Abstract
Cognitive impairment is prevalent in heart failure (HF), though substantial variability in the pattern of cognitive impairment is found across studies. To clarify the nature of cognitive impairment in HF, we examined longitudinal trajectories across multiple domains of cognition in HF patients using latent growth class modeling. 115 HF patients completed a neuropsychological battery at baseline, 3-months and 12-months. Participants also completed the Beck Depression Inventory-II (BDI-II). Latent class growth analyses revealed a three-class model for attention/executive function, four-class model for memory, and a three-class model for language. The slope for attention/executive function and language remained stable, while improvements were noted in memory performance. Education and BDI-II significantly predicted the intercept for attention/executive function and language abilities. The BDI-II also predicted baseline memory. The current findings suggest that multiple performance-based classes of neuropsychological test performance exist within cognitive domains, though case-controlled prospective studies with extended follow-ups are needed to fully elucidate changes and predictors of cognitive function in HF.
Keywords: Heart failure, trajectories, cognitive function, longitudinal, cognitive profile
Introduction
Heart failure (HF) affects an estimated 6 million Americans and nearly 600,000 new cases are diagnosed each year (Heidenreich et al., 2011; Roger et al., 2012). HF is associated with many poor outcomes, including elevated mortality (Roger et al., 2004), recurrent hospitalizations (Rosamond et al., 2008; Jencks, Williams, & Coleman, 2009), decreased quality of life (Bennett et al., 2003), and loss of functional independence (Alosco et al., 2012). Persons with HF are also at risk for adverse neurological changes, including Alzheimer’s disease, vascular dementia, and abnormal findings on neuroimaging (Qiu et al., 2006; Roman, 2005; Vogels et al., 2007). Milder forms of cognitive impairment affect up to 75% of HF patients (Sauve, Lewis, Blankenbiller, Rickabaugh, & Pressler, 2009; Vogels, Scheltens, Schroeder-Tanka, & Weinstein, 2007).
Despite these findings, the specific pattern of cognitive deficits varies across studies (Hoth, Poppas, Moser, Paul, & Cohen, 2008; Festa et al., 2011; Beer et al., 2009; Jerskey et al., 2009). Indeed, relative to controls HF patients frequently exhibit reductions in executive function, memory, and psychomotor speed (Pressler et al., 2010). However, such deficits are inconsistently observed throughout the literature in this population. For instance, some studies have shown HF patients to exhibit impaired memory, while others have failed to find such a relationship (Grubb, Simpson, & Fox, 2000; Vogels et al., 2007). The few prospective studies examining neurocognitive outcomes in HF are also conflicting. Some provide evidence for progressive decline in global cognition, attention/executive function, and memory while others indicate stability or even improvements in domains of attention and executive function over time (Stanek et al., 2009; Hjelm et al., 2012; van den hurk et al., 2011; Almeida et al., 2012).
In light of these findings, it is possible that longitudinal patterns of change in cognitive test scores in HF patients differ across domains and vary over time as a function of other common medical conditions that accompany HF. For example, a number of factors have been shown to contribute to variability of neuropsychological test performance in HF patients. The most prominent among them are age, sex, HF severity (i.e., left ventricular ejection fraction; LVEF), depression, and medical comorbidity (i.e., diabetes, hypertension) (Miller et al., 2012; Zuccala et al., 2005; Alosco et al., 2012; Garcia et al., 2012; Jefferson et al., 2011; Festa et al., 2011).
The current study investigated the longitudinal trajectories of multiple cognitive domains in older adults with HF using latent growth class modeling. This method allows not only to gauge the true mean change over time but also to evaluate variance (i.e. individual differences) in change. Follow-up analyses identified factors that predicted change, including demographic factors (i.e., sex, education), cardiac function (i.e., left ventricular ejection), and depression.
Methods
Participants
The current sample consisted of 115 consecutively enrolled persons with HF from a longitudinal project examining neurocognitive function in older adults with HF. All participants were between the ages of 50–85 years of age, English-speaking, and were New York Heart Association (NYHA) class II or III at the time of enrollment. Exclusion criteria included history of significant neurological disorder (e.g. dementia, stroke, multiple sclerosis), head injury with more than 10 minutes loss of consciousness, severe psychiatric disorder, substance abuse and/or dependence, and renal failure. Participants averaged 69.84 (SD = 9.39) years of age, were 35.7% female, and 84.3% Caucasian. Medical record review indicated the current sample demonstrated an average left ventricular ejection fraction (LVEF) of 42.43 (SD = 15.89). See Table 1 for baseline demographic and clinical characteristics.
Table 1.
Demographic and Clinical Characteristics of 115 Older Adults with Heart Failure
| Demographic Characteristics | Median (Range) | |
|---|---|---|
| Age, mean (SD) | 69.84 (9.39) | 70.00 (50 to 85) |
| Years of Education, mean (SD) | 13.61 (2.60) | 13.00 (4 to 22) |
| AMNART, mean (SD) | 111.58 (10.40) | 112.92 (86.05 to 130.50) |
| Female (%) | 35.7 | -- |
| Race (% Caucasian; % African American; % Native American/Alaskan Eskimo; % Caucasian and Native American/Alaskan Eskimo) |
84.3; 10.5; 3.5; 1.7 | -- |
| Race (% Caucasian) | 84.3 | -- |
| Medical Characteristics | ||
| Overall Sample LVEF, mean (SD) | 42.43 (15.89) | 42.43 (12 to 79) |
| Diabetes (% yes) | 34.8 | -- |
| Hypertension (% yes) | 68.7 | -- |
| MI (% yes) | 51.3 | -- |
| BDI-II, mean (SD) | 7.39(6.74) | 6.00 (0 to 34) |
LVEF = Left Ventricular Ejection Fraction; MI = Myocardial Infarction; BDI-II = Beck Depression Inventory-II
Measures
Cognitive Function
Based on past work, all neuropsychological tests used in the current study demonstrate strong psychometric properties, including excellent reliability and validity (Strauss, Spreen, & Sherman, 2006). The following neuropsychological tests were administered to assess cognitive function in the current sample:
Attention/Executive Function: Trail Making Test A (Spreen & Strauss, 1991), Digit Symbol Coding (Smith, 1983), Trail Making Test B (Dikmen, Heaton, Grant, & Temkin, 1999), Letter Number Sequencing (LNS) (Wechsler, 1997), Frontal Assessment Battery (Dubois, Slachevsky, Litvan, & Pillon, 2000), Stroop Color Word Interference Effect (Lezak, Howieson, & Loring, 2004; Utl & Graf, 1997).
