Abstract
Background
Among participants in the Secondary Prevention of Small Subcortical Strokes randomized trial, we sought to identify patients with high vs. low rates of recurrent ischemic stroke and to assess effects of aggressive blood pressure control and dual antiplatelet therapy according to risk status.
Methods
Multivariable analyses of 3020 participants with recent MRI-defined lacunar strokes followed for a mean of 3.7 years with 243 recurrent ischemic strokes. Results: Prior symptomatic lacunar stroke or TIA (HR 2.2, 95%CI 1.6,2.9), diabetes (HR 2.0, 95%CI 1.5,2.5), Black race (HR 1.7, 95%CI 1.3,2.3) and male sex (HR 1.5, 95%CI 1.1,1.9) were each independently predictive of recurrent ischemic stroke. Recurrent ischemic stroke occurred at a rate of 4.3%/yr (95% CI 3.3, 5.5) in patients with prior symptomatic lacunar stroke or TIA (15% of the cohort), 3.1%/yr (95%CI 2.6, 3.9) in those with >1 of the other 3 risk factors (27% of the cohort), and 1.3%/yr (95%CI 1.0,1.7) in those with 0 to 1 risk factors (58% of the cohort). There were no significant interactions between treatment effects and stroke risk status.
Conclusions
In this large, carefully followed cohort of patients with recent lacunar stroke and aggressive blood pressure management, prior symptomatic lacunar ischemia, diabetes, Black race and male sex independently predicted ischemic stroke recurrence. The effects of blood pressure targets and dual antiplatelet therapy were similar across the spectrum of independent risk factors and recurrence risk.
Keywords: lacunar infarct, cerebral small vessel disease, prognosis, recurrent stroke
Introduction
Small subcortical brain infarcts, commonly known as lacunar strokes, comprise about 25% of ischemic strokes.(1-4) Most result from intrinsic cerebral small artery disease. Independent risk factors for stroke recurrence in patients with lacunar strokes have not been well delineated in the existing small studies.(5-8)
The Secondary Prevention of Small Subcortical Strokes (SPS3) randomized trial included 3020 patients with recent MRI-defined lacunar strokes and compared dual antiplatelet therapy with clopidogrel plus aspirin vs. aspirin alone and, separately, two target goals of systolic blood pressure treatment. Here, independent risk factors for recurrent ischemic stroke in SPS3 participants are identified, and the effects of the two treatment interventions are assessed according to the risk of stroke recurrence.(9)
Methods
The rationale, design, participant characteristics, and main results of the SPS3 trial have been reported elsewhere.(10-13) In brief, SPS3 was a randomized, multicenter clinical trial conducted in 82 clinical centers in North America, Latin America, and Spain. Patients ≥ 30 years with a recent (≤180 days) symptomatic lacunar stroke who were without surgically-amenable ipsilateral carotid artery disease or major-risk cardioembolic sources were eligible and randomized simultaneously in a 2-by-2 factorial design, either to single or dual antiplatelet therapy (double-blind) and to one of two target levels of systolic blood pressure control (<130 mmHg vs. 130-149 mmHg, open-label). Participants with a clinical lacunar syndrome were required to meet MRI criteria that included a diffusion-weighted imaging lesion ≤ 2.0 cm in size with confirmatory apparent diffusion coefficient image or a well-delineated focal hyperintensity ≤ 2.0 cm in size on FLAIR or T2 that corresponded to the clinical syndrome. In addition, MRI evidence of a recent or remote cortical infarct, large (>1.5 cm) subcortical infarct, or prior intracerebral hemorrhage excluded participation (the presence of microbleeds was not an exclusion). MRI eligibility was determined by local investigators, with images submitted for central interpretation by a neuroradiologist. Salient additional exclusion criteria included disabling stroke (modified Rankin Scale ≥4).
Patients with prior lacunar stroke or TIA required a clinical episode antecedent to the qualifying event that was consistent with a classic subcortical ischemic stroke syndrome, and not based solely on neuroimaging findings. Diabetics were those with a history of diabetes mellitus at the time of the qualifying stroke (91%) plus those with diabetes diagnosed at the time of stroke (fasting serum glucose ≥120 mg/dL) or initiation of antidiabetic medications during the first 3 months of follow-up (9%). Blood pressure at entry was categorized based on the average of screening systolic blood pressure measurements taken at least one week apart, adjusted by adding 5 mmHg for each antihypertensive medication (up to a maximum of 4) at the time of the measurement. Normotensive was defined as <120 mmHg, pre-hypertensive as 120-<140 mmHg, stage I hypertension as 140-<160 mmHg, and stage II hypertension as ≥160 mmHg.
