Abstract
The adolescent brain is a period of dynamic development making it vulnerable to environmental factors such as drug exposure. Of the illicit drugs, cannabis is most used by teenagers since it is perceived by many to be of little harm. This perception has led to a growing number of states approving its legalization and increased accessibility. Most of the debates and ensuing policies regarding cannabis were done without consideration of its impact on one of the most vulnerable population, namely teens, or without consideration of scientific data. We provide an overview of the endocannabinoid system in relation to adolescent cannabis exposure and provide insights regarding factors such as genetics and behavioral traits that confer risk for subsequent addiction. While it is clear that more systematic scientific studies are needed to understand the long-term impact of adolescent cannabis exposure on brain and behavior, the current evidence suggests that it has a far-reaching influence on adult addictive behaviors particularly for certain subsets of vulnerable individuals.
Keywords: marijuana, cannabinoid, opioid neuropeptide, nucleus accumbens, prefrontal cortex
1. Introduction
Adolescence is an important stage of behavioral maturation and brain development during which the high degree of neuroplasticity that occurs in this ontogenetic period places the adolescent brain at particular risk to environmental factors such as drug exposure. Marijuana (Cannabis sativa) continues to be the illicit drug most commonly used by teenagers in the United States as well as in other Western societies (Johnston et al., 2012; SAMHSA, 2011). Although cannabis is not as highly addictive as other substances, such as heroin and cocaine, cannabis-dependent individuals still greatly outnumber those reporting dependence on other illicit drugs and the number of people seeking treatment for cannabis dependence continues to increase yearly (SAMHSA, 2011).
Despite these facts, there is a growing perception, particularly in adolescents and young adults (Kilmer et al., 2007; Lopez-Quintero and Neumark, 2010), that cannabis is ‘harmless’ especially when compared to other abused substances like nicotine (tobacco) and alcohol that are legal. Reasons cited for this perception include the consideration that cannabis-associated mortality is lower than tobacco and alcohol, which are associated with cancer and overdose/vehicular accidents, respectively. In addition, cannabinoids provide medicinal benefits (Hermanson and Marnett, 2011; Hill et al., 2012) in contrast to tobacco and alcohol, which have no medical indications. These and other considerations have contributed to the decriminalization, or even legalization, of cannabis in a number of states within the USA. Economic factors have also been suggested as a rationalization for legalization as a potential source of tax revenue for state governments. Despite some cogent arguments in the current debates regarding legalization and increased availability of cannabis, most of the discussion and policies have been made without significant consideration of scientific data.
Growing evidence suggests a differential effect of cannabis exposure on the human brain based on the age of exposure, but the question remains as to the potential long-term mental health consequences of cannabis exposure in teens. Few scientific studies have systematically investigated the long-term impact of cannabis use in relation to the developing teenage brain, the population most crucial to the current debates. Nevertheless, the available data to date, as discussed in this review, suggest that adolescent cannabis exposure induces significant protracted effects suggestive of enhanced vulnerability to addiction and psychiatric disorders in later life, at least in certain subsets of individuals.
2. Neurobiology of the endocannabinoid system
The main psychoactive component of cannabis, Δ9-tetrahydrocannabinol (THC), acts primarily via cannabinoid receptors (CBRs) — CB1R and CB2R (Gerard et al., 1991; Griffin et al., 2000; Matsuda et al., 1990; Munro et al., 1993). The CB1R is one of the most abundant G-protein-coupled receptor in the brain (Herkenham et al., 1990; Herkenham et al., 1991a) and is Gi/o-coupled, suppressing neurotransmitter release (Howlett et al., 2002). The expression of CB1R is most pronounced within the basal ganglia, cerebellum, cerebral cortex, hippocampus and amygdala (Biegon and Kerman, 2001; Glass et al., 1997; Herkenham et al., 1990; Herkenham et al., 1991b; Mailleux et al., 1992; Pettit et al., 1998; Wang et al., 2003) (Fig. 1), consistent with cannabis exerting significant effects on motor function, cognition, and emotional regulation. Recent evidence, though initially controversial, suggests that CB2R is also expressed within the central nervous system in immune cells as well as glia and potentially neurons (Gong et al., 2006; Lanciego et al., 2011; Onaivi et al., 2006; Van Sickle et al., 2005). Nevertheless, the broad and abundant expression of CB1R in neuronal circuits relevant to addiction and psychiatric disorders still place a prominent emphasis on cannabis’ modulation of this CBR subtype in relation to psychiatric vulnerability.
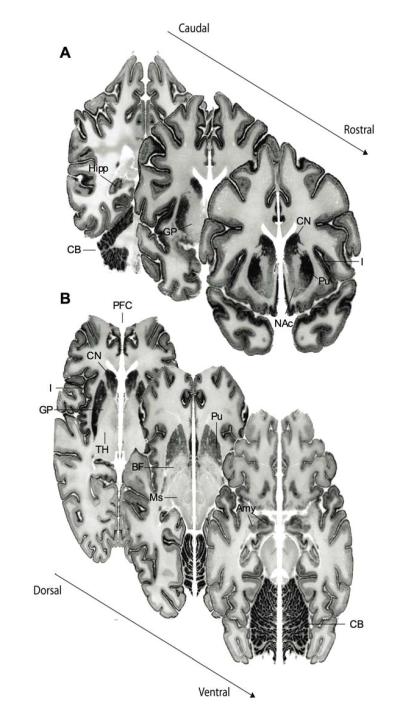
Figure 1.
Cannabinoid receptor mRNA (CNR1) expression in the human brain emphasizes this gene’s abundant expression in cerebral cortex – such as insular cortex (I) and prefrontal cortex (PFC) – as well as the caudate nucleus (CN), putamen (Pu), nucleus accumbens (NAc), hippocampus (Hipp), amygdala (Amy), and cerebellum (CB). Absent-to-low mRNA expression is notable in the thalamus (T), basal forebrain (BF), globus pallidus (GP), and midbrain (Ms).
Imaging studies of rodents (Verdurand et al., 2011) and human subjects (Mato et al., 2003) suggest global increases in CB1R throughout early life into adolescence, at which period adult levels are generally maintained (Belue et al., 1995; McLaughlin et al., 1994; Rodriguez de Fonseca et al., 1993), but there are also reports of reduced CB1R expression from juvenile to adulthood that mirrors developmental changes in CB1R-mediated signaling (Heng et al., 2011). Some of the inconsistencies regarding the ontogenic pattern of the CB1R may be due to regional, as opposed to global, developmental differences in the receptor development in addition to differences in mRNA, receptor protein or receptor binding being studied. Most preclinical investigations to date that have examined the neurodevelopment of CB1R have focused on the striatum and prefrontal cortical brain regions which are key components of neuronal circuits implicated in addiction and related psychiatric disorders. Reward, motivated behavior, decision-making, habit formation and motor function are mediated by the prefrontal cortex as well as components of the dorsal (associative- and sensorimotor-related) and ventral (limbic-related) striatal areas making these brains regions relevant to adolescent cannabis exposure.
With respect to cortical development, remodeling of excitatory connections in the prefrontal cortex is a key feature of adolescent neurodevelopment. For instance, there is significant pruning of excitatory synapses that parallels the delayed maturity of cognitive behaviors such as inhibitory control and working memory. The CB1R is highly abundant in the prefrontal cortex, primarily localized on large cholecystokinin interneurons (Marsicano and Lutz, 1999), but molecular and functional evidence suggest that CB1R is also present in a subset of pyramidal neurons (Hill et al., 2007; Marsicano and Lutz, 1999; Matsuda et al., 1993). Interestingly, the most pronounced and progressive cortical alteration observed on CB1R expression and CB1R-mediated functional signaling evident during adolescent development in rodents is within the medial prefrontal and other limbic/associative cortices as compared to sensorimotor cortices (Heng et al., 2011). Whether such developmental fluctuations of the CB1R directly relate to plasticity and the synaptic remodeling that occurs in the prefrontal cortex during adolescence is unknown.