Memory: The California Verbal Learning Test-II (CVLT-II) short delay free recall, long delay free recall, and total recognition hits (Delis, Kramer, Kaplan, & Ober, 2000). The alternate form of this measure was given at the 3-month follow-up and the standard form at baseline and 12-months. Both forms demonstrate similar and strong psychometric properties (Woods et al., 2006).
Language: Boston Naming Test (BNT) (Hawkins et al., 1993), and Animal Fluency (Morris et al., 1989).
Depressive Symptomatology
The Beck Depression Inventory-II (BDI-II) was administered to assess self-reported depressive symptomatology. The BDI-II is a commonly used checklist of depressive symptoms that exhibits strong psychometric properties in persons with medical conditions (i.e., test-re-test reliability of r = .93 to r = .96, and an internal consistency of r = .54 to r = .74) (Arnau, Meagher, Norris, & Bramson, 2001; Beck, Steer, & Brown, 1996). BDI-II scores range from 0–63 with increased score indicative of increased symptomatology.
Estimated Premorbid Intelligence
The American National Adult Reading Test (AMNART) assessed premorbid intelligence. The AMNART asks individuals to read a list of irregularly pronounced words and is a reliable estimate of intelligence in medical populations (Blair & Spreen, 1989; Friend & Grattan, 1998; Uttl, 2002).
Physical Fitness
The 2-minute step test (2MST) assessed physical fitness levels in the current sample to help further quantify HF severity over time (Jones & Rikli, 2002). The 2MST requires participants to lift his/her knees to a marked target set on the wall set at the midpoint between the kneecap and crest of the iliac for a 2-minute period. Greater step count is associated with better physical fitness. The 2MST has recently been shown to correlate with metabolic equivalents in a sample of older adults with HF (Garcia et al., in press).
Demographic Characteristics
Medical and demographic characteristics were ascertained through participant self-report and medical record review.
Procedures
The local Institutional Review Board (IRB) approved the study procedures and all participants provided written informed consent prior to study enrollment. During a baseline assessment, participants completed demographic and psychosocial self-report measures, including the BDI-II. Participants were also administered a brief neuropsychological test battery to assess cognitive function. The same procedures were repeated at 3-months and 12-months.
Statistical Analyses
To facilitate clinical interpretation and minimize discrepancy within scales all raw scores of neuropsychological measures assessing cognitive function were transformed to T-scores (a distribution with a mean of 50, and a standard deviation of 10) using existing normative data correcting for age, and sex in the case of memory. Composite scores for attention/executive, memory, and language were means of the T-scores within each cognitive domain. Participants with missing data for all three-time points on any of the cognitive domains were excluded from the analyses (n = 1). Additional missing data for the domains (<1%) across the time points was handled using maximum likelihood estimation in MPlus 5.0. Cases with missing data for LVEF (n =4) and BDI-II (n = 1) were handled using mean imputation.
A latent class growth analysis (LCGA) using MPlus 5.0 software was performed to determine trajectories of cognitive test performance in each domain (i.e., attention, executive function, memory, and language) over the three time points (i.e., baseline, 3 months, and 12-months). LCGA was chosen because it statistically places individuals into homogenous groups based on their trajectories of cognitive function, thus allowing us to determine the presence and number of subgroups of trajectories within each cognitive domain for the sample (Nagin, 1999). We tested two-to four class models for each domain. To determine the number of classes that best fit the data for each cognitive domain we examined the Bayesian Information Criterion (BIC), Entropy, the Lo Mendel Rubin Likelihood Ratio Test (LMR RT), and practical usefulness. Specific model fit criterion included: the lowest BIC, Entropy closest to 1, and the LMR RT was also used to compare fit across models with a significant LMR RT (i.e., p < .05) reflective of better fit for the current model rather than dropping a class from the model. Practical usefulness included examination of graphs to determine different intercepts and trajectory shapes (Muthen & Muthen, 2000; Nagin, 2005; Hix-Small, Duncan, Duncan, & Okut, 2004; Beyers & Seiffge-Krenke, 2007). Finally, conditional latent growth analyses were performed to determine whether demographic and clinical characteristics predicted the intercept and slope for each domain.
Results
Medical Status
In terms of HF severity, among those participants that completed the 2MST at baseline and the 12-month follow-up (N = 94), repeated measures analyses showed physical fitness levels remained stable over time (F(1,93) = 0.95, p = 0.33; Baseline M(SD) = 59.39 (21.34) versus 61.64 (24.25) at 12-months). Baseline medical comorbities were also prevalent in this sample, as 34.8% and 68.7% of the sample had a diagnostic history of diabetes and hypertension, respectively. See Table 1. At the 12-month follow-up, medical comorbid status was rather unchanged with 34.8% and 70.4% of participants with a diagnostic history of diabetes and hypertension, respectively.
Unconditional Latent Class Growth Analyses
See Table 2 for correlations of cognitive domains across each time point. We first examined model fit across four different classes of trajectories for each cognitive domain. See Table 3 for a summary of model fit statistics for attention/executive function, memory, and language. Of note, T-score cutoffs consistent with clinical convention were used to descriptively characterize the classes identified within each cognitive domain. Refer to Table 4 for neuropsychological test performance across the three time points.
Table 2.
Correlation Matrix Among the Cognitive Domains Across Each Time Point
| MemBL | Mem_3 | Mem_12 | Atten/EFBL | Atten/EF_3 | Atten/EF_12 | LangBL | Lang_3 | |
|---|---|---|---|---|---|---|---|---|
| Mem BL | -- | -- | -- | -- | -- | -- | -- | -- |
| Mem_3 | .63** | -- | -- | -- | -- | -- | -- | -- |
| Mem_12 | .65** | .61** | -- | -- | -- | -- | -- | -- |
| Atten/EFBL | .32** | .38** | .42** | -- | -- | -- | -- | -- |
| Atten/EF_3 | .25** | .38** | .40** | .84** | -- | -- | -- | -- |
| Atten/EF_12 | .27** | .35** | .49** | .83** | .81** | -- | -- | -- |
| LangBL | .23* | .33** | .28** | .58** | .64** | .59** | -- | -- |
| Lang_3 | .16 | .33** | .16 | .51** | .61** | .50** | .84** | -- |
| Lang 12 | .19* | .37** | .26** | .63** | .70** | .68** | .83** | .79** |
Note.
p < .05;
p < .01;
Correlations are based on complete data for the domains at each time point.
