Abstract
Complex motivated behavioral processes, such as those that can go awry following substance abuse and other neuropsychiatric disorders, are mediated by a distributive network of neurons that reside throughout the brain. Neural circuits within the amygdala regions, such as the basolateral amygdala (BLA), and downstream targets such as the bed nucleus of the stria terminalis (BNST), are critical neuroanatomical structures for orchestrating emotional behavioral responses that may influence motivated actions such as the reinstatement of drug seeking behavior. Here, we review the functional neurocircuitry of the BLA and the BNST, and discuss how these circuits may guide maladaptive behavioral processes such as those seen in addiction. Thus, further study of the functional connectivity within these brain regions and others may provide insight for the development of new treatment strategies for substance use disorders.
Introduction
The amygdala, located within the medial temporal lobe, is divided into at least 13 distinct subnuclei; the most clearly defined being the basolateral amygdala (BLA), the lateral amygdala (LA), and the central amygdala (CeA) (Amaral and Price 1984; Amunts et al. 2005). The CeA connects the amygdala proper with the extended amygdala, located between the amygdala and the nucleus accumbens (NAc). The extended amygdala is comprised of the bed nucleus of the stria terminalis (BNST) as well as other interconnected nuclei such as the dorsal substantia innominata (Cassell et al. 1999).
Primary functions of the amygdala include emotional learning and regulation (Phelps and LeDoux 2005), memory formation (Packard and Cahill 2001), and reward processing (Baxter and Murray 2002). The BNST is made up of a vast array of cell types including GABAergic and glutamatergic efferent populations, as well as GABAergic and cholinergic interneurons (Ju and Swanson 1989; Ju et al. 1989). Overlapping with these populations are cells expressing an assortment of neuropeptides including NPY, CRF, enkephalin, dynorphin, and substance P (Kozicz et al. 1997). The BNST is involved in sustained fear behaviors (Walker and Davis 2008; Walker et al. 2009), anxiety-like behaviors (Walker and Davis 1997; Cecchi et al. 2002; Walker and Davis 2008), and stress induced reinstatement of drug seeking (Erb et al. 2000; Erb et al. 2001; Erb et al. 2001; Wang et al. 2001).
Human neuroimaging studies have provided strong evidence for the role of the amygdala and extended amygdala structures in drug and alcohol addiction. A meta-analysis of data collected from fMRI and PET studies revealed that the amygdala and nucleus accumbens (NAc), an area that receives dense innervation from the amygdala, show the most robust neural activation in response to drug-associated cues (Chase et al. 2011). Additionally, two functional fMRI studies found correlations between NAc activity and drug cravings (Kufahl et al. 2005; Risinger et al. 2005). Changes in the amygdala have also been associated with alcohol use disorders. An MRI study found that the amygdala is smaller in children of parents with alcohol use disorders (Hill et al. 2001). Furthermore, gray matter is decreased in the medial prefrontal cortex (mPFC), a region that receives strong innervation from the amygdala, in patients with alcohol use disorders (Pfefferbaum et al. 1998).
In this review we outline amygdala and BNST afferent and efferent connectivity, as well as animal studies that have implicated these circuits in the development and maintenance of drug addiction.
Role of BLA in addiction
The amygdala is thought to be necessary for attributing emotional value to cues that predict salient events. The BLA, in particular, has an integral role in processing affective states (Phelps and LeDoux 2005). Importantly, the BLA has been implicated as a critical modulator of reinstatement of drug seeking in rodents (Fuchs et al. 2005). Lesions of the BLA disrupt cue-induced reinstatement of cocaine self-administration (Meil and See 1997), conditioned reinforcement for a natural reward (Burns et al. 1993), and disrupts the acquisition of cocaine seeking during a second order schedule of reinforcement (Whitelaw et al. 1996). However, BLA lesions alone do not disrupt self-administration for cocaine (Meil and See 1997) or natural reinforcers (Corbit and Balleine 2005), suggesting the BLA plays a critical role in secondary reinforcement of natural rewards and drugs of abuse.
BLA afferents
The BLA receives strong innervation from the thalamus, hippocampus, and medial prefrontal cortex (Fig 1A) (Ottersen 1982; Albanese and Minciacchi 1983; van Vulpen and Verwer 1989). The BLA also receives dopaminergic innervation from the ventral tegmental area (VTA, Fig 1A) (Albanese and Minciacchi 1983), and this circuit may underlie behavioral changes in drug addiction. Dopamine receptor antagonist in the BLA blocks cued reinstatement of drug seeking behavior (See et al. 2001). Likewise, studies have shown increases in extracellular dopamine in the BLA during the presentation of a cue that predicts a cocaine reinforcer (Weiss et al. 2000). Dopamine receptor activation in vivo increases the firing rate of fast-spiking interneurons, suggesting that dopamine release in the BLA decreases the firing rate of BLA projection neurons (Rosenkranz and Grace 1999). Thus, dopamine's role in cued reinstatement in the BLA is may be through suppressing BLA output.
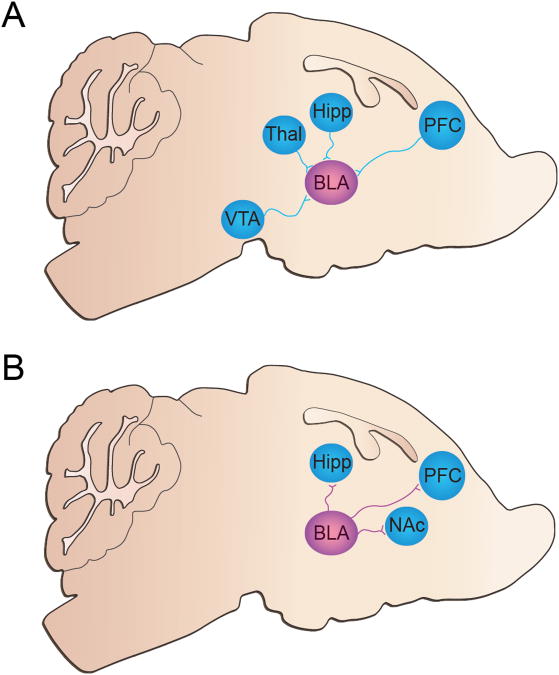
Figure 1.
Schematic detailing BLA afferent and efferent connectivity (A) The BLA receives inputs from the ventral tegmental area (VTA), thalamus (Thal), hippocampus (Hipp), and prefrontal cortex (PFC). (B) The BLA sends projections to the Hipp, PFC, and nucleus accumbens (NAc).
BLA efferents
BLA projections to the nucleus accumbens
Neurotransmission between the BLA and NAc is critically involved in reward-seeking behavior and conditioned reinforcement (Everitt et al. 1999). The BLA sends a dense glutamatergic projection to inhibitory medium spiny neurons in the shell and core of the NAc (Fig. 1B) (Kelley et al. 1982; French and Totterdell 2003; Britt et al. 2012). Interestingly, other striatal sub-regions such as the dorsal medial and dorsal lateral striatum receive substantially less BLA innervation (Stuber et al. 2011; Britt et al. 2012). This implies that BLA inputs to striatum are preferential for ventral striatal regions known to be critical in driving motivated behavioral state. Further, BLA terminals in the NAc have lower glutamatergic release probability than other glutamatergic inputs, such as the prefrontal cortex and ventral hippocampus (Britt et al. 2012), suggesting that this circuit may function as a high-pass filter, implying that burst firing of BLA neurons may be necessary to activate postsynaptic NAc neurons.
Recent studies have demonstrated that BLA excitatory drive onto NAc neurons facilitates reward-seeking behavior. Using a disconnection experiment, Ambroggi et al. (2008) found that the interaction of BLA and dopamine afferents in the NAc facilitates reward-seeking behavior in a discriminative stimulus response task. While both BLA and NAc neurons responded to cues that predicted a reward, the BLA neuronal responses preceded those of the NAc responses, suggesting that the neuronal activity of BLA neurons is driving the NAc neurons to promote reward-seeking behavior (Ambroggi et al. 2008). Supporting this hypothesis, the authors found that intact BLA-to-NAc circuitry is necessary for the dopamine-mediated incentive cue-induced neuronal responses in the NAc (Ambroggi et al. 2008). Using optogenetics, we found that found that excitatory transmission from the BLA to the NAc is both sufficient and necessary for reward seeking behavior (Stuber et al. 2011). In vivo optical stimulation of BLA glutamatergic terminals in the NAc promoted self-stimulation, while optical inhibition of this pathway reduced reward-seeking for sucrose. We also found that the reinforcing effects of BLA terminal stimulation in the NAc require dopamine signaling in the NAc (Stuber et al. 2011), further highlighting a critical interaction between BLA glutamate release and dopamine signaling within the NAc.
Neurotransmission between the BLA and NAc is also involved in reward-seeking behavior for drugs of abuse. Studies have indicated that intact BLA-to-NAc circuitry is necessary for cocaine-seeking behavior (Di Ciano and Everitt 2004), and is critical in modulating opioid reward salience (Lintas et al. 2012). Collectively, these studies support the hypothesis that BLA excitatory drive onto NAc postsynaptic neurons is reinforcing, and that the connectivity between the BLA and NAc facilitates behavioral responding to salient cues.
Along with directly regulating medium spiny neurons in the NAc, BLA neurons also modulate dopamine terminal release in the NAc. In anesthetized experiments, electrical stimulation of the BLA resulted in dopamine efflux in the NAc (Floresco et al. 1998). In vivo, BLA modulation of dopamine terminals in the NAc is likely important for encoding a cue that predicts a reinforcer (Jones et al. 2010).