These analyses focused on the risk of recurrent ischemic stroke, in contrast to the primary outcome of the SPS3 trials, which also included intracranial hemorrhage (12% of primary events).(12,13) Recurrent ischemic stroke was clinically defined as a focal neurological deficit of sudden onset persisting for greater than 24 hours with hemorrhage excluded by acute neuro-imaging. All strokes were reviewed by a central adjudication committee that was unaware of treatment assignment and that additionally classified ischemic strokes by presumed mechanism based on available diagnostic studies. The antiplatelet trial was terminated four months prior to the blood pressure trial.(12,13) Here, the additional 12 ischemic strokes (eight assigned aspirin and four assigned aspirin plus clopidogrel) which occurred during the four month interval as well as two ischemic strokes (both assigned to aspirin alone) which occurred during the antiplatelet trial but not identified prior to the publication of the main results.(12) For the analyses of predictors of ischemic stroke, the 238 (98%) recurrent ischemic strokes were combined with 5 (2%) strokes thought to be ischemic but lacking neuroimaging. Recurrent lacunar infarcts (n=136) comprised 56% of ischemic strokes, and independent predictors of this subgroup are separately assessed.
Patient characteristics selected for inclusion in the analysis were the clinical characteristics reported in a previously published description of participant features (12) plus body mass index, stage of hypertension, and estimated glomerular filtration rate.(14) To identify patient characteristics independently predictive of ischemic stroke, the cohort was randomly divided into two groups of equal size, i.e. derivation and validation cohorts. Clinical features independently associated with ischemic stroke were identified in the derivation cohort using forward stepwise Cox proportional hazards modeling techniques (likelihood ratio test), both without and with stratification by assigned treatment groups to confirm that treatment assignment did not influence identification of predictors. Independent factors were then combined by inspection in the derivation cohort to yield a risk stratification scheme that was then applied to the validation cohort. Cumulative recurrent stroke rates were compared across risk strata by fitting a Kaplan-Meier curve (log rank test). Annualized stroke rates were calculated with person-years as the denominator with 95% confidence intervals (CIs) computed assuming the Poisson distribution. Absolute differences and CIs in stroke rates between groups were also computed assuming the Poisson distribution. All analyses followed an intention-to-treat paradigm. All tests were two-sided, and statistical significance was accepted at the 0.05 level, with no adjustment made for multiple comparisons. Analyses were performed with SPSS version 20.
Results
The mean age (standard deviation) of the cohort was 63 (11) years, 63% were men, and hypertension, diabetes and ischemic heart disease were present in 75%, 37% and 10%, respectively.(Table 1) Most participants (65%) were from North America; 51% were White, 30% Hispanic, and 16% Black. During 11,046 total years of follow-up (mean follow-up 3.7 years, range 0 to 8.6 years), there were 243 recurrent ischemic strokes (annualized rate of 2.2%/yr). Relative risk reductions by the randomized interventions for recurrent ischemic stroke were 19% (95% CI -4,37) for dual antiplatelet therapy vs. aspirin alone and 15% (95%CI -9,34) for lower (<130 mmHg) vs. higher (130-149 mmHg) systolic blood pressure target, with no evidence of treatment interaction (p = 0.3).
Table 1. Features associated with recurrent ischemic stroke.