Abbreviations: Mem = Memory; Atten/EF = Attention and Executive Function; Lang = Language; BL = Baseline; 3 = 3-Month Follow-up; 12 = 12-Month Follow-up
Table 3.
Summary of Model Fit Indices of the Latent Class Growth Analyses for Cognitive Function(N= 115)
|
Attention/Executive Function |
BIC | Entropy | LRT | 1 | 2 | 3 | 4 |
|---|---|---|---|---|---|---|---|
| Two Class | 2220 | .90 | .03 | 71.63 | 28.37 | -- | -- |
| Three Class | 2161 | .93 | .02 | 6.21 | 27.95 | 65.85 | -- |
| Four Class | 2144 | .83 | .17 | 6.09 | 22.77 | 34.22 | 36.92 |
| Memory | |||||||
| Two Class | 2467 | .75 | .002 | 53.38 | 46.62 | -- | -- |
| Three Class | 2420 | .76 | .71 | 31.27 | 56.03 | 12.70 | -- |
| Four Class | 2447 | .80 | .003 | 38.45 | 9.20 | 8.26 | 44.09 |
| Language | |||||||
| Two Class | 2475 | .96 | .0008 | 13.00 | 87.00 | -- | -- |
| Three Class | 2427 | .84 | .12 | 27.19 | 12.12 | 60.70 | -- |
| Four Class | 2408 | .89 | .07 | 10.73 | 60.98 | 2.58 | 25.71 |
BIC = Bayesian Information Criterion; LRT = Lo Mendel Rubin Likelihood Ratio Test
Table 4.
Baseline, 3-Month, and 12-Month T-Score Neuropsychological Test Performance among Older Adults with Heart Failure
| Baseline M(SD) |
3-month M(SD) |
12-month M(SD) |
|
|---|---|---|---|
|
Attention/Executive Function |
|||
| TMTA | 50.69(9.99) | 51.07(12.11) | 49.92(13.11) |
| TMTB | 44.88(15.98) | 46.66(13.36) | 45.62(15.63) |
| Digit Symbol | 47.76(9.35) | 49.59(10.07) | 49.13(9.70) |
| LNS | 50.94(9.09) | 51.59(8.11) | 51.44(9.64) |
| FAB | 42.43(21.11) | 47.23(16.18) | 43.22(19.79) |
| Stroop | 49.81(7.19) | 51.41(7.46) | 50.50(7.40) |
| Memory | |||
| CVLT SDFR | 47.26(11.05) | 49.60(11.67) | 52.22(11.22) |
| CVLT LDFR | 46.96(10.88) | 47.96(11.68) | 51.70(11.03) |
| CVLT Total Hits | 43.57(12.79) | 45.13(11.62) | 47.26(11.15) |
| Language | |||
| BNT | 50.36(12.92) | 51.91(12.83) | 50.16(16.96) |
| Animals | 54.59(11.58) | 56.32(12.21) | 53.72(11.82) |
Note. Averages were based on complete data for each time point.
Abbreviations—TMTA = Trail Making Test A; TMTB = Trail Making Test B; LNS = Letter Number Sequencing; FAB = Frontal Assessment Battery; Stroop = Stroop Color Word Interference Effect; CVLT = California Verbal Learning Test; SDFR = Short Delay Free Recall; LDFR = Long Delay Free Recall; BNT = Boston Naming Test.
Attention/Executive Function
A three-class model for attention/executive function was chosen to best fit the data for several statistical reasons. The three-class model exhibited a lower BIC and higher entropy than the two-class model. Additionally, the LMR-RT p-value was < .05 suggesting that a model with one less class would fit the data significantly worse. Although the four-class model had a smaller BIC value than the three-class model, entropy was lower for three classes and the LMR-RT was not statistically significant for the four-class model. The three-class model also demonstrated the most practical usefulness. See Figure 1.
Figure 1.
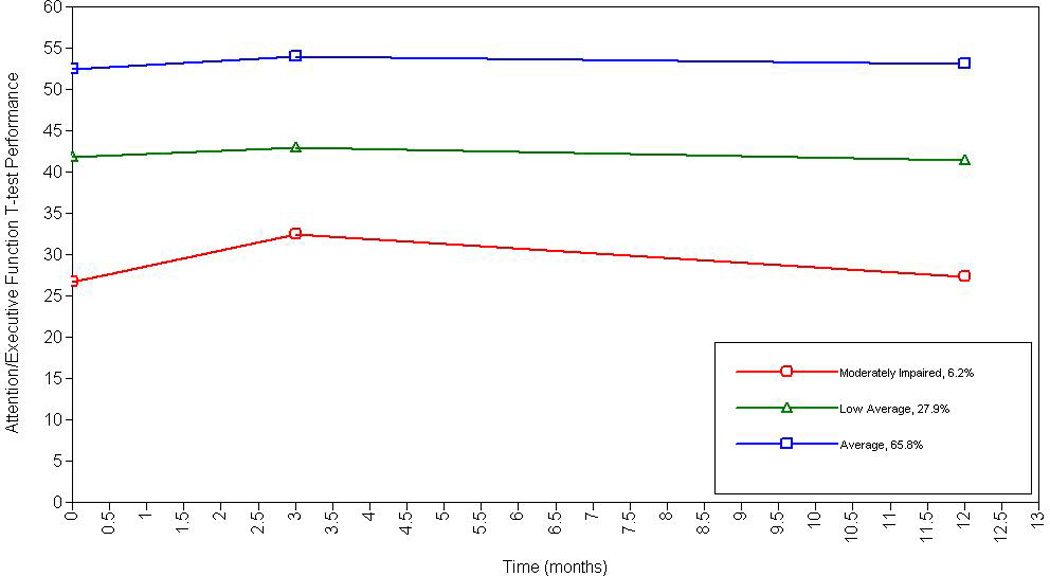
Longitudinal Trajectories of Attention and Executive Function Among Older Adults with Heart Failure
Specifically, according to clinical convention for T-score classification, HF patients demonstrated the following trajectory categorization that remained stable over time: moderately impaired performance on tests of attention/executive function (6.2%; intercept = 29.50 (p < .001), low average performance (27.9%; intercept = 42.39, p < .01), and average attention/executive function performance (65.8%; intercept = 53.10, p < .001). Of note, the slope remained stable for each class over the three-time points: moderately impaired (β = −.08, p = .81), low average (β = −.06, p = .51), and average (β = .02, p = .62).