The studies discussed above demonstrate that BLA excitatory drive onto NAc GABAergic neurons, and BLA modulation of dopamine terminal release, is critical for reward-seeking behavior and attributing emotional value to reward-predicting cues. While BLA glutamate release may directly regulate presynaptic dopamine release in the NAc, it is also likely that BLA inputs indirectly control dopamine release by activating NAc projection neurons that feed back to the ventral midbrain (Xia et al. 2011; Watabe-Uchida et al. 2012). Due to the significant role that this circuit has on reward-seeking behavior, is also likely a critical circuit that becomes deregulated during the development of drug addiction.
BLA projections to the hippocampus
Along with robust projections to the NAc, the BLA also sends a dense excitatory projection to hippocampal regions (Fig 1B). Areas that receive the strongest innervations from the BLA include the entorhinal cortex, the ventral subiculum, and the perirhinal cortex (Pitkanen et al. 2000). Most of the studies investigating BLA-to-hippocampus circuitry have focused on fear conditioning and functional changes in the circuit following exposure to aversive stimuli. Exposure to various uncontrollable and unpredictable stressors has been shown to disrupt LTP in the CA1 region of the hippocampus (Shors et al. 1989; Diamond and Rose 1994; Xu et al. 1997). BLA lesions block this stress-induced impairment in LTP (Kim et al. 2005). Consistent with these findings, another study found that contextual fear conditioning increased arc and c-fos, products of immediate-early genes, expression in the hippocampus, which was significantly reduced following BLA pharmacological inactivation (Huff et al. 2006). Immediate early genes are thought to represent changes in synaptic strength, suggesting that amygdala drive onto hippocampal neurons may modulate synapses necessary for processing aversive memories. Fear conditioning reliably induces a physiological stress response (Rodrigues et al. 2009), and exposure to stress plays a vital role in drug abuse and relapse (Sinha 2001). Thus, neural circuits that show physiological changes in response to fear conditioning and stress are likely key modulators of stress-induced relapse.
In addition to stress, cues paired with drug self-administration contribute to relapse like behavior (Weiss et al. 2001). A recent study suggests that the BLA-to-hippocampus pathway may also play a role in cue-induced relapse. Using a disconnection procedure, Wells et al. (2011) found that intact connectivity between the BLA and the dorsal hippocampus is necessary for cocaine memory reconsolidation and subsequent cue-induced cocaine self-administration. Collectively, these studies demonstrate that amygdalar modulation of the hippocampus is critical for both aversive and appetitive emotional memories, both of which can become pathological in drug addiction.
BLA projections to the mPFC
The medial prefrontal cortex (mPFC) is likely critical in processing emotionally salient information, as it facilitates to promote appropriate behavioral responding to these cues. Deregulation of the mPFC is thought to occur in many neuropsychiatric disorders, such as schizophrenia, depression, mood and anxiety disorders, as well as addiction (Kalivas 2009; Benes 2010; Goto et al. 2010; Price and Drevets 2010). The mPFC, comprised of glutamatergic pyramidal neurons and GABAergic interneurons, forms extensive connections with distributed subcortical brain regions including a functional reciprocal connection with the BLA (Fig 1B). Excitatory projections from glutamatergic neurons in the BLA synapse preferentially onto glutamatergic and some GABAergic postsynaptic targets within layers 2/3/5 of the infralimbic, prelimbic, and orbitofrontal regions of the mPFC (Bacon et al. 1996; Peters et al. 2009). The infralimbic region of the mPFC also sends glutamatergic projections back to GABAergic cells of the CeA and to intercalated (ITC) cell masses within the amygdala, while the prelimbic region of the mPFC projects primarily to pyramidal cells in the BLA (Pare and Smith 1993; McDonald et al. 1996). Studies have implicated the BLA-to-mPFC pathway as integral for processing information about cues related to appetitive and aversive environmental stimuli that come to drive conditioned behavioral responses (Quirk and Mueller 2008).
A hallmark of many neuropsychiatric disorders, including drug addiction, is cognitive inflexibility and exaggerated behavioral responses to emotionally salient environmental cues. Analogously, mice that learn an appetitive Pavlovian conditioning task display high inflexible stimulus-driven behavior following extended training. Studies in rats have shown that extinction of conditioned fear, a form of cognitive flexibility whereby a new learned association is formed that overrides a previous stimulus-driven behavioral response, depends on an intact mPFC (Morgan et al. 1993; Morgan and LeDoux 1995; Morgan and LeDoux 1999), and that communication between the amygdala and mPFC suppresses conditioned fear responses (Garcia et al. 1999). Concerning the extinction of inflexible responses to appetitive stimuli, a neural circuit including regions of the mPFC and BLA has been heavily implicated in the extinction of drug-seeking behaviors (Fuchs et al. 2007; Peters et al. 2008; Fuchs et al. 2009). Another feature of drug addiction that this neural circuit is thought to be critically involved in is impulsive, stimulus-driven behavioral responses to drug-related cues. Supporting this hypothesis, functional disruption of the BLA-to-mPFC significantly increased risky-choice decision-making in rats, mirroring the impulsivity seen in addiction (Churchwell et al. 2009; St Onge et al. 2012).
Taken together, these results demonstrate the prominent role of the BLA-mPFC pathway in the proper processing of emotional learning in response to salient cues in the environment.
Role of BNST in addiction
The BNST is considered to be a connective center between stress regions, including the BLA, CeA, medial amygdala (MeA) and the paraventricular nucleus of the hypothalamus (PVN), and brain reward centers, such as the ventral tegmental area (VTA) and nucleus accumbens (NAc) (Silverman et al. 1981; Brog et al. 1993; Georges and Aston-Jones 2001; Georges and Aston-Jones 2002; Jalabert et al. 2009). Importantly, the BNST is a critical modulator of addiction-like behavior (Aston-Jones and Druhan 1999; Leri et al. 2002). Chronic morphine administration increases ΔFosB levels in the BNST (Nunez et al. 2010). Pharmacological inactivation of the BNST reduces both cue- and stress-induced reinstatement of cocaine seeking (McFarland et al. 2004; Buffalari and See 2011). Multiple electrophysiological studies have demonstrated robust synaptic plasticity can be induced within the BNST, which is altered by drugs of abuse (Weitlauf et al. 2004). For example, cocaine self-administration increases AMPA/NMDA ratio, a marker of excitatory synaptic strength, in the BNST (Dumont et al. 2005). Additionally, chronic intermittent ethanol exposure increases NMDA receptor efficacy via an upregulation in NR2B, which in turn potentiates the induction of long term potentiation (LTP) (Kash et al. 2009; Wills et al. 2012). Taken together, these data suggest that chronic drug administration can increase excitatory synaptic drive into the BNST. However, because the BNST is a heterogeneous mix of different cell types and sub nuclei, parceling out the discrete neurons and subcircuits that are enhanced by drug of abuse has presented a challenge.
BNST afferents
Glutamatergic and GABAergic inputs to the BNST
The BNST receives dense glutamatergic and GABAergic innervation from widespread brain regions. Major excitatory inputs to the BNST include several cortical projections including the caudal infralimbic cortex, prelimbic cortex, insula cortex, entrorhinal cortex, and caudal orbital PFC (Fig 2A) (McDonald 1998). Additional glutamate inputs arise from the hippocampus via the ventral subiculum, the BLA, parabrachial nucleus, the accessory olfactory bulb and the main olfactory bulb (Fig 2A) (Cullinan et al. 1993; McDonald 1998). A recent study examined the role of BLA glutamatergic inputs to the BNST on anxiety-like behavior (Kim et al. 2013). Surprisingly, photostimulation of BLA glutamatergic inputs to the anterodorsal (ad) BNST resulted in the reduction of behavioral anxiety as well as respiratory rate. Conversely, photoinhibition of the same pathway caused behavioral anxiogenesis and increased respiratory rate.
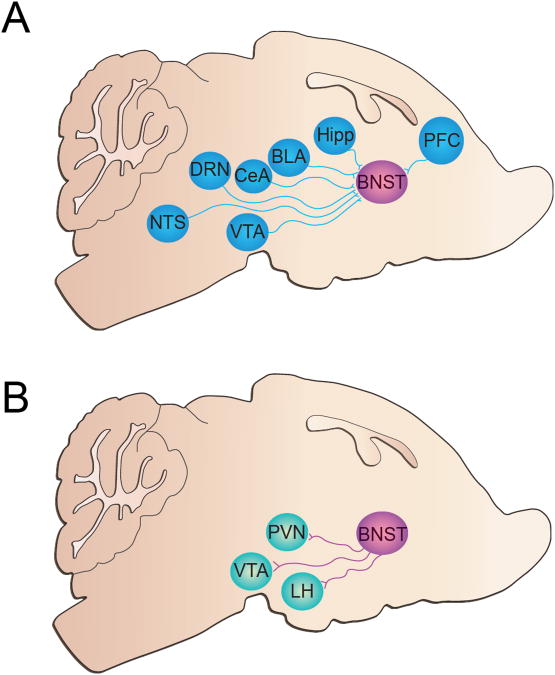
Figure 2.
Schematic detailing BNST afferent and efferent connectivity (A) The BNST receives inputs from the ventral tegmental area (VTA), nucleus tractus solitaries (NTS), dorsal raphe nucleus (DRN), central amygdala (CeA), basolateral amygdala (BLA), hippocampus (Hipp), and prefrontal cortex (PFC). (B) The BNST sends projections to the paraventricular nucleus of the hypothalamus (PVN), VTA, and lateral hypothalamus (LH).