In the striatum, CB1R are localized in the medium spiny GABAergic neurons that constitute the major output pathways — striatonigral and striatopallidal (Fig. 2). These receptors are expressed on both striatonigral ‘direct’ and striatopallidal ‘indirect’ pathways which mediate ‘Go’ facilitatory (positive reward/choice) and ‘NoGo’ (avoidance learning/inhibitory control) behaviors, respectively, relevant to motor function and decision-making processes (Durieux et al., 2009; Frank et al., 2007; Klein et al., 2007; Sano et al., 2003). While no study thus far has characterized the pattern and abundance of CB1R in the different output pathways during development, it is known that CB1R expression is dynamic during the course of adolescent development in different brain regions. For example, in vivo (Verdurand et al., 2011) and in vitro (Belue et al., 1995) imaging of the rat brain that revealed global enhanced CB1R in the cortex, also showed increased CB1R in other brain structures including the striatum during the transition from early adolescence to adulthood. However, other investigators have provided significant evidence for reduced CB1R expression and mRNA levels from juvenile to adulthood (Van Waes et al., 2012). Moreover, examination of CB1R protein expression restricted to the adolescent developmental window suggests significant CB1R differences even within distinct compartments of the nucleus accumbens (Ellgren et al., 2008). During adolescence, CB1R protein decreases in the nucleus accumbens shell yet concomitantly increases in the core compartment. This suggests that distinct time periods during adolescence may have different sensitivity to cannabis exposure relevant to mesolimbic striatal function.
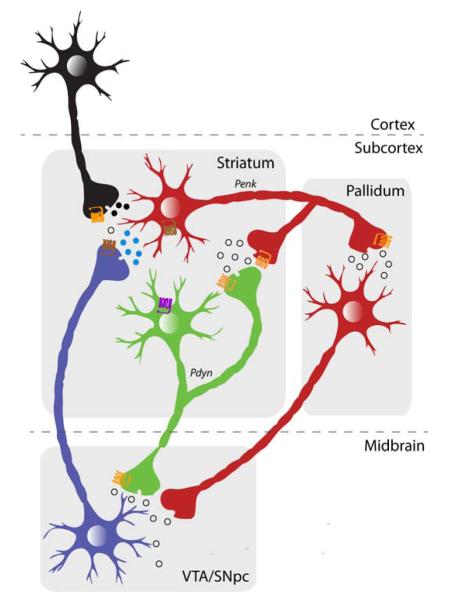
Figure 2.
Schematic illustration of the striatonigral ‘Go’ andstriatopallidal ‘NoGo’ pathways. These medium spiny output neurons are distinguishable based on their targets and subcellular markers, namely the expression of D1R (purple) and D2R (brown), respectively. Both cell-types, however, express CB1R (orange). This dissociation is based mainly on the dorsal striatal circuit, but a similar organization, particularly with respect to the ‘NoGo’ pathway, exists for the ventral striatal circuit.
Given anatomical and functional relationships between the prefrontal cortex and the striatum, it is not surprising that these regions are coordinated in regard to development of the endocannabinoid (eCB) system. There appears to be a direct correlation between the developmental trajectory of CB1R expression in specific cortical regions with their projection to distinct striatal subregions. For example, striatal subregions with high levels of CB1R expression (dorsolateral sensorimotor regions) receive input primarily from cortical areas with relatively low CB1R levels (motor, somatosensory) (Van Waes et al., 2012). In contrast, striatal subregions with low mRNA expression of Cnr1, such as the associative ventromedial dorsal striatum and limbic nucleus accumbens, receive afferents from cortical areas with greater CB1R expression (cingulate, insular). These findings emphasize a strong inverse relationship between cortical and striatal CB1R expression in frontostriatal circuits, suggesting a functional orchestration vulnerable to adolescent cannabis exposure.
In addition to CBRs, other components of the eCB system, such as the endogenous cannabinoids anandamide and 2-arachidonoylglycerol (2-AG), have significant developmental implications. Indeed, eCB signaling plays a key role in hardwiring of the brain during prenatal ontogeny, regulating synaptogenesis and target selection for the development of neuronal pathways (Harkany et al., 2008; Mulder et al., 2008). During later developmental stages, the eCBs are well-documented regulators of synaptic plasticity (Katona and Freund, 2008). Within adolescent ontogeny, anandamide and 2-AG are dynamically altered in the striatum and prefrontal cortex, as for instance, 2-AG is reduced from early to late adolescence in these regions (Ellgren et al., 2008). There is also a continuous increase of anandamide in the prefrontal cortex over the course of adolescence. The fact that the eCB system is dynamically altered during adolescence in brain areas central to reward, decision-making and motivation suggests that cannabis exposure during this critical developmental phase may have long-term influence on behaviors linked to the mesocorticolimbic system. Clearly, however, the limited studies to date are incomplete, so there still remains a large gap of knowledge regarding the adolescent ontogeny of the eCB system.
3. Cannabis and ‘gateway’ effects
A major aspect of the debate regarding adolescent cannabis use is whether it increases the use of other addictive substances such as heroin and cocaine later in life, a phenomenon known as the gateway hypothesis. Clinical and epidemiological studies have documented a significant link between repeated early cannabis exposure and an increased risk of other illicit drug use (Agrawal et al., 2004; Brook et al., 1999a; Fergusson and Boden, 2008; Fergusson and Horwood, 2000; Hall and Lynskey, 2005; Kandel, 1975; Yamaguchi and Kandel, 1984). Altogether, the data suggest that use of ‘heavy’ drugs is almost systematically preceded by cannabis use, and that risk is correlated with the intensity of cannabis exposure. Moreover, cannabis use also appears more deleterious when its onset occurs in younger versus older adolescents in regard to adjustment in transition from adolescence to young adulthood, education attainment, employment, delinquency and ability to conform to adult role (Brook et al., 1999a; Brook et al., 1999b; Fergusson and Boden, 2008; Fergusson et al., 2002; Lynskey et al., 2003; Tucker et al., 2006). Despite these findings, a major caveat of human studies is the difficulty of demonstrating a causal relationship between adolescent cannabis use and subsequent behavioral disturbances, especially when considering the influence of genetic and environmental factors alongside other aspects such as polysubstance use (Cleveland and Wiebe, 2008; Fergusson et al., 2006; Kandel et al., 2006; Lessem et al., 2006; Maccoun, 2006; Tarter et al., 2006). Given these complexities, animal models are a valuable tool to obtain direct insights about the relationship between early cannabis exposure and behavioral disruptions.
Many rodent investigations exploring the potential gateway effects of cannabis have primarily studied synthetic cannabinoid agonists that differ in pharmacological properties to THC. Nevertheless, studies examining adolescent exposure to cannabinoid agonists or THC provide evidence of enhanced intake and sensitivity later in life to opiate drugs (Biscaia et al., 2008; Ellgren et al., 2007; Tomasiewicz et al., 2012). In our experimental rat model that mimics the more periodic use of most adolescent cannabis users, adult male rats with low-to-moderate THC exposure during adolescence exhibit enhanced heroin self-administration behavior (Ellgren et al., 2007). Cannabinoid-opioid interactions have also been documented by studies showing that developmental exposure to cannabinoid agonists increases heroin-induced conditioned place preference (Biscaia et al., 2008; Singh et al., 2006). Short adolescent exposure to cannabinoid agonist, WIN 55,212-2, has also been reported to instead induce tolerance to morphine (Pistis et al., 2004).
Animal models make it possible to identify neuroadaptations that may contribute to the behavioral vulnerability related to adolescent cannabis. Intriguingly, many experimental animal studies to date have implicated the striatopallidal circuit in association with developmental cannabinoid exposure (Corchero et al., 1998; Corchero et al., 1999; Ellgren et al., 2007; Morel et al., 2009; Perez-Rosado et al., 2000; Spano et al., 2007; Valverde et al., 2001). This theory is based on consistent alterations of striatal dopamine D2 receptors (Drd2) and proenkephalin (Penk) mRNA expression both of which are preferentially co-expressed within striatopallidal medium spiny neurons (Gerfen et al., 1990; Gerfen and Young III, 1988; Le Moine et al., 1990). In our model of adolescent THC exposure, reduced Drd2 and Penk (Ellgren et al., 2007; Tomasiewicz et al., 2012) mRNA levels were observed within the nucleus accumbens of adult animals (Fig. 3). Reduced D2R, the protein encoded by Drd2, has long been a characteristic neurobiological feature of addiction vulnerability. In vivo positron emission tomography (PET) evidence has consistently demonstrated that subjects with substance abuse have less available D2R in the striatum (Heinz et al., 2004; Volkow et al., 2001; Volkow et al., 2004; Volkow et al., 1999; Wang et al., 1997), findings that animal models have shown to be linked to enhanced drug self-administration vulnerability (Morgan et al., 2002; Nader et al., 2006). Over-expression of Drd2 in the ventral striatum attenuates cocaine intake (Thanos et al., 2008), and D2R binding in this region in cocaine-naïve rats negatively predicts future cocaine-seeking behavior (Michaelides et al., 2012). In addition to adolescent THC exposure, prenatal THC also leads to dysregulation of the Drd2 gene in adulthood (DiNieri et al., 2011). That developmental THC exposure reduces Drd2 mRNA expression in the striatum, and affects related behavioral traits, support the hypothesis that developmental cannabis may induce a neurobiological state of addiction vulnerability.