Memory
A four-class model for memory best fit the data. The four-class model exhibited the highest entropy, and a statistically significant LMR-RT p-value (< .01). This model also exhibited a lower BIC value than the two-class model; the three-class model had a lower entropy value and a non-significant LMR-RT p-value.
The four class model also demonstrated the most practical usefulness for memory test performance, as there were four distinct trajectories categorized as follows: Average memory performance (38.4%; intercept = 51.10, p < .001), mild to moderately impaired memory performance (9.2%; intercept = 31.56, p < .001), high average memory performance (8.3%; intercept = 61.73, p < .001), and low average memory performance (44.1%; intercept = 42.07, p < .001). The slope for the average (β = .39, p < .001) and low average performers (β = .32, p < .01) indicated that these HF persons significantly improved over-time and approached high average and average performance by 12-months, respectively. See Figure 2. Although not significant at the .05 level, the slope indicated trends for improvements in performance for the mild to moderately impaired group (β = .38, p = .09) and the high average performers (β = .35, p = .07).
Figure 2.
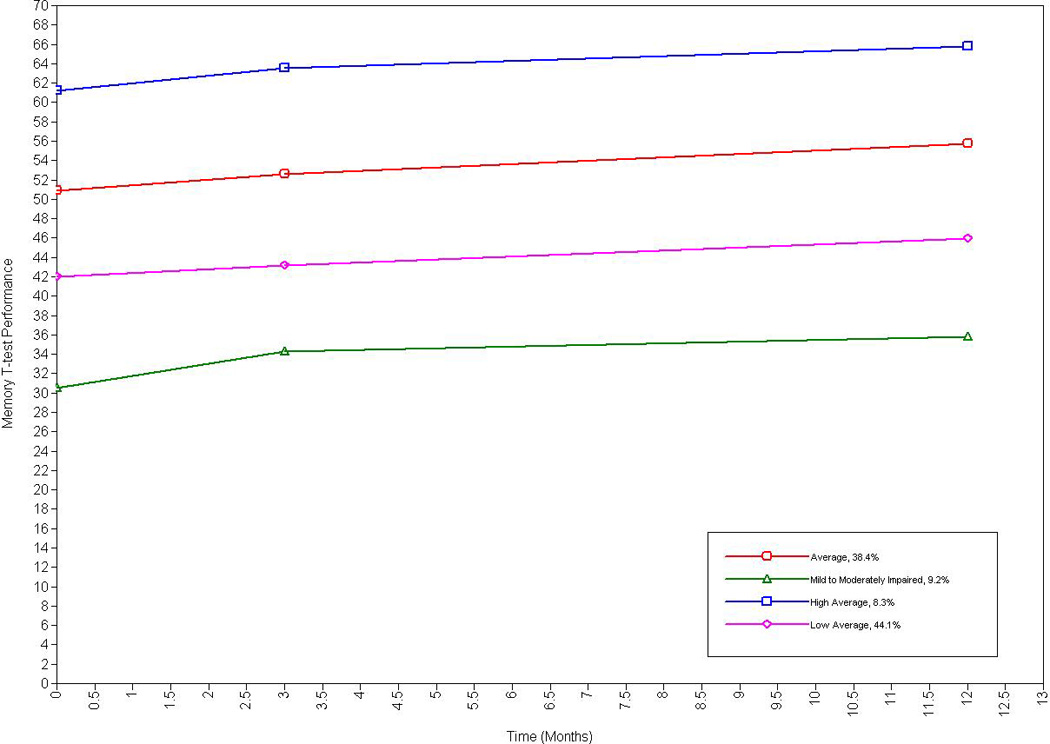
Longitudinal Trajectories of Memory Among Older Adults with Heart Failure
Language
A three-class model for language best fit the data. Although the two-class model demonstrated the highest entropy and a significant LMR-RT and there was a non-significant LMR-RT for the three-class model, the three-class model exhibited a lower BIC value and demonstrated the most practical usefulness. See Figure 3.
Figure 3.
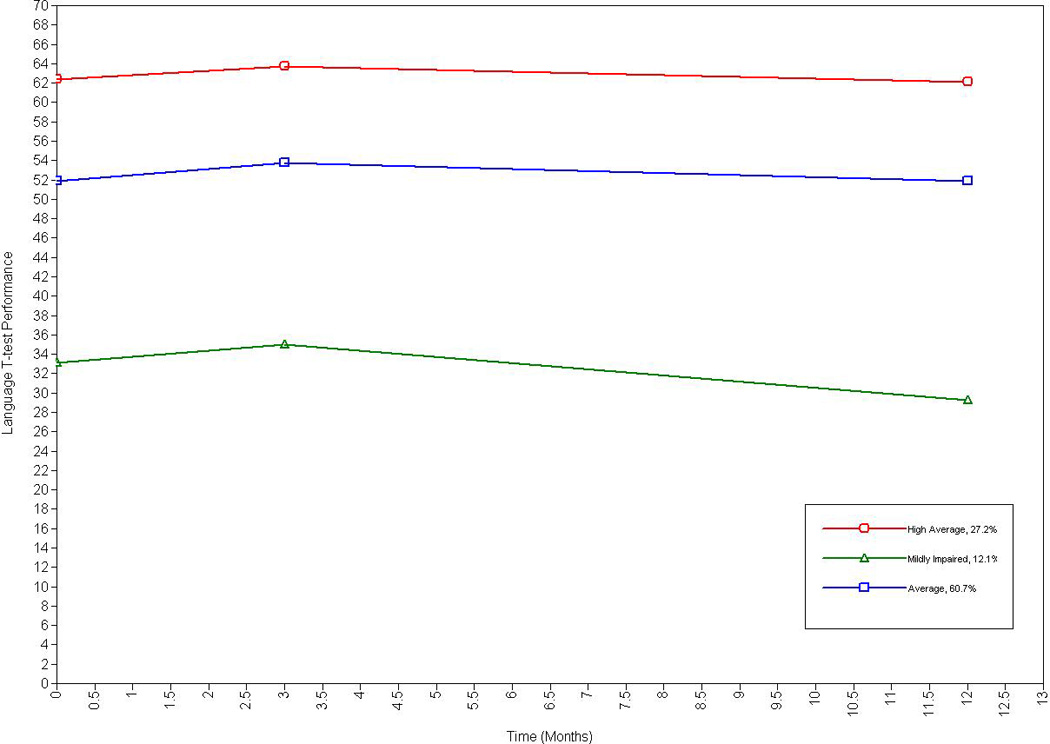
Longitudinal Trajectories of Language Abilities Among Older Adults with Heart Failure
The three-class model had a proportionate number of individuals in three distinct classes, specifically: High average performers (27.2%; intercept = 62.99, p < .001), mildly impaired performers (12.1%; intercept = 34.38, p < .001), and average language abilities (60.7%, intercept = 52.69, p < .001). The slope remained stable over the time points for the high average (β = −.04, p = .69), mildly impaired (β = −.36, p = .13), and average trajectories (β = −.03, p = .66).