| No recurrent ischemic stroke (n = 2777) | Recurrent ischemic stroke (n = 243) | Univariate hazard ratio (95% CI) | |
|---|---|---|---|
|
| |||
| Age, mean (sd) | 63 (11) | 62 (11) | 1.0 (0.9, 1.1)* |
|
| |||
| Male, % | 62 | 70 | 1.4 (1.1, 1.8) |
|
| |||
| Race/ethnic group, % | |||
| White | 51 | 48 | reference group |
| Hispanic | 31 | 22 | 0.8 (0.6, 1.1) |
| Black | 15 | 26 | 1.7 (1.2, 2.2) |
| Other/multiple | 2 | 4 | 1.5 (0.8, 2.9) |
|
| |||
| Region, % | |||
| US or Canada | 64 | 79 | reference group |
| Latin America | 24 | 14 | 0.6 (0.4, 0.9) |
| Spain | 13 | 7 | 0.6 (0.4, 1.0) |
|
| |||
| Body mass index, kg/m2, mean (sd) | 29 (7) | 29 (6) | 1.0 (0.8, 1.2)* |
|
| |||
| Education, years, % | |||
| 0-4 | 11 | 7 | 0.8 (0.5, 1.3) |
| 5-8 | 13 | 14 | 1.0 (0.7, 1.5) |
| 9-12 | 37 | 43 | 1.2 (0.9, 1.7) |
| any college | 36 | 35 | reference group |
|
| |||
| Current tobacco smoker, % | 20 | 24 | 1.3 (1.0, 1.8) |
|
| |||
| Alcohol use (≥7 drinks/week), % | 13 | 8 | 0.6 (0.4, 1.0) |
|
| |||
| History of HTN, % | 74 | 81 | 1.5 (1.1, 2.0) |
|
| |||
| Screening blood pressure, mean (sd) | 143(19)/78(11) | 144(19)/78(11) | 1.0 (1.0, 1.1)*/ 0.9 (0.8, 1.1)* |
|
| |||
| Severity of HTNˆ, % | |||
| normotensive | 2 | 3 | 1.3 (0.6, 2.8) |
| pre-HTN | 18 | 19 | reference group |
| stage I | 41 | 41 | 1.0 (0.7, 1.5) |
| stage II | 39 | 37 | 1.0 (0.7, 1.5) |
|
| |||
| Diabetes, % | 35 | 52 | 2.0 (1.6, 2.6) |
|
| |||
| Ischemic heart disease, % | 10 | 15 | 1.5 (1.0, 2.1) |
|
| |||
| eGFR (ml/min/1.73m2) mean (sd) | 81 (20) | 81 (21) | 1.0 (0.9, 1.1)* |
|
| |||
| Prior symptomatic lacunar stroke or TIA, % | 14 | 28 | 2.3 (1.7, 3.0) |
|
| |||
| Location of qualifying event, % | |||
| anterior | 52 | 50 | reference group |
| thalamic | 22 | 23 | 1.0 (0.8, 1.4) |
| posterior | 26 | 27 | 1.0 (0.8, 1.4) |
|
| |||
| Aspirin use at time of qualifying event, % | 28 | 42 | 1.7 (1.3, 2.2) |
|
| |||
| Statins at time of randomization, % | 69 | 64 | 0.9 (0.7, 1.1) |
HTN = hypertension; eGFR = estimated glomerular filtration rate using the CKD-Epi equation (14); US = United States; sd = standard deviation.
per 10 unit increase
See Methods section for definition of severity of hypertension.
Factors associated with recurrent stroke
Male sex, Black race, diabetes, and prior symptomatic lacunar stroke or TIA were each independently predictive of recurrent ischemic stroke in the derivation cohort (each p ≤ 0.01), and these risk factors (each p ≤ 0.05) except male sex (p = 0.2) were confirmed in the validation cohort.(Table 2) In the entire cohort, prior symptomatic lacunar stroke or TIA (HR 2.2, 95%CI 1.6, 2.9), diabetes (HR 2.0, 95%CI 1.5,2.5), Black race (HR 1.7, 95%CI 1.3,2.3) and male sex (HR 1.5, 95%CI 1.1,1.9) were independently predictive of ischemic stroke recurrence. There was no significant interaction between either randomized intervention and any of the independent risk factors of recurrent ischemic stroke (each p for interaction ≥ 0.3). Analysis with stratification by treatment group yielded the same set of predictors in the model, as did analyses according to the duration of observation after trial entry.(Supplement Table 1)
Table 2. Factors associated with recurrent ischemic stroke: Multivariable analysis.