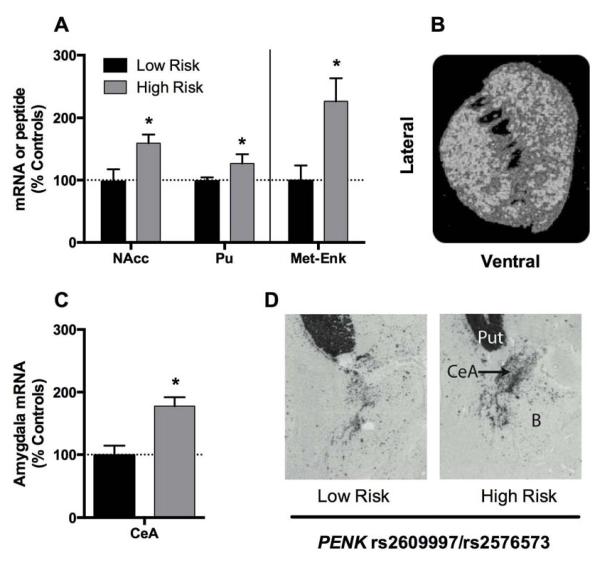
Figure 3.
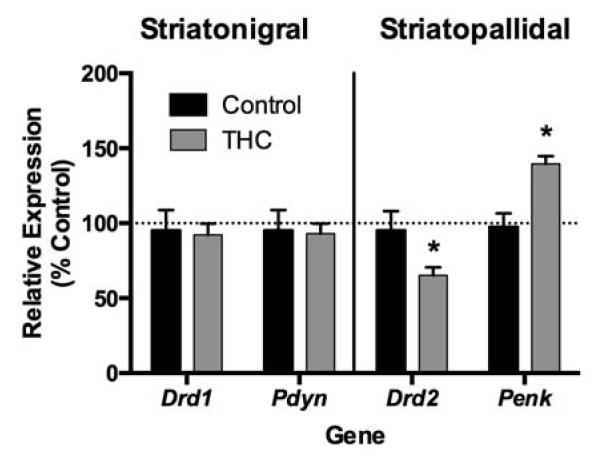
Periodic low-to-moderate THC exposure during adolescence (1.5 mg/kg every third day between postnatal days 28 and 49) alters Drd2 and Penk mRNA expression in the adult nucleus accumbens. These genes are strongly enriched in striatopallidal neurons of the nucleus accumbens (*p < 0.05).
The finding of impaired Penk gene expression in cannabis-exposed subjects is perhaps not surprising given the tight neurobiological interactions between the opioid and eCB systems. Opioid neuropeptide receptors and CB1R are coexpressed on similar neurons in the striatum, share similar G-protein coupled signaling mechanisms and appear functionally interdependent (Blume et al., 2013; Canals and Milligan, 2008). Of the opioid neuropeptides, enkephalin, encoded by the Penk gene, directly regulates hedonic states (Kelley et al., 2002; Skoubis et al., 2005). We recently documented a direct causal link between regulation of ventral striatal Penk mRNA expression and heroin self-administration behavior. Overexpression of the Penk gene in the nucleus accumbens shell by use of viral-mediated manipulation enhanced heroin self-administration and heroin-seeking behavior in animals naïve to THC, whereas in contrast, knocking down the Penk gene in THC-exposed rats reduced heroin intake behavior (Tomasiewicz et al., 2012). Altogether, these and other findings (Spano et al., 2010; Vigano et al., 2005) suggest the tight interaction between cannabinoids and the opioid system could contribute to the development of opiate abuse in adults with previous exposure to THC during adolescence.
In contrast to the effects noted for opiates, the impact of early cannabinoid exposure on the subsequent sensitivity to stimulant drugs have yielded inconsistent findings. While some studies failed to find significant behavioral differences in response to amphetamine later in life (Ellgren et al., 2004), others report that adolescent cannabinoids enhance cocaine-induced motor behavior (Dow-Edwards and Izenwasser, 2012). Moreover, both increased (Higuera-Matas et al., 2008) and deceased self-administration of cocaine (Panlilio et al., 2007) have been reported in adult animals with adolescent cannabinoid exposure. Differences in experimental factors, such as the duration and frequency of exposure, dose and formulation of the cannabinoid, and even gender likely contribute to these inconsistencies. For example, adolescent administration of the CB1R agonist CP 55,940 was reported to increase cocaine self-administration primarily in females, not males (Higuera-Matas et al., 2008). These findings emphasize the need to systematically probe factors such as the magnitude and duration of cannabinoid exposure, adolescent period of exposure and gender in order to help expand insights about individual risk factors contributing to the gateway effects of adolescent cannabis.
4. Genetic and behavioral traits contribute to individual vulnerability
Although animal studies demonstrate protracted behavioral and neurobiological effects of adolescent THC exposure into adulthood, there remains the fact that not all teenage cannabis users develop future addictions or psychiatric disorders. In fact, despite its common use, only a subset of teens (~25%) and young adults (~19%) using cannabis progress to abuse or dependence (SAMHSA, 2011). Indeed, for most teenagers, cannabis is a terminus with no further use of that or other illicit drugs as they mature into full adulthood, suggesting that there are differences in individual vulnerability. Humans vary tremendously for instance in regards to environment, behavioral traits, genetics, and cultural norms. While these and other factors play significant roles in complex disorders as addiction, understanding the contribution of each factor is as much a challenge as determining their interactions to risk..
4.1 Behavioral traits and personality
Cannabis users are generally characterized by apathy, loss of goal-motivated behavior and negative mood states, and dependent subjects report more negative affect, neuroticism, aggressivity and impulsivity (Dorard et al., 2008; Hyman and Sinha, 2009; Jutras-Aswad et al., 2012; Zvolensky et al., 2007). Negative affect in cannabis-dependent subjects is also related to the severity of dependence insomuch that this personality trait correlates with years of cannabis use, implying that long-term use of cannabis also worsens negative affect. Such findings are consistent with the hypothesis that in attempts to ‘self-medicate’, a user’s continued consumption of cannabis itself exacerbates underlying negative traits (Arendt et al., 2007). Rat studies also substantiate these human findings by documenting that exposure to CB1R agonists during adolescence induces long-term increases in anxiety- and depression-like behaviors as adults (Biscaia et al., 2003; Ciccocioppo et al., 2002). Indeed, a growing body of evidence suggests that cannabis exposure in humans during adolescence is linked to the development of symptoms characteristic of mood and anxiety disorders (Fergusson et al., 2002; Hayatbakhsh et al., 2007; Patton et al., 2002).
While the use of cannabis itself appears to lead to negative affect, which could contribute to subsequent drug abuse as individuals try to self-medicate, even young cannabis dependent subjects without a long history of drug use show high neuroticism/anxiety and depression traits, implying a preexisting negative emotional affect in these users (Dorard et al., 2008). As such, there may be subsets of individuals with at-risk behavioral traits that contribute to self-medication ultimately leading to dependence. Self-medication due to a preexisting vulnerable state is also evident in regard to psychosis risk. Cannabis use is high among people with psychosis (Koskinen et al., 2010; van Gastel et al., 2013) and it has been documented that psychotic symptoms are evident in subjects who have never used cannabis before the onset of psychotic symptoms, which also predicts future cannabis use (Ferdinand et al., 2005). This suggests that current cannabis-dependent subjects may have underlying psychiatric disorders that contributed to self-medication and that through repeated use, led to dependence. Thus, while cannabis may itself increase drug addiction and psychiatric vulnerability, pre-existing prodromal states (or disease vulnerability) may initially promote the initiation and continuation of cannabis use.
4.2 Heritable genetic factors
A growing number of family, twin and adoption studies have shown that cannabis use disorder is strongly heritable (30-80%) (Agrawal and Lynskey, 2009; Kendler et al., 2000; Kendler and Prescott, 1998; Maes et al., 1999; McGue et al., 2000; Miles et al., 2001; Rhee et al., 2003; Tsuang et al., 1998; van den Bree et al., 1998). Most studies examining cannabis dependence and genetic risk have used a candidate gene approach. Thus far, genes encoding CB1R (CNR1) and the fatty acid amide hydrolase (FAAH), an enzyme responsible for the hydrolysis of the eCB anadamide, have been shown to modulate cannabis dependence risk (Agrawal et al., 2009; Hopfer et al., 2006; Tyndale et al., 2007).
