial outcomes more strongly in persons of low, compared to high, activity MAOA genotype (as in Caspi et al(1)). Moreover, analyses stratified by category of early life adversity showed this finding accounted for principally by the interaction of MAOA and childhood maltreatment (P = 8.2 × 10−7), and therefore, where environmental risk most closely matched the sentinel study(1). The interaction with childhood maltreatment also proved robust to sensitivity analyses and generalized across studies of either cross-sectional or longitudinal design and studies in which maltreatment exposure was assessed by family (self, parent) report only or included independent informant sources. It is noteworthy, too, that MAOA variation interacted with childhood maltreatment to predict outcomes referenced to both childhood/adolescence and adulthood; dependent measures of both continuous and categorical distribution; and both violent and non-violent antisocial behaviors. The latter finding suggests that the low activity MAOA genotype heightens maltreatment-dependent risk for a range of conduct problems, and not aggression or criminal violence specifically.
These findings are consistent with a broader literature on early risk factors for antisocial behavior, in which maltreatment indicators like domestic violence, physical abuse, neglect and parental rejection figure prominently(52-60). They also accord with findings of a recent twin study, in which maltreatment increased children’s risk for conduct problems as a function of “latent” genetic risk, defined by twin-pair zygosity and co-twin diagnostic status for conduct disorder(61). Although biological mechanisms underlying these associations remain unknown, environmental insults like maltreatment presumably compound or otherwise interact with neurobehavioral correlates of MAOA to augment aggressive or antisocial potential. Relatedly, individuals of low, compared to high, activity MAOA genotype have performed more poorly on some executive processing tasks, such as tests of working memory and attentional control, and exhibited reduced task-dependent activations of frontal brain regions supporting these processes(62-66). The low activity MAOA genotype has been linked as well to altered neural responses to affective stimuli, including enhanced amygdala reactions to facial expressions of emotion or emotion recall; lesser engagement of prefrontal regulatory regions; and disrupted functional and effective (top-down) connectivity within corticolimbic circuitry of emotion processing(63, 67-69). It is possible that early maltreatment either exacerbates these neural deficits or engenders antagonistic and antisocial motivations that are abetted by MAOA-modulated impairments in inhibitory control.
In contrast to studies of childhood maltreatment, MAOA genotype did not moderate the aggregate effects of other early life adversities in males. The collection of environmental risks sampled in these investigations was quite variable, however, and it may be premature to conclude that MAOA interacts only with maltreatment to affect antisocial outcomes. For instance, risk associated with maternal prenatal smoking varied by MAOA genotype in each of the two studies that examined this variable (weighted P = .024)(25, 31). We should note that all but one attempted replication included in the prior meta-analysis by Taylor and Kim-Cohen(11) were categorized here as studies of maltreatment. The one such investigation that we placed instead in the category of other adversities used a measure of family relationships that, although encompassing abuse, emphasized a broader range of difficulties (e.g., separations, disability of self or sibling, marital problems and parental psychopathology)(18). Nonetheless, this study found the interaction of MAOA genotype and family difficulties to also predict male aggression, so that if included in meta-analysis with studies focusing more explicitly on maltreatment, the outcome does not differ (P = 4.3 × 10−7).
Unlike males, MAOA variation did not interact with early life adversities across 12 female cohorts, but like males, the interaction was significant in studies of childhood maltreatment alone. Yet among girls who were maltreated, the high, not low, activity MAOA genotype was more strongly associated with antisocial outcomes, although the interaction was weak and lost significance with deletion of some individual studies. Still, it is interesting that a combination of high activity MAOA genotype and environmental risk predicted delinquent behavior in several studies of adolescent girls and across different groups of investigators23,31,36,38. Elsewhere, symptoms of dysthymia have been found greater in women who were maltreated in childhood and carry the MAOA high activity (4-repeat) variant than among women of homozygous, low activity genotype(70). At present, it is unclear how a reversal of allelic association between males and females might be explained, and existing literature provides few clues. Incomplete X-inactivation at the MAOA locus could conceivably produce a different expression profile in women, possibly yielding a sex difference in MAOA product.50,51 Also potentially relevant to MAOA expression is some evidence that CpG residues in the MAOA promoter are hypermethylated in women, compared to men, and that differential methylation may be greatest among women of low activity MAOA genotype(71). A third possibility is that MAOA interacts with sex differences in perinatal androgen exposure to affect brain development or that gonadal hormones modulate genotype-dependent variation in MAOA expression in adolescence(26, 70). These suggestions are highly speculative, however, and do not yet provide a clear mechanism for a bidirectional association of MAOA genotype with antisocial behavior among maltreated cohorts of different sexes. Nonetheless, additional research may be warranted to determine if the findings suggesting heightened susceptibility for the high activity genotype in females emerge more prominently in a larger literature.