| Feature | Derivation cohort Hazard ratio (95%CI) | Validation cohort Hazard ratio (95%CI) | All participants Hazard ratio (95%CI) |
|---|---|---|---|
| Male sex | 1.7 (1.1, 2.6) | 1.3 (0.9, 1.9) | 1.5 (1.1, 1.9) |
| Black race | 2.0 (1.4, 3.0) | 1.5 (1.0, 2.3) | 1.7 (1.3, 2.3) |
| Diabetes | 2.1 (1.5, 3.0) | 1.9 (1.3, 2.7) | 2.0 (1.5, 2.5) |
| Prior symptomatic lacunar stroke or TIA | 2.5 (1.7, 3.7) | 1.9 (1.3, 2.8) | 2.2 (1.6, 2.9) |
Exclusion of prior symptomatic lacunar stroke or TIA from the model did not appreciably change the hazard ratio estimates for the remaining three predictors.(Supplement Table 2a). Omitting prior symptomatic lacunar stroke or TIA as a potential risk factor and repeating the analyses in both the derivation and validation cohorts resulted in identification of the same three predictors plus aspirin use at the time of qualifying stroke became significantly predictive of recurrence in the derivation cohort. (Supplement Table 2b)
Risk stratification for recurrent stroke and effect of interventions by risk status
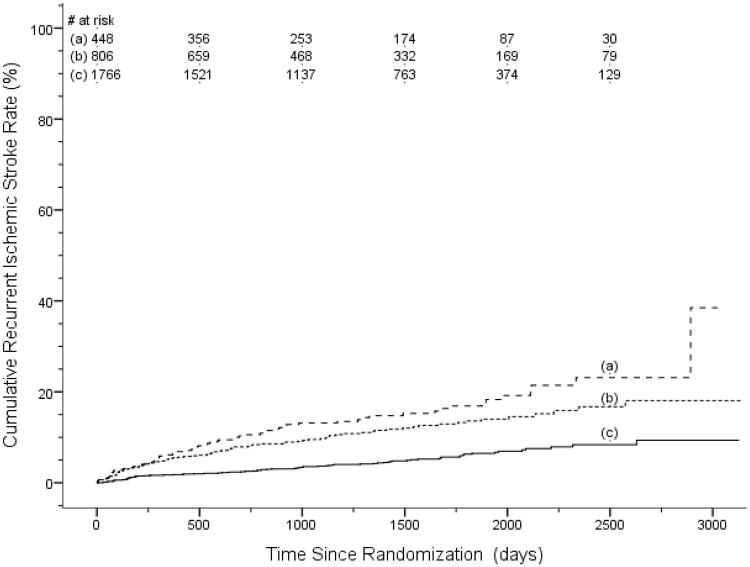
Considering the 4 independent predictors of recurrent ischemic stroke,(Table 2) patients with prior symptomatic lacunar stroke or TIA had the highest rates of recurrent stroke, 4.3%/yr (95% CI 3.3,5.5).(Table 3, Figure 1) Among those without prior symptomatic lacunar stroke or TIA, patients with 2 or 3 of diabetes, male sex and Black race (27% of the cohort) had a recurrent stroke rate of 3.1 %/yr (95%CI 2.6,3.9). Patients with none or a single risk factor of diabetes, male sex or Black race (58% of the cohort) had recurrent stroke rate of 1.3%/yr (95% CI 1.0,1.7).(Table 3) No subgroup of patients had a significant reduction in recurrent stroke rate by either randomized intervention. (Table 4)
Table 3. Risk stratification for recurrent ischemic stroke*.
| Risk Stratum | n | % of cohort | Annualized rate of recurrent ischemic stroke (% per pt-yr) (95%CI) |
|---|---|---|---|
|
| |||
| Moderate to High risk: | |||
| - Prior symptomatic stroke or TIAˆ | 448 | 15 | 4.3 (3.3, 5.5) |
| - No prior symptomatic stroke or TIA and 2 or more of male, Black race or diabetes | 806 | 27 | 3.1 (2.6, 3.9) |
|
| |||
| Low risk: | |||
| No prior symptomatic stroke or TIAˆ | |||
| - any 1 of male, Black race or diabetes | 1256 | 42 | 1.4 (1.1, 1.7) |
| - no risk factors | 510 | 17 | 1.1 (0.7, 1.7) |
|
| |||
| Rates associated with individual independent predictors | |||
|
| |||
| Prior symptomatic stroke or TIA | |||
| - all | 448 | 15 | 4.3 (3.3, 5.5) |
| - only independent risk factor | 72 | 2 | 4.1 (2.3, 7.4) |
|
| |||
| Male sex | |||
| - all | 1622 | 54 | 2.2 (1.8, 2.6) |
| - only independent risk factor | 897 | 30 | 1.4 (1.1, 1.9) |
|
| |||
| Black race | |||
| - all | 406 | 13 | 2.9 (2.2, 3.9) |
| - only independent risk factor | 102 | 3 | 1.0 (0.3, 2.8) |
|
| |||
| Diabetes | |||
| - all | 914 | 30 | 2.7 (2.2, 3.4) |
| - only independent risk factor | 257 | 9 | 1.2 (0.7, 2.2) |
Risk factors are prior symptomatic stroke or transient ischemic attack (TIA), male sex, Black race and diabetes.
Includes 4 participants for whom prior symptomatic lacunar stroke or TIA status was unknown.