Given the effects of adolescent THC on striatopallidal-related genes, namely Drd2 and Penk expression, in the rodent models, (Corchero et al., 1998; Corchero et al., 1999; Ellgren et al., 2007; Morel et al., 2009; Perez-Rosado et al., 2000; Spano et al., 2007; Valverde et al., 2001; Wang et al., 2006), it was of interest to explore DRD2 and PENK SNPs in humans in relation to cannabis dependence. The DRD2 Taq1A polymorphism, which has been studied in multiple addiction disorders, did not associate with cannabis use (Creemers et al., 2011; Jutras-Aswad et al., 2012; Sakai et al., 2007). However, considering the link between DRD2 and inhibitory control, we probed their interacting association with cannabis dependence risk. A number of neurocognitive studies have documented that DRD2 SNPs predict avoidance-based decisions in healthy subjects, in line with this gene’s association with the striatopallidal NoGo pathway (Frank and Hutchison, 2009; Frank et al., 2007; Klein et al., 2007). Our results confirmed that negative reinforcement learning, linked with the ability to avoid maladaptative choices in a probabilistic learning task, was indeed associated with the DRD2 rs6277 SNP (Jutras-Aswad et al., 2012). For this DRD2 SNP, however, both cannabis users and controls exhibited the same association with negative reinforcement. Interestingly, negative affect (high anxiety/neuroticism trait) which was prominent in cannabis users significantly modulated the association between DRD2 and PENK genotypes with cannabis dependence. This interaction was most apparent for PENK SNPs (rs2609997 and rs2576573), with neuroticism/anxiety trait explaining approximately 15% to 20% of the association between genotype and cannabis dependence (Jutras-Aswad et al., 2012).
Recent evidence from animal models have demonstrated a direct role of the nucleus accumbens Penk striatopallidal pathway in mediating behavioral responses associated with aversive behavior (Hikida et al., 2010). In the human brain, there is a significant association of PENK SNPs (rs2609997 and rs2576573) with mRNA expression of this gene in the nucleus accumbens and dorsal striatum as well as with striatal metenkephalin peptide levels (Jutras-Aswad et al., 2012). These findings emphasize the transcriptional and translational functional relationships of polymorphisms of the PENK gene (Fig. 4A). In addition to the striatum, PENK mRNA amygdala expression is also related to PENK genotype which is interesting given that the amygdala plays a prominent role in negative mood states and enkephalinergic neurons in the central amygdala are critically involved in anxiety and stress responsivity (Kang et al., 2000; Kung et al., 2010) (Fig. 4B).
Figure 4.
PENK SNPs (rs2609997 and rs2576573) are associated with PENK expression in the human brain. High-risk alleles for cannabis dependence of the rs2609997 and rs2576573 PENK SNPs associate with elevated mRNA expression and met-enkephalin peptide (met-enk) levels in the human striatum (A). Similarly, these alleles associate with elevated mRNA expression in central amygdala nucleus (B). (High-risk genotypes = C/C + C/T for rs2609997 or A/A + A/G for rs2576573; low-risk genotypes = T/T for rs2609997 or G/G for rs2576573. *p < 0.05.)
4.3 Interactions between genetics and behavioral traits
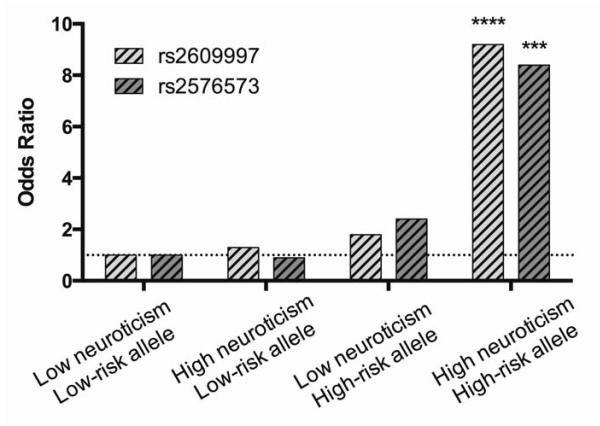
It is clear that multiple factors can converge to contribute to vulnerability. For example, while PENK genotype and anxiety/neuroticism trait are individually associated with cannabis dependence, there was a strong synergism between high-risk genotypes and the negative affect trait that enhanced cannabis dependence risk 8-9-fold (Jutras-Aswad et al., 2012) (Fig. 5). This synergistic interaction was also evident in another population (homogenous Caucasian Greek army conscripts) in which aspects of cigarette use could be explored separately from cannabis use (Jutras-Aswad et al., 2012). The finding that cannabis dependence is significantly enhanced in individuals with both high neuroticism/anxiety and risk genotypes emphasizes the important synergistic contribution of negative emotional traits and genetics to vulnerability. Thus, developmental cannabis exposure may confer susceptibility primarily in those individuals with underlying genetic and behavioral trait (Fig. 6). Both clinical reports and research studies suggest that coping with stress and negative mood states is a common motive for use among heavy abusers (Hyman and Sinha, 2009), which would be consistent with self-medicating even subthreshold anxiety and negative affect induced by PENK dysfunction. Cannabis exposure and negative affect may thus interact in a complex way such that cannabis is used to cope with subthreshold symptoms, but paradoxically further increases these symptoms in the long term.
Figure 5.
Synergistic Contribution of negative affect trait and PENK variants to cannabis dependence vulnerability. (High-risk genotype = C/C + C/T for rs2609997 or A/A + A/G for rs2576573; low-risk genotype = T/T for rs2609997 or G/G for rs2576573. ***p < 0.01; ****p < 0.001. Modified from Jutras-Aswad et al., 2012.)
Figure 6.
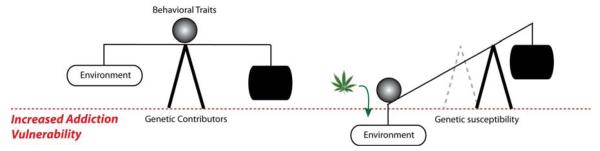
Schematic overview of the interaction between environmental factors, genetics and behavioral traits that together contribute to complex neuropsychiatric disorders like addiction. Vulnerability involves a delicate balance between factors that promote and protect against disease, and adolescent THC, an environmental factor, may tip this balance in teens with high-risk genotypes and behavioral traits.
5. Summary
Different lines of evidence suggest a link between adolescent THC and subsequent vulnerability to addiction and psychiatric risk. Yet, it is clear that more scientific evidence is critically needed to fully understand this relationship considering the multiple factors that appear to influence this trajectory. While some neurobiological insights have been obtained, it is clear that additional information is needed to fully understand the dynamic neurodevelopment of distinct components of the eCB and related neuronal systems, and the impact of cannabis upon these systems during adolescent ontogeny. The mechanisms by which cannabis may disrupt the functional organization of brain structures such as the striatum and prefrontal cortex during adolescent development as well as specific behavioral phenotypes are still unknown. Aside from the direct pharmacological effects of the drug on brain development, individual factors contribute tremendously to the complexity of the relationship between adolescent cannabis exposure and addiction risk (Fig. 6). The apparent synergistic interactions of genetics and negative affective trait suggest that genetic screens should begin to consider behavioral endophenotypes since the gene-addiction risk relationship is not direct. The possibility to identify vulnerable adolescents is essential for early intervention of cannabis dependence and related psychiatric disorders. Overall, it is impossible to ignore the evidence that cannabis/THC is not harmless to the developing brain, but there remain large gaps of knowledge that need to be filled in order to help inform public policy, thereby enhancing teenagers’ well-being and their mental health later in life.
Highlights.