To recapitulate, our meta-analysis confirms the first seminal study of GxE interaction reported by Caspi et al(1). We find that MAOA variation moderates effects of early life adversity on males’ aggressive and antisocial behaviors, and this interaction is attributable to studies that, like the initial report, delimit adversity to experiences of maltreatment, such as physical and sexual abuse, harsh discipline, neglect, assault or other ill-treatment. Significance of the weighted z-score for direct replications (i.e., maltreatment studies) is substantial (P = 8.2 × 10−7) and supported by a sizable failsafe ratio. Of course, even a positive meta-analysis does not exhaust validity challenges or vouchsafe a true association. For instance, publication bias may be indicated if reported replications aggregate among smaller rather than larger, better powered studies or in early, but not later, studies. Here however, we found the interaction of MAOA genotype and childhood maltreatment no less likely to replicate in studies with sample sizes larger (or smaller) than Caspi et al(1) or in studies published later (in the last three years) or prior to 2010. Indeed, the interaction also proves significant (P = 5.1 × 10−4) if restricted only to maltreatment studies not included in the preceding (positive) meta-analysis of Taylor and Kim-Cohen(11). Finally, considering the recent, parallel meta-analysis of 5-HTTLPR variation, life stress and depression(7) alongside our study suggests that the two novel investigations spawning these literatures(1-2) reflect not only prominent, but also durable, examples of GxE interaction. Both analyses also highlight points of methodology affecting study outcomes, such as type of early adversity (here) or, in studies of 5-HTTLPR and depression, stressor type and quality of measurement, which may usefully guide future research aimed at elucidation of etiologic mechanisms. Whether these two instances reflect a more general role of GxE interactions in the genesis of major psychopathologies is unknown, however, and awaits the emergence of comparable literatures addressed to other disorders, genes, and environmental risk factors.
Acknowledgements
This work was supported in part by NIH grant PO1 HL040962 [SBM] and NSF IGERT 0549352 [ALB].
Footnotes
Disclosure Amy L. Byrd and Stephen B. Manuck report no biomedical financial interests or potential conflicts of interest.