The main source of GABAergic drive to the BNST comes from the CeA to which it sends reciprocal projections (Fig 2A) (Dong et al. 2001; Li et al. 2012). Using a pharmacological inactivation approach, Walker and Davis (1997) demonstrated that the CeA is involved in conditioned fear, while the BNST is important in mediating unconditioned fear responses. However, subsequent studies have revealed that the duration of exposure to a learned cue regulates which part of the extended amygdala governs the expression of fear behavior. While brief exposure to a cue recruited CeA, sustained presentation of a cue (on the order of several minutes) recruited the BNSTs involvement in the behavioral manifestation of the learned fear response (Walker and Davis 2008). Furthermore, pharmacological studies with CRFR1 antagonists suggest that the feedback circuitry between the BNST and CeA is actively involved in the sustained fear system (BNST) inhibiting the phasic fear system (CeA) (Walker et al. 2009). Therefore, alterations in this circuitry, perhaps following exposure to drugs of abuse, could lead to dramatic psychopathologies by increasing behavioral responding to perceived vs. actual threats.
Dopaminergic inputs to the BNST
The dorsal lateral (dl) dlBNST receives dopaminergic input from the VTA and PAG (Fig 2A) (Meloni et al. 2006). Microdialysis studies have shown that all major drugs of abuse have the ability to release dopamine within the BNST, and recent studies have shown that dopamine is released in the BNST to natural rewards as well as predictive cues associated with intracranial self-stimulation of the medial forebrain bundle (Park et al. 2012; Park et al. 2012). Furthermore, dopamine signaling within the dlBNST in part mediates the reinforcing effects of multiple drugs of abuse (Carboni et al. 2000). In vivo or in vitro cocaine increases glutamatergic efficacy at synapses in the dlBNST via a presynaptic DR1 to CRFR1 dependent mechanism (Kash et al. 2008). Coincidentally, dopamine reduces inhibitory signaling in the oval nucleus of the BNST via D2 receptors (Krawczyk et al. 2011). Following cocaine self-administration, but not yoked cocaine or self-administration of sucrose, however, there is a switch in this mechanism whereby dopamine signaling now results in a D1 receptor dependent increase in inhibitory transmission that persists through an extensive abstinence period (Krawczyk et al. 2011).
Noradrenergic inputs to the BNST
The BNST also receives a strong projection of noradrenergic (NE) neurons from the nucleus of the tractus solitarius (NTS – A2 cell group), the ventral lateral medulla (VLM – A1 cell group) via the ventral noradrenergic bundle (VNAB) (Forray and Gysling 2004), and the locus coeruleus (LC) (Fig 2A) (Myers et al. 2005). NE signaling (from the VNAB) within the BNST influences several addiction-related behaviors including conditioned place aversion to a chamber previously paired with opiate withdrawal and stress-induced reinstatement to drug seeking (Delfs et al. 2000; Erb et al. 2000; Wang et al. 2001). In addition NE signaling in the BNST mediates stress-induced anxiety-like behavior (Cecchi et al. 2002).
Noradrenaline modulates BNST GABAergic and glutamatergic signaling in a complex manor. Signaling at α2 adrenergic receptors (α2-ARs) can inhibit both GABAergic and glutamatergic projections (Egli et al. 2005; Shields et al. 2009). Meanwhile, signaling at α1- and β-ARs can increase both GABAergic and glutamatergic transmission through presynaptic mechanisms (Dumont and Williams 2004; Egli et al. 2005; McElligott et al. 2010; Nobis et al. 2011). Interestingly, NE signaling in brain slices from morphine dependent rats increased IPSC frequency via a β-AR dependent mechanism, while activation of α1-ARs increased frequency in both morphine dependent and control groups (Dumont and Williams 2004). Moreover, activation of α1-ARs in the BNST can induce a long term depression (LTD) of glutamatergic signaling (McElligott and Winder 2008). Although also coupled to Gq G-protein receptors, this LTD is expressed and maintained by different mechanism than the mGluR5 LTD discussed above, and is not sensitive to treatment with cocaine but induced by chronic stress (McElligott et al. 2010). This suggests that the two LTDs may be expressed on different synapses. While it is currently unknown precisely how these synaptic mechanisms contribute to the reinstatement and learned aversion discussed above, plastic alterations downstream of NE signaling remains an intriguing possibility for learned drug and/or withdrawal associations.
Serotonergic input to the BNST
The dorsal BNST receives serotonergic (5-HT) input via the dorsal raphe (Fig 2A) (Phelix et al. 1992). Interestingly, recent studies from the Rainnie group have shown a differential distribution of 5-HT receptors across multiple cell types within the anterolateral BNST and that unpredictable stress alters the expression of 5-HT receptors within these neurons (in particular decreases in 5-HT1A, increases in 5-HT1B and 5-HT7) (Guo et al. 2009). Congruent with this, 5-HT1A activation in the BNST has been shown to reduce anxiety-like behavior (Levita et al. 2004), while 5-HT2C agonist in the BNST results in anxiogenesis (Fox et al. 2008), suggesting opposing roles for these receptors in BNST function. Very recent studies have suggested that enhanced serotonergic signaling selectively within the BNST potentiates fear learning (Ravinder et al. 2013). Although 5-HT signaling in the BNST has not been examined with respect to addiction behaviors, the co-morbidity between addiction and anxiety disorders (Koob 2009) would suggest that this modulatory system could strongly impact drug-seeking behaviors.
Peptidergic input to the BNST
Several neuropeptides including corticotrophin releasing factor (CRF), neuropeptide Y (NPY), dynorphin, and orexin also innervate and modulate activity within the BNST and have been implicated in features of drug addiction. Early lesion studies suggest that the BNST receives CRF from the CeA (Sakanaka et al. 1986), however this has yet to be tested using more modern genetic approaches. Furthermore, the BNST contains CRF neurons that have the potential to release CRF locally within the nucleus (Shepard et al. 2006). Infusions of CRF directly into the BNST promote stress-induced cocaine reinstatement, whereas intra-BNST administration of CRF antagonists blocks reinstatement of cocaine seeking and morphine conditioned place preference following foot shock (Erb and Stewart 1999; Leri et al. 2002; Wang et al. 2006). CRF mRNA is increased in the dorsal BNST following foot-shock induced reinstatement of heroin seeking (Shalev et al. 2001). Moreover, increases in extracellular CRF are observed in the BNST during acute withdrawal from ethanol (Olive et al. 2002).
Akin to CRF, several regions that express NPY have been shown to project to the BNST (for example the medial amygdala, CeA, and NTS); however, the BNST also contains NPY immunoreactive cells, which could release NPY locally (Sakanaka et al. 1986; Ishizaki et al. 2003). Within the BNST, NPY has been shown to constrain GABA release via a NPY-Y2 receptor mechanism (Kash and Winder 2006). Interestingly, this modulation is disrupted following chronic stress in a stress/anxiety prone mouse strain but not a resilient strain (Pleil et al. 2012).
The endogenous opiate dynorphin is expressed in BNST neurons and also in fibers that innervated the BNST from the CeA (Marchant et al. 2007). Systemic administration of a kappa opioid receptor (KOR) selective agonist increases metabolism in the BNST (Hooker et al. 2009). Additionally, KOR activation reduces GABAergic transmission from the CeA, via a novel presynaptic mechanism involving the recruitment of extracellular signal regulated kinase (ERK), suggesting that KORs are expressed on presynaptic CeA afferents in the BNST (Li et al. 2012). These data, along with evidence supporting a synergistic effect of dynorphin and CRF systems (Bruchas et al. 2009), suggest that dynorphin signaling in the BNST may promote anxiogenesis.
Orexin-A modulation in the BNST increases anxiety-like behaviors and depolarize neurons via a NMDAR dependent mechanism (Lungwitz et al. 2012). The BNST also receives a dense orexinergic projection from the lateral hypothalamus (Baldo et al. 2003). Yohimbine, while classically thought to inhibit α2-adrenergic receptors, impairs extinction from cocaine conditioned place preference via an orexin 1 receptor (OX(1)-R) dependent mechanism. Moreover, yohimbine can depress excitatory transmission in the BNST via an OX(1)-R dependent mechanism (Conrad et al. 2012).
BNST efferents
BNST projections to the Ventral Tegmental Area
The BNST sends both a GABAergic and glutamatergic projection to the VTA, as observed in retrograde tracing and electrophysiological studies (Fig 2B) (Cullinan et al. 1993; Georges and Aston-Jones 2001; Georges and Aston-Jones 2002; Jalabert et al. 2009), although a recent study using retrograde tracing and electron microscopy techniques showed that this projection is primarily GABAergic (Kudo et al. 2012). Recently, we demonstrated that these GABAergic and glutamatergic BSNT projection neurons synapse onto putative VTA GABA neurons (Jennings et al. 2013). Kudo et al. (2012) confirmed this finding anatomically by demonstrating that BNST GABAergic and glutamatergic terminals made synaptic contact predominantly with tyrosine hydroxylase negative and GAD67 positive dendrites within the VTA. VTA GABAergic neurons robustly inhibit VTA dopaminergic neurons (van Zessen et al. 2012), and thus these BNST projections are likely inhibiting (glutamatergic projection) or exciting (GABAergic projection) VTA dopaminergic neurons. Our lab also found that activation of BNST GABAergic terminals in the VTA is rewarding and anxiolytic, while activation of BNST glutamatergic terminals in the VTA is aversive and anxiogenic (Jennings et al. 2013). Importantly, we showed that direct photoinhibition of VTA GABAergic neurons resulted in both rewarding and anxiolytic phenotypes, which recapitulated the findings from our BNST-VTA GABAergic terminal behavioral manipulations. Additionally, we optically identified VTA-projecting BNST glutamatergic and GABAergic neurons in vivo and showed that these two distinct neuronal populations displayed opposing firing profiles in response to an aversive foot-shock stimulus. We found that VTA-projecting BNST glutamatergic neurons displayed a net enhancement of activity, whereas the firing rate of VTA-projecting BNST GABAergic neurons was predominately suppressed. Interestingly, the firing profiles of both VTA-projecting GABAergic and glutamatergic BNST neurons were similar between the foot-shock sessions and re-exposure to the context in the absence of the foot-shock stressor. Collectively, these data indicate that multiple BNST subcircuits to the VTA may play a prominent role in modulating different components of drug addiction.