Demographic and Clinical Predictors of Cognitive Function
A final set of conditional latent growth analyses in Mplus was conducted to determine whether sex (1 = male; 2 = females), education, LVEF, and/or depressive symptomatology (as assessed by the BDI-II) predicted the intercept for each domain and the slope in the case of memory. Sex was not examined as a predictor of memory, as this domain was already adjusted for sex using normative data. This was also true for age for all cognitive domains. Predictors of the slope for attention/executive function and language abilities were not examined, as the slope for these domains remained stable across time (see above).
Analyses revealed education and the BDI-II significantly predicted the intercept for attention/executive function [education (β = .56, p = .001); BDI-II (β = −.15, p = .02)] and language abilities [education (β = 1.94, p < .001); BDI-II (β = −.28, p = .01)]. HF patients with fewer years of education and higher scores on the BDI-II demonstrated poorer baseline performance on neuropsychological tests assessing these domains. LVEF and sex did not emerge as significant predictors of baseline attention/executive function and language performance in this sample (p > .05).
HF participants with higher BDI-II scores also exhibited poorer baseline memory performance (β = −.37, p < .001), though did not predict slope of memory across the time points (β = −.001, p = .92). Of note, follow-up repeated measure ANOVA analysis showed that BDI-II scores did not significantly change from baseline to 12-months (F(1,114) = .13, p = .72; baseline M(SD) = 7.39(6.71), 12-month M(SD) = 7.57 (6.60). LVEF and education did not significantly predict the intercept or slope for memory (p > .05 for all).
Discussion
Past studies have shown considerable variability in the pattern of cognitive deficits in HF patients. The current study sought to clarify the trajectories of cognitive function for attention/executive function, memory, and language in HF patients over a 12-month period and found evidence for both stability and/or improvement on cognitive testing. Interestingly, multiple performance-level cognitive trajectories existed within each domain and our findings suggest depressive symptomatology and education may contribute to the variability observed in cognitive function in HF. Several aspects of these findings warrant brief discussion.
The finding that performance-level subgroups of trajectories existed within each cognitive domain is similar to past work among HF patients (Riegel, Lee, Glaser, Moelter, 2012)—though that study examined only a six month time period using limited measures of cognitive function. Our findings indicate that the level of cognitive impairment in HF is not uniform and may be unique on the individual level. Indeed, this study suggests that lower education level and greater depressive symptomatology are important risk factors for cognitive impairment in HF patients. This pattern is consistent with the extant evidence for the protective role of cognitive reserve against neuropathological insult (Alosco et al., 2012) and the adverse effects of depression on cognitive function in HF (Garcia et al., 2011). However, prospective studies that directly compare HF patients to controls are needed to fully elucidate the impact of depression and cognitive reserve on cognitive function, including their sensitivity to the detection of cognitive impairment.
The current study also showed that HF patients demonstrated improved testing on memory tasks over time. This finding is in contrast to the research showing declines in memory and risk for Alzheimer’s disease in this population (Qiu et al., 2006; Hjelm et al., 2012). The exact reason for improved memory in this study is not entirely clear, though test-retest effects over the course of the year is one possibility. For instance, the base rates of test-retest scores on the CVLT-II are significantly higher for both improvements and stability relative to decline in healthy adults (Woods et al., 2006). However, it is noted that the long-term follow-up and current use of alternate test versions at each time point notably diminishes practice effects on the CVLT-II (Woods et al, 2006). Similarly, past work also shows largely unchanged memory performance (as assessed by the CVLT-II) over time in HF relative to controls (Almeida et al., 2012). Taken together, it is also possible that improved memory in this sample may be related to factors such as an increase in treatment adherence (e.g. increase in exercise, change in diet) (Zuccala et al., 2005; Tanne et al., 2005). Future studies with control and comparison groups are needed to confirm our findings and clarify the trajectory of memory change over time in HF.
Interestingly, we also found stable performances over time on measures of attention and executive function. These findings are consistent with past work examining cognitive function in HF patients over a 6-month time period (Riegel et al., 2012). While the lack of improvement in these domains may be reflective of impairment, the more long-term follow-up used in this study (i.e., 12-months) limits this possibility. It is plausible that the 12-month period may not have been a long enough time period to capture decline in these domains in HF. For instance, other studies provide evidence for cognitive decline in HF at a two-year follow up (Almeida et al., 2012). Prospective studies examining cognitive function in HF for over extended periods of time are needed to further elucidate the pattern and rate of cognitive change in this population.
The association between depressive sympomtatology and cognitive impairment in the current study deserves further discussion given the elevated prevalence rates of depression in HF (Skotzko et al., 2000). Indeed, this study found depression was associated with reduced baseline cognitive function in each domain, which is consistent with past cross-sectional studies examining this link in HF (Garcia et al., 2011; Pullicino et al., 2008). Depression in HF may represent a neuropsychiatric change secondary to underlying brain pathology, including white matter lesions of the frontal brain regions (Almeida et al., 2005) and such mechanisms are also thought to underlie cognitive impairment in HF (Hoth, 2010). Our findings showed that depression remained stable over time, which indeed may be attributed to minimal changes in neuropathology or disease severity. In contrast, compared to cardiac and healthy controls, recent work shows that depressive symptomatology increases in severity over time in HF, but did not correspond to changes in cognitive function (Almeida et al., 2013). These findings suggest that the effects of depression on cognition in HF may be more complicated than typically believed (Almeida et al., 2013). Prospective studies examining the course and mechanisms of depression in HF is strongly encouraged, particularly since depressive symptomatology is associated with increased risk for re-hospitalizatoin in this population (Silver, 2010; Zuluage et al., 2010).