References
- 1.Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, et al. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297:851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- 2.Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, et al. Influence of Life Stress on Depression: Moderation by a Polymorphism in the 5-HTT Gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 3.Munafò MR, Durrant C, Lewis G, Flint J. Gene × environment interactions at the serotonin transporter locus. Biological Psychiatry. 2009;65:211–219. doi: 10.1016/j.biopsych.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 4.Risch N, Herrell R, Lehner T, Liang KY, Eaves L, Hoh J, et al. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: A meta-analysis. JAMA: The Journal of the American Medical Association. 2009;301:2462–2471. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rutter M, Thapar A, Pickles A. Gene-environment interactions: Biologically valid pathway or artifact? Archives of General Psychiatry. 2009;66:1287–1289. doi: 10.1001/archgenpsychiatry.2009.167. [DOI] [PubMed] [Google Scholar]
- 6.Uher R, McGuffin P. The moderation by the serotonin transporter gene of environmental adversity in the etiology of depression: 2009 update. Molecular Psychiatry. 2010;15 doi: 10.1038/mp.2009.123. [DOI] [PubMed] [Google Scholar]
- 7.Karg K, Burmeister M, Shedden K, Sen S. The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited: Evidence of genetic moderation. Archives of General Psychiatry. 2011;68:444–454. doi: 10.1001/archgenpsychiatry.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sabol SZ, Hu S, Hamer D. A functional polymorphism in the monoamine oxidase A gene promoter. Human Genetics. 1998;103:273–279. doi: 10.1007/s004390050816. [DOI] [PubMed] [Google Scholar]
- 9.Denney RM, Koch H, Craig IW. Association between monoamine oxidase A activity in human male skin fibroblasts and genotype of the MAOA promoter-associated variable number tandem repeat. Human Genetics. 1999;195:542–551. doi: 10.1007/s004399900183. [DOI] [PubMed] [Google Scholar]
- 10.Deakert J, Catalano M, Syagailo YV, Bosi M, Okladnova O, Di Bella D, et al. Excess of high activity monoamine oxidase A gene promoter alleles in females patients with panic disorder. Human Molecular Genetics. 1999;8:621–624. doi: 10.1093/hmg/8.4.621. [DOI] [PubMed] [Google Scholar]
- 11.Taylor A, Kim-Cohen J. Meta-analysis of gene-environment interactions in developmental psychopathology. Dev Psychopathol. 2007;19:1029–1037. doi: 10.1017/S095457940700051X. [DOI] [PubMed] [Google Scholar]
- 12.Foley DL, Eaves LJ, Wormley B, Silberg JL, Maes HH, Kuhn J, et al. Childhood adversity, monoamine oxidase a genotype, and risk for conduct disorder. Arch Gen Psychiatry. 2004;61:738–744. doi: 10.1001/archpsyc.61.7.738. [DOI] [PubMed] [Google Scholar]
- 13.Haberstick BC, Lessem JM, Hopfer CJ, Smolen A, Ehringer MA, Timberlake D, et al. Monoamine oxidase A (MAOA) and antisocial behaviors in the presence of childhood and adolescent maltreatment. Am J Med Genet B Neuropsychiatr Genet. 2005;135:59–64. doi: 10.1002/ajmg.b.30176. [DOI] [PubMed] [Google Scholar]
- 14.Huizinga D, Haberstick BC, Smolen A, Menard S, Young SE, Corley RP, et al. Childhood Maltreatment, Subsequent Antisocial Behavior, and the Role of Monoamine Oxidase A Genotype. Biological Psychiatry. 2006;60:677–683. doi: 10.1016/j.biopsych.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 15.Kim-Cohen J, Caspi A, Taylor A, Williams B, Newcombe R, Craig IW, et al. MAOA, maltreatment, and gene-environment interaction predicting children’s mental health: New evidence and a meta-analysis. Mol Psychiatry. 2006;11:903–913. doi: 10.1038/sj.mp.4001851. [DOI] [PubMed] [Google Scholar]
