Abstract
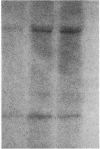
In addition to the contractile proteins actin and myosin, contractile filaments of striated muscle contain other proteins that are important for regulating the structure and the interaction of the two force-generating proteins. In the thin filaments, troponin and tropomyosin form a Ca-sensitive trigger that activates normal contraction when intracellular Ca is elevated. In the thick filament, there are several myosin-binding proteins whose functions are unclear. Among these is the myosin-binding protein C (MBP-C). The cardiac isoform contains four phosphorylation sites under the control of cAMP and calmodulin-regulated kinases, whereas the skeletal isoform contains only one such site, suggesting that phosphorylation in cardiac muscle has a specific regulatory function. We isolated natural thick filaments from cardiac muscle and, using electron microscopy and optical diffraction, determined the effect of phosphorylation of MBP-C on cross bridges. The thickness of the filaments that had been treated with protein kinase A was increased where cross bridges were present. No change occurred in the central bare zone that is devoid of cross bridges. The intensity of the reflections along the 43-nm layer line, which is primarily due to the helical array of cross bridges, was increased, and the distance of the first peak reflection from the meridian along the 43-nm layer line was decreased. The results indicate that phosphorylation of MBP-C (i) extends the cross bridges from the backbone of the filament and (ii) increases their degree of order and/or alters their orientation. These changes could alter rate constants for attachment to and detachment from the thin filament and thereby modify force production in activated cardiac muscle.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gautel M., Zuffardi O., Freiburg A., Labeit S. Phosphorylation switches specific for the cardiac isoform of myosin binding protein-C: a modulator of cardiac contraction? EMBO J. 1995 May 1;14(9):1952–1960. doi: 10.1002/j.1460-2075.1995.tb07187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzell H. C. Effects of phosphorylated and unphosphorylated C-protein on cardiac actomyosin ATPase. J Mol Biol. 1985 Nov 5;186(1):185–195. doi: 10.1016/0022-2836(85)90268-2. [DOI] [PubMed] [Google Scholar]
- Hartzell H. C., Glass D. B. Phosphorylation of purified cardiac muscle C-protein by purified cAMP-dependent and endogenous Ca2+-calmodulin-dependent protein kinases. J Biol Chem. 1984 Dec 25;259(24):15587–15596. [PubMed] [Google Scholar]
- Hofmann P. A., Lange J. H., 3rd Effects of phosphorylation of troponin I and C protein on isometric tension and velocity of unloaded shortening in skinned single cardiac myocytes from rats. Circ Res. 1994 Apr;74(4):718–726. doi: 10.1161/01.res.74.4.718. [DOI] [PubMed] [Google Scholar]
- Holubarsch C. H., Hasenfuss G., Just H., Blanchard E. M., Mulieri L. A., Alpert N. R. Modulation of myothermal economy of isometric force generation by positive inotropic interventions in the guinea pig myocardium. Cardioscience. 1990 Mar;1(1):33–41. [PubMed] [Google Scholar]
- Huxley H. E., Brown W. The low-angle x-ray diagram of vertebrate striated muscle and its behaviour during contraction and rigor. J Mol Biol. 1967 Dec 14;30(2):383–434. doi: 10.1016/s0022-2836(67)80046-9. [DOI] [PubMed] [Google Scholar]
- Irving M., St Claire Allen T., Sabido-David C., Craik J. S., Brandmeier B., Kendrick-Jones J., Corrie J. E., Trentham D. R., Goldman Y. E. Tilting of the light-chain region of myosin during step length changes and active force generation in skeletal muscle. Nature. 1995 Jun 22;375(6533):688–691. doi: 10.1038/375688a0. [DOI] [PubMed] [Google Scholar]