Several studies have demonstrated that drugs of abuse perturb the BNST-VTA neural circuit. VTA-projecting BNST neurons show increases in c-Fos activation during stress-induced reinstatement of cocaine seeking (Briand et al. 2010). Additionally, bilateral disconnection experiments, in which the BNST and VTA are both inhibited by pharmacological methods, results in the reduction of a cocaine conditioned place preference (Sartor and Aston-Jones 2012). Chronic morphine can increase evoked VTA EPSCs caused by electrical stimulation of the BNST (Dumont et al. 2008). The BNST also sends a CRFergic projection to the VTA (Rodaros et al. 2007) and a recent study showed that application of CRF could increase the frequency of spontaneous excitatory postsynaptic currents on VTA-projecting BNST neurons using patch clamp electrophysiology. This effect was abolished during withdrawal from chronic, intermittent ethanol exposure (Silberman et al. 2013).
BNST projections to the Lateral hypothalamus
The lateral hypothalamus (LH) also receives a substantial projection from the BNST (Fig 2B) (Dong and Swanson 2004). Similar to the BNST, the LH is a heterogeneous structure consisting of a diverse array of neurotransmitters and neuropeptides including neuropeptide S, orexin, and melanin concentrating hormone (de Lecea et al. 1998; Sakurai et al. 1998). Importantly, the LH has been identified as an area of the brain critical for drug-seeking behavior (Aston-Jones and Harris 2004). For example, cocaine self-administration increases excitatory drive on LH neurons (Yeoh et al. 2012). Additionally, lesions of the LH will prevent the learning of a morphine conditioned place preference (Harris et al. 2007). A recent study also demonstrated a role for the BNST-LH neural circuit in addiction-like behavior. LH-projecting BNST neurons display increased c-Fos activation during cocaine conditioned place preference (Sartor and Aston-Jones 2012). It has been hypothesized that BNST afferents synapse onto orexin neurons within the LH, which is intriguing because these neurons play a prominent role in drugs of abuse (Sakurai et al. 2005; Yoshida et al. 2006). Elevated c-Fos levels in LH orexin neurons are observed during morphine withdrawal as well as after a morphine conditioned place preference task in dependent animals (Georgescu et al. 2003; Aston-Jones and Harris 2004). However, pharmacological inactivation of the BNST resulted in increased Fos induction in LH orexin neurons after a cocaine conditioned place preference task, indicating that this projection may not be perturbed during drug administration (Sartor and Aston-Jones 2012). Recently, Kim et al. (2013) found that photostimulation of adBNST inputs to the LH resulted in behavioral anxiolysis without altering respiratory rate or reward related behaviors, indicating that this projection modulates a specific component of the anxiety-like state.
BNST projections to the Paraventricular nucleus
The paraventricular nucleus of the hypothalamus (PVN) is a brain region involved in the maintenance of homeostasis and is a critical component of the hypothalamic-pituitary-adrenal (HPA) axis. As described by Swanson and Sawchenko (1983), the PVN can be delineated into two cell groups based on their projection targets: magnocellular and parvocellular neurons. Magnocellular neurons are primarily oxytocinergic and vasopressinergic, which project to the posterior pituitary whereas parvocellular neurons contain CRF, vasopressin, and thyrotropin-releasing hormone (TRH) and project to the anterior pituitary and spinal cord (Swanson and Sawchenko 1983; Barberis and Tribollet 1996). Importantly, the PVN receives a substantial GABAergic, as well as a CRFergic projection, from the BNST (Fig 2B) (Roland and Sawchenko 1993; Champagne et al. 1998; Dong et al. 2001; Dong and Swanson 2006). Through the use of anatomical tracing studies, it appears the main GABAergic projections to the parvocellular region of the PVN are not from the BNST (Roland and Sawchenko 1993). Therefore, it is hypothesized that BNST GABAergic neurons synapse primarily onto neurons within the magnocellular region of the PVN. Based on its integral role in HPA axis maintenance, the PVN has been implicated in modulating addictive drugs. Stressors, including drugs of abuse, cause activation of the HPA axis, which in turn leads to the release of adrenocorticotropic hormone (ACTH) and glucocorticoids (O'Connor et al. 2000). CRF signaling within the PVN plays an important role in HPA axis homeostasis as acute stressors, such as a cocaine injection, can increase PVN CRF mRNA (Rivier and Lee 1994; Rotllant et al. 2007). Additionally, opiates, nicotine, and amphetamine can increase c-Fos levels in PVN CRF neurons (Laorden et al. 2000) (Matta et al. 1997) (Rotllant et al. 2007). Based on the prevailing literature, it appears that the BNST neurons may synapse onto CRFergic cells within the PVN. Thus, it is likely that drug administration increases BNST CRFergic drive onto PVN CRF neurons.
Based on its numerous downstream projection targets to critical brain structures that mediate drugs of abuse, the BNST appears to be a crucial modulator of addiction. While the majority of the studies discussed above have focused on general BNST circuit projections and function, the advent of optogenetic strategies to dissect discrete BNST neural circuit elements will shed light on their intimate connection with drugs of abuse.
Concluding remarks
While the studies discussed above imply a critical role for BLA and BNST connectivity in different aspects of drug addiction, there remains a great lack of understanding for which specific inputs or outputs are being activated or modulated and under what particular behavioral conditions. Further studies of the functional connectivity within these circuits, including the use of innovative techniques such as pharmacogenetic and optogenetic tools, may provide clinically relevant insight into the fundamental neural circuit mechanisms underlying addiction.
Table 1. Overview of BLA and BNST connectivity.
| Circuit | Approach used | Projection type | Reference |
|---|---|---|---|
| VTA → BLA | Tracing | Dopaminergic | (Albanese and Minciacchi 1983) |
| Thal → BLA | Tracing | Unknown | (van Vulpen and Verwer 1989) |
| Hipp → BLA | Tracing | Glutamatergic | (Ottersen 1982) |
| mPFC → BLA | Tracing | Glutamatergic | (Ottersen 1982) |
| BLA → NAc | Tracing, Optogenetics, behavior | Glutamatergic | (Kelley et al. 1982; Stuber et al. 2011) |
| BLA → Hipp | Tracing | Glutamatergic | (Pitkanen et al. 2000) |
| BLA → BNST | Optogenetics, behavior | Glutamatergic | (Kim et al. 2013) |
| BLA → mPFC | Electron microscopy | Glutamatergic | (Bacon et al. 1996) |
| Cortex → BNST | Tracing, electron microscopy, electrophysiology | Glutamatergic | (McDonald 1998) |
| CeA → BNST | Tracing, optogenetics | GABAergic | (Dong et al. 2001; Li et al. 2012) |
| Hipp → BNST | Tracing | Glutamtergic | (Cullinan et al. 1993) |
| PFC → BNST | Tracing | Glutamatergic | (McDonald 1998) |
| VTA/PAG → BNST | Pharmacology | Dopaminergic | (Meloni et al. 2006) |
| NTS,VLM,VNAB → BNST | Anatomical, neurochemical, behavior | Noradrenergic | (Forray and Gysling 2004) |
| DRN → BNST | Electron microscopy | Serotonergic | (Phelix et al. 1992) |
| BNST → VTA | Tracing, optogenetics, behavior | Glutamatergic And GABAergic | (Georges and Aston-Jones, 2001; 2002; Jalabert et al., 2009; Jennings et al., 2013; Kim et al., 2013; Kudo et al., 2012) |
| BNST → LH | Tracing | Unknown | (Dong and Swanson 2004) |
| BNST → PVN | Tracing | GABAergic, CRFergic | (Roland and Sawchenko 1993; Champagne et al. 1998; Dong et al. 2001; Dong and Swanson 2006) |
Highlights.
We discuss basolateral amygdala circuitry and implications for drug addiction.
We discuss bed nucleus of the stria terminalis circuitry and its relevance to drug addiction.
Dissecting the amygdala connectivity may be critical for further treatments for addiction.