- 16.Nilsson KW, Sjoberg RL, Damberg M, Leppert J, Ohrvik J, Alm PO, et al. Role of monoamine oxidase A genotype and psychosocial factors in male adolescent criminal activity. Biol Psychiatry. 2006;59:121–127. doi: 10.1016/j.biopsych.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 17.Widom CS, Brzustowicz LM. MAOA and the “Cycle of Violence:” Childhood Abuse and Neglect, MAOA Genotype, and Risk for Violent and Antisocial Behavior. Biological Psychiatry. 2006;60:684–689. doi: 10.1016/j.biopsych.2006.03.039. [DOI] [PubMed] [Google Scholar]
- 18.Frazzetto G, Di Lorenzo G, Carola V, Proietti L, Sokolowska E, Siracusano A, et al. Early Trauma and Increased Risk for Physical Aggression during Adulthood: The Moderating Role of MAOA Genotype. PLoS ONE. 2007;2:e486. doi: 10.1371/journal.pone.0000486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van der Vegt EJM, Oostra BA, Arias-Vásquez A, van der Ende J, Verhulst FC, Tiemeier H. High activity of Monoamine oxidase A is associated with externalizing behaviour in maltreated and nonmaltreated adoptees. Psychiatric Genetics. 2009;19:209–211. doi: 10.1097/YPG.0b013e32832a5084. [DOI] [PubMed] [Google Scholar]
- 20.Beach SRH, Brody GH, Gunter TD, Packer H, Wernett P, Philibert RA. Child maltreatment moderates the association of MAOA with symptoms of depression and antisocial personality disorder. Journal of Family Psychology. 2010;24:12–20. doi: 10.1037/a0018074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Derringer J, Krueger RF, Irons D, Iacono WG. Harsh Discipline, Childhood Sexual Assault, and MAOA Genotype: An Investigation of Main and Interactive Effects on Diverse Clinical Externalizing Outcomes. Behavior Genetics. 2010;40:639–648. doi: 10.1007/s10519-010-9358-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edwards AC, Dodge KA, Latendresse SJ, Lansford JE, Bates JE, Pettit GS, et al. MAOA-uVNTR and early physical discipline interact to influence delinquent behavior. Journal of Child Psychology and Psychiatry. 2010;51:679–687. doi: 10.1111/j.1469-7610.2009.02196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Åslund C, Nordquist N, Comasco E, Leppert J, Oreland L, Nilsson K. Maltreatment, MAOA, and Delinquency: Sex Differences in Gene–Environment Interaction in a Large Population-Based Cohort of Adolescents. Behavior Genetics. 2011;41:262–272. doi: 10.1007/s10519-010-9356-y. [DOI] [PubMed] [Google Scholar]
- 24.Cicchetti D, Rogosch FA, Thibodeau EL. The effects of child maltreatment on early signs of antisocial behavior: Genetic moderation by tryptophan hydroxylase, serotonin transporter, and monoamine oxidase A genes. Development and Psychopathology. 2012;24:907–928. doi: 10.1017/S0954579412000442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fergusson DM, Boden JM, Horwood LJ, Miller A, Kennedy MA. Moderating role of the MAOA genotype in antisocial behaviour. The British journal of psychiatry : the journal of mental science. 2012;200:116–123. doi: 10.1192/bjp.bp.111.093328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sjöerg RL, Nilsson KW, Wargelius HL, Leppert J, Lindström L, Oreland L. Adolescent girls and criminal activity: Role of MAOA-LPR genotype and psychosocial factors. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2007;144B:159–164. doi: 10.1002/ajmg.b.30360. [DOI] [PubMed] [Google Scholar]
- 27.Vanyukov MM, Maher BS, Devlin B, Kirillova GP, Kirisci L, Yu LM, et al. The MAOA promoter polymorphism, disruptive behavior disorders, and early onset substance use disorder: gene-environment interaction. Psychiatric Genetics. 2007;17:323–332. doi: 10.1097/YPG.0b013e32811f6691. 310.1097/YPG.1090b1013e32811f36691. [DOI] [PubMed] [Google Scholar]