Adolescents undergo dynamic brain development increasing drug vulnerability
Cannabis is most used by teenagers since it is perceived to be of little harm
Adolescent cannabis exposure significantly impacts the endocannabinoid system
Cannabis exposure, genetics and behavioral traits increased addiction vulnerability
Acknowledgements
This work was funded by NIDA DA030359 and DA023214.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Agrawal A, Lynskey MT. Candidate genes for cannabis use disorders: findings, challenges and directions. Addiction. 2009;104:518–532. doi: 10.1111/j.1360-0443.2009.02504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal A, Neale MC, Prescott CA, Kendler KS. Cannabis and other illicit drugs: comorbid use and abuse/dependence in males and females. Behav Genet. 2004;34:217–228. doi: 10.1023/B:BEGE.0000017868.07829.45. [DOI] [PubMed] [Google Scholar]
- Agrawal A, Wetherill L, Dick DM, Xuei X, Hinrichs A, Hesselbrock V, Kramer J, Nurnberger JI, Jr., Schuckit M, Bierut LJ, Edenberg HJ, Foroud T. Evidence for association between polymorphisms in the cannabinoid receptor 1 (CNR1) gene and cannabis dependence. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:736–740. doi: 10.1002/ajmg.b.30881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt M, Rosenberg R, Fjordback L, Brandholdt J, Foldager L, Sher L, Munk-Jorgensen P. Testing the self-medication hypothesis of depression and aggression in cannabis-dependent subjects. Psychol Med. 2007;37:935–945. doi: 10.1017/S0033291706009688. [DOI] [PubMed] [Google Scholar]
- Belue RC, Howlett AC, Westlake TM, Hutchings DE. The ontogeny of cannabinoid receptors in the brain of postnatal and aging rats. Neurotoxicol Teratol. 1995;17:25–30. doi: 10.1016/0892-0362(94)00053-g. [DOI] [PubMed] [Google Scholar]
- Biegon A, Kerman IA. Autoradiographic study of pre- and postnatal distribution of cannabinoid receptors in human brain. Neuroimage. 2001;14:1463–1468. doi: 10.1006/nimg.2001.0939. [DOI] [PubMed] [Google Scholar]
- Biscaia M, Fernandez B, Higuera-Matas A, Miguens M, Viveros MP, Garcia-Lecumberri C, Ambrosio E. Sex-dependent effects of periadolescent exposure to the cannabinoid agonist CP-55,940 on morphine self-administration behaviour and the endogenous opioid system. Neuropharmacology. 2008;54:863–873. doi: 10.1016/j.neuropharm.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Biscaia M, Marin S, Fernandez B, Marco EM, Rubio M, Guaza C, Ambrosio E, Viveros MP. Chronic treatment with CP 55,940 during the peri-adolescent period differentially affects the behavioural responses of male and female rats in adulthood. Psychopharmacology (Berl) 2003;170:301–308. doi: 10.1007/s00213-003-1550-7. [DOI] [PubMed] [Google Scholar]
- Blume LC, Bass CE, Childers SR, Dalton GD, Roberts DC, Richardson JM, Xiao R, Selley DE, Howlett AC. Striatal CB1 and D2 receptors regulate expression of each other, CRIP1A and delta opioid systems. J Neurochem. 2013;124:808–820. doi: 10.1111/jnc.12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook JS, Balka EB, Whiteman M. The risks for late adolescence of early adolescent marijuana use. Am J Public Health. 1999a;89:1549–1554. doi: 10.2105/ajph.89.10.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook JS, Richter L, Whiteman M, Cohen P. Consequences of adolescent marijuana use: incompatibility with the assumption of adult roles. Genet Soc Gen Psychol Monogr. 1999b;125:193–207. [PubMed] [Google Scholar]
- Canals M, Milligan G. Constitutive activity of the cannabinoid CB1 receptor regulates the function of co-expressed Mu opioid receptors. J Biol Chem. 2008;283:11424–11434. doi: 10.1074/jbc.M710300200. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Antonelli L, Biondini M, Perfumi M, Pompei P, Massi M. Memory impairment following combined exposure to delta(9)-tetrahydrocannabinol and ethanol in rats. Eur J Pharmacol. 2002;449:245–252. doi: 10.1016/s0014-2999(02)01999-4. [DOI] [PubMed] [Google Scholar]
- Cleveland HH, Wiebe RP. Understanding the association between adolescent marijuana use and later serious drug use: gateway effect or developmental trajectory? Dev Psychopathol. 2008;20:615–632. doi: 10.1017/S0954579408000308. [DOI] [PubMed] [Google Scholar]
- Corchero J, Garcia-Gil L, Manzanares J, Fernandez-Ruiz JJ, Fuentes JA, Ramos JA. Perinatal delta9-tetrahydrocannabinol exposure reduces proenkephalin gene expression in the caudate-putamen of adult female rats. Life Sci. 1998;63:843–850. doi: 10.1016/s0024-3205(98)00341-5. [DOI] [PubMed] [Google Scholar]
- Corchero J, Romero J, Berrendero F, Fernandez-Ruiz J, Ramos JA, Fuentes JA, Manzanares J. Time-dependent differences of repeated administration with Delta9-tetrahydrocannabinol in proenkephalin and cannabinoid receptor gene expression and G-protein activation by mu-opioid and CB1-cannabinoid receptors in the caudate-putamen. Brain Res Mol Brain Res. 1999;67:148–157. doi: 10.1016/s0169-328x(99)00053-4. [DOI] [PubMed] [Google Scholar]
- Creemers HE, Harakeh Z, Dick DM, Meyers J, Vollebergh WA, Ormel J, Verhulst FC, Huizink AC. DRD2 and DRD4 in relation to regular alcohol and cannabis use among adolescents: does parenting modify the impact of genetic vulnerability? The TRAILS study. Drug Alcohol Depend. 2011;115:35–42. doi: 10.1016/j.drugalcdep.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNieri JA, Wang X, Szutorisz H, Spano SM, Kaur J, Casaccia P, Dow-Edwards D, Hurd YL. Maternal cannabis use alters ventral striatal dopamine D2 gene regulation in the offspring. Biological psychiatry. 2011;70:763–769. doi: 10.1016/j.biopsych.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorard G, Berthoz S, Phan O, Corcos M, Bungener C. Affect dysregulation in cannabis abusers: a study in adolescents and young adults. European child & adolescent psychiatry. 2008;17:274–282. doi: 10.1007/s00787-007-0663-7. [DOI] [PubMed] [Google Scholar]
- Dow-Edwards D, Izenwasser S. Pretreatment with Delta9-tetrahydrocannabinol (THC) increases cocaine-stimulated activity in adolescent but not adult male rats. Pharmacology, biochemistry, and behavior. 2012;100:587–591. doi: 10.1016/j.pbb.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durieux PF, Bearzatto B, Guiducci S, Buch T, Waisman A, Zoli M, Schiffmann SN, de Kerchove d’Exaerde A. D2R striatopallidal neurons inhibit both locomotor and drug reward processes. Nat Neurosci. 2009;12:393–395. doi: 10.1038/nn.2286. [DOI] [PubMed] [Google Scholar]
- Ellgren M, Artmann A, Tkalych O, Gupta A, Hansen HS, Hansen SH, Devi LA, Hurd YL. Dynamic changes of the endogenous cannabinoid and opioid mesocorticolimbic systems during adolescence: THC effects. Eur Neuropsychopharmacol. 2008;18:826–834. doi: 10.1016/j.euroneuro.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellgren M, Hurd YL, Franck J. Amphetamine effects on dopamine levels and behavior following cannabinoid exposure during adolescence. Eur J Pharmacol. 2004;497:205–213. doi: 10.1016/j.ejphar.2004.06.048. [DOI] [PubMed] [Google Scholar]
- Ellgren M, Spano SM, Hurd YL. Adolescent cannabis exposure alters opiate intake and opioid limbic neuronal populations in adult rats. Neuropsychopharmacology. 2007;32:607–615. doi: 10.1038/sj.npp.1301127. [DOI] [PubMed] [Google Scholar]