Acknowledgments
We are supported by The Whitehall Foundation, NARSAD, The Foundation of Hope, and NIH grants DA029325 and DA032750 (G.D.S.), AA018610 and AA007573 (D.R.S.), and NS007431 and DA034472 (A.M.S.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albanese A, Minciacchi D. Organization of the ascending projections from the ventral tegmental area: a multiple fluorescent retrograde tracer study in the rat. The Journal of comparative neurology. 1983;216(4):406–420. doi: 10.1002/cne.902160406. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Price JL. Amygdalo-cortical projections in the monkey (Macaca fascicularis) The Journal of comparative neurology. 1984;230(4):465–496. doi: 10.1002/cne.902300402. [DOI] [PubMed] [Google Scholar]
- Ambroggi F, Ishikawa A, et al. Basolateral amygdala neurons facilitate reward-seeking behavior by exciting nucleus accumbens neurons. Neuron. 2008;59(4):648–661. doi: 10.1016/j.neuron.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amunts K, Kedo O, et al. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anatomy and embryology. 2005;210(5-6):343–352. doi: 10.1007/s00429-005-0025-5. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Druhan J. Behavioural pharmacology. Breaking the chain of addiction. Nature. 1999;400(6742):317–319. doi: 10.1038/22432. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Harris GC. Brain substrates for increased drug seeking during protracted withdrawal. Neuropharmacology. 2004;47(1):167–179. doi: 10.1016/j.neuropharm.2004.06.020. [DOI] [PubMed] [Google Scholar]
- Bacon SJ, Headlam AJ, et al. Amygdala input to medial prefrontal cortex (mPFC) in the rat: a light and electron microscope study. Brain research. 1996;720(1-2):211–219. doi: 10.1016/0006-8993(96)00155-2. [DOI] [PubMed] [Google Scholar]
- Baldo BA, Daniel RA, et al. Overlapping distributions of orexin/hypocretin- and dopamine-beta-hydroxylase immunoreactive fibers in rat brain regions mediating arousal, motivation, and stress. The Journal of comparative neurology. 2003;464(2):220–237. doi: 10.1002/cne.10783. [DOI] [PubMed] [Google Scholar]
- Barberis C, Tribollet E. Vasopressin and oxytocin receptors in the central nervous system. Crit Rev Neurobiol. 1996;10(1):119–154. doi: 10.1615/critrevneurobiol.v10.i1.60. [DOI] [PubMed] [Google Scholar]
- Baxter MG, Murray EA. The amygdala and reward. Nature reviews. Neuroscience. 2002;3(7):563–573. doi: 10.1038/nrn875. [DOI] [PubMed] [Google Scholar]
- Benes FM. Amygdalocortical circuitry in schizophrenia: from circuits to molecules. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2010;35(1):239–257. doi: 10.1038/npp.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briand LA, Vassoler FM, et al. Ventral tegmental afferents in stress-induced reinstatement: the role of cAMP response element-binding protein. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2010;30(48):16149–16159. doi: 10.1523/JNEUROSCI.2827-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britt JP, Benaliouad F, et al. Synaptic and behavioral profile of multiple glutamatergic inputs to the nucleus accumbens. Neuron. 2012;76(4):790–803. doi: 10.1016/j.neuron.2012.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brog JS, Salyapongse A, et al. The patterns of afferent innervation of the core and shell in the accumbens part of the rat ventral striatum: immunohistochemical detection of retrogradely transported fluoro-gold. The Journal of comparative neurology. 1993;338(2):255–278. doi: 10.1002/cne.903380209. [DOI] [PubMed] [Google Scholar]
- Bruchas MR, Land BB, et al. CRF1-R activation of the dynorphin/kappa opioid system in the mouse basolateral amygdala mediates anxiety-like behavior. PloS one. 2009;4(12):e8528. doi: 10.1371/journal.pone.0008528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffalari DM, See RE. Inactivation of the bed nucleus of the stria terminalis in an animal model of relapse: effects on conditioned cue-induced reinstatement and its enhancement by yohimbine. Psychopharmacology. 2011;213(1):19–27. doi: 10.1007/s00213-010-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns LH, Robbins TW, et al. Differential effects of excitotoxic lesions of the basolateral amygdala, ventral subiculum and medial prefrontal cortex on responding with conditioned reinforcement and locomotor activity potentiated by intra-accumbens infusions of D-amphetamine. Behavioural brain research. 1993;2;55:167–183. doi: 10.1016/0166-4328(93)90113-5. [DOI] [PubMed] [Google Scholar]
- Carboni E, Silvagni A, et al. Stimulation of in vivo dopamine transmission in the bed nucleus of stria terminalis by reinforcing drugs. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2000;20(20):RC102. doi: 10.1523/JNEUROSCI.20-20-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassell MD, Freedman LJ, et al. The intrinsic organization of the central extended amygdala. Annals of the New York Academy of Sciences. 1999;877:217–241. doi: 10.1111/j.1749-6632.1999.tb09270.x. [DOI] [PubMed] [Google Scholar]
- Cecchi M, Khoshbouei H, et al. Modulatory effects of norepinephrine in the lateral bed nucleus of the stria terminalis on behavioral and neuroendocrine responses to acute stress. Neuroscience. 2002;112(1):13–21. doi: 10.1016/s0306-4522(02)00062-3. [DOI] [PubMed] [Google Scholar]
- Champagne D, Beaulieu J, et al. CRFergic innervation of the paraventricular nucleus of the rat hypothalamus: a tract-tracing study. Journal of neuroendocrinology. 1998;10(2):119–131. doi: 10.1046/j.1365-2826.1998.00179.x. [DOI] [PubMed] [Google Scholar]
- Chase HW, Eickhoff SB, et al. The neural basis of drug stimulus processing and craving: an activation likelihood estimation meta-analysis. Biological psychiatry. 2011;70(8):785–793. doi: 10.1016/j.biopsych.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchwell JC, Morris AM, et al. Interactions between the prefrontal cortex and amygdala during delay discounting and reversal. Behavioral neuroscience. 2009;123(6):1185–1196. doi: 10.1037/a0017734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad KL, Davis AR, et al. Yohimbine depresses excitatory transmission in BNST and impairs extinction of cocaine place preference through orexin-dependent, norepinephrine-independent processes. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2012;37(10):2253–2266. doi: 10.1038/npp.2012.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Balleine BW. Double dissociation of basolateral and central amygdala lesions on the general and outcome-specific forms of pavlovian-instrumental transfer. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25(4):962–970. doi: 10.1523/JNEUROSCI.4507-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullinan WE, Herman JP, et al. Ventral subicular interaction with the hypothalamic paraventricular nucleus: evidence for a relay in the bed nucleus of the stria terminalis. The Journal of comparative neurology. 1993;332(1):1–20. doi: 10.1002/cne.903320102. [DOI] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95(1):322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfs JM, Zhu Y, et al. Noradrenaline in the ventral forebrain is critical for opiate withdrawal-induced aversion. Nature. 2000;403(6768):430–434. doi: 10.1038/35000212. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Direct interactions between the basolateral amygdala and nucleus accumbens core underlie cocaine-seeking behavior by rats. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2004;24(32):7167–7173. doi: 10.1523/JNEUROSCI.1581-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond DM, Rose GM. Stress impairs LTP and hippocampal-dependent memory. Annals of the New York Academy of Sciences. 1994;746:411–414. doi: 10.1111/j.1749-6632.1994.tb39271.x. [DOI] [PubMed] [Google Scholar]
- Dong HW, Petrovich GD, et al. Basic organization of projections from the oval and fusiform nuclei of the bed nuclei of the stria terminalis in adult rat brain. The Journal of comparative neurology. 2001;436(4):430–455. doi: 10.1002/cne.1079. [DOI] [PubMed] [Google Scholar]
- Dong HW, Swanson LW. Organization of axonal projections from the anterolateral area of the bed nuclei of the stria terminalis. J Comp Neurol. 2004;468(2):277–298. doi: 10.1002/cne.10949. [DOI] [PubMed] [Google Scholar]
- Dong HW, Swanson LW. Projections from bed nuclei of the stria terminalis, dorsomedial nucleus: implications for cerebral hemisphere integration of neuroendocrine, autonomic, and drinking responses. J Comp Neurol. 2006;494(1):75–107. doi: 10.1002/cne.20790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont EC, Mark GP, et al. Self-administration enhances excitatory synaptic transmission in the bed nucleus of the stria terminalis. Nat Neurosci. 2005;8(4):413–414. doi: 10.1038/nn1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont EC, Rycroft BK, et al. Morphine produces circuit-specific neuroplasticity in the bed nucleus of the stria terminalis. Neuroscience. 2008;153(1):232–239. doi: 10.1016/j.neuroscience.2008.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont EC, Williams JT. Noradrenaline triggers GABAA inhibition of bed nucleus of the stria terminalis neurons projecting to the ventral tegmental area. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2004;24(38):8198–8204. doi: 10.1523/JNEUROSCI.0425-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egli RE, Kash TL, et al. Norepinephrine modulates glutamatergic transmission in the bed nucleus of the stria terminalis. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2005;30(4):657–668. doi: 10.1038/sj.npp.1300639. [DOI] [PubMed] [Google Scholar]
- Erb S, Hitchcott PK, et al. Alpha-2 adrenergic receptor agonists block stress-induced reinstatement of cocaine seeking. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2000;23(2):138–150. doi: 10.1016/S0893-133X(99)00158-X. [DOI] [PubMed] [Google Scholar]
- Erb S, Salmaso N, et al. A role for the CRF-containing pathway from central nucleus of the amygdala to bed nucleus of the stria terminalis in the stress-induced reinstatement of cocaine seeking in rats. Psychopharmacology. 2001;158(4):360–365. doi: 10.1007/s002130000642. [DOI] [PubMed] [Google Scholar]