- 28.Hart D, Marmorstein NR. Neighborhoods and genes and everything in between: Understanding adolescent aggression in social and biological contexts. Development and Psychopathology. 2009;21:961–973. doi: 10.1017/S0954579409000510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beaver KM, DeLisi M, Vaughn MG, Barnes JC. Monoamine oxidase A genotype is associated with gang membership and weapon use. Comprehensive Psychiatry. 2010;51:130–134. doi: 10.1016/j.comppsych.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 30.Enoch MA, Steer CD, Newman TK, Gibson N, Goldman D. Early life stress, MAOA, and gene-environment interactions predict behavioral disinhibition in children. Genes, Brain and Behavior. 2010;9:65–74. doi: 10.1111/j.1601-183X.2009.00535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wakschlag LS, Kistner EO, Pine DS, Biesecker G, Pickett KE, Skol AD, et al. Interaction of prenatal exposure to cigarettes and MAOA genotype in pathways to youth antisocial behavior. Molecular Psychiatry. 2010;15:928–937. doi: 10.1038/mp.2009.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee SS. Deviant Peer Affiliation and Antisocial Behavior: Interaction with Monoamine Oxidase A (MAOA) Genotype. Journal of Abnormal Child Psychology. 2011;39:321–332. doi: 10.1007/s10802-010-9474-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weder N, Yang B, Douglas-Palumberi H, Massey J, Krystal JH, Gelernter J, et al. MAOA Genotype, Maltreatment, and Aggressive Behavior: The Changing Impact of Genotype at Varying Levels of Trauma. Biological Psychiatry. 2009;65:417–424. doi: 10.1016/j.biopsych.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reti IM, Xu JZ, Yanofski J, McKibben J, Uhart M, Cheng YJ, et al. Monoamine oxidase A regulates antisocial personality in whites with no history of physical abuse. Comprehensive Psychiatry. 2011;52:188–194. doi: 10.1016/j.comppsych.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sjöberg RL, Ducci F, Barr CS, Newman TK, Dell’Osso L, Virkkunen M, et al. A Non-Additive Interaction of a Functional MAO-A VNTR and Testosterone Predicts Antisocial Behavior. Neuropsychopharmacology. 2008;33:425–430. doi: 10.1038/sj.npp.1301417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prom-Wormley EC, Eaves LJ, Foley DL, Gardner CO, Archer KJ, Wormley BK, et al. Monoamine oxidase A and childhood adversity as risk factors for conduct disorder in females. Psychological Medicine. 2009;39:579–590. doi: 10.1017/S0033291708004170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ducci F, Enoch M, Hodgkinson C, Xu K, Catena M, Robin RW, et al. Interaction between a functional MAOA locus and childhood sexual abuse predicts alcoholism and antisocial personality disorder in adult women. Molecular Psychiatry. 2008;13:334–347. doi: 10.1038/sj.mp.4002034. [DOI] [PubMed] [Google Scholar]
- 38.McGrath L, Mustanski B, Metzger A, Pine D, Kistner-Griffin E, Cook E, et al. A latent modeling approach to genotype–phenotype relationships: Maternal problem behavior clusters, prenatal smoking, and MAOA genotype. Archives of Women’s Mental Health. 2012;15:269–282. doi: 10.1007/s00737-012-0286-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Whitlock MC. Combining probability from independent tests: The weighted Z-method is superior to Fisher’s approach. Journal of Evolutionary Biology. 2005;18:1368–1373. doi: 10.1111/j.1420-9101.2005.00917.x. [DOI] [PubMed] [Google Scholar]
- 40.Hedges LV, Olkin I. Statistical Methods for Meta-Analysis. Academic; New York, NY: 1985. [Google Scholar]
- 41.Richards JB, Waterworth D, O’Rahilly S, Hivert M-F, Loos RJF, Perry JRB, et al. A Genome-Wide Association Study Reveals Variants in ARL15 that Influence Adiponectin Levels. PLoS Genet. 2009;5:e1000768. doi: 10.1371/journal.pgen.1000768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hwang D, Rust AG, Ramsey S, Smith JJ, Leslie DM, Weston AD, et al. A data integration methodology for systems biology. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:17296–17301. doi: 10.1073/pnas.0508647102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Young SE, Smolen A, Hewitt JK, Haberstick BC, Stallings MC, Corley RP, et al. Interaction Between MAO-A Genotype and Maltreatment in the Risk for Conduct Disorder: Failure to Confirm in Adolescent Patients. American Journal of Psychiatry. 2006;163:1019–1025. doi: 10.1176/ajp.2006.163.6.1019. [DOI] [PubMed] [Google Scholar]