- Ferdinand RF, Sondeijker F, van der Ende J, Selten JP, Huizink A, Verhulst FC. Cannabis use predicts future psychotic symptoms, and vice versa. Addiction. 2005;100:612–618. doi: 10.1111/j.1360-0443.2005.01070.x. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Boden JM. Cannabis use and later life outcomes. Addiction. 2008;103:969–976. doi: 10.1111/j.1360-0443.2008.02221.x. discussion 977-968. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Boden JM, Horwood LJ. Cannabis use and other illicit drug use: testing the cannabis gateway hypothesis. Addiction. 2006;101:556–569. doi: 10.1111/j.1360-0443.2005.01322.x. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Horwood LJ. Does cannabis use encourage other forms of illicit drug use? Addiction. 2000;95:505–520. doi: 10.1046/j.1360-0443.2000.9545053.x. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Horwood LJ, Swain-Campbell N. Cannabis use and psychosocial adjustment in adolescence and young adulthood. Addiction. 2002;97:1123–1135. doi: 10.1046/j.1360-0443.2002.00103.x. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Hutchison K. Genetic contributions to avoidance-based decisions: striatal D2 receptor polymorphisms. Neuroscience. 2009;164:131–140. doi: 10.1016/j.neuroscience.2009.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MJ, Moustafa AA, Haughey HM, Curran T, Hutchison KE. Genetic triple dissociation reveals multiple roles for dopamine in reinforcement learning. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:16311–16316. doi: 10.1073/pnas.0706111104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerard CM, Mollereau C, Vassart G, Parmentier M. Molecular cloning of a human cannabinoid receptor which is also expressed in testis. Biochem J. 1991;279(Pt 1):129–134. doi: 10.1042/bj2790129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, Enber TM, Susel Z, Chase TN, Monsma FJ, Mahan LC, Sibley DR. D1 and D2 dopamine receptor regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Young WS., III Distributution of striatonigral and striatopallidal peptidergic neurons in both patch and matrix compartments: an in situ hybridization histochemistry and fluorescent retrograde tracing study. Brain Res. 1988;460:161–167. doi: 10.1016/0006-8993(88)91217-6. [DOI] [PubMed] [Google Scholar]
- Glass M, Dragnunow M, Faull RL. Cannabinoid receptors in the human brain: a detailed anatomical quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neurosci. 1997;77:299–318. doi: 10.1016/s0306-4522(96)00428-9. [DOI] [PubMed] [Google Scholar]
- Gong JP, Onaivi ES, Ishiguro H, Liu QR, Tagliaferro PA, Brusco A, Uhl GR. Cannabinoid CB2 receptors: immunohistochemical localization in rat brain. Brain Res. 2006;1071:10–23. doi: 10.1016/j.brainres.2005.11.035. [DOI] [PubMed] [Google Scholar]
- Griffin G, Tao Q, Abood ME. Cloning and pharmacological characterization of the rat CB(2) cannabinoid receptor. J Pharmacol Exp Ther. 2000;292:886–894. [PubMed] [Google Scholar]
- Hall WD, Lynskey M. Is cannabis a gateway drug? Testing hypotheses about the relationship between cannabis use and the use of other illicit drugs. Drug Alcohol Rev. 2005;24:39–48. doi: 10.1080/09595230500126698. [DOI] [PubMed] [Google Scholar]
- Harkany T, Keimpema E, Barabas K, Mulder J. Endocannabinoid functions controlling neuronal specification during brain development. Mol Cell Endocrinol. 2008;286:S84–90. doi: 10.1016/j.mce.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Hayatbakhsh MR, Najman JM, Jamrozik K, Mamun AA, Alati R, Bor W. Cannabis and anxiety and depression in young adults: a large prospective study. J Am Acad Child Adolesc Psychiatry. 2007;46:408–417. doi: 10.1097/chi.0b013e31802dc54d. [DOI] [PubMed] [Google Scholar]
- Heinz A, Siessmeier T, Wrase J, Hermann D, Klein S, Grusser SM, Flor H, Braus DF, Buchholz HG, Grunder G, Schreckenberger M, Smolka MN, Rosch F, Mann K, Bartenstein P. Correlation between dopamine D(2) receptors in the ventral striatum and central processing of alcohol cues and craving. Am J Psychiatry. 2004;161:1783–1789. doi: 10.1176/appi.ajp.161.10.1783. [DOI] [PubMed] [Google Scholar]
- Heng L, Beverley JA, Steiner H, Tseng KY. Differential developmental trajectories for CB1 cannabinoid receptor expression in limbic/associative and sensorimotor cortical areas. Synapse. 2011;65:278–286. doi: 10.1002/syn.20844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M, Lynn A, Little M, Johnson M, Melvin L, De Costa B, Rice K. Cannabinoid receptor localization in brain. Proc. Natl. Acad. Sci. USA. 1990;87:1932. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, de Costa BR, Richfield EK. Neuronal localization of cannabinoid receptors in the basal ganglia of the rat. Brain Res. 1991a;547:267–274. doi: 10.1016/0006-8993(91)90970-7. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1991b;11:563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermanson DJ, Marnett LJ. Cannabinoids, endocannabinoids, and cancer. Cancer Metastasis Rev. 2011;30:599–612. doi: 10.1007/s10555-011-9318-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuera-Matas A, Soto-Montenegro ML, del Olmo N, Miguens M, Torres I, Vaquero JJ, Sanchez J, Garcia-Lecumberri C, Desco M, Ambrosio E. Augmented acquisition of cocaine self-administration and altered brain glucose metabolism in adult female but not male rats exposed to a cannabinoid agonist during adolescence. Neuropsychopharmacology. 2008;33:806–813. doi: 10.1038/sj.npp.1301467. [DOI] [PubMed] [Google Scholar]
- Hikida T, Kimura K, Wada N, Funabiki K, Nakanishi S. Distinct roles of synaptic transmission in direct and indirect striatal pathways to reward and aversive behavior. Neuron. 2010;66:896–907. doi: 10.1016/j.neuron.2010.05.011. [DOI] [PubMed] [Google Scholar]
- Hill AJ, Williams CM, Whalley BJ, Stephens GJ. Phytocannabinoids as novel therapeutic agents in CNS disorders. Pharmacol Ther. 2012;133:79–97. doi: 10.1016/j.pharmthera.2011.09.002. [DOI] [PubMed] [Google Scholar]
- Hill EL, Gallopin T, Ferezou I, Cauli B, Rossier J, Schweitzer P, Lambolez B. Functional CB1 receptors are broadly expressed in neocortical GABAergic and glutamatergic neurons. J Neurophysiol. 2007;97:2580–2589. doi: 10.1152/jn.00603.2006. [DOI] [PubMed] [Google Scholar]
- Hopfer CJ, Young SE, Purcell S, Crowley TJ, Stallings MC, Corley RP, Rhee SH, Smolen A, Krauter K, Hewitt JK, Ehringer MA. Cannabis receptor haplotype associated with fewer cannabis dependence symptoms in adolescents. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:895–901. doi: 10.1002/ajmg.b.30378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, Felder CC, Herkenham M, Mackie K, Martin BR, Mechoulam R, Pertwee RG. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- Hyman SM, Sinha R. Stress-related factors in cannabis use and misuse: implications for prevention and treatment. J Subst Abuse Treat. 2009;36:400–413. doi: 10.1016/j.jsat.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national results on adolescent drug use: Overview of key findings, 2011. Institute for Social Research, The University of Michigan; Ann Arbor: 2012. [Google Scholar]
- Jutras-Aswad D, Jacobs MM, Yiannoulos G, Roussos P, Bitsios P, Nomura Y, Liu X, Hurd YL. Cannabis-dependence risk relates to synergism between neuroticism and proenkephalin SNPs associated with amygdala gene expression: case-control study. PloS one. 2012;7:e39243. doi: 10.1371/journal.pone.0039243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel D. Stages in adolescent involvement in drug use. Science. 1975;190:912–914. doi: 10.1126/science.1188374. [DOI] [PubMed] [Google Scholar]
- Kandel DB, Yamaguchi K, Klein LC. Testing the Gateway Hypothesis. Addiction. 2006;101:470–472. doi: 10.1111/j.1360-0443.2006.01426.x. discussion 474-476. [DOI] [PubMed] [Google Scholar]
- Kang W, Wilson SP, Wilson MA. Overexpression of proenkephalin in the amygdala potentiates the anxiolytic effects of benzodiazepines. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2000;22:77–88. doi: 10.1016/S0893-133X(99)00090-1. [DOI] [PubMed] [Google Scholar]
- Katona I, Freund TF. Endocannabinoid signaling as a synaptic circuit breaker in neurological disease. Nat Med. 2008;14:923–930. doi: 10.1038/nm.f.1869. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Bakshi VP, Haber SN, Steininger TL, Will MJ, Zhang M. Opioid modulation of taste hedonics within the ventral striatum. Physiol Behav. 2002;76:365–377. doi: 10.1016/s0031-9384(02)00751-5. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Neale MC, Prescott CA. Illicit psychoactive substance use, heavy use, abuse, and dependence in a US population-based sample of male twins. Arch Gen Psychiatry. 2000;57:261–269. doi: 10.1001/archpsyc.57.3.261. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA. Cannabis use, abuse, and dependence in a population-based sample of female twins. Am J Psychiatry. 1998;155:1016–1022. doi: 10.1176/ajp.155.8.1016. [DOI] [PubMed] [Google Scholar]