The current study is limited in several ways. Most importantly, the lack of control group in the current study limits generalizability of our findings due to the possibility of practice effects. However, practice effects likely did not fully account for our results given the long-term intervals between test administrations and the use of alternate test versions for memory tasks. Prospective case-controlled studies with an extended follow-up (e.g., 3–5years) would clarify the nature of cognitive decline in HF and also elucidate whether HF accelerates the aging process. Moreover, the lack of change over time in attention/executive function as well as language limited the investigation on predictors of change for these domains. The no changes in cognitive functioning for these domains over time may in part be because they were not studied for a long enough period or because the tests used were not sensitive enough to measure subtle changes. Thus, prospective studies with longer follow-up periods and healthy controls would help to clarify the key predictors and modifiers of cognitive function, including depression. For instance, because of the lack of control group in the current study, it also remains unclear whether lower education and increased depressive symptomatology impacts cognition in HF beyond what is typically found in other healthy and clinical populations. However, it is noted that past work demonstrates HF patients exhibit greater depressive symptoms than controls (Almeida et al., 2013), highlighting a strong need for further research.
Finally, we were unable to get baseline testing before cognitive deficits or depressive symptomatology, making it unclear the interaction of this relationship from a functional and structural perspective. Future studies should either use more specific measures of cognition or examine cognition for longer periods of time in this population to better understand the trajectories of cognitive decline. Future studies should also examine neuroimaging in HF over time to determine to whether HF patients distinct cognitive profiles is consistent with neuropathology.
In brief summary, the current study revealed evidence for stability and improvement in cognitive function over time. Furthermore, significant variability existed within each cognitive domain (i.e., attention/executive function, memory, and language) suggesting that cognitive impairment may be distinct across HF patients and affected by factors such as depression and education. Future research that uses healthy control and other medical comparison groups is needed to better understand both the long-term trajectories of cognition in HF patients as well as other potential predictors of change for intervention.
Acknowledgments
Support for this work included National Institutes of Health (NIH) grants DK075119 and HLO89311. Dr. Naftali Raz is supported by National Institutes of Health (NIH) grant R37 AG011230. The authors have no competing interests to report.
References
- Almeida JR, Alves TC, Wajngarten M, Rays J, Castro CC, Cordeiro Q, Busatto GF. Late-life depression, heart failure and frontal white matter hyperintensity: A structural magnetic resonance imaging study. Brazailian Journal of Medical and Biological Research. 2005;38:431–436. doi: 10.1590/s0100-879x2005000300014. [DOI] [PubMed] [Google Scholar]
- Almeida OP, Beer C, Lautenschlager NT, Arnolda L, Alfonso H, Flicker L. Two year course of cognitive function and mood in adults with congestive heart failure and coronary artery disease: the Heart-Mind Study. International Psychogeriatrics. 2012;24:38–47. doi: 10.1017/S1041610211001657. [DOI] [PubMed] [Google Scholar]
- Almeida OP, Garrido GJ, Etherton-Beer C, Lautenschlager NT, Arnolda L, Alfonso H, Flicker L. Brain and mood changes over 2 years in healthy controls and adults with heart failure and ischaemic heart disease. Eur J Heart Fail. 2013 doi: 10.1093/eurjhf/hft029. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Alosco ML, Spitznagel MB, Raz N, Cohen R, Sweet LH, van Dulmen M, Gunstad J. Cognitive reserve moderates the association between heart failure and cognitive impairment. Journal of Clinical and Experimental Neuropsychology. 2012;34:1–10. doi: 10.1080/13803395.2011.614596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alosco ML, Spitznagel MB, Cohen R, Sweet LH, Colbert LH, Josephson R, Gunstad J. Cognitive impairment is independently associated with reduced instrumental ADLs in persons with heart failure. Journal of Cardiovascular Nursing. 2012;27:44–50. doi: 10.1097/JCN.0b013e318216a6cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alosco ML, Spitznagel MB, van Dulmen M, Raz N, Cohen R, Sweet LH, Gunstad J. The additive effects of type-2 diabetes on cognitive function in older adults with heart failure. Cardiology Research and Practice. 2012 doi: 10.1155/2012/348054. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnau R, Meagher M, Norris M, Bramson R. Psychometric evaluation of the Beck Depression Inventory-II with primary care medical patients. Health Psychology. 2001;20:112–119. doi: 10.1037//0278-6133.20.2.112. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Beck Depression Inventory. 2nd ed. San Antonio, TX: The Psychological Corporation; 1996. [Google Scholar]
- Beer C, Ebenezer E, Fenner S, Lautenschlager NT, Arnolda L, Flicker L, Almeida OP. Contributors to cognitive impairment in congestive heart failure: a pilot case-controlled study. Internal Medicine Journal. 2009;39:600–605. doi: 10.1111/j.1445-5994.2008.01790.x. [DOI] [PubMed] [Google Scholar]
- Bennett SJ, Oldridge NB, Eckert G, Embree JL, Browning S, Hou N, Murray MD. Comparison of quality of life measures in heart failure. Nursing Research. 2003;52:207–216. doi: 10.1097/00006199-200307000-00001. [DOI] [PubMed] [Google Scholar]
- Beyers W, Seiffge-Krenke I. Are friends and romantic partners the “best medicine”? How the quality of other close relations mediates the impact of changing family relationships on adjustment. International Journal of Behavioral Development. 2007;31:559–568. [Google Scholar]
- Blair J, Spreen O. Predicting premorbid IQ: a revision of the National Adult Reading Test. The Clinical Neuropsychologist. 1989;3:129–136. [Google Scholar]
- Delis D, Kramer J, Kaplan E, Ober B. Manual. San Antonio (TX): Psychological Corporation; 2000. California Verbal Learning Test-Second Edition: Adult Version. [Google Scholar]