Figure 1. Kaplan-Meier curves of recurrent ischemic stroke according the presence of independent risk factors.
(a) prior symptomatic lacunar stroke or TIA, (b) no prior symptomatic lacunar stroke or TIA and 2-3 other risk factors, (c) no prior symptomatic lacunar stroke or TIA and 0-1 other risk factors. Other risk factors are male sex, Black race, and diabetes. Recurrent ischemic stroke rates are significantly different overall (log rank test, p< 0.001) with rates in groups (a) and (b) each significantly different from (c) (p < 0.001). Rates in group (a) and (b) were not significantly different (p = 0.06).
Table 4. Absolute rate reductions in recurrent ischemic stroke according to treatment assignment by the presence of independent risk factors.
| Absolute rate difference (% per pt-yr) | Annualized rate (% per pt-yr) | |||
|---|---|---|---|---|
|
| ||||
| n | Aspirin + placebo vs. aspirin + clopidogrel* | Higher vs. lower blood pressure target* | ||
|
| ||||
| Sex | ||||
| female | 1118 | 0.6 (−0.2, 1.4) | 0.06 (−0.8, 0.9) | 1.8 (1.4, 2.2) |
| male | 1902 | 0.4 (−0.4, 1.1) | 0.5 (−0.2, 1.2) | 2.5 (2.1, 2.9) |
|
| ||||
| Race | ||||
| Non-black | 2528 | 0.5 (−0.04, 1.1) | 0.2 (−0.4, 0.8) | 2.0 (1.7, 2.2) |
| Black | 492 | −0.04 (−1.7, 1.6) | 1.4 (−0.3, 3.0) | 3.4 (2.6, 4.3) |
|
| ||||
| Diabetes | ||||
| No diabetes | 1915 | 0.4 (−0.2, 0.9) | 0.2 (−0.4, 0.8) | 1.6 (1.3, 1.9) |
| Diabetes present | 1105 | 0.5 (−0.7, 1.6) | 0.7 (−0.4, 1.9) | 3.3 (2.8, 4.0) |
|
| ||||
| Prior symptomatic lacunar ischemia | ||||
| No# | 2572 | 0.4 (−0.1, 1.0) | 0.5 (−0.04, 1.1) | 1.9 (1.6, 2.2) |
| Yes | 448 | 0.8 (−1.2, 2.9) | −0.3 (−2.5, 1.8) | 4.3 (3.4, 5.5) |
pt-yr = patient year
RateAspirin+placebo − Rateaspirin + clopidogrel; Rate130-149 mmHg − Rate<130 mmHg
Includes four participants for whom prior symptomatic lacunar stroke or TIA status was unknown.
Factors associated with recurrent lacunar strokes
Of classifiable ischemic stroke recurrences, 70% (136/194) were deemed recurrent lacunar strokes by the central adjudication committee (insufficient data were available to classify ischemic stroke subtype for 49 recurrent ischemic strokes). Significant independent predictors of recurrent lacunar strokes were identical to those for all recurrent ischemic strokes, except that aspirin use at the time of qualifying stroke was statistically significant in the derivation, but not the validation or overall.(Supplement Tables 3 and 4)
Discussion
These analyses of the largest, most carefully followed cohort of patients with MRI-defined lacunar infarction revealed 4 independent predictors of recurrent ischemic stroke: Prior symptomatic lacunar stroke or TIA (HR = 2.2), diabetes (HR = 2.0) and Black race (HR = 1.7) were the strongest and most consistent independent risk factors, with male sex (HR = 1.5) weaker and less consistent.(Table 2) The randomized interventions (i.e. higher vs. lower blood pressure management targets and dual antiplatelet therapy vs. aspirin) performed similarly in patient subgroups with independent risk factors for recurrent ischemic stroke, with no interaction with risk status.(Table 4) Consequently, the best estimates of the effect of the randomized interventions in subgroups defined by risk of recurrence are those for all participants.