- Kilmer JR, Hunt SB, Lee CM, Neighbors C. Marijuana use, risk perception, and consequences: is perceived risk congruent with reality? Addict Behav. 2007;32:3026–3033. doi: 10.1016/j.addbeh.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Klein TA, Neumann J, Reuter M, Hennig J, von Cramon DY, Ullsperger M. Genetically determined differences in learning from errors. Science. 2007;318:1642–1645. doi: 10.1126/science.1145044. [DOI] [PubMed] [Google Scholar]
- Koskinen J, Lohonen J, Koponen H, Isohanni M, Miettunen J. Rate of cannabis use disorders in clinical samples of patients with schizophrenia: a meta-analysis. Schizophr Bull. 2010;36:1115–1130. doi: 10.1093/schbul/sbp031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung JC, Chen TC, Shyu BC, Hsiao S, Huang AC. Anxiety- and depressive-like responses and c-fos activity in preproenkephalin knockout mice: oversensitivity hypothesis of enkephalin deficit-induced posttraumatic stress disorder. J Biomed Sci. 2010;17:29. doi: 10.1186/1423-0127-17-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanciego JL, Barroso-Chinea P, Rico AJ, Conte-Perales L, Callen L, Roda E, Gomez-Bautista V, Lopez IP, Lluis C, Labandeira-Garcia JL, Franco R. Expression of the mRNA coding the cannabinoid receptor 2 in the pallidal complex of Macaca fascicularis. J Psychopharmacol. 2011;25:97–104. doi: 10.1177/0269881110367732. [DOI] [PubMed] [Google Scholar]
- Le Moine C, Normand E, Guitteny AF, Fouque B, Teoule R, Bloch B. Dopamine receptor gene expression by enkephalin neurons in rat forebrain. Proc. Natl. Acad. Sci. USA. 1990;87:230–234. doi: 10.1073/pnas.87.1.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessem JM, Hopfer CJ, Haberstick BC, Timberlake D, Ehringer MA, Smolen A, Hewitt JK. Relationship between adolescent marijuana use and young adult illicit drug use. Behav Genet. 2006;36:498–506. doi: 10.1007/s10519-006-9064-9. [DOI] [PubMed] [Google Scholar]
- Lopez-Quintero C, Neumark Y. Effects of risk perception of marijuana use on marijuana use and intentions to use among adolescents in Bogota, Colombia. Drug Alcohol Depend. 2010;109:65–72. doi: 10.1016/j.drugalcdep.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Lynskey MT, Coffey C, Degenhardt L, Carlin JB, Patton G. A longitudinal study of the effects of adolescent cannabis use on high school completion. Addiction. 2003;98:685–692. doi: 10.1046/j.1360-0443.2003.00356.x. [DOI] [PubMed] [Google Scholar]
- Maccoun RJ. Competing accounts of the gateway effect: the field thins, but still no clear winner. Addiction. 2006;101:473–474. doi: 10.1111/j.1360-0443.2006.01428.x. discussion 474-476. [DOI] [PubMed] [Google Scholar]
- Maes HH, Woodard CE, Murrelle L, Meyer JM, Silberg JL, Hewitt JK, Rutter M, Simonoff E, Pickles A, Carbonneau R, Neale MC, Eaves LJ. Tobacco, alcohol and drug use in eight- to sixteen-year-old twins: the Virginia Twin Study of Adolescent Behavioral Development. J Stud Alcohol. 1999;60:293–305. doi: 10.15288/jsa.1999.60.293. [DOI] [PubMed] [Google Scholar]
- Mailleux P, Parmentier M, Vanderhaeghen JJ. Distribution of cannabinoid receptor messenger RNA in the human brain: an in situ hybridization histochemistry with oligonucleotides. Neurosci Lett. 1992;143:200–204. doi: 10.1016/0304-3940(92)90265-9. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Lutz B. Expression of the cannabinoid receptor CB1 in distinct neuronal subpopulations in the adult mouse forebrain. The European journal of neuroscience. 1999;11:4213–4225. doi: 10.1046/j.1460-9568.1999.00847.x. [DOI] [PubMed] [Google Scholar]
- Mato S, Del Olmo E, Pazos A. Ontogenetic development of cannabinoid receptor expression and signal transduction functionality in the human brain. The European journal of neuroscience. 2003;17:1747–1754. doi: 10.1046/j.1460-9568.2003.02599.x. [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Bonner TI, Lolait SJ. Localization of cannabinoid receptor mRNA in rat brain. J Comp Neurol. 1993;327:535–550. doi: 10.1002/cne.903270406. [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- McGue M, Elkins I, Iacono WG. Genetic and environmental influences on adolescent substance use and abuse. Am J Med Genet. 2000;96:671–677. doi: 10.1002/1096-8628(20001009)96:5<671::aid-ajmg14>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- McLaughlin CR, Martin BR, Compton DR, Abood ME. Cannabinoid receptors in developing rats: detection of mRNA and receptor binding. Drug and Alcohol Dependence. 1994;36:27–31. doi: 10.1016/0376-8716(94)90006-x. [DOI] [PubMed] [Google Scholar]
- Michaelides M, Thanos PK, Kim R, Cho J, Ananth M, Wang GJ, Volkow ND. PET imaging predicts future body weight and cocaine preference. Neuroimage. 2012;59:1508–1513. doi: 10.1016/j.neuroimage.2011.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles DR, van den Bree MB, Gupman AE, Newlin DB, Glantz MD, Pickens RW. A twin study on sensation seeking, risk taking behavior and marijuana use. Drug Alcohol Depend. 2001;62:57–68. doi: 10.1016/s0376-8716(00)00165-4. [DOI] [PubMed] [Google Scholar]
- Morel LJ, Giros B, Dauge V. Adolescent exposure to chronic delta-9-tetrahydrocannabinol blocks opiate dependence in maternally deprived rats. Neuropsychopharmacology. 2009;34:2469–2476. doi: 10.1038/npp.2009.70. [DOI] [PubMed] [Google Scholar]
- Morgan D, Grant KA, Gage HD, Mach RH, Kaplan JR, Prioleau O, Nader SH, Buchheimer N, Ehrenkaufer RL, Nader MA. Social dominance in monkeys: dopamine D2 receptors and cocaine self-administration. Nat Neurosci. 2002;5:169–174. doi: 10.1038/nn798. [DOI] [PubMed] [Google Scholar]
- Mulder J, Aguado T, Keimpema E, Barabas K, Ballester Rosado CJ, Nguyen L, Monory K, Marsicano G, Di Marzo V, Hurd YL, Guillemot F, Mackie K, Lutz B, Guzman M, Lu HC, Galve-Roperh I, Harkany T. Endocannabinoid signaling controls pyramidal cell specification and long-range axon patterning. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:8760–8765. doi: 10.1073/pnas.0803545105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Nader MA, Morgan D, Gage HD, Nader SH, Calhoun TL, Buchheimer N, Ehrenkaufer R, Mach RH. PET imaging of dopamine D2 receptors during chronic cocaine self-administration in monkeys. Nat Neurosci. 2006 doi: 10.1038/nn1737. [DOI] [PubMed] [Google Scholar]
- Onaivi ES, Ishiguro H, Gong JP, Patel S, Perchuk A, Meozzi PA, Myers L, Mora Z, Tagliaferro P, Gardner E, Brusco A, Akinshola BE, Liu QR, Hope B, Iwasaki S, Arinami T, Teasenfitz L, Uhl GR. Discovery of the presence and functional expression of cannabinoid CB2 receptors in brain. Annals of the New York Academy of Sciences. 2006;1074:514–536. doi: 10.1196/annals.1369.052. [DOI] [PubMed] [Google Scholar]
- Panlilio LV, Solinas M, Matthews SA, Goldberg SR. Previous exposure to THC alters the reinforcing efficacy and anxiety-related effects of cocaine in rats. Neuropsychopharmacology. 2007;32:646–657. doi: 10.1038/sj.npp.1301109. [DOI] [PubMed] [Google Scholar]
- Patton GC, Coffey C, Carlin JB, Degenhardt L, Lynskey M, Hall W. Cannabis use and mental health in young people: cohort study. Bmj. 2002;325:1195–1198. doi: 10.1136/bmj.325.7374.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Rosado A, Manzanares J, Fernandez-Ruiz J, Ramos JA. Prenatal Delta(9)-tetrahydrocannabinol exposure modifies proenkephalin gene expression in the fetal rat brain: sex-dependent differences. Brain Res Dev Brain Res. 2000;120:77–81. doi: 10.1016/s0165-3806(99)00170-4. [DOI] [PubMed] [Google Scholar]
- Pettit DA, Harrison MP, Olson JM, Spencer RF, Cabral GA. Immunohistochemical localization of the neural cannabinoid receptor in rat brain. J Neurosci Res. 1998;51:391–402. doi: 10.1002/(SICI)1097-4547(19980201)51:3<391::AID-JNR12>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Pistis M, Perra S, Pillolla G, Melis M, Muntoni AL, Gessa GL. Adolescent exposure to cannabinoids induces long-lasting changes in the response to drugs of abuse of rat midbrain dopamine neurons. Biological psychiatry. 2004;56:86–94. doi: 10.1016/j.biopsych.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Rhee SH, Hewitt JK, Young SE, Corley RP, Crowley TJ, Stallings MC. Genetic and environmental influences on substance initiation, use, and problem use in adolescents. Arch Gen Psychiatry. 2003;60:1256–1264. doi: 10.1001/archpsyc.60.12.1256. [DOI] [PubMed] [Google Scholar]