- Dikmen S, Heaton R, Grant I, Temkin N. Test-retest reliability of the Expanded Halstead-Reitan Neuropsychological Test Battery. Journal of the International Neuropsychological Society. 1999;5:346–356. [PubMed] [Google Scholar]
- Dubois B, Slachevsky A, Litvan I, Pillon B. The FAB: a frontal assessment battery at bedside. Neurology. 2000;55:1621–1626. doi: 10.1212/wnl.55.11.1621. [DOI] [PubMed] [Google Scholar]
- Festa JR, Jia X, Cheung K, Marchidann A, Schmidt M, Shapiro PA, Lazar RM. Association of low ejection fraction with impaired verbal memory in older patients with heart failure. Archives of Neurology. 2011;68:1021–1026. doi: 10.1001/archneurol.2011.163. [DOI] [PubMed] [Google Scholar]
- Friend L, Grattan K. Use of the North American Adult Reading Test to estimate premorbid intellectual functioning in patients with multiple sclerosis. Journal of Clinical and Experimental Neuropsychology. 1998;6:846–851. doi: 10.1076/jcen.20.6.846.1110. [DOI] [PubMed] [Google Scholar]
- Garcia S, Spitznagel MB, Cohen R, Raz N, Sweet L, Colbert L, Gunstad J. Depression is associated with cognitive dysfunction in older adults with heart failure. Cardiovascular Psychiatry and Neurology. 2012 doi: 10.1155/2011/368324. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia S, Alosco ML, Spitznagel MB, Cohen R, Raz N, Sweet L, Josephson R, Hughes J, Rosneck J, Oberle LM, Gunstad J. Cardiovascular Fitness Predicts Cognitive Performance in Heart Failure Patients Enrolled in Cardiac Rehabilitation. BMC Cardiovascular Disorders. in press doi: 10.1186/1471-2261-13-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb NR, Simpson C, Fox KA. Memory function in patients with stable, moderate to severe cardiac failure. American Heart Journal. 2000;140:E1–E5. doi: 10.1067/mhj.2000.106647. [DOI] [PubMed] [Google Scholar]
- Hawkins LA, Killian S, Firek A, Kashner TM, Firek CJ, Silvet H. Cognitive impairment and medication adherence in outpatients with heart failure. Heart & Lung. 2012 doi: 10.1016/j.hrtlng.2012.06.001. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Hawkins KA, Sledge WH, Orlean JE, Quinlan DM, Rakfeldt J, Huffman RE. Normative implications of the relationship between reading vocabulary and Boston Naming Test performance. Archives of Clinical Neuropsychology. 1993;8:525–537. [PubMed] [Google Scholar]
- Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD. Council on Cardiovascular Surgery and Anesthesia, and interdisciplinary Council on Quality of Care and Outcomes Research Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123:933–944. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- Hix-Small H, Duncan TE, Duncan SC, Okut H. A multivariate associative finite growth mixture modeling approach examining adolescent alcohol and marijuana use. Journal of Psychopathology and Behavioral Assessment. 2004;26:255–270. [Google Scholar]
- Hjelm C, Dahl A, Brostrom A, Martensson J, Johansson B, Stromberg A. The influence of heart failure on longitudinal changes in cognition among individuals 80 years of age and older. Journal of Clinical Nursing. 2012;7–8:994–1003. doi: 10.1111/j.1365-2702.2011.03817.x. [DOI] [PubMed] [Google Scholar]
- Hoth KF. Heart Failure and Cognitive function. In: Cohen RA, Gunstad J, editors. Neuropsychology and Cardiovascular Disease. Oxford (NY): Oxford university press; 2010. [Google Scholar]
- Hoth KF, Poppas A, Moser DJ, Paul RH, Cohen RA. Cardiac Dysfunction and cognition in older adults with heart failure. Cognitive and Behavioral Neurology. 2008;21:65–72. doi: 10.1097/WNN.0b013e3181799dc8. [DOI] [PubMed] [Google Scholar]
- Hoth KF, Poppas A, Ellison KE, Paul RH, Sokobin A, Cho Y, Cohen RA. Link between change in cognition and left ventricular function following cardiac resynchronization therapy. Journal of Cardiopulmonary Prevention and Rehabilitation. 2010;30:401–408. doi: 10.1097/HCR.0b013e3181e1739a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson AL, Himali JJ, Au R, Seshadri S, Decarli C, O’Donnell CJ, Benjamin EJ. Relation of left ventricular ejection fraction to cognitive aging (from the Framingham Heart Study) American Journal of Cardiology. 108:1346–1351. doi: 10.1016/j.amjcard.2011.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jencks SF, Williams MV, Coleman EA. Rehospitalization among patients in Medicare fee-for-service program. New England Journal of Medicine. 2009;360:1418–1428. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- Jerskey BA, Cohen RA, Jefferson AL, Hoth KF, Haley AP, Gunstad JJ, Poppas A. Sustained attention is associated with left ventricular ejection fraction in older adults with heart failure. Journal of the International Neuropsychological Society. 2009;15:137–141. doi: 10.1017/S1355617708090073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C, Rikli RE. Measuring functional fitness of older adults. The Journal on Active Aging, March April. 2002:24–30. [Google Scholar]
- Lezak MD, Howieson DB, Loring DW.Neuropsychological assessment 20044thNew York (NY)Oxford University Press [Google Scholar]
- Miller LA, Spitznagel MB, Alosco ML, Cohen RA, Raz N, Sweet LH, Gunstad J. Cognitive profiles in heart failure: a cluster analytic approach. Journal of Clinical and Experimental Neuropsychology. 2012;34:509–520. doi: 10.1080/13803395.2012.663344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J, Heyman A, Mohs R, Hughes JP, van Belle G, Fillenbaum G, Clark C. The consortium to establish a registry for Alzheimer’s disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimers disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- Muthén BO, Muthén LK. Integrating person-centered and variable-centered analyses: Growth mixture modeling with latent trajectory classes. Alcoholism: Clinical and Experimental Research. 2000;24:882–891. [PubMed] [Google Scholar]
- Nagin DS. Group-based modeling of development. Cambridge, MA: Harvard University Press; 2005. [Google Scholar]
- Nagin DS. Analyzing developmental trajectories: A semiparametirc, group-based approach. Psychological Methods. 1999;4:139–157. doi: 10.1037/1082-989x.6.1.18. [DOI] [PubMed] [Google Scholar]