- Ishikawa T., Yanagisawa M., Kimura S., Goto K., Masaki T. Positive inotropic action of novel vasoconstrictor peptide endothelin on guinea pig atria. Am J Physiol. 1988 Oct;255(4 Pt 2):H970–H973. doi: 10.1152/ajpheart.1988.255.4.H970. [DOI] [PubMed] [Google Scholar]
- Jones L. G., Rozich J. D., Tsutsui H., Cooper G., 4th Endothelin stimulates multiple responses in isolated adult ventricular cardiac myocytes. Am J Physiol. 1992 Nov;263(5 Pt 2):H1447–H1454. doi: 10.1152/ajpheart.1992.263.5.H1447. [DOI] [PubMed] [Google Scholar]
- Kensler R. W., Levine R. J. An electron microscopic and optical diffraction analysis of the structure of Limulus telson muscle thick filaments. J Cell Biol. 1982 Feb;92(2):443–451. doi: 10.1083/jcb.92.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensler R. W., Stewart M. Frog skeletal muscle thick filaments are three-stranded. J Cell Biol. 1983 Jun;96(6):1797–1802. doi: 10.1083/jcb.96.6.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim M. S., Walsh M. P. Phosphorylation of skeletal and cardiac muscle C-proteins by the catalytic subunit of cAMP-dependent protein kinase. Biochem Cell Biol. 1986 Jul;64(7):622–630. doi: 10.1139/o86-086. [DOI] [PubMed] [Google Scholar]
- Matsubara I., Millman B. M. X-ray diffraction patterns from mammalian heart muscle. J Mol Biol. 1974 Feb 5;82(4):527–536. doi: 10.1016/0022-2836(74)90246-0. [DOI] [PubMed] [Google Scholar]
- Matsubara I. X-ray diffraction studies of the heart. Annu Rev Biophys Bioeng. 1980;9:81–105. doi: 10.1146/annurev.bb.09.060180.000501. [DOI] [PubMed] [Google Scholar]
- McClellan G., Weisberg A., Winegrad S. Effect of endothelin-1 on actomyosin ATPase activity. Implications for the efficiency of contraction. Circ Res. 1996 Jun;78(6):1044–1050. doi: 10.1161/01.res.78.6.1044. [DOI] [PubMed] [Google Scholar]
- McClellan G., Weisberg A., Winegrad S. cAMP can raise or lower cardiac actomyosin ATPase activity depending on alpha-adrenergic activity. Am J Physiol. 1994 Aug;267(2 Pt 2):H431–H442. doi: 10.1152/ajpheart.1994.267.2.H431. [DOI] [PubMed] [Google Scholar]
- Okagaki T., Weber F. E., Fischman D. A., Vaughan K. T., Mikawa T., Reinach F. C. The major myosin-binding domain of skeletal muscle MyBP-C (C protein) resides in the COOH-terminal, immunoglobulin C2 motif. J Cell Biol. 1993 Nov;123(3):619–626. doi: 10.1083/jcb.123.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayment I., Rypniewski W. R., Schmidt-Bäse K., Smith R., Tomchick D. R., Benning M. M., Winkelmann D. A., Wesenberg G., Holden H. M. Three-dimensional structure of myosin subfragment-1: a molecular motor. Science. 1993 Jul 2;261(5117):50–58. doi: 10.1126/science.8316857. [DOI] [PubMed] [Google Scholar]
- Strang K. T., Sweitzer N. K., Greaser M. L., Moss R. L. Beta-adrenergic receptor stimulation increases unloaded shortening velocity of skinned single ventricular myocytes from rats. Circ Res. 1994 Mar;74(3):542–549. doi: 10.1161/01.res.74.3.542. [DOI] [PubMed] [Google Scholar]
- Venema R. C., Kuo J. F. Protein kinase C-mediated phosphorylation of troponin I and C-protein in isolated myocardial cells is associated with inhibition of myofibrillar actomyosin MgATPase. J Biol Chem. 1993 Feb 5;268(4):2705–2711. [PubMed] [Google Scholar]
- Winegrad S., Weisberg A., Lin L. E., McClellan G. Adrenergic regulation of myosin adenosine triphosphatase activity. Circ Res. 1986 Jan;58(1):83–95. doi: 10.1161/01.res.58.1.83. [DOI] [PubMed] [Google Scholar]
- de Tombe P. P., Stienen G. J. Protein kinase A does not alter economy of force maintenance in skinned rat cardiac trabeculae. Circ Res. 1995 May;76(5):734–741. doi: 10.1161/01.res.76.5.734. [DOI] [PubMed] [Google Scholar]