- Erb S, Shaham Y, et al. Stress-induced relapse to drug seeking in the rat: role of the bed nucleus of the stria terminalis and amygdala. Stress. 2001;4(4):289–303. doi: 10.3109/10253890109014753. [DOI] [PubMed] [Google Scholar]
- Erb S, Stewart J. A role for the bed nucleus of the stria terminalis, but not the amygdala, in the effects of corticotropin-releasing factor on stress-induced reinstatement of cocaine seeking. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1999;19(20):RC35. doi: 10.1523/JNEUROSCI.19-20-j0006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Parkinson JA, et al. Associative processes in addiction and reward. The role of amygdala-ventral striatal subsystems. Annals of the New York Academy of Sciences. 1999;877:412–438. doi: 10.1111/j.1749-6632.1999.tb09280.x. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Yang CR, et al. Basolateral amygdala stimulation evokes glutamate receptor-dependent dopamine efflux in the nucleus accumbens of the anaesthetized rat. The European journal of neuroscience. 1998;10(4):1241–1251. doi: 10.1046/j.1460-9568.1998.00133.x. [DOI] [PubMed] [Google Scholar]
- Forray MI, Gysling K. Role of noradrenergic projections to the bed nucleus of the stria terminalis in the regulation of the hypothalamic-pituitary-adrenal axis. Brain Res Brain Res Rev. 2004;47(1-3):145–160. doi: 10.1016/j.brainresrev.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Fox JH, Hammack SE, et al. Exercise is associated with reduction in the anxiogenic effect of mCPP on acoustic startle. Behavioral neuroscience. 2008;122(4):943–948. doi: 10.1037/0735-7044.122.4.943. [DOI] [PubMed] [Google Scholar]
- French SJ, Totterdell S. Individual nucleus accumbens-projection neurons receive both basolateral amygdala and ventral subicular afferents in rats. Neuroscience. 2003;119(1):19–31. doi: 10.1016/s0306-4522(03)00150-7. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Bell GH, et al. Basolateral amygdala involvement in memory reconsolidation processes that facilitate drug context-induced cocaine seeking. The European journal of neuroscience. 2009;30(5):889–900. doi: 10.1111/j.1460-9568.2009.06888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Eaddy JL, et al. Interactions of the basolateral amygdala with the dorsal hippocampus and dorsomedial prefrontal cortex regulate drug context-induced reinstatement of cocaine-seeking in rats. The European journal of neuroscience. 2007;26(2):487–498. doi: 10.1111/j.1460-9568.2007.05674.x. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, et al. The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2005;30(2):296–309. doi: 10.1038/sj.npp.1300579. [DOI] [PubMed] [Google Scholar]
- Garcia R, Vouimba RM, et al. The amygdala modulates prefrontal cortex activity relative to conditioned fear. Nature. 1999;402(6759):294–296. doi: 10.1038/46286. [DOI] [PubMed] [Google Scholar]
- Georges F, Aston-Jones G. Potent regulation of midbrain dopamine neurons by the bed nucleus of the stria terminalis. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2001;21(16):RC160. doi: 10.1523/JNEUROSCI.21-16-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges F, Aston-Jones G. Potent regulation of midbrain dopamine neurons by the bed nucleus of the stria terminalis. J Neurosci. 2001;21(16):RC160. doi: 10.1523/JNEUROSCI.21-16-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges F, Aston-Jones G. Activation of ventral tegmental area cells by the bed nucleus of the stria terminalis: a novel excitatory amino acid input to midbrain dopamine neurons. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2002;22(12):5173–5187. doi: 10.1523/JNEUROSCI.22-12-05173.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgescu D, Zachariou V, et al. Involvement of the lateral hypothalamic peptide orexin in morphine dependence and withdrawal. J Neurosci. 2003;23(8):3106–3111. doi: 10.1523/JNEUROSCI.23-08-03106.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y, Yang CR, et al. Functional and dysfunctional synaptic plasticity in prefrontal cortex: roles in psychiatric disorders. Biological psychiatry. 2010;67(3):199–207. doi: 10.1016/j.biopsych.2009.08.026. [DOI] [PubMed] [Google Scholar]
- Guo JD, Hammack SE, et al. Bi-directional modulation of bed nucleus of stria terminalis neurons by 5-HT: molecular expression and functional properties of excitatory 5-HT receptor subtypes. Neuroscience. 2009;164(4):1776–1793. doi: 10.1016/j.neuroscience.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, et al. Lateral hypothalamic orexin neurons are critically involved in learning to associate an environment with morphine reward. Behav Brain Res. 2007;183(1):43–51. doi: 10.1016/j.bbr.2007.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, De Bellis MD, et al. Right amygdala volume in adolescent and young adult offspring from families at high risk for developing alcoholism. Biological psychiatry. 2001;49(11):894–905. doi: 10.1016/s0006-3223(01)01088-5. [DOI] [PubMed] [Google Scholar]
- Hooker JM, Patel V, et al. Metabolic changes in the rodent brain after acute administration of salvinorin A. Molecular imaging and biology: MIB: the official publication of the Academy of Molecular Imaging. 2009;11(3):137–143. doi: 10.1007/s11307-008-0192-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff NC, Frank M, et al. Amygdala regulation of immediate-early gene expression in the hippocampus induced by contextual fear conditioning. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2006;26(5):1616–1623. doi: 10.1523/JNEUROSCI.4964-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaki K, Honma S, et al. Gene expression of neuropeptide Y in the nucleus of the solitary tract is activated in rats under restricted daily feeding but not under 48-h food deprivation. The European journal of neuroscience. 2003;17(10):2097–2105. doi: 10.1046/j.1460-9568.2003.02672.x. [DOI] [PubMed] [Google Scholar]
- Jalabert M, Aston-Jones G, et al. Role of the bed nucleus of the stria terminalis in the control of ventral tegmental area dopamine neurons. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(8):1336–1346. doi: 10.1016/j.pnpbp.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalabert M, Aston-Jones G, et al. Role of the bed nucleus of the stria terminalis in the control of ventral tegmental area dopamine neurons. Progress in neuro-psychopharmacology & biological psychiatry. 2009;33(8):1336–1346. doi: 10.1016/j.pnpbp.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings JH, Sparta DR, et al. Distinct extended amygdala circuits for divergent motivational states. Nature. 2013;496(7444):224–228. doi: 10.1038/nature12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JL, Day JJ, et al. Basolateral amygdala modulates terminal dopamine release in the nucleus accumbens and conditioned responding. Biological psychiatry. 2010;67(8):737–744. doi: 10.1016/j.biopsych.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju G, Swanson LW. Studies on the cellular architecture of the bed nuclei of the stria terminalis in the rat: I. Cytoarchitecture. The Journal of comparative neurology. 1989;280(4):587–602. doi: 10.1002/cne.902800409. [DOI] [PubMed] [Google Scholar]
- Ju G, Swanson LW, et al. Studies on the cellular architecture of the bed nuclei of the stria terminalis in the rat: II. Chemoarchitecture. The Journal of comparative neurology. 1989;280(4):603–621. doi: 10.1002/cne.902800410. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nature reviews Neuroscience. 2009;10(8):561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- Kash TL, Baucum AJ, 2nd, et al. Alcohol exposure alters NMDAR function in the bed nucleus of the stria terminalis. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2009;34(11):2420–2429. doi: 10.1038/npp.2009.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kash TL, Nobis WP, et al. Dopamine enhances fast excitatory synaptic transmission in the extended amygdala by a CRF-R1-dependent process. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2008;28(51):13856–13865. doi: 10.1523/JNEUROSCI.4715-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kash TL, Winder DG. Neuropeptide Y and corticotropin-releasing factor bi-directionally modulate inhibitory synaptic transmission in the bed nucleus of the stria terminalis. Neuropharmacology. 2006;51(5):1013–1022. doi: 10.1016/j.neuropharm.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Domesick VB, et al. The amygdalostriatal projection in the rat--an anatomical study by anterograde and retrograde tracing methods. Neuroscience. 1982;7(3):615–630. doi: 10.1016/0306-4522(82)90067-7. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Koo JW, et al. Amygdalar inactivation blocks stress-induced impairments in hippocampal long-term potentiation and spatial memory. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2005;25(6):1532–1539. doi: 10.1523/JNEUROSCI.4623-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Adhikari A, et al. Diverging neural pathways assemble a behavioural state from separable features in anxiety. Nature. 2013;496(7444):219–223. doi: 10.1038/nature12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Dynamics of neuronal circuits in addiction: reward, antireward, and emotional memory. Pharmacopsychiatry. 2009;42(1):S32–41. doi: 10.1055/s-0029-1216356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozicz T, Vigh S, et al. Axon terminals containing PACAP- and VIP-immunoreactivity form synapses with CRF-immunoreactive neurons in the dorsolateral division of the bed nucleus of the stria terminalis in the rat. Brain research. 1997;767(1):109–119. doi: 10.1016/s0006-8993(97)00737-3. [DOI] [PubMed] [Google Scholar]