- Pressler SJ, Subramanian U, Kareken D, Perkins SM, Gradus-Pizlo I, Sauve MJ, Shaw RM. Cognitive deficits in chronic heart failure. Nursing Research. 2010;59:127–139. doi: 10.1097/NNR.0b013e3181d1a747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullicino PM, Wadley VG, McClure LA, Safford MM, Lazar RM, Klapholz M, Howard G. Factors contributing to global cognitive impairment in heart failure: Results from a population-based cohort. Journal of Cardiac Failure. 2008;14:290–295. doi: 10.1016/j.cardfail.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu C, Winblad B, Marengoni A, Klarin I, Fastbom J, Fratglioni L. Heart failure and risk of dementia and Alzheimer disease: a population-based cohort study. Archives of Internal Medicine. 2006;166:1003–1008. doi: 10.1001/archinte.166.9.1003. [DOI] [PubMed] [Google Scholar]
- Riegel B, Lee CS, Glaser D, Moelter ST. Patterns of change in cognitive function over six months in adults with chronic heart failure. Cardiology Research and Practice. 2012 doi: 10.1155/2012/631075. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roger VL, Weston SA, Redfield MM, Hellermann-Homan JP, Killian J, Yawn BP, Jacobsen SJ. Trends in heart failure incidence and survival in a community-based population. Journal of the American Medical Association. 2004;292:344–350. doi: 10.1001/jama.292.3.344. [DOI] [PubMed] [Google Scholar]
- Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB. American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics—2012 update. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman G. Vascular dementia prevention: a risk factor analysis. Cerebrovascular Diseases. 2005;20:91–100. doi: 10.1159/000089361. [DOI] [PubMed] [Google Scholar]
- Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N. American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics—2008 update: A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–e146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- Silver MA. Depression and heart failure: an overview of what we know and don’t know. Cleveland Clinic Journal of Medicing. 2010;77:S7–S11. doi: 10.3949/ccjm.77.s3.02. [DOI] [PubMed] [Google Scholar]
- Skotzko CE, Krichten C, Zietowski G, Alves L, Freudenberger R, Robinson S, Gottlieb SS. Depression is common and precludes accurate assessment of functional status in elderly patients with congestive heart failure. Journal of Cardiac Failure. 6:300–305. doi: 10.1054/jcaf.2000.19222. [DOI] [PubMed] [Google Scholar]
- Smith A. Clinical psychological practice and principals of neuropsychological assessment. In: Walker C, editor. Handbook of clinical psychology: Theory, Research, and practice. Homewood (IL): Dorsey Press; 1983. [Google Scholar]
- Spreen O, Strauss EA. Compendium of Neuropsychological Tests. New York: Oxford University Press; 1991. [Google Scholar]
- Stanek KM, Gunstad J, Paul RH, Poppas A, Jefferson AL, Sweet LH, Cohen RA. Longitudinal cognitive performance in older adults with cardiovascular disease: evidence for improvement in heart failure. Journal of Cardiovascular Nursing. 2009;24:192–197. doi: 10.1097/JCN.0b013e31819b54de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss E, Sherman EMS, Spreen O. A compendium of neuropsychological tests: Administration, norms, and commentary. 3rd Ed. New York, NY: Oxford University Press; 2006. [Google Scholar]
- Suave MJ, Lewis WR, Blankenbiller M, Rickabaugh B, Pressler SJ. Cognitive impairments in chronic heart failure: a case controlled study. Journal of Cardiac Failure. 2009;15:1–10. doi: 10.1016/j.cardfail.2008.08.007. [DOI] [PubMed] [Google Scholar]
- Tanne D, Freimark D, Poreh A, Merzeliak O, Bruck B, Schwammenthal Y, Adler Y. Cognitive functions in severe congestive heart failure before and after an exercise training program. International Journal of Cardiology. 2005;103:145–149. doi: 10.1016/j.ijcard.2004.08.044. [DOI] [PubMed] [Google Scholar]
- Utl B, Graf P. Color-Word Stroop test performance acrossthe adult life span. Journal of Clinical and Experimental Neuropsychology. 1997;19:405–420. doi: 10.1080/01688639708403869. [DOI] [PubMed] [Google Scholar]
- Uttl B. North American Adult Reading Test: age norms, reliability, and validity. Journal of Clinical and Experimental Neuropsychology. 2002;24:1123–1137. doi: 10.1076/jcen.24.8.1123.8375. [DOI] [PubMed] [Google Scholar]
- Van den Hurk K, Reijmer YD, van den Berg E, Alssema M, Nijpels G, Kostense PJ, Biessels GJ. Heart failure and cognitive function in the general population: the Hoorn Study. European Journal of Heart Failure. 2011;13:1362–1369. doi: 10.1093/eurjhf/hfr138. [DOI] [PubMed] [Google Scholar]
- Vogels RL, Oosterman JM, van Harten B, Scheltens P, van der Flier WM, Schroeder-Tanka JM, Weinstein HC. Profile of cognitive impairment in chronic heart failure. Journal of the American Geriatrics Society. 2007;55:1764–1770. doi: 10.1111/j.1532-5415.2007.01395.x. [DOI] [PubMed] [Google Scholar]
- Vogels RLC, van der Flier WM, van Harten B, Gouw AA, Scheltens P, Schroeder-Tanka JM, Weinstein HC. Brain magnetic resonance imaging abnormalities in patients with heart failure. European Journal of Heart Failure. 2007;9:1003–1009. doi: 10.1016/j.ejheart.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Vogels RL, Scheltens P, Schroeder-Tanka JM, Weinstein HC. Cognitive impairment in heart failure: A systematic review of the literature. European Journal of Heart Failure. 2007;9:440–449. doi: 10.1016/j.ejheart.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler Adult Intelligence Scale-Third Edition. San Antonio (TX): The Psychological Corporation; 1997. [Google Scholar]
- Woods SP, Delis DC, Scott JC, Kramer JH, Holdnack JA. The Calirfornia Verbal Learning Test—second edition: Test-retest reliability, practice effects, and reliable change indices for the standard and alternate forms. Archives of Clinical Neuropsychology. 2006;21:413–420. doi: 10.1016/j.acn.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Zuccala G, Marzetti E, Cesari M, Lo Monaco MR, Antonica L, Cocchi A, Carbonin P, Bernabel R. Correlates of cognitive impairment among patients with heart failure: Results of a multicenter survey. American Journal of Medicine. 2005;118:406–502. doi: 10.1016/j.amjmed.2005.01.030. [DOI] [PubMed] [Google Scholar]
- Zuluaga MC, Guallar-Catillon P, Rodriguez-Pascual C, Conde-Herrera M, Conthe P, Rodriguez-Artalejo F. Mechanisms of the association between depressive symptoms and long-term mortality in heart failure. American Heart Journal. 2010;159:231–237. doi: 10.1016/j.ahj.2009.11.011. [DOI] [PubMed] [Google Scholar]