- Rodriguez de Fonseca F, Ramos JA, Bonnin A, Fernández-Ruiz JJ. Presence of cannabinoid binding sites in the brain from early postnatal ages. Neuroreport. 1993;4:135–138. doi: 10.1097/00001756-199302000-00005. [DOI] [PubMed] [Google Scholar]
- Sakai JT, Hopfer CJ, Hartman C, Haberstick BC, Smolen A, Corley RP, Stallings MC, Young SE, Timberlake D, Hewitt JK, Crowley TJ. Test of association between TaqIA A1 allele and alcohol use disorder phenotypes in a sample of adolescent patients with serious substance and behavioral problems. Drug Alcohol Depend. 2007;88:130–137. doi: 10.1016/j.drugalcdep.2006.10.002. [DOI] [PubMed] [Google Scholar]
- SAMHSA . Results from the 2010 National Survey on Drug Use and Health: National Findings. Office of Applied Studies; Rockville, MD: 2011. NSDUH Series H-41, HHS Publication No. (SMA) 11-4658. [Google Scholar]
- Sano H, Yasoshima Y, Matsushita N, Kaneko T, Kohno K, Pastan I, Kobayashi K. Conditional ablation of striatal neuronal types containing dopamine D2 receptor disturbs coordination of basal ganglia function. J Neurosci. 2003;23:9078–9088. doi: 10.1523/JNEUROSCI.23-27-09078.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh ME, McGregor IS, Mallet PE. Perinatal exposure to delta(9)-tetrahydrocannabinol alters heroin-induced place conditioning and fosimmunoreactivity. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2006;31:58–69. doi: 10.1038/sj.npp.1300770. [DOI] [PubMed] [Google Scholar]
- Skoubis PD, Lam HA, Shoblock J, Narayanan S, Maidment NT. Endogenous enkephalins, not endorphins, modulate basal hedonic state in mice. The European journal of neuroscience. 2005;21:1379–1384. doi: 10.1111/j.1460-9568.2005.03956.x. [DOI] [PubMed] [Google Scholar]
- Spano MS, Ellgren M, Wang X, Hurd YL. Prenatal cannabis exposure increases heroin seeking with allostatic changes in limbic enkephalin systems in adulthood. Biological psychiatry. 2007;61:554–563. doi: 10.1016/j.biopsych.2006.03.073. [DOI] [PubMed] [Google Scholar]
- Spano MS, Fadda P, Fratta W, Fattore L. Cannabinoid-opioid interactions in drug discrimination and self-administration: effect of maternal, postnatal, adolescent and adult exposure to the drugs. Curr Drug Targets. 2010;11:450–461. doi: 10.2174/138945010790980295. [DOI] [PubMed] [Google Scholar]
- Tarter RE, Vanyukov M, Kirisci L, Reynolds M, Clark DB. Predictors of marijuana use in adolescents before and after licit drug use: examination of the gateway hypothesis. Am J Psychiatry. 2006;163:2134–2140. doi: 10.1176/ajp.2006.163.12.2134. [DOI] [PubMed] [Google Scholar]
- Thanos PK, Michaelides M, Benveniste H, Wang GJ, Volkow ND. The effects of cocaine on regional brain glucose metabolism is attenuated in dopamine transporter knockout mice. Synapse. 2008;62:319–324. doi: 10.1002/syn.20503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasiewicz HC, Jacobs MM, Wilkinson MB, Wilson SP, Nestler EJ, Hurd YL. Proenkephalin mediates the enduring effects of adolescent cannabis exposure associated with adult opiate vulnerability. Biological psychiatry. 2012;72:803–810. doi: 10.1016/j.biopsych.2012.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuang MT, Lyons MJ, Meyer JM, Doyle T, Eisen SA, Goldberg J, True W, Lin N, Toomey R, Eaves L. Co-occurrence of abuse of different drugs in men: the role of drug-specific and shared vulnerabilities. Arch Gen Psychiatry. 1998;55:967–972. doi: 10.1001/archpsyc.55.11.967. [DOI] [PubMed] [Google Scholar]
- Tucker JS, Ellickson PL, Collins RL, Klein DJ. Are drug experimenters better adjusted than abstainers and users?: a longitudinal study of adolescent marijuana use. J Adolesc Health. 2006;39:488–494. doi: 10.1016/j.jadohealth.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Tyndale RF, Payne JI, Gerber AL, Sipe JC. The fatty acid amide hydrolase C385A (P129T) missense variant in cannabis users: studies of drug use and dependence in Caucasians. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:660–666. doi: 10.1002/ajmg.b.30491. [DOI] [PubMed] [Google Scholar]
- Valverde O, Noble F, Beslot F, Dauge V, Fournie-Zaluski MC, Roques BP. Delta9-tetrahydrocannabinol releases and facilitates the effects of endogenous enkephalins: reduction in morphine withdrawal syndrome without change in rewarding effect. Eur J Neurosci. 2001;13:1816–1824. doi: 10.1046/j.0953-816x.2001.01558.x. [DOI] [PubMed] [Google Scholar]
- van den Bree MB, Johnson EO, Neale MC, Pickens RW. Genetic and environmental influences on drug use and abuse/dependence in male and female twins. Drug Alcohol Depend. 1998;52:231–241. doi: 10.1016/s0376-8716(98)00101-x. [DOI] [PubMed] [Google Scholar]
- van Gastel WA, Maccabe JH, Schubart CD, Vreeker A, Tempelaar W, Kahn RS, Boks MP. Cigarette smoking and cannabis use are equally strongly associated with psychotic-like experiences: a cross-sectional study in 1929 young adults. Psychol Med. 2013:1–9. doi: 10.1017/S0033291713000202. [DOI] [PubMed] [Google Scholar]
- Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, Stella N, Makriyannis A, Piomelli D, Davison JS, Marnett LJ, Di Marzo V, Pittman QJ, Patel KD, Sharkey KA. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science. 2005;310:329–332. doi: 10.1126/science.1115740. [DOI] [PubMed] [Google Scholar]
- Van Waes V, Beverley JA, Siman H, Tseng KY, Steiner H. CB1 Cannabinoid Receptor Expression in the Striatum: Association with Corticostriatal Circuits and Developmental Regulation. Front Pharmacol. 2012;3:21. doi: 10.3389/fphar.2012.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdurand M, Nguyen V, Stark D, Zahra D, Gregoire MC, Greguric I, Zavitsanou K. Comparison of Cannabinoid CB(1) Receptor Binding in Adolescent and Adult Rats: A Positron Emission Tomography Study Using [F]MK-9470. Int J Mol Imaging. 2011;2011:548123. doi: 10.1155/2011/548123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigano D, Rubino T, Parolaro D. Molecular and cellular basis of cannabinoid and opioid interactions. Pharmacology, biochemistry, and behavior. 2005;81:360–368. doi: 10.1016/j.pbb.2005.01.021. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M, Logan J, Franceschi D, Gatley J, Hitzemann R, Gifford A, Wong C, Pappas N. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry. 2001;158:2015–2021. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Swanson JM. Dopamine in drug abuse and addiction: results from imaging studies and treatment implications. Molecular psychiatry. 2004;9:557–569. doi: 10.1038/sj.mp.4001507. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Gifford A, Hitzemann R, Ding YS, Pappas N. Prediction of reinforcing responses to psychostimulants in humans by brain dopamine D2 receptor levels. Am J Psychiatry. 1999;156:1440–1443. doi: 10.1176/ajp.156.9.1440. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Fowler JS, Logan J, Abumrad NN, Hitzemann RJ, Pappas NS, Pascani K. Dopamine D2 receptor availability in opiate-dependent subjects before and after naloxone-precipitated withdrawal. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 1997;16:174–182. doi: 10.1016/S0893-133X(96)00184-4. [DOI] [PubMed] [Google Scholar]
- Wang X, Dow-Edwards D, Andersen V, Minkoff H, Hurd YL. Discrete opioid gene expression impairment in the human fetal brain associated with maternal marijuana use. Pharmacogenomics J. 2006;6 doi: 10.1038/sj.tpj.6500375. [DOI] [PubMed] [Google Scholar]
- Wang X, Dow-Edwards D, Keller E, Hurd YL. Preferential limbic expression of the cannabinoid receptor mRNA in the human fetal brain. Neuroscience. 2003;118:681–694. doi: 10.1016/s0306-4522(03)00020-4. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K, Kandel DB. Patterns of drug use from adolescence to young adulthood: III. Predictors of progression. Am J Public Health. 1984;74:673–681. doi: 10.2105/ajph.74.7.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvolensky MJ, Vujanovic AA, Bernstein A, Bonn-Miller MO, Marshall EC, Leyro TM. Marijuana use motives: A confirmatory test and evaluation among young adult marijuana users. Addict Behav. 2007;32:3122–3130. doi: 10.1016/j.addbeh.2007.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]