It is important to note that these analyses were done in a clinical trial cohort with aggressive blood pressure management, high adherence to antiplatelet therapy, and high prevalence of statin therapy that likely contributed to the relatively low ischemic stroke recurrence rate (2.2%/yr). Neither baseline blood pressure nor classification at entry as normotension, prehypertension, class I hypertension and class II hypertension emerged as independently predictive of recurrent ischemic stroke. However, 90% of participants were categorized as hypertensive at study entry, and excellent blood pressure control during follow-up (systolic blood pressure at the last follow-up visit averaged 131 mmHg among all patients)(12) may have confounded identification of blood pressure at entry as an independent predictor of ischemic stroke recurrence. It was our a priori anecdotal clinical impression that Hispanics with lacunar infarcts had an especially high risk of stroke recurrence. This was not borne-out by these analyses, despite a particularly high frequency of diabetes (42%) among Hispanic participants.(11)
Previous studies of predictors of stroke recurrence in patients with lacunar infarcts have involved smaller numbers of lacunar stroke patients with fewer recurrent strokes.(Table 5) Diabetes has most consistently been associated with recurrent strokes, with hypertension predictive of recurrent stroke in two prior studies.(5,7,8) As in our analysis, age did not emerge from previous studies as an independent predictor of recurrent stroke.
Table 5. Previous studies of clinical predictors of stroke recurrence in patients with lacunar stroke*.
| Study | Design (n) | Statistically Significant Predictors |
|---|---|---|
| Predictors by univariable analysis | ||
| Gandolfo et al. (1986)(20) | Prospective study of 107 lacunar stroke pts with 24 recurrent strokes (at least 2 hemorrhages; m. age = 65 yrs | none |
| Clavier et al. (1994)(18) | Prospective stroke unit case series of 172 lacunar stroke pts with 16 recurrent strokes (14 ischemic); m. age NR. | none |
| Samuelsson et al. (1996)(19) | Inpatient case series of 81 pts with first lacunar stroke and 20 recurrent strokes; m. age = 66 yrs. | age (RR = NR; p < 0.02)# |
| Soda et al. (2004)(8)ˆ | Prospective hospital-based study of 198 Japanese lacunar stroke pts with 12 recurrent strokes; m. age = 70 yrs. | prior stroke (RR 3.4, 95%CI 1.1,11) diabetes (RR 3.4, 95% CI 1.0,11) |
| Mok et al (2009)(21) | 75 Chinese pts with small vessel disease selected from 106 consecutive stroke unit pts with lacunar stroke; of 12 recurrent strokes, 5 were hemorrhages; m age = 71 yrs. | age (HR 1.1, 95%CI 1.0,1.2) NIHSS (HR 1.3, (94%CI 1.1,1.6) systolic BP (HR 1.02, 95%CI 1.0,1.04) hyperhomocystinemia (HR 9.1, 95%CI 1.8, 47) |
| Predictors by multivariable analysis | ||
| Salgado et al. (1996)(6) | Prospective case series of 145 lacunar stroke pts with 30 recurrent strokes; m. age = 65 yrs. | none |
| Staaf et al. (2001)(5)+ | Prospective case series of 178 pts with pure motor lacunar strokes with 42 recurrent strokes; m. age = 73 yrs. | diabetes (p = 0.02) hypertension (p = 0.03) |
| Arboix et al. (2007)(7) | Cross-sectional analysis of a prospective hospital-based registry comparing 573 pts with first lacunar stroke with 122 pts with recurrent lacunar stroke; m.age = 74 yrs. | hypertension (OR 2.0, 95%CI 1.2,3.3) diabetes (OR 1.6, 95%CI 1.1,2.5) hyperlipidemia (OR 0.5, 95%CI 0.3,0.9) |
| SPS3 (current study)(2013) | Clinical trial cohort of 3020 pts with lacunar stroke with 243 recurrent ischemic strokes; m. age = 63 yrs. | male sex (HR1.5 , 95%CI 1.1,1.9) Black race (HR 1.7, 95%CI 1.3,2.3) diabetes (HR 2.0, 95%CI 1.5,2.5) prior lacunar stroke/TIA (HR 2.2, 95%CI 1.6,2.9) |
Pts = patients; m. age = mean age; BP = blood pressure; yrs = years; OR = odds ratio; RR = relative risk; HR = hazard ratio; NR = not reported.
Studies published since 1980 in which the diagnosis of lacunar stroke based on brain imaging; imaging predictors were not considered. An additional study based on MRI evidence of prior silent lacunes reported diabetes and elevated hematocrit to be independently associated with multiple lacunar strokes.(22)
Adjusted for age and sex.
Prior stroke was not included as a variable.
The OR for male sex was 2.1, for diabetes 2.0 and hypertension 0.5; none statistically significant.