- Krawczyk M, Georges F, et al. Double-dissociation of the catecholaminergic modulation of synaptic transmission in the oval bed nucleus of the stria terminalis. Journal of neurophysiology. 2011;105(1):145–153. doi: 10.1152/jn.00710.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawczyk M, Sharma R, et al. A switch in the neuromodulatory effects of dopamine in the oval bed nucleus of the stria terminalis associated with cocaine self-administration in rats. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31(24):8928–8935. doi: 10.1523/JNEUROSCI.0377-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo T, Uchigashima M, et al. Three types of neurochemical projection from the bed nucleus of the stria terminalis to the ventral tegmental area in adult mice. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2012;32(50):18035–18046. doi: 10.1523/JNEUROSCI.4057-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kufahl PR, Li Z, et al. Neural responses to acute cocaine administration in the human brain detected by fMRI. NeuroImage. 2005;28(4):904–914. doi: 10.1016/j.neuroimage.2005.06.039. [DOI] [PubMed] [Google Scholar]
- Laorden ML, Castells MT, et al. Activation of c-fos expression in hypothalamic nuclei by mu- and kappa-receptor agonists: correlation with catecholaminergic activity in the hypothalamic paraventricular nucleus. Endocrinology. 2000;141(4):1366–1376. doi: 10.1210/endo.141.4.7407. [DOI] [PubMed] [Google Scholar]
- Leri F, Flores J, et al. Blockade of stress-induced but not cocaine-induced reinstatement by infusion of noradrenergic antagonists into the bed nucleus of the stria terminalis or the central nucleus of the amygdala. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2002;22(13):5713–5718. doi: 10.1523/JNEUROSCI.22-13-05713.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levita L, Hammack SE, et al. 5-hydroxytryptamine1A-like receptor activation in the bed nucleus of the stria terminalis: electrophysiological and behavioral studies. Neuroscience. 2004;128(3):583–596. doi: 10.1016/j.neuroscience.2004.06.037. [DOI] [PubMed] [Google Scholar]
- Li C, Pleil KE, et al. Presynaptic inhibition of gamma-aminobutyric acid release in the bed nucleus of the stria terminalis by kappa opioid receptor signaling. Biological psychiatry. 2012;71(8):725–732. doi: 10.1016/j.biopsych.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lintas A, Chi N, et al. Inputs from the basolateral amygdala to the nucleus accumbens shell control opiate reward magnitude via differential dopamine D1 or D2 receptor transmission. The European journal of neuroscience. 2012;35(2):279–290. doi: 10.1111/j.1460-9568.2011.07943.x. [DOI] [PubMed] [Google Scholar]
- Lungwitz EA, Molosh A, et al. Orexin-A induces anxiety-like behavior through interactions with glutamatergic receptors in the bed nucleus of the stria terminalis of rats. Physiology & behavior. 2012;107(5):726–732. doi: 10.1016/j.physbeh.2012.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant NJ, Densmore VS, et al. Coexpression of prodynorphin and corticotrophin-releasing hormone in the rat central amygdala: evidence of two distinct endogenous opioid systems in the lateral division. The Journal of comparative neurology. 2007;504(6):702–715. doi: 10.1002/cne.21464. [DOI] [PubMed] [Google Scholar]
- Matta SG, Valentine JD, et al. Nicotinic activation of CRH neurons in extrahypothalamic regions of the rat brain. Endocrine. 1997;7(2):245–253. doi: 10.1007/BF02778147. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Cortical pathways to the mammalian amygdala. Progress in neurobiology. 1998;55(3):257–332. doi: 10.1016/s0301-0082(98)00003-3. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F, et al. Projections of the medial and lateral prefrontal cortices to the amygdala: a Phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience. 1996;71(1):55–75. doi: 10.1016/0306-4522(95)00417-3. [DOI] [PubMed] [Google Scholar]
- McElligott ZA, Klug JR, et al. Distinct forms of Gq-receptor-dependent plasticity of excitatory transmission in the BNST are differentially affected by stress. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(5):2271–2276. doi: 10.1073/pnas.0905568107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElligott ZA, Winder DG. Alpha1-adrenergic receptor-induced heterosynaptic long-term depression in the bed nucleus of the stria terminalis is disrupted in mouse models of affective disorders. Neuropsychopharmacology. 2008;33(10):2313–2323. doi: 10.1038/sj.npp.1301635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Davidge SB, et al. Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. J Neurosci. 2004;24(7):1551–1560. doi: 10.1523/JNEUROSCI.4177-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meil WM, See RE. Lesions of the basolateral amygdala abolish the ability of drug associated cues to reinstate responding during withdrawal from self-administered cocaine. Behavioural brain research. 1997;87(2):139–148. doi: 10.1016/s0166-4328(96)02270-x. [DOI] [PubMed] [Google Scholar]
- Meloni EG, Gerety LP, et al. Behavioral and anatomical interactions between dopamine and corticotropin-releasing factor in the rat. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2006;26(14):3855–3863. doi: 10.1523/JNEUROSCI.4957-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan MA, LeDoux JE. Differential contribution of dorsal and ventral medial prefrontal cortex to the acquisition and extinction of conditioned fear in rats. Behavioral neuroscience. 1995;109(4):681–688. doi: 10.1037//0735-7044.109.4.681. [DOI] [PubMed] [Google Scholar]
- Morgan MA, LeDoux JE. Contribution of ventrolateral prefrontal cortex to the acquisition and extinction of conditioned fear in rats. Neurobiology of learning and memory. 1999;72(3):244–251. doi: 10.1006/nlme.1999.3907. [DOI] [PubMed] [Google Scholar]
- Morgan MA, Romanski LM, et al. Extinction of emotional learning: contribution of medial prefrontal cortex. Neuroscience letters. 1993;163(1):109–113. doi: 10.1016/0304-3940(93)90241-c. [DOI] [PubMed] [Google Scholar]
- Myers EA, Banihashemi L, et al. The anxiogenic drug yohimbine activates central viscerosensory circuits in rats. J Comp Neurol. 2005;492(4):426–441. doi: 10.1002/cne.20727. [DOI] [PubMed] [Google Scholar]
- Nobis WP, Kash TL, et al. beta-Adrenergic receptors enhance excitatory transmission in the bed nucleus of the stria terminalis through a corticotrophin-releasing factor receptor-dependent and cocaine-regulated mechanism. Biological psychiatry. 2011;69(11):1083–1090. doi: 10.1016/j.biopsych.2010.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez C, Martin F, et al. Induction of FosB/DeltaFosB in the brain stress system-related structures during morphine dependence and withdrawal. J Neurochem. 2010;114(2):475–487. doi: 10.1111/j.1471-4159.2010.06765.x. [DOI] [PubMed] [Google Scholar]
- O'Connor TM, O'Halloran DJ, et al. The stress response and the hypothalamic-pituitary-adrenal axis: from molecule to melancholia. QJM. 2000;93(6):323–333. doi: 10.1093/qjmed/93.6.323. [DOI] [PubMed] [Google Scholar]
- Olive MF, Koenig HN, et al. Elevated extracellular CRF levels in the bed nucleus of the stria terminalis during ethanol withdrawal and reduction by subsequent ethanol intake. Pharmacol Biochem Behav. 2002;72(1-2):213–220. doi: 10.1016/s0091-3057(01)00748-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottersen OP. Connections of the amygdala of the rat. IV: Corticoamygdaloid and intraamygdaloid connections as studied with axonal transport of horseradish peroxidase. The Journal of comparative neurology. 1982;205(1):30–48. doi: 10.1002/cne.902050104. [DOI] [PubMed] [Google Scholar]
- Packard MG, Cahill L. Affective modulation of multiple memory systems. Current opinion in neurobiology. 2001;11(6):752–756. doi: 10.1016/s0959-4388(01)00280-x. [DOI] [PubMed] [Google Scholar]
- Pare D, Smith Y. The intercalated cell masses project to the central and medial nuclei of the amygdala in cats. Neuroscience. 1993;57(4):1077–1090. doi: 10.1016/0306-4522(93)90050-p. [DOI] [PubMed] [Google Scholar]
- Park J, Bucher ES, et al. Opposing Catecholamine Changes in the Bed Nucleus of the Stria Terminalis During Intracranial Self-Stimulation and Its Extinction. Biological psychiatry. 2012 doi: 10.1016/j.biopsych.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Wheeler RA, et al. Catecholamines in the bed nucleus of the stria terminalis reciprocally respond to reward and aversion. Biological psychiatry. 2012;71(4):327–334. doi: 10.1016/j.biopsych.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Kalivas PW, et al. Extinction circuits for fear and addiction overlap in prefrontal cortex. Learning & memory. 2009;16(5):279–288. doi: 10.1101/lm.1041309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, LaLumiere RT, et al. Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2008;28(23):6046–6053. doi: 10.1523/JNEUROSCI.1045-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, et al. A controlled study of cortical gray matter and ventricular changes in alcoholic men over a 5-year interval. Archives of general psychiatry. 1998;55(10):905–912. doi: 10.1001/archpsyc.55.10.905. [DOI] [PubMed] [Google Scholar]
- Phelix CF, Liposits Z, et al. Monoamine innervation of bed nucleus of stria terminalis: an electron microscopic investigation. Brain research bulletin. 1992;28(6):949–965. doi: 10.1016/0361-9230(92)90218-m. [DOI] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48(2):175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Pikkarainen M, et al. Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat. A review. Annals of the New York Academy of Sciences. 2000;911:369–391. doi: 10.1111/j.1749-6632.2000.tb06738.x. [DOI] [PubMed] [Google Scholar]
- Pleil KE, Lopez A, et al. Chronic stress alters neuropeptide Y signaling in the bed nucleus of the stria terminalis in DBA/2J but not C57BL/6J mice. Neuropharmacology. 2012;62(4):1777–1786. doi: 10.1016/j.neuropharm.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2010;35(1):192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2008;33(1):56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravinder S, Burghardt NS, et al. A role for the extended amygdala in the fear-enhancing effects of acute selective serotonin reuptake inhibitor treatment. Translational psychiatry. 2013;3:e209. doi: 10.1038/tp.2012.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risinger RC, Salmeron BJ, et al. Neural correlates of high and craving during cocaine self-administration using BOLD fMRI. NeuroImage. 2005;26(4):1097–1108. doi: 10.1016/j.neuroimage.2005.03.030. [DOI] [PubMed] [Google Scholar]