- 44.Reif A, Rosler M, Freitag CM, Schneider M, Eujen A, Kissling C, et al. Nature and Nurture Predispose to Violent Behavior: Serotonergic Genes and Adverse Childhood Environment. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301359. [DOI] [PubMed] [Google Scholar]
- 45.Tikkanen R, Ducci F, Goldman D, Holi M, Lindberg N, Tiihonen J, et al. MAOA Alters the Effects of Heavy Drinking and Childhood Physical Abuse on Risk for Severe Impulsive Acts of Violence Among Alcoholic Violent Offenders. Alcoholism: Clinical and Experimental Research. 2010;34:853–860. doi: 10.1111/j.1530-0277.2010.01157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang YY, Cate SP, Battistuzzi C, Oquendo MA, Brent D, Mann JJ. An association between a functional polymorphism in the monoamine oxidase a gene promoter, impulsive traits and early abuse experiences. Neuropsychopharmacology. 2004;29:1498–1505. doi: 10.1038/sj.npp.1300455. [DOI] [PubMed] [Google Scholar]
- 47.Kinnally EL, Huang Y, Haverly R, Burke AK, Galfalvy H, Brent DP, et al. Parental care moderates the influence of MAOA-uVNTR genotype and childhood stressors on trait impulsivity and aggression in adult women. Psychiatric Genetics. 2009;19:126–133. doi: 10.1097/YPG.0b013e32832a50a7. 110.1097/YPG.1090b1013e32832a32850a32837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nilsson KW, Wargelius H-L, Sjóberg RL, Leppert J, Oreland L. The MAO-A gene, platelet MAO-B activity and psychosocial environment in adolescent female alcohol-related problem behaviour. Drug and Alcohol Dependence. 2008;93:51–62. doi: 10.1016/j.drugalcdep.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 49.Prichard Z, Mackinnon A, Jorm AF, Easteal S. No evidence for interaction between MAOA and childhood adversity for antisocial behavior. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2008;147B:228–232. doi: 10.1002/ajmg.b.30581. [DOI] [PubMed] [Google Scholar]
- 50.Benjamin D, Van Badel I, Craig I. A novel expression based approach for assessing the inactivation status of human X-linked genes. European Journal of Human Genetics. 2000;8:103–108. doi: 10.1038/sj.ejhg.5200427. [DOI] [PubMed] [Google Scholar]
- 51.Carrel L, Willard H. X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature. 2005;434:400–404. doi: 10.1038/nature03479. [DOI] [PubMed] [Google Scholar]
- 52.Jaffee SR, Caspi A, Moffitt TE, Taylor A. Physical maltreatment victim to antisocial child: Evidence of an environmentally mediated process. Journal of Abnormal Psychology. 2004;113:44–55. doi: 10.1037/0021-843X.113.1.44. [DOI] [PubMed] [Google Scholar]
- 53.Fergusson DM, Horwood JL. Exposure to interparental violence in childhood and psychosocial adjustment in young adulthood. Child Abuse and Neglect. 1998;22:339–357. doi: 10.1016/s0145-2134(98)00004-0. [DOI] [PubMed] [Google Scholar]
- 54.Jaffee SR, Moffitt TE, Caspi A, Taylor A, Arseneault L. Influence of adult domestic violence on children’s internalizing and externalizing problems: An environmentally informative twin study. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41:1095–1103. doi: 10.1097/00004583-200209000-00010. [DOI] [PubMed] [Google Scholar]
- 55.Caspi A, Moffitt TE, Morgan J, Rutter M, Taylor A, Arseneault L, et al. Maternal expressed emotion predicts children’s antisocial behavior problems: Using monozygotic-twin differences to identify environmental effects on behavioral development. Developmental Psychology. 2004;40:149–161. doi: 10.1037/0012-1649.40.2.149. [DOI] [PubMed] [Google Scholar]
- 56.Farrington DP, Hawkins JD. Predicting participation, early onset and later persistence in officially recorded offending. Criminal Behaviour and Mental Health. 1991;1:1–33. [Google Scholar]
- 57.Luntz BK, Widom CS. Antisocial personality disorder in abused and neglected children grown up. American Journal of Psychiatry. 1994;151:670–674. doi: 10.1176/ajp.151.5.670. [DOI] [PubMed] [Google Scholar]