Independent predictors for recurrent ischemic stroke identified in this cohort of patients with recent lacunar infarcts overlap in part with well-defined predictors of stroke in patients with atrial fibrillation: prior stroke/TIA and diabetes.(15) However, age and female sex are important and consistent predictors of stroke in atrial fibrillation patients, supporting distinct stroke mechanisms.(15,16) Prior symptomatic lacunar ischemia, the most powerful predictor to emerge from these analyses, is not a patho-etiologic cause of recurrence per se, but more likely a marker of severity of underlying cerebral small artery disease. Prior symptomatic lacunar ischemia was strongly correlated with diabetes (p=0.004) and a history of hypertension (p<0.001) in the SPS3 cohort.(Supplement Table 5) However, omitting prior symptomatic lacunar stroke or TIA as a potential risk factor neither strengthened the association between the remaining risk predictors with recurrent ischemic stroke nor resulted in any of the hypertension-related variables becoming predictive.(Supplememnt Tables 2a and 2b). Hence, we hypothesize that prior symptomatic lacunar stroke or TIA is a surrogate for important, as yet unidentified, factors that were not captured in these analyses that may offer clues to stroke pathogenesis and prevention. The presence of asymptomatic lacunar infarcts detected by neuroimaging may further contribute to stratification of the risk of recurrent stroke (17) and will be explored in subsequent analyses of the SPS3 cohort.
Despite vigorous treatments aimed at secondary stroke prevention employed in these participants closely followed in a clinical trial (and hence exceeding that anticipated in most clinical practice settings), these analyses identified 42% of patients with recent lacunar stroke with substantial (>3%/yr) rates of ischemic stroke recurrence.(Table 3) For this large fraction of patients with lacunar stroke, exploring additional novel stroke prevention strategies is warranted.
Supplementary Material
Acknowledgments
Funding Source: National Institute of Neurological Disorders and Stroke (U01 NS38529-04A1)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Robert G. Hart, Department of Medicine (Neurology), McMaster University, Hamilton, Ontario, Canada
Lesly A. Pearce, Biostatistics Consultant, Minot, North Dakota USA
Majid F. Bakheet, Department of Neurology, Taiba University, Medina, Saudi Arabia
Oscar Benavente, Department of Neurology, University of British Columbia, Vancouver, British Columbia, Canada
Robin A. Conwit, National Institute of Neurological Disorders and Stroke, Rockville, Maryland USA
Leslie A. McClure, Department of Biostatistics, University of Alabama at Birmingham, Birmingham, Alabama USA
Robert L. Talbert, Department of Clinical Pharmacy, University of Texas, Austin, Texas USA
David C. Anderson, Department of Neurology, University of Minnesota, Minneapolis, Minnesota USA
References
- 1.Norrving B. Long-term prognosis after lacunar infarction. Lancet Neurol. 2003;2:38–45. doi: 10.1016/s1474-4422(03)00352-1. [DOI] [PubMed] [Google Scholar]
- 2.Bogousslavsky J, Van Melle G, Regli F. The Lausanne Stroke Registry: analysis of 1,000 consecutive patients with first stroke. Stroke. 1988;19:1083–92. doi: 10.1161/01.str.19.9.1083. [DOI] [PubMed] [Google Scholar]
- 3.Kolominsky-Rabas PL, Weber M, Gefeller O, Neundoerfer B, Heuschmann PU. Epidemiology of ischemic stroke subtypes according to TOAST criteria: incidence, recurrence, and long-term survival in ischemic stroke subtypes: a population-based study. Stroke. 2001;32:2735–40. doi: 10.1161/hs1201.100209. [DOI] [PubMed] [Google Scholar]
- 4.Wolfe CDA, Rudd AG, Howard R, Coshall C, Stewart J, Lawrence E, et al. Incidence and case fatality rates of stroke subtypes in a multiethnic population: the South London Stroke Register. J Neurol Neurosurg Psych. 2002;72:211–6. doi: 10.1136/jnnp.72.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Staaf G, Lindgren A, Norrving B. Pure motor stroke from presumed lacunar infarct Long-term prognosis for survival and risk of recurrent stroke. Stroke. 2001;32:2592–6. doi: 10.1161/hs1101.098355. [DOI] [PubMed] [Google Scholar]