- Rivier C, Lee S. Stimulatory effect of cocaine on ACTH secretion: role of the hypothalamus. Mol Cell Neurosci. 1994;5(2):189–195. doi: 10.1006/mcne.1994.1021. [DOI] [PubMed] [Google Scholar]
- Rodaros D, Caruana DA, et al. Corticotropin-releasing factor projections from limbic forebrain and paraventricular nucleus of the hypothalamus to the region of the ventral tegmental area. Neuroscience. 2007;150(1):8–13. doi: 10.1016/j.neuroscience.2007.09.043. [DOI] [PubMed] [Google Scholar]
- Rodrigues SM, LeDoux JE, et al. The influence of stress hormones on fear circuitry. Annual review of neuroscience. 2009;32:289–313. doi: 10.1146/annurev.neuro.051508.135620. [DOI] [PubMed] [Google Scholar]
- Roland BL, Sawchenko PE. Local origins of some GABAergic projections to the paraventricular and supraoptic nuclei of the hypothalamus in the rat. The Journal of comparative neurology. 1993;332(1):123–143. doi: 10.1002/cne.903320109. [DOI] [PubMed] [Google Scholar]
- Rosenkranz JA, Grace AA. Modulation of basolateral amygdala neuronal firing and afferent drive by dopamine receptor activation in vivo. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1999;19(24):11027–11039. doi: 10.1523/JNEUROSCI.19-24-11027.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotllant D, Nadal R, et al. Differential effects of stress and amphetamine administration on Fos-like protein expression in corticotropin releasing factor-neurons of the rat brain. Dev Neurobiol. 2007;67(6):702–714. doi: 10.1002/dneu.20345. [DOI] [PubMed] [Google Scholar]
- Sakanaka M, Shibasaki T, et al. Distribution and efferent projections of corticotropin-releasing factor-like immunoreactivity in the rat amygdaloid complex. Brain research. 1986;382(2):213–238. doi: 10.1016/0006-8993(86)91332-6. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92(4):573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Nagata R, et al. Input of orexin/hypocretin neurons revealed by a genetically encoded tracer in mice. Neuron. 2005;46(2):297–308. doi: 10.1016/j.neuron.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Sartor GC, Aston-Jones G. Regulation of the ventral tegmental area by the bed nucleus of the stria terminalis is required for expression of cocaine preference. Eur J Neurosci. 2012;36(11):3549–3558. doi: 10.1111/j.1460-9568.2012.08277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See RE, Kruzich PJ, et al. Dopamine, but not glutamate, receptor blockade in the basolateral amygdala attenuates conditioned reward in a rat model of relapse to cocaine-seeking behavior. Psychopharmacology. 2001;154(3):301–310. doi: 10.1007/s002130000636. [DOI] [PubMed] [Google Scholar]
- Shalev U, Morales M, et al. Time-dependent changes in extinction behavior and stress-induced reinstatement of drug seeking following withdrawal from heroin in rats. Psychopharmacology (Berl) 2001;156(1):98–107. doi: 10.1007/s002130100748. [DOI] [PubMed] [Google Scholar]
- Shepard JD, Schulkin J, et al. Chronically elevated corticosterone in the amygdala increases corticotropin releasing factor mRNA in the dorsolateral bed nucleus of stria terminalis following duress. Behavioural brain research. 2006;174(1):193–196. doi: 10.1016/j.bbr.2006.07.019. [DOI] [PubMed] [Google Scholar]
- Shields AD, Wang Q, et al. alpha2A-adrenergic receptors heterosynaptically regulate glutamatergic transmission in the bed nucleus of the stria terminalis. Neuroscience. 2009;163(1):339–351. doi: 10.1016/j.neuroscience.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Seib TB, et al. Inescapable versus escapable shock modulates long-term potentiation in the rat hippocampus. Science. 1989;244(4901):224–226. doi: 10.1126/science.2704997. [DOI] [PubMed] [Google Scholar]
- Silberman Y, Matthews RT, et al. A corticotropin releasing factor pathway for ethanol regulation of the ventral tegmental area in the bed nucleus of the stria terminalis. J Neurosci. 2013;33(3):950–960. doi: 10.1523/JNEUROSCI.2949-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman AJ, Hoffman DL, et al. The descending afferent connections of the paraventricular nucleus of the hypothalamus (PVN) Brain Res Bull. 1981;6(1):47–61. doi: 10.1016/s0361-9230(81)80068-8. [DOI] [PubMed] [Google Scholar]
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology. 2001;158(4):343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- St Onge JR, Stopper CM, et al. Separate prefrontal-subcortical circuits mediate different components of risk-based decision making. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2012;32(8):2886–2899. doi: 10.1523/JNEUROSCI.5625-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Sparta DR, et al. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature. 2011;475(7356):377–380. doi: 10.1038/nature10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- van Vulpen EH, Verwer RW. Organization of projections from the mediodorsal nucleus of the thalamus to the basolateral complex of the amygdala in the rat. Brain research. 1989;500(1-2):389–394. doi: 10.1016/0006-8993(89)90337-5. [DOI] [PubMed] [Google Scholar]
- van Zessen R, Phillips JL, et al. Activation of VTA GABA neurons disrupts reward consumption. Neuron. 2012;73(6):1184–1194. doi: 10.1016/j.neuron.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Davis M. Double dissociation between the involvement of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in startle increases produced by conditioned versus unconditioned fear. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1997;17(23):9375–9383. doi: 10.1523/JNEUROSCI.17-23-09375.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Davis M. Role of the extended amygdala in short-duration versus sustained fear: a tribute to Dr. Lennart Heimer. Brain structure & function. 2008;213(1-2):29–42. doi: 10.1007/s00429-008-0183-3. [DOI] [PubMed] [Google Scholar]
- Walker DL, Miles LA, et al. Selective participation of the bed nucleus of the stria terminalis and CRF in sustained anxiety-like versus phasic fear-like responses. Progress in neuro-psychopharmacology & biological psychiatry. 2009;33(8):1291–1308. doi: 10.1016/j.pnpbp.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Fang Q, et al. Region-specific effects of brain corticotropin-releasing factor receptor type 1 blockade on footshock-stress- or drug-priming-induced reinstatement of morphine conditioned place preference in rats. Psychopharmacology. 2006;185(1):19–28. doi: 10.1007/s00213-005-0262-6. [DOI] [PubMed] [Google Scholar]
- Wang X, Cen X, et al. Noradrenaline in the bed nucleus of the stria terminalis is critical for stress-induced reactivation of morphine-conditioned place preference in rats. European journal of pharmacology. 2001;432(2-3):153–161. doi: 10.1016/s0014-2999(01)01487-x. [DOI] [PubMed] [Google Scholar]
- Watabe-Uchida M, Zhu L, et al. Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron. 2012;74(5):858–873. doi: 10.1016/j.neuron.2012.03.017. [DOI] [PubMed] [Google Scholar]
- Weiss F, Ciccocioppo R, et al. Compulsive drug-seeking behavior and relapse. Neuroadaptation, stress, and conditioning factors. Annals of the New York Academy of Sciences. 2001;937:1–26. doi: 10.1111/j.1749-6632.2001.tb03556.x. [DOI] [PubMed] [Google Scholar]
- Weiss F, Maldonado-Vlaar CS, et al. Control of cocaine-seeking behavior by drug-associated stimuli in rats: effects on recovery of extinguished operant-responding and extracellular dopamine levels in amygdala and nucleus accumbens. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(8):4321–4326. doi: 10.1073/pnas.97.8.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitlauf C, Egli RE, et al. High-frequency stimulation induces ethanol-sensitive long-term potentiation at glutamatergic synapses in the dorsolateral bed nucleus of the stria terminalis. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2004;24(25):5741–5747. doi: 10.1523/JNEUROSCI.1181-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells AM, Lasseter HC, et al. Interaction between the basolateral amygdala and dorsal hippocampus is critical for cocaine memory reconsolidation and subsequent drug context-induced cocaine-seeking behavior in rats. Learning & memory. 2011;18(11):693–702. doi: 10.1101/lm.2273111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitelaw RB, Markou A, et al. Excitotoxic lesions of the basolateral amygdala impair the acquisition of cocaine-seeking behaviour under a second-order schedule of reinforcement. Psychopharmacology. 1996;127(3):213–224. [PubMed] [Google Scholar]
- Wills TA, Klug JR, et al. GluN2B subunit deletion reveals key role in acute and chronic ethanol sensitivity of glutamate synapses in bed nucleus of the stria terminalis. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(5):E278–287. doi: 10.1073/pnas.1113820109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y, Driscoll JR, et al. Nucleus accumbens medium spiny neurons target non-dopaminergic neurons in the ventral tegmental area. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31(21):7811–7816. doi: 10.1523/JNEUROSCI.1504-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Anwyl R, et al. Behavioural stress facilitates the induction of long-term depression in the hippocampus. Nature. 1997;387(6632):497–500. doi: 10.1038/387497a0. [DOI] [PubMed] [Google Scholar]
- Yeoh JW, James MH, et al. Cocaine potentiates excitatory drive in the perifornical/lateral hypothalamus. J Physiol. 2012;590(Pt 16):3677–3689. doi: 10.1113/jphysiol.2012.230268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, McCormack S, et al. Afferents to the orexin neurons of the rat brain. J Comp Neurol. 2006;494(5):845–861. doi: 10.1002/cne.20859. [DOI] [PMC free article] [PubMed] [Google Scholar]