- 58.Dodge KA, Sherrill MR. The interaction of nature and nurture in antisocial behavior. In: Flannery DJ, Vazsonyi AT, Waldman ID, editors. The Cambridge handbook of violent behavior and aggression. Cambridge University Press; New York: 2007. pp. 215–242. [Google Scholar]
- 59.Lansford JE, Dodge KA, Pettit GS, Bates JE, Crozier J, Kaplow J. A 12-year prospective study of the long-term effects of early child physical maltreatment on psychological, behavioral, and academic problems in adolescence. Archives of Pediatrics and Adolescent Medicine. 2002;156:824–830. doi: 10.1001/archpedi.156.8.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McCord J. Questioning the value of punishment. Social Problems. 1992;38 [Google Scholar]
- 61.Jaffee SR, Caspi A, Moffitt TE, Dodge KA, Rutter M, Taylor A, et al. Nature x nurture: Genetic vulnerabilities interact with physical maltreatment to promote conduct problems. Development and Psychopathology. 2005;17:67–84. doi: 10.1017/s0954579405050042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fan J, Fossella J, Sommer T, Wu Y, Posner MI. Mapping the genetic variation of executive attention onto brain activity. Proceedings of the National Academy of Sciences. 2003;100:7406–7411. doi: 10.1073/pnas.0732088100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Meyer-Lindenberg A, Buckholtz JW, Kolachana B, R Hariri A, Pezawas L, Blasi G, et al. Neural mechanisms of genetic risk for impulsivity and violence in humans. Proceedings of the National Academy of Sciences: USA. 2006;103:6269–6274. doi: 10.1073/pnas.0511311103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cerasa A, Gioia MC, Fera FP, L., Kiguori M, Lanza P, Muglia M, et al. Ventro-lateral prefrontal activity during working memory is modulated by MAO A genetic variation. Brain Research. 2008;1201:114–121. doi: 10.1016/j.brainres.2008.01.048. [DOI] [PubMed] [Google Scholar]
- 65.Passamonti L, Fera F, Magariello A, Cerasa A, Gioia MC, Muglia M, et al. Monoamine oxidase-A genetic variations influence brain activity associated with inhibitory control: New insight into the neural correlates of impulsivity. Biological Psychiatry. 2006;59:334–340. doi: 10.1016/j.biopsych.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 66.Enge S, Fleischhauer M, Lesch K-P, Reif A, Strobel A. Serotonergic modulation in executive functioning: Linking genetic variations to working memory performance. Neuropsychologia. 2011;49:3776–3785. doi: 10.1016/j.neuropsychologia.2011.09.038. [DOI] [PubMed] [Google Scholar]
- 67.Buckholtz JW, Meyer-Lindenberg A. MAOA and the neurogenetic architecture of human aggression. Trends in Neurosciences. 2008;31:120–129. doi: 10.1016/j.tins.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 68.Buckholtz JW, Callicott JH, Kolachana B, Hariri AR, Goldberg TE, Genderson M, et al. Genetic variation in MAOA modulates ventromedial prefrontal circuitry mediating individual differences in human personality. Molecular Psychiatry. 2008;13:313–324. doi: 10.1038/sj.mp.4002020. [DOI] [PubMed] [Google Scholar]
- 69.Lee B-T, Ham BJ. Monoamine oxidase A-uVNTR genotype affects limbic brain activity in response to affective facial stimuli. NeuroReport. 2008;19:515–519. doi: 10.1097/WNR.0b013e3282f94294. [DOI] [PubMed] [Google Scholar]
- 70.Nikulina V, Widom CS, Brzustowicz LM. Child abuse and neglect, MAOA, and mental health outcomes: A prospective examination. Biological Psychiatry. 2012;71:350–357. doi: 10.1016/j.biopsych.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Philibert RA, Gunter TD, Beach SRH, Brody GH, Madan A. MAOA methylation is associated with nicotine and alcohol dependence in women. American Journal of Medical Genetics Part B (Neuropsychiatric Genetics) 2008;147B:565–570. doi: 10.1002/ajmg.b.30778. [DOI] [PMC free article] [PubMed] [Google Scholar]