- 6.Salgado AV, Ferro JM, Gouveia-Oliveira A. Long-term prognosis of first-ever lacunar stroke. Stroke. 1996;27:661–6. doi: 10.1161/01.str.27.4.661. [DOI] [PubMed] [Google Scholar]
- 7.Arboix A, Font A, Garro C, Garcia-Eroles L, Comes E, Massons J. Recurrent lacunar infarction following a previous lacunar stroke: a clinical study of 122 patients. J Neurol Neurosurg Psych. 2007;78:1392–4. doi: 10.1136/jnnp.2007.119776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soda T, Nakayasu J, Maeda M, Kusumi M, Kowa H, Awaki E, Saito J, Nakashima K. Stroke recurrence within the first year followig cerebral infarction – Tottori University Lacunar Infarction Prognosis Study (TULIPS) Acta Neurol Scand. 2004;110:343–9. doi: 10.1111/j.1600-0404.2004.00290.x. [DOI] [PubMed] [Google Scholar]
- 9.Wang R, Lagakos SW, Ware JH, et al. Statistics in Medicine – reporting of subgroup analyses in clinical trials. N Engl J Med. 2007;357:2189–92. doi: 10.1056/NEJMsr077003. [DOI] [PubMed] [Google Scholar]
- 10.Benavente OR, White C, Pearce LA, Pergola P, Roldan A, Benavente MF, Coffey C, McClure L, Szychowski JM, Conwit R, Heberling PA, Howard G, Bazan C, Vidal-Pergola G, Talbert RL, Hart RG for the SPS3 Investigators. Secondary Prevention of Small Subcortical Strokes (SPS3) Study. International J Stroke. 2011;6:164–75. doi: 10.1111/j.1747-4949.2010.00573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.White CL, Szychowski JM, Roldan A, Benavente MF, Pretell EJ, Del Brutto OH, et al. Clinical features and racial/ethnic differences among 3020 participants in the Secondary Prevention of Small Subcortical Stroke trial. J Stroke Cerebrovas Dis. 2012 doi: 10.1016/j.jstrokecerebrovasdis.2012.03.002. on-line April 17th. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Writing Group for the SPS3 Investigators. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med. 2012;367:817–25. doi: 10.1056/NEJMoa1204133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.SPS3 Investigators. Effects of blood pressure targets in patients with recent lacunar stroke. Lancet. 2013 doi: 10.1016/S0140-6736(13)60852-1. accepted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levey AS, Stevens LA, Schmid CH, Zhang Y, Castro AF, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J for the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stroke Risk in Atrial Fibrillation Working Group. Independent predictors of stroke in patients with atrial fibrillation: A systematic review. Neurology. 2007;69:546–54. doi: 10.1212/01.wnl.0000267275.68538.8d. [DOI] [PubMed] [Google Scholar]
- 16.Hart RG, Eikelboom JW, Pearce LA. Sex, stroke, and atrial fibrillation. Arch Neurol. 2012 doi: 10.1001/archneurol.2012.2691. online Sept 24th, 2012. [DOI] [PubMed] [Google Scholar]
- 17.de Jong G, Kessels F, Lodder J. Two types of lacunar infarcts. Further arguments from a study on prognosis. Stroke. 2002;33:2072–6. doi: 10.1161/01.str.0000022807.06923.a3. [DOI] [PubMed] [Google Scholar]
- 18.Clavier I, Hommel M, Besson G, Noèlle B, Ferjus Perret JE. Long-term prognosis of symptomatic lacunar infarcts: a hospital-based study. Stroke. 1994;25:2005–9. doi: 10.1161/01.str.25.10.2005. [DOI] [PubMed] [Google Scholar]
- 19.Samuelsson M, Lindell D, Norrving B. Presumed pathogenetic mechanisms of recurrent stroke after lacunar infarction. Cerebrovas Dis. 1996;6:128–136. [Google Scholar]
- 20.Gandolfo G, Moretti C, Dall'Agata D, Primavera A, Brusa G, Loeb C. Long-term prognosis of patients with lacunar syndromes. Acta Neurol Scand. 1986;74:224–9. doi: 10.1111/j.1600-0404.1986.tb07859.x. [DOI] [PubMed] [Google Scholar]
- 21.Mok VCT, Lau AYL, Wong A, Lam WWM, Chan A, Leung H, et al. Long-term prognosis of Chinese patients with lacunar infarct associated with small vessel disease: a five-year longitudinal study. Internat J Stroke. 2009;4:81–8. doi: 10.1111/j.1747-4949.2009.00262.x. [DOI] [PubMed] [Google Scholar]
- 22.Arauz A, Murillo L, Cantu C, Barinagarrementeria F, Higuera J. Prospective study of single and multiple lacunar infarcts using magnetic resonance imaging: risk factors, recurrence, and outcome in 175 consecutive cases. Stroke. 2003;34:2453–8. doi: 10.1161/01.STR.0000090351.41662